Modulation of Master Transcription Factor Expression of Nile Tilapia Leukocytes via Cholinergic Pathways
Abstract
1. Introduction
2. Results and Discussion
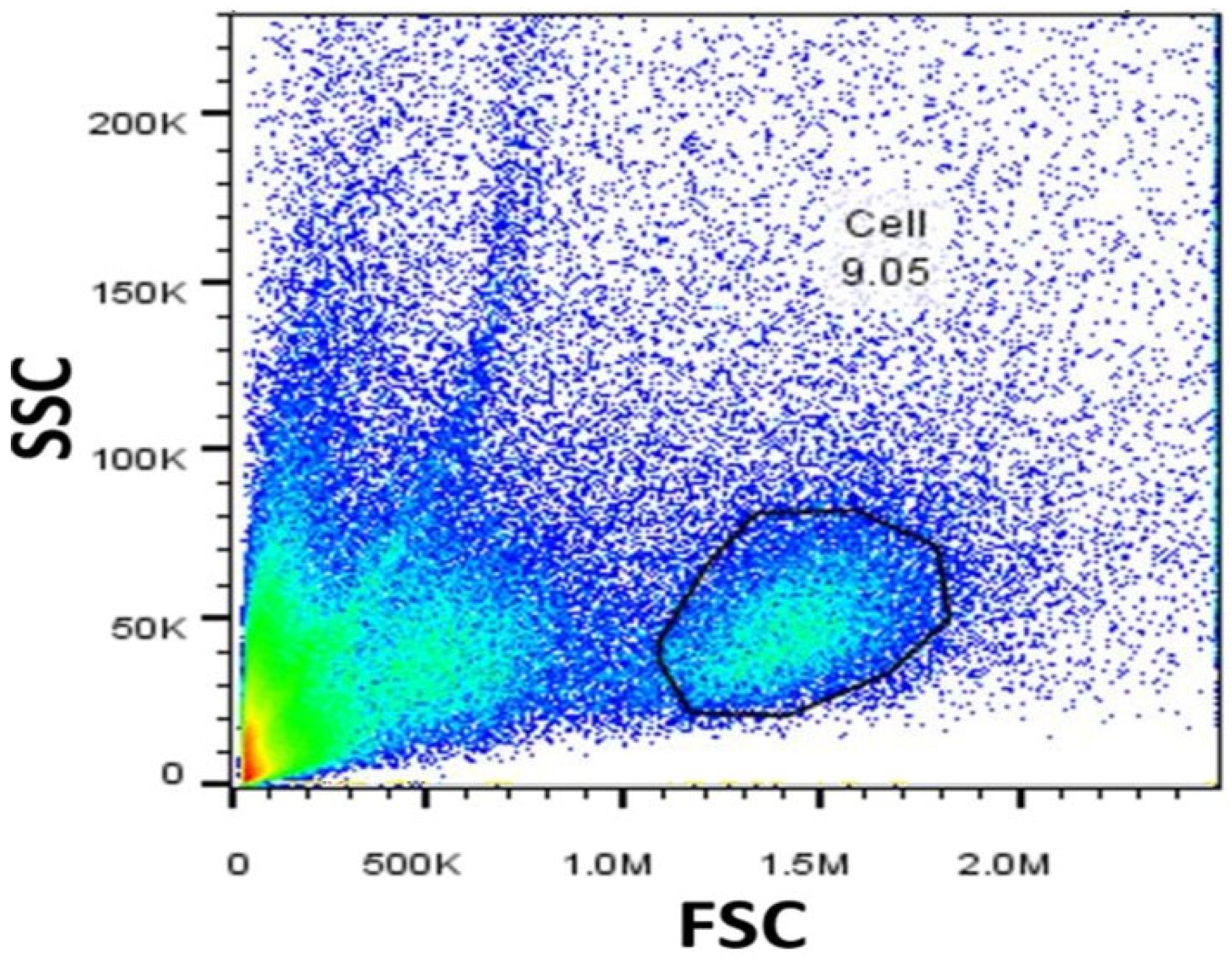
2.1. Characterization of SMNCs by Flow Cytometry
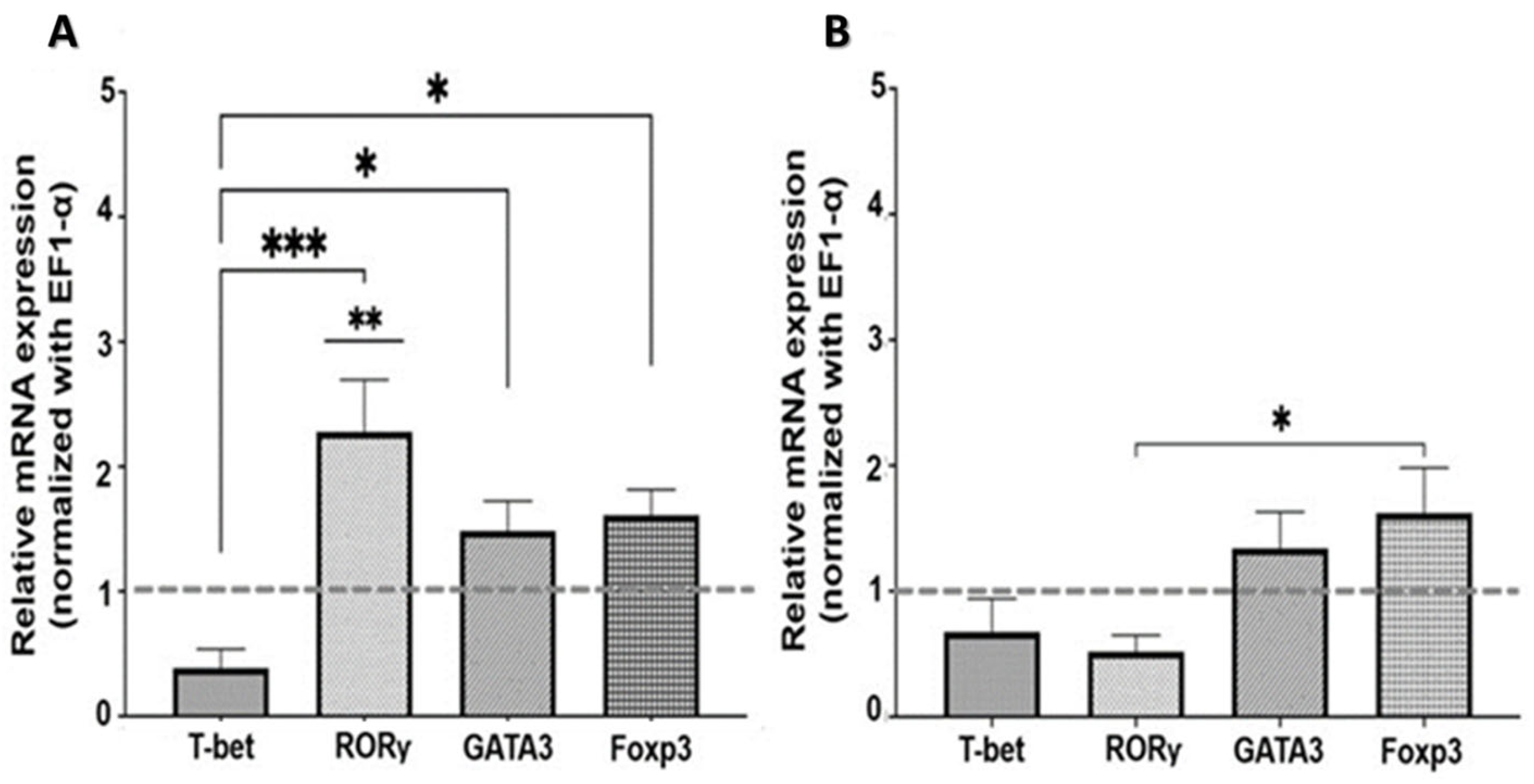
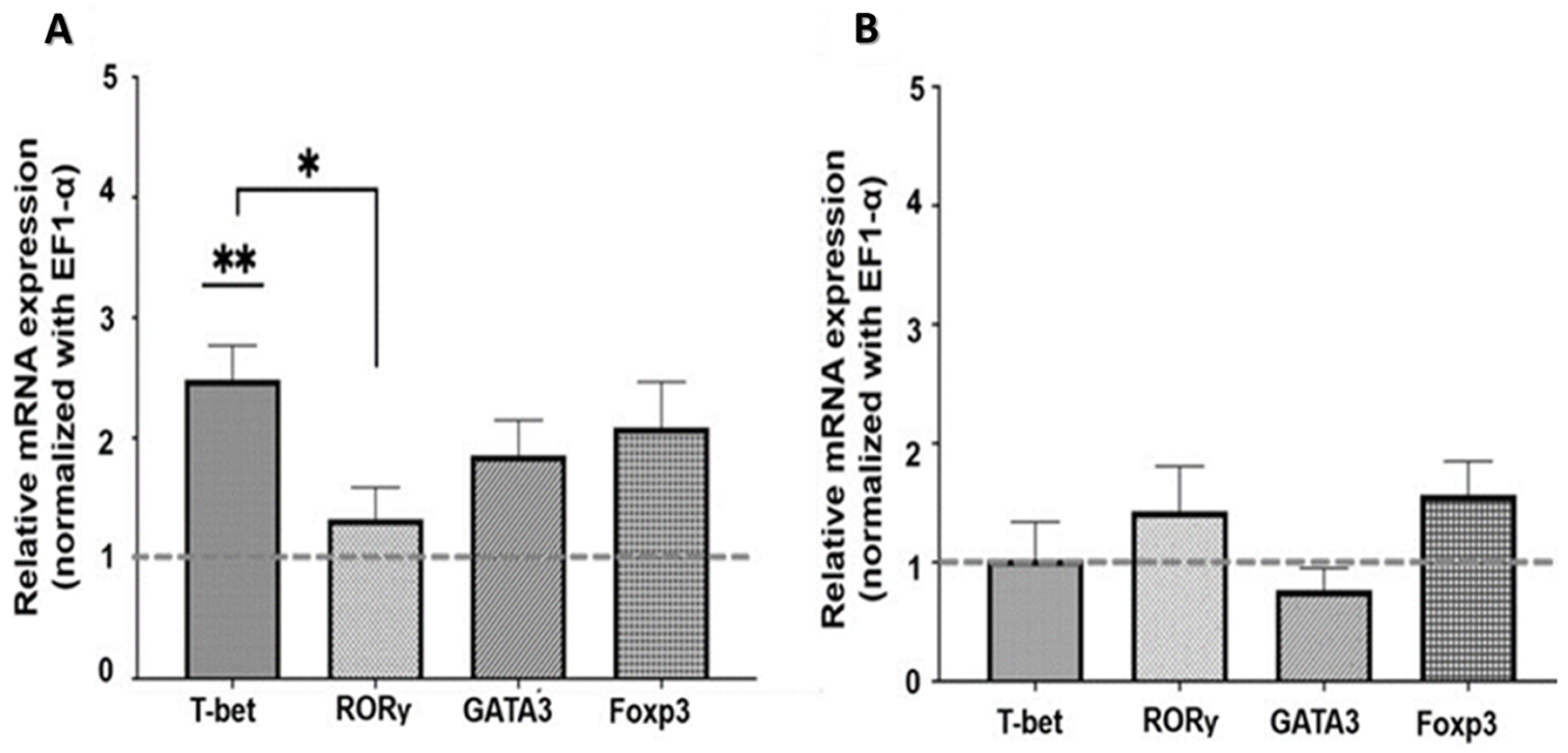
2.2. Expression of Transcription Factors in SMNCs Exposed to Cholinergic Agonists and Antagonists
2.2.1. Nicotinic Pathway
2.2.2. Muscarinic Pathway
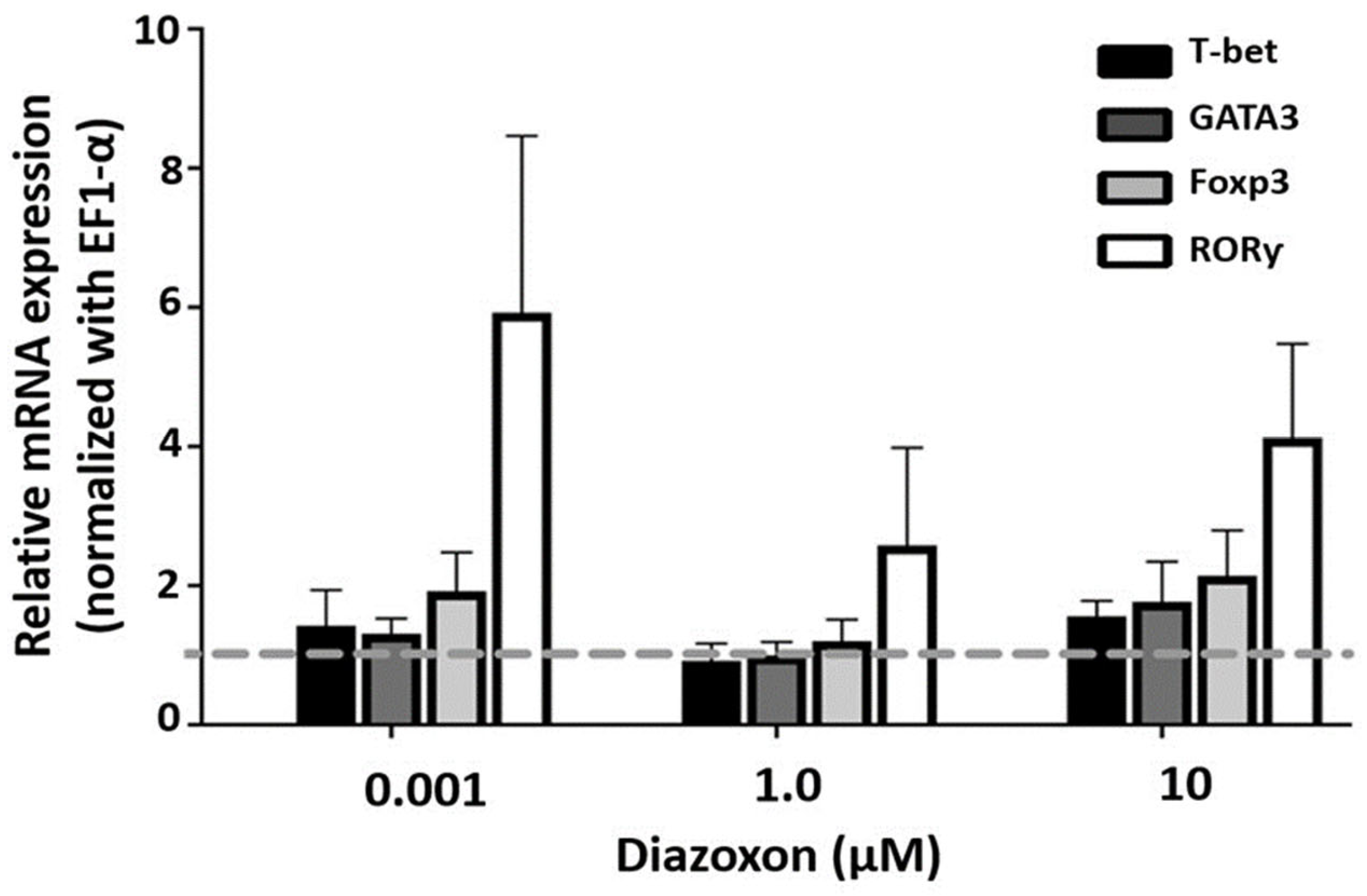
2.3. Expression of Transcription Factors in SMNCs Exposed to Diazoxon
3. Material and Methods
3.1. Experimental Fish
3.2. Isolation of Spleen Mononuclear Cells (SMNCs)
3.3. In Vitro Exposure to Diazoxon and Cholinergic Agonists and Antagonists
3.4. Master Transcription Factors Expression
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laing, K.J.; Zou, J.J.; Purcell, M.K.; Phillips, R.; Secombes, C.J.; Hansen, J.D. Evolution of the CD4 Family: Teleost Fish Possess Two Divergent Forms of CD4 in Addition to Lymphocyte Activation Gene-3. J. Immunol. 2006, 177, 3939–3951. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Ramirez-Gomez, F.; Greene, W.; Rego, K.; Hansen, J.D.; Costa, G.; Kataria, P.; Bromage, E.S. Discovery and Characterization of Secretory IgD in Rainbow Trout: Secretory IgD Is Produced through a Novel Splicing Mechanism. J. Immunol. 2012, 188, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J.O. Fishing for mammalian paradigms in the teleost immune system. Nat. Immunol. 2013, 14, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Scapigliati, G.; Fausto, A.M.; Picchietti, S. Fish lymphocytes: An evolutionary equivalent of mammalian innate-like lymphocytes? Front. Immunol. 2018, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.; Goto, K.; Akune, I.; Aoka, S.; Kondo, H.; Hirono, I. CD4 and CD8 homologues in Japanese flounder, Paralichthys olivaceus: Differences in the expressions and localizations of CD4-1, CD4-2, CD8α and CD8β. Dev. Comp. Immunol. 2013, 39, 293–301. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4 +T cells: Differentiation and functions. J. Immunol. Res. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Holland, J.W.; Martin, S.A.M.; Secombes, C.J. Sequence and expression analysis of two T helper master transcription factors, T-bet and GATA3, in rainbow trout Oncorhynchus mykiss and analysis of their expression during bacterial and parasitic infection. Fish Shellfish Immunol. 2010, 29, 705–715. [Google Scholar] [CrossRef]
