Peimine Alleviates DSS-Induced Colitis by Modulating Gut Microbiota and Attenuating Inflammation and Oxidative Stress
Abstract
1. Introduction
2. Results
2.1. Network Pharmacology and Molecular Docking Identify Key Targets and Pathways for Peimine in UC
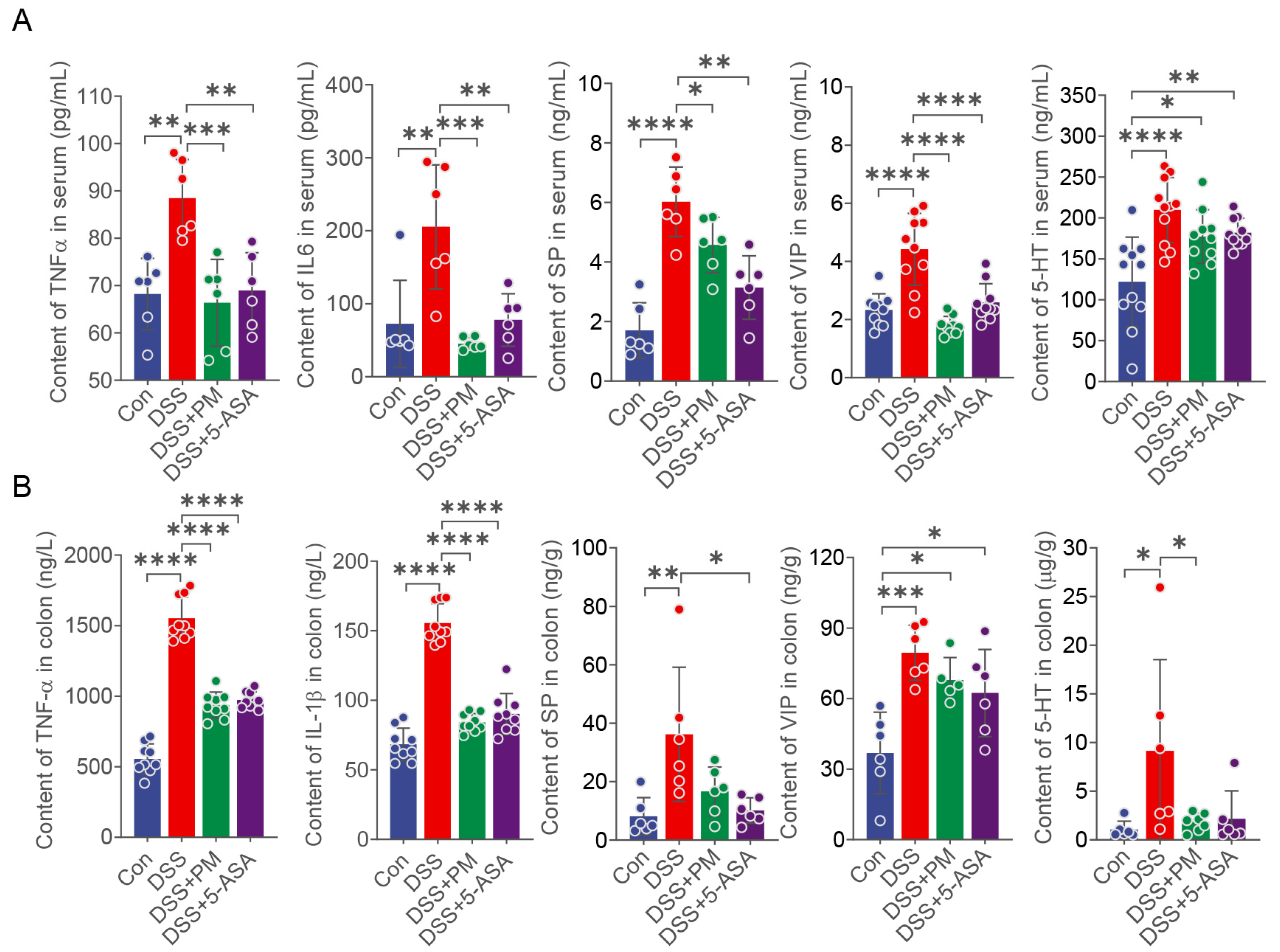
2.2. PM Attenuates DSS-Induced Colitis via Suppressing Inflammation and Restoring Intestinal Barrier Integrity
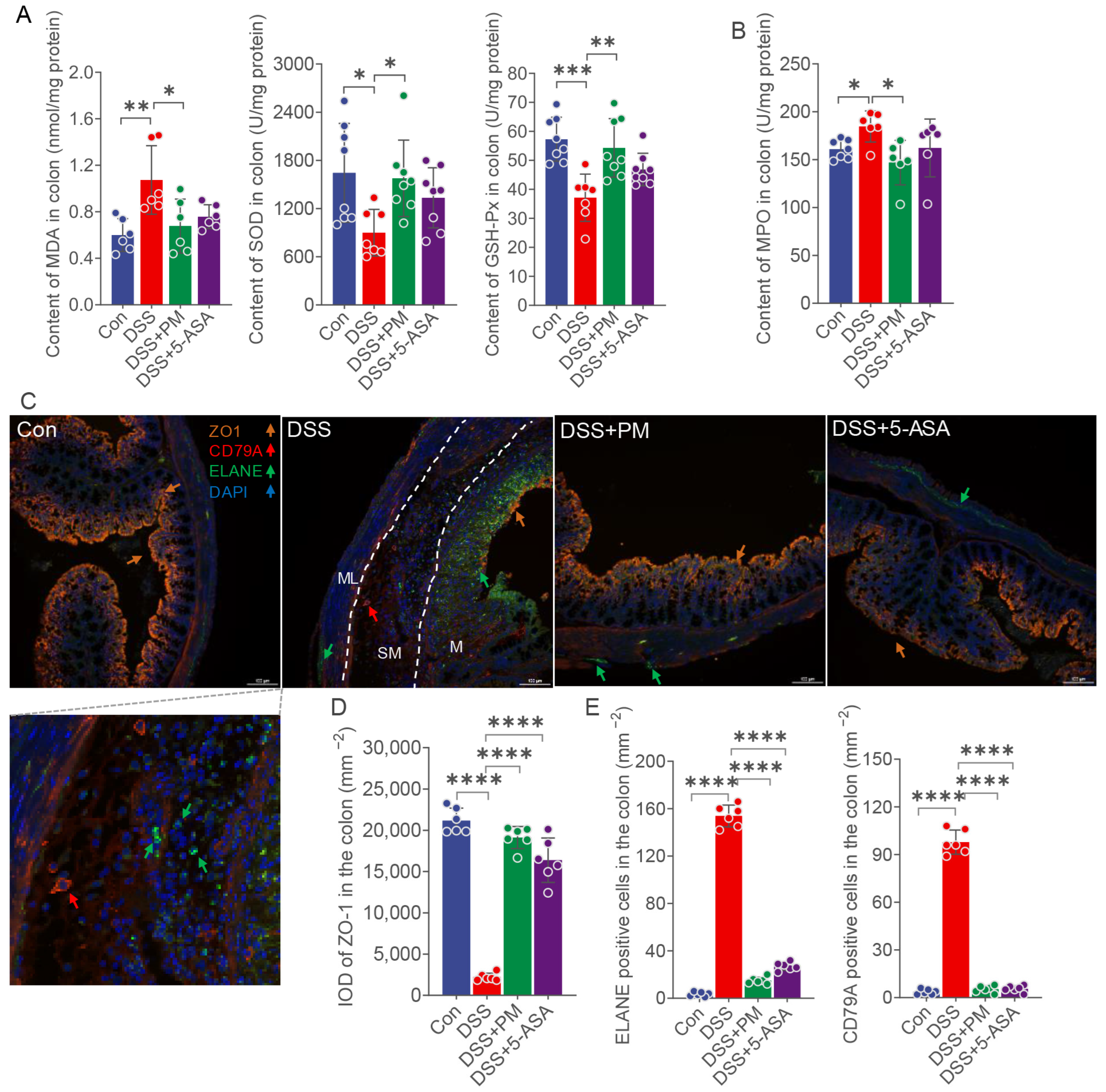
2.3. PM Attenuates Oxidative Stress and Restores Mucosal Barrier Integrity
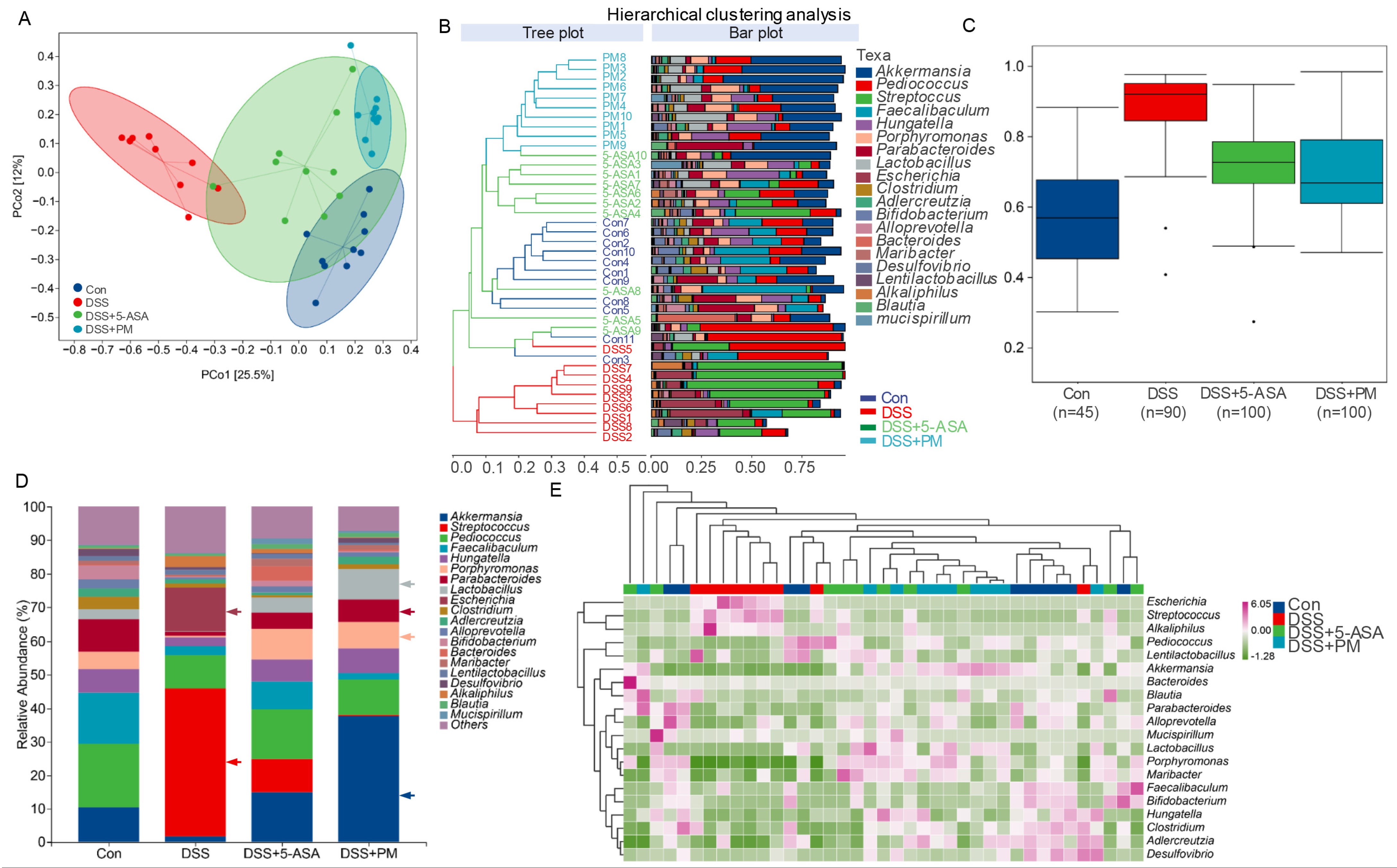
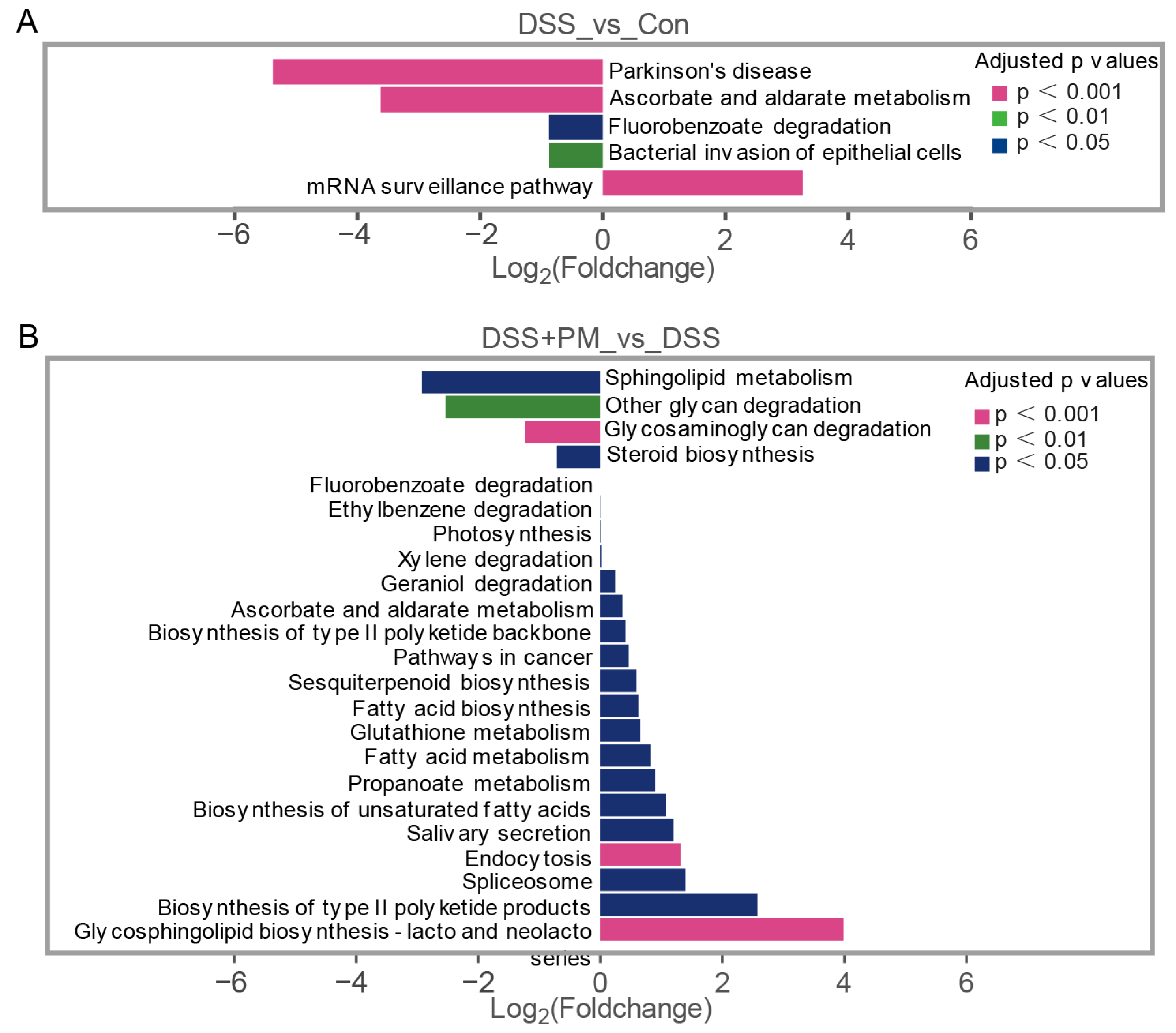
2.4. PM Restores Gut Microbiota Homeostasis and Metabolic Function in Colitis
2.5. PM Restores DSS-Induced Impairment of Beneficial Short-Chain Fatty Acid Production
3. Discussion
4. Materials and Methods
4.1. Network Pharmacological Analysis
4.2. Mice and Experiment Design
4.3. Histological Analysis
4.4. Periodic Acid-Schiff Stain
4.5. Quantification of Inflammatory Cytokines and Antioxidant Indexes in the Colon and Serum
4.6. Microbial Amplicon Sequencing and Data Analysis
4.7. Short-Chain Fatty Acid (SCFA) Quantitative Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, B.; Sun, Y.; Li, J.; Shen, P.; Hu, L.; Liu, G.; Wang, J.; Duan, L.; Zhan, S.; et al. Incidence of Inflammatory Bowel Disease in Urban China: A Nationwide Population-based Study. Clin. Gastroenterol. Hepatol. 2023, 21, 3379–3386.e29. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Chauhan, S.; Harwansh, R.K. Recent advances in nanocarrier systems for ulcerative colitis: A new era of targeted therapy and biomarker integration. J. Drug Deliv. Sci. Technol. 2024, 93, 105466. [Google Scholar] [CrossRef]
