Untargeted Metabolomics Identifies Faecal Filtrate-Derived Metabolites That Disrupt Clostridioides difficile Metabolism and Confer Gut Barrier Cytoprotection
Abstract
1. Introduction
2. Results
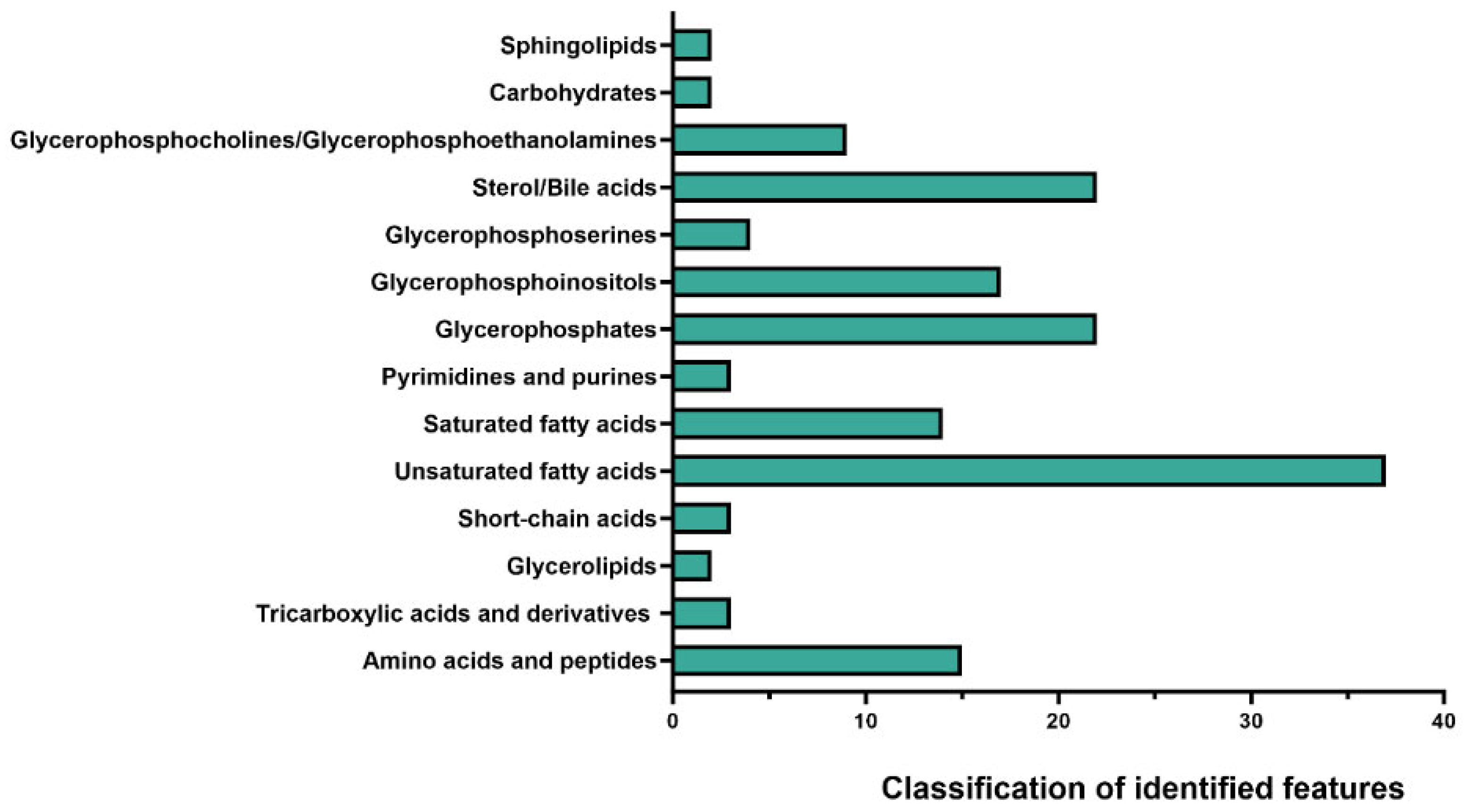
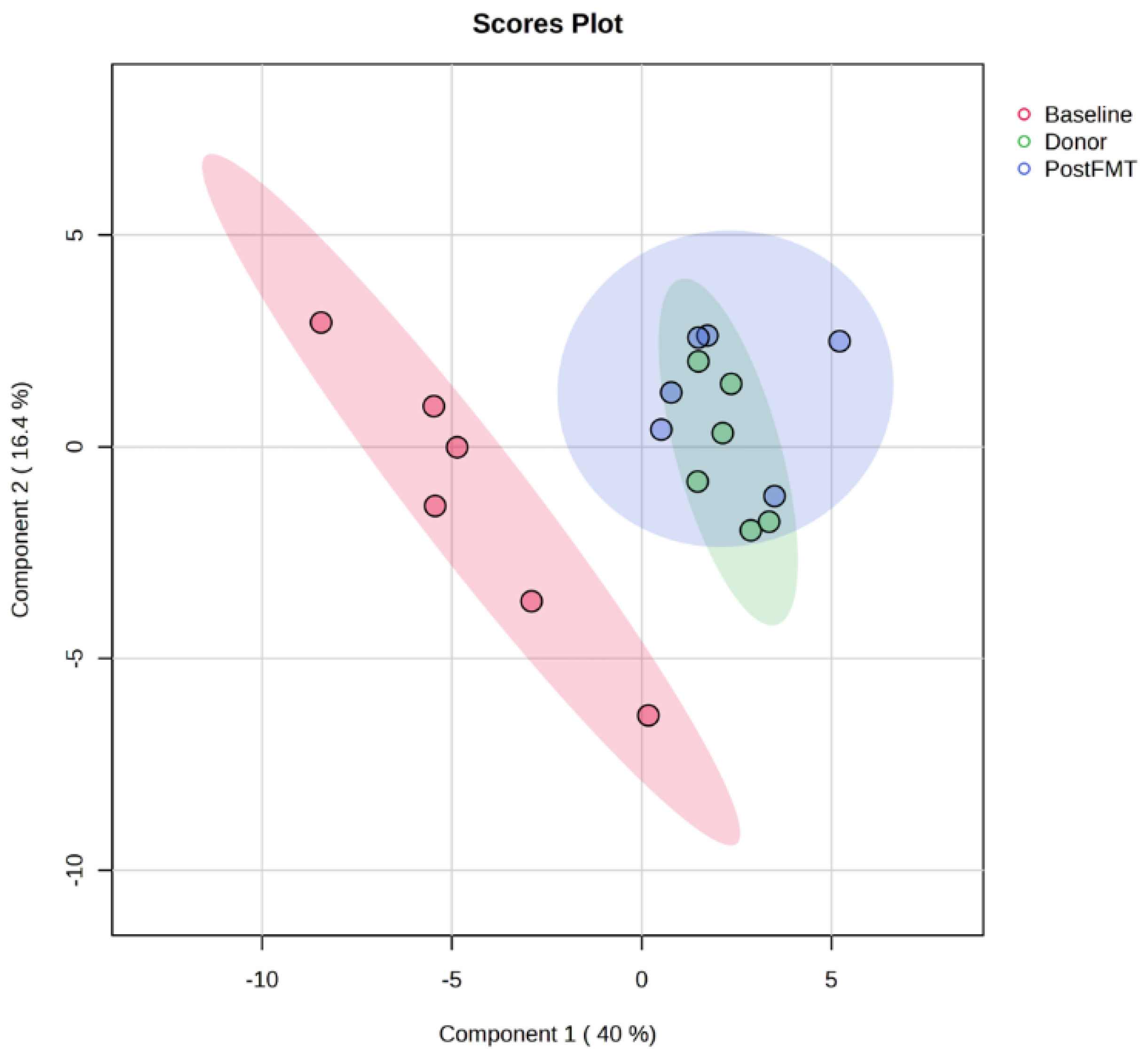
2.1. Distinct Metabolomic Profiles in Pre-FMT, Post-FMT, and Donor Samples by OrbiSIMS
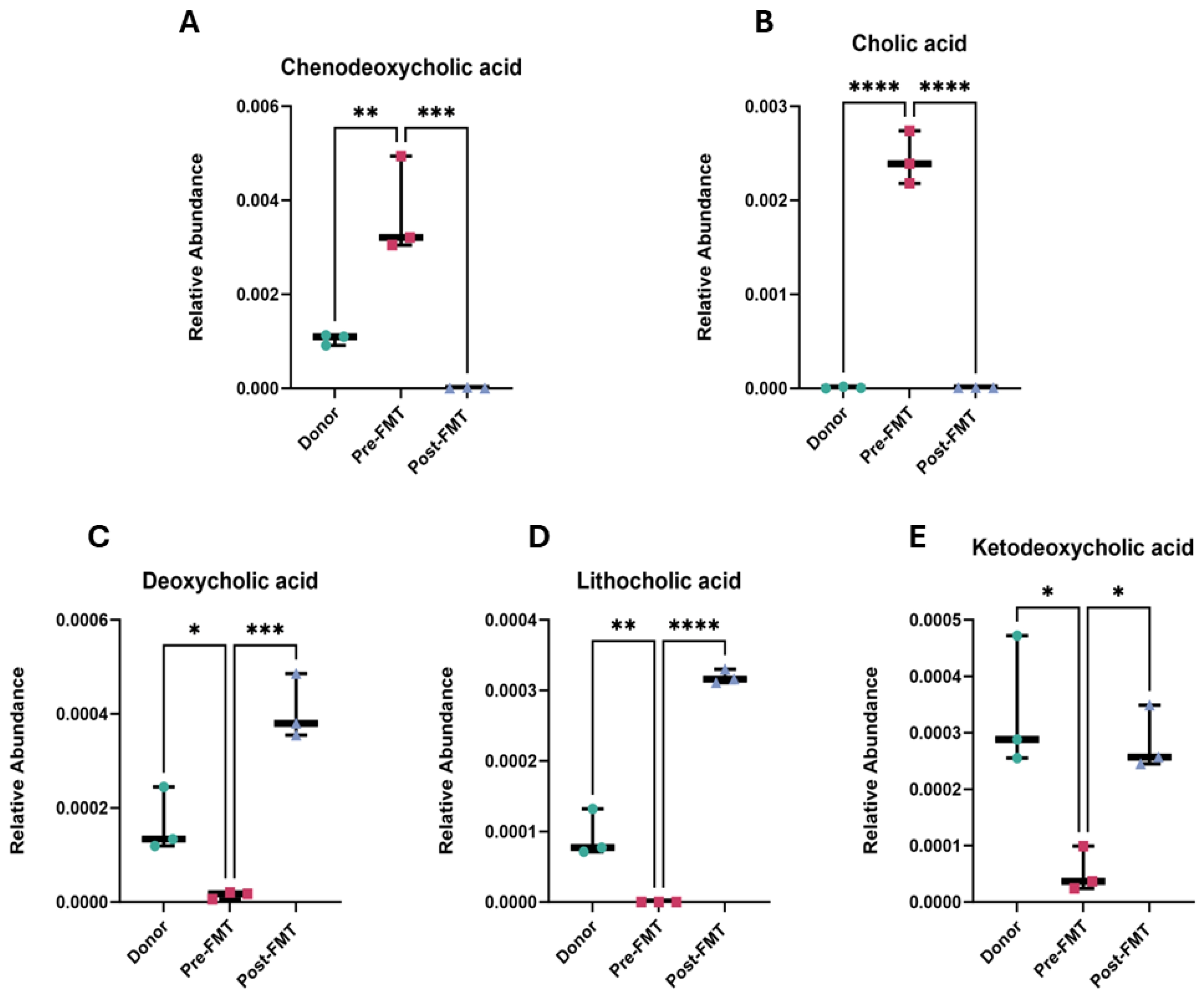
2.2. Elevated Primary Bile Acids and Amino Acids in Pre-FMT Stool Samples
2.3. Enrichment of Amino Acids in Pre-FMT Samples
2.4. Pathway Analysis of FMT-Induced Metabolic Reprogramming
2.5. FMT Alters Glyoxylate and Dicarboxylate Metabolism in rCDI Patients
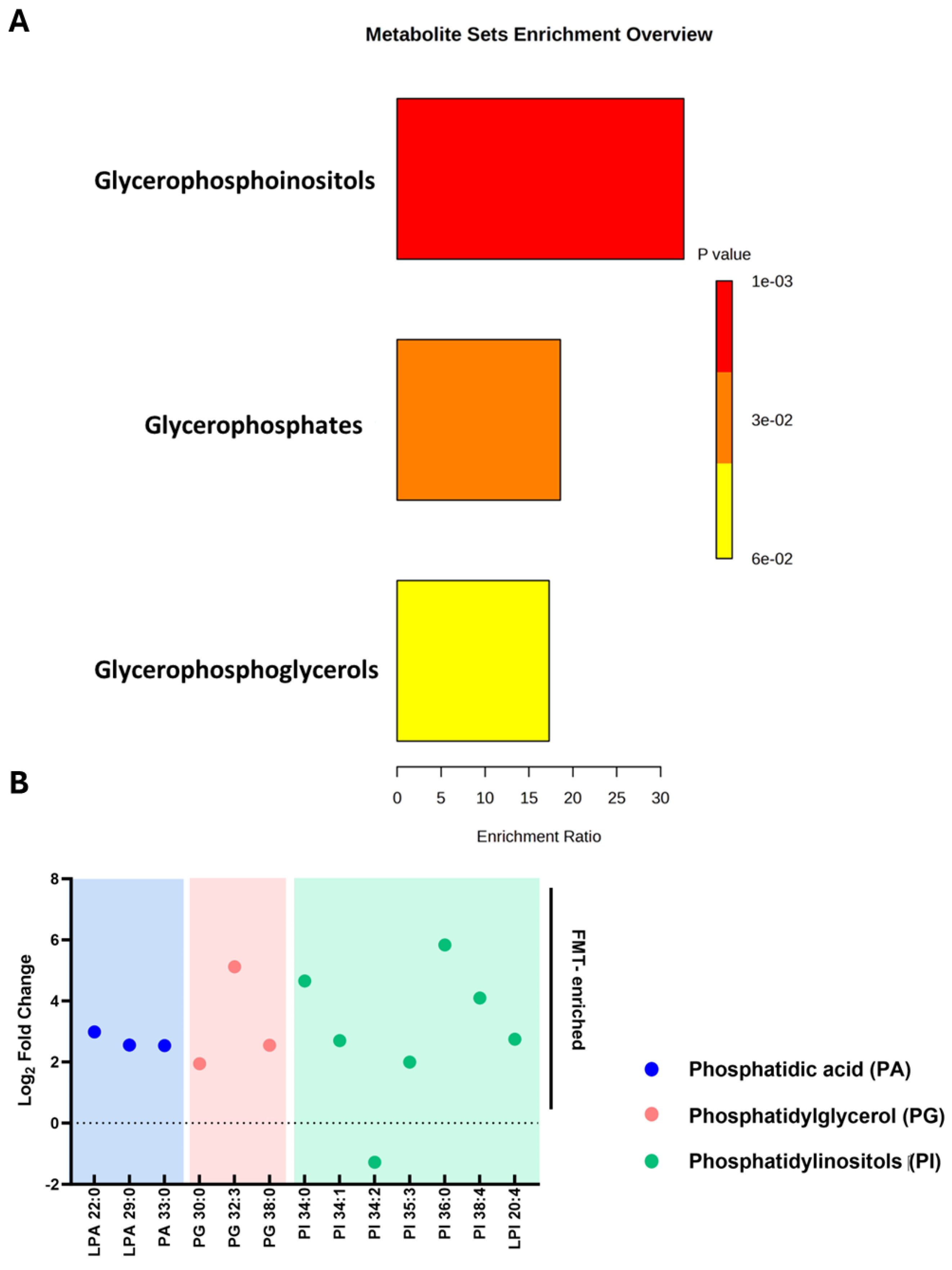
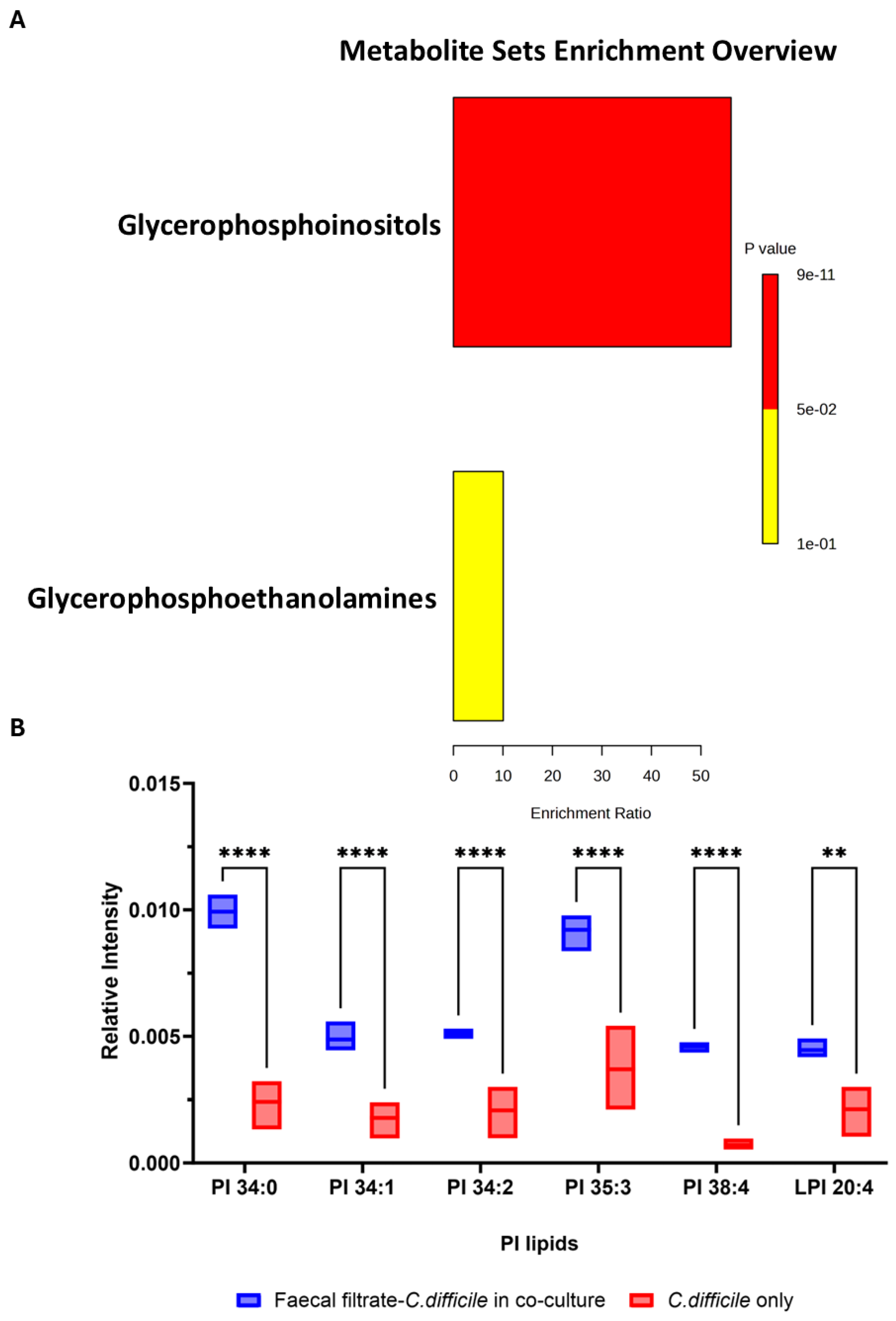
2.6. Restoration of Phosphatidylinositol Lipid Metabolism After FMT
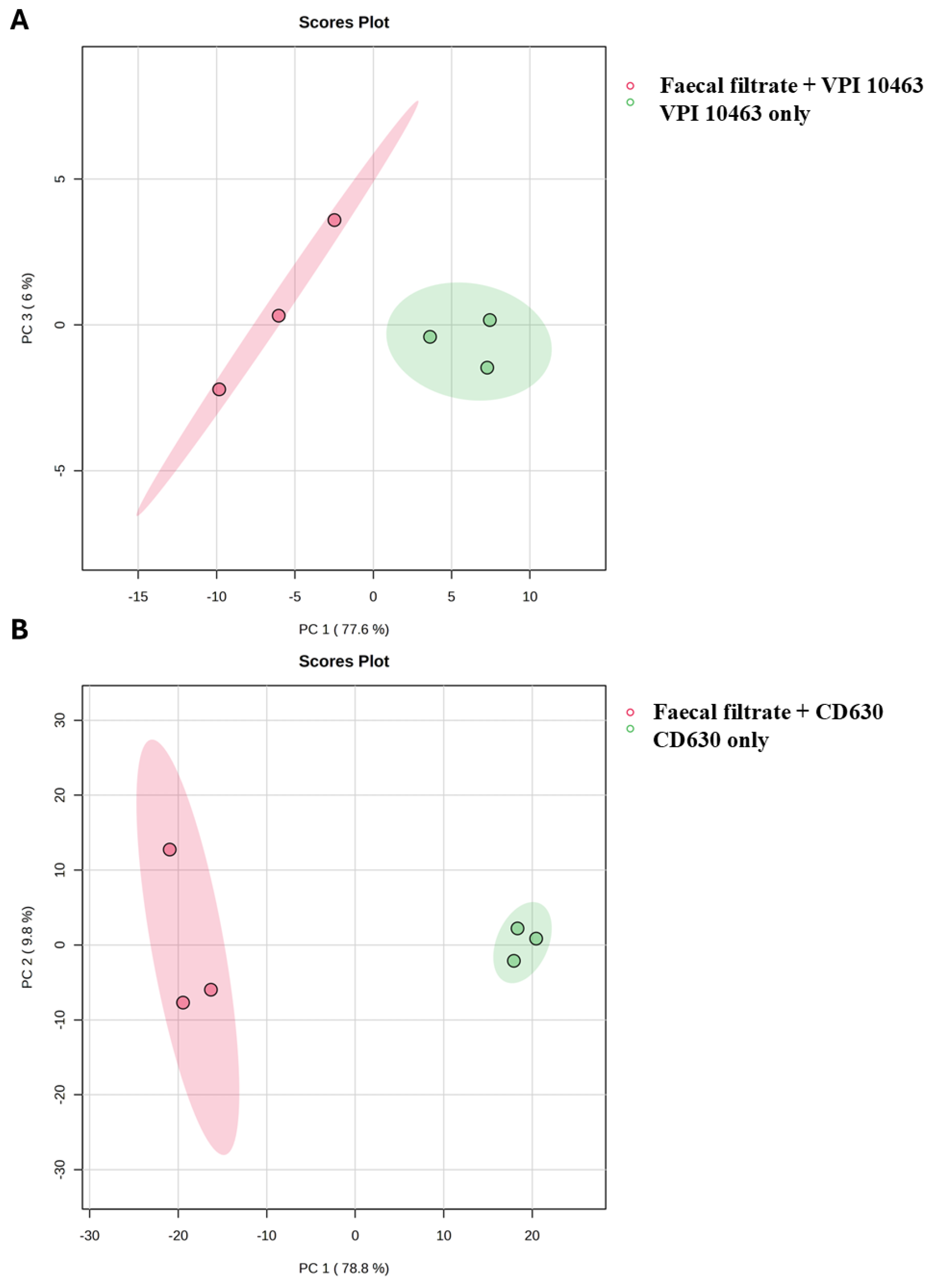
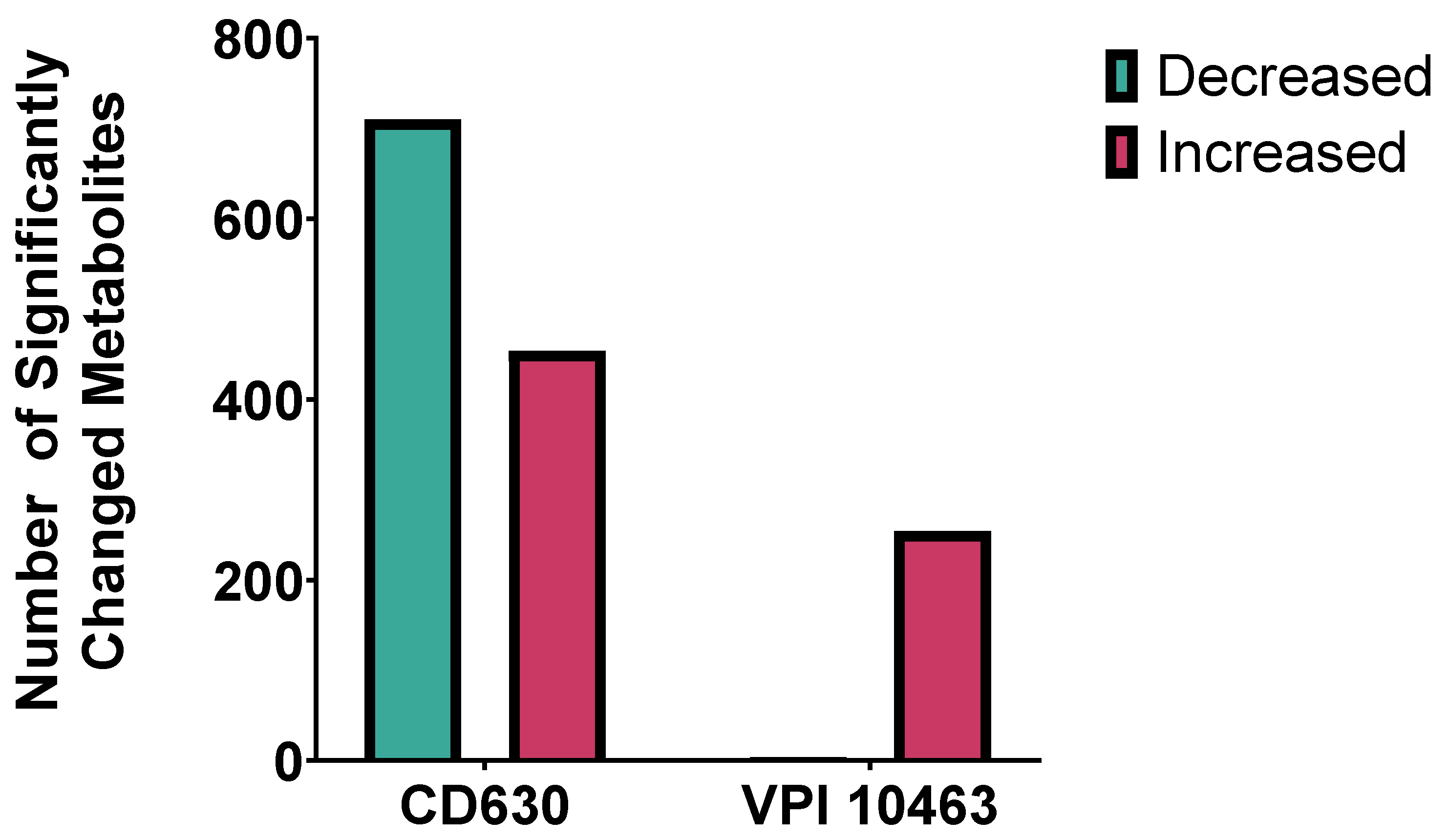
2.7. Faecal Filtrate Treatment Induces Strain-Specific Metabolic Reprogramming in C. difficile
2.8. Strain-Specific Metabolic Responses in VPI 10463 and CD630
2.9. Faecal Filtrate Co-Culture Alters Lipid Metabolism in C. difficile
2.10. Faecal Filtrate Modulates Virulence-Associated Metabolites in C. difficile
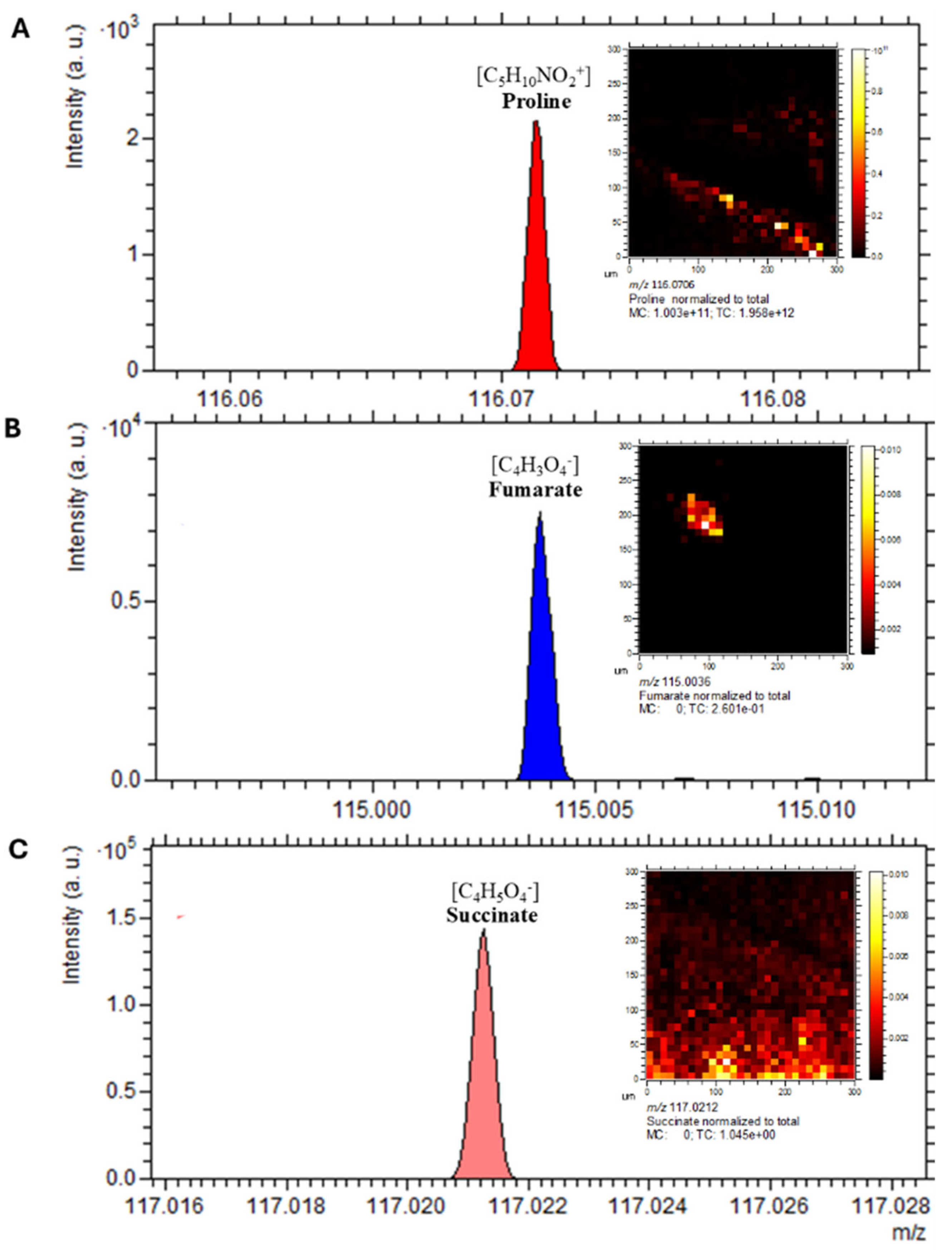
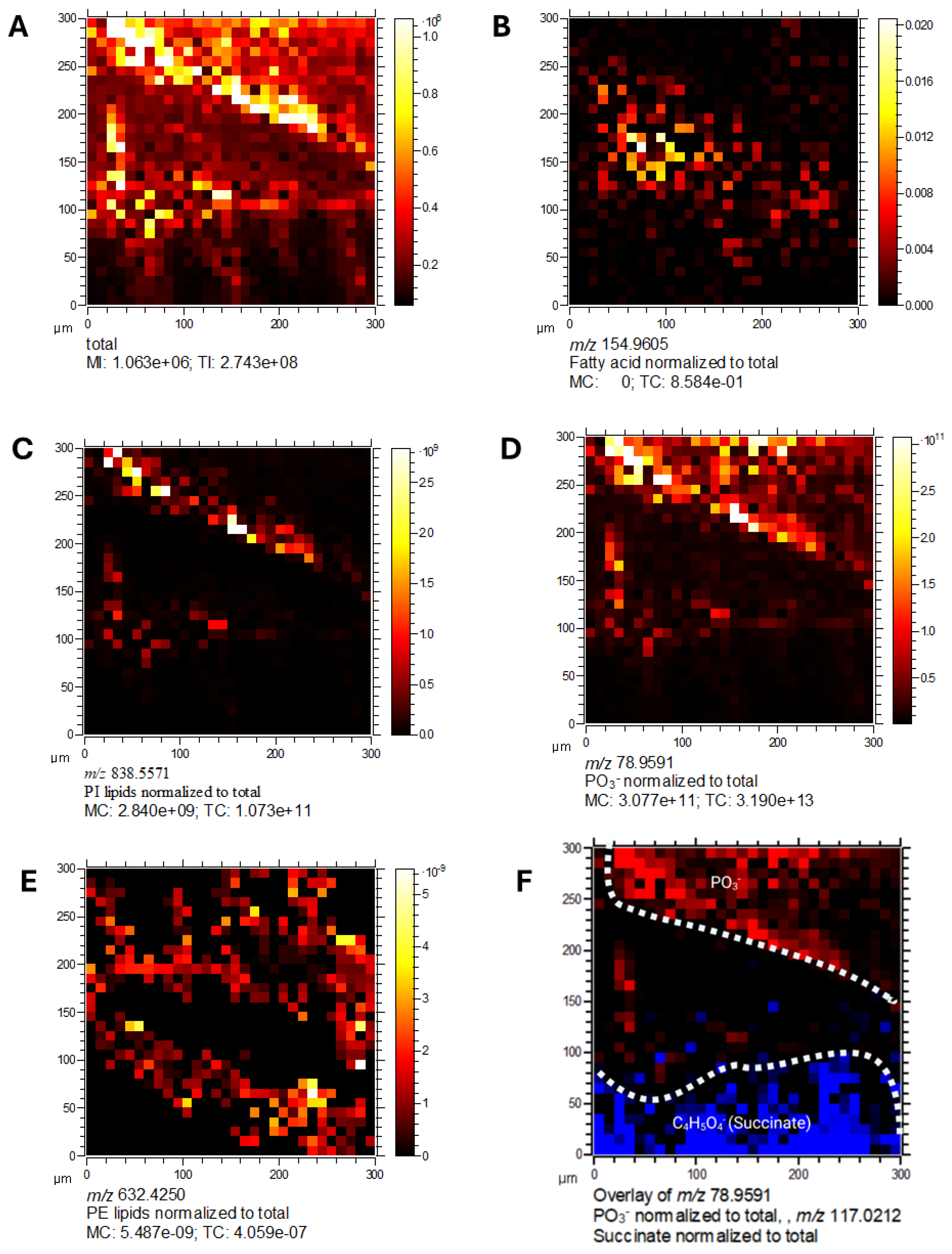
2.11. Spatial Localisation of Lipid Species in C. difficile
2.12. Metabolic Compartmentalisation Revealed by Spatial Imaging
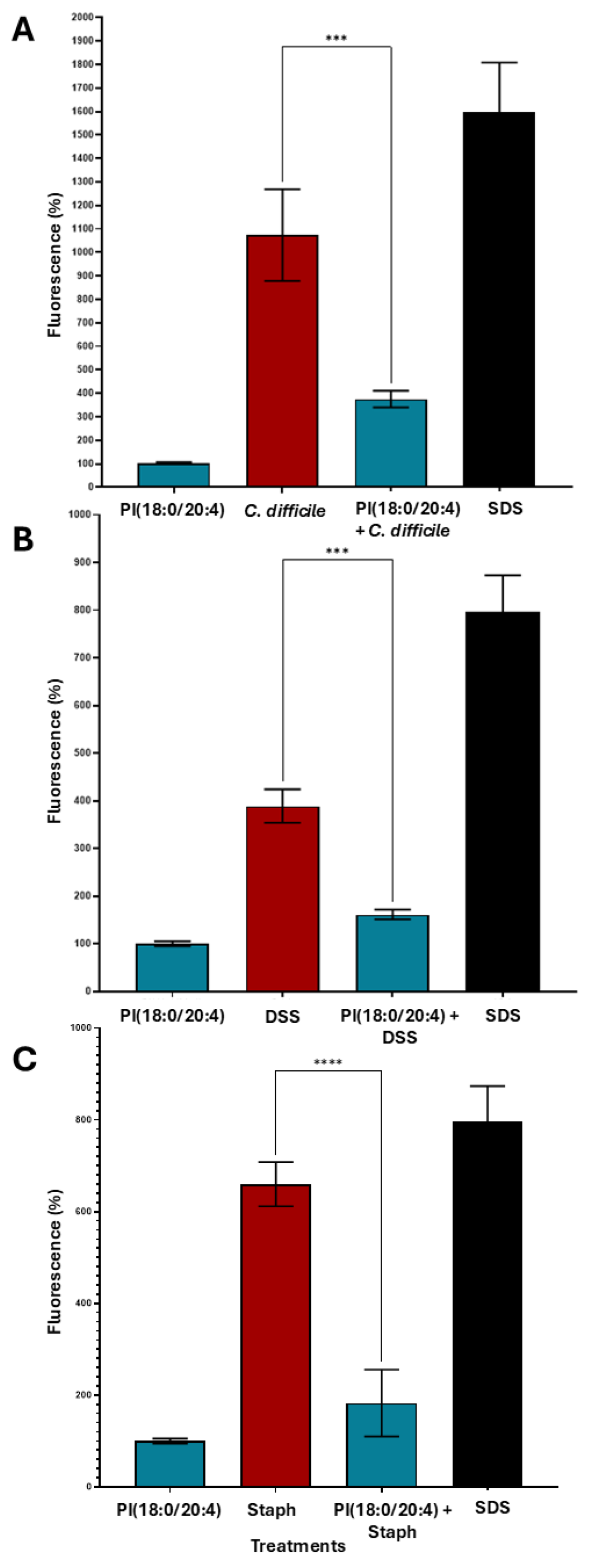
2.13. Metabolite-Specific Cytoprotective Effects of Phosphatidylinositols Against Toxin-Induced Epithelial Barrier Damage
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Preparation of Donor-Derived Faecal Filtrates for 3D OrbiSIMS
4.3. Metabolite Preparation and Treatment
4.4. Preparation of Caco-2 Transwell Monolayers
4.5. Barrier Integrity Measurements and Toxin Exposure
