1. Introduction
The gut microbiota is a crucial regulator of host immunity, redox balance, and inflammatory signaling. Polyphenols from plant-derived foods and herbal preparations are known to interact closely with intestinal microorganisms. Because of their high molecular weight and low intestinal absorption, these compounds undergo extensive microbial biotransformation in the colon, yielding smaller, more bioavailable metabolites that can exert systemic biological effects. This metabolic interplay, often referred to as the polyphenol–microbiota–immune axis, is increasingly recognized as a key determinant of the biological activity of tannin-rich herbal medicines [
1,
2,
3].
Tormentillae rhizoma (the rhizome of
Potentilla erecta L.) has a long history of use in European folk medicine, especially for the treatment of diarrhea, mucosal irritation, and inflammatory conditions of the gastrointestinal tract [
4,
5]. In Germany, tormentil rhizome is traditionally consumed as a digestive tincture, while in Ukraine, a honey-based tincture is popular as a household remedy for intestinal discomfort and inflammation [
6]. Despite this long-standing traditional use, the molecular basis of tormentil’s biological activity and the contribution of its gut microbiota-derived metabolites remain insufficiently characterized [
7].
Phytochemically, tormentil rhizome is exceptionally rich in polyphenolic compounds, particularly condensed and hydrolysable tannins (up to 20–25% of dry mass), including catechin oligomers, procyanidins, agrimoniin, and ellagic acid derivatives [
8,
9]. In addition to tannins, the rhizome contains notable amounts of pentacyclic triterpenes, such as tormentil triterpenes (tormentic acid, myrianthic acid, and cecropiacic acid) and their esters [
6]. These lipophilic constituents have been shown to exhibit anti-inflammatory, antioxidant, and wound-healing properties, partly by inhibition of cyclooxygenase (COX) and lipoxygenase (LOX) enzymes, modulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, as well as upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant defense mechanisms. Together, these polyphenolic and triterpenoid constituents form a complex phytochemical matrix responsible for the broad pharmacological potential of tormentil rhizome [
5,
10,
11].
Recent studies have demonstrated that tormentil rhizome exhibits notable anti-inflammatory activity both in vitro and in vivo. Agrimoniin-rich fractions obtained from a 50% ethanol extract and enriched in agrimoniin, the main ellagitannin of
P. erecta, inhibited COX-2 expression and reduced prostaglandin E
2 (PGE
2) production in UVB- or interleukin-1
α (IL-1
α)-stimulated HaCaT keratinocytes. In a subsequent clinical study, topical application of the same fraction significantly decreased UVB-induced erythema in healthy volunteers, confirming its anti-inflammatory potential in acute skin inflammation models [
12].
Although these effects were not evaluated directly in immune cells, they provide strong evidence of downstream modulation of inflammatory mediators. Consistent anti-inflammatory activity has also been reported for other
Potentilla species. Ethanol extracts of
P. supina and
P. paradoxa suppressed nitric oxide (NO), PGE
2, tumor necrosis factor alpha (TNF-
α), and interleukin-6 (IL-6) production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages by downregulating inducible nitric oxide synthase (iNOS) and COX-2 expression and inhibiting NF-κB and activator protein 1 (AP-1) signaling. Both extracts also reduced inflammation in vivo in mouse models of endotoxemia and gastritis [
13,
14]. Likewise,
P. discolor ethanol extract suppressed NO, TNF-
α, and monocyte chemoattractant protein-1 (MCP-1) secretion and decreased iNOS mRNA expression in macrophages [
15]. These findings indicate that ethanol extracts across the
Potentilla genus consistently attenuate macrophage-mediated inflammatory responses through the NF-κB/AP-1 → iNOS/COX-2 → NO/PGE
2/TNF-
α/IL-6 axis, providing a strong mechanistic rationale for
P. erecta. Moreover, metabolites derived from ellagitannins, particularly urolithins formed by gut microbiota, have demonstrated anti-inflammatory effects in human and murine immune cells, including THP-1 (human monocytic leukemia cell line)- and RAW 264.7-derived macrophages, by attenuating TNF-
α production and enhancing interleukin-10 (IL-10) secretion through activation of extracellular signal-regulated kinase 1/2 (ERK1/2) signaling. Notably, their glucuronide conjugates were inactive in these models [
16]. Similarly, urolithins, the gut microbiota-derived metabolites of ellagitannins, have been shown to exert strong anti-inflammatory and antioxidant effects. Urolithin A suppresses NF-κB and MAPK signaling, reducing the expression of COX-2 and iNOS and the release of pro-inflammatory mediators such as TNF-
α, IL-6, and PGE
2 while enhancing antioxidant enzyme activity. These findings suggest that microbial conversion of ellagitannins may significantly contribute to the anti-inflammatory potential of
P. erecta extracts [
12,
17,
18,
19,
20].
