Does Folcisteine (NATCA) Play a Role in Facilitating Seed Germination, Root Development, and Elevating Root AsA-GSH Cycle Efficiency Under Combined Copper–Cadmium Stress in Maize?
Abstract
1. Introduction
2. Results
2.1. Evaluation of Germination and Copper–Cadmium Tolerance Parameters During Stress
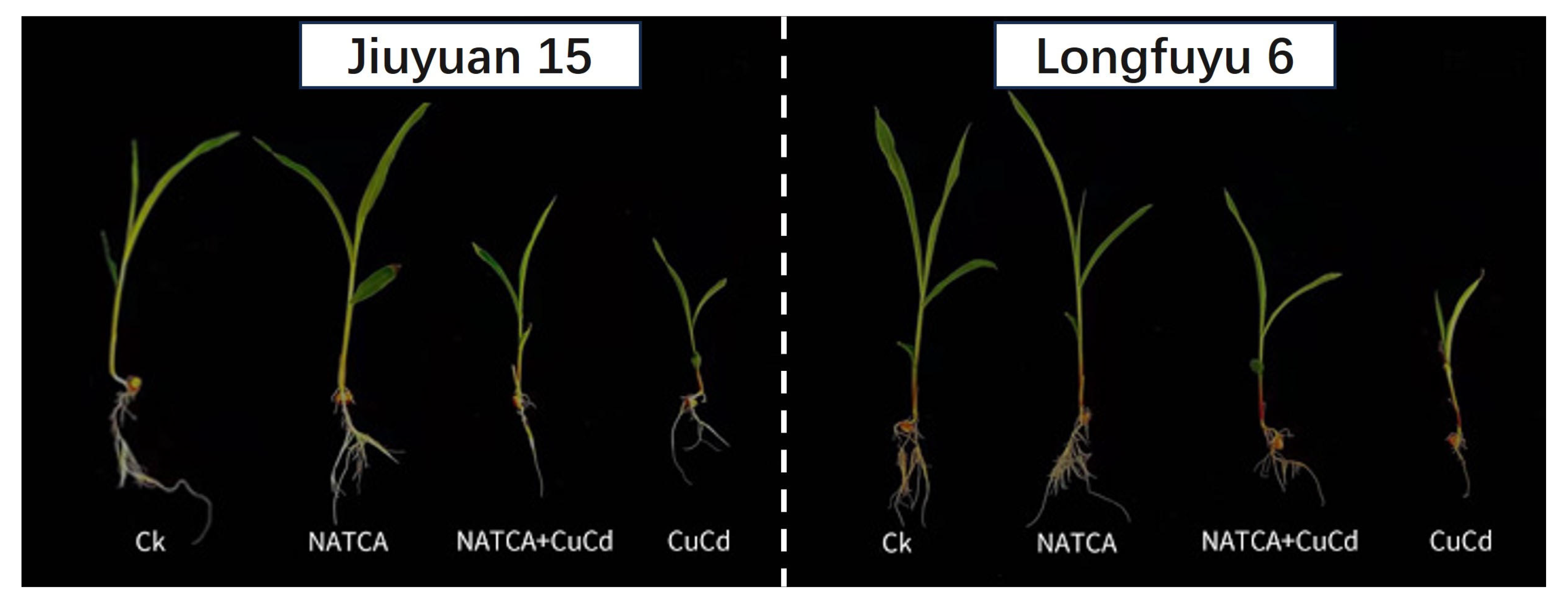
2.2. Growth Phenotype of Maize Seedlings
2.3. Dry and Fresh Weight, Moisture Content, and Natural Saturation Deficit
2.4. Root Characteristic Parameters
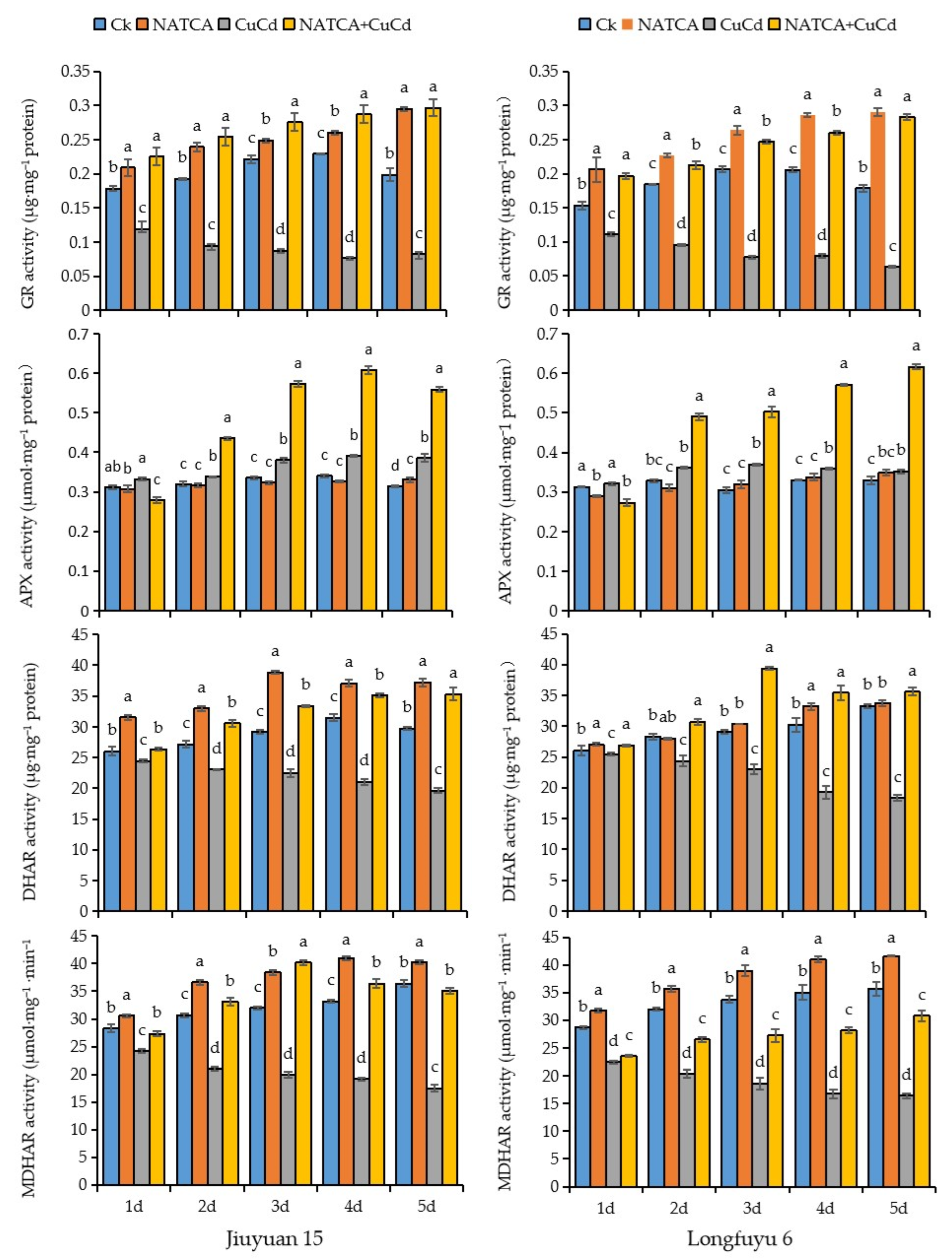
2.5. Activities of Key Enzymes in the Root Ascorbate–Glutathione (AsA-GSH) Cycle in Maize Root
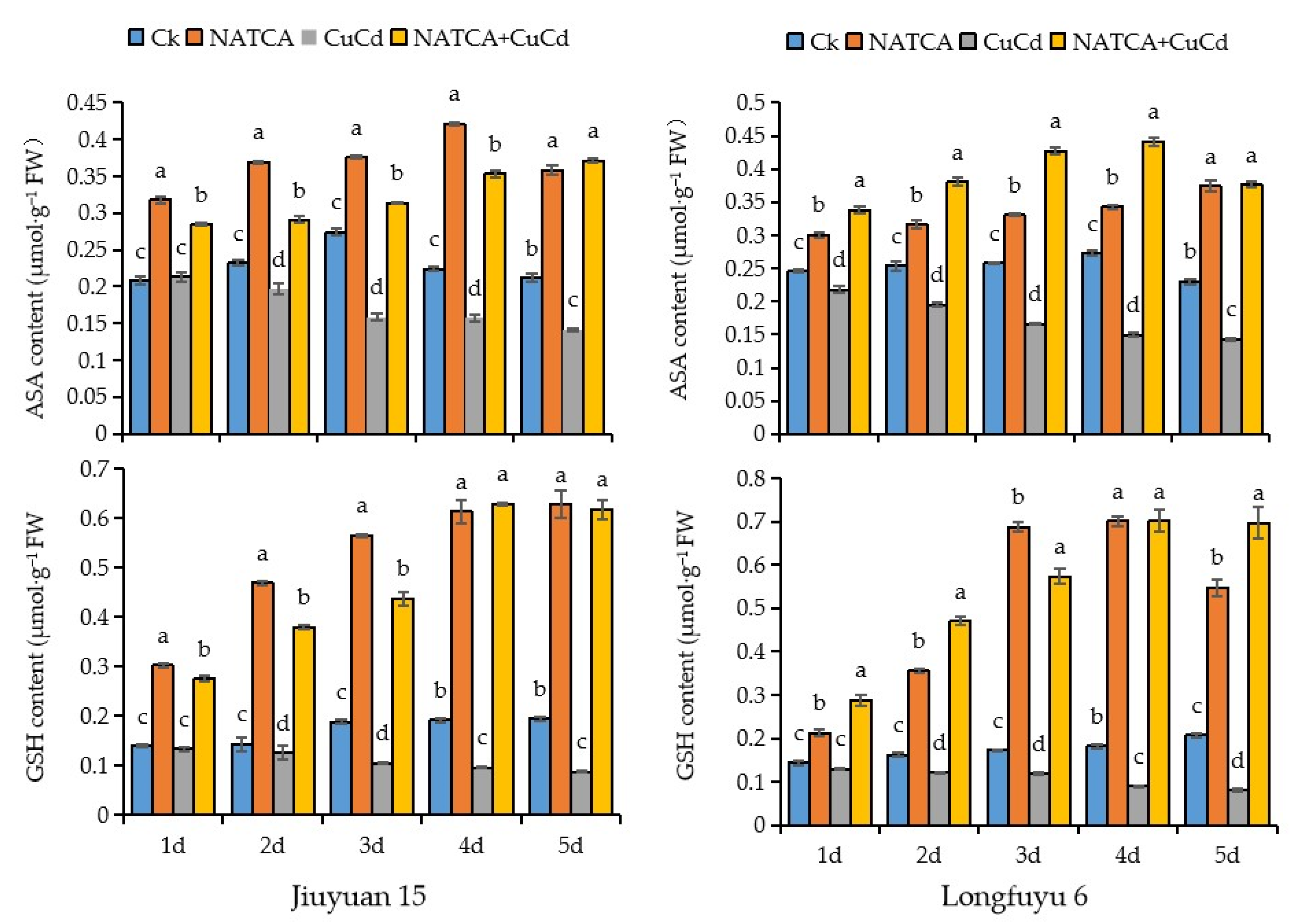
2.6. Contents of Ascorbic Acid (AsA) and Glutathione (GSH) in Maize Root
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Growth Condition of Seed Germination
4.3. Growth Condition of Seedling Hydroponic Cultivation
4.4. Determination of Seedling Growth Phenotypic
4.5. Determination of Root Characteristic Parameters
4.6. Determination of GR Activities
4.7. Determination of APX Activities
4.8. Determination of DHAR Activities
4.9. Determination of MDHAR Activities
4.10. Determination of AsA Contents
4.11. Determination of GSH Contents
4.12. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eijsackers, H.; Reinecke, A.; Reinecke, S.; Maboeta, M. Heavy Metal Threats to Plants and Soil Life in Southern Africa: Present Knowledge and Consequences for Ecological Risk Assessment. Rev. Environ. Contam. Toxicol. 2019, 249, 29–70. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wu, C.N.; Zhong, W.J.; Hou, Z.L.; Liu, Y.; Du, S.T.; Jin, C.Y. Effects of dicyandiamide on cadmium accumulation in pakchoi under instant soluble nitrogen fertilizers. Chin. J. Appl. Ecol. 2020, 31, 3093–3100. [Google Scholar] [CrossRef]
- Hu, W.; Wang, H.; Dong, L.; Huang, B.; Borggaard, O.K.; Bruun Hansen, H.C.; He, Y.; Holm, P.E. Source Identification of Heavy Metals in Peri-Urban Agricultural Soils of Southeast China: An Integrated Approach. Environ. Pollut. 2018, 237, 650–661. [Google Scholar] [CrossRef]
- Zhao, R.F.; Cheng, B.; Hua, X.Z.; Wang, S.; Wang, Z.; Lyu, W. Evaluation and Distribution Characteristics of Heavy Metal Pollution in Soil of Xinzhou Irrigation District. N. Hortic. 2021, 6, 81–88. [Google Scholar] [CrossRef]
- Yan, M. Evaluation and Analysis of Heavy Metal Pollution in Metal Mining Areas. Chin. Resour. Compr. Util. 2021, 39, 148–150. [Google Scholar] [CrossRef]
- Xu, H.Z.; Wu, D.T.; Li, G.S.; Wu, L.T.; Ye, C.F.; Guo, B.; Ma, J.W.; Ye, Z.Q.; Liu, D. Input and output balance of cadmium (Cd) in cultivated land with moderate pollution in Songyang County. J. Zhejiang AF Univ. 2021, 38, 1231–1237. [Google Scholar]
- Aladesanmi, O.T.; Oroboade, J.G.; Osisiogu, C.P.; Osewole, A.O. Bioaccumulation Factor of Selected Heavy Metals in Zea mays. J. Health Pollut. 2019, 9, 191207. [Google Scholar] [CrossRef]
- Chen, N.C.; Zheng, Y.J.; He, X.F.; Li, X.F.; Zhang, X.X. Analysis of the Report on the national general survey of soil contamination. J. Agro-Environ. Sci. 2017, 36, 1689–1692. [Google Scholar] [CrossRef]
