Evaluation of the Telomere Length in Patients with Spinal Muscular Atrophy
Abstract
1. Introduction
2. Results
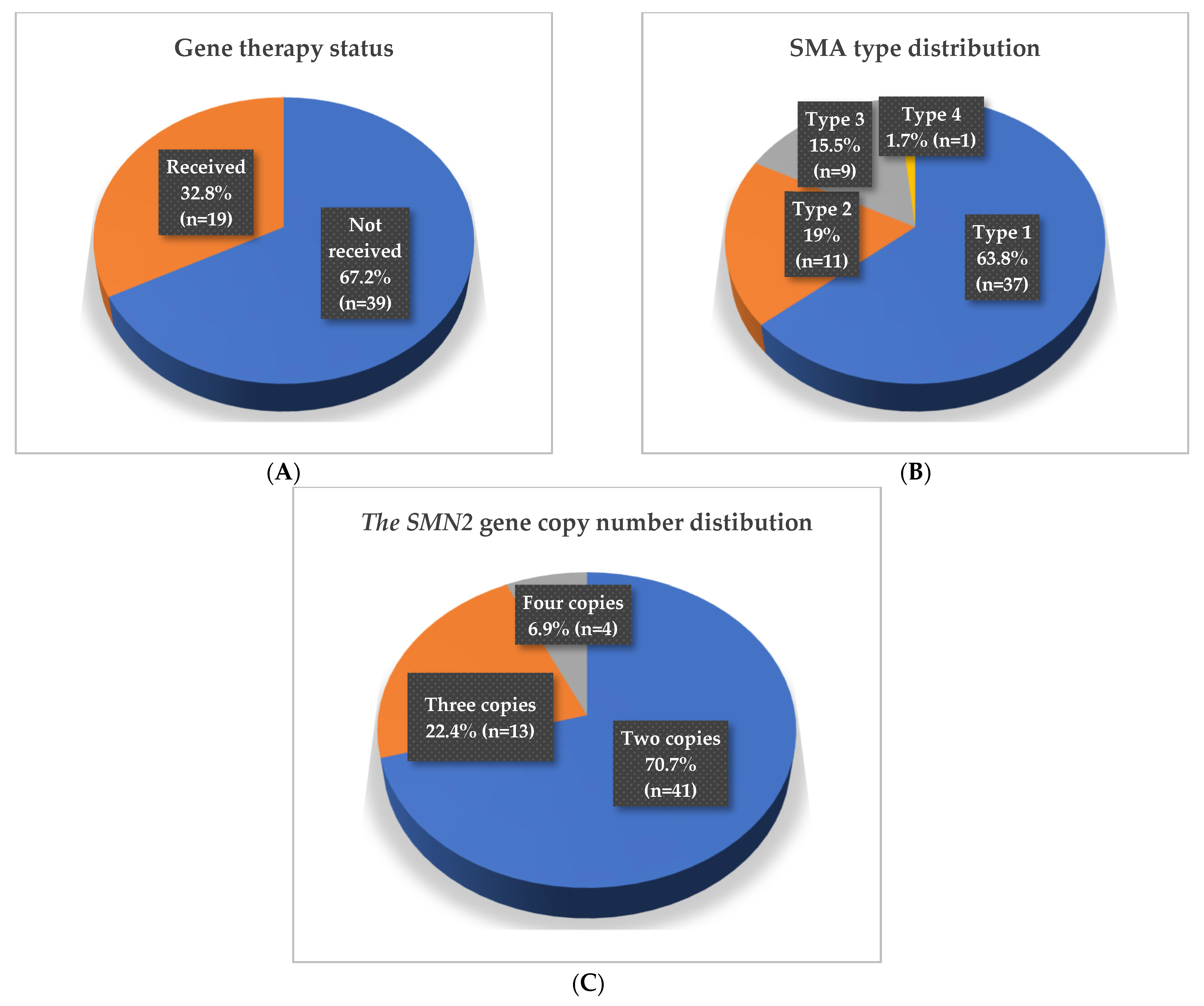
2.1. Clinical and Demographic Characteristics of the Study Cohort
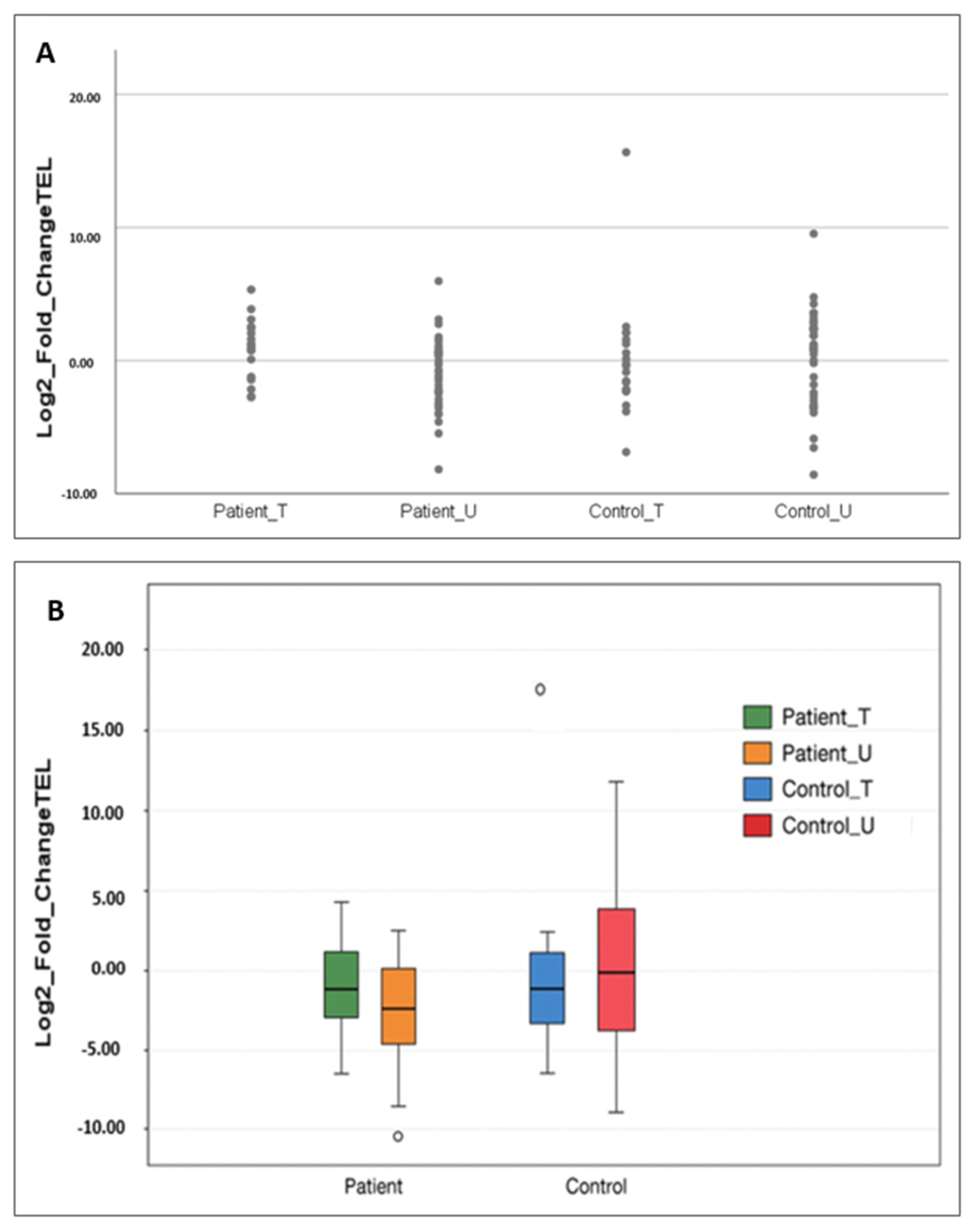
2.2. Telomere Length Evaluation in Patients and Controls
2.2.1. Comparison by Gene Replacement Therapy Status
2.2.2. Comparison by SMA Type
2.2.3. Comparison by the SMN2 Gene Copy Number
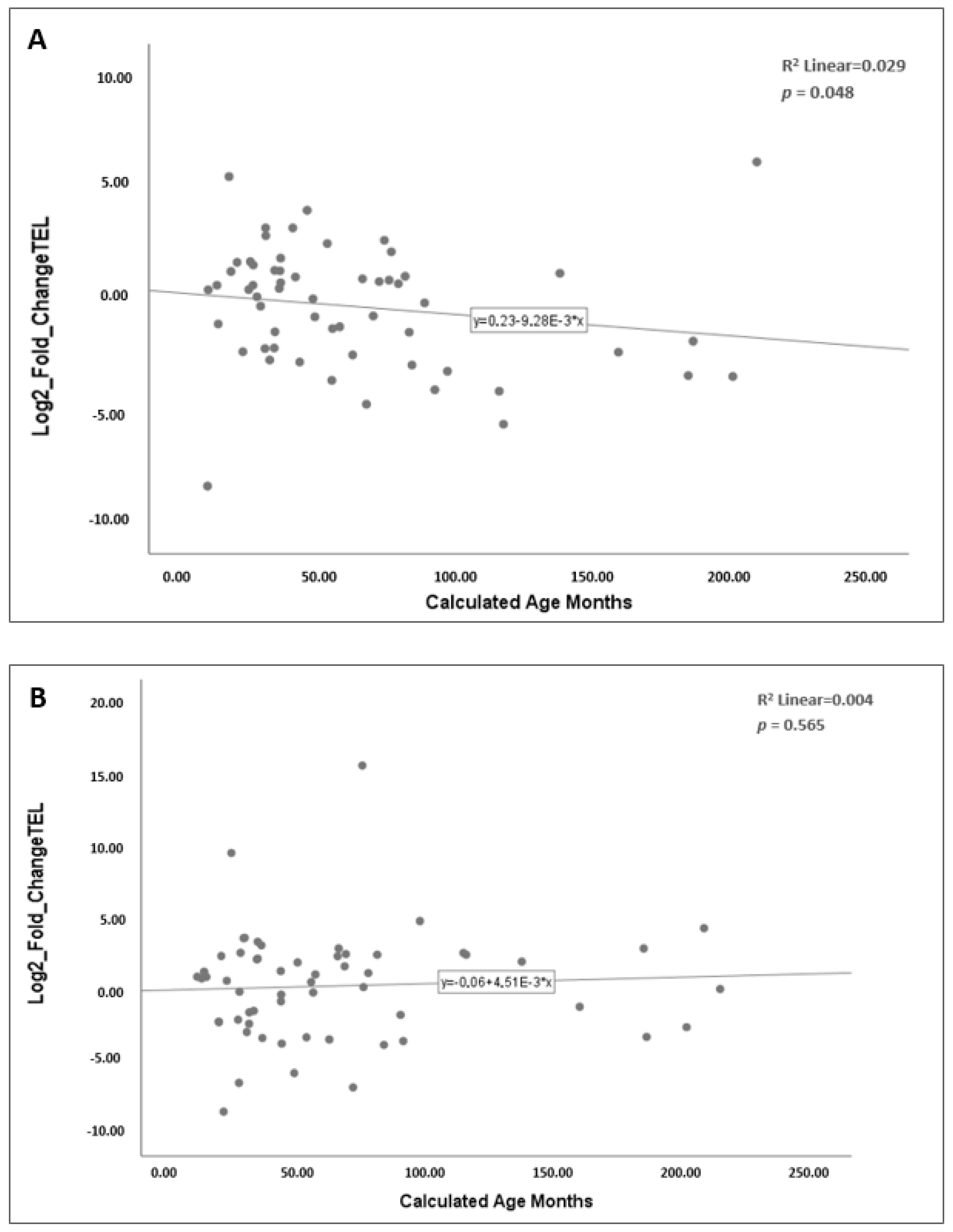
2.2.4. Correlation of Telomere Length with Age
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Sample Collection and Study Population
4.2. Genomic DNA Isolation
4.3. Quantification of Relative Telomere Length
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMA | Spinal Muscular Atrophy |
| PCR | Polymerase Chain Reaction |
| SMN | Survival Motor Neuron |
| RNA | Ribonucleic Acid |
| mRNA | Messenger RNA |
| AAV | Adeno-Associated Viral Vector |
| snRNP | Small Nuclear Ribonucleoprotein |
| DNA | Deoxyribonucleic Acid |
| qPCR | Quantitative Polymerase Chain Reaction |
| MMQPCR | Monochrome Multiplex Quantitative Polymerase Chain Reaction |
| Ct | Threshold Cycle |
| ALS | Amyotrophic Lateral Sclerosis |
| EDTA | Ethylenediamine Tetraacetic Acid |
References
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef]
- Mercuri, E.; Sumner, C.J.; Muntoni, F.; Darras, B.T.; Finkel, R.S. Spinal muscular atrophy. Nat. Rev. Dis. Primers 2022, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Ebeling, M.; Baird, J.; Bendels, S.; Bylund, J.; Chen, K.S.; Denk, N.; Feng, Z.; Green, L.; Guerard, M.; et al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 6501–6517. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Cooper, K.; Nalbant, G.; Sutton, A.; Harnan, S.; Thokala, P.; Chilcott, J.; McNeill, A.; Bessey, A. Systematic Review of Presymptomatic Treatment for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 56. [Google Scholar] [CrossRef]
