Unlocking the Cu-Co Interplay: Electrodeposited Spinel Co2CuO4 as a High-Performance Hydrogen Evolution Catalyst
Abstract
1. Introduction
2. Results
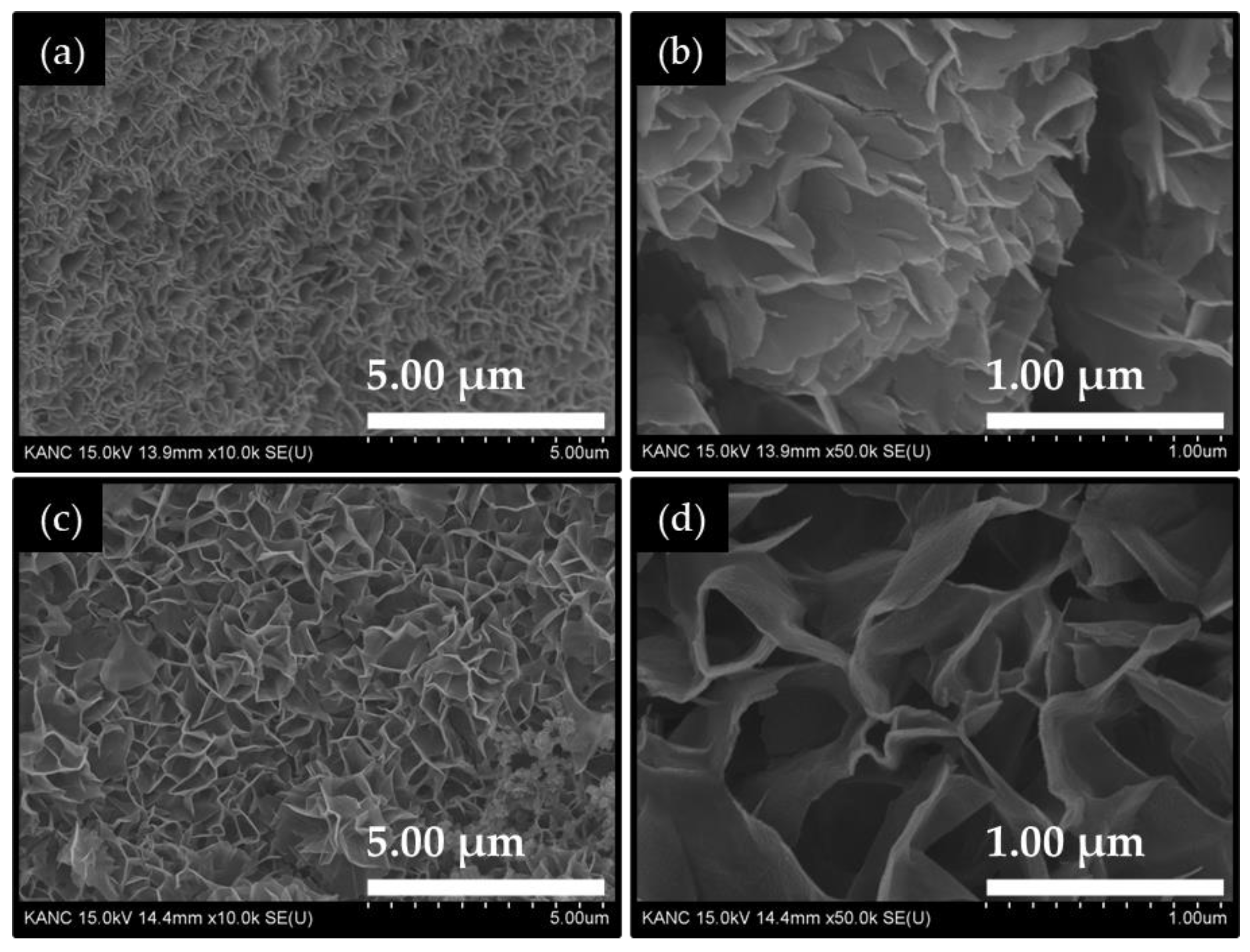
2.1. Morphological and Compositional Properties of Co2CuO4 and Co3O4 Films
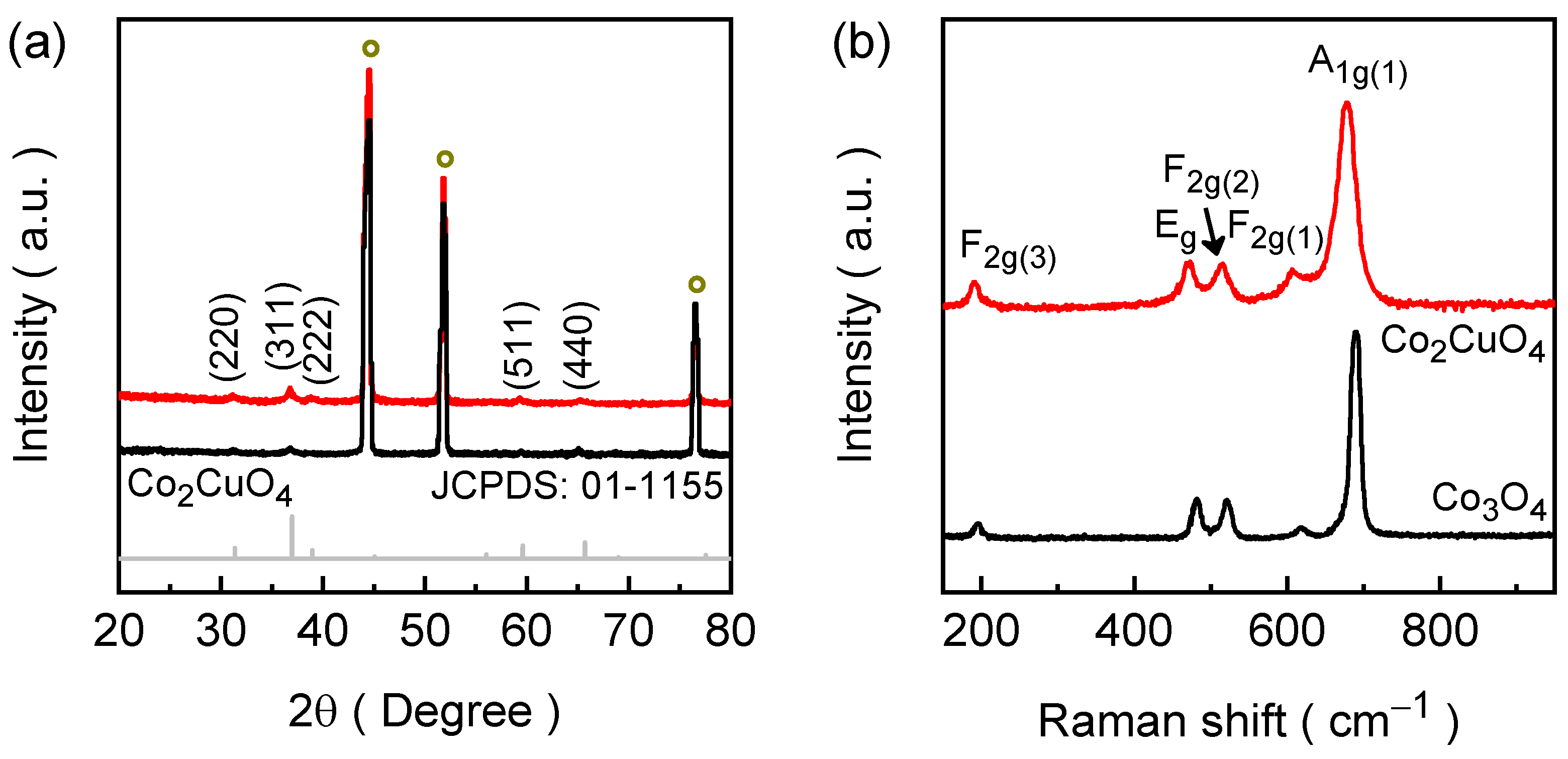
2.2. Crystallographic and Bonding Properties of Co2CuO4 and Co3O4 Films
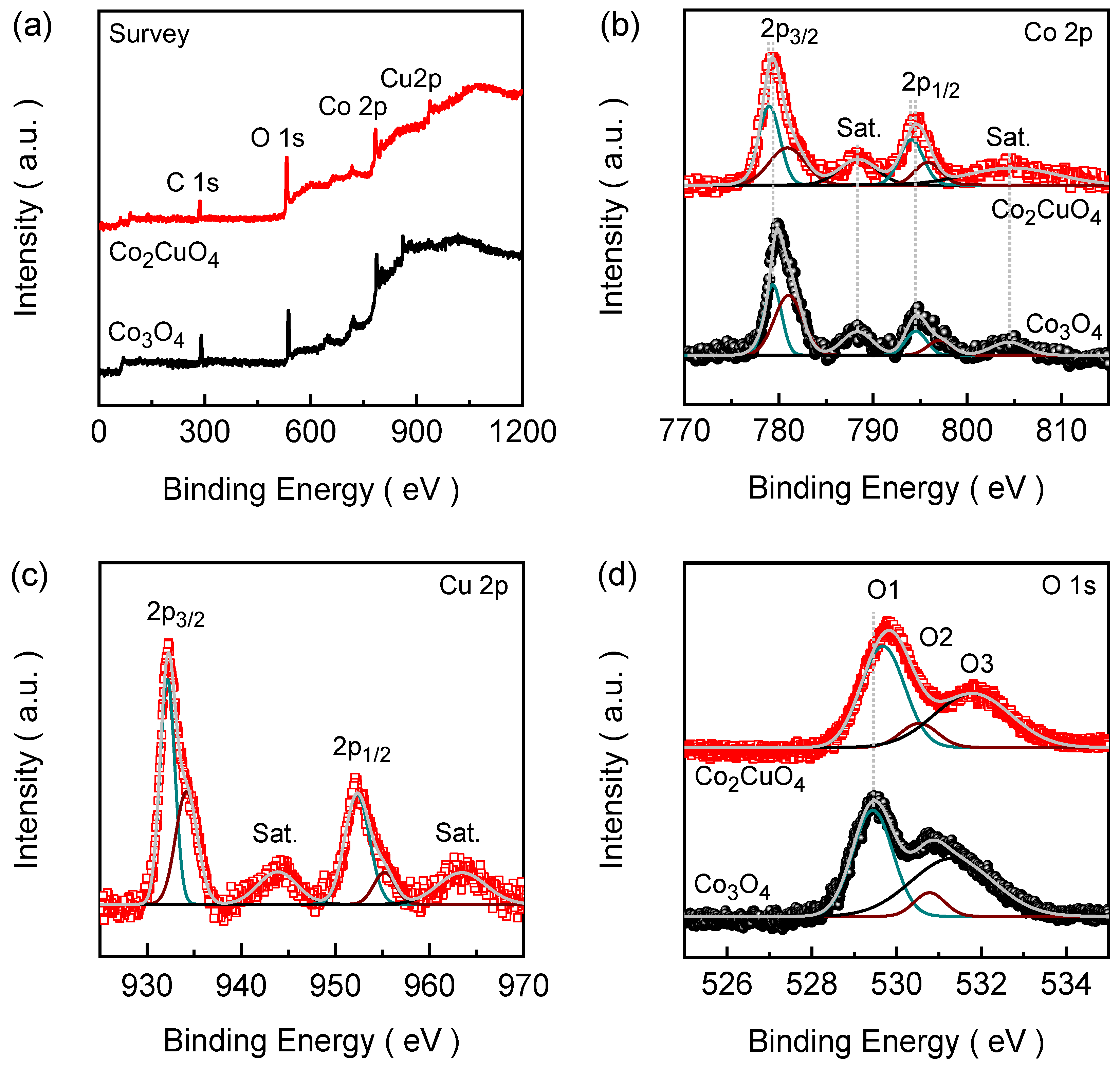
2.3. Chemical Bonding States of Co2CuO4 than Co3O4 Films
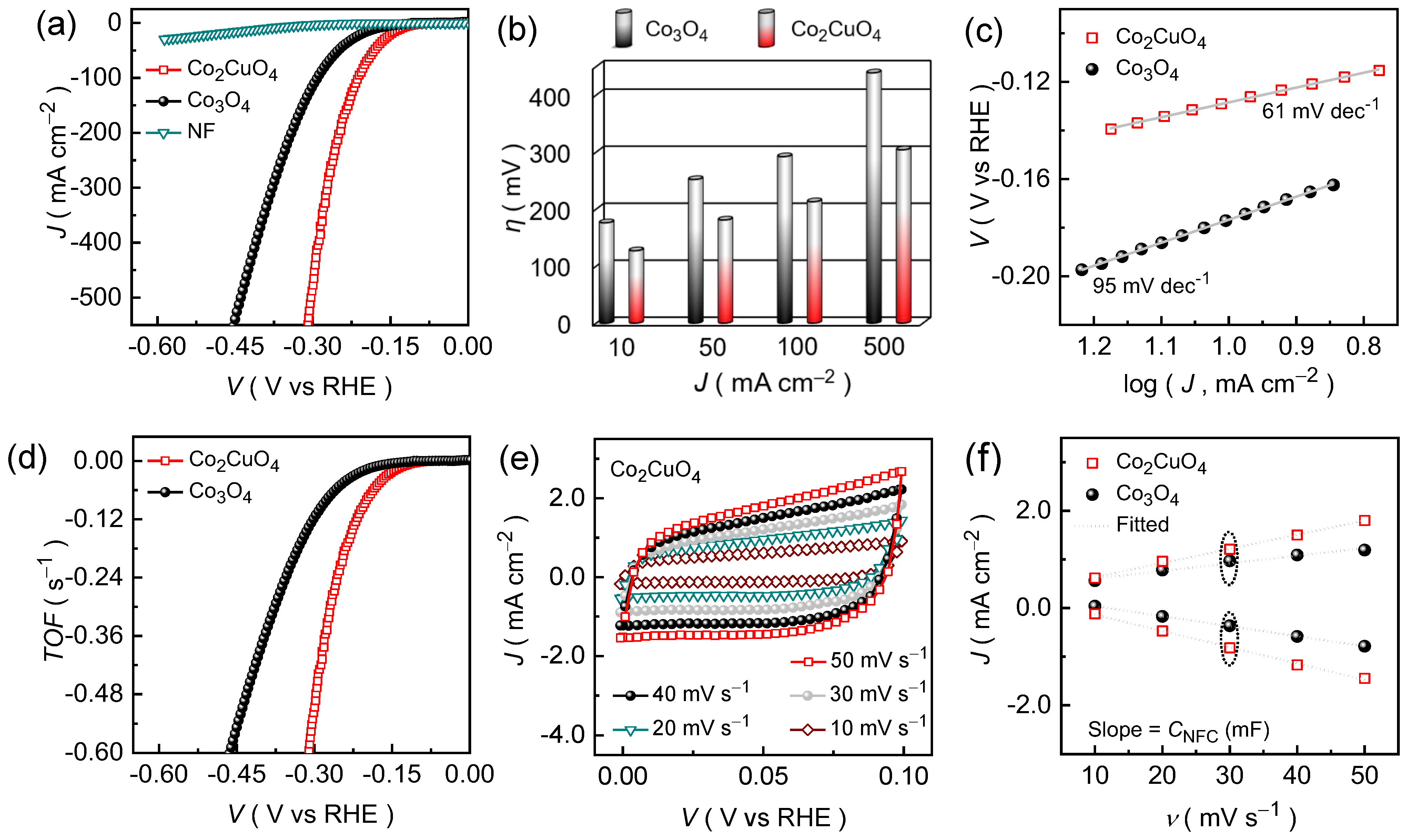
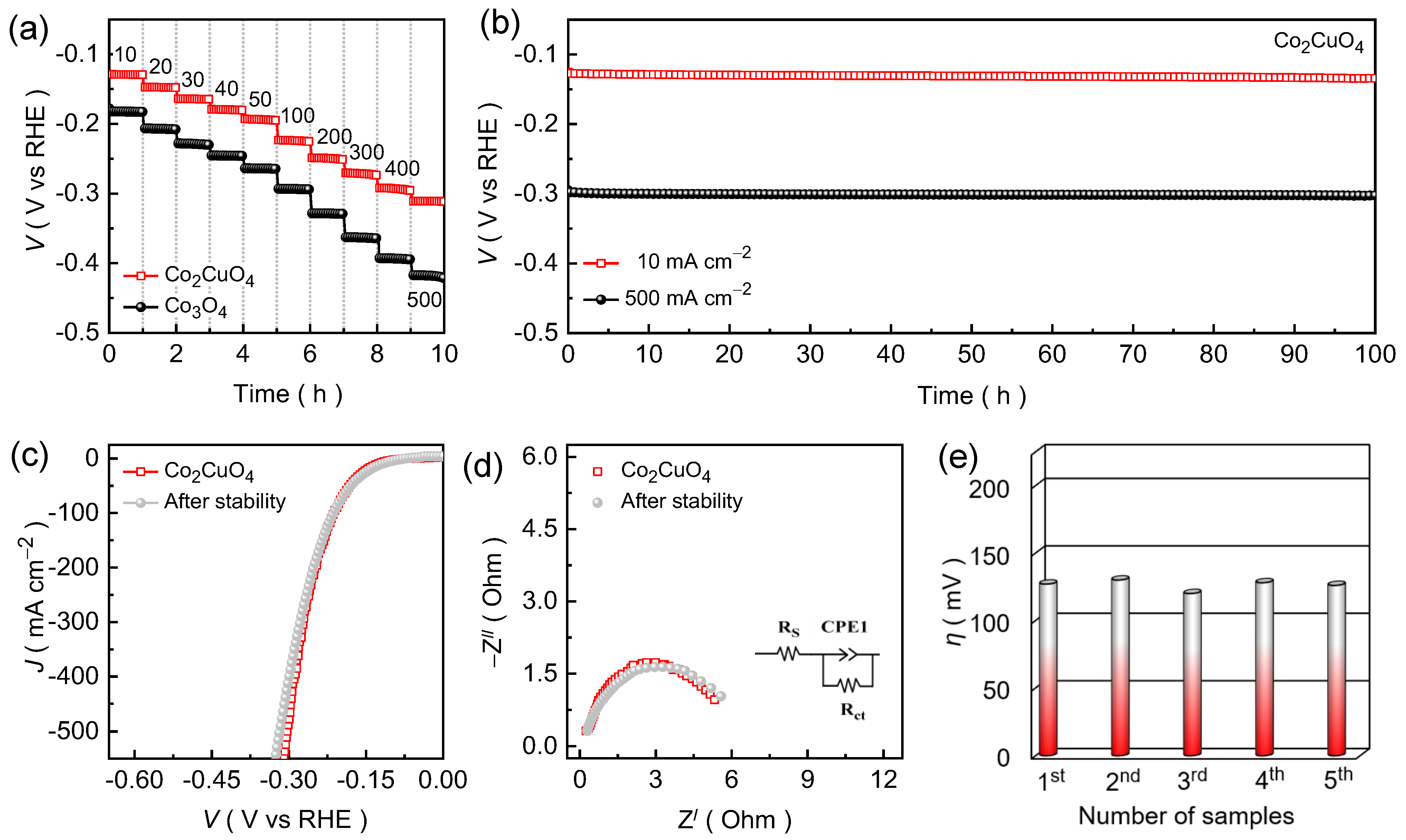
2.4. Electrochemical Properties of Co2CuO4 than Co3O4 Electrode Films
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Co3O4 and Co2CuO4 Electrodes
3.3. Material Characterization
3.4. Electrochemical Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HER | Hydrogen evolution reaction |
| Pt | Platinum |
| FE-SEM | Field-emission scanning electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| SCE | Saturated calomel electrode |
| RHE | Reversible hydrogen electrode |
| EIS | Electrochemical impedance spectroscopy |
| JNFC | Non-faradaic current density |
| CNFC | Non-faradaic capacitance |
| ECSA | electrochemically active surface area |
| CV | Cyclic voltammetry |
| LSV | Linear sweep voltammetry |
| TOF | Turnover frequency |
| η | Overpotential |
| Rct | Charge-transfer resistance |
| F | Faraday’s constant |
| A | Geometric area of the electrode |
| N | Number of moles of active catalyst |
| n | Number of electrons transferred per hydrogen molecule |
References
- Evro, S.; Oni, B.A.; Tomomewo, O.S. Carbon neutrality and hydrogen energy systems. Int. J. Hydrogen Energy 2024, 78, 1449–1467. [Google Scholar] [CrossRef]
- Boretti, A.; Pollet, B.G. Hydrogen economy: Paving the path to a sustainable, low-carbon future. Int. J. Hydrogen Energy 2024, 93, 307–319. [Google Scholar] [CrossRef]
