Neural Cues and Genomic Clues: NGS Insights into Neurogenic Sarcopenia and Muscle Atrophy
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Eligibility Criteria
2.3. Data Extraction and Synthesis
2.4. Risk of Bias and Limitations
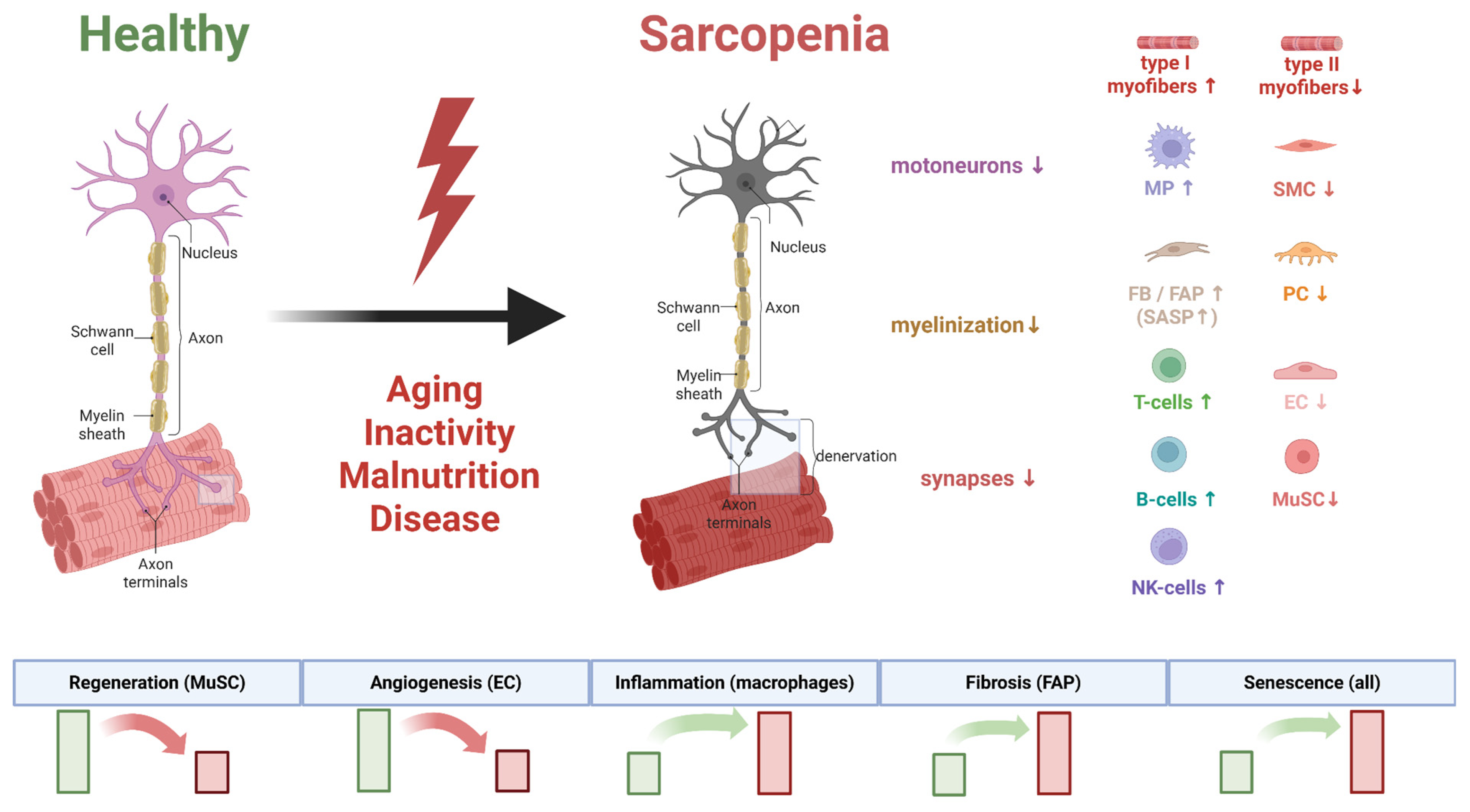
3. The Neurogenic Paradigm in Sarcopenia Pathogenesis: From Synaptic Dysfunction to Systemic Neurodegeneration
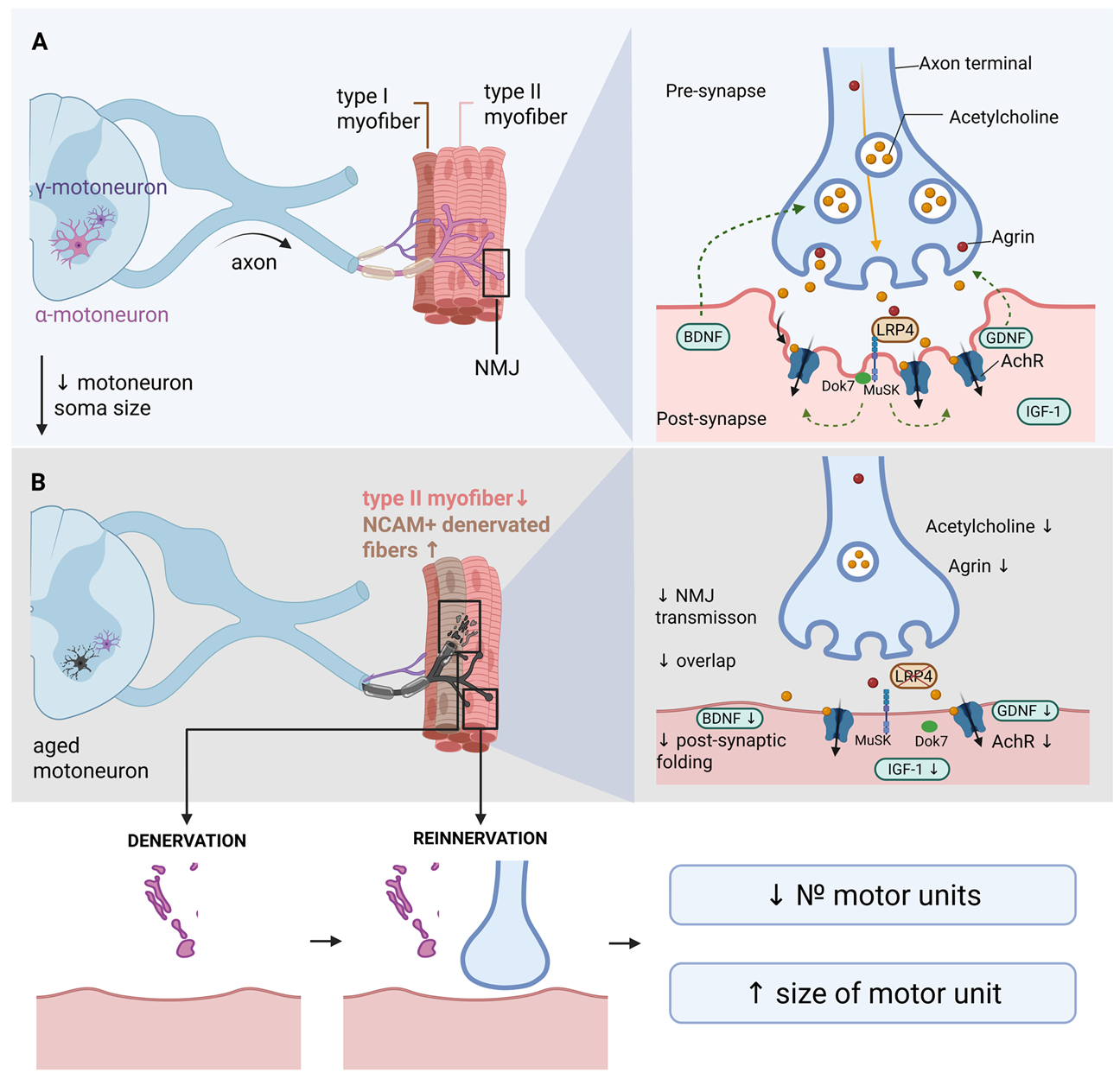
3.1. Architecture of the Neuromuscular Axis and Its Remodeling in Aging and Sarcopenia
3.2. Molecular Determinants of Neurogenic Muscle Atrophy: Neurotrophic Factors and Signaling Cascades
3.2.1. Brain-Derived Neurotrophic Factor (BDNF) in the Maintenance of NMJ Structure and Function
3.2.2. Neurotrophin-4 (NT-4) and TrkB Signaling in Maintaining Neuromuscular Synapse Stability
3.2.3. NGF and NT-3: Additional Regulators of the Neuromuscular Axis Neuritrophin-3 (NT-3)
3.2.4. Nerve Growth Factor (NGF)
3.2.5. GDNF and Its Role in Supporting the Neuromuscular System
3.2.6. IGF-1 as a Key Anabolic Factor and Potential Biomarker of Sarcopenia
3.2.7. Regulatory Role of Irisin in Muscle Homeostasis and Sarcopenia Development
3.3. Sarcopenia as a Component of the Neurodegenerative Disease Continuum: Shared Pathogenic Mechanisms with Alzheimer’s and Parkinson’s Diseases
3.3.1. Alzheimer’s Disease
3.3.2. Parkinson’s Disease
3.4. Summary: The Neuromuscular Junction as a Critical Link in the Pathogenesis of Muscle Failure
4. Genomic Landscapes Unveiled by NGS: Insights into Neurogenic Sarcopenia
4.1. Genetic Determinants of Sarcopenia Development and Progression
4.2. Epigenetic Regulation in Sarcopenia: The Role of DNA Methylation and Post-Translational Histone Modifications
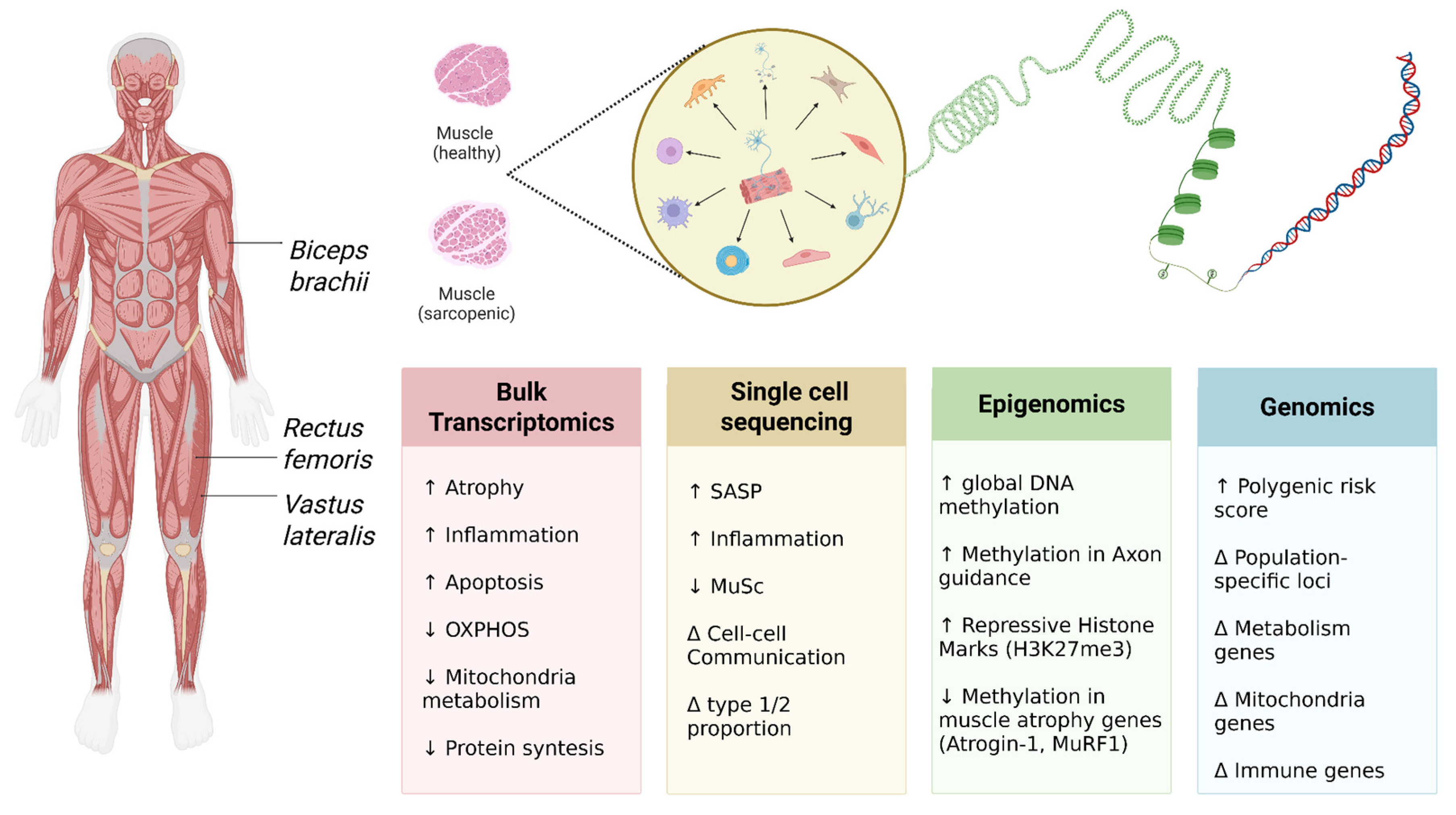
5. Transcriptomic Signatures from NGS: Dysregulated Pathways in Neuromuscular Crosstalk
5.1. Systemic Analysis of Muscle Tissue Transcriptome: Dysregulation of Key Signaling Pathways and Biomarker Identification
5.2. Analysis of Cellular Heterogeneity by Single-Cell Sequencing: The Contribution of Cellular Populations to Sarcopenia Development
6. Translational Implications and Future Directions: From NGS Data to Clinical Applications
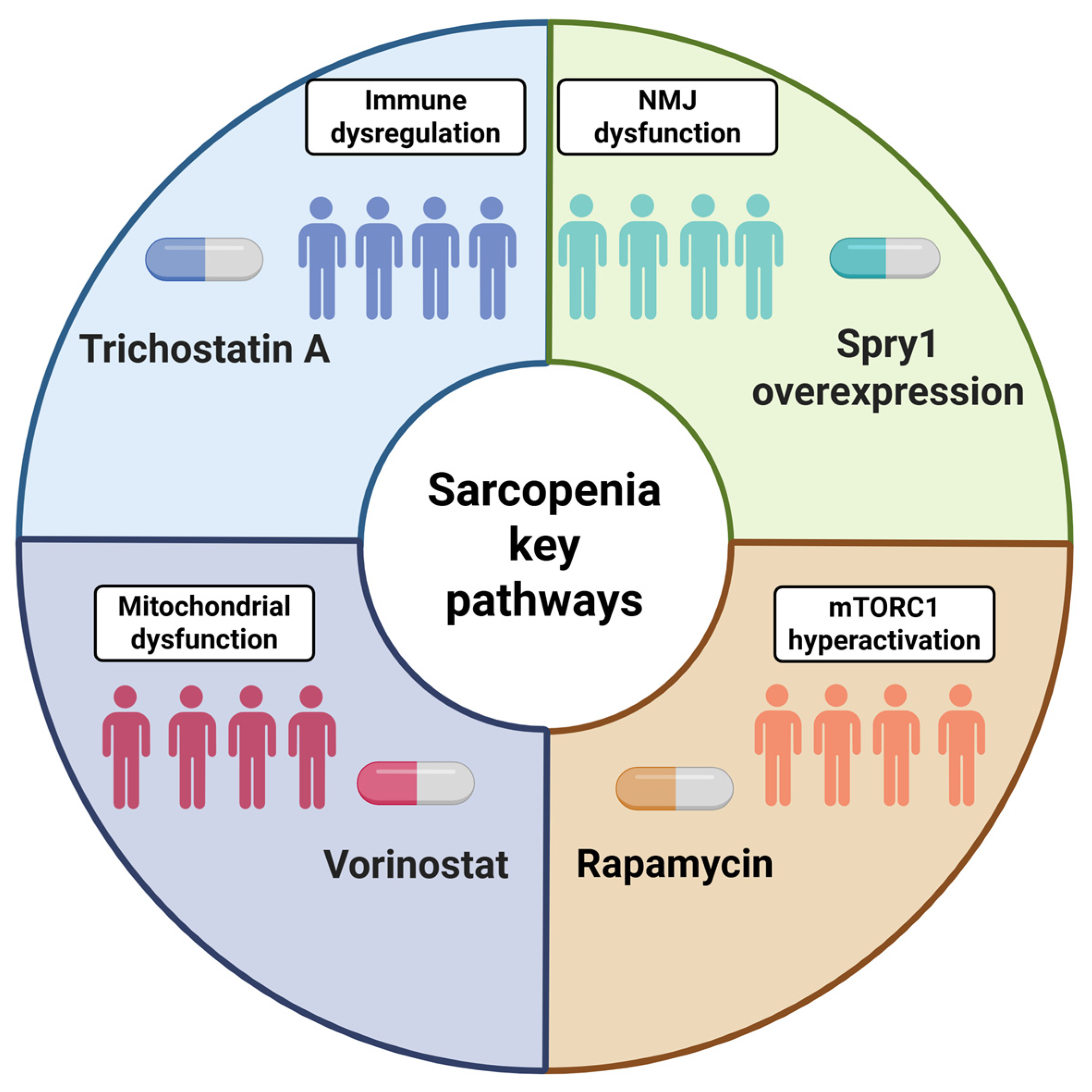
6.1. Contemporary Experimental Models: Platforms for Modeling Sarcopenia In Vitro and In Vivo
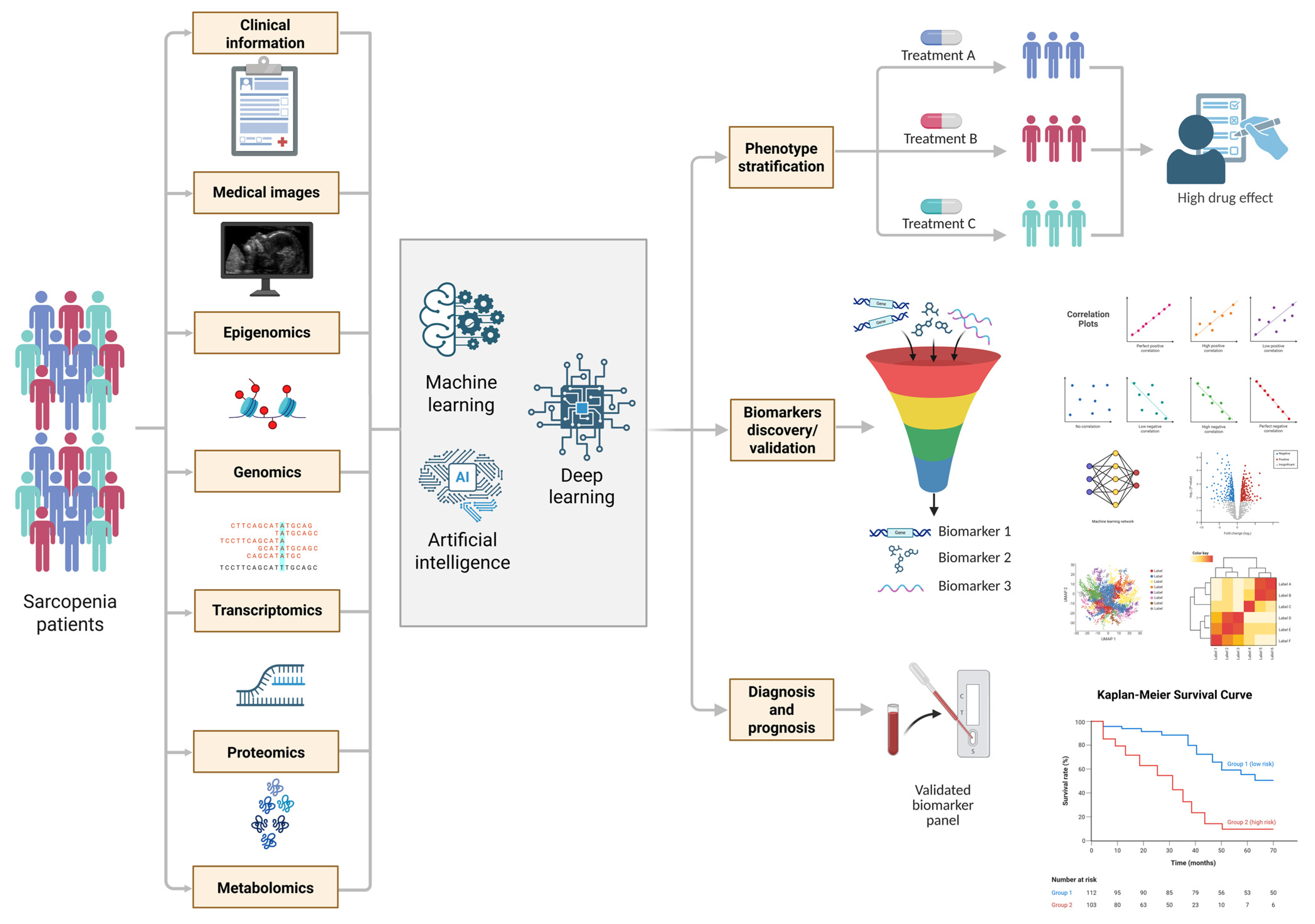
6.2. Biomarker Identification Strategies: Integration of Multi-Omics Data for Diagnosis and Treatment
6.3. Personalized Medicine Technologies: Application of Artificial Intelligence and Machine Learning for Analyzing Multidimensional Datasets in Sarcopenia
7. Clinical Implications and Translational Outlook
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 Code for Sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of Sarcopenia: Prevalence, Risk Factors, and Consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Beaudart, C.; Alcazar, J.; Aprahamian, I.; Batsis, J.A.; Yamada, Y.; Prado, C.M.; Reginster, J.-Y.; Sanchez-Rodriguez, D.; Lim, W.S.; Sim, M.; et al. Health Outcomes of Sarcopenia: A Consensus Report by the Outcome Working Group of the Global Leadership Initiative in Sarcopenia (GLIS). Aging Clin. Exp. Res. 2025, 37, 100. [Google Scholar] [CrossRef]
- Xu, J.; Wan, C.S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia Is Associated with Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Demonceau, C.; Reginster, J.-Y.; Locquet, M.; Cesari, M.; Cruz Jentoft, A.J.; Bruyère, O. Sarcopenia and Health-Related Quality of Life: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1228–1243. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and Its Association with Falls and Fractures in Older Adults: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- Darvishi, A.; Nikkhah, A.; Shafiee, G.; Daroudi, R.; Heshmat, R. Economic Burden of Sarcopenia-Related Disability in the Elderly Population: A Study in Iran. BMC Res. Notes 2024, 17, 319. [Google Scholar] [CrossRef]
- Xu, H.; Brown, J.L.; Bhaskaran, S.; Van Remmen, H. Reactive Oxygen Species in the Pathogenesis of Sarcopenia. Free Radic. Biol. Med. 2025, 227, 446–458. [Google Scholar] [CrossRef]
- Morawin, B.; Tylutka, A.; Bielewicz, F.; Zembron-Lacny, A. Diagnostics of Inflammaging in Relation to Sarcopenia. Front. Public Health 2023, 11, 1162385. [Google Scholar] [CrossRef]
- Boccardi, V. Sarcopenia: A Dive into Metabolism to Promote a Multimodal, Preventive, and Regenerative Approach. Mech. Ageing Dev. 2024, 219, 111941. [Google Scholar] [CrossRef] [PubMed]
- Paez, H.G.; Pitzer, C.R.; Alway, S.E. Age-Related Dysfunction in Proteostasis and Cellular Quality Control in the Development of Sarcopenia. Cells 2023, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Kostrominova, T.Y. Skeletal Muscle Denervation: Past, Present and Future. Int. J. Mol. Sci. 2022, 23, 7489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shen, C.; Li, L.; Wu, H.; Xing, G.; Dong, Z.; Jing, H.; Chen, W.; Zhang, H.; Tan, Z.; et al. Sarcoglycan Alpha Mitigates Neuromuscular Junction Decline in Aged Mice by Stabilizing LRP4. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 8860–8873. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. The Recent Understanding of the Neurotrophin’s Role in Skeletal Muscle Adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696. [Google Scholar] [CrossRef]
- Cui, C.; Hu, Y.; Wong, R.M.Y.; Zhang, N.; Guan, Y.; Cheung, W.-H. Exploring Motor Unit and Neuromuscular Junction Dysfunction in Aging and Sarcopenia: Insights from Electromyography in Systematic Review. GeroScience 2025. [Google Scholar] [CrossRef]
- Lai, Y.; Ramírez-Pardo, I.; Isern, J.; An, J.; Perdiguero, E.; Serrano, A.L.; Li, J.; García-Domínguez, E.; Segalés, J.; Guo, P.; et al. Multimodal Cell Atlas of the Ageing Human Skeletal Muscle. Nature 2024, 629, 154–164. [Google Scholar] [CrossRef]
- Sahinyan, K.; Blackburn, D.M.; Simon, M.-M.; Lazure, F.; Kwan, T.; Bourque, G.; Soleimani, V.D. Application of ATAC-Seq for Genome-Wide Analysis of the Chromatin State at Single Myofiber Resolution. eLife 2022, 11, e72792. [Google Scholar] [CrossRef]
- Ran, S.; He, X.; Jiang, Z.-X.; Liu, Y.; Zhang, Y.-X.; Zhang, L.; Gu, G.-S.; Pei, Y.; Liu, B.-L.; Tian, Q.; et al. Whole-Exome Sequencing and Genome-Wide Association Studies Identify Novel Sarcopenia Risk Genes in Han Chinese. Mol. Genet. Genomic Med. 2020, 8, e1267. [Google Scholar] [CrossRef]
- Jones, G.; Trajanoska, K.; Santanasto, A.J.; Stringa, N.; Kuo, C.-L.; Atkins, J.L.; Lewis, J.R.; Duong, T.; Hong, S.; Biggs, M.L.; et al. Genome-Wide Meta-Analysis of Muscle Weakness Identifies 15 Susceptibility Loci in Older Men and Women. Nat. Commun. 2021, 12, 654. [Google Scholar] [CrossRef]
- Pigna, E.; Renzini, A.; Greco, E.; Simonazzi, E.; Fulle, S.; Mancinelli, R.; Moresi, V.; Adamo, S. HDAC4 Preserves Skeletal Muscle Structure Following Long-Term Denervation by Mediating Distinct Cellular Responses. Skelet. Muscle 2018, 8, 6. [Google Scholar] [CrossRef]
- Perez, K.; Ciotlos, S.; McGirr, J.; Limbad, C.; Doi, R.; Nederveen, J.P.; Nilsson, M.I.; Winer, D.A.; Evans, W.; Tarnopolsky, M.; et al. Single Nuclei Profiling Identifies Cell Specific Markers of Skeletal Muscle Aging, Frailty, and Senescence. Aging 2022, 14, 9393–9422. [Google Scholar] [CrossRef] [PubMed]
- Bączyk, M.; Manuel, M.; Roselli, F.; Zytnicki, D. Diversity of Mammalian Motoneurons and Motor Units. In Vertebrate Motoneurons; O’Donovan, M.J., Falgairolle, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 131–150. ISBN 978-3-031-07167-6. [Google Scholar]
- Tomlinson, B.E.; Irving, D. The Numbers of Limb Motor Neurons in the Human Lumbosacral Cord throughout Life. J. Neurol. Sci. 1977, 34, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.; Sieck, G. Hypoglossal Motor Neuron Loss and Degeneration Underpins Intrinsic and Extrinsic Tongue Muscle Sarcopenia. Physiology 2023, 38, 5730104. [Google Scholar] [CrossRef]
- Kostka, T. Quadriceps Maximal Power and Optimal Shortening Velocity in 335 Men Aged 23–88 Years. Eur. J. Appl. Physiol. 2005, 95, 140–145. [Google Scholar] [CrossRef]
- Siddiqi, A.; Arjunan, S.P.; Kumar, D.K. Age Related Neuromuscular Changes in sEMG of m. Tibialis Anterior Using Higher Order Statistics (Gaussianity & Linearity Test). In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 3638–3641. [Google Scholar] [CrossRef]
- Jahanian, S.; Pareja-Cajiao, M.; Gransee, H.M.; Sieck, G.C.; Mantilla, C.B. Autophagy Markers LC3 and P62 in Aging Lumbar Motor Neurons. Exp. Gerontol. 2024, 194, 112483. [Google Scholar] [CrossRef]
- Pollock, N.; Macpherson, P.C.; Staunton, C.A.; Hemmings, K.; Davis, C.S.; Owen, E.D.; Vasilaki, A.; Van Remmen, H.; Richardson, A.; McArdle, A.; et al. Deletion of Sod1 in Motor Neurons Exacerbates Age-Related Changes in Axons and Neuromuscular Junctions in Mice. eNeuro 2023, 10, ENEURO.0086-22.2023. [Google Scholar] [CrossRef]
