“Feeding the Rhythm”—Effects of Food and Nutrients on Daily Cortisol Secretion: From Molecular Mechanisms to Clinical Impact
Abstract
1. Introduction
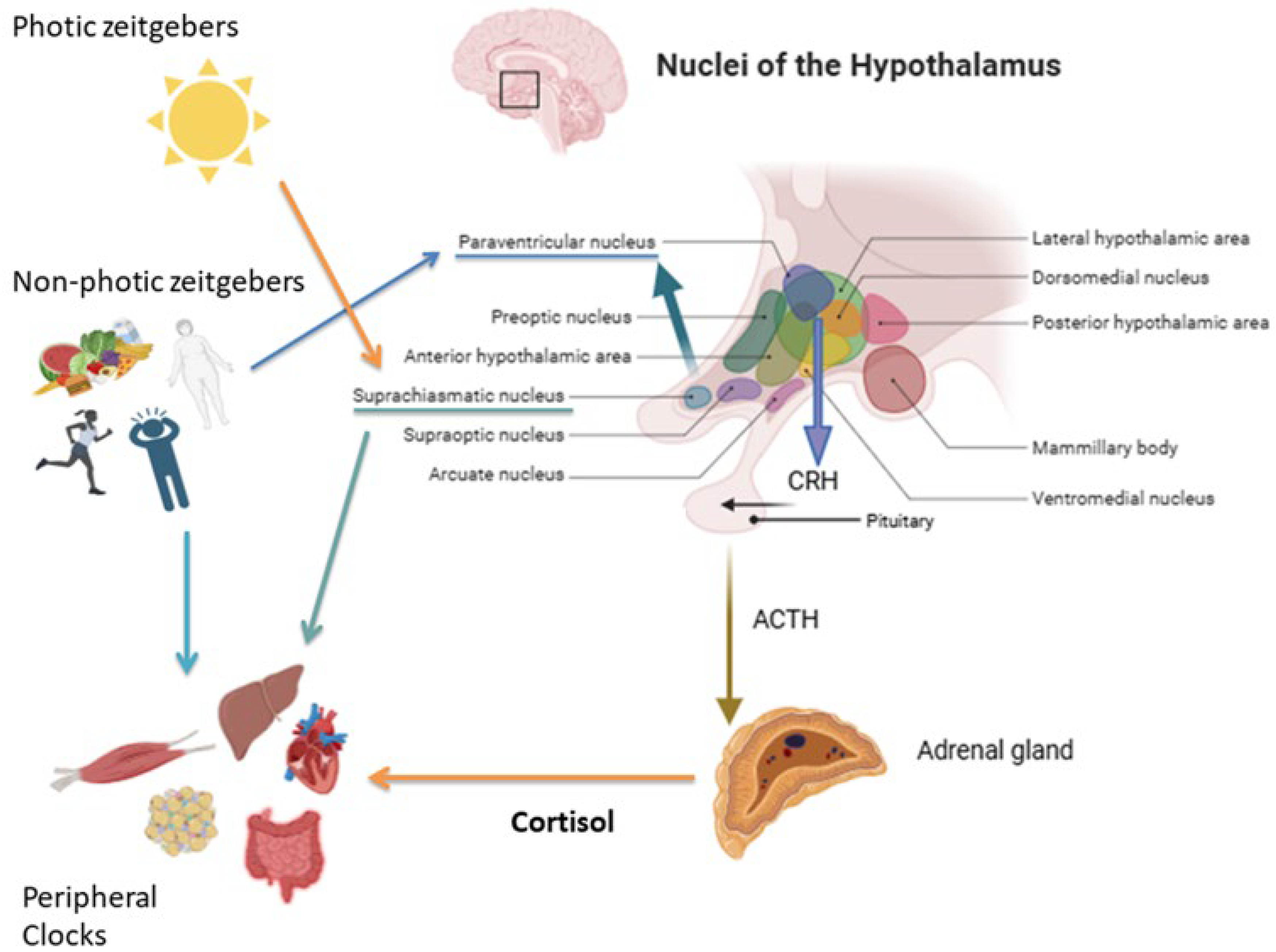
2. Cortisol Daily Rhythm: Physiological Mechanisms
2.1. Neuroendocrine Regulation of Cortisol Secretion
2.2. Role of Nutrients on Clock Genes Expression
2.3. Physical Exercise: The Timing of Physical Activity in the Regulation of HPA Axis
3. Nutritional Regulation of Cortisol Secretion
3.1. Effects of Meal Timing and Frequency on Cortisol Secretion (Chrononutrition)
3.2. Nutrient Composition and HPA Axis Activity
3.3. Dietary Pattern and Daily Cortisol Secretion
3.4. Role of Gut Microbiota in Modulating Cortisol Rhythms
4. Disruption of Cortisol Secretion Rhythm in Human Diseases
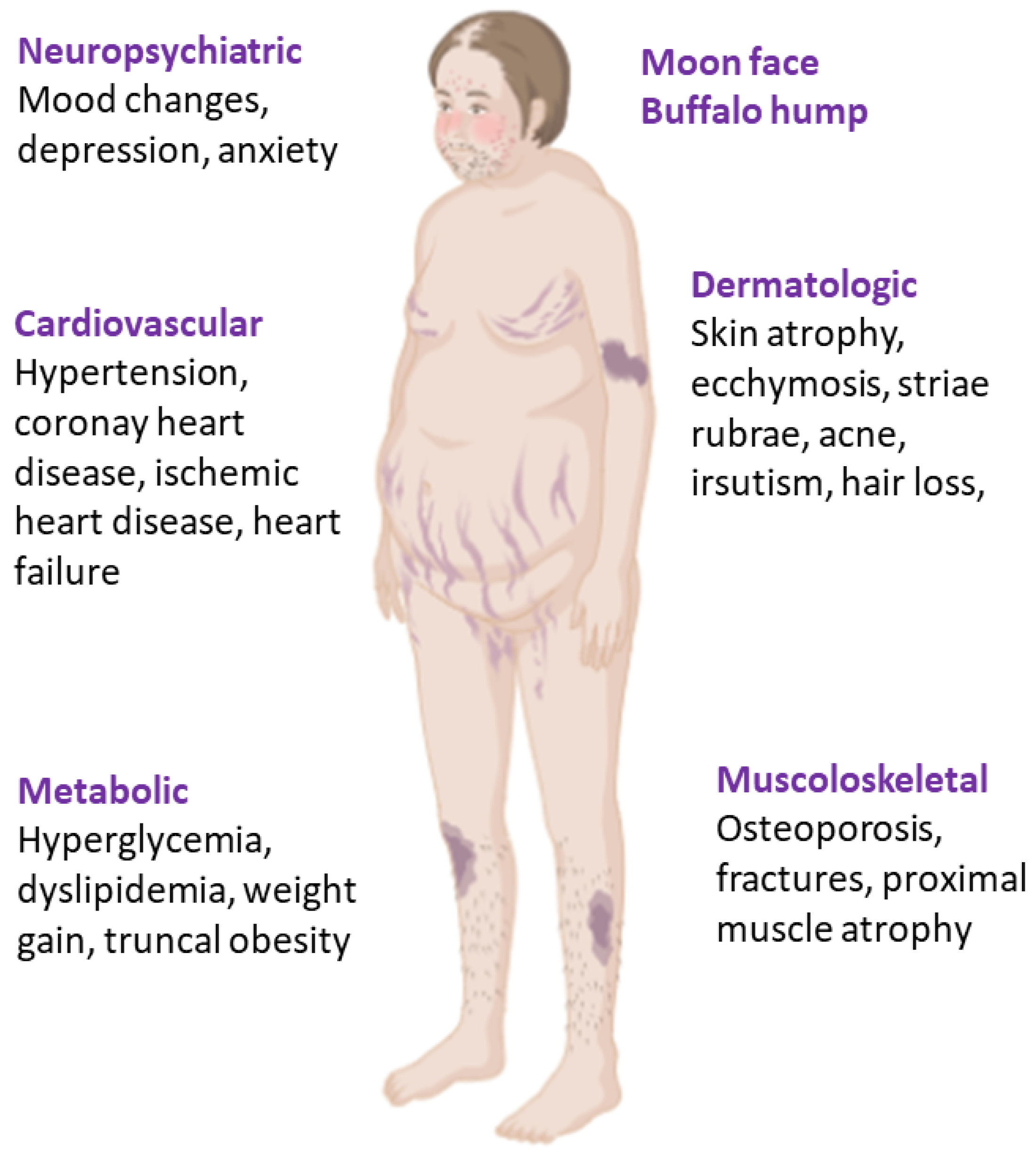
4.1. Endogenous Cushing’s Syndrome
4.2. Metabolic Syndrome
4.3. Psychiatric and Behavioral Disorders
4.4. Cortisol Daily Rhythm Disruption in Shift Workers and Chronic Jet Lag
5. Strategies for Cortisol Rhythm Restoration
5.1. Personalized Nutritional Suggestions: Practical Considerations
5.2. Pharmacological Modulation on HPA Axis
5.2.1. Drugs Targeting Cortisol Secretion
5.2.2. Drugs Acting on Metabolic Complications and Supplements
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halberg, F. Physiologic 24-hour periodicity; general and procedural considerations with reference to the adrenal cycle. Int. Z. Vitaminforsch. Beih. 1959, 10, 225–296. [Google Scholar] [PubMed]
- Mohd Azmi, N.A.S.; Juliana, N.; Azmani, S.; Mohd Effendy, N.; Abu, I.F.; Mohd Fahmi Teng, N.I.; Das, S. Cortisol on circadian rhythm and its effect on cardiovascular system. Int. J. Environ. Res. Public Health 2021, 18, 676. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.C.; Wood, S.A.; Kershaw, Y.M.; Bate, E.; Lightman, S.L. Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. J. Neuroendocrinol. 2006, 18, 526–533. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Owens, J.A.; Voultsios, A.; Boden, M.J.; Varcoe, T.J. Metabolic homeostasis in mice with disrupted clock gene expression in peripheral tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1528–R1537. [Google Scholar] [CrossRef]
- Murray, A.; Tharmalingam, S.; Khurana, S.; Lalonde, C.; Nguyen, P.; Tai, T.C. Effect of prenatal glucocorticoid exposure on circadian rhythm gene expression in the brains of adult rat offspring. Cells 2022, 11, 1613. [Google Scholar] [CrossRef]
- Weaver, D.R. Introduction to circadian rhythms and mechanisms of circadian oscillations. In Circadian Clocks: Role in Health and Disease; Gumz, M.L., Ed.; Springer: New York, NY, USA, 2016; pp. 1–55. [Google Scholar]
- Bussell, K. Keeping time. Nat. Rev. Mol. Cell Biol. 2003, 4, 90. [Google Scholar] [CrossRef]
- Begemann, K.; Rawashdeh, O.; Olejniczak, I.; Pilorz, V.; de Assis, L.V.M.; Osorio-Mendoza, J.; Oster, H. Endocrine regulation of circadian rhythms. npj Biol. Timing Sleep 2025, 2, 10. [Google Scholar] [CrossRef]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of acth and cortisol secretion and implications for disease. Endocr. Rev. 2020, 41, bnaa002. [Google Scholar] [CrossRef]
- Quante, M.; Mariani, S.; Weng, J.; Marinac, C.R.; Kaplan, E.R.; Rueschman, M.; Mitchell, J.A.; James, P.; Hipp, J.A.; Cespedes Feliciano, E.M.; et al. Zeitgebers and their association with rest-activity patterns. Chronobiol. Int. 2019, 36, 203–213. [Google Scholar] [CrossRef]
- Son, G.H.; Chung, S.; Kim, K. The adrenal peripheral clock: Glucocorticoid and the circadian timing system. Front. Neuroendocrinol. 2011, 32, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Minnetti, M.; Hasenmajer, V.; Pofi, R.; Venneri, M.A.; Alexandraki, K.I.; Isidori, A.M. Fixing the broken clock in adrenal disorders: Focus on glucocorticoids and chronotherapy. J. Endocrinol. 2020, 246, R13–R31. [Google Scholar] [CrossRef] [PubMed]
- Focke, C.M.B.; Iremonger, K.J. Rhythmicity matters: Circadian and ultradian patterns of hpa axis activity. Mol. Cell. Endocrinol. 2020, 501, 110652. [Google Scholar] [CrossRef] [PubMed]
- Charmandari, E.; Chrousos, G.P.; Lambrou, G.I.; Pavlaki, A.; Koide, H.; Ng, S.S.; Kino, T. Peripheral clock regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS ONE 2011, 6, e25612. [Google Scholar] [CrossRef]