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Lu, J.; Cheng, L.; Wu, D.; Li, L.; Zhang, S.; Lai, X.; Xu, L. The relationship between telomere length and aging-related diseases. Clin. Exp. Med. 2025, 25, 72. [Google Scholar] [CrossRef]
- Greider, C.W.; Blackburn, E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Machyna, M.; Heyn, P.; Neugebauer, K.M. Cajal bodies: Where form meets function. Wiley Interdiscip. Rev. RNA 2013, 4, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Hebert, M.D. SMN and coilin negatively regulate dyskerin association with telomerase RNA. Biol. Open 2016, 5, 726–735. [Google Scholar] [CrossRef]
- Bachand, F.; Boisvert, F.M.; Cote, J.; Richard, S.; Autexier, C. The product of the survival of motor neuron (SMN) gene is a human telomerase-associated protein. Mol. Biol. Cell 2002, 13, 3192–3202. [Google Scholar] [CrossRef]
- Lansdorp, P.M. Telomeres, stem cells, and hematology. Blood 2008, 111, 1759–1766. [Google Scholar] [CrossRef]
- Hassan, R.; Bhat, G.R.; Mir, F.A.; Ganie, H.A.; Mushtaq, I.; Bhat, M.A.; Asimi, R.P.; Afroze, D. Concomitant telomere attrition is associated with spinal muscular atrophy in highly inbred region of North India: Unraveling the thread in Kashmir region. BMC Med. Genom. 2024, 17, 275. [Google Scholar] [CrossRef]
- Martin, N.A.; McLester-Davis, L.W.Y.; Roy, T.R.; Magruder, M.G.; Hastings, W.J.; Drury, S.S. Monochrome Multiplex Quantitative PCR Telomere Length Measurement. J. Vis. Exp. JoVE 2024, 205, e66545. [Google Scholar] [CrossRef]
- Friedrich, U.; Schwab, M.; Griese, E.U.; Fritz, P.; Klotz, U. Telomeres in neonates: New insights in fetal hematopoiesis. Pediatr. Res. 2001, 49, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Yu, J.; Basanta-Henry, P.; Brou, L.; Berga, S.L.; Fortunato, S.J.; Taylor, R.N. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS ONE 2012, 7, e31136. [Google Scholar] [CrossRef]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Vasu, V.; Turner, K.J.; George, S.; Greenall, J.; Slijepcevic, P.; Griffin, D.K. Preterm infants have significantly longer telomeres than their term born counterparts. PLoS ONE 2017, 12, e0180082. [Google Scholar] [CrossRef]