- Guo, Y.; Jones, E.J.; Škarabot, J.; Inns, T.B.; Phillips, B.E.; Atherton, P.J.; Piasecki, M. Common Synaptic Inputs and Persistent Inward Currents of Vastus Lateralis Motor Units Are Reduced in Older Male Adults. GeroScience 2024, 46, 3249–3261. [Google Scholar] [CrossRef]
- Deschenes, M.R. Motor Unit and Neuromuscular Junction Remodeling with Aging. Curr. Aging Sci. 2011, 4, 209–220. [Google Scholar] [CrossRef]
- Balanyà-Segura, M.; Polishchuk, A.; Just-Borràs, L.; Cilleros-Mañé, V.; Silvera, C.; Ardévol, A.; Tomàs, M.; Lanuza, M.A.; Hurtado, E.; Tomàs, J. Molecular Adaptations of BDNF/NT-4 Neurotrophic and Muscarinic Pathways in Ageing Neuromuscular Synapses. Int. J. Mol. Sci. 2024, 25, 8018. [Google Scholar] [CrossRef] [PubMed]
- Kern, H.; Carraro, U.; Loefler, S.; Hofer, C.; Zampieri, S.; Mayr, W.; Boncompagni, S.; Protasi, F.; Rizzuto, R.; Sandri, M.; et al. Functional Electrical Stimulation of Skeletal Muscles in Aging and Premature Aging. In Rehabilitation Medicine for Elderly Patients; Masiero, S., Carraro, U., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 93–103. ISBN 978-3-319-57406-6. [Google Scholar]
- Covault, J.; Sanes, J.R. Neural Cell Adhesion Molecule (N-CAM) Accumulates in Denervated and Paralyzed Skeletal Muscles. Proc. Natl. Acad. Sci. USA 1985, 82, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Slack, J.R.; Hopkins, W.G.; Williams, M.N. Nerve Sheaths and Motoneurone Collateral Sprouting. Nature 1979, 282, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.L.; Farah, M.H. Axonal Regeneration and Sprouting as a Potential Therapeutic Target for Nervous System Disorders. Neural Regen. Res. 2021, 16, 1901–1910. [Google Scholar] [CrossRef]
- Paul, T.A.; Macpherson, P.C.; Janetzke, T.L.; Davis, C.S.; Jackson, M.J.; McArdle, A.; Brooks, S.V. Older Mice Show Decreased Regeneration of Neuromuscular Junctions Following Lengthening Contraction-Induced Injury. GeroScience 2023, 45, 1899–1912. [Google Scholar] [CrossRef]
- Nitkin, R.M.; Smith, M.A.; Magill, C.; Fallon, J.R.; Yao, Y.M.; Wallace, B.G.; McMahan, U.J. Identification of Agrin, a Synaptic Organizing Protein from Torpedo Electric Organ. J. Cell Biol. 1987, 105, 2471–2478. [Google Scholar] [CrossRef]
- Tsim, K.W.K.; Ruegg, M.A.; Escher, G.; Kröger, S.; McMahan, U.J. cDNA That Encodes Active Agrin. Neuron 1992, 8, 677–689. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Nhieu, J.; Liu, P.-Y.; Le, G.; Lee, D.J.; Wei, C.-W.; Lin, Y.-W.; Oh, S.-H.; Lowe, D.; Wei, L.-N. CRABP1-CaMKII-Agrn Regulates the Maintenance of Neuromuscular Junction in Spinal Motor Neuron. Cell Death Differ. 2022, 29, 1744–1756. [Google Scholar] [CrossRef]
- Magill-Solc, C.; McMahan, U.J. Motor Neurons Contain Agrin-like Molecules. J. Cell Biol. 1988, 107, 1825–1833. [Google Scholar] [CrossRef]
- Kim, N.; Stiegler, A.L.; Cameron, T.O.; Hallock, P.T.; Gomez, A.M.; Huang, J.H.; Hubbard, S.R.; Dustin, M.L.; Burden, S.J. Lrp4 Is a Receptor for Agrin and Forms a Complex with MuSK. Cell 2008, 135, 334–342. [Google Scholar] [CrossRef]
- Tu, W.-Y.; Xu, W.; Bai, L.; Liu, J.; Han, Y.; Luo, B.; Wang, B.; Zhang, K.; Shen, C. Local Protein Synthesis at Neuromuscular Synapses Is Required for Motor Functions. Cell Rep. 2024, 43, 114661. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, A.; Risson, V.; Simonet, T.; Roussange, F.; Lacoste, N.; Ribault, S.; Carras, J.; Theuriet, J.; Girard, E.; Grosjean, I.; et al. Severe Congenital Myasthenic Syndromes Caused by Agrin Mutations Affecting Secretion by Motoneurons. Acta Neuropathol. 2022, 144, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Cui, C.; Liu, C.; Long, Y.; Wong, R.M.Y.; Chai, S.; Qin, L.; Rubin, C.; Yip, B.H.K.; Xu, Z.; et al. Prevention of Age-Related Neuromuscular Junction Degeneration in Sarcopenia by Low-Magnitude High-Frequency Vibration. Aging Cell 2024, 23, e14156. [Google Scholar] [CrossRef] [PubMed]
- Schellino, R.; Boido, M.; Vrijbloed, J.W.; Fariello, R.G.; Vercelli, A. Synergistically Acting on Myostatin and Agrin Pathways Increases Neuromuscular Junction Stability and Endurance in Old Mice. Aging Dis. 2024, 15, 893–910. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Dong, X.; Hui, T.; Yan, M.; Ren, D.; Zou, S.; Wang, S.; Fei, E.; Zhang, W.; et al. Deficiency of Skeletal Muscle Agrin Contributes to the Pathogenesis of Age-Related Sarcopenia in Mice. Cell Death Dis. 2024, 15, 201. [Google Scholar] [CrossRef]
- Pratt, J.; Whitton, L.; Ryan, A.; Juliusdottir, T.; Dolan, J.; Conroy, J.; Narici, M.; De Vito, G.; Boreham, C. Genes Encoding Agrin (AGRN) and Neurotrypsin (PRSS12) Are Associated with Muscle Mass, Strength and Plasma C-Terminal Agrin Fragment Concentration. GeroScience 2023, 45, 1289–1302. [Google Scholar] [CrossRef]
- Qaisar, R.; Karim, A.; Iqbal, M.S.; Ahmad, F.; Hussain, M.A. Tracking the Plasma C-Terminal Agrin Fragment as a Biomarker of Neuromuscular Decline in 18- to 87-Year-Old Men. Mol. Diagn. Ther. 2024, 28, 611–620. [Google Scholar] [CrossRef]
- Cui, J.; Wu, S.; Wang, J.; Wang, Y.; Su, Y.; Xu, D.; Liu, Y.; Gao, J.; Jing, X.; Bai, W. Visualizing the Morphological Characteristics of Neuromuscular Junction in Rat Medial Gastrocnemius Muscle. J. Vis. Exp. 2022, 17, 183. [Google Scholar] [CrossRef]
- Südhof, T.C. The Presynaptic Active Zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef]
- Zong, Y.; Jin, R. Structural Mechanisms of the Agrin-LRP4-MuSK Signaling Pathway in Neuromuscular Junction Differentiation. Cell. Mol. Life Sci. 2013, 70, 3077–3088. [Google Scholar] [CrossRef]
- De Harven, E.; Coers, C. Electron Microscope Study of the Human Neuromuscular Junction. J. Biophys. Biochem. Cytol. 1959, 6, 7–10. [Google Scholar] [CrossRef]
- Geng, L.; Zhang, H.L.; Peng, H.B. The Formation of Acetylcholine Receptor Clusters Visualized with Quantum Dots. BMC Neurosci. 2009, 10, 80. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kouzaki, K.; Sasaki, K.; Nakazato, K. Alterations in Neuromuscular Junction Morphology with Ageing and Endurance Training Modulate Neuromuscular Transmission and Myofibre Composition. J. Physiol. 2025, 603, 107–125. [Google Scholar] [CrossRef]
- Li, Y.; Case, E.H.; Blanchard, C.; Monteleone, A.; Gandhi, M.; Jaie, A.; Badawi, Y.; Meriney, S.D. Ageing-Induced Weakness of Mouse NMJs Is Associated with Reduced Active Zone Density, Synaptic Event Kinetics and Presynaptic Calcium Entry. J. Physiol. 2025, 603, 6445–6465. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Li, L.; Sathyamurthy, A.; Xiong, W.-C.; Mei, L. Schwann Cells in Neuromuscular Junction Formation and Maintenance. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 9770–9781. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Alvarez, S.; Izeta, A. Terminal Schwann Cell Aging: Implications for Age-Associated Neuromuscular Dysfunction. Aging Dis. 2021, 12, 494–514. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.L.; Avila, M.F.; Suneby, E.; Juros, D.; O’Young, A.; Peres da Silva, J.; Valdez, G. Cellular and Molecular Evidence That Synaptic Schwann Cells Contribute to Aging of Mouse Neuromuscular Junctions. Aging Cell 2023, 22, e13981. [Google Scholar] [CrossRef]
- Perez-Gonzalez, A.P.; Provost, F.; Rousse, I.; Piovesana, R.; Benzina, O.; Darabid, H.; Lamoureux, B.; Wang, Y.S.; Arbour, D.; Robitaille, R. Functional Adaptation of Glial Cells at Neuromuscular Junctions in Response to Injury. Glia 2022, 70, 1605–1629. [Google Scholar] [CrossRef]
- Rentería, I.; García-Suárez, P.C.; Fry, A.C.; Moncada-Jiménez, J.; Machado-Parra, J.P.; Antunes, B.M.; Jiménez-Maldonado, A. The Molecular Effects of BDNF Synthesis on Skeletal Muscle: A Mini-Review. Front. Physiol. 2022, 13, 934714. [Google Scholar] [CrossRef]
- Arosio, B.; Calvani, R.; Ferri, E.; Coelho-Junior, H.J.; Carandina, A.; Campanelli, F.; Ghiglieri, V.; Marzetti, E.; Picca, A. Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle-Brain Axis. Nutrients 2023, 15, 1853. [Google Scholar] [CrossRef]
- Clow, C.; Jasmin, B.J. Brain-Derived Neurotrophic Factor Regulates Satellite Cell Differentiation and Skeltal Muscle Regeneration. Mol. Biol. Cell 2010, 21, 2182–2190. [Google Scholar] [CrossRef]
- Bulgay, C.; Zorba, E.; Kazan, H.H.; Bayraktar, I.; Uca, M.; Ergün, M.A.; John, G.; Yusupov, R.A.; Sultanov, R.I.; Semenova, E.A.; et al. BDNF Coexpresses with MTOR and Is Associated with Muscle Fiber Size, Lean Mass and Power-Related Traits. Eur. J. Appl. Physiol. 2025, 125, 2781–2792. [Google Scholar] [CrossRef]