- Nader, N.; Chrousos, G.P.; Kino, T. Circadian rhythm transcription factor clock regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. FASEB J. 2009, 23, 1572–1583. [Google Scholar] [CrossRef]
- Stavreva, D.A.; Wiench, M.; John, S.; Conway-Campbell, B.L.; McKenna, M.A.; Pooley, J.R.; Johnson, T.A.; Voss, T.C.; Lightman, S.L.; Hager, G.L. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat. Cell Biol. 2009, 11, 1093–1102. [Google Scholar] [CrossRef]
- Reddy, T.E.; Gertz, J.; Crawford, G.E.; Garabedian, M.J.; Myers, R.M. The hypersensitive glucocorticoid response specifically regulates period 1 and expression of circadian genes. Mol. Cell Biol. 2012, 32, 3756–3767. [Google Scholar] [CrossRef]
- Lehmann, M.; Haury, K.; Oster, H.; Astiz, M. Circadian glucocorticoids throughout development. Front. Neurosci. 2023, 17, 1165230. [Google Scholar] [CrossRef]
- Andreadi, A.; Andreadi, S.; Todaro, F.; Ippoliti, L.; Bellia, A.; Magrini, A.; Chrousos, G.P.; Lauro, D. Modified cortisol circadian rhythm: The hidden toll of night-shift work. Int. J. Mol. Sci. 2025, 26, 2090. [Google Scholar] [CrossRef]
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes. Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.K. Feeding rhythms and the circadian regulation of metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.S.; Ratcliffe, W.F.; Grenett, M.H.; Brewer, R.A.; Gamble, K.L.; Young, M.E. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int. J. Obes. 2013, 37, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef]
- Oike, H. Modulation of circadian clocks by nutrients and food factors. Biosci. Biotechnol. Biochem. 2017, 81, 863–870. [Google Scholar] [CrossRef]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef]
- Gangitano, E.; Gnessi, L.; Lenzi, A.; Ray, D. Chronobiology and metabolism: Is ketogenic diet able to influence circadian rhythm? Front. Neurosci. 2021, 15, 756970. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 2016, 65, 714–727. [Google Scholar] [CrossRef]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal frequency and timing are associated with changes in body mass index in adventist health study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar] [CrossRef]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Night eating syndrome and nocturnal snacking: Association with obesity, binge eating and psychological distress. Int. J. Obes. 2007, 31, 1722–1730. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Okano, T.; Kokame, K.; Shirotani-Ikejima, H.; Miyata, T.; Fukada, Y. Glucose down-regulates per1 and per2mrna levels and induces circadian gene expression in cultured rat-1 fibroblasts. J. Biol. Chem. 2002, 277, 44244–44251. [Google Scholar] [CrossRef]
- Garaulet, M.; Smith, C.E.; Gomez-Abellan, P.; Ordovas-Montanes, M.; Lee, Y.C.; Parnell, L.D.; Arnett, D.K.; Ordovas, J.M. Rev-erb-alpha circadian gene variant associates with obesity in two independent populations: Mediterranean and North American. Mol. Nutr. Food Res. 2014, 58, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Camblor Murube, M.; Borregon-Rivilla, E.; Colmenarejo, G.; Aguilar-Aguilar, E.; Martinez, J.A.; Ramirez De Molina, A.; Reglero, G.; Loria-Kohen, V. Polymorphism of clock gene rs3749474 as a modulator of the circadian evening carbohydrate intake impact on nutritional status in an adult sample. Nutrients 2020, 12, 1142. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Rodríguez-Barranco, M.; Alcalá-Santiago, Á.; Gálvez-Navas, J.M.; Huerta, J.M.; Amiano, P.; Lasheras, C.; Moreno-Iribas, C.; Jimenez-Zabala, A.; Chirlaque, M.D.; et al. Nutritional profile of the diet according to circadian clock genes in the european prospective investigation into cancer and nutrition (epic) chronodiet study. Clin. Nutr. 2025, 49, 165–177. [Google Scholar] [CrossRef]
- Mennitti, C.; Farina, G.; Imperatore, A.; De Fonzo, G.; Gentile, A.; La Civita, E.; Carbone, G.; De Simone, R.R.; Di Iorio, M.R.; Tinto, N.; et al. How Does Physical Activity Modulate Hormone Responses? Biomolecules 2024, 14, 1418. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Hypothalamic-pituitary-adrenal (hpa) axis functioning in overtraining syndrome: Findings from endocrine and metabolic responses on overtraining syndrome (eros)-eros-hpa axis. Sports Med. Open 2017, 3, 45. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Weltman, J.Y.; Pieper, K.S.; Weltman, A.; Hartman, M.L. Cortisol and growth hormone responses to exercise at different times of day. J. Clin. Endocrinol. Metab. 2001, 86, 2881–2889. [Google Scholar] [CrossRef]