- Tian, H.; Wen, Z.; Chen, J.; Zhao, C.; Yang, C.; Guo, Y.; Sun, B. Alleviation of DSS-induced ulcerative colitis by pomelo peel polysaccharides: Exploration for the potential mechanism from comprehensive analysis of multi-omics. Food Biosci. 2025, 65, 105955. [Google Scholar] [CrossRef]
- Cottone, M.; Renna, S.; Modesto, I.; Orlando, A. Is 5-ASA Still the Treatment of Choice for Ulcerative Colitis? Curr. Drug Targets 2011, 12, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Geng, J.; Meng, H. Glucocorticoid receptor modulates dendritic cell function in ulcerative colitis. Histol. Histopathol. 2020, 35, 1379–1389. [Google Scholar]
- Le Berre, C.; Roda, G.; Nedeljkovic Protic, M.; Danese, S.; Peyrin-Biroulet, L. Modern use of 5-aminosalicylic acid compounds for ulcerative colitis. Expert Opin. Biol. Ther. 2020, 20, 363–378. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Lan, D.; Niu, J.; Miao, J.; Dong, X.; Yang, G.; Zhang, F.; Cao, Y.; Wang, K.; et al. Differential expression of serum microRNAs in glucocorticoid-resistant patients with ulcerative colitis. Int. J. Clin. Exp. Pathol. 2018, 11, 936–946. [Google Scholar]
- Sands, B.E. Immunosuppressive drugs in ulcerative colitis: Twisting facts to suit theories? Gut 2006, 55, 437–441. [Google Scholar] [CrossRef]