- Ahuja, P.; Ng, C.F.; Pang, B.P.S.; Chan, W.S.; Tse, M.C.L.; Bi, X.; Kwan, H.-L.R.; Brobst, D.; Herlea-Pana, O.; Yang, X.; et al. Muscle-Generated BDNF (Brain Derived Neurotrophic Factor) Maintains Mitochondrial Quality Control in Female Mice. Autophagy 2022, 18, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Lu, B. Pro-Region of Neurotrophins: Role in Synaptic Modulation. Neuron 2003, 39, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.R.A.F.; Casarotto, P.C.; Resstel, L.; Joca, S.R.L. Beyond Good and Evil: A Putative Continuum-Sorting Hypothesis for the Functional Role of proBDNF/BDNF-Propeptide/mBDNF in Antidepressant Treatment. Neurosci. Biobehav. Rev. 2018, 90, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Aby, K.; Antony, R.; Eichholz, M.; Srinivasan, R.; Li, Y. Enhanced Pro-BDNF-p75NTR Pathway Activity in Denervated Skeletal Muscle. Life Sci. 2021, 286, 120067. [Google Scholar] [CrossRef]
- Chan, W.S.; Ng, C.F.; Pang, B.P.S.; Hang, M.; Tse, M.C.L.; Iu, E.C.Y.; Ooi, X.C.; Yang, X.; Kim, J.K.; Lee, C.W.; et al. Exercise-Induced BDNF Promotes PPARδ-Dependent Reprogramming of Lipid Metabolism in Skeletal Muscle during Exercise Recovery. Sci. Signal. 2024, 17, eadh2783. [Google Scholar] [CrossRef]
- Naimo, M.A.; Varanoske, A.N.; Hughes, J.M.; Pasiakos, S.M. Skeletal Muscle Quality: A Biomarker for Assessing Physical Performance Capabilities in Young Populations. Front. Physiol. 2021, 12, 706699. [Google Scholar] [CrossRef]
- Won, C.W.; Kim, M.; Shin, H.E. From a Solitary Blood-Derived Biomarker to Combined Biomarkers of Sarcopenia: Experiences from the Korean Frailty and Aging Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2025, 80, glae237. [Google Scholar] [CrossRef]
- Miki, A.; Aihara, M.; Kawaguchi, H.; Hirose, N.; Hagiwara, H. Effects of Inactivity and Exercise Intervention on Brain-Derived Neurotrophic Factor in Mice: Comparison of Kinetics in Serum, Skeletal Muscle, and Brain. Biomed. Res. 2024, 45, 163–172. [Google Scholar] [CrossRef]
- Garcia, N.; Tomàs, M.; Santafe, M.M.; Lanuza, M.A.; Besalduch, N.; Tomàs, J. Localization of Brain-Derived Neurotrophic Factor, Neurotrophin-4, Tropomyosin-Related Kinase b Receptor, and P75 NTR Receptor by High-Resolution Immunohistochemistry on the Adult Mouse Neuromuscular Junction. J. Peripher. Nerv. Syst. 2010, 15, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, O.; Parsadanian, A.S.; Sendtner, M.; Thoenen, H. Expression of Neurotrophins in Skeletal Muscle: Quantitative Comparison and Significance for Motoneuron Survival and Maintenance of Function. J. Neurosci. Res. 1995, 42, 21–33. [Google Scholar] [CrossRef]
- Kulakowski, S.A.; Parker, S.D.; Personius, K.E. Reduced TrkB Expression Results in Precocious Age-like Changes in Neuromuscular Structure, Neurotransmission, and Muscle Function. J. Appl. Physiol. 2011, 111, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R.; Wilson, M.H. Age-Related Differences in Synaptic Plasticity Following Muscle Unloading. J. Neurobiol. 2003, 57, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Sirago, G.; Candia, J.; Franchi, M.V.; Sarto, F.; Monti, E.; Toniolo, L.; Reggiani, C.; Giacomello, E.; Zampieri, S.; Hartnell, L.M.; et al. Upregulation of Sarcolemmal Hemichannels and Inflammatory Transcripts with Neuromuscular Junction Instability during Lower Limb Unloading in Humans. Biology 2023, 12, 431. [Google Scholar] [CrossRef]
- Andreatta, R.D.; Stemple, J.C.; Seward, T.S.; McMullen, C.A. Subcutaneous Neurotrophin 4 Infusion Using Osmotic Pumps or Direct Muscular Injection Enhances Aging Rat Laryngeal Muscles. J. Vis. Exp. 2017, 2017, 55837. [Google Scholar] [CrossRef]
- Duricki, D.A.; Drndarski, S.; Bernanos, M.; Wood, T.; Bosch, K.; Chen, Q.; Shine, H.D.; Simmons, C.; Williams, S.C.R.; McMahon, S.B.; et al. Stroke Recovery in Rats after 24-Hour-Delayed Intramuscular Neurotrophin-3 Infusion. Ann. Neurol. 2019, 85, 32–46. [Google Scholar] [CrossRef]
- Xu, X.; Song, L.; Li, Y.; Guo, J.; Huang, S.; Du, S.; Li, W.; Cao, R.; Cui, S. Neurotrophin-3 Promotes Peripheral Nerve Regeneration by Maintaining a Repair State of Schwann Cells after Chronic Denervation via the TrkC/ERK/c-Jun Pathway. J. Transl. Med. 2023, 21, 733. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, H.; Yang, L.; Zhao, Y.; Ma, C.; Zhang, C. Effects of Neurotrophin-3 Plasmids on Myocyte Apoptosis and Ca2+-ATPase Content in the Muscle After Nerve Injury in Rats. Neurophysiology 2015, 47, 442–447. [Google Scholar] [CrossRef]
- Yalvac, M.E.; Amornvit, J.; Chen, L.; Shontz, K.M.; Lewis, S.; Sahenk, Z. AAV1.NT-3 Gene Therapy Increases Muscle Fiber Diameter through Activation of mTOR Pathway and Metabolic Remodeling in a CMT Mouse Model. Gene Ther. 2018, 25, 129–138. [Google Scholar] [CrossRef]
- Jun, L.; Ding, X.-W.; Robinson, M.; Jafari, H.; Knight, E.; Geetha, T.; Greene, M.W.; Babu, J.R. Targeting Molecular Mechanisms of Obesity- and Type 2 Diabetes Mellitus-Induced Skeletal Muscle Atrophy with Nerve Growth Factor. Int. J. Mol. Sci. 2024, 25, 4307. [Google Scholar] [CrossRef]
- Morcuende, S.; Muñoz-Hernández, R.; Benítez-Temiño, B.; Pastor, A.M.; de la Cruz, R.R. Neuroprotective Effects of NGF, BDNF, NT-3 and GDNF on Axotomized Extraocular Motoneurons in Neonatal Rats. Neuroscience 2013, 250, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.E.; Phillips, H.S.; Pollock, R.A.; Davies, A.M.; Lemeulle, C.; Armanini, M.; Simmons, L.; Moffet, B.; Vandlen, R.A.; Simpson LC corrected to Simmons, L.; et al. GDNF: A Potent Survival Factor for Motoneurons Present in Peripheral Nerve and Muscle. Science 1994, 266, 1062–1064, Erratum in Science 1995, 267, 777. [Google Scholar] [CrossRef] [PubMed]
- Saarma, M. GDNF—A Stranger in the TGF-Beta Superfamily? Eur. J. Biochem. 2000, 267, 6968–6971. [Google Scholar] [CrossRef]
- Magill, C.K.; Moore, A.M.; Yan, Y.; Tong, A.Y.; MacEwan, M.R.; Yee, A.; Hayashi, A.; Hunter, D.A.; Ray, W.Z.; Johnson, P.J.; et al. The Differential Effects of Pathway-versus Target-Derived Glial Cell Line-Derived Neurotrophic Factor on Peripheral Nerve Regeneration. J. Neurosurg. 2010, 113, 102–109. [Google Scholar] [CrossRef]
- Doherty, C.; Lodyga, M.; Correa, J.; Di Ciano-Oliveira, C.; Plant, P.J.; Bain, J.R.; Batt, J. Utilization of the Rat Tibial Nerve Transection Model to Evaluate Cellular and Molecular Mechanisms Underpinning Denervation-Mediated Muscle Injury. Int. J. Mol. Sci. 2024, 25, 1847. [Google Scholar] [CrossRef]
- Rhymes, E.R.; Tosolini, A.P.; Fellows, A.D.; Mahy, W.; McDonald, N.Q.; Schiavo, G. Bimodal Regulation of Axonal Transport by the GDNF-RET Signalling Axis in Healthy and Diseased Motor Neurons. Cell Death Dis. 2022, 13, 584. [Google Scholar] [CrossRef]
- Ohnishi, Y.-I.; Iwatsuki, K.; Yoshimine, T. Depletion of Glial Cell Line-Derived Neurotrophic Factor by Disuse Muscle Atrophy Exacerbates the Degeneration of Alpha Motor Neurons in Caudal Regions Remote from the Spinal Cord Injury. Neurosci. Med. 2014, 05, 214–221. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, F.; Min, S.; Chen, J.; Yang, J.; Wang, X. Glial Cell Line-Derived Neurotrophic Factor (GDNF) Attenuates the Peripheral Neuromuscular Dysfunction without Inhibiting the Activation of Spinal Microglia/Monocyte. BMC Geriatr. 2018, 18, 110. [Google Scholar] [CrossRef]
- Suzuki, H.; Hase, A.; Miyata, Y.; Arahata, K.; Akazawa, C. Prominent Expression of Glial Cell Line-Derived Neurotrophic Factor in Human Skeletal Muscle. J. Comp. Neurol. 1998, 402, 303–312. [Google Scholar] [CrossRef]
- Karim, A.; Iqbal, M.S.; Muhammad, T.; Qaisar, R. Evaluation of Sarcopenia Using Biomarkers of the Neuromuscular Junction in Parkinson’s Disease. J. Mol. Neurosci. 2022, 72, 820–829. [Google Scholar] [CrossRef]
- Ganse, B.; Bosutti, A.; Drey, M.; Degens, H. Sixty Days of Head-down Tilt Bed Rest with or without Artificial Gravity Do Not Affect the Neuromuscular Secretome. Exp. Cell Res. 2021, 399, 112463. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 Isoforms on Muscle Growth and Sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef] [PubMed]
- Nyul-Toth, A.; Shanmugarama, S.; Patai, R.; Gulej, R.; Faakye, J.; Nagy, D.; Nagykaldi, M.; Kiss, T.; Csipo, T.; Milan, M.; et al. Endothelial IGF-1R Deficiency Disrupts Microvascular Homeostasis, Impairing Skeletal Muscle Perfusion and Endurance: Implications for Age-Related Sarcopenia. GeroScience 2025, 47, 4187–4204. [Google Scholar] [CrossRef] [PubMed]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Gellhaus, B.; Böker, K.O.; Schilling, A.F.; Saul, D. Therapeutic Consequences of Targeting the IGF-1/PI3K/AKT/FOXO3 Axis in Sarcopenia: A Narrative Review. Cells 2023, 12, 2787. [Google Scholar] [CrossRef]