- Barrientos, C.; Moris, S.; Arias, D.; Pecchi, G.; Ibarra, J.; Ramírez, G.; Gidi, L. New Paste Electrode Based on Copper and Gallium Mixed Metal Oxides-Decorated CNT for Highly Electrocatalyzed Hydrogen Evolution Reaction. Int. J. Mol. Sci. 2025, 26, 9057. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kalauni, K.; Pahwa, R. A review of water electrolysis technologies with insights into optimization and numerical simulations. Int. J. Hydrogen Energy 2025, 140, 694–727. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kalauni, K.; Pahwa, R. Water Electrolysis Technologies and Their Modeling Approaches: A Comprehensive Review. Eng 2025, 6, 81. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Cho, S.; Im, H.; Jana, A. Enhanced Catalytic Activity of CuO@CuS Core–Shell Structure for Highly Efficient HER Application. Nanomaterials 2024, 14, 1941. [Google Scholar] [CrossRef] [PubMed]
- Irshad, H.; Zia, M.; Al-Hajri, R.; Khattak, Z.A.K.; Al-Abri, M.; Ahmad, N.; Younus, H.A. Electrocatalysts for hydrogen and oxygen evolution reactions under neutral/near-neutral conditions: Summary and challenges. Int. J. Hydrogen Energy 2025, 137, 1009–1041. [Google Scholar] [CrossRef]
- Thalji, M.R.; Mahmoudi, F.; Bachas, L.G.; Park, C. MXene-Based Electrocatalysts for Water Splitting: Material Design, Surface Modulation, and Catalytic Performance. Int. J. Mol. Sci. 2025, 26, 8019. [Google Scholar] [CrossRef]
- Jarząbek-Karnas, M.; Bojarska, Z.; Klemczak, P.; Werner, Ł.; Makowski, Ł. Advanced Hybrid Nanocatalysts for Green Hydrogen: Carbon-Supported MoS2 and ReS2 as Noble Metal Alternatives. Int. J. Mol. Sci. 2025, 26, 6640. [Google Scholar] [CrossRef]
- Bergedahl, M.; Narea, P.; Llanos, J.; Pulido, R.; Naveas, N.; Amo-Ochoa, P.; Zamora, F.; Delgado, G.E.; Madrid, F.M.G.; León, Y.; et al. Synthesis, Crystal Structures, Hirshfeld Surface Analysis, Computational Investigations, Thermal Properties, and Electrochemical Analysis of Two New Cu(II) and Co(II) Coordination Polymers with the Ligand 5-Methyl-1-(pyridine-4-yl-methyl)-1H-1,2,3-triazole-4-carboxylate. Int. J. Mol. Sci. 2025, 26, 1671. [Google Scholar]