- Mitra, S.; Alnabulsi, A.; Secombes, C.J.; Bird, S. Identification and characterization of the transcription factors involved in T-cell development, t-bet, stat6 and foxp3, within the zebrafish, Danio rerio. FEBS J. 2010, 277, 128–147. [Google Scholar] [CrossRef]
- Gorissen, M.; de Vrieze, E.; Flik, G.; Huising, M.O. STAT genes display differential evolutionary rates that correlate with their roles in the endocrine and immune system. J. Endocrinol. 2011, 209, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shang, N.; Feng, H.; Guo, Q.; Dai, H. Molecular cloning of grass carp (Ctenopharyngodon idellus) T-bet and GATA-3, and their expression profiles with IFN-γ in response to grass carp reovirus (GCRV) infection. Fish Physiol. Biochem. 2013, 39, 793–805. [Google Scholar] [CrossRef]
- Ho, I.C.; Pai, S.Y. GATA-3—Not just for Th2 cells anymore. Cell Mol. Immunol. 2007, 4, 15–29. [Google Scholar] [PubMed]
- Kumari, J.; Bogwald, J.; Dalmo, R.A. Transcription factor GATA-3 in Atlantic salmon (Salmo salar): Molecular characterization, promoter activity and expression analysis. Mol. Immunol. 2009, 46, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4+ T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Gunimaladevi, I.; Savan, R.; Sakai, M. Identification, cloning and characterization of interleukin-17 and its family from zebrafish. Fish Shellfish Immunol. 2006, 21, 393–403. [Google Scholar] [CrossRef]
- Laing, K.J.; Hansen, J.D. Fish T cells: Recent advances through genomics. Dev. Comp. Immunol. 2011, 35, 1282–1295. [Google Scholar] [CrossRef]
- Monte, M.M.; Wang, T.; Holland, J.W.; Zou, J.; Secombes, C.J. Cloning and characterization of rainbow trout interleukin-17A/F2 (IL-17A/F2) and IL-17 receptor a: Expression during infection and bioactivity of recombinant IL-17A/F2. Infect. Immun. 2013, 81, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Malek, T.R. Cellular and molecular determinants for the development of natural and induced regulatory T cells. Hum. Immunol 2012, 73, 773–782. [Google Scholar] [CrossRef]
- Wohlfert, E.A.; Grainger, J.R.; Bouladoux, N.; Konkel, J.E.; Oldenhove, G.; Ribeiro, C.H.; Hall, J.A.; Yagi, R.; Naik, S.; Bhairavabhotla, R.; et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J. Clin. Investig. 2011, 121, 4503–4515. [Google Scholar] [CrossRef]