- Day, C.S.; Buranapanitkit, B.; Riano, F.A.; Tomaino, M.M.; Somogyi, G.; Sotereanos, D.G.; Kuroda, R.; Huard, J. Insulin Growth Factor-1 Decreases Muscle Atrophy Following Denervation. Microsurgery 2002, 22, 144–151. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Giacinti, C.; Pelosi, L.; Nicoletti, C.; Winn, N.; Barberi, L.; Molinaro, M.; Rosenthal, N.; Musarò, A. Muscle Expression of a Local Igf-1 Isoform Protects Motor Neurons in an ALS Mouse Model. J. Cell Biol. 2005, 168, 193–199. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Aucello, M.; Molinaro, M.; Musarò, A. Local Expression of mIgf-1 Modulates Ubiquitin, Caspase and CDK5 Expression in Skeletal Muscle of an ALS Mouse Model. Neurol. Res. 2008, 30, 131–136. [Google Scholar] [CrossRef]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between Sarcopenia and Levels of Growth Hormone and Insulin-like Growth Factor-1 in the Elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef]
- Brzeszczyński, F.; Hamilton, D.; Bończak, O.; Brzeszczyńska, J. Systematic Review of Sarcopenia Biomarkers in Hip Fracture Patients as a Potential Tool in Clinical Evaluation. Int. J. Mol. Sci. 2024, 25, 13433. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin Ameliorates Age-Associated Sarcopenia and Metabolic Dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, I.; Coccurello, R. Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology. Int. J. Mol. Sci. 2024, 25, 13480. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kong, I.D. Irisin Prevents Dexamethasone-Induced Atrophy in C2C12 Myotubes. Pflugers Arch. 2020, 472, 495–502. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Zhu, S.; Liu, H.; Xu, S. Irisin Protects Musculoskeletal Homeostasis via a Mitochondrial Quality Control Mechanism. Int. J. Mol. Sci. 2024, 25, 10116. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.-S.; Kim, N.; Kong, I.D. Circulating Irisin Levels as a Predictive Biomarker for Sarcopenia: A Cross-Sectional Community-Based Study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef]
- Park, H.-S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.-K. The Novel Myokine Irisin: Clinical Implications and Potential Role as a Biomarker for Sarcopenia in Postmenopausal Women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Zhu, M.; Wen, X.; Jin, J.; Wang, H.; Lv, D.; Zhao, S.; Wu, X.; Jiao, J. Myokines and Biomarkers of Frailty in Older Inpatients with Undernutrition: A Prospective Study. J. Frailty Aging 2024, 13, 82–90. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jang, I.-Y.; Jung, H.-W.; Park, S.J.; Lee, J.Y.; Choi, E.; Lee, Y.S.; Lee, E.; Kim, B.-J. Serum Irisin Level Is Independent of Sarcopenia and Related Muscle Parameters in Older Adults. Exp. Gerontol. 2022, 162, 111744. [Google Scholar] [CrossRef]
- Adilakshmi, P.; Suganthi, V.; Rao, K.S.; Mahendran, K.B. Effect of High-Intensity Resistance Training Versus Endurance Training on Irisin and Adipomyokine Levels in Healthy Individuals: An 8-Week Interventional Study. Cureus 2023, 15, e46483. [Google Scholar] [CrossRef]
- Rezaei, S.; Eslami, R.; Tartibian, B. The Effects of TRX Suspension Training on Sarcopenic Biomarkers and Functional Abilities in Elderlies with Sarcopenia: A Controlled Clinical Trial. BMC Sports Sci. Med. Rehabil. 2024, 16, 58. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Zheng, J.; Zheng, Z.; Li, J. Fndc5/Irisin Mediates the Benefits of Aerobic Exercise Intervention on Aging-Associated Sarcopenia in Mice. Eur. Geriatr. Med. 2025, 16, 1081–1089. [Google Scholar] [CrossRef]
- Kim, J.; Suh, S.-I.; Park, Y.J.; Kang, M.; Chung, S.J.; Lee, E.S.; Jung, H.N.; Eo, J.S.; Koh, S.-B.; Oh, K.; et al. Sarcopenia Is a Predictor for Alzheimer’s Continuum and Related Clinical Outcomes. Sci. Rep. 2024, 14, 21074. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, S.; Zheng, Q.; Miao, J.; Guo, J. Prevalence and Correlation of Sarcopenia with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2025, 20, e0318920. [Google Scholar] [CrossRef] [PubMed]
- Tintignac, L.A.; Brenner, H.-R.; Rüegg, M.A. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C. Neural Mechanisms of Age-Related Loss of Muscle Performance and Physical Function. J. Gerontol. A. Biol. Sci. Med. Sci. 2023, 78, 8–13. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Zou, W.-J.; Lee, D.; Mei, L.; Xiong, W.-C. APP in the Neuromuscular Junction for the Development of Sarcopenia and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 7809. [Google Scholar] [CrossRef]
- Miao, Y.; Xie, L.; Song, J.; Cai, X.; Yang, J.; Ma, X.; Chen, S.; Xie, P. Unraveling the Causes of Sarcopenia: Roles of Neuromuscular Junction Impairment and Mitochondrial Dysfunction. Physiol. Rep. 2024, 12, e15917. [Google Scholar] [CrossRef]
- Xu, H.; Bhaskaran, S.; Piekarz, K.M.; Ranjit, R.; Bian, J.; Kneis, P.; Ellis, A.; Bhandari, S.; Rice, H.C.; Van Remmen, H. Age Related Changes in Muscle Mass and Force Generation in the Triple Transgenic (3xTgAD) Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 876816. [Google Scholar] [CrossRef]
- Gillon, A.; Steel, C.; Cornwall, J.; Sheard, P. Increased Nuclear Permeability Is a Driver for Age-Related Motoneuron Loss. GeroScience 2020, 42, 833–847. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Sin, D.S.; Lim, J.-Y. Newly Diagnosed Sarcopenia and Alzheimer’s Disease in an Older Patient with Chronic Inflammation. Ann. Geriatr. Med. Res. 2019, 23, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Rygiel, K.A.; Picard, M.; Turnbull, D.M. The Ageing Neuromuscular System and Sarcopenia: A Mitochondrial Perspective. J. Physiol. 2016, 594, 4499–4512. [Google Scholar] [CrossRef] [PubMed]
- Chabi, B.; Ljubicic, V.; Menzies, K.J.; Huang, J.H.; Saleem, A.; Hood, D.A. Mitochondrial Function and Apoptotic Susceptibility in Aging Skeletal Muscle. Aging Cell 2008, 7, 2–12. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial Dysfunction in Alzheimer’s Disease: Role in Pathogenesis and Novel Therapeutic Opportunities. Br. J. Pharmacol. 2019, 176, 3489–3507. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Zhao, C.; Pang, S.; Lu, J.; Chan, P. α-Synuclein Aggregation Causes Muscle Atrophy through Neuromuscular Junction Degeneration. J. Cachexia Sarcopenia Muscle 2023, 14, 226–242. [Google Scholar] [CrossRef]
- Gui, M.; Lv, L.; Hu, S.; Qin, L.; Wang, C. Sarcopenia in Parkinson’s Disease: From Pathogenesis to Interventions. Metabolism. 2025, 169, 156272. [Google Scholar] [CrossRef]
- Hegde, M.; Vasquez, V.; Kodavati, M.; Mitra, J.; Vendula, I.; Hamilton, D.; Garruto, R.; Rao, K.S. Mitochondria-Targeted Oligomeric α-Synuclein Induces TOM40 Degradation and Mitochondrial Dysfunction in Parkinson’s Disease and Parkinsonism-Dementia of Guam. Res. Sq. 2024, preprint. [Google Scholar] [CrossRef]
- Ibebunjo, C.; Chick, J.M.; Kendall, T.; Eash, J.K.; Li, C.; Zhang, Y.; Vickers, C.; Wu, Z.; Clarke, B.A.; Shi, J.; et al. Genomic and Proteomic Profiling Reveals Reduced Mitochondrial Function and Disruption of the Neuromuscular Junction Driving Rat Sarcopenia. Mol. Cell. Biol. 2013, 33, 194–212. [Google Scholar] [CrossRef]
- Schellenberg, G.D.; Blue, E.E. Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA A Decade of Gene Discovery by the Alzheimer’s Disease Sequencing Project. Alzheimers Dement. 2022, 18, e066434. [Google Scholar] [CrossRef]
- Kablan, A.; Silan, F.; Ozdemir, O. Re-Evaluation of Genetic Variants in Parkinson’s Disease Using Targeted Panel and Next-Generation Sequencing. Twin Res. Hum. Genet. 2023, 26, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Summa, S.; Ittiwut, C.; Kulsirichawaroj, P.; Paprad, T.; Likasitwattanakul, S.; Sanmaneechai, O.; Boonsimma, P.; Suphapeetiporn, K.; Shotelersuk, V. Utilisation of Exome Sequencing for Muscular Disorders in Thai Paediatric Patients: Diagnostic Yield and Mutational Spectrum. Sci. Rep. 2023, 13, 1376. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Xiao, Y.; Zhou, C.; Zhang, H.; Wang, J.; Zeng, Y. NGS-Based Targeted Sequencing Identified Six Novel Variants in Patients with Duchenne/Becker Muscular Dystrophy from Southwestern China. BMC Med. Genomics 2023, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Warr, A.; Robert, C.; Hume, D.; Archibald, A.; Deeb, N.; Watson, M. Exome Sequencing: Current and Future Perspectives. G3 Genes Genomes Genet. 2015, 5, 1543–1550. [Google Scholar] [CrossRef]
- Rabbani, B.; Tekin, M.; Mahdieh, N. The Promise of Whole-Exome Sequencing in Medical Genetics. J. Hum. Genet. 2014, 59, 5–15. [Google Scholar] [CrossRef]
- Lelieveld, S.H.; Spielmann, M.; Mundlos, S.; Veltman, J.A.; Gilissen, C. Comparison of Exome and Genome Sequencing Technologies for the Complete Capture of Protein-Coding Regions. Hum. Mutat. 2015, 36, 815–822. [Google Scholar] [CrossRef]
- Burdick, K.J.; Cogan, J.D.; Rives, L.C.; Robertson, A.K.; Koziura, M.E.; Brokamp, E.; Duncan, L.; Hannig, V.; Pfotenhauer, J.; Vanzo, R.; et al. Limitations of Exome Sequencing in Detecting Rare and Undiagnosed Diseases. Am. J. Med. Genet. A 2020, 182, 1400–1406. [Google Scholar] [CrossRef]