- Gupta, S.; Fernandes, R.; Patel, R.; Spreitzer, M.; Patel, N. A review of cobalt-based catalysts for sustainable energy and environmental applications. Appl. Catal. A Gen. 2023, 661, 119254. [Google Scholar] [CrossRef]
- Chen, X.H.; Li, T.; Li, X.; Lei, J.; Li, N.B.; Luo, H.Q. Oxyanion-Coordinated Co-Based Catalysts for Optimized Hydrogen Evolution: The Feedback of Adsorbed Anions to the Catalytic Activity and Mechanism. ACS Catal. 2023, 13, 6721–6729. [Google Scholar] [CrossRef]
- Huang, C.; Qin, P.; Luo, Y.; Ruan, Q.; Liu, L.; Wu, Y.; Li, Q.; Xu, Y.; Liu, R.; Chu, P.K. Recent progress and perspective of cobalt-based catalysts for water splitting: Design and nanoarchitectonics. Mater. Today Energy 2022, 23, 100911. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Zhu, S.-L.; Han, X.-Q.; Wang, D.-C.; Zhang, J.-C.; Huai, X.-D.; Li, X.; Zhang, F.-Y.; Xiang, Z.; Zhang, W.-Z. Engineering Cationic Vacancies in Octahedral Sites of Co3O4 for High-Efficiency Oxygen Evolution. Energy Fuels 2023, 37, 8523–8530. [Google Scholar] [CrossRef]
- Ibarra, J.; Aguirre, M.J.; del Río, R.; Henriquez, R.; Faccio, R.; Dalchiele, E.A.; Arce, R.; Ramírez, G. α-Fe2O3/, Co3O4/, and CoFe2O4/MWCNTs/Ionic Liquid Nanocomposites as High-Performance Electrocatalysts for the Electrocatalytic Hydrogen Evolution Reaction in a Neutral Medium. Int. J. Mol. Sci. 2024, 25, 7043. [Google Scholar] [CrossRef]
- Li, G.; Liu, F.; Ma, W.; Li, H.; Li, S. Surface Modification of a Lignin-Derived Carbon-Supported Co-Based Metal/Oxide Nanostructure for Alkaline Water Splitting. Molecules 2023, 28, 5648. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.T.; Mujtaba, M.M.; Thakre, K.; Momin, Z.H.; Ansari, A.S.; Cho, S.; Ahmed, A.T.A. From granules to nanosheets: Unlocking the energy potential of CoFe2O4 for highly-efficient asymmetric energy storage applications. J. Energy Storage 2025, 140, 119168. [Google Scholar] [CrossRef]