- Wei, J.; Yu, L.; Sun, L.; Zhang, X.; Li, M.; Qi, W.; Zhou, L.; Wang, D. Molecular cloning and expression analysis of foxp3 from nile tilapia. Vet. Immunol. Immunopathol. 2013, 155, 48–56. [Google Scholar] [CrossRef]
- Ashfaq, H.; Soliman, H.; Saleh, M.; El-Matbouli, M. CD4: A vital player in the teleost fish immune system. Vet. Res. 2019, 50, 1. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Díaz-Resendíz, K.J.G.; Ventura-Ramón, G.H.; Girón-Pérez, M.I. Muscarinic acetylcholine receptor expression in brain and immune cells of Oreochromis niloticus. J. Neuroimmunol. 2019, 328, 105–107. [Google Scholar] [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, K.; Kawashima, K. Expression and function of the cholinergic system in immune cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Mashimo, M.; Moriwaki, Y.; Misawa, H.; Kawashima, K.; Fuji, T. Regulation of immune functions by non- neuronal acetylcholine (Ach) via muscarinic and nicotinic ach receptors. Int. J. Mol. Sci. 2021, 22, 6818. [Google Scholar] [CrossRef] [PubMed]
- Poet, T.S.; Kousba, A.A.; Dennison, S.L.; Timchalk, C. Physiologically based pharmacokinetic/pharmacodynamic model for the organophosphorus pesticide diazinon. Neurotoxicology 2004, 25, 1013–1030. [Google Scholar] [CrossRef]
- Durmaz, H.; Sevgiler, Y.; Üner, N. Tissue-specific antioxidative and neurotoxic responses to diazinon in Oreochromis niloticus. Pestic. Biochem. Physiol. 2006, 84, 215–226. [Google Scholar] [CrossRef]
- Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Díaz-Resendiz, K.J.G.; Ventura-Ramón, G.H.; Pavón, L.; Becerril-Villanueva, E.; Bauer, M.E.; Bottaso, O.; Girón-Pérez, M.I. Modulation of the extraneuronal cholinergic system on main innate response leukocytes. J. Neuroimmunol. 2019, 327, 22–35. [Google Scholar] [CrossRef]
- Covantes-Rosales, C.E.; Trujillo-Lepe, A.M.; Díaz-Reséndiz, K.J.G.; Toledo-Ibarra, G.A.; Ventura-Ramón, G.H.; Ortiz-Lazareno, P.C.; Girón-Pérez, M.I. Phagocytosis and ROS production as biomarkers in Nile tilapia (Oreochromis niloticus) leukocytes by exposure to organophosphorus pesticides. Fish Shellfish Immunol. 2019, 84, 189–195. [Google Scholar]