4.6. 3D OrbiSIMS Data Analysis
4.7. rCDI Participant Stool Samples
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feuerstadt, P.; Theriault, N.; Tillotson, G. The burden of CDI in the United States: A multifactorial challenge. BMC Infect. Dis. 2023, 23, 132. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. eClinicalMedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef] [PubMed]
- Yadegar, A.; Pakpoor, S.; Ibrahim, F.F.; Nabavi-Rad, A.; Cook, L.; Walter, J.; Seekatz, A.M.; Wong, K.; Monaghan, T.M.; Kao, D. Beneficial effects of fecal microbiota transplantation in recurrent Clostridioides difficile infection. Cell Host Microbe 2023, 31, 695–711. [Google Scholar] [CrossRef]
- Ott, S.J.; Waetzig, G.H.; Rehman, A.; Moltzau-Anderson, J.; Bharti, R.; Grasis, J.A.; Cassidy, L.; Tholey, A.; Fickenscher, H.; Seegert, D.; et al. Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients with Clostridium difficile Infection. Gastroenterology 2017, 152, 799–811.e7. [Google Scholar] [CrossRef]
- Kao, D.; Wong, K.; Lee, C.; Steiner, T.; Franz, R.; McDougall, C.; Silva, M.; Schmidt, T.S.B.; Walter, J.; Loebenberg, R.; et al. Effects of lyophilised faecal filtrate compared with lyophilised donor stool on Clostridioides difficile recurrence: A multicentre, randomised, double-blinded, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2025, 10, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Young, V.B. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 2012, 20, 313–319. [Google Scholar] [CrossRef]
- Yang, S.Y.; Han, S.M.; Lee, J.Y.; Kim, K.S.; Lee, J.E.; Lee, D.W. Advances in the integration of metabolomics and metagenomics for human gut microbiome and their clinical applications. TrAC Trends Anal. Chem. 2023, 167, 117248. [Google Scholar] [CrossRef]