- Winter, T.R.; Borkowski, L.; Zeier, J.; Rostás, M. Heavy Metal Stress Can Prime for Herbivore—Induced Plant Volatile Emission. Plant Cell Environ. 2012, 35, 1287–1298. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.P.; Su, H.; Sui, H.J.; Shi, X.L.; Li, X.Y.; Yang, H.; Zheng, Z.Z. Soil heavy metal background values and pollution degree in southern Songnen Plain of Heilongjiang Province. Agric. Res. Arid. Areas 2018, 36, 230–236. [Google Scholar] [CrossRef]
- Li, H.X.; Zhao, W.J.; Wu, Y.F.; Liu, Z.H.; Wang, Q.B.; Fu, D.F.; Sun, X.X. Heavy Metal Contents and Pollution Assessment of the Soil of Natural Wetlands in Heilongjiang Sanjiang Natural Reserve. Wetl. Sci. Manag. 2018, 14, 38–41. [Google Scholar] [CrossRef]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, M.; Madawala, H.M.S.P.; Ok, Y.S.; Vithanage, M. Heavy Metal-Induced Oxidative Stress on Seed Germination and Seedling Development: A Critical Review. Environ. Geochem. Health 2019, 41, 1813–1831, Erratum in Environ. Geochem. Health 2019, 41, 1635. [Google Scholar] [CrossRef] [PubMed]
- Mihoub, A.; Chaoui, A.; El Ferjani, E. Changements Biochimiques Induits Par Le Cadmium et Le Cuivre Au Cours de La Germination Des Graines de Petit Pois (Pisum sativum L.). Comptes Rendus Biol. 2004, 328, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Wang, Z.Y.; Zhang, Z.F.; Tian, H.; Xiong, J.B.; Lu, J.Y.; Liu, Y. Study on Effects of Copper Stress on Seed Germination and Root Growth of White Clover. Seed 2020, 39, 13–16. [Google Scholar] [CrossRef]
- Pang, G.F.; Meng, Q.D.; Zhao, H.Y.; Qi, M.Y.; Lin, H.; Hu, J. Effects of Cd, Pb and Their Combined Stresses on Seed Germination and Seedling Root Growth of Wheat. Mod. Agric. Sci. Technol. 2020, 3, 21–22+24. [Google Scholar]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-Induced Changes in Antioxidative Systems, Hydrogen Peroxide Content, and Differentiation in Scots Pine Roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef]
- Karmous, I.; Khadija, J.; Chaoui, A.; El Ferjani, E. Proteolytic Activities in Phaseolus Vulgaris Cotyledons under Copper Stress. Physiol. Mol. Biol. Plants 2012, 18, 337–343. [Google Scholar] [CrossRef]
- Siddiqui, M.M.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Mahmood, T. Toxic Effects of Heavy Metals (Cd, Cr and Pb) on Seed Germination and Growth and DPPH-Scavenging Activity in Brassica rapa Var. turnip. Toxicol. Ind. Health 2014, 30, 238–249. [Google Scholar] [CrossRef]
- Pokorska-Niewiada, K.; Rajkowska-Myśliwiec, M.; Protasowicki, M. Acute Lethal Toxicity of Heavy Metals to the Seeds of Plants of High Importance to Humans. Bull. Environ. Contam. Toxicol. 2018, 101, 222–228. [Google Scholar] [CrossRef]
- Staszak, A.M.; Małecka, A.; Ciereszko, I.; Ratajczak, E. Differences in Stress Defence Mechanisms in Germinating Seeds of Pinus sylvestris Exposed to Various Lead Chemical Forms. PLoS ONE 2020, 15, e0238448. [Google Scholar] [CrossRef]
- Xie, L.; Hao, P.; Cheng, Y.; Ahmed, I.M.; Cao, F. Effect of Combined Application of Lead, Cadmium, Chromium and Copper on Grain, Leaf and Stem Heavy Metal Contents at Different Growth Stages in Rice. Ecotoxicol. Environ. Saf. 2018, 162, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Z.; Li, Y.P.; Jiang, Y.J.; Jia, C.H.; Jiang, S.M.; Yian, X.P.; Qin, Z.X.; Luo, J. Effect of Cadmium and Lead Combined Stress on Growth of Mulberry Saplings and Contents of Heavy Metal in Mulberry leaf. Acta Sericol. Sin. 2018, 44, 665–671. [Google Scholar] [CrossRef]