- Kim, N.; Ka, S.; Park, J. Effects of exercise timing and intensity on physiological circadian rhythm and sleep quality: A systematic review. Phys. Act. Nutr. 2023, 27, 52–63. [Google Scholar] [CrossRef]
- Morales-Palomo, F.; Moreno-Cabanas, A.; Alvarez-Jimenez, L.; Mora-Gonzalez, D.; Ortega, J.F.; Mora-Rodriguez, R. Efficacy of morning versus afternoon aerobic exercise training on reducing metabolic syndrome components: A randomized controlled trial. J. Physiol. 2024, 602, 6463–6477. [Google Scholar] [CrossRef]
- Augsburger, G.R.; Sobolewski, E.J.; Escalante, G.; Graybeal, A.J. Circadian regulation for optimizing sport and exercise performance. Clocks Sleep 2025, 7, 18. [Google Scholar] [CrossRef]
- Arroyo Tardio, P.; Baldini, G.; Seelig, E. Food-induced cortisol secretion is comparable in lean and obese male subjects. Endocr. Connect. 2023, 12, e230126. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Panda, S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Beretoulis, S.; Deere, A.; Radenkovic, D. The window matters: A systematic review of time restricted eating strategies in relation to cortisol and melatonin secretion. Nutrients 2021, 13, 2525. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef]
- Gibson, E.L.; Checkley, S.; Papadopoulos, A.; Poon, L.; Daley, S.; Wardle, J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom. Med. 1999, 61, 214–224. [Google Scholar] [CrossRef]
- Bogdan, A.; Bouchareb, B.; Touitou, Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal-time as a synchronizer in humans? Life Sci. 2001, 68, 1607–1615. [Google Scholar] [CrossRef]
- Vasaghi-Gharamaleki, B.; Mirzaii-Dizgah, I. Unstimulated whole saliva cortisol levels during ramadan in iranian muslims. J. Contemp. Dent. Pract. 2014, 15, 341–344. [Google Scholar] [CrossRef]
- Brini, S.; Marzouki, H.; Ouerghi, N.; Ouergui, I.; Castagna, C.; Bouassida, A. Effects of ramadan observance combined with two training programs on plasma lipids and testosterone/cortisol ratio in male senior basketball players. Med. Dello Sport 2019, 72, 47–58. [Google Scholar] [CrossRef]
- Witbracht, M.; Keim, N.L.; Forester, S.; Widaman, A.; Laugero, K. Female breakfast skippers display a disrupted cortisol rhythm and elevated blood pressure. Physiol. Behav. 2015, 140, 215–221. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.J.; Mann, T.; Vinas, D.; Hunger, J.M.; Dejager, J.; Taylor, S.E. Low calorie dieting increases cortisol. Psychosom. Med. 2010, 72, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.T.; Tinsley, G.M.; Alesi, M.G.; Hester, G.M.; Olmos, A.A.; Serafini, P.R.; Modjeski, A.S.; Mangine, G.T.; King, K.; Savage, S.N.; et al. Four weeks of time-restricted feeding combined with resistance training does not differentially influence measures of body composition, muscle performance, resting energy expenditure, and blood biomarkers. Nutrients 2020, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Delarue, J.; Matzinger, O.; Binnert, C.; Schneiter, P.; Chioléro, R.; Tappy, L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003, 29, 289–295. [Google Scholar] [CrossRef]
- Abbasi, B.; Kimiagar, M.; Sadeghniiat, K.; Shirazi, M.M.; Hedayati, M.; Rashidkhani, B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J. Res. Med. Sci. 2012, 17, 1161–1169. [Google Scholar]
- Dhabhar, F.S. Stress-induced enhancement of cell-mediated immunity. Ann. N. Y. Acad. Sci. 1998, 840, 359–372. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Polito, R.; Messina, G.; Valenzano, A.; Scarinci, A.; Villano, I.; Monda, M.; Cibelli, G.; Porro, C.; Pisanelli, D.; Monda, V.; et al. The role of very low calorie ketogenic diet in sympathetic activation through cortisol secretion in male obese population. J. Clin. Med. 2021, 10, 4230. [Google Scholar] [CrossRef]