- Szelinger, S.; Pearson, J.V.; Craig, D.W. Microarray-Based Genome-Wide Association Studies Using Pooled DNA. In Disease Gene Identification. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 700, pp. 49–60. [Google Scholar] [CrossRef]
- Kitsios, G.D.; Zintzaras, E. Genome-Wide Association Studies: Hypothesis-“free” or “Engaged”? Transl. Res. J. Lab. Clin. Med. 2009, 154, 161–164. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and Limitations of Genome-Wide Association Studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Zappala, Z.; Montgomery, S.B. Non-Coding Loss-of-Function Variation in Human Genomes. Hum. Hered. 2016, 81, 78–87. [Google Scholar] [CrossRef]
- Palmer, C.; Pe’er, I. Statistical Correction of the Winner’s Curse Explains Replication Variability in Quantitative Trait Genome-Wide Association Studies. PLoS Genet. 2017, 13, e1006916. [Google Scholar] [CrossRef]
- Ran, S.; Zhang, Y.-X.; Liu, L.; Jiang, Z.-X.; He, X.; Liu, Y.; Shen, H.; Tian, Q.; Pei, Y.-F.; Deng, H.-W.; et al. Association of 3p27.1 Variants with Whole Body Lean Mass Identified by a Genome-Wide Association Study. Sci. Rep. 2020, 10, 4293. [Google Scholar] [CrossRef]
- Wu, S.-E.; Chen, W.L. A Genome-Wide Association Study Identifies Novel Risk Loci for Sarcopenia in a Taiwanese Population. J. Inflamm. Res. 2021, 14, 5969–5980. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yoo, H.J.; Kim, Y.A.; Lee, J.H.; Lee, Y.; Kwon, S.-H.; Seo, Y.J.; Lee, S.H.; Koh, J.-M.; Ji, Y.; et al. Unveiling Genetic Variants for Age-Related Sarcopenia by Conducting a Genome-Wide Association Study on Korean Cohorts. Sci. Rep. 2022, 12, 3501. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, X.; Li, R.; Lu, Q.; Ba, Y.; Fang, J.; Liu, Y.; Li, R.; Liu, Y.; Wang, Y.; et al. Identifying Genetic Determinants of Sarcopenia-Related Traits: A Mendelian Randomization Study of Druggable Genes. Metabolism 2024, 160, 155994. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.-F.; Chen, T.; Gu, X.-J.; Su, W.-M.; Jiang, Z.; Lu, S.-J.; Cao, B.; Chi, L.-Y.; Gao, X.; Chen, Y.-P. Systematic Druggable Genome-Wide Mendelian Randomization Identifies Therapeutic Targets for Sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 1324–1334. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L. Drug Risks Associated with Sarcopenia: A Real-World and GWAS Study. BMC Pharmacol. Toxicol. 2024, 25, 84. [Google Scholar] [CrossRef]
- Ran, S.; Lin, X.; Wang, S.; Li, Z.; Liu, B. Multi-Trait Genome-Wide Analysis Identified 20 Novel Loci for Sarcopenia-Related Traits in UK Biobank. Calcif. Tissue Int. 2025, 116, 10. [Google Scholar] [CrossRef]
- Zhang, X.; Su, K.-J.; Banerjee, B.; Eres, I.; Hsu, Y.-H.; Crandall, C.J.; Donaka, R.; Han, Z.; Jackson, R.D.; Liu, H.; et al. Multi-Ancestry Whole Genome Sequencing Analysis of Lean Body Mass. Genome Biol. 2025, 26, 106. [Google Scholar] [CrossRef]
- Furutani, M.; Kimura, T.; Fukunaga, K.; Suganuma, M.; Takemura, M.; Matsui, Y.; Satake, S.; Nakano, Y.; Mushiroda, T.; Niida, S.; et al. Identification of a Risk Allele at SLC41A3 and a Protective Allele HLA-DPB1*02:01 Associated with Sarcopenia in Japanese. Gerontology 2025, 71, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Association between Polygenetic Risk Scores Related to Sarcopenia Risk and Their Interactions with Regular Exercise in a Large Cohort of Korean Adults. Clin. Nutr. Edinb. Scotl. 2021, 40, 5355–5364. [Google Scholar] [CrossRef] [PubMed]
- Semenova, E.A.; Pranckevičienė, E.; Bondareva, E.A.; Gabdrakhmanova, L.J.; Ahmetov, I.I. Identification and Characterization of Genomic Predictors of Sarcopenia and Sarcopenic Obesity Using UK Biobank Data. Nutrients 2023, 15, 758. [Google Scholar] [CrossRef] [PubMed]
- Coppedè, F. The Potential of Epigenetic Therapies in Neurodegenerative Diseases. Front. Genet. 2014, 5, 220. [Google Scholar] [CrossRef]
- Coppedè, F. Epigenetics of Neuromuscular Disorders. Epigenomics 2020, 12, 2125–2139. [Google Scholar] [CrossRef]
- Zou, Z.; Ohta, T.; Miura, F.; Oki, S. ChIP-Atlas 2021 Update: A Data-Mining Suite for Exploring Epigenomic Landscapes by Fully Integrating ChIP-Seq, ATAC-Seq and Bisulfite-Seq Data. Nucleic Acids Res. 2022, 50, W175–W182. [Google Scholar] [CrossRef]
- Tsitkov, S.; Valentine, K.; Kozareva, V.; Donde, A.; Frank, A.; Lei, S.; Van Eyk, J.E.; Finkbeiner, S.; Rothstein, J.D.; Thompson, L.M.; et al. Disease Related Changes in ATAC-Seq of iPSC-Derived Motor Neuron Lines from ALS Patients and Controls. Nat. Commun. 2024, 15, 3606. [Google Scholar] [CrossRef]
- He, S.; Qu, Q.; Chen, X.; Zhao, L.; Jiao, Z.; Wan, Z.; Kwok, H.F.; Qu, S. Downregulation of Ambra1 by Altered DNA Methylation Exacerbates Dopaminergic Neuron Damage in a Fenpropathrin-Induced Parkinson-like Mouse Model. Ecotoxicol. Environ. Saf. 2024, 271, 115995. [Google Scholar] [CrossRef]
- Wang, Q.; Lan, X.; Ke, H.; Xu, S.; Huang, C.; Wang, J.; Wang, X.; Huang, T.; Wu, X.; Chen, M.; et al. Histone β-Hydroxybutyrylation Is Critical in Reversal of Sarcopenia. Aging Cell 2024, 23, e14284. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Yang, X.; Liang, C.; Raza, S.H.A.; Pan, Y.; Zhang, K.; Zan, L. Integration of RNA-Seq and ATAC-Seq Identifies Muscle-Regulated Hub Genes in Cattle. Front. Vet. Sci. 2022, 9, 925590. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, S.; Shi, L.; Zhou, A.; Lin, X.; Zhang, J.; Zhu, X.; Huang, L.; Li, K. Integrated ATAC-Seq and RNA-Seq Analysis of In Vitro Cultured Skeletal Muscle Satellite Cells to Understand Changes in Cell Proliferation. Cells 2024, 13, 1031. [Google Scholar] [CrossRef]
- Li, J.-W.; Shen, Z.-K.; Lin, Y.-S.; Wang, Z.-Y.; Li, M.-L.; Sun, H.-X.; Wang, Q.; Zhao, C.; Xu, J.-S.; Lu, X.; et al. DNA Methylation of Skeletal Muscle Function-Related Secretary Factors Identifies FGF2 as a Potential Biomarker for Sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 1209–1217. [Google Scholar] [CrossRef]
- Gim, J.-A.; Lee, S.-Y.; Kim, S.C.; Baek, K.-W.; Seo, S.H.; Yoo, J.-I. Relationship between DNA Methylation Changes and Skeletal Muscle Mass. BMC Genomic Data 2023, 24, 48. [Google Scholar] [CrossRef]
- Xuekelati, S.; Maimaitiwusiman, Z.; Bai, X.; Xiang, H.; Li, Y.; Wang, H. Sarcopenia Is Associated with Hypomethylation of TWEAK and Increased Plasma Levels of TWEAK and Its Downstream Inflammatory Factor TNF-α in Older Adults: A Case-Control Study. Exp. Gerontol. 2024, 188, 112390. [Google Scholar] [CrossRef]
- Antoun, E.; Garratt, E.S.; Taddei, A.; Burton, M.A.; Barton, S.J.; Titcombe, P.; Westbury, L.D.; Baczynska, A.; Migliavacca, E.; Feige, J.N.; et al. Epigenome-Wide Association Study of Sarcopenia: Findings from the Hertfordshire Sarcopenia Study (HSS). J. Cachexia Sarcopenia Muscle 2022, 13, 240–253. [Google Scholar] [CrossRef]
- Voisin, S.; Jacques, M.; Landen, S.; Harvey, N.R.; Haupt, L.M.; Griffiths, L.R.; Gancheva, S.; Ouni, M.; Jähnert, M.; Ashton, K.J.; et al. Meta-Analysis of Genome-Wide DNA Methylation and Integrative Omics of Age in Human Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2021, 12, 1064–1078. [Google Scholar] [CrossRef]
- Turner, D.C.; Gorski, P.P.; Maasar, M.F.; Seaborne, R.A.; Baumert, P.; Brown, A.D.; Kitchen, M.O.; Erskine, R.M.; Dos-Remedios, I.; Voisin, S.; et al. DNA Methylation across the Genome in Aged Human Skeletal Muscle Tissue and Muscle-Derived Cells: The Role of HOX Genes and Physical Activity. Sci. Rep. 2020, 10, 15360. [Google Scholar] [CrossRef]
- He, L.; Khanal, P.; Morse, C.I.; Williams, A.; Thomis, M. Differentially Methylated Gene Patterns between Age-Matched Sarcopenic and Non-Sarcopenic Women. J. Cachexia Sarcopenia Muscle 2019, 10, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Sung, Y.; Song, W. Machine Learning-Based Identification of Diagnostic Biomarkers for Korean Male Sarcopenia Through Integrative DNA Methylation and Methylation Risk Score: From the Korean Genomic Epidemiology Study (KoGES). J. Korean Med. Sci. 2024, 39, e200. [Google Scholar] [CrossRef] [PubMed]
- Kmiołek, T.; Filipowicz, G.; Bogucka, D.; Wajda, A.; Ejma-Multański, A.; Stypińska, B.; Modzelewska, E.; Kaliberda, Y.; Radkowski, M.; Targowski, T.; et al. Aging and the Impact of Global DNA Methylation, Telomere Shortening, and Total Oxidative Status on Sarcopenia and Frailty Syndrome. Immun. Ageing 2023, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Z.; Wang, X.; Wang, D.; Zhang, T.; Zhang, Y.; Wang, X.; Chen, Z. Identification of Novel Pathways and Immune Profiles Related to Sarcopenia. Front. Med. 2023, 10, 928285. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, G.; Zhang, F.; Yu, D.; Jia, X.; Ma, B.; Chen, W.; Cai, X.; Mao, L.; Zhuang, C.; et al. Identification of a Novel Immune-Related Transcriptional Regulatory Network in Sarcopenia. BMC Geriatr. 2023, 23, 463. [Google Scholar] [CrossRef]