- D’Amico, F.; Parigi, T.L.; Bonovas, S.; Peyrin-Biroulet, L.; Danese, S. Long-term safety of approved biologics for ulcerative colitis. Expert Opin. Drug Saf. 2020, 19, 807–816. [Google Scholar] [CrossRef]
- Pugliese, D.; Felice, C.; Papa, A.; Gasbarrini, A.; Rapaccini, G.L.; Guidi, L.; Armuzzi, A. Anti TNF-α therapy for ulcerative colitis: Current status and prospects for the future. Expert Rev. Clin. Immunol. 2017, 13, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Kavanagh, D.O.; Winter, D.C. Modern surgery for ulcerative colitis. Updates Surg 2020, 72, 325–333. [Google Scholar] [CrossRef]
- Ryan, D.P.; Doody, D.P. Surgical options in the treatment of ulcerative colitis. Semin. Pediatr. Surg. 2017, 26, 379–383. [Google Scholar] [CrossRef]
- Han, D.; Guan, X.; Zhu, F.; Yang, Q.; Su, D. Oral aged garlic (Allium sativum) alleviates ulcerative colitis in mice by improving gut homeostasis. Food Funct. 2024, 15, 8935–8951. [Google Scholar] [CrossRef]
- Liu, C.; Zhen, D.; Du, H.; Gong, G.; Wu, Y.; Ma, Q.; Quan, Z. Synergistic anti-inflammatory effects of peimine, peiminine, and forsythoside a combination on LPS-induced acute lung injury by inhibition of the IL-17-NF-κB/MAPK pathway activation. J. Ethnopharmacol. 2022, 295, 115343. [Google Scholar] [CrossRef]
- Chen, K.; Lv, Z.; Zhou, C.; Liang, S.; Huang, W.; Wang, Z.; Zhu, W.; Wang, Y.; Jing, X.; Lin, H.; et al. Peimine suppresses interleukin-1β-induced inflammation via MAPK downregulation in chondrocytes. Int. J. Mol. Med. 2019, 43, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Dong, H.; Zheng, W.; Feng, S.; Tian, Y.; Zhao, P.; Ma, J.; Ren, Z.; Xie, Y. Effective-constituent compatibility-based analysis of Bufei Yishen formula, a traditional herbal compound as an effective treatment for chronic obstructive pulmonary disease. J. Integr. Med. 2020, 18, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Deng, S.; Chen, M.; Deng, C.; Gu, W.; Wang, S.; Tang, D. Huang-Lian-Jie-Du Decoction alleviates diabetic encephalopathy by regulating inflammation and pyroptosis via suppression of AGEs/RAGE/NF-κB pathways. J. Ethnopharmacol. 2025, 337, 118787. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, L.; Xu, M.; Jiang, P.; Zhang, K. Moringin alleviates DSS-induced ulcerative colitis in mice by regulating Nrf2/NF-κB pathway and PI3K/AKT/mTOR pathway. Int. Immunopharmacol. 2024, 134, 112241. [Google Scholar] [CrossRef]
- Ma, Y.; Lang, X.; Yang, Q.; Han, Y.; Kang, X.; Long, R.; Du, J.; Zhao, M.; Liu, L.; Li, P.; et al. Paeoniflorin promotes intestinal stem cell-mediated epithelial regeneration and repair via PI3K-AKT-mTOR signalling in ulcerative colitis. Int. Immunopharmacol. 2023, 119, 110247. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.; Stappenbeck, T.S. IL-6 Stimulates Intestinal Epithelial Proliferation and Repair after Injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, C.; Wu, S.; Ma, J.; Liu, Y.; Li, W.; Wang, T.; Yang, L.; Cheng, K.; Zhuang, R. Knockout of IL-6 mitigates cold water-immersion restraint stress-induced intestinal epithelial injury and apoptosis. Front. Immunol. 