Precision Medicine Advances in Chronic Lung Diseases
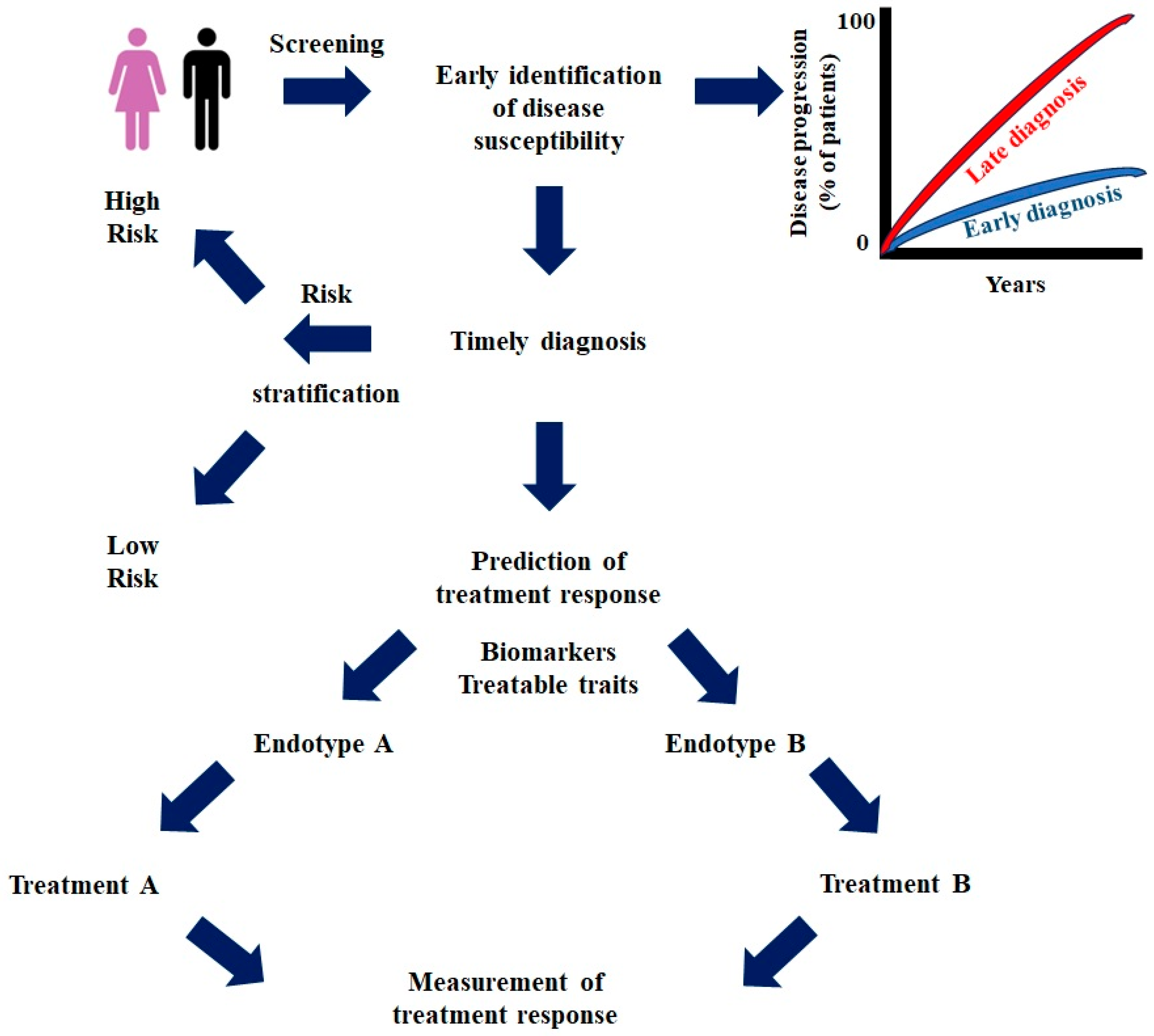
1. Introduction
2. Lung Cancer
3. Bronchial Asthma
4. Chronic Obstructive Pulmonary Disease
5. Bronchiectasis
6. Interstitial Lung Diseases
7. Future Perspectives and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Apalowo, O.E.; Walt, H.K.; Alaba, T.E.; Komakech, J.J.; Schilling, M.W. Exploring the Potential Roles of SLC39A8 and POC5 Missense Variants in the Association Between Body Composition, Beverage Consumption, and Chronic Lung Diseases: A Two-Sample Mendelian Randomization Study. Int. J. Mol. Sci. 2025, 26, 7799. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wu, K.H.; Guo, B.C.; Lin, W.Y.; Chang, Y.J.; Wei, C.W.; Lin, M.J.; Wu, H.P. Personalized Medicine in Severe Asthma: From Biomarkers to Biologics. Int. J. Mol. Sci. 2023, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Voutidou, S.; Eleftheriadis, D.; Drakopanagiotakis, F.; Papanikolaou, I.C.; Steiropoulos, P. Pathogenetic Mechanisms Linking Sarcoidosis to Lymphoma. Int. J. Mol. Sci. 2025, 26, 594. [Google Scholar] [CrossRef] [PubMed]
- Precision Medicine. Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/precision-medicine (accessed on 1 November 2025).
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef]
- Jeon, H.; Wang, S.; Song, J.; Gill, H.; Cheng, H. Update 2025: Management of Non-Small-Cell Lung Cancer. Lung 2025, 203, 53. [Google Scholar] [CrossRef] [PubMed]
- Su, P.L.; Furuya, N.; Asrar, A.; Rolfo, C.; Li, Z.; Carbone, D.P.; He, K. Recent advances in therapeutic strategies for non-small cell lung cancer. J. Hematol. Oncol. 2025, 18, 35. [Google Scholar] [CrossRef]
- van der Leest, P.; Rozendal, P.; Rifaela, N.; van der Wekken, A.J.; Kievit, H.; de Jager, V.D.; Sidorenkov, G.; van Kempen, L.C.; Hiltermann, T.J.N.; Schuuring, E. Detection of actionable mutations in circulating tumor DNA for non-small cell lung cancer patients. Commun. Med. 2025, 5, 204. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohe, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Wu, Y.L.; Dziadziuszko, R.; Ahn, J.S.; Barlesi, F.; Nishio, M.; Lee, D.H.; Lee, J.S.; Zhong, W.; Horinouchi, H.; Mao, W.; et al. Alectinib in Resected ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 1265–1276. [Google Scholar] [CrossRef]
- Bouchard, N.; Daaboul, N. Lung Cancer: Targeted Therapy in 2025. Curr. Oncol. 2025, 32, 146. [Google Scholar] [CrossRef]
- Zanchetta, C.; De Marchi, L.; Macerelli, M.; Pelizzari, G.; Costa, J.; Aprile, G.; Cortiula, F. Antibody-Drug Conjugates in Non-Small Cell Lung Cancer: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2024, 26, 221. [Google Scholar] [CrossRef]