- Turner, S.; Wong, H.P.; Rai, J.; Hartshorne, G.M. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol. Hum. Reprod. 2010, 16, 685–694. [Google Scholar] [CrossRef]
- Holmes, D.K.; Bellantuono, I.; Walkinshaw, S.A.; Alfirevic, Z.; Johnston, T.A.; Subhedar, N.V.; Chittick, R.; Swindell, R.; Wynn, R.F. Telomere length dynamics differ in foetal and early post-natal human leukocytes in a longitudinal study. Biogerontology 2009, 10, 279–284. [Google Scholar] [CrossRef]
- Youngren, K.; Jeanclos, E.; Aviv, H.; Kimura, M.; Stock, J.; Hanna, M.; Skurnick, J.; Bardeguez, A.; Aviv, A. Synchrony in telomere length of the human fetus. Hum. Genet. 1998, 102, 640–643. [Google Scholar] [CrossRef]
- Akkad, A.; Hastings, R.; Konje, J.C.; Bell, S.C.; Thurston, H.; Williams, B. Telomere length in small-for-gestational-age babies. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Factor-Litvak, P.; Susser, E.; Kezios, K.; McKeague, I.; Kark, J.D.; Hoffman, M.; Kimura, M.; Wapner, R.; Aviv, A. Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics 2016, 137, e20153927. [Google Scholar] [CrossRef] [PubMed]
- Njajou, O.T.; Cawthon, R.M.; Damcott, C.M.; Wu, S.H.; Ott, S.; Garant, M.J.; Blackburn, E.H.; Mitchell, B.D.; Shuldiner, A.R.; Hsueh, W.C. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 12135–12139. [Google Scholar] [CrossRef] [PubMed]
- Nordfjäll, K.; Larefalk, A.; Lindgren, P.; Holmberg, D.; Roos, G. Telomere length and heredity: Indications of paternal inheritance. Proc. Natl. Acad. Sci. USA 2005, 102, 16374–16378. [Google Scholar] [CrossRef]