- Geier, B.; Sogin, E.; Michellod, D.; Janda, M.; Kompauer, M.; Spengler, B.; Dubilier, N.; Liebeke, M. Spatial metabolomics of in situ host–microbe interactions at the micrometre scale. Nat. Microbiol. 2020, 5, 498–510. [Google Scholar] [CrossRef]
- Karu, N.; Deng, L.; Slae, M.; Guo, A.C.; Sajed, T.; Huynh, H.; Wine, E.; Wishart, D.S. A review on human fecal metabolomics: Methods, applications and the human fecal metabolome database. Anal. Chim. Acta 2018, 1030, 1–24. [Google Scholar] [CrossRef]
- Ratnasekhar, C.H.; Rathor, P.; Rakwal, P.; Verma, A.K.; Khan, S. Chapter One-Analytical Platforms in Metabolomics of Health and Disease. In Comprehensive Analytical Chemistry; Ratnasekhar, C.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 104, pp. 1–20. [Google Scholar]
- Griffiths, R.L. Surface-sampling mass spectrometry and imaging: Direct analysis of bacterial species. Surf. Interface Anal. 2021, 53, 999–1005. [Google Scholar] [CrossRef]
- Passarelli, M.K.; Pirkl, A.; Moellers, R.; Grinfeld, D.; Kollmer, F.; Havelund, R.; Newman, C.F.; Marshall, P.S.; Arlinghaus, H.; Alexander, M.R.; et al. The 3D OrbiSIMS—Label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat. Methods 2017, 14, 1175–1183. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018, 67, 634. [Google Scholar] [CrossRef]
- Mullish, B.H.; Allegretti, J.R. The contribution of bile acid metabolism to the pathogenesis of Clostridioides difficile infection. Therap Adv. Gastroenterol. 2021, 14, 17562848211017724. [Google Scholar] [CrossRef]
- Mullish, B.H.; Martinez-Gili, L.; Chekmeneva, E.; Correia, G.D.S.; Lewis, M.R.; Horneffer-Van Der Sluis, V.; Roberts, L.A.; McDonald, J.A.K.; Pechlivanis, A.; Walters, J.R.F.; et al. Assessing the clinical value of faecal bile acid profiling to predict recurrence in primary Clostridioides difficile