- Aqueel Ahmed, A.T.; Hou, B.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Cha, S.; Inamdar, A.I.; Kim, H.; et al. Self-Assembled Nanostructured CuCo2O4 for Electrochemical Energy Storage and the Oxygen Evolution Reaction via Morphology Engineering. Small 2018, 14, 1800742. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, L.; Liu, R.; Zhang, C.; Mak, C.H.; Zou, X.; Shen, H.-H.; Leu, S.-Y.; Hsu, H.-Y. A review on morphology engineering for highly efficient and stable hybrid perovskite solar cells. J. Mater. Chem. A 2018, 6, 12842–12875. [Google Scholar] [CrossRef]
- Chen, K.; Sun, C.; Xue, D. Morphology engineering of high performance binary oxide electrodes. Phys. Chem. Chem. Phys. 2015, 17, 732–750. [Google Scholar] [CrossRef]
- Tan, F.; Dong, N.; He, J.; Yuan, L.; Lin, Z.; Liu, X.; Yang, X.; Li, B. Morphology Engineering of Aggregation-Induced Emission-Based Metal–Organic Frameworks Templated with Cellulose Nanocrystals for Lactic Acid Detection. ACS Mater. Lett. 2025, 7, 2041–2048. [Google Scholar] [CrossRef]
- Timmerman, M.A.; Xia, R.; Le, P.T.P.; Wang, Y.; ten Elshof, J.E. Metal Oxide Nanosheets as 2D Building Blocks for the Design of Novel Materials. Chem. Eur. J. 2020, 26, 9084–9098. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Kang, J.; Betzler, S.; Czarnik, C.; Zhang, X.; Ophus, C.; Yu, C.; Bustillo, K.; Pan, M.; et al. Formation of two-dimensional transition metal oxide nanosheets with nanoparticles as intermediates. Nat. Mater. 2019, 18, 970–976. [Google Scholar] [CrossRef]