- Kong, L.; Valdivia, D.O.; Simon, C.M.; Hassinan, C.W.; Delestrée, N.; Ramos, D.M.; Park, J.H.; Pilato, C.M.; Xu, X.; Crowder, M.; et al. Impaired prenatal motor axon development necessitates early therapeutic intervention in severe SMA. Sci. Transl. Med. 2021, 13, eabb6871. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—A literature review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Alajjuri, M.A.; Abusamra, R.; Mundada, V.; Narayan, O. Real-World Data in Children with Spinal Muscular Atrophy Type 1 on Long-Term Ventilation Receiving Gene Therapy: A Prospective Cohort Study. Adv. Respir. Med. 2024, 92, 338–347. [Google Scholar] [CrossRef]
- Hebert, M.D.; Szymczyk, P.W.; Shpargel, K.B.; Matera, A.G. Coilin forms the bridge between Cajal bodies and SMN, the spinal muscular atrophy protein. Genes Dev. 2001, 15, 2720–2729. [Google Scholar] [CrossRef]
- Jády, B.E.; Bertrand, E.; Kiss, T. Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal. J. Cell Biol. 2004, 164, 647–652. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Saffrey, M.J.; Cameron, K.; et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 2012, 11, 996–1004. [Google Scholar] [CrossRef]
- Grodstein, F.; van Oijen, M.; Irizarry, M.C.; Rosas, H.D.; Hyman, B.T.; Growdon, J.H.; De Vivo, I. Shorter telomeres may mark early risk of dementia: Preliminary analysis of 62 participants from the nurses’ health study. PLoS ONE 2008, 3, e1590. [Google Scholar] [CrossRef]
- Thomas, P.; O’Callaghan, N.J.; Fenech, M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech. Ageing Dev. 2008, 129, 183–190. [Google Scholar] [CrossRef]
- Guan, J.Z.; Maeda, T.; Sugano, M.; Oyama, J.; Higuchi, Y.; Suzuki, T.; Makino, N. A percentage analysis of the telomere length in Parkinson’s disease patients. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 467–473. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Annunziata, A.; Fiorentino, G.; Manfellotto, F.; D’Alessandro, R.; Marino, R.; Borra, M.; Biffali, E. Telomerase expression in amyotrophic lateral sclerosis (ALS) patients. J. Hum. Genet. 2014, 59, 555–561. [Google Scholar] [CrossRef]