- Díaz-Resendiz, K.J.G.; Bernal-Ortega, J.A.; Covantes-Rosales, C.E.; Ortiz-Lazareno, P.C.; Toledo-Ibarra, G.A.; Ventura-Ramón, G.H.; Girón-Pérez, M.I. In-vitro effect of diazoxon, a metabolite of diazinon, on proliferation, signal transduction, and death induction in mononuclear cells of Nile tilapia fish (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 105, 8–15. [Google Scholar] [CrossRef]
- Toledo-Ibarra, G.A.; Girón-Pérez, M.I.; Covantes-Rosales, C.E.; Ventura-Ramón, G.H.; Pérez-Sánchez, G.; López-Torres, A.; Díaz-Resendiz, K.J.G.; Becerril-Villanueva, E.; Pavón, L. Alterations in the non-neuronal cholinergic system induced by in-vitro exposure to diazoxon in spleen mononuclear cells of Nile tilapia (O. niloticus). Fish Shellfish Immunol. 2021, 108, 134–141. [Google Scholar] [CrossRef]
- Qian, J.; Galitovskiy, V.; Chernyavsky, A.I.; Marchenko, S.; Grando, S.A. Plasticity of the murine spleen T-cell cholinergic receptors and their role in in vitro differentiation of naïve CD4 T cells toward the Th1, Th2 and Th17 lineages. Genes Immun. 2011, 12, 222–230. [Google Scholar] [CrossRef]
- Grando, S.A.; Kawashima, K.; Wessler, I. A historic perspective on the current progress in elucidation of the biologic significance of non-neuronal acetylcholine. Int. Immunopharmacol. 2020, 81, 106289. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Villaça, Y.; Filgueiras, C.C.; Manhães, A.C. Developmental aspects of the cholinergic system. Behav. Brain Res. 2011, 221, 367–378. [Google Scholar] [CrossRef]
- Deiana, S.; Platt, B.; Riedel, G. The cholinergic system and spatial learning. Behav. Brain Res. 2011, 221, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Monte, M.M.; Wang, T.; Costa, M.M.; Harun, N.O.; Secombes, C.J. Cloning and expression analysis of two ROR-γ homologues (ROR-γa1 and ROR-γa2) in rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol. 2012, 33, 365–374. [Google Scholar] [CrossRef]
- Kono, T.; Korenaga, H.; Sakai, M. Genomics of fish IL-17 ligand and receptors: A review. Fish Shellfish Immunol. 2011, 31, 635–643. [Google Scholar] [CrossRef]
- Feng, S.; Zhou, H.; Wang, Y.; Qiu, X.; Zhang, A.; Wang, X. Novel functions of grass carp three p40 isoforms as modulators of Th17 signature cytokine expression in head kidney leukocytes. Fish Shellfish Immunol. 2020, 98, 995–1000. [Google Scholar] [CrossRef]