Neutrophils and macrophages are pivotal components of innate immunity and play essential roles in the initiation, amplification, and resolution of inflammation. Activated neutrophils rapidly generate reactive oxygen species (ROS) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, release proteolytic enzymes, form neutrophil extracellular traps (NETs), and secrete cytokines such as interleukin-8 (IL-8) and TNF-
α [
21,
22,
23]. Macrophages, including THP-1-derived macrophages, coordinate inflammatory signaling by phagocytosis and secretion of cytokines (IL-1
β, IL-6, TNF-
α) and regulate redox homeostasis and apoptosis through multiple intracellular signaling networks. Dysregulation of these processes leads to persistent inflammation and tissue damage, contributing to the pathogenesis of chronic inflammatory and autoimmune diseases [
24,
25,
26].
Although several studies have reported antioxidant, anti-inflammatory, and cytoprotective properties of tormentil rhizome extracts [
4,
5], the contribution of gut microbiota-derived metabolites and triterpenoid components to the modulation of immune cell function remains poorly defined. Moreover, no studies have yet compared the effects of the native ethanolic extract and its gut microbiota-derived metabolites on human innate immune cells. Published studies mainly concern other
Potentilla species or focus exclusively on ellagitannin fractions, without considering metabolite fractions containing other compound classes. To address this gap, the study focused on two complementary models of innate immune response. Human neutrophils were selected as a model of acute inflammation due to their rapid response to inflammatory stimuli, involving ROS generation, cytokine release, and regulated cell death [
23]. THP-1-derived macrophages, in contrast, represent a model of chronic or sustained inflammation and cytokine-driven activation, allowing for evaluation of metabolic activity and mediator secretion [
27]. Together, these two models provide a comprehensive view of how tormentil rhizome and its gut microbiota-derived metabolites influence key processes in innate immune regulation.
The aim of this study was to evaluate and compare the immunomodulatory effects of the ethanolic extract of tormentil rhizome (EtTR) and its gut microbiota-derived metabolites (TRGMs) on human innate immune cells, specifically neutrophils and THP-1-derived macrophages, to elucidate their potential roles in modulating inflammation.
3. Discussion
Because neutrophils and macrophages play distinct but complementary roles in the inflammatory response, the experimental design and readouts were adapted to their biological characteristics. Neutrophils are short-lived, non-adherent immune cells that respond rapidly to pro-inflammatory stimuli through oxidative burst and cytokine release [
21,
22,
23]. Therefore, their functional activity was evaluated by assessing cell viability and death mode (apoptosis/necrosis), ROS generation, and the secretion of IL-8, IL-1
β, and TNF-
α, which are key markers of acute neutrophil activation. In contrast, THP-1-derived macrophages are adherent, metabolically active cells that represent a model of sustained inflammatory signaling [
27]. Accordingly, cytotoxicity was determined using the MTT assay, reflecting mitochondrial activity, while cytokine secretion was analyzed for MCP-1, IL-6, and TNF-
α—typical mediators of macrophage-driven inflammatory and chemotactic responses. This complementary experimental approach enabled the evaluation of both early oxidative and late cytokine-mediated events in innate immune activation, providing a comprehensive overview of the immunomodulatory potential of tormentil rhizome extract and its microbial metabolites.