2022, 13, 936689. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Liu, P.; Bian, Y.; Fan, Y.; Zhong, J.; Liu, Z. Protective Effect of Naringin on In Vitro Gut-Vascular Barrier Disruption of Intestinal Microvascular Endothelial Cells Induced by TNF-α. J. Agric. Food Chem. 2020, 68, 168–175. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, E.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Y.; Yuan, Y.; Guo, J.; Li, H.; Li, Q.; Liu, S. Liposome-embedded SOD attenuated DSS-induced ulcerative colitis in mice by ameliorating oxidative stress and intestinal barrier dysfunction. Food Funct. 2023, 14, 4392–4405. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chen, H.; Chen, X.; Guo, C. The Roles of Neutrophil-Derived Myeloperoxidase (MPO) in Diseases: The New Progress. Antioxidants 2024, 13, 132. [Google Scholar] [CrossRef]
- Prasad, A.; Rossi, C.; Manoharan, R.R.; Sedlářová, M.; Cangeloni, L.; Rathi, D.; Tamasi, G.; Pospíšil, P.; Consumi, M. Bioactive Compounds and Their Impact on Protein Modification in Human Cells. Int. J. Mol. Sci. 2022, 23, 7424. [Google Scholar] [CrossRef]
- Di Sebastiano, P.; Grossi, L.; Di Mola, F.F.; Angelucci, D.; Friess, H.; Marzio, L.; Innocenti, P.; Buchler, M.W. SR140333, a Substance P Receptor Antagonist, Influences Morphological and Motor Changes in Rat Experimental Colitis. Dig. Dis. Sci. 1999, 44, 439–444. [Google Scholar] [CrossRef]