infection. Aliment. Pharmacol. Ther. 2022, 56, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Aston, C.; Kelly, M.L.; Griffin, R. Towards Development of a Non-Toxigenic Clostridioides difficile Oral Spore Vaccine against Toxigenic C. difficile. Pharmaceutics 2022, 14, 1086. [Google Scholar] [CrossRef]
- Arcay, R.; Barceló-Nicolau, M.; Suárez, L.; Martín, L.; Reigada, R.; Höring, M.; Liebisch, G.; Garrido, C.; Cabot, G.; Vílchez, H.; et al. Gut microbiome and plasma lipidome analysis reveals a specific impact of Clostridioides difficile infection on intestinal bacterial communities and sterol metabolism. mBio 2024, 15, e0134724. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.B.; Li, S.S.; Maistrenko, O.M.; Akanni, W.; Coelho, L.P.; Dolai, S.; Fullam, A.; Glazek, A.M.; Hercog, R.; Herrema, H.; et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nat. Med. 2022, 28, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.L.; Pieper, D.H.; Stallmach, A.; Steube, A.; Vital, M.; Reck, M.; Wagner-Döbler, I. Engraftment of essential functions through multiple fecal microbiota transplants in chronic antibiotic-resistant pouchitis—A case study using metatranscriptomics. Microbiome 2023, 11, 269. [Google Scholar] [CrossRef]
- Mullish, B.H.; McDonald, J.A.K.; Pechlivanis, A.; Allegretti, J.R.; Kao, D.; Barker, G.F.; Kapila, D.; Petrof, E.O.; Joyce, S.A.; Gahan, C.G.M.; et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut 2019, 68, 1791. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking Gut Microbiome and Lipid Metabolism: Moving beyond Associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef]
- Heaver, S.L.; Le, H.H.; Tang, P.; Baslé, A.; Mirretta Barone, C.; Vu, D.L.; Waters, J.L.; Marles-Wright, J.; Johnson, E.L.; Campopiano, D.J.; et al. Characterization of inositol lipid metabolism in gut-associated Bacteroidetes. Nat. Microbiol. 2022, 7, 986–1000. [Google Scholar] [CrossRef]