- Khadela, A.; Megha, K.; Shah, V.B.; Soni, S.; Shah, A.C.; Mistry, H.; Bhatt, S.; Merja, M. Exploring the Potential of Antibody-Drug Conjugates in Targeting Non-Small Cell Lung Cancer Biomarkers. Clin. Med. Insights Oncol. 2024, 18, 11795549241260534. [Google Scholar] [CrossRef]
- Cho, B.C.; Lu, S.; Felip, E.; Spira, A.I.; Girard, N.; Lee, J.S.; Lee, S.H.; Ostapenko, Y.; Danchaivijitr, P.; Liu, B.; et al. Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2024, 391, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.; Singh, H.; Larkins, E.; Drezner, N.; Ricciuti, B.; Mishra-Kalyani, P.; Tang, S.; Beaver, J.A.; Awad, M.M. Impact of Increasing PD-L1 Levels on Outcomes to PD-1/PD-L1 Inhibition in Patients with NSCLC: A Pooled Analysis of 11 Prospective Clinical Trials. Oncologist 2024, 29, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.D.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Wang, C.; Lu, S.; Felip, E.; Swanson, S.J.; Brahmer, J.R.; et al. Overall Survival with Neoadjuvant Nivolumab plus Chemotherapy in Lung Cancer. N. Engl. J. Med. 2025, 393, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Severe Adult Asthmas: Integrating Clinical Features, Biology, and Therapeutics to Improve Outcomes. Am. J. Respir. Crit. Care Med. 2021, 203, 809–821. [Google Scholar] [CrossRef]
- Corren, J.; Casale, T.; Deniz, Y.; Ashby, M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J. Allergy Clin. Immunol. 2003, 111, 87–90. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkstrom, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Siergiejko, Z.; Swiebocka, E.; Smith, N.; Peckitt, C.; Leo, J.; Peachey, G.; Maykut, R. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Curr. Med. Res. Opin. 2011, 27, 2223–2228. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Georas, S.N.; Khurana, S. Biologics in severe asthma: A state-of-the-art review. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2025, 34, 240088. [Google Scholar] [CrossRef]
- Seluk, L.; Davis, A.E.; Rhoads, S.; Wechsler, M.E. Novel asthma treatments: Advancing beyond approved novel step-up therapies for asthma. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2025, 134, 9–18. [Google Scholar] [CrossRef]
- Bourdin, A.; Brusselle, G.; Couillard, S.; Fajt, M.L.; Heaney, L.G.; Israel, E.; McDowell, P.J.; Menzies-Gow, A.; Martin, N.; Mitchell, P.D.; et al. Phenotyping of Severe Asthma in the Era of Broad-Acting Anti-Asthma Biologics. J. Allergy Clin. Immunol. Pract. 2024, 12, 809–823. [Google Scholar] [CrossRef]