- Koon, H.W.; Shih, D.; Karagiannides, I.; Zhao, D.; Fazelbhoy, Z.; Hing, T.; Xu, H.; Lu, B.; Gerard, N.; Pothoulakis, C. Substance P Modulates Colitis-Asscociated Fibrosis. Am. J. Pathol. 2010, 177, 2300–2309. [Google Scholar] [CrossRef]
- Delgado, M.; Pozo, D.; Ganea, D. The Significance of Vasoactive Intestinal Peptide in Immunomodulation. Pharmacol. Rev. 2004, 56, 249–290. [Google Scholar] [CrossRef]
- Gonzalez–Rey, E.; Delgado, M. Therapeutic Treatment of Experimental Colitis with Regulatory Dendritic Cells Generated with Vasoactive Intestinal Peptide. Gastroenterology 2006, 131, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Miyake, H.; Alganabi, M.; Janssen Lok, M.; O’Connell, J.S.; Lee, C.; Li, B.; Pierro, A. Vasoactive intestinal peptide decreases inflammation and tight junction disruption in experimental necrotizing enterocolitis. J. Pediatr. Surg. 2019, 54, 2520–2523. [Google Scholar] [CrossRef]
- Wu, X.; Conlin, V.S.; Morampudi, V.; Ryz, N.R.; Nasser, Y.; Bhinder, G.; Bergstrom, K.S.; Yu, H.B.; Waterhouse, C.C.M.; Buchan, A.M.J.; et al. Vasoactive Intestinal Polypeptide Promotes Intestinal Barrier Homeostasis and Protection Against Colitis in Mice. PLoS ONE 2015, 10, e0125225. [Google Scholar] [CrossRef]
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef]