- Ghosh, S.; Sethy, S. Effect of Heavy Metals on Germination of Seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Berkelaar, E.; Hale, B. The Relationship between Root Morphology and Cadmium Accumulation in Seedlings of Two Durum Wheat Cultivars. Can. J. Bot. 2000, 78, 381–387. [Google Scholar] [CrossRef]
- Farrell, R.E.; McArthur, D.F.E.; Van Rees, K.C.J. Net Cd2+ Flux at the Root Surface of Durum Wheat (Triticum turgidum L. var. durum) Cultivars in Relation to Cultivar Differences in Cd Accumulation. Can. J. Plant Sci. 2005, 85, 103–107. [Google Scholar] [CrossRef]
- Xia, S.; Deng, R.; Zhang, Z.; Liu, C.; Shi, G. Variations in the Accumulation and Translocation of Cadmium among Pak Choi Cultivars as Related to Root Morphology. Environ. Sci. Pollut. Res. 2016, 23, 9832–9842. [Google Scholar] [CrossRef]
- Yu, H.; Guo, J.; Li, Q.; Zhang, X.; Huang, H.; Huang, F.; Yang, A.; Li, T. Characteristics of Cadmium Immobilization in the Cell Wall of Root in a Cadmium-Safe Rice Line (Oryza sativa L.). Chemosphere 2020, 241, 125095. [Google Scholar] [CrossRef]
- Yu, Z.H.; Li, H.F.; Zhu, W.Q. The Inhibitory Effects of Cu2+ Stress on Seed Germination, Seedling Growth and Root Elongation for Forage Grasses. Environ. Impact Assess. 2011, 33, 19–23. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculik, M.; White, P.J. Root Responses to Cadmium in the Rhizosphere: A Review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Hoy, K.S.; Uppal, J.S.; Le, X.C. Editorial: Effect of root anatomy and apoplastic barrier development on cadmium uptake in rice. J. Environ. Sci. 2019, 79, 361–363. [Google Scholar] [CrossRef]
- Yu, H.; Wang, K.; Huang, H.; Zhang, X.; Li, T. The Regulatory Role of Root in Cadmium Accumulation in a High Cadmium-Accumulating Rice Line (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 25432–25441. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Xu, R.K. Acute Toxicity of Cu2+/Cd2+ to Maize as Related to Chemical Forms of the Metals on Whole Plant Roots. J. Ecol. Rural Environ. 2021, 37, 387–393. [Google Scholar] [CrossRef]
- Zhang, C.Q. Study on Rhizosphere Characteristics and Root Responses of Malva sinensis Cavan. Under Cadium Stress. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2017. [Google Scholar]
- Peng, G.Y.; Hu, L.; Huang, C.; Yang, K.; Wan, W.; Huang, C.G. Transcriptome Analysis of Response to Heavy Metal Copper Stress in Setcreasea purpurea Root Tissue. Biotechnol. Bull. 2022, 38, 83–94. [Google Scholar] [CrossRef]
- Sang, J.S. Copper Speciation in the Rhizosphere of Apple and Its Effect on Nutrient Uptake and Distribution in Young Trees. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2022. [Google Scholar]