- Dawkins, J.J.; Allegretti, J.R.; Gibson, T.E.; McClure, E.; Delaney, M.; Bry, L.; Gerber, G.K. Gut metabolites predict Clostridioides difficile recurrence. Microbiome 2022, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.K.; Williams, S.A.; Bindra, G.K.; Lay, F.T.; Poon, I.K.H.; Hulett, M.D. Phosphoinositides: Multipurpose cellular lipids with emerging roles in cell death. Cell Death Differ. 2019, 26, 781–793. [Google Scholar] [CrossRef]
- Vessichelli, M.; Mariggiò, S.; Varone, A.; Zizza, P.; Di Santo, A.; Amore, C.; Dell’Elba, G.; Cutignano, A.; Fontana, A.; Cacciapuoti, C.; et al. The natural phosphoinositide derivative glycerophosphoinositol inhibits the lipopolysaccharide-induced inflammatory and thrombotic responses. J. Biol. Chem. 2017, 292, 12828–12841. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37.e6. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174. [Google Scholar] [CrossRef]
- McMillan, A.S.; Zhang, G.; Dougherty, M.K.; McGill, S.K.; Gulati, A.S.; Baker, E.S.; Theriot, C.M. Metagenomic, metabolomic, and lipidomic shifts associated with fecal microbiota transplantation for recurrent Clostridioides difficile infection. mSphere 2024, 9, e0070624. [Google Scholar] [CrossRef]
- Laurent, B.; Self, W.T.; Sonenshein, A.L. Proline-Dependent Regulation of Clostridium difficile Stickland Metabolism. J. Bacteriol. 2013, 195, 844–854. [Google Scholar]
- Cersosimo, L.M.; Graham, M.; Monestier, A.; Pavao, A.; Worley, J.N.; Peltier, J.; Dupuy, B.; Bry, L. Central in vivo mechanisms by which C. difficile’s proline reductase drives efficient metabolism, growth, and toxin production. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ferreyra, J.A.; Wu, K.J.; Hryckowian, A.J.; Bouley, D.M.; Weimer, B.C.; Sonnenburg, J.L. Gut Microbiota-Produced Succinate Promotes C. difficile Infection after Antibiotic Treatment or Motility Disturbance. Cell Host Microbe 2014, 16, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Auria, E.; Deschamps, J.; Briandet, R.; Dupuy, B. Extracellular succinate induces spatially organized biofilm formation in Clostridioides difficile. Biofilm 2023, 5, 100125. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef]
- Neumann-Schaal, M.; Jahn, D.; Schmidt-Hohagen, K. Metabolism the Difficile Way: The Key to the Success of the Pathogen Clostridioides difficile. Front. Microbiol. 2019, 10, 219. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Antharam, V.C.; McEwen, D.C.; Garrett, T.J.; Dossey, A.T.; Li, E.C.; Kozlov, A.N.; Mesbah, Z.; Wang, G.P. An Integrated Metabolomic and Microbiome Analysis Identified Specific Gut Microbiota Associated with Fecal Cholesterol and Coprostanol in Clostridium difficile Infection. PLoS ONE 2016, 11, e0148824. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- Gianetto-Hill, C.M.; Vancuren, S.J.; Daisley, B.; Renwick, S.; Wilde, J.; Schroeter, K.; Daigneault, M.C.; Allen-Vercoe, E. The Robogut: A Bioreactor Model of the Human Colon for Evaluation of Gut Microbial Community Ecology and Function. Curr. Protoc. 2023, 3, e737. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Clevers, H. Organoids and organs-on-chips: Insights into human gut-microbe interactions. Cell Host Microbe 2021, 29, 867–878. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 2019, 3, 520–531, Erratum in Nat. Biomed Eng. 2019, 3, 583. [Google Scholar] [CrossRef]
- Ng, R.W.; Dharmaratne, P.; Wong, S.; Hawkey, P.; Chan, P.; Ip, M. Revisiting the donor screening protocol of faecal microbiota transplantation (FMT): A systematic review. Gut 2024, 73, 1029. [Google Scholar] [CrossRef]