- Baimova, J.; Polyakova, P.; Shcherbinin, S. Effect of the Structure Morphology on the Mechanical Properties of Crumpled Graphene Fiber. Fibers 2021, 9, 85. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zheng, Y.; Zhang, Y.; Hu, X.; Xu, T. Construction of CuCo2O4@CuCo2O4 hierarchical nanowire arrays grown on Ni foam for high-performance supercapacitors. RSC Adv. 2017, 7, 3983–3991. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, W.; Lin, S.; Zhang, L.; Li, W.; Zhuo, Q.; Yang, W.; Luo, Y.; Qian, Q.; Chen, Q. Morphology effect of In2O3/Co3O4 on Co–In2O3 interface formation to drive the hydrogenation of CO2 to methanol. Int. J. Hydrogen Energy 2024, 90, 42–51. [Google Scholar] [CrossRef]
- Madadi, M.; Salarizadeh, P.; Rohani Moghadam, M.; Bazmandegan-Shamili, A. CuCo2O4 supported graphene quantum dots as a new and promising catalyst for methanol oxidation reaction. J. Electroanal. Chem. 2023, 941, 117532. [Google Scholar] [CrossRef]
- Lin, X.; Xue, X.; Du, J. Electrochemical glucose-to-formic acid conversion coupled with alkaline hydrogen production over nanostructured CuCo2O4 catalysts. J. Mater. Chem. A 2024, 12, 32095–32103. [Google Scholar] [CrossRef]
- Koniakhin, S.V.; Utesov, O.I.; Yashenkin, A.G. Raman peak shift and broadening in crystalline nanoparticles with lattice impurities. Diam. Relat. Mater. 2024, 146, 111182. [Google Scholar] [CrossRef]
- UmaSudharshini, A.; Bououdina, M.; Venkateshwarlu, M.; Dhamodharan, P.; Manoharan, C. Solvothermal synthesis of Cu-doped Co3O4 nanosheets at low reaction temperature for potential supercapacitor applications. Appl. Phys. A 2021, 127, 353. [Google Scholar] [CrossRef]
- Mayer, B.; Uhlenbrock, S.; Neumann, M. XPS satellites in transition metal oxides. J. Electron Spectrosc. Relat. Phenom. 1996, 81, 63–67. [Google Scholar] [CrossRef]