- Li, W.; Guo, S.X.; Xing, L.J.; Zhai, Y.L.; Li, W.B.; Li, M.; Liu, R.J. Synergistic utilization of arbuscular mycorrhizal fungi and Festuca elata for remediating cadmium-contaminated soils. Mycosystema 2021, 40, 2785–2799. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Srivastava, P.K.; Prasad, S.M. Cadmium Toxicity and Its Amelioration by Kinetin in Tomato Seedlings Vis-à-Vis Ascorbate-Glutathione Cycle. J. Photochem. Photobiol. B 2018, 178, 76–84. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zhao, X.; Min, J.; He, J. Effect of Microwave Curing on Antimicrobial Activity of Chitosan Biguanidine Hydrochloride Treated Wool Fabrics. J. Text. Inst. 2011, 102, 801–807. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Bernard, F.; Seif, M.; Latifi, M.; Hassani, B.; Didaran, F.; Bosacchi, M.; Rezadoost, H.; Li, T. γ-Aminobutyric Acid Confers Cadmium Tolerance in Maize Plants by Concerted Regulation of Polyamine Metabolism and Antioxidant Defense Systems. Sci. Rep. 2020, 10, 3356. [Google Scholar] [CrossRef]
- Małkowski, E.; Sitko, K.; Szopiński, M.; Gieroń, Ż.; Pogrzeba, M.; Kalaji, H.M.; Zieleźnik-Rusinowska, P. Hormesis in Plants: The Role of Oxidative Stress, Auxins and Photosynthesis in Corn Treated with Cd or Pb. Int. J. Mol. Sci. 2020, 21, 2099. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Qi, J.; Dang, P.; Xia, T. Cadmium Activates ZmMPK3-1 and ZmMPK6-1 via Induction of Reactive Oxygen Species in Maize Roots. Biochem. Biophys. Res. Commun. 2019, 516, 747–752. [Google Scholar] [CrossRef]
- Fiala, R.; Repka, V.; Čiamporová, M.; Martinka, M.; Pavlovkin, J. The Effect of Cadmium-Nickel Interactions on Superoxide Production, Cell Viability and Membrane Potential (EM) in Roots of Two Maize Cultivars. Acta Biol. Hung. 2015, 66, 192–204. [Google Scholar] [CrossRef]
- Ding, S.; Ma, C.; Shi, W.; Liu, W.; Lu, Y.; Liu, Q.; Luo, Z.-B. Exogenous Glutathione Enhances Cadmium Accumulation and Alleviates Its Toxicity in Populus × Canescens. Tree Physiol. 2017, 37, 1697–1712. [Google Scholar] [CrossRef]
- Zayneb, C.; Bassem, K.; Zeineb, K.; Grubb, C.D.; Noureddine, D.; Hafedh, M.; Amine, E. Physiological Responses of Fenugreek Seedlings and Plants Treated with Cadmium. Environ. Sci. Pollut. Res. 2015, 22, 10679–10689. [Google Scholar] [CrossRef]
- Yetişsin, F.; Kurt, F. Gallic Acid (GA) Alleviating Copper (Cu) Toxicity in Maize (Zea mays L.) Seedlings. Int. J. Phytoremediation 2020, 22, 420–426. [Google Scholar] [CrossRef]
- Roy, D.; Adhikari, S.; Adhikari, A.; Ghosh, S.; Azahar, I.; Basuli, D.; Hossain, Z. Impact of CuO Nanoparticles on Maize: Comparison with CuO Bulk Particles with Special Reference to Oxidative Stress Damages and Antioxidant Defense Status. Chemosphere 2022, 287, 131911. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, Y.C.; Niu, Y.Q.; Zhou, H.; An, Y.L.; Jin, Q.J.; Wang, Y.L. Effects of ethylene and NO on AsA-GSH in lotus under cadmium stress. Chin. J. Appl. Ecol. 2018, 29, 3433–3440. [Google Scholar] [CrossRef]
- Lin, X.P.; Shi, M.T.; Chen, W.; Lu, T.T.; Ni, X.C. Effects of cadmium stress on active oxygen metabolism in leaves of balsam pear. J. Fujian Agric. For. Univ. 2010, 39, 143–146. [Google Scholar] [CrossRef]
- Shi, G.Y.; Yu, Z.Q.; Shi, W.L. Research Progress on Mechanism and Application of Exogenous Substances in Phytoremediation of Heavy Metal Contaminated Soil. Ecol. Environ. Sci. 2021, 30, 655–666. [Google Scholar] [CrossRef]