- Guarnotta, V.; Emanuele, F.; Amodei, R.; Giordano, C. Very low-calorie ketogenic diet: A potential application in the treatment of hypercortisolism comorbidities. Nutrients 2022, 14, 2388. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Camajani, E.; Sojat, A.S.; Marina, L.; Savastano, S.; Colao, A.; Caprio, M.; Muscogiuri, G. Effects of very low-calorie ketogenic diet on hypothalamic-pituitary-adrenal axis and renin-angiotensin-aldosterone system. J. Endocrinol. Investig. 2023, 46, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.K.; Packard, A.E.B.; Larson, K.R.; Stout, J.; Fourman, S.M.; Thompson, A.M.K.; Ludwick, K.; Habegger, K.M.; Stemmer, K.; Itoh, N.; et al. Dietary manipulations that induce ketosis activate the hpa axis in male rats and mice: A potential role for fibroblast growth factor-21. Endocrinology 2018, 159, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Sprague, J.E.; Arbeláez, A.M. Glucose counterregulatory responses to hypoglycemia. Pediatr. Endocrinol. Rev. PER 2011, 9, 463–475. [Google Scholar] [PubMed]
- Xiang, C.; Sun, Y.; Luo, Y.; Xie, C.; Huang, W.; Jones, K.L.; Horowitz, M.; Sun, Z.; Rayner, C.K.; Ma, J.; et al. Gastric emptying is slower in women than men with type 2 diabetes and impacts on postprandial glycaemia. Diabetes Obes. Metab. 2024, 26, 3119–3127. [Google Scholar] [CrossRef]
- Huang, W.; Xie, C.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Sex differences in the plasma glucagon responses to a high carbohydrate meal and a glucose drink in type 2 diabetes. Diabetes Res. Clin. Pract. 2024, 214, 111769. [Google Scholar] [CrossRef]
- Xie, C.; Huang, W.; Sun, Y.; Xiang, C.; Trahair, L.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Disparities in the glycemic and incretin responses to intraduodenal glucose infusion between healthy young men and women. J. Clin. Endocrinol. Metab. 2023, 108, e712–e719. [Google Scholar] [CrossRef]
- Karim, Z.; Attmane-Elakeb, A.; Bichara, M. Renal handling of nh4+ in relation to the control of acid-base balance by the kidney. J. Nephrol. 2002, 15, S128–S134. [Google Scholar]
- McCarty, M.F. Acid-base balance may influence risk for insulin resistance syndrome by modulating cortisol output. Med. Hypotheses 2005, 64, 380–384. [Google Scholar] [CrossRef]
- Komori, T. The effects of phosphatidylserine and omega-3 fatty acid-containing supplement on late life depression. Ment. Illn. 2015, 7, 5647. [Google Scholar] [CrossRef]
- Mayer, E.A. The neurobiology of stress and gastrointestinal disease. Gut 2000, 47, 861–869. [Google Scholar] [CrossRef]
- Shibata, C.; Muratsubaki, T.; Shibata, S.; Aizawa, E.; Watanabe, S.; Kanazawa, M.; Fukudo, S. A randomized controlled trial of environmental richness on gastrointestinal symptoms, salivary cortisol, and gut microbiota in early childhood. Sci. Rep. 2025, 15, 8493. [Google Scholar] [CrossRef]
- Ravenda, S.; Mancabelli, L.; Gambetta, S.; Barbetti, M.; Turroni, F.; Carnevali, L.; Ventura, M.; Sgoifo, A. Heart rate variability, daily cortisol indices and their association with psychometric characteristics and gut microbiota composition in an Italian community sample. Sci. Rep. 2025, 15, 8584. [Google Scholar] [CrossRef]
- Keskitalo, A.; Aatsinki, A.K.; Kortesluoma, S.; Pelto, J.; Korhonen, L.; Lahti, L.; Lukkarinen, M.; Munukka, E.; Karlsson, H.; Karlsson, L. Gut microbiota diversity but not composition is related to saliva cortisol stress response at the age of 2.5 months. Stress 2021, 24, 551–560. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Dalile, B.; Fuchs, A.; La Torre, D.; Vervliet, B.; Van Oudenhove, L.; Verbeke, K. Colonic butyrate administration modulates fear memory but not the acute stress response in men: A randomized, triple-blind, placebo-controlled trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 131, 110939. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Dettenborn, L.; Kirschbaum, C. The cortisol awakening response (car): Facts and future directions. Int. J. Psychophysiol. 2009, 72, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.K.; Kumari, M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 2009, 34, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Fekedulegn, D.B.; Andrew, M.E.; Burchfiel, C.M.; Violanti, J.M.; Hartley, T.A.; Charles, L.E.; Miller, D.B. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom. Med. 2007, 69, 651–659. [Google Scholar] [CrossRef]
- Nieman, L.K.; Castinetti, F.; Newell-Price, J.; Valassi, E.; Drouin, J.; Takahashi, Y.; Lacroix, A. Cushing syndrome. Nat. Rev. Dis. Primers 2025, 11, 4. [Google Scholar] [CrossRef]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.; Colao, A. Complications of cushing’s syndrome: State of the art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef]