- Glauben, R.; Batra, A.; Fedke, I.; Zeitz, M.; Lehr, H.A.; Leoni, F.; Mascagni, P.; Fantuzzi, G.; Dinarello, C.A.; Siegmund, B. Histone Hyperacetylation Is Associated with Amelioration of Experimental Colitis in Mice. J. Immunol. 2006, 176, 5015–5022. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Xuan, B.; Li, D.; Yin, N.; Ning, L.; Zhou, Y.; Yan, Y.; Tong, T.; Zhu, X.; et al. Disruption of CerS6-mediated sphingolipid metabolism by FTO deficiency aggravates ulcerative colitis. Gut 2024, 73, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, C.; Sun, Y. Pharmacological mechanisms and therapeutic potential of Puerarin in post-stroke rehabilitation: Insights from network pharmacology and experimental validation. Biochem. Biophys. Res. Commun. 2025, 785, 152610. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Mehmood, K.; Adnan, M.; Khan, M.A.; Eltoum, N.E.; Krishnan, A.; Baig, M.S. Multi-targeted molecular docking, pharmacokinetics, and drug-likeness evaluation of okra-derived ligand abscisic acid targeting signaling proteins involved in the development of diabetes. Molecules 2021, 26, 5957. [Google Scholar] [CrossRef] [PubMed]

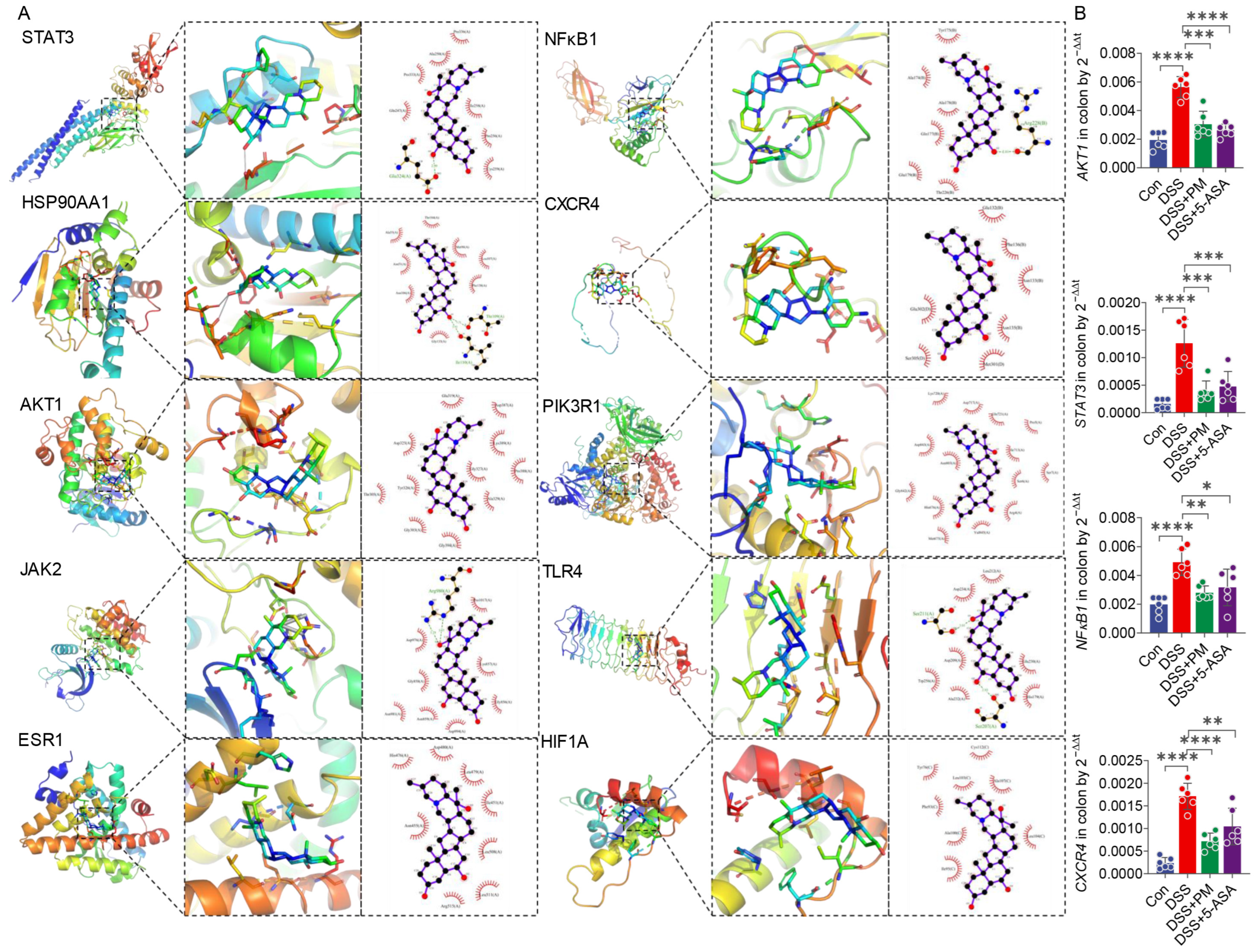


| Name | Degree 1 | Closeness Centrality 2 | Betweenness Centrality 3 | Binding Energy (kcal/mol) | Predicted Inhibitory Constant (pKi) |
|---|---|---|---|---|---|
| STAT3 | 28 | 0.68 | 0.21 | −8.1 ± 0.0 | 5.94 ± 0.00 μM |
| HSP90AA1 | 24 | 0.63 | 0.10 | −7.7 ± 0.2 | 5.65 ± 0.13 μM |
| AKT1 | 23 | 0.63 | 0.06 | −9.4 ± 0.0 | 6.89 ± 0.00 μM |
| JAK2 | 23 | 0.63 | 0.08 | −8.2 ± 0.0 | 6.01 ± 0.00 μM |
| ESR1 | 19 | 0.58 | 0.06 | −8.1 ± 0.0 | 5.94 ± 0.00 μM |
| NFKB1 | 17 | 0.57 | 0.06 | −7.5 ± 0.0 | 5.50 ± 0.00 μM |