- Yao, F.N.; Wan, C.; Liu, J.P.; Xiu, W.C.; Zheng, X.F. Research on Biological Activity of Acetyl-Thiazolidine-4-Carboxylic Acid in Seedling Stage of Several Crops. Mod. Agrochem. 2021, 20, 62–64. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Palma, J.M.; Gómez, M.; Del Río, L.A.; Sandalio, L.M. Cadmium Causes the Oxidative Modification of Proteins in Pea Plants. Plant Cell Environ. 2002, 25, 677–686. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and Detection of Reactive Oxygen Species (ROS), Lipid Peroxidation, and Electrolyte Leakage in Plants. In Plant Stress Tolerance; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 291–297. [Google Scholar] [CrossRef]
- Li, W.; Khan, M.A.; Yamaguchi, S.; Kamiya, Y. Effects of Heavy Metals on Seed Germination and Early Seedling Growth of Arabidopsis Thaliana. Plant Growth Regul. 2005, 46, 45–50. [Google Scholar] [CrossRef]
- Deng, B.; Yang, K.; Zhang, Y.; Li, Z. Can Heavy Metal Pollution Defend Seed Germination against Heat Stress? Effect of Heavy Metals (Cu2+, Cd2+ and Hg2+) on Maize Seed Germination under High Temperature. Environ. Pollut. 2016, 216, 46–52. [Google Scholar] [CrossRef]
- Xu, Z.M.; He, K.; Lin, T.; Chen, A.L.; Zhu, T.; Ding, G.C. Effect of Copper and Cadmium Combined Stress on Seed Germination Characteristics of Five Ornamental Grasses. Shandong Agric. Sci. 2019, 51, 59–64. [Google Scholar] [CrossRef]
- Gao, Q.J.; Zhang, F.E.; Kong, D.Y.; Bao, W.M.; Zhang, W.W. Effects of Cu2+ and Pb2+ Stress on Seed Germination of Two Varieties of Cockscombs. J. Northeast. For. Univ. 2021, 49, 36–40. [Google Scholar] [CrossRef]
- Li, H.Y.; Wu, Y.; Yang, R.; Wang, Z.Y.; Xiao, J.; Gao, C.H. Creening and Identification on Corn Varieties with Resistance to Heavy Metal Cd in Germination Stage. Hortic. Seed 2017, 61–65. [Google Scholar] [CrossRef]
- Liu, J.X.; Bai, Z.Z.; Wang, R.M.; Liu, L.Z.; Zhang, Z.H.; Wen, R.Y. Germination Characteristics and Accumulation Effects of Adzuki Bean under Heavy Metal Stress. Crops 2019, 6, 182–186. [Google Scholar] [CrossRef]
- Khan, A.A. Cytokinins: Permissive Role in Seed Germination: With Other Plant Hormones, Cytokinins Regulate Germination and Dormancy by a Novel Mechanism. Science 1971, 171, 853–859. [Google Scholar] [CrossRef]
- Song, S.Q.; Liu, J.; Yang, H.; Zhang, W.H.; Zhang, Q.; Gao, J.D. Research Progress in Seed Development, Dormancy and Germination Regulated by Cytokinin. Chin. Bull. Bot. 2021, 56, 218–231. [Google Scholar] [CrossRef]
- Kollárová, K.; Kusá, Z.; Vatehová-Vivodová, Z.; Lišková, D. The Response of Maize Protoplasts to Cadmium Stress Mitigated by Silicon. Ecotoxicol. Environ. Saf. 2019, 170, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Asilian, E.; Ghasemi-Fasaei, R.; Ronaghi, A.; Sepehri, M.; Niazi, A. Chemical- and Microbial-Enhanced Phytoremediation of Cadmium-Contaminated Calcareous Soil by Maize. Toxicol. Ind. Health 2019, 35, 378–386. [Google Scholar] [CrossRef]
- Vatehová, Z.; Malovíková, A.; Kollárová, K.; Kučerová, D.; Lišková, D. Impact of Cadmium Stress on Two Maize Hybrids. Plant Physiol. Biochem. 2016, 108, 90–98. [Google Scholar] [CrossRef]