- Bengtsson, D.; Ragnarsson, O.; Berinder, K.; Dahlqvist, P.; Edén Engström, B.; Ekman, B.; Höybye, C.; Järås, J.; Valdemarsson, S.; Burman, P.; et al. Increased mortality persists after treatment of cushing’s disease: A matched nationwide cohort study. J. Endocr. Soc. 2022, 6, bvac045. [Google Scholar] [CrossRef]
- Paes, T.; Feelders, R.A.; Hofland, L.J. Epigenetic mechanisms modulated by glucocorticoids with a focus on cushing syndrome. J. Clin. Endocrinol. Metab. 2024, 109, e1424–e1433. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.T.; Vogel, F.; Zopp, S.; Marchant Seiter, T.; Rubinstein, G.; Berr, C.M.; Künzel, H.; Beuschlein, F.; Reincke, M. Whom should we screen for cushing syndrome? The endocrine society practice guideline recommendations 2008 revisited. J. Clin. Endocrinol. Metab. 2022, 107, e3723–e3730. [Google Scholar] [CrossRef] [PubMed]
- Hasenmajer, V.; Sbardella, E.; Sciarra, F.; Simeoli, C.; Pivonello, C.; Ceccato, F.; Pofi, R.; Minnetti, M.; Rizzo, F.; Ferrari, D.; et al. Circadian clock disruption impairs immune oscillation in chronic endogenous hypercortisolism: A multi-level analysis from a multicentre clinical trial. EBioMedicine 2024, 110, 105462. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef]
- Savas, M.; Mehta, S.; Agrawal, N.; van Rossum, E.F.C.; Feelders, R.A. Approach to the patient: Diagnosis of cushing syndrome. J. Clin. Endocrinol. Metab. 2022, 107, 3162–3174. [Google Scholar] [CrossRef]
- Raff, H.; Carroll, T. Cushing’s syndrome: From physiological principles to diagnosis and clinical care. J. Physiol. 2015, 593, 493–506. [Google Scholar] [CrossRef]
- Soares, V.R.; Silva Martins, C.; Martinez, E.Z.; Araujo, L.D.; Roa, S.L.R.; Silva, L.R.; Moreira, A.C.; De Castro, M. Peripheral clock system circadian abnormalities in cushing’s disease. Chronobiol. Int. 2020, 37, 867–876. [Google Scholar] [CrossRef]
- Angelousi, A.; Nasiri-Ansari, N.; Karapanagioti, A.; Kyriakopoulos, G.; Aggeli, C.; Zografos, G.; Choreftaki, T.; Parianos, C.; Kounadi, T.; Alexandraki, K.; et al. Expression of clock-related genes in benign and malignant adrenal tumors. Endocrine 2020, 68, 650–659. [Google Scholar] [CrossRef]
- Van Aken, M.O.; Pereira, A.M.; van Thiel, S.W.; van den Berg, G.; Frölich, M.; Veldhuis, J.D.; Romijn, J.A.; Roelfsema, F. Irregular and frequent cortisol secretory episodes with preserved diurnal rhythmicity in primary adrenal cushing’s syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 1570–1577. [Google Scholar] [CrossRef]
- Saini, J.; Singh, S.; Ebbehoj, A.; Zhang, C.D.; Nathani, R.; Fell, V.; Atkinson, E.; Achenbach, S.; Rivard, A.; Singh, R.; et al. Steroid profiling and circadian cortisol secretion in patients with mild autonomous cortisol secretion: A cross-sectional study. J. Clin. Endocrinol. Metab. 2025, 110, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Bolte, E.; Tremblay, J.; Dupre, J.; Poitras, P.; Fournier, H.; Garon, J.; Garrel, D.; Bayard, F.; Taillefer, R.; et al. Gastric inhibitory polypeptide-dependent cortisol hypersecretion—A new cause of cushing’s syndrome. N. Engl. J. Med. 1992, 327, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Gerl, H.; Rohde, W.; Biering, H.; Schulz, N.; Lochs, H. Food-dependent cushing syndrome of long standing with mild clinical features. Dtsch. Med. Wochenschr. 2000, 125, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Athyros, V.G.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. J. Clin. Endocrinol. Metab. 2009, 94, 2692–2701. [Google Scholar] [CrossRef]
- Hackett, R.A.; Kivimäki, M.; Kumari, M.; Steptoe, A. Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the whitehall II cohort study. J. Clin. Endocrinol. Metab. 2016, 101, 619–625. [Google Scholar] [CrossRef]
- Jang, Y.M.; Lee, E.J.; Kim, D.L.; Kim, S.K.; Song, K.H. The association between midnight salivary cortisol and metabolic syndrome in korean adults. Diabetes Metab. J. 2012, 36, 245–250. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Alexander, N.; Bornstein, S.R.; Gao, W.; Miller, R.; Stark, S.; Bosch, J.A.; Fischer, J.E. Cortisol in hair and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2573–2580. [Google Scholar] [CrossRef]
- Garcez, A.; Leite, H.M.; Weiderpass, E.; Paniz, V.M.V.; Watte, G.; Canuto, R.; Olinto, M.T.A. Basal cortisol levels and metabolic syndrome: A systematic review and meta-analysis of observational studies. Psychoneuroendocrinology 2018, 95, 50–62. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, W.; Wu, S.; Liu, Q.; Li, X. Evidence for disruption of diurnal salivary cortisol rhythm in childhood obesity: Relationships with anthropometry, puberty and physical activity. BMC Pediatr. 2020, 20, 381. [Google Scholar] [CrossRef]