| CXCR4 | 17 | 0.57 | 0.15 | −7.2 ± 0.1 | 5.31 ± 0.08 μM |
| PIK3R1 | 17 | 0.55 | 0.04 | −9.6 ± 0.0 | 7.04 ± 0.00 μM |
| TLR4 | 16 | 0.56 | 0.08 | −7.5 ± 0.3 | 5.53 ± 0.18 μM |
| HIF1A | 16 | 0.55 | 0.02 | −7.6 ± 0.4 | 5.55 ± 0.30 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Han, D.; Sha, H.; Yao, M.; Zhang, J.; Zhang, G.; Wu, Y.; Su, D.; Yang, Q. Peimine Alleviates DSS-Induced Colitis by Modulating Gut Microbiota and Attenuating Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 11203. https://doi.org/10.3390/ijms262211203
Guan X, Han D, Sha H, Yao M, Zhang J, Zhang G, Wu Y, Su D, Yang Q. Peimine Alleviates DSS-Induced Colitis by Modulating Gut Microbiota and Attenuating Inflammation and Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(22):11203. https://doi.org/10.3390/ijms262211203
Chicago/Turabian StyleGuan, Xuke, Deping Han, Haojie Sha, Moyue Yao, Jiaying Zhang, Guangyao Zhang, Yibing Wu, Dingding Su, and Qing Yang. 2025. "Peimine Alleviates DSS-Induced Colitis by Modulating Gut Microbiota and Attenuating Inflammation and Oxidative Stress" International Journal of Molecular Sciences 26, no. 22: 11203. https://doi.org/10.3390/ijms262211203
APA StyleGuan, X., Han, D., Sha, H., Yao, M., Zhang, J., Zhang, G., Wu, Y., Su, D., & Yang, Q. (2025). Peimine Alleviates DSS-Induced Colitis by Modulating Gut Microbiota and Attenuating Inflammation and Oxidative Stress. International Journal of Molecular Sciences, 26(22), 11203. https://doi.org/10.3390/ijms262211203