- Edney, M.K.; Kotowska, A.M.; Spanu, M.; Trindade, G.F.; Wilmot, E.; Reid, J.; Barker, J.; Aylott, J.W.; Shard, A.G.; Alexander, M.R.; et al. Molecular Formula Prediction for Chemical Filtering of 3D OrbiSIMS Datasets. Anal. Chem. 2022, 94, 4703–4711. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef]
- Chen, Y.; Li, E.-M.; Xu, L.-Y. Guide to Metabolomics Analysis: A Bioinformatics Workflow. Metabolites 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Karp, P.D.; Paley, S.; Caspi, R.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Moore, L.R.; Subhraveti, P.; Gama-Castro, S.; Tierrafria, V.H.; et al. The EcoCyc Database (2023). EcoSal Plus 2023, 11, eesp-0002-2023. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]


| Metabolite | Fold Change (FC) | log2(FC) | p-Value | VIP |
|---|---|---|---|---|
| (S)-malate | 37.276 | 5.2202 | 0.002504 | 1.051211 |
| cis-aconitate | 10.632 | 3.4103 | 0.000248 | 1.083543 |
| citrate | 72.491 | 6.1797 | 0.000338 | 1.0771 |
| Deoxy uridine | 9.5446 | 3.2547 | 0.000632 | 1.070974 |
| D-glucopyranose | 327.66 | 8.3561 | 0.000315 | 1.079193 |
| Guanine | 19.083 | 4.2543 | 0.000704 | 1.070274 |
| Lysine | 734.13 | 9.5199 | 0.000248 | 1.083333 |
| Phenylalanine | 10.308 | 3.3657 | 0.000886 | 1.067918 |
| Proline | 49.838 | 5.6392 | 0.000236 | 1.085902 |
| Thymidine | 5.8103 | 2.5386 | 0.000113 | 1.058325 |
| Thymine | 14.014 | 3.8088 | 0.000315 | 1.079273 |
| Tryptophan | 55.978 | 5.8068 | 0.000315 | 1.08043 |
| Uracil | 13.239 | 3.7267 | 0.000406 | 1.076506 |
| Uridine | 72.225 | 6.1744 | 0.000248 | 1.081858 |
| Pathway | FDR-Adjusted p-Value |
|---|---|
| Glyoxylate and dicarboxylate metabolism | 0.000359 |
| Glycerophosphoinositol pathway | 0.000645 |