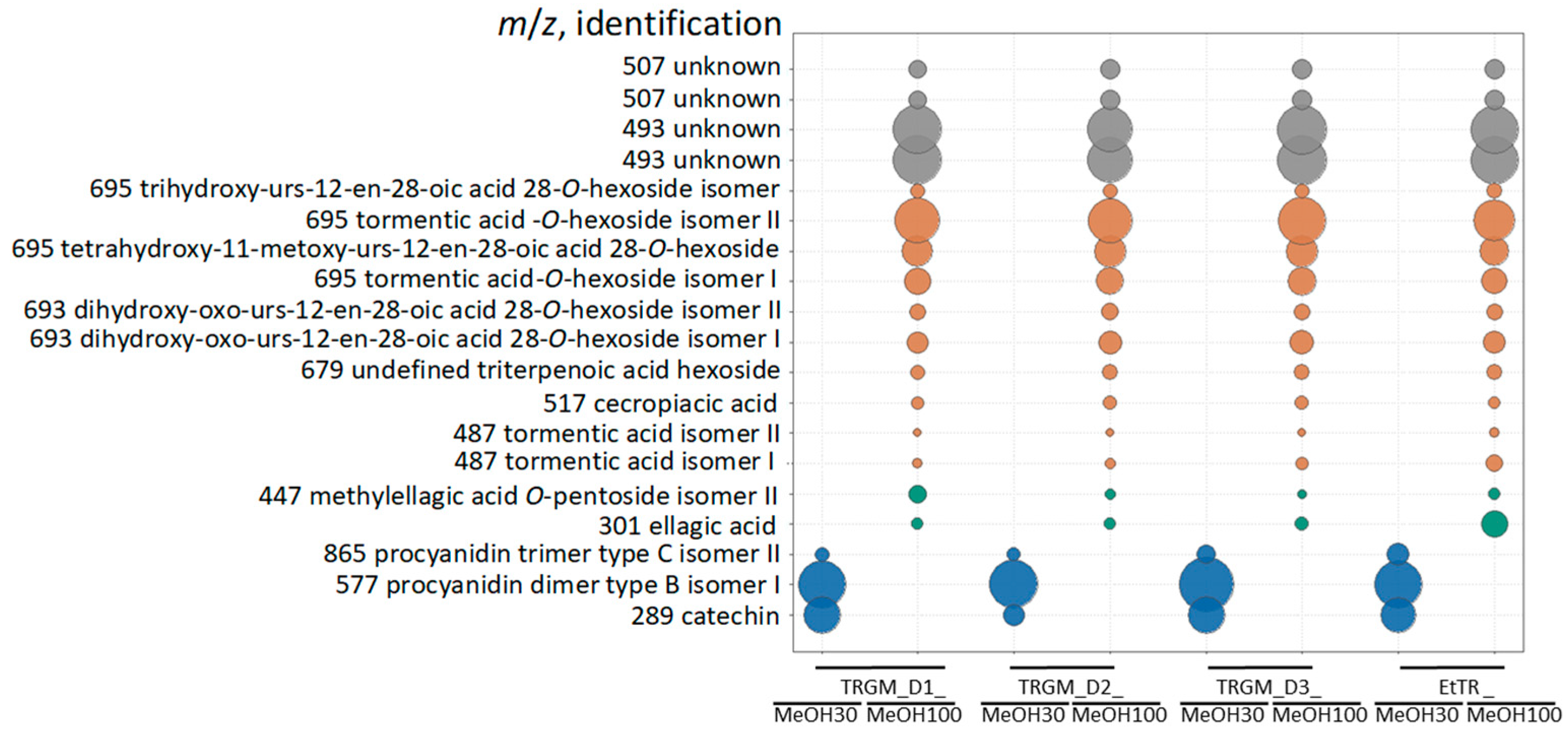
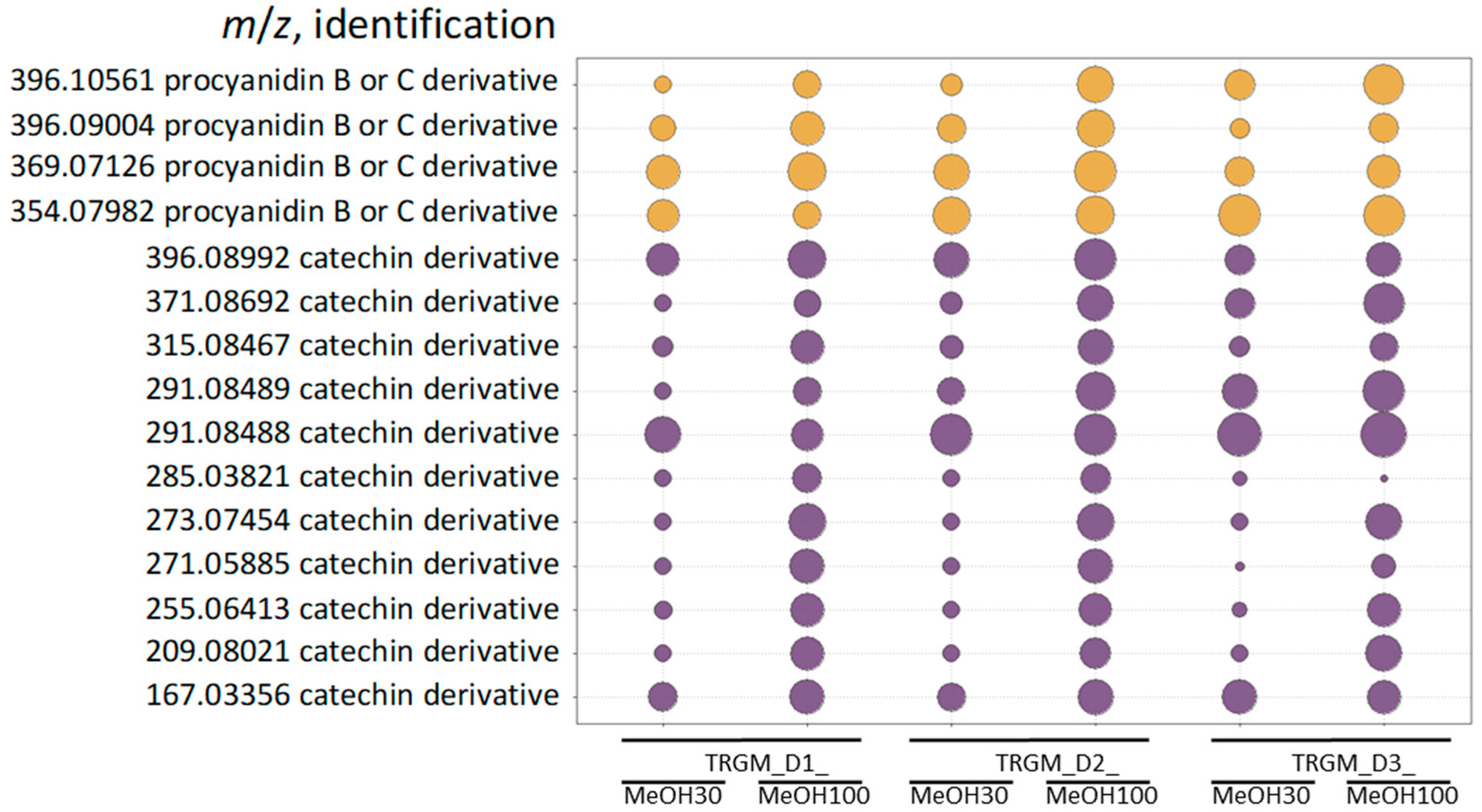
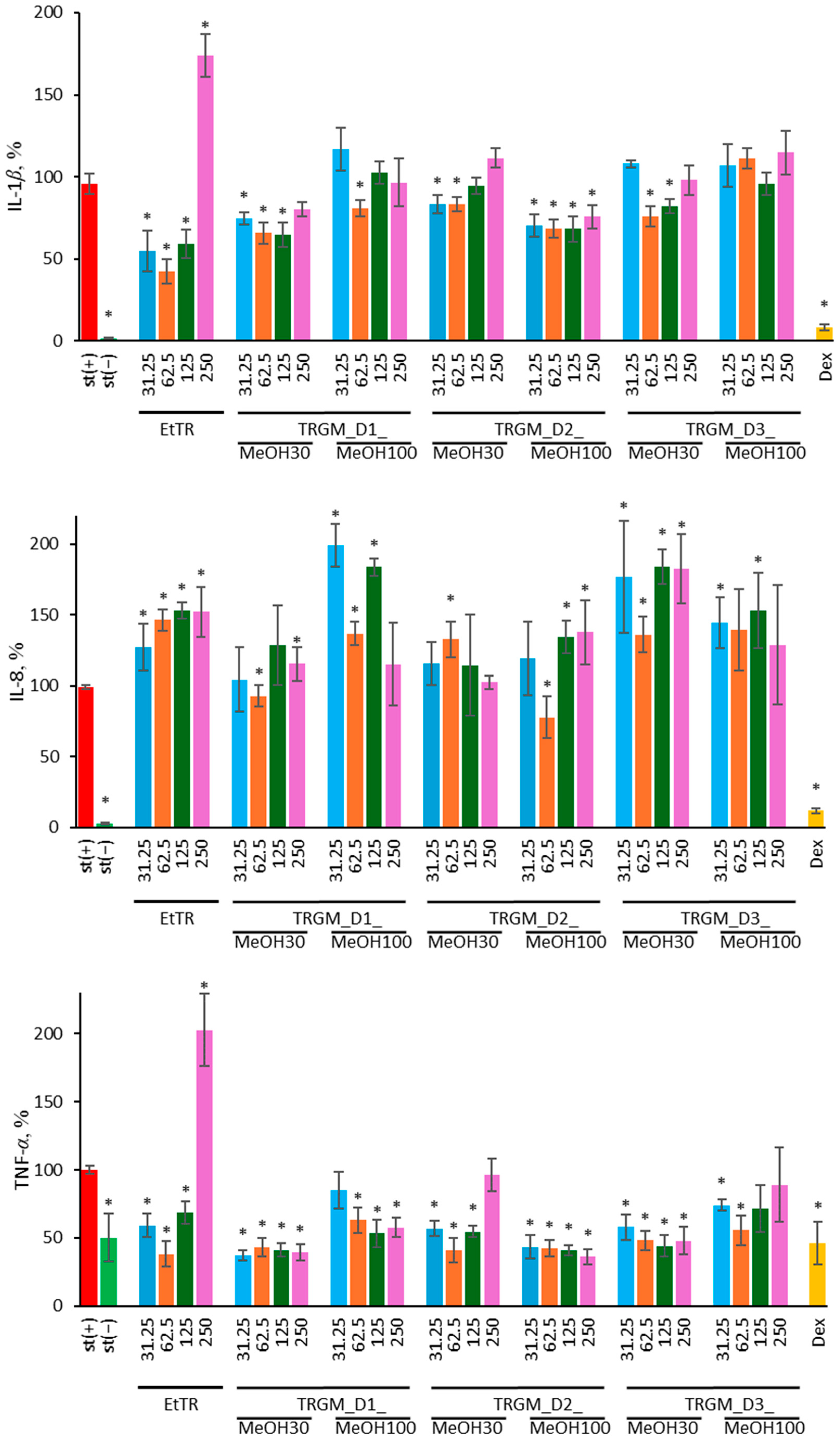
The biological activity of the tormentil rhizome extract and its gut microbiota–derived metabolites reflected their distinct chemical composition. The ethanolic extract, dominated by catechins, procyanidins, and triterpenoid acids, exhibited strong antioxidant and anti-inflammatory properties, consistent with the high abundance of redox-active polyphenols and membrane-stabilizing triterpenes [
5,
10,
11,
12]. Its ability to reduce ROS generation and suppress IL-1
β and TNF-
α secretion in LPS-stimulated neutrophils indicates direct modulation of early inflammatory signaling, likely through inhibition of NADPH oxidase-dependent oxidative burst and attenuation of NF-κB activation. Similar antioxidant and anti-inflammatory effects of catechin- and procyanidin-rich fractions were previously reported for other