- Krzesłowska, M. The Cell Wall in Plant Cell Response to Trace Metals: Polysaccharide Remodeling and Its Role in Defense Strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Yang, C.; Qiu, W.; Chen, Z.; Chen, W.; Li, Y.; Zhu, J.; Rahman, S.U.; Han, Z.; Jiang, Y.; Yang, G.; et al. Phosphorus Influence Cd Phytoextraction in Populus Stems via Modulating Xylem Development, Cell Wall Cd Storage and Antioxidant Defense. Chemosphere 2020, 242, 125154. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, Y.; Huang, H.; Zhan, J.; Wang, K.; Li, T. The Predominant Role of Pectin in Binding Cd in the Root Cell Wall of a High Cd Accumulating Rice Line (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 206, 111210. [Google Scholar] [CrossRef]
- Vatehová-Vivodová, Z.; Kollárová, K.; Malovíková, A.; Lišková, D. Maize Shoot Cell Walls under Cadmium Stress. Environ. Sci. Pollut. Res. 2018, 25, 22318–22322. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Fernández-Fuego, D.; Bertrand, A.; González, A. Strategies for Cd Accumulation in Dittrichia viscosa (L.) Greuter: Role of the Cell Wall, Non-Protein Thiols and Organic Acids. Plant Physiol. Biochem. 2014, 78, 63–70. [Google Scholar] [CrossRef]
- Sun, J.; Cui, J.; Luo, C.; Gao, L.; Chen, Y.; Shen, Z. Contribution of Cell Walls, Nonprotein Thiols, and Organic Acids to Cadmium Resistance in Two Cabbage Varieties. Arch. Environ. Contam. Toxicol. 2013, 64, 243–252. [Google Scholar] [CrossRef]
- Hu, J.; Wu, S.; Wu, F.; Leung, H.M.; Lin, X.; Wong, M.H. Arbuscular Mycorrhizal Fungi Enhance Both Absorption and Stabilization of Cd by Alfred Stonecrop (Sedum alfredii Hance) and Perennial Ryegrass (Lolium perenne L.) in a Cd-Contaminated Acidic Soil. Chemosphere 2013, 93, 1359–1365. [Google Scholar] [CrossRef]
- Cui, X.M.; Hu, M.M.; Wang, J.Q.; Zhu, G.Y.P.; Lou, Y.H. The alleviating effect of humic acid on copper cadmium composite stress. In Proceedings of the Summary of the 20th Meeting of the Soil Environment Professional Committee of the Chinese Soil Society and the Seminar on Farmland Soil Pollution and Remediation, Hefei, China, 5 August 2018; p. 28. [Google Scholar]
- Wang, Z.W.; Li, H.; Liang, S.H.; Chu, X.; Sun, X.W. Effects of Cadmium on Growth and Some Physiological of Oriental Melon Seedlings. Acta Agric. Boreali-Sin. 2020, 35, 81–88. [Google Scholar]
- Zhang, L.H.; Li, P.J.; Li, X.M.; Meng, X.L.; Xu, C.B. Effects of cadmium stress on the growth and physiological characteristics of wheat seedlings. Chin. J. Ecol. 2005, 24, 458–460. [Google Scholar]
- Ma, B.; Zhang, J.; Wei, Y.Y.; Xie, S.M. Effects of Lanthanum on the Growth and Physiological Characteristics of Miscanthus sacchariflorus under Single Stress of Copper and Cadmium. J. Anhui Norm Univ. (Nat. Sci.) 2020, 43, 467–474. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Penna, S.; Nguyen, D.V.; Tran, L.-S.P. Multifaceted Roles of Aquaporins as Molecular Conduits in Plant Responses to Abiotic Stresses. Crit. Rev. Biotechnol. 2014, 36, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Chai, T.; Wen, Z.; Zhang, H. Indian Mustard Aquaporin Improves Drought and Heavy-Metal Resistance in Tobacco. Mol. Biotechnol. 2008, 40, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kronzucker, H.J.; Shi, W. Root Developmental Adaptation to Fe Toxicity: Mechanisms and Management. Plant Signal. Behav. 2016, 11, e1117722. [Google Scholar] [CrossRef]