| Valine, leucine and isoleucine biosynthesis | 0.004422 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 0.004422 |
| Alanine, aspartate and glutamate metabolism | 0.013322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qassadi, F.I.; Johnson, C.; Robinson, K.; Griffin, R.; Polytarchou, C.; Kao, D.; Kim, D.-H.; Griffiths, R.L.; Zhu, Z.; Monaghan, T.M. Untargeted Metabolomics Identifies Faecal Filtrate-Derived Metabolites That Disrupt Clostridioides difficile Metabolism and Confer Gut Barrier Cytoprotection. Int. J. Mol. Sci. 2025, 26, 11221. https://doi.org/10.3390/ijms262211221
Qassadi FI, Johnson C, Robinson K, Griffin R, Polytarchou C, Kao D, Kim D-H, Griffiths RL, Zhu Z, Monaghan TM. Untargeted Metabolomics Identifies Faecal Filtrate-Derived Metabolites That Disrupt Clostridioides difficile Metabolism and Confer Gut Barrier Cytoprotection. International Journal of Molecular Sciences. 2025; 26(22):11221. https://doi.org/10.3390/ijms262211221
Chicago/Turabian StyleQassadi, Fatimah I., Charlotte Johnson, Karen Robinson, Ruth Griffin, Christos Polytarchou, Dina Kao, Dong-Hyun Kim, Rian L. Griffiths, Zheying Zhu, and Tanya M. Monaghan. 2025. "Untargeted Metabolomics Identifies Faecal Filtrate-Derived Metabolites That Disrupt Clostridioides difficile Metabolism and Confer Gut Barrier Cytoprotection" International Journal of Molecular Sciences 26, no. 22: 11221. https://doi.org/10.3390/ijms262211221
APA StyleQassadi, F. I., Johnson, C., Robinson, K., Griffin, R., Polytarchou, C., Kao, D., Kim, D.-H., Griffiths, R. L., Zhu, Z., & Monaghan, T. M. (2025). Untargeted Metabolomics Identifies Faecal Filtrate-Derived Metabolites That Disrupt Clostridioides difficile Metabolism and Confer Gut Barrier Cytoprotection. International Journal of Molecular Sciences, 26(22), 11221. https://doi.org/10.3390/ijms262211221