- Ho, C.-T.; Weng, T.-H.; Wang, C.-Y.; Yen, S.-J.; Yew, T.-R. Tunable band gaps of Co3−xCuxO4 nanorods with various Cu doping concentrations. RSC Adv. 2014, 4, 20053–20057. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, S.; Zhu, J.; Wang, Z.; Chen, S.; Qi, J.; Wang, H. Dual-Engineering Tailored Co3O4 Hollow Microspheres Assembled by Nanosheets for Boosting Oxygen Evolution Reaction. Molecules 2025, 30, 2181. [Google Scholar] [CrossRef]
- Kalasina, S.; Kongsawatvoragul, K.; Phattharasupakun, N.; Phattharaphuti, P.; Sawangphruk, M. Cobalt oxysulphide/hydroxide nanosheets with dual properties based on electrochromism and a charge storage mechanism. RSC Adv. 2020, 10, 14154–14160. [Google Scholar] [CrossRef]
- Sun, J.; Tian, X.; Xu, C.; Chen, H. Porous CuCo2O4 microtubes as a promising battery-type electrode material for high-performance hybrid supercapacitors. J. Mater. 2021, 7, 1358–1368. [Google Scholar] [CrossRef]
- Tang, J.; Ge, Y.; Shen, J.; Ye, M. Facile synthesis of CuCo2S4 as a novel electrode material for ultrahigh supercapacitor performance. Chem. Commun. 2016, 52, 1509–1512. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Sun, C.; Zhao, X.; Xu, C.; Li, Z.; Hou, Y.; Lei, L.; Yang, B.; Duan, X. Oriented generation of 1O2 from peroxymonosulfate via Co3O4 facet engineering. Appl. Catal. B Environ. 2025, 364, 124854. [Google Scholar] [CrossRef]
- Behera, A.; Seth, D.; Agarwal, M.; Haider, M.A.; Bhattacharyya, A.J. Exploring Cu-Doped Co3O4 Bifunctional Oxygen Electrocatalysts for Aqueous Zn–Air Batteries. ACS Appl. Mater. Interfaces 2024, 16, 17574–17586. [Google Scholar] [CrossRef]
- Talha Aqueel Ahmed, A.; Ho Lee, C.; Saad Ansari, A.; Pawar, S.M.; Han, J.; Park, S.; Shin, G.; Yeon, S.; Cho, S.; Seol, J.; et al. Hybridized heterostructure of CoS and MoS2 nanoparticles for highly-efficient and robust bifunctional water electrolysis. Appl. Surf. Sci. 2022, 592, 153196. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Seol, J.H.; Seok, J.H.; Jana, A.; Meena, A.; Cho, S.; Sree, V.G.; Park, Y.; Lee, S.U.; Im, H. Boosting the electrocatalytic performance of CuCo2S4 via surface-state engineering for ampere current water electrolysis applications. Appl. Surf. Sci. 2025, 680, 161353. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Sree, V.G.; Meena, A.; Inamdar, A.I.; Im, H.; Cho, S. In Situ Transformed CoOOH@Co3S4 Heterostructured Catalyst for Highly Efficient Catalytic OER Application. Nanomaterials 2024, 14, 1732. [Google Scholar] [CrossRef]
- Hanan, A.; Lakhan, M.N.; Shu, D.; Hussain, A.; Ahmed, M.; Soomro, I.A.; Kumar, V.; Cao, D. An efficient and durable bifunctional electrocatalyst based on PdO and Co2FeO4 for HER and OER. Int. J. Hydrogen Energy 2023, 48, 19494–19508. [Google Scholar] [CrossRef]
- Aqueel Ahmed, A.T.; Pawar, S.M.; Inamdar, A.I.; Kim, H.; Im, H. A Morphologically Engineered Robust Bifunctional CuCo2O4 Nanosheet Catalyst for Highly Efficient Overall Water Splitting. Adv. Mater. Interfaces 2020, 7, 1901515. [Google Scholar] [CrossRef]
- Tan, S.; Ji, Y.; Ren, F.; Chen, F.; Ouyang, W. Improved energy conversion and storage performance enabled by hierarchical zigzag-like P-doped CuCo2O4 nanosheets based 3D electrode materials. Int. J. Hydrogen Energy 2022, 47, 9248–9260. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Wang, Z.; Kang, Y.-M.; Bando, Y.; Yamauchi, Y. Elaborately assembled core-shell structured metal sulfides as a bifunctional catalyst for highly efficient electrochemical overall water splitting. Nano Energy 2018, 47, 494–502. [Google Scholar] [CrossRef]
- Wang, K.; Lin, Z.; Tang, Y.; Tang, Z.; Tao, C.-L.; Qin, D.-D.; Tian, Y. Selenide/sulfide heterostructured NiCo2Se4/NiCoS4 for oxygen evolution reaction, hydrogen evolution reaction, water splitting and Zn-air batteries. Electrochim. Acta 2021, 368, 137584. [Google Scholar] [CrossRef]
- Wang, C.; Jiu, H.; Zhang, L.; Song, W.; Zhang, Y.; Wei, H.; Xu, Q.; Che, S.; Guo, Z.; Qin, Y. Bifunctional CuCo2O4/CoOOH as a synergistic catalyst supported on nickel foam for alkaline overall water splitting. J. Alloys Compd. 2022, 929, 167367. [Google Scholar] [CrossRef]