- Tarum, J.; Ball, G.; Gustafsson, T.; Altun, M.; Santos, L. Artificial Neural Network Inference Analysis Identified Novel Genes and Gene Interactions Associated with Skeletal Muscle Aging. J. Cachexia Sarcopenia Muscle 2024, 15, 2143–2155. [Google Scholar] [CrossRef]
- Liang, S.; Liu, D.; Xiao, Z.; Greenbaum, J.; Shen, H.; Xiao, H.; Deng, H. Repurposing Approved Drugs for Sarcopenia Based on Transcriptomics Data in Humans. Pharm. Basel Switz. 2023, 16, 607. [Google Scholar] [CrossRef]
- Liu, W.; Klose, A.; Forman, S.; Paris, N.D.; Wei-LaPierre, L.; Cortés-Lopéz, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; et al. Loss of Adult Skeletal Muscle Stem Cells Drives Age-Related Neuromuscular Junction Degeneration. eLife 2017, 6, e26464. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Chen, B.; Wang, W.; Zhang, L.; Ji, Y.; Chen, Z.; Ni, X.; Shen, Y.; Sun, H. Transcriptome Sequencing and Analysis Reveals the Molecular Mechanism of Skeletal Muscle Atrophy Induced by Denervation. Ann. Transl. Med. 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.J.; Börsch, A.; Lin, S.; Thürkauf, M.; Weihrauch, M.; Reinhard, J.R.; Delezie, J.; Battilana, F.; Wang, X.; Kaiser, M.S.; et al. The Neuromuscular Junction Is a Focal Point of mTORC1 Signaling in Sarcopenia. Nat. Commun. 2020, 11, 4510. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.; Jing, H.; Lai, X. Neuromuscular Junction-Specific Genes Screening by Deep RNA-Seq Analysis. Cell Biosci. 2021, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, M.; Chen, J.; Nerella, R.; Vyavahare, S.; Kumar, S.; Isales, C.M.; Hamrick, M.; Adusumilli, S.; Fulzele, S. Sex-Specific Alteration in Human Muscle Transcriptome with Age. GeroScience 2023, 45, 1303–1316. [Google Scholar] [CrossRef]
- Altab, G.; Merry, B.J.; Beckett, C.W.; Raina, P.; Lopes, I.; Goljanek-Whysall, K.; de Magalhães, J.P. Unravelling the Transcriptomic Symphony of Muscle Ageing: Key Pathways and Hub Genes Altered by Ageing and Caloric Restriction in Rat Muscle Revealed by RNA Sequencing. BMC Genom. 2025, 26, 29. [Google Scholar] [CrossRef]
- Kim, S.; Ayan, B.; Shayan, M.; Rando, T.A.; Huang, N.F. Skeletal Muscle-on-a-Chip in Microgravity as a Platform for Regeneration Modeling and Drug Screening. Stem Cell Rep. 2024, 19, 1061–1073. [Google Scholar] [CrossRef]
- Koike, H.; Manabe, I.; Oishi, Y. Mechanisms of Cooperative Cell-Cell Interactions in Skeletal Muscle Regeneration. Inflamm. Regen. 2022, 42, 48. [Google Scholar] [CrossRef]
- Henrot, P.; Blervaque, L.; Dupin, I.; Zysman, M.; Esteves, P.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Cellular Interplay in Skeletal Muscle Regeneration and Wasting: Insights from Animal Models. J. Cachexia Sarcopenia Muscle 2023, 14, 745–757. [Google Scholar] [CrossRef]
- Dos Santos, M.; Shah, A.M.; Zhang, Y.; Bezprozvannaya, S.; Chen, K.; Xu, L.; Lin, W.; McAnally, J.R.; Bassel-Duby, R.; Liu, N.; et al. Opposing Gene Regulatory Programs Governing Myofiber Development and Maturation Revealed at Single Nucleus Resolution. Nat. Commun. 2023, 14, 4333. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zhou, Q.; Liu, X.; Qiao, Y.; Xie, T.; Sun, H.; Ong, M.T.-Y.; Wang, H. Multiomics and Cellular Senescence Profiling of Aging Human Skeletal Muscle Uncovers Maraviroc as a Senotherapeutic Approach for Sarcopenia. Nat. Commun. 2025, 16, 6207. [Google Scholar] [CrossRef] [PubMed]
- Ulintz, P.J.; Larouche, J.; Mohiuddin, M.; Macias, J.C.; Kurpiers, S.J.; Liu, W.; Choi, J.J.; Brown, L.A.; Markworth, J.F.; de Silva, K.; et al. Single Cell Deconstruction of Muscle Stem Cell Heterogeneity During Aging Reveals Sensitivity to the Neuromuscular Junction. bioRxiv 2020. [Google Scholar] [CrossRef]
- De Micheli, A.J.; Spector, J.A.; Elemento, O.; Cosgrove, B.D. A Reference Single-Cell Transcriptomic Atlas of Human Skeletal Muscle Tissue Reveals Bifurcated Muscle Stem Cell Populations. Skelet. Muscle 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, A.B.; Smith, G.R.; Raue, U.; Begue, G.; Minchev, K.; Ruf-Zamojski, F.; Nair, V.D.; Wang, X.; Zhou, L.; Zaslavsky, E.; et al. Single-Cell Transcriptional Profiles in Human Skeletal Muscle. Sci. Rep. 2020, 10, 229. [Google Scholar] [CrossRef]
- Barruet, E.; Garcia, S.M.; Striedinger, K.; Wu, J.; Lee, S.; Byrnes, L.; Wong, A.; Xuefeng, S.; Tamaki, S.; Brack, A.S.; et al. Functionally Heterogeneous Human Satellite Cells Identified by Single Cell RNA Sequencing. eLife 2020, 9, e51576. [Google Scholar] [CrossRef]
- Shen, L.; Zong, Y.; Zhao, J.; Yang, Y.; Li, L.; Li, N.; Gao, Y.; Xie, X.; Bao, Q.; Jiang, L.; et al. Characterizing the Skeletal Muscle Immune Microenvironment for Sarcopenia: Insights from Transcriptome Analysis and Histological Validation. Front. Immunol. 2024, 15, 1414387. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, R.; Kuang, X.; Yu, C.; Niu, S.; Du, Y.; Lu, D.; Li, S.; Teng, Z.; Lu, S. Single-Cell RNA-Seq Reveals Interferon-Induced Guanylate-Binding Proteins Are Linked with Sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2985–2998. [Google Scholar] [CrossRef]
- Ham, A.S.; Lin, S.; Tse, A.; Thürkauf, M.; McGowan, T.J.; Jörin, L.; Oliveri, F.; Rüegg, M.A. Single-Nuclei Sequencing of Skeletal Muscle Reveals Subsynaptic-Specific Transcripts Involved in Neuromuscular Junction Maintenance. Nat. Commun. 2025, 16, 2220. [Google Scholar] [CrossRef]
- YAFFE, D.; SAXEL, O. Serial Passaging and Differentiation of Myogenic Cells Isolated from Dystrophic Mouse Muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Yaffe, D. Retention of Differentiation Potentialities during Prolonged Cultivation of Myogenic Cells. Proc. Natl. Acad. Sci. USA 1968, 61, 477–483. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.-B.; Park, D.-H.; Kang, J.-H. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Contartese, D.; Di Sarno, L.; Borsari, V.; Fini, M.; Giavaresi, G. In Vitro Models of Cell Senescence: A Systematic Review on Musculoskeletal Tissues and Cells. Int. J. Mol. Sci. 2023, 24, 15617. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.B.; Pratley, R.; Ossowski, V. Human Primary Myoblast Cell Cultures from Non-Diabetic Insulin Resistant Subjects Retain Defects in Insulin Action. J. Clin. Investig. 1996, 98, 2346–2350. [Google Scholar] [CrossRef]
- Son, Y.H.; Kim, W.J.; Shin, Y.J.; Lee, S.-M.; Lee, B.; Lee, K.-P.; Lee, S.H.; Kim, K.J.; Kwon, K.-S. Human Primary Myoblasts Derived from Paraspinal Muscle Reflect Donor Age as an Experimental Model of Sarcopenia. Exp. Gerontol. 2023, 181, 112273. [Google Scholar] [CrossRef]
- Rajabian, N.; Shahini, A.; Asmani, M.; Vydiam, K.; Choudhury, D.; Nguyen, T.; Ikhapoh, I.; Zhao, R.; Lei, P.; Andreadis, S.T. Bioengineered Skeletal Muscle as a Model of Muscle Aging and Regeneration. Tissue Eng. Part A 2021, 27, 74–86. [Google Scholar] [CrossRef]
- Faustino Martins, J.-M.; Fischer, C.; Urzi, A.; Vidal, R.; Kunz, S.; Ruffault, P.-L.; Kabuss, L.; Hube, I.; Gazzerro, E.; Birchmeier, C.; et al. Self-Organizing 3D Human Trunk Neuromuscular Organoids. Cell Stem Cell 2020, 27, 498. [Google Scholar] [CrossRef]
- Yang, J.; Qian, S.; Chen, M.; Wang, L.; Wang, Y.; Liu, J.; Xi, C.; Yang, Y.; Li, Y.; Gao, C.; et al. Skeletal Muscle, Neuromuscular Organoids and Assembloids: A Scoping Review. eBioMedicine 2025, 118, 105825. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Kim, G. Micro/Nano-Hierarchical Scaffold Fabricated Using a Cell Electrospinning/3D Printing Process for Co-Culturing Myoblasts and HUVECs to Induce Myoblast Alignment and Differentiation. Acta Biomater. 2020, 107, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Sakar, M.S.; Neal, D.; Boudou, T.; Borochin, M.A.; Li, Y.; Weiss, R.; Kamm, R.D.; Chen, C.S.; Asada, H.H. Formation and Optogenetic Control of Engineered 3D Skeletal Muscle Bioactuators. Lab. Chip 2012, 12, 4976–4985. [Google Scholar] [CrossRef] [PubMed]
- Afshar Bakooshli, M.; Lippmann, E.S.; Mulcahy, B.; Iyer, N.; Nguyen, C.T.; Tung, K.; Stewart, B.A.; van den Dorpel, H.; Fuehrmann, T.; Shoichet, M.; et al. A 3D Culture Model of Innervated Human Skeletal Muscle Enables Studies of the Adult Neuromuscular Junction. eLife 2019, 8, e44530. [Google Scholar] [CrossRef]
- Parafati, M.; Giza, S.; Shenoy, T.S.; Mojica-Santiago, J.A.; Hopf, M.; Malany, L.K.; Platt, D.; Moore, I.; Jacobs, Z.A.; Kuehl, P.; et al. Human Skeletal Muscle Tissue Chip Autonomous Payload Reveals Changes in Fiber Type and Metabolic Gene Expression Due to Spaceflight. NPJ Microgravity 2023, 9, 77. [Google Scholar] [CrossRef]
- Xie, W.-Q.; He, M.; Yu, D.-J.; Wu, Y.-X.; Wang, X.-H.; Lv, S.; Xiao, W.-F.; Li, Y.-S. Mouse Models of Sarcopenia: Classification and Evaluation. J. Cachexia Sarcopenia Muscle 2021, 12, 538–554. [Google Scholar] [CrossRef]
- Kerr, H.L.; Krumm, K.; Anderson, B.; Christiani, A.; Strait, L.; Li, T.; Irwin, B.; Jiang, S.; Rybachok, A.; Chen, A.; et al. Mouse Sarcopenia Model Reveals Sex- and Age-Specific Differences in Phenotypic and Molecular Characteristics. J. Clin. Investig. 2024, 134, e172890. [Google Scholar] [CrossRef]