- Hirose, T.; Smith, R.J.; Jetten, A.M. ROR-γ: The Third Member of ROR/RZR Orphan Receptor Subfamily That Is Highly Expressed in Skeletal Muscle. Biochem. Biophys. Res. Commun. 1994, 205, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Kameswaran, V.; Zhang, Y.; Zheng, S.; Sun, J.; Wang, J.; DeVirgiliis, J.; Liou, H.C.; Beg, A.A.; Chen, Y.H. The Th17 immune response is controlled by the Rel-RORγ-RORγT transcriptional axis. J. Exp. Med. 2011, 208, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Bøgwald, J.; Dalmo, R.A.; Zhang, W.; Hu, Y.H. Th17 master transcription factors RORα and RORγ regulate the expression of IL-17C, IL-17D and IL-17F in Cynoglossus semilaevis. Dev. Comp. Immunol. 2016, 55, 169–178. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Xu, S.; Ni, H.; Chen, H.; Dai, Q. The interaction between STAT3 and nAChRa1 interferes with nicotine-induced atheroslerosis via Akt/mTOR signaling cascade. Aging 2019, 11, 8120–8138. [Google Scholar] [CrossRef]
- Rostamzadeh, D.; Yousefi, M.; Haghshenas, M.R.; Ahmadi, M.; Dolati, S.; Babaloo, Z. mTOR Signaling pathway as a master regulator of memory CD8+ T-cells, Th17, and NK cells development and their functional properties. J. Cell Physiol. 2019, 234, 12353–12368. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Nagai, S.; Ikejiri, A.; Ohtani, M.; Ichiyama, K.; Baba, Y.; Yamada, T.; Egami, S.; Hoshii, T.; Hirao, A.; et al. PI3K-Akt-mTORC1-S6K1/2 Axis Controls Th17 Differentiation by Regulating Gfi1 Expression and Nuclear Translocation of RORγ. Cell Rep. 2012, 1, 360–373. [Google Scholar] [CrossRef]
- Zhou, J.; Wulfkuhle, J.; Zhang, H.; Gu, P.; Yang, Y.; Deng, J.; Margolick, J.B.; Liotta, L.A.; Petricoin, E., 3rd; Zhang, Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. USA 2007, 104, 16158–16163, Erratum in Proc. Natl. Acad. Sci. USA 2007, 104, 19655. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, Z.; Chen, B.; Han, J.; Gao, Y.; Zhang, J.; Zhao, W.; Wang, X.; Dai, J. Nogo-66 promotes the differentiation of neural progenitors into astroglial lineage cells through mTOR-STAT3 pathway. PLoS ONE 2008, 3, e1856. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Tan, L.; Xu, Y.; Xie, X.; Zhao, Y. The Regulatory Effects of mTOR Complexes in the Differentiation and Function of CD4+ T Cell Subsets. J. Immunol. Res. 2020, 2020, 3406032. [Google Scholar] [CrossRef]
- Toledo-Ibarra, G.A.; Díaz-Resendiz, K.J.G.; Pavón-Romero, L.; Rojas-García, A.E.; Medina-Díaz, I.M.; Girón-Pérez, M.I. Effects of diazinon on the lymphocytic cholinergic system of Nile tilapia fish (Oreochromis niloticus). Vet Immunol Immunopathol. 2016, 176, 58–63. [Google Scholar] [CrossRef]