- Al Khleifat, A.; Iacoangeli, A.; Shatunov, A.; Fang, T.; Sproviero, W.; Jones, A.R.; Opie-Martin, S.; Morrison, K.E.; Shaw, P.J.; Shaw, C.E.; et al. Telomere length is greater in ALS than in controls: A whole genome sequencing study. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 229–234. [Google Scholar] [CrossRef]
- Eitan, E.; Tichon, A.; Gazit, A.; Gitler, D.; Slavin, S.; Priel, E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol. Med. 2012, 4, 313–329. [Google Scholar] [CrossRef]
- Harley, J.; Santosa, M.M.; Ng, C.Y.; Grinchuk, O.V.; Hor, J.H.; Liang, Y.; Lim, V.J.; Tee, W.W.; Ong, D.S.T.; Ng, S.Y. Telomere shortening induces aging-associated phenotypes in hiPSC-derived neurons and astrocytes. Biogerontology 2024, 25, 341–360. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Müller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365, Erratum in Nature 2011, 475, 254. [Google Scholar] [CrossRef]
- Ye, Q.; Apsley, A.T.; Etzel, L.; Hastings, W.J.; Kozlosky, J.T.; Walker, C.; Wolf, S.E.; Shalev, I. Telomere length and chronological age across the human lifespan: A systematic review and meta-analysis of 414 study samples including 743,019 individuals. Ageing Res. Rev. 2023, 90, 102031. [Google Scholar] [CrossRef]
- René, C.A.; Parks, R.J. Expanding the Availability of Onasemnogene Abeparvovec to Older Patients: The Evolving Treatment Landscape for Spinal Muscular Atrophy. Pharmaceutics 2023, 15, 1764. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, G.; Burghes, A.H.M.; Hsieh, C.; Do, J.; Chu, B.T.T.; Perry, S.; Barkho, B.; Kaufmann, P.; Sproule, D.M.; Feltner, D.E.; et al. Biodistribution of onasemnogene abeparvovec DNA, mRNA and SMN protein in human tissue. Nat. Med. 2021, 27, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.R.; Zhang, R.; Johnstone, A.J.; Garner, R.; Eichelberger, E.J.; Lepez, S.; Yi, V.; Stevens, V.; Poxson, R.; Schwartz, R.; et al. Whole blood survival motor neuron protein levels correlate with severity of denervation in spinal muscular atrophy. Muscle Nerve 2020, 62, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Okur Altındaş, B. Spinal Musküler Atrofi Hastalarında Telomer Uzunluğunun Değerlendirilmesi. Residency Thesis, Necmettin Erbakan University, Konya, Türkiye, 2025. [Google Scholar]