- Dube, S.; Norby, B.J.; Pattan, V.; Carter, R.E.; Basu, A.; Basu, R. 11β-hydroxysteroid dehydrogenase types 1 and 2 activity in subcutaneous adipose tissue in humans: Implications in obesity and diabetes. J. Clin. Endocrinol. Metab. 2015, 100, E70–E76. [Google Scholar] [CrossRef]
- Rask, E.; Walker, B.R.; Soderberg, S.; Livingstone, D.E.; Eliasson, M.; Johnson, O.; Andrew, R.; Olsson, T. Tissue-specific changes in peripheral cortisol metabolism in obese women: Increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J. Clin. Endocrinol. Metab. 2002, 87, 3330–3336. [Google Scholar] [CrossRef]
- Jagannath, A.; Peirson, S.N.; Foster, R.G. Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr. Opin. Neurobiol. 2013, 23, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Federenko, I.; Hellhammer, D.H.; Kirschbaum, C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology 2000, 25, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kim, Y.C.; Jeong, J.H. Bipolar disorder, circadian rhythm and clock genes. Clin. Psychopharmacol. Neurosci. 2024, 22, 211–221. [Google Scholar] [CrossRef]
- Morelli, V.; Ghielmetti, A.; Caldiroli, A.; Grassi, S.; Siri, F.M.; Caletti, E.; Mucci, F.; Aresta, C.; Passeri, E.; Pugliese, F.; et al. Mental health in patients with adrenal incidentalomas: Is there a relation with different degrees of cortisol secretion? J. Clin. Endocrinol. Metab. 2021, 106, e130–e139. [Google Scholar] [CrossRef]
- Findling, J.W.; Raff, H. Recognition of nonneoplastic hypercortisolism in the evaluation of patients with cushing syndrome. J. Endocr. Soc. 2023, 7, bvad087. [Google Scholar] [CrossRef]
- Hinojosa-Amaya, J.M.; González-Colmenero, F.D.; Alvarez-Villalobos, N.A.; Salcido-Montenegro, A.; Quintanilla-Sánchez, C.; Moreno-Peña, P.J.; Manzanares-Gallegos, D.M.; Gutiérrez-Dávila, L.F.; Castillo-Morales, P.L.; García-Campa, M.; et al. The conundrum of differentiating cushing’s syndrome from non-neoplastic hypercortisolism: A systematic review and meta-analysis. Pituitary 2024, 27, 345–359. [Google Scholar] [CrossRef]
- Herxheimer, A.; Waterhouse, J. The prevention and treatment of jet lag. BMJ 2003, 326, 296–297. [Google Scholar] [CrossRef]
- Clow, A.; Hucklebridge, F.; Stalder, T.; Evans, P.; Thorn, L. The cortisol awakening response: More than a measure of hpa axis function. Neurosci. Biobehav. Rev. 2010, 35, 97–103. [Google Scholar] [CrossRef]
- Li, J.; Bidlingmaier, M.; Petru, R.; Pedrosa Gil, F.; Loerbroks, A.; Angerer, P. Impact of shift work on the diurnal cortisol rhythm: A one-year longitudinal study in junior physicians. J. Occup. Med. Toxicol. 2018, 13, 23. [Google Scholar] [CrossRef]
- Bostock, S.; Steptoe, A. Influences of early shift work on the diurnal cortisol rhythm, mood and sleep: Within-subject variation in male airline pilots. Psychoneuroendocrinology 2013, 38, 533–541. [Google Scholar] [CrossRef]
- Paragliola, R.M.; Corsello, A.; Troiani, E.; Locantore, P.; Papi, G.; Donnini, G.; Pontecorvi, A.; Corsello, S.M.; Carrozza, C. Cortisol circadian rhythm and jet-lag syndrome: Evaluation of salivary cortisol rhythm in a group of eastward travelers. Endocrine 2021, 73, 424–430. [Google Scholar] [CrossRef]
- Adam, T.C.; Epel, E.S. Stress, eating and the reward system. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, H.; Niki, T.; Shiiya, T. Feeding behavior and gene expression of appetite-related neuropeptides in mice lacking for neuropeptide y y5 receptor subclass. World J. Gastroenterol. 2008, 14, 6312–6317. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azmi, N.A.S.; Juliana, N.; Mohd Fahmi Teng, N.I.; Azmani, S.; Das, S.; Effendy, N. Consequences of circadian disruption in shift workers on chrononutrition and their psychosocial well-being. Int. J. Environ. Res. Public Health 2020, 17, 2043. [Google Scholar] [CrossRef]
- Bergendahl, M.; Vance, M.L.; Iranmanesh, A.; Thorner, M.O.; Veldhuis, J.D. Fasting as a metabolic stress paradigm selectively amplifies cortisol secretory burst mass and delays the time of maximal nyctohemeral cortisol concentrations in healthy men. J. Clin. Endocrinol. Metab. 1996, 81, 692–699. [Google Scholar] [CrossRef]