- Bai, X.Y. Effects and Mechanisms of Nitric Oxide and Salicylic Acid on Alleviating Cd and Cu Stress in Ryegrass. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2016. [Google Scholar]
- Zhou, J.N.; Yang, C.; Song, Z.Y.; He, C.F.; He, J.H.; Huang, W.L.; Dang, Z. Influences of tetracycline and cadmium on rice roots: Growth and root exudates. Acta Sci. Circumstantiae 2021, 41, 1518–1528. [Google Scholar] [CrossRef]
- Zheng, A.Z.; Song, W.Y. Effects of Cu on Seeds Germination and Seedling Early Growth of Cucumber and Corn. North. Hortic. 2009, 9, 7–10. [Google Scholar]
- Agarwal, K.; Sharma, A.; Talukder, G. Copper Toxicity in Plant Cellular Systems. Nucleus 1987, 30, 131–158. [Google Scholar]
- Jiang, W.; Liu, D.; Li, H. Effects of Cu2+ on Root Growth, Cell Division, and Nucleolus of Helianthus annuus L. Sci. Total Environ. 2000, 256, 59–65. [Google Scholar] [CrossRef]
- Yuan, H.M.; Liu, W.C.; Jin, Y.; Lu, Y.T. Role of ROS and Auxin in Plant Response to Metal-Mediated Stress. Plant Signal. Behav. 2013, 8, e24671. [Google Scholar] [CrossRef]
- Finger-Teixeira, A.; Lucio Ferrarese, M.D.L.; Ricardo Soares, A.; Da Silva, D.; Ferrarese-Filho, O. Cadmium-Induced Lignification Restricts Soybean Root Growth. Ecotoxicol. Environ. Saf. 2010, 73, 1959–1964. [Google Scholar] [CrossRef]
- Rodriguez-Salus, M.; Bektas, Y.; Schroeder, M.; Knoth, C.; Vu, T.; Roberts, P.; Kaloshian, I.; Eulgem, T. The Synthetic Elicitor 2-(5-Bromo-2-Hydroxy-Phenyl)-Thiazolidine-4-Carboxylic Acid Links Plant Immunity to Hormesis. Plant Physiol. 2016, 170, 444–458. [Google Scholar] [CrossRef]
- Speiser, A.; Silbermann, M.; Dong, Y.; Haberland, S.; Uslu, V.V.; Wang, S.; Bangash, S.A.K.; Reichelt, M.; Meyer, A.J.; Wirtz, M.; et al. Sulfur Partitioning between Glutathione and Protein Synthesis Determines Plant Growth. Plant Physiol. 2018, 177, 927–937. [Google Scholar] [CrossRef]
- Bonet, B.; Corcoll, N.; Guasch, H. Antioxidant Enzyme Activities as Biomarkers of Zn Pollution in Fluvial Biofilms. Ecotoxicol. Environ. Saf. 2012, 80, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of Nitric Oxide in Cadmium Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
| Variety | Treatment | Germination Vigor (%) | Germination Rate (%) | Germination Index | Relative Damage Degree | Tolerance Index |
|---|---|---|---|---|---|---|
| Jiuyuan 15 | Ck | 92.00 ± 0.12 a | 96.00 ± 0.16 a | 26.44 ± 0.12 b | ||
| NATCA | 97.33 ± 0.06 a | 97.33 ± 0.67 a | 31.00 ± 5.67 a | |||
| CuCd | 70.00 ± 0.02 c | 76.00 ± 0.02 b | 18.19 ± 2.33 d | 0.20 ± 0.34 | 0.69 ± 0.01 | |
| NATCA + CuCd | 88.00 ± 0.23 b | 91.33 ± 0.31 a | 23.86 ± 2.31 c | 0.05 ± 0.36 | 0.90 ± 0.02 | |
| Longfuyu 6 | Ck | 54.00 ± 0.24 b | 94.67 ± 1.76 ab | 15.78 ± 4.18 b | ||
| NATCA | 68.00 ± 0.02 a | 97.33 ± 0.91 a | 18.66 ± 9.01 a | |||
| CuCd | 20.67 ± 0.18 d | 72.67 ± 0.55 c | 8.39 ± 5.46 c | 0.23 ± 3.23 | 0.53 ± 0.04 | |
| NATCA + CuCd | 35.33 ± 0.03 c | 90.67 ± 0.13 b | 14.27 ± 2.88 b | 0.05 ± 0.02 | 0.90 ± 0.02 |