| Participants (n, %) | Mean Age (Months) ± SD | Median Age (Months) | Minimum (Months) | Maximum (Months) |
|---|---|---|---|---|
| Patient Group (n = 58) | - | 44.9 | 7.5 | 208.3 |
| Untreated (n = 39) | - | 53.1 | 7.5 | 208.3 |
| Treated (n = 19) | 42.1 ± 19.1 | - | 15.3 | 74.7 |
| SMA type 1 (n = 37, 63.8%) | - | 34.2 | 7.5 | 114.1 |
| Treated (n = 18, 48.6%) | 42.8 ± 18.4 | - | - | - |
| Untreated (n = 19, 51.4%) | - | 33.6 | 7.5 | 114.1 |
| SMA type 2 (n = 11, 19%) | 75.3 ± 52.9 | - | - | - |
| Treated (n = 1, 9.1%) | 30.2 | - | - | - |
| Untreated (n = 10, 90.9%) | 79.9 ± 53.5 | - | - | - |
| SMA type 3 (n = 9, 15.5%) | 113.7 ± 72.8 | - | - | - |
| SMA type 4 (n = 1, 1.7%) | 20.4 | - | - | - |
| Two SMN2 copies (n = 41, 70.7%) | - | 38.6 | 7.5 | 183.2 |
| Treated (n = 18, 43.9%) | 42.8 ± 19.5 | - | - | - |
| Untreated (n = 23, 56.1%) | - | 34.2 | 7.5 | 183.2 |
| Three SMN2 copies (n = 13, 22.4%) | - | 65.6 | 26.9 | 185 |
| Treated (n = 1, 7.7%) | 30.2 | - | - | - |
| Untreated (n = 12, 92.3%) | 72.8 ± 44.5 | - | - | - |
| Four SMN2 copies (n = 4, 6.9%) | 146.5 ± 86.9 | - | - | - |
| Control Group (n = 58) | - | 43.5 | 8 | 212.5 |
| TOTAL | - | 44.9 | 7.5 | 212.5 |
| Group | Comparison Group | p Value |
|---|---|---|
| Patient group (n = 58) | Control group | 0.346 * |
| Untreated patients (n = 39) | Their matched controls | 0.029 * |
| Treated patients (n = 19) | Their matched controls | 0.108 * |
| Treated patients (n = 19) | Untreated patients (n = 39) | 0.012 + |
| Group | Comparison Group | p Value |
|---|---|---|
| SMA type 1 patients (n = 37) | Matched controls | 0.846 * |
| Untreated (n = 19) | Matched controls | 0.14 * |
| Treated (n = 18) | Matched controls | 0.09 * |