- Chiba, Y.; Shimada, A.; Kumagai, N.; Yoshikawa, K.; Ishii, S.; Furukawa, A.; Takei, S.; Sakura, M.; Kawamura, N.; Hosokawa, M. The Senescence-Accelerated Mouse (SAM): A Higher Oxidative Stress and Age-Dependent Degenerative Diseases Model. Neurochem. Res. 2009, 34, 679–687. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F. The Senescence-Accelerated Prone Mouse (SAMP8): A Model of Age-Related Cognitive Decline with Relevance to Alterations of the Gene Expression and Protein Abnormalities in Alzheimer’s Disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef]
- Guo, A.Y.; Leung, K.S.; Siu, P.M.F.; Qin, J.H.; Chow, S.K.H.; Qin, L.; Li, C.Y.; Cheung, W.H. Muscle Mass, Structural and Functional Investigations of Senescence-Accelerated Mouse P8 (SAMP8). Exp. Anim. 2015, 64, 425–433. [Google Scholar] [CrossRef]
- Le Bacquer, O.; Combe, K.; Patrac, V.; Ingram, B.; Combaret, L.; Dardevet, D.; Montaurier, C.; Salles, J.; Giraudet, C.; Guillet, C.; et al. 4E-BP1 and 4E-BP2 Double Knockout Mice Are Protected from Aging-Associated Sarcopenia. J. Cachexia Sarcopenia Muscle 2019, 10, 696–709. [Google Scholar] [CrossRef]
- Lozier, N.R.; Kopchick, J.J.; de Lacalle, S. Relative Contributions of Myostatin and the GH/IGF-1 Axis in Body Composition and Muscle Strength. Front. Physiol. 2018, 9, 1418. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389.e6. [Google Scholar] [CrossRef] [PubMed]
- Sataranatarajan, K.; Pharaoh, G.; Brown, J.L.; Ranjit, R.; Piekarz, K.M.; Street, K.; Wren, J.D.; Georgescu, C.; Kinter, C.; Kinter, M.; et al. Molecular Changes in Transcription and Metabolic Pathways Underlying Muscle Atrophy in the CuZnSOD Null Mouse Model of Sarcopenia. GeroScience 2020, 42, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.J.; Benian, G.M. Animal Models of Sarcopenia. Aging Cell 2020, 19, e13223. [Google Scholar] [CrossRef] [PubMed]
- Hettwer, S.; Dahinden, P.; Kucsera, S.; Farina, C.; Ahmed, S.; Fariello, R.; Drey, M.; Sieber, C.C.; Vrijbloed, J.W. Elevated Levels of a C-Terminal Agrin Fragment Identifies a New Subset of Sarcopenia Patients. Exp. Gerontol. 2013, 48, 69–75. [Google Scholar] [CrossRef]
- Drey, M.; Sieber, C.C.; Bauer, J.M.; Uter, W.; Dahinden, P.; Fariello, R.G.; Vrijbloed, J.W. C-Terminal Agrin Fragment as a Potential Marker for Sarcopenia Caused by Degeneration of the Neuromuscular Junction. Exp. Gerontol. 2013, 48, 76–80. [Google Scholar] [CrossRef]
- Chiariello, A.; Conte, G.; Rossetti, L.; Trofarello, L.; Salvioli, S.; Conte, M. Different Roles of Circulating and Intramuscular GDF15 as Markers of Skeletal Muscle Health. Front. Endocrinol. 2024, 15, 1404047. [Google Scholar] [CrossRef]
- Heezen, L.G.M.; Abdelaal, T.; van Putten, M.; Aartsma-Rus, A.; Mahfouz, A.; Spitali, P. Spatial Transcriptomics Reveal Markers of Histopathological Changes in Duchenne Muscular Dystrophy Mouse Models. Nat. Commun. 2023, 14, 4909. [Google Scholar] [CrossRef]
- Hsu, J.-E.; Ruiz, L.; Hwang, Y.; Guzman, S.; Cho, C.-S.; Cheng, W.; Si, Y.; Macpherson, P.; Schrank, M.; Jun, G.; et al. High-Resolution Spatial Transcriptomic Atlas of Mouse Soleus Muscle: Unveiling Single Cell and Subcellular Heterogeneity in Health and Denervation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lee, N.; Kim, Y.; Choi, S.; Kim, Y.; Son, Y.; Choi, S.; Kang, Y.Y.; Young Jeon, J.; Jin Han, S.; Jin Kim, H.; et al. 1636-P: The Effects of MS-275 Derivative in Diabetic Skeletal Muscle Atrophy. Diabetes 2023, 72, 1636-P. [Google Scholar] [CrossRef]
- Phongsavanh, M.; Bizot, F.; Saoudi, A.; Gastaldi, C.; Le Coz, O.; Tensorer, T.; Brisebard, E.; Garcia, L.; Goyenvalle, A. Valproic Acid Improves Antisense-Mediated Exon-Skipping Efficacy in Mdx Mice. Int. J. Mol. Sci. 2025, 26, 2583. [Google Scholar] [CrossRef] [PubMed]
- Habibian, J.; Ferguson, B. Lysine Deacetylases (KDACs) Regulate PKCõ Phosphorylation in Response to Muscle Atrophy. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Cai, Q.; Sahu, R.; Ueberschlag-Pitiot, V.; Souali-Crespo, S.; Charvet, C.; Silem, I.; Cottard, F.; Ye, T.; Taleb, F.; Metzger, E.; et al. LSD1 Inhibition Circumvents Glucocorticoid-Induced Muscle Wasting of Male Mice. Nat. Commun. 2024, 15, 3563. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Kuwahara, T.; Tajika, M.; Tanaka, T.; Yamada, K.; Shimizu, M.; Niwa, Y.; Yamaguchi, R. Artificial Intelligence for Body Composition Assessment Focusing on Sarcopenia. Sci. Rep. 2025, 15, 1324. [Google Scholar] [CrossRef]
- Zhang, D.; Kang, H.; Sun, Y.; Liu, J.Y.W.; Lee, K.-S.; Song, Z.; Khaw, J.V.; Yeung, J.; Peng, T.; Lam, S.-K.; et al. Rectus Femoris Muscle Segmentation on Ultrasound Images of Older Adults Using Automatic Segment Anything Model, nnU-Net and U-Net-A Prospective Study of Hong Kong Community Cohort. Bioengineering 2024, 11, 1291. [Google Scholar] [CrossRef]
- Zupo, R.; Moroni, A.; Castellana, F.; Gasparri, C.; Catino, F.; Lampignano, L.; Perna, S.; Clodoveo, M.L.; Sardone, R.; Rondanelli, M. A Machine-Learning Approach to Target Clinical and Biological Features Associated with Sarcopenia: Findings from Northern and Southern Italian Aging Populations. Metabolites 2023, 13, 565. [Google Scholar] [CrossRef]
- Liang, Q.-Y.; Wang, J.; Yang, Y.-F.; Zhao, K.; Luo, R.-L.; Tian, Y.; Li, F.-X. Machine Learning to Identify Potential Biomarkers for Sarcopenia in Liver Cirrhosis. World J. Hepatol. 2025, 17, 105332. [Google Scholar] [CrossRef]
- Chung, H.; Jo, Y.; Ryu, D.; Jeong, C.; Choe, S.-K.; Lee, J. Artificial-Intelligence-Driven Discovery of Prognostic Biomarker for Sarcopenia. J. Cachexia Sarcopenia Muscle 2021, 12, 2220–2230. [Google Scholar] [CrossRef]
- Lin, S.; Chen, C.; Cai, X.; Yang, F.; Fan, Y. Development and Verification of a Combined Diagnostic Model for Sarcopenia with Random Forest and Artificial Neural Network. Comput. Math. Methods Med. 2022, 2022, 2957731. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Hu, X.; Zhang, Y. Epigenetic Characterization of Sarcopenia-Associated Genes Based on Machine Learning and Network Screening. Eur. J. Med. Res. 2024, 29, 54. [Google Scholar] [CrossRef]
- Reza, M.S.; Qiu, C.; Lin, X.; Su, K.-J.; Liu, A.; Zhang, X.; Gong, Y.; Luo, Z.; Tian, Q.; Nwadiugwu, M.; et al. An Attention-Aware Multi-Task Learning Framework Identifies Candidate Targets for Drug Repurposing in Sarcopenia. J. Cachexia Sarcopenia Muscle 2025, 16, e13661. [Google Scholar] [CrossRef]
- Fatima, R.; Kim, Y.; Baek, S.; Suram, R.P.; An, S.-J.L.; Hong, Y. C-Terminal Agrin Fragment as a Biomarker for Sarcopenia: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2025, 16, e13707. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Lorenzi, M.; Martone, A.M.; Tosato, M.; Drey, M.; D’Angelo, E.; Capoluongo, E.; Russo, A.; Bernabei, R.; et al. Serum Levels of C-Terminal Agrin Fragment (CAF) Are Associated with Sarcopenia in Older Multimorbid Community-Dwellers: Results from the ilSIRENTE Study. Exp. Gerontol. 2016, 79, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Drey, M.; Grösch, C.; Neuwirth, C.; Bauer, J.M.; Sieber, C.C. The Motor Unit Number Index (MUNIX) in Sarcopenic Patients. Exp. Gerontol. 2013, 48, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Drey, M.; Krieger, B.; Sieber, C.C.; Bauer, J.M.; Hettwer, S.; Bertsch, T. DISARCO Study Group Motoneuron Loss Is Associated with Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 435–439. [Google Scholar] [CrossRef]
- Nishimune, H.; Stanford, J.A.; Mori, Y. Role of Exercise in Maintaining the Integrity of the Neuromuscular Junction. Muscle Nerve 2014, 49, 315–324. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1α Isoform Induced by Resistance Training Regulates Skeletal Muscle Hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef]
- Rooks, D.; Praestgaard, J.; Hariry, S.; Laurent, D.; Petricoul, O.; Perry, R.G.; Lach-Trifilieff, E.; Roubenoff, R. Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study. J. Am. Geriatr. Soc. 2017, 65, 1988–1995. [Google Scholar] [CrossRef]
- Rooks, D.; Swan, T.; Goswami, B.; Filosa, L.A.; Bunte, O.; Panchaud, N.; Coleman, L.A.; Miller, R.R.; Garcia Garayoa, E.; Praestgaard, J.; et al. Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2020836. [Google Scholar] [CrossRef]
- Dungan, C.M.; Figueiredo, V.C.; Wen, Y.; VonLehmden, G.L.; Zdunek, C.J.; Thomas, N.T.; Mobley, C.B.; Murach, K.A.; Brightwell, C.R.; Long, D.E.; et al. Senolytic Treatment Rescues Blunted Muscle Hypertrophy in Old Mice. GeroScience 2022, 44, 1925–1940. [Google Scholar] [CrossRef]
- Ozes, B.; Tong, L.; Myers, M.; Moss, K.; Ridgley, A.; Sahenk, Z. AAV1.NT-3 Gene Therapy Prevents Age-Related Sarcopenia. Aging 2023, 15, 1306–1329. [Google Scholar] [CrossRef]

| Factor | Mechanism | Role |