| Cultivars | Treatment | Fresh Weight (g·plant−1) | Dry Weight (g·plant−1) | Water Content (%) | Natural Saturation Deficit (%) |
|---|---|---|---|---|---|
| Jiuyuan 15 | CK | 6.15 ± 0.06 b | 0.51 ± 0.01 b | 91.78 ± 0.09 a | 2.38 ± 0.01 d |
| NATCA | 7.17 ± 0.15 a | 0.56 ± 0.01 a | 92.24 ± 0.01 a | 3.95 ± 0.01 c | |
| CuCd | 3.49 ± 0.03 d | 0.50 ± 0.04 b | 85.76 ± 0.05 b | 28.34 ± 0.01 a | |
| NATCA + CuCd | 4.36 ± 0.11 c | 0.57 ± 0.01 a | 86.91 ± 0.01 b | 7.70 ± 0.02 b | |
| Longfuyu 6 | CK | 5.24 ± 0.03 b | 0.61 ± 0.04 b | 87.48 ± 0.05 b | 7.28 ± 0.02 c |
| NATCA | 6.04 ± 0.04 a | 0.66 ± 0.07 a | 90.00 ± 0.03 a | 12.71 ± 0.06 b | |
| CuCd | 3.31 ± 0.01 d | 0.46 ± 0.01 d | 84.03 ± 0.02 c | 26.24 ± 0.05 a | |
| NATCA + CuCd | 3.66 ± 0.01 c | 0.53 ± 0.04 c | 87.39 ± 0.07 b | 13.19 ± 0.06 b |
| Cultivars | Treatment | Length (cm) | Average Diameter (cm) | Surface Area (cm2) | Volume (cm3) | Root Tips |
|---|---|---|---|---|---|---|
| Jiuyuan 15 | Ck | 8.69 ± 0.53 c | 1.09 ± 0.06 a | 3.03 ± 0.04 c | 0.08 ± 0.05 ab | 170.00 ± 6.08 a |
| NATCA | 14.93 ± 0.90 a | 1.11 ± 0.03 a | 4.60 ± 0.02 a | 0.11 ± 0.02 a | 49.00 ± 8.50 c | |
| CuCd | 7.28 ± 0.28 c | 0.98 ± 0.07 a | 2.49 ± 0.18 d | 0.07 ± 0.02 b | 28.67 ± 4.48 c | |
| NATCA + CuCd | 11.41 ± 0.54 b | 1.05 ± 0.03 a | 3.78 ± 0.28 b | 0.10 ± 0.08 ab | 78.00 ± 6.08 b | |
| Longfuyu 6 | Ck | 16.07 ± 1.02 ab | 1.00 ± 0.09 bc | 5.46 ± 0.29 a | 0.14 ± 0.05 b | 214.00 ± 18.33 a |
| NATCA | 17.33 ± 0.93 a | 1.16 ± 0.04 a | 5.61 ± 0.23 a | 0.16 ± 0.04 a | 101.00 ± 9.29 b | |
| CuCd | 11.61 ± 0.61 c | 0.95 ± 0.08 c | 4.24 ± 0.03 b | 0.12 ± 0.04 b | 37.00 ± 2.31 c | |
| NATCA + CuCd | 13.66 ± 0.45 bc | 1.11 ± 0.02 ab | 4.70 ± 0.17 b | 0.13 ± 0.04 b | 96.00 ± 4.36 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Zhao, M.; Hou, K.; Wei, J.; Liu, Z.; Wang, R.; Zhou, Y.; Gu, W. Does Folcisteine (NATCA) Play a Role in Facilitating Seed Germination, Root Development, and Elevating Root AsA-GSH Cycle Efficiency Under Combined Copper–Cadmium Stress in Maize? Int. J. Mol. Sci. 2025, 26, 11220. https://doi.org/10.3390/ijms262211220
Dong L, Zhao M, Hou K, Wei J, Liu Z, Wang R, Zhou Y, Gu W. Does Folcisteine (NATCA) Play a Role in Facilitating Seed Germination, Root Development, and Elevating Root AsA-GSH Cycle Efficiency Under Combined Copper–Cadmium Stress in Maize? International Journal of Molecular Sciences. 2025; 26(22):11220. https://doi.org/10.3390/ijms262211220
Chicago/Turabian StyleDong, Ling, Meng Zhao, Kangbo Hou, Jingwen Wei, Ziwen Liu, Runze Wang, Yu Zhou, and Wanrong Gu. 2025. "Does Folcisteine (NATCA) Play a Role in Facilitating Seed Germination, Root Development, and Elevating Root AsA-GSH Cycle Efficiency Under Combined Copper–Cadmium Stress in Maize?" International Journal of Molecular Sciences 26, no. 22: 11220. https://doi.org/10.3390/ijms262211220
APA StyleDong, L., Zhao, M., Hou, K., Wei, J., Liu, Z., Wang, R., Zhou, Y., & Gu, W. (2025). Does Folcisteine (NATCA) Play a Role in Facilitating Seed Germination, Root Development, and Elevating Root AsA-GSH Cycle Efficiency Under Combined Copper–Cadmium Stress in Maize? International Journal of Molecular Sciences, 26(22), 11220. https://doi.org/10.3390/ijms262211220