- Li, K.; Shen, X.; Qiu, H.; Zhao, T.; Ai, K.; Li, C.; Zhang, Y.; Li, K.; Duan, M.; Wei, X.; et al. S6K1/S6 axis-regulated lymphocyte activation is important for adaptive immune response of Nile tilapia. Fish Shellfish Immunol. 2020, 106, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, X.; Zhang, L.; Chi, H.; Yang, J. Raptor/mTORC1 Acts as a Modulatory Center to Regulate Anti-bacterial Immune Response in Rockfish. Front. Immunol. 2019, 10, 2953. [Google Scholar] [CrossRef]
- D’Angelo, C.; Costantini, E.; Salvador, N.; Marchioni, M.; Di Nicola, M.; Greig, N.H.; Reale, M. nAChRs gene expression and neuroinflammation in APPswe/PS1dE9 transgenic mouse. Sci. Rep. 2021, 11, 9711. [Google Scholar]
- Torrealba, D.; Balasch, J.C.; Criado, M.; Tort, L.; Mackenzie, S.; Roher, N. Functional evidence for the inflammatory reflex in teleosts: A novel α7 nicotinic acetylcholine receptor modulates the macrophage response to dsRNA. Dev. Comp. Immunol. 2018, 84, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Filippini, P.; Cesario, A.; Fini, M.; Locatelli, F.; Rutella, S. The Yin and Yang of Non-Neuronal α7-Nicotinic Receptors in Inflammation and Autoimmunity. Curr. Drug. Targets 2013, 13, 644–655. [Google Scholar] [CrossRef]
- Moon, J.; Lee, S.Y.; Choi, J.W.; Lee, A.R.; Yoo, J.H.; Moon, S.J.; Park, S.H.; Cho, M.L. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J. Transl. Med. 2021, 19, 192, Correction in J. Transl. Med. 2021, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Razani-Boroujerdi, S.; Behl, M.; Hahn, F.F.; Pena-Philippides, J.C.; Hutt, J.; Sopori, M.L. Role of muscarinic receptors in the regulation of immune and inflammatory responses. J. Neuroimmunol. 2008, 194, 83–88. [Google Scholar] [CrossRef]
- Maeda, S.; Qu, Q.; Robertson, M.J.; Skiniotis, G.; Kobilka, B.K. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 2019, 364, 552–557. [Google Scholar] [CrossRef]
- Langmead, C.J.; Watson, J.; Reavill, C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008, 117, 232–243. [Google Scholar] [CrossRef]