- Maglio, A.; Vitale, C.; Pelaia, C.; D’Amato, M.; Ciampo, L.; Sferra, E.; Molino, A.; Pelaia, G.; Vatrella, A. Severe Asthma Remissions Induced by Biologics Targeting IL5/IL5r: Results from a Multicenter Real-Life Study. Int. J. Mol. Sci. 2023, 24, 2455. [Google Scholar] [CrossRef] [PubMed]
- Sheng-Kai Ma, K.; Brumbaugh, B.; Saff, R.R.; Phipatanakul, W.; Yun-Chen Tsai, S.; Westmeijer, M.; Holt, A.; Ebriani, J.; Camargo, C.A., Jr.; Chen, S.T. Dupilumab and lymphoma risk among patients with asthma: A population-based cohort study. Eur. Respir. J. 2025, 66, 2500139. [Google Scholar] [CrossRef]
- Dupin, C.; Valéry, S.; Guilleminault, L.; Devouassoux, G.; Merveilleau, M.; Russier, M.; Mourin, G.; Pradelli, J.; Bonniaud, P.; Le Brun, M.; et al. Articular manifestations related to anti-interleukin-5 therapies in severe asthma: A case series. ERJ Open Res. 2024, 10, 00935-2023. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Gogali, A.; Bartziokas, K.; Kostikas, K. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. 2021, 7, 00309-2020. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, A.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Colice, G.; Griffiths, J.M.; Almqvist, G.; Skarby, T.; Piechowiak, T.; Kaur, P.; Bowen, K.; Hellqvist, A.; Mo, M.; et al. SOURCE: A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir. Res. 2020, 21, 264. [Google Scholar] [CrossRef]
- Cosio, B.G.; Iglesias, A.; Shafiek, H.; Mosteiro, M.; Escribano, I.; Toledo-Pons, N.; Valera, J.L.; Gomez Bellvert, C.; Perez de Llano, L. The Role of Bronchial Biopsy in the Prediction of Response to Biologic Therapy in Severe Uncontrolled Asthma: A Prospective Study. Chest 2025, 167, 945–955. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Calverley, P.M.A.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Hurst, J.R.; Miravitlles, M.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Prevention of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2017, 50, 1602265. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; Patel, N.; et al. Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. N. Engl. J. Med. 2024, 390, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med. 2023, 389, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Sciurba, F.C.; Criner, G.J.; Christenson, S.A.; Martinez, F.J.; Papi, A.; Roche, N.; Bourbeau, J.; Korn, S.; Bafadhel, M.; Han, M.K.; et al. Mepolizumab to Prevent Exacerbations of COPD with an Eosinophilic Phenotype. N. Engl. J. Med. 2025, 392, 1710–1720. [Google Scholar] [CrossRef]
- Efficacy and Safety of Benralizumab in Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COPD) with a History of Frequent Exacerbations (RESOLUTE). Available online: https://clinicaltrials.gov/study/NCT04053634 (accessed on 19 April 2025).