|---|---|---|
| BDNF | Signaling via TrkB and p75NTR receptors; modifies the presynaptic exocytotic machinery (phosphorylation of Munc18-1, SNAP-25) and influences downstream kinases (PKC-βI/PKCε) | Involved in maintaining NMJ integrity and function; altered levels are associated with sarcopenia markers in humans and age-related NMJ changes in animal models |
| NT-4 | Shares the receptor pathway with BDNF via TrkB and p75NTR, influencing presynaptic exocytosis and the activity of PKC-dependent pathways | A postsynaptically released factor involved in neuroprotection and regulation of neurotransmission at the NMJ; an altered BDNF/NT-4 ratio is noted with aging |
| NGF | Acts through high-affinity TrkA and p75NTR receptors; supports neuronal survival and differentiation | Influences neuronal survival and motoneuron status during aging; identified as a positive regulator of muscle mass |
| NT-3 | Acts through high-affinity TrkC/TrkA and p75NTR receptors; supports neuronal survival and differentiation | |
| GDNF | Acts via the Ret/GFRα receptor system, stimulating pro-survival neuronal signals and maintaining synaptic stability | Supports motoneuron survival and helps preserve innervation; implicated in neuromuscular plasticity |
| NRG4 | Adipokine-like action; improves insulin sensitivity and indirectly contributes to the preservation of muscle mass | Levels decrease with age; administration of recombinant NRG4 in aged mice improves signs of sarcopenia and metabolic disorders |
| IGF-1 | Activation of IGF-1R → PI3K/AKT mechanisms stimulates protein synthesis and suppresses catabolic pathways | Anabolic factor promoting muscle cell growth and survival; protects against age-related loss of mass and strength |
| Irisin | Secreted myokine with anti-inflammatory and neuroprotective effects; modulates mitochondrial function and metabolism | Positive regulator of muscle function; its levels correlate with physical performance in the elderly |
| Link to Parkinson’s Disease (PD) | Link to Alzheimer’s Disease (AD) | Specific Processes and Manifestations | Pathogenic Mechanism |
|---|---|---|---|
| Pathological α-synuclein aggregation in motor neuron axons and NMJs, directly disrupting their structure and function, leading to denervation. | Altered NMJ structure (fragmentation, partial denervation) in AD models (3xTgAD mice). Accumulation of APP and β-amyloid in muscle tissue. | Muscle fiber denervation, NMJ degeneration, motor neuron dysfunction. | Neurogenic Mechanisms and Neuromuscular Junction (NMJ) Dysfunction |
| α-Synuclein impairs the mitochondrial importer TOM40. Decreased expression of oxidative phosphorylation genes in muscle. Sharp increase in ROS production. | Disrupted neuronal energy homeostasis, ROS accumulation. Mitochondrial dysfunction in skeletal muscle leads to reduced strength and apoptosis activation. | Disrupted dynamics, biogenesis, and mitophagy; accumulation of mtDNA damage; reduced energy production. | Mitochondrial Dysfunction |
| Oxidative stress and inflammation create a self-sustaining pathological cycle that enhances α-synuclein aggregation and muscle tissue damage. | Pro-inflammatory mediators in AD exert systemic effects on skeletal muscle, creating a vicious cycle that exacerbates sarcopenia. | Chronic low-grade inflammation (inflammaging); release of pro-inflammatory cytokines. | Neuroinflammation/Systemic Inflammation |
| Key role of α-synuclein: its aggregates in NMJs impair acetylcholine release and increase abnormal mitochondria count. | Accumulation of β-amyloid and APP in muscle tissue, contributing to dysfunction. | Accumulation and aggregation of specific pathological proteins with direct toxic effects. | Pathological Protein Aggregates |
| α-Synuclein reduces basal respiratory and glycolytic capacity of MuSCs, impairing their migration and fusion, critically compromising muscle regeneration. | May be a consequence of the general neurogenic and inflammatory context. | Dysfunction of muscle satellite cells (MuSCs), impaired metabolism, proliferation, and fusion capacity. | Impaired Regenerative Potential |
| NA | Identified as a key mechanism of age-related motor neuron loss. Physical activity can modulate this process. | Loss of nuclear membrane integrity, increased permeability, accumulation of toxic proteins in the nucleus. | Impaired Nucleocytoplasmic Transport |
| Notes/Replication | Phenotype Assessed | Biological Function/Pathway | Key Variant(s)/Gene(s) | Design and Sample Size | Study/Population |
|---|---|---|---|---|---|
| Sub-GWS; replicated in UK Biobank | WLBM | Ubiquitin–proteasome; lipid metabolism | rs740681 (FZR1), SOAT2 | WES | Han Chinese (n = 101) [137] |
| Significant in discovery, nominal in replication | WLBM | Actin signaling, glycoprotein biosynthesis, ATPase | rs3732593 (3p27.1; MCF2L2, B3GNT5, ATP11B) | GWAS | Framingham Heart Study (n = 6004) [138,139] |
| PRS ≥ 4 alleles: very high OR (630.6) | Clinical diagnosis | Cholesterol binding, apoptosis | rs10282247 (OSBPL3), rs7022373 (ACER2) | GWAS (clin. sarcopenia) | Taiwanese elderly [140] |
| eQTL confirmed expression in skeletal muscle | LBM, ASM | mRNA destabilization, immune signaling | rs1187118, rs3768582 (RPS10, NUDT3, NCF2, SMG7, ARPC5) | GWAS meta-analysis | Korean cohorts (n = 6961) [141] |
| 20 novel loci identified | Grip strength, walking speed | Glycogen synthesis, myogenesis, Ca2+ homeostasis | PPP1R3A, ZBTB38, ATP2A1 | MTAG + TWAS | UK Biobank (n = 217,822) [145] |
| Partial overlap with strength traits | Weakness (clinical) | Immune regulation, TGF-β signaling | HLA-DQA1, GDF5 | GWAS | Muscle weakness GWAS (n = 256,523) [146] |
| Functional validation in model organisms | Muscle mass | Sex-specific loci; mitochondrial/structural pathways | LINC01661/PRMT6, RCC2P8/COL25A1, DMAC1, EMP2, SSUH2 | WGS | Multi-ancestry WGS (n = 10,729) [147] |
| Protective allele in NE Asians | Sarcopenia diagnosis | Myogenic differentiation, immune protection | SLC41A3, HLA-DPB102:01 | WGS | Japanese cohort (n = 129) [148] |
| OR ≈ 1.98; physical activity mitigates risk | Sarcopenia risk | Lipid metabolism, cytoskeleton, signaling | FADS2, MYO10, KCNQ5, DOCK5, LRP1B | PRS | Korean case–control (1368 vs. 15,472) [149] |
| Significance and Future Directions | AI/ML Methods | Data Type |
|---|---|---|
| Laid the groundwork for diagnostic automation and identification of clinical predictors of sarcopenia | Deep learning architectures (U-Net, nnU-Net, AutoSAM); semantic segmentation (DeepLabV3+ with EfficientNetV2-L); image classification (EfficientNetV2-L); ensemble methods (Random Forest) | Clinical Data and Medical Images |
| Opened prospects for early detection and prediction of sarcopenia as a complication of other diseases based on metabolic profiles | LASSO, SVM-RFE и RF | Metabolomic Data |
| Highlighted the necessity of accounting for population characteristics. Deepened understanding of molecular mechanisms via key signaling pathways | Specialized neural network architectures (DSnet-v1); ensemble methods (Random Forest, XGBoost, AdaBoost) followed by deep neural network training | Transcriptomic Data |
| Provided a molecular basis for understanding the pathogenetic mechanisms of sarcopenia | LASSO, SVM-RFE | Epigenetic Data |
| The most promising direction. Enables identification of novel drug targets and repurposing candidates, unraveling complex molecular interaction networks | MTA-MO (Multi-Task Attention-aware for Multi-Omics data); ANNi (Artificial Neural Network Inference) | Integrative Multi-Omics Data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupriyanova, D.; Bilyalov, A.; Filatov, N.; Brovkin, S.; Shestakov, D.; Bodunova, N.; Gusev, O. Neural Cues and Genomic Clues: NGS Insights into Neurogenic Sarcopenia and Muscle Atrophy. Int. J. Mol. Sci. 2025, 26, 11185. https://doi.org/10.3390/ijms262211185
Kupriyanova D, Bilyalov A, Filatov N, Brovkin S, Shestakov D, Bodunova N, Gusev O. Neural Cues and Genomic Clues: NGS Insights into Neurogenic Sarcopenia and Muscle Atrophy. International Journal of Molecular Sciences. 2025; 26(22):11185. https://doi.org/10.3390/ijms262211185
Chicago/Turabian StyleKupriyanova, Darya, Airat Bilyalov, Nikita Filatov, Sergei Brovkin, Dmitrii Shestakov, Natalia Bodunova, and Oleg Gusev. 2025. "Neural Cues and Genomic Clues: NGS Insights into Neurogenic Sarcopenia and Muscle Atrophy" International Journal of Molecular Sciences 26, no. 22: 11185. https://doi.org/10.3390/ijms262211185
APA StyleKupriyanova, D., Bilyalov, A., Filatov, N., Brovkin, S., Shestakov, D., Bodunova, N., & Gusev, O. (2025). Neural Cues and Genomic Clues: NGS Insights into Neurogenic Sarcopenia and Muscle Atrophy. International Journal of Molecular Sciences, 26(22), 11185. https://doi.org/10.3390/ijms262211185