- Seo, J.S.; Kim, M.S.; Park, E.M.; Ahn, S.J.; Kim, N.Y.; Jung, S.H.; Kim, J.W.; Lee, H.H.; Chung, J.K. Cloning and characterization of muscarinic receptor genes from the nile tilapia (Oreochromis niloticus). Mol. Cells 2009, 27, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Szabo, S.J.; Sullivan, B.M.; Sternmann, C.; Satoskar, A.R.; Sleckman, B.P.; Glimcher, L.H. Distinct effects of T-bet in Th1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science 2002, 295, 338–342. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 2012, 91, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Darby, M.; Schnoeller, C.; Vira, A.; Culley, F.J.; Bobat, S.; Logan, E.; Kirstein, F.; Wess, J.; Cunningham, A.F.; Brombacher, F.; et al. The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection. PLoS Pathog. 2015, 11, e1004636, Correction in PLoS Pathog. 2015, 11, e1004727. [Google Scholar] [CrossRef]
- Díaz-Reséndiz, K.J.G.; Toledo-Ibarra, G.A.; Girón-Pérez, M.I. Modulation of immune response by organophosphorus pesticides: Fishes as a potential model in immunotoxicology. J. Immunol. Res. 2015, 2015, 213836. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Resendiz, K.J.G.; Ortiz-Lazareno, P.C.; Covantes-Rosales, C.E.; Trujillo-Lepe, A.M.; Ventura-Ramón, G.H.; Girón-Pérez, M.I. Effect of diazinon, an organophosphate pesticide, on signal transduction and death induction in mononuclear cells of Nile tilapia fish (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 89, 12–17. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Kurokawa, M.; Ozaki, N.; Nara, K.; Atou, K.; Takada, E.; Kamochi, H.; Suzzuki, N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin. Exp. Immunol. 2006, 146, 116–123. [Google Scholar] [CrossRef]
- Lein, P.J.; Fryer, A.D. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol. Sci. 2005, 83, 166–176. [Google Scholar] [CrossRef]
- Sellick, J. Enhancing the protection of animals used for scientific purposes. J. Environ. Law Manag. 2011, 23, 75–82. [Google Scholar]
- Wilson, J.M.; Bunte, R.M.; Carty, A.J. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 785–789. [Google Scholar]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 6, pdb.prot5439. [Google Scholar]
- Yao, Y.Y.; Chen, D.D.; Cui, Z.W.; Zhang, X.Y.; Zhou, Y.Y.; Guo, X.; Li, A.H.; Zhang, Y.A. Oral vaccination of tilapia against Streptococcus agalactiae using Bacillus subtilis spores expressing Sip. Fish Shellfish Immunol. 2019, 86, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.G.; Wang, X.L.; Tian, J.; Liu, W.; Wu, F.; Jiang, M.; Wen, H. Evaluation of reference genes for quantitative real-time RT-PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus). Gene 2013, 527, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene | Forward | Reverse | Reference |