- Long-Term Efficacy and Safety of Tozorakimab in Participants with Chronic Obstructive Pulmonary Disease with a History of Exacerbations (PROSPERO). (PROSPERO). Available online: https://clinicaltrials.gov/study/NCT05742802 (accessed on 18 April 2025).
- Rabe, K.F.; Martinez, F.J.; Bhatt, S.P.; Kawayama, T.; Cosio, B.G.; Mroz, R.M.; Boomsma, M.M.; Goulaouic, H.; Nivens, M.C.; Djandji, M.; et al. AERIFY-1/2: Two phase 3, randomised, controlled trials of itepekimab in former smokers with moderate-to-severe COPD. ERJ Open Res. 2024, 10, 00718-2023. [Google Scholar] [CrossRef]
- A Study to Evaluate Astegolimab in Participants with Chronic Obstructive Pulmonary Disease (ARNASA). Available online: https://clinicaltrials.gov/study/NCT05595642 (accessed on 18 April 2025).
- A Study to Evaluate the Long-Term Safety of Astegolimab in Participants with Chronic Obstructive Pulmonary Disease (COPD). Available online: https://clinicaltrials.gov/study/NCT05878769 (accessed on 18 April 2025).
- Anzueto, A.; Barjaktarevic, I.Z.; Siler, T.M.; Rheault, T.; Bengtsson, T.; Rickard, K.; Sciurba, F. Ensifentrine, a Novel Phosphodiesterase 3 and 4 Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease: Randomized, Double-Blind, Placebo-controlled, Multicenter Phase III Trials (the ENHANCE Trials). Am. J. Respir. Crit. Care Med. 2023, 208, 406–416. [Google Scholar] [CrossRef]
- Dransfield, M.; Marchetti, N.; Kalhan, R.; Reyner, D.; Dixon, A.L.; Rheault, T.; Rickard, K.A.; Anzueto, A. Ensifentrine in COPD patients taking long-acting bronchodilators: A pooled post-hoc analysis of the ENHANCE-1/2 studies. Chronic Respir. Dis. 2025, 22, 14799731251314874. [Google Scholar] [CrossRef]
- Mahler, D.A.; Bhatt, S.P.; Rheault, T.; Reyner, D.; Bengtsson, T.; Dixon, A.; Rickard, K.; Singh, D. Effect of ensifentrine on dyspnea in patients with moderate-to-severe chronic obstructive pulmonary disease: Pooled analysis of the ENHANCE trials. Expert Rev. Respir. Med. 2024, 18, 645–654. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Calverley, P.M.; Rabe, K.F. Roflumilast: A review of its use in the treatment of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 81–90. [Google Scholar] [CrossRef]
- Matera, M.G.; Cazzola, M.; Page, C. Prospects for COPD treatment. Curr. Opin. Pharmacol. 2021, 56, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bakakos, A.; Sotiropoulou, Z.; Anagnostopoulos, N.; Vontetsianos, A.; Cholidou, K.; Papaioannou, A.I.; Bartziokas, K. Anti-inflammatory agents for the management of COPD-Quo Vadis? Respir. Med. 2025, 248, 108396. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A., Jr.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C.; et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 365, 689–698, Correction in N. Engl. J. Med. 2012, 366, 1356. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Russell, R.E.K.; Mahmood, H.R.; Krassowska, K.; Melhorn, J.; Mwasuku, C.; Pavord, I.D.; Bermejo-Sanchez, L.; Howell, I.; Mahdi, M.; et al. Treating eosinophilic exacerbations of asthma and COPD with benralizumab (ABRA): A double-blind, double-dummy, active placebo-controlled randomised trial. Lancet Respir. Med. 2025, 13, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Raboso, B.; Pou, C.; Abril, R.; Erro, M.; Sanchez, C.; Manzano, C.; Zamarron, E.; Suarez-Cuartin, G.; Gonzalez, J. Bronchiectasis. Open Respir. Arch. 2024, 6, 100339. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Burgel, P.R.; Daley, C.L.; De Soyza, A.; Haworth, C.S.; Mauger, D.; Loebinger, M.R.; McShane, P.J.; Ringshausen, F.C.; Blasi, F.; et al. Phase 3 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N. Engl. J. Med. 2025, 392, 1569–1581. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Haworth, C.S.; Flume, P.; Long, M.B.; Burgel, P.R.; Dimakou, K.; Blasi, F.; Herrero-Cortina, B.; Dhar, R.; Chotirmall, S.H.; et al. European Respiratory Society Clinical Practice Guideline for the Management of Adult Bronchiectasis. Eur. Respir. J. 2025; 2501126, online ahead of print. [Google Scholar] [CrossRef]
- Chotirmall, S.H.; Chalmers, J.D. The Precision Medicine Era of Bronchiectasis. Am. J. Respir. Crit. Care Med. 2024, 210, 24–34. [Google Scholar] [CrossRef]
- Karampitsakos, T.; Tourki, B.; Herazo-Maya, J.D. The Dawn of Precision Medicine in Fibrotic Interstitial Lung Disease. Chest 2024, 167, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Koppelman, G.H. Biologic Therapies for Severe Asthma. N. Engl. J. Med. 2022, 386, 157–171. [Google Scholar] [CrossRef]
- Karampitsakos, T.; Juan-Guardela, B.M.; Tzouvelekis, A.; Herazo-Maya, J.D. Precision medicine advances in idiopathic pulmonary fibrosis. EBioMedicine 2023, 95, 104766. [Google Scholar] [CrossRef]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 2011, 364, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.D.; Lee, J.S.; Kozlitina, J.; Noth, I.; Devine, M.S.; Glazer, C.S.; Torres, F.; Kaza, V.; Girod, C.E.; Jones, K.D.; et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: An observational cohort study with independent validation. Lancet Respir. Med. 2014, 2, 557–565. [Google Scholar] [CrossRef]
- Noth, I.; Zhang, Y.; Ma, S.F.; Flores, C.; Barber, M.; Huang, Y.; Broderick, S.M.; Wade, M.S.; Hysi, P.; Scuirba, J.; et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: A genome-wide association study. The Lancet. Respir. Med. 2013, 1, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Herazo-Maya, J.D.; Sun, J.; Molyneaux, P.L.; Li, Q.; Villalba, J.A.; Tzouvelekis, A.; Lynn, H.; Juan-Guardela, B.M.; Risquez, C.; Osorio, J.C.; et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: An international, multicentre, cohort study. Lancet Respir. Med. 2017, 5, 857–868. [Google Scholar] [CrossRef]
- Scott, M.K.D.; Quinn, K.; Li, Q.; Carroll, R.; Warsinske, H.; Vallania, F.; Chen, S.; Carns, M.A.; Aren, K.; Sun, J.; et al. Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: A retrospective, multicentre cohort study. Lancet Respir. Med. 2019, 7, 497–508. [Google Scholar] [CrossRef]