- Sharma, A.; Vella, A. Glucose metabolism in cushing’s syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 140–145. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal timing regulates the human circadian system. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef]
- Guarnotta, V.; Amodei, R.; Di Gaudio, F.; Giordano, C. Nutritional intervention in cushing’s disease: The ketogenic diet’s effects on metabolic comorbidities and adrenal steroids. Nutrients 2023, 15, 4647. [Google Scholar] [CrossRef]
- Ceccato, F.; Zilio, M.; Barbot, M.; Albiger, N.; Antonelli, G.; Plebani, M.; Watutantrige-Fernando, S.; Sabbadin, C.; Boscaro, M.; Scaroni, C. Metyrapone treatment in cushing’s syndrome: A real-life study. Endocrine 2018, 62, 701–711. [Google Scholar] [CrossRef]
- Colao, A.; Petersenn, S.; Newell-Price, J.; Findling, J.W.; Gu, F.; Maldonado, M.; Schoenherr, U.; Mills, D.; Salgado, L.R.; Biller, B.M.; et al. A 12-month phase 3 study of pasireotide in cushing’s disease. N. Engl. J. Med. 2012, 366, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Dzialach, L.; Respondek, W.; Witek, P. Real-world experience with pasireotide-lar in cushing’s disease: Single-center 12-month observational study. J. Clin. Med. 2025, 14, 2794. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Bonaventura, I.; Simeoli, C.; Tomaselli, A.; Vincenzi, L.; De Alcubierre, D.; Sciarra, F.; Rizzo, F.; Cerroni, L.; Di Paola, N.; et al. Chronotherapy with once-daily osilodrostat improves cortisol rhythm, quality of life, and sleep in cushing’s syndrome. J. Clin. Endocrinol. Metab. 2025, 110, 3525–3537. [Google Scholar] [CrossRef] [PubMed]
- Barnea, M.; Haviv, L.; Gutman, R.; Chapnik, N.; Madar, Z.; Froy, O. Metformin affects the circadian clock and metabolic rhythms in a tissue-specific manner. Biochim. Biophys. Acta 2012, 1822, 1796–1806, Erratum in Biochim. Biophys. Acta 2013, 1832, 696. [Google Scholar] [CrossRef]
- Herxheimer, A.; Petrie, K.J. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst. Rev. 2002, 2010, CD001520. [Google Scholar] [CrossRef]
- Gu, C.; Brereton, N.; Schweitzer, A.; Cotter, M.; Duan, D.; Borsheim, E.; Wolfe, R.R.; Pham, L.V.; Polotsky, V.Y.; Jun, J.C. Metabolic effects of late dinner in healthy volunteers-a randomized crossover clinical trial. J. Clin. Endocrinol. Metab. 2020, 105, 2789–2802. [Google Scholar] [CrossRef]
- Lowden, A.; Holmback, U.; Akerstedt, T.; Forslund, J.; Lennernas, M.; Forslund, A. Performance and sleepiness during a 24 h wake in constant conditions are affected by diet. Biol. Psychol. 2004, 65, 251–263. [Google Scholar] [CrossRef]
- Van der Rhee, M.; Oosterman, J.E.; Wopereis, S.; van der Horst, G.T.J.; Chaves, I.; Dolle, M.E.T.; Burdorf, A.; van Kerkhof, L.W.M.; der Holst, H.M.L. Personalized sleep and nutritional strategies to combat adverse effects of night shift work: A controlled intervention protocol. BMC Public Health 2024, 24, 2555. [Google Scholar] [CrossRef]
- Teng, N.; Ngelayang, E.; Said, N.M.; Juliana, N.; Mohd Azmi, N.A.S.; Chen, L.W.; Loy, S.L. A qualitative study on nutrition and well-being of healthcare shift workers. Sci. Rep. 2025, 15, 30512. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Murad, M.H.; Newell-Price, J.; Savage, M.O.; Tabarin, A.; Endocrine, S. Treatment of cushing’s syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 2807–2831. [Google Scholar] [CrossRef]
- Debono, M.; Harrison, R.F.; Chadarevian, R.; Gueroult, C.; Abitbol, J.L.; Newell-Price, J. Resetting the abnormal circadian cortisol rhythm in adrenal incidentaloma patients with mild autonomous cortisol secretion. J. Clin. Endocrinol. Metab. 2017, 102, 3461–3469. [Google Scholar] [CrossRef]
- Findling, J.W.; Fleseriu, M.; Newell-Price, J.; Petersenn, S.; Pivonello, R.; Kandra, A.; Pedroncelli, A.M.; Biller, B.M. Late-night salivary cortisol may be valuable for assessing treatment response in patients with cushing’s disease: 12-month, phase III pasireotide study. Endocrine 2016, 54, 516–523. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.J.; Huang, W.; Spencer-Segal, J.L.; Yuen, K.C.J.; Dacus, K.C.; Padgett, J.; Babler, E.K.; Das, A.K.; Campos, C.; et al. Osilodrostat treatment of cushing syndrome in real-world clinical practice: Findings from the illustrate study. J. Endocr. Soc. 2025, 9, bvaf046. [Google Scholar] [CrossRef]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.; Rajaratnam, S.M.; Van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011, 96, E463–E472. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I. Therapeutic applications of melatonin. Ther. Adv. Endocrinol. Metab. 2013, 4, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Campino, C.; Valenzuela, F.J.; Torres-Farfan, C.; Reynolds, H.E.; Abarzua-Catalan, L.; Arteaga, E.; Trucco, C.; Guzman, S.; Valenzuela, G.J.; Seron-Ferre, M. Melatonin exerts direct inhibitory actions on acth responses in the human adrenal gland. Horm. Metab. Res. 2011, 43, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Hasannia, E.; Derakhshanpour, F.; Vakili, M.A. Effects of melatonin on salivary levels of cortisol and sleep quality of hemodialysis patients: A randomized clinical trial. Iran. J. Psychiatry 2021, 16, 305–311. [Google Scholar] [CrossRef]
- Pachimsawat, P.; Ratanachamnong, P.; Jantaratnotai, N. Exogenous melatonin’s effect on salivary cortisol and amylase: A randomized controlled trial. Pharmacol. Res. Perspect. 2024, 12, e1205. [Google Scholar] [CrossRef]
| Strategy | Specific Intervention | Rationale | Clinical Evidence |
|---|---|---|---|
| Chrononutrition | Early time-restricted eating, avoiding late-night meals | Aligns feeding cycles with central pacemakers, improves glycemic control, reduces evening cortisol elevations | Beneficial in healthy subjects and MetS; BMI reduction [115,117,118] |
| Low-glycemic, low-fat meals in hypercortisolism | Lower postprandial cortisol excursion | Clinical rationale in CS and MACS [119] | |
| Shift workers: avoid food intake between midnight–6 a.m., prefer protein-rich light meals if eating at night | Reduces daily misalignment, improves alertness/satiety | Observational data [117] | |
| Jet lag: adapt meal timing to destination (prioritize breakfast, avoid late evening meals) | Improves daily realignment | Practical clinical advice [120] | |
| Ketogenic diet (KD/VLCKD) | Improves body composition and metabolic parameters | Evidence in obese and CD patients; improves metabolic comorbidities [121] | |
| Pharmacological (only in CS) | Ketoconazole, metyrapone | Inhibit steroidogenesis; lowers mean cortisol | Does not fully restore rhythm; evening metyrapone may help in MACS [122] |
| Pasireotide | Reduces ACTH and late-night cortisol | Conflicting data [123,124] | |
| Osilodrostat (evening dosing) | Inhibits steroidogenesis; improves daily profile, QoL, sleep | Promising evidence in CD patient [125] | |
| Pharmacological (metabolic/supplements) | Metformin (evening modified release) | Modulates peripheral clocks | Preclinical evidence; not yet confirmed in human trials [126] |
| Melatonin (0.5–5 mg at bedtime) | Modulates central and adrenal clocks, blunts ACTH-induced steroidogenesis | Effective in jet lag [127] | |
| Probiotics, SCFA-promoting diets, Mediterranean diet, Omega-3 fatty acids, and vitamin C | Reduce stress-related cortisol responses and HPA reactivity in selected contexts | Supportive but modest evidence [70,71,75,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paragliola, R.M.; Marchetti, M.; Montagna, C.; Corsello, S.M.; Peluso, G. “Feeding the Rhythm”—Effects of Food and Nutrients on Daily Cortisol Secretion: From Molecular Mechanisms to Clinical Impact. Int. J. Mol. Sci. 2025, 26, 11230. https://doi.org/10.3390/ijms262211230
Paragliola RM, Marchetti M, Montagna C, Corsello SM, Peluso G. “Feeding the Rhythm”—Effects of Food and Nutrients on Daily Cortisol Secretion: From Molecular Mechanisms to Clinical Impact. International Journal of Molecular Sciences. 2025; 26(22):11230. https://doi.org/10.3390/ijms262211230
Chicago/Turabian StyleParagliola, Rosa Maria, Marco Marchetti, Costanza Montagna, Salvatore Maria Corsello, and Gianfranco Peluso. 2025. "“Feeding the Rhythm”—Effects of Food and Nutrients on Daily Cortisol Secretion: From Molecular Mechanisms to Clinical Impact" International Journal of Molecular Sciences 26, no. 22: 11230. https://doi.org/10.3390/ijms262211230
APA StyleParagliola, R. M., Marchetti, M., Montagna, C., Corsello, S. M., & Peluso, G. (2025). “Feeding the Rhythm”—Effects of Food and Nutrients on Daily Cortisol Secretion: From Molecular Mechanisms to Clinical Impact. International Journal of Molecular Sciences, 26(22), 11230. https://doi.org/10.3390/ijms262211230