|---|---|---|---|
| T-bet | 5′CCTCCTCATCTTCTACATCAC 3 | 5′CCTCTTCTTGTTTTCACCCAC3′ | Yao et al., 2019 [73] |
| GATA-3 | 5′CTGGAGGGGAGCAAAGGAAT3′ | 5′CGTGAAGAGGTGTGGACTGG3′ | Yao et al., 2019 [73] |
| Foxp3 | 5′GCAGCCTCAGGTTACCACTG3′ | 5′AAGGAGGCCTGATGTTGTTG3′ | Wei et al., 2013 [21] |
| RORγ | 5′CCATGGAGGTGGTTCTGGTC 3′ | 5′CAGGGCACTGAAGAGAGCAA 3′ | Primer-BLAST (NCBI) |
| EF1-α | 5′CAAGGAAATCCGTCGTGGATAC3′ | 5′ACGGCGAAACGACCGAGGGG3′ | Yang et al., 2013 [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girón-Pérez, M.I.; Ramírez-Ibarra, K.M.; Covantes-Rosales, C.E.; Girón-Pérez, D.A.; Razura-Carmona, F.F.; Contis-Montes de Oca, A.; Morales-Montor, J.; Pavón, L.; Toledo-Ibarra, G.A. Modulation of Master Transcription Factor Expression of Nile Tilapia Leukocytes via Cholinergic Pathways. Int. J. Mol. Sci. 2025, 26, 11206. https://doi.org/10.3390/ijms262211206
Girón-Pérez MI, Ramírez-Ibarra KM, Covantes-Rosales CE, Girón-Pérez DA, Razura-Carmona FF, Contis-Montes de Oca A, Morales-Montor J, Pavón L, Toledo-Ibarra GA. Modulation of Master Transcription Factor Expression of Nile Tilapia Leukocytes via Cholinergic Pathways. International Journal of Molecular Sciences. 2025; 26(22):11206. https://doi.org/10.3390/ijms262211206
Chicago/Turabian StyleGirón-Pérez, Manuel Ivan, Kenia María Ramírez-Ibarra, Carlos Eduardo Covantes-Rosales, Daniel Alberto Girón-Pérez, Francisco Fabián Razura-Carmona, Arturo Contis-Montes de Oca, Jorge Morales-Montor, Lenin Pavón, and Gladys Alejandra Toledo-Ibarra. 2025. "Modulation of Master Transcription Factor Expression of Nile Tilapia Leukocytes via Cholinergic Pathways" International Journal of Molecular Sciences 26, no. 22: 11206. https://doi.org/10.3390/ijms262211206
APA StyleGirón-Pérez, M. I., Ramírez-Ibarra, K. M., Covantes-Rosales, C. E., Girón-Pérez, D. A., Razura-Carmona, F. F., Contis-Montes de Oca, A., Morales-Montor, J., Pavón, L., & Toledo-Ibarra, G. A. (2025). Modulation of Master Transcription Factor Expression of Nile Tilapia Leukocytes via Cholinergic Pathways. International Journal of Molecular Sciences, 26(22), 11206. https://doi.org/10.3390/ijms262211206