- Karampitsakos, T.; Torrisi, S.; Antoniou, K.; Manali, E.; Korbila, I.; Papaioannou, O.; Sampsonas, F.; Katsaras, M.; Vasarmidi, E.; Papakosta, D.; et al. Increased monocyte count and red cell distribution width as prognostic biomarkers in patients with Idiopathic Pulmonary Fibrosis. Respir. Res. 2021, 22, 140. [Google Scholar] [CrossRef]
- Kreuter, M.; Lee, J.S.; Tzouvelekis, A.; Oldham, J.M.; Molyneaux, P.L.; Weycker, D.; Atwood, M.; Kirchgaessler, K.U.; Maher, T.M. Monocyte Count as a Prognostic Biomarker in Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Adegunsoye, A.; Oldham, J.M.; Kozlitina, J.; Garcia, N.; Poonawalla, M.; Strykowski, R.; Linderholm, A.L.; Ley, B.; Ma, S.-F.; et al. Telomere Length and Immunosuppression in Non-Idiopathic Pulmonary Fibrosis Interstitial Lung Disease. Eur. Respir. J. 2023, 62, 2300441. [Google Scholar] [CrossRef]
- Oldham, J.M.; Ma, S.F.; Martinez, F.J.; Anstrom, K.J.; Raghu, G.; Schwartz, D.A.; Valenzi, E.; Witt, L.; Lee, C.; Vij, R.; et al. TOLLIP, MUC5B, and the Response to N-Acetylcysteine among Individuals with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Kim, J.S.; Cooper, C.B.; Lasky, J.A.; Murray, S.; Oldham, J.M.; Raghu, G.; Flaherty, K.R.; Spino, C.; Noth, I.; et al. Design and rationale for the prospective treatment efficacy in IPF using genotype for NAC selection (PRECISIONS) clinical trial. BMC Pulm. Med. 2022, 22, 475. [Google Scholar] [CrossRef]
- Whalen, W.; Berger, K.; Kim, J.S.; Simmons, W.; Ma, S.F.; Kaner, R.J.; Martinez, F.J.; Anstrom, K.J.; Parfrey, H.; Maher, T.M.; et al. TOLLIP SNP and Antimicrobial Treatment Effect in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2024, 210, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.; Sun, H.; Gulati, M.; Herazo-Maya, J.D.; Chen, Y.; Osafo-Addo, A.; Brandsdorfer, C.; Winkler, J.; Blaul, C.; Faunce, J.; et al. Extracellular Mitochondrial DNA Is Generated by Fibroblasts and Predicts Death in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 1571–1581. [Google Scholar] [CrossRef]
- Tzilas, V.; Bouros, D.; Ryu, J.H. In pursuit of personalized medicine in fibrotic interstitial lung diseases. Divide and conquer. Pulmonology 2024, 30, 101–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakakos, A.; Tzilas, V.; Loukides, S.; Bakakos, P.; Karampitsakos, T. Precision Medicine Advances in Chronic Lung Diseases. Int. J. Mol. Sci. 2025, 26, 11243. https://doi.org/10.3390/ijms262311243
Bakakos A, Tzilas V, Loukides S, Bakakos P, Karampitsakos T. Precision Medicine Advances in Chronic Lung Diseases. International Journal of Molecular Sciences. 2025; 26(23):11243. https://doi.org/10.3390/ijms262311243
Chicago/Turabian StyleBakakos, Agamemnon, Vasilios Tzilas, Stelios Loukides, Petros Bakakos, and Theodoros Karampitsakos. 2025. "Precision Medicine Advances in Chronic Lung Diseases" International Journal of Molecular Sciences 26, no. 23: 11243. https://doi.org/10.3390/ijms262311243
APA StyleBakakos, A., Tzilas, V., Loukides, S., Bakakos, P., & Karampitsakos, T. (2025). Precision Medicine Advances in Chronic Lung Diseases. International Journal of Molecular Sciences, 26(23), 11243. https://doi.org/10.3390/ijms262311243