| Treated (n = 18) | Untreated (n = 19) | 0.032 + |
| SMA type 2 patients (n = 11) | Matched controls | 0.004 * |
| Untreated (n = 10) | Matched controls | 0.002 * |
| SMA type 3 patients (n = 9) | Matched controls | 0.757 * |
| Group | Comparison Group | p Value * |
|---|---|---|
| Patients with two SMN2 copies (n = 41) | Matched controls | 0.613 |
| Untreated (n = 23) | Matched controls | 0.024 |
| Treated (n = 18) | Matched controls | 0.9 |
| Treated (n = 18) | Untreated (n = 23) | 0.01 |
| Patients with three SMN2 copies (n = 13) | Matched controls | 0.521 |
| Untreated (n = 12) | Matched controls | 0.273 |
| Patients with four SMN2 copies (n = 4) | Matched controls | 0.773 |
| Primer Name | Nucleotide Sequence (5′ → 3′) |
|---|---|
| TEL-F | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT |
| TEL-R | GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT |
| IFNB1-F | TGGCACAACAGGTAGTAGGCGACAC |
| IFNB1-R | GCACAACAGGAGAGCAATTTGGAGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okur Altındaş, B.; Öktem, S.; Çarman, K.B.; Yıldırım, M.S. Evaluation of the Telomere Length in Patients with Spinal Muscular Atrophy. Int. J. Mol. Sci. 2025, 26, 11223. https://doi.org/10.3390/ijms262211223
Okur Altındaş B, Öktem S, Çarman KB, Yıldırım MS. Evaluation of the Telomere Length in Patients with Spinal Muscular Atrophy. International Journal of Molecular Sciences. 2025; 26(22):11223. https://doi.org/10.3390/ijms262211223
Chicago/Turabian StyleOkur Altındaş, Betül, Sedat Öktem, Kürşat Bora Çarman, and Mahmut Selman Yıldırım. 2025. "Evaluation of the Telomere Length in Patients with Spinal Muscular Atrophy" International Journal of Molecular Sciences 26, no. 22: 11223. https://doi.org/10.3390/ijms262211223
APA StyleOkur Altındaş, B., Öktem, S., Çarman, K. B., & Yıldırım, M. S. (2025). Evaluation of the Telomere Length in Patients with Spinal Muscular Atrophy. International Journal of Molecular Sciences, 26(22), 11223. https://doi.org/10.3390/ijms262211223