- Zequine, C.; Wang, F.; Li, X.; Guragain, D.; Mishra, S.R.; Siam, K.; Kahol, P.K.; Gupta, R.K. Nanosheets of CuCo2O4 As a High-Performance Electrocatalyst in Urea Oxidation. Appl. Sci. 2019, 9, 793. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Sekar, S.; Khadtare, S.S.; Rochman, N.T.; Chinna, B.; Ansari, A.S. Anion-exchange synthesis of an MnCo2S4 electrocatalyst towards facilitated ultralong hydrogen evolution reaction in acidic and alkaline media. CrystEngComm 2024, 26, 215–222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Momin, M.M.; Ansari, A.S.; Cho, S.; Lee, Y.; Lee, S.; Ahmed, A.T.A. Unlocking the Cu-Co Interplay: Electrodeposited Spinel Co2CuO4 as a High-Performance Hydrogen Evolution Catalyst. Int. J. Mol. Sci. 2025, 26, 11226. https://doi.org/10.3390/ijms262211226
Sekar S, Momin MM, Ansari AS, Cho S, Lee Y, Lee S, Ahmed ATA. Unlocking the Cu-Co Interplay: Electrodeposited Spinel Co2CuO4 as a High-Performance Hydrogen Evolution Catalyst. International Journal of Molecular Sciences. 2025; 26(22):11226. https://doi.org/10.3390/ijms262211226
Chicago/Turabian StyleSekar, Sankar, M. Mujtaba Momin, Abu Saad Ansari, Sangeun Cho, Youngmin Lee, Sejoon Lee, and Abu Talha Aqueel Ahmed. 2025. "Unlocking the Cu-Co Interplay: Electrodeposited Spinel Co2CuO4 as a High-Performance Hydrogen Evolution Catalyst" International Journal of Molecular Sciences 26, no. 22: 11226. https://doi.org/10.3390/ijms262211226
APA StyleSekar, S., Momin, M. M., Ansari, A. S., Cho, S., Lee, Y., Lee, S., & Ahmed, A. T. A. (2025). Unlocking the Cu-Co Interplay: Electrodeposited Spinel Co2CuO4 as a High-Performance Hydrogen Evolution Catalyst. International Journal of Molecular Sciences, 26(22), 11226. https://doi.org/10.3390/ijms262211226