Potentilla species, including
P. supina and
P. paradoxa, where ethanol extracts reduced NO and PGE
2 production and suppressed iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages through NF-κB and AP-1 inhibition. Among the metabolite fractions, biological effects corresponded closely to their chemical profiles. Fractions eluted with 30% MeOH were enriched in catechins and low-molecular-weight procyanidins, whereas the 100% MeOH fractions contained mainly ellagic acid derivatives and glycosylated triterpenoids such as tormentic acid
O-hexoside and its isomers. The strongest suppression of ROS and pro-inflammatory cytokines (IL-1
β and TNF-
α) was observed for TRGM_D1_MeOH30 and TRGM_D2_MeOH100, aligning with their high content of catechin-type polyphenols and triterpenoid conjugates. In contrast, fractions poorer in these constituents (e.g., D1_MeOH100 and D3_MeOH30) showed weaker or inconsistent effects, whereas D3_MeOH100, enriched in triterpenoids but containing fewer catechins, displayed a biphasic response with mild stimulation at higher concentrations. These findings suggest that both phenolic and triterpenoid components contribute to the anti-inflammatory action, but their relative ratio and polarity determine the overall biological outcome. The stimulation of IL-8 secretion observed for several samples, particularly at higher concentrations, may reflect a context-dependent, redox-linked chemokine response rather than a straightforward pro-inflammatory effect. Upregulation of IL-8 through EGFR/NF-κB-dependent modulation by polyphenols, including resveratrol, has been documented in epithelial models (HaCaT and NHEK cells stimulated with TNF-
α), whereas in innate immune systems, the response appears more variable and depends on the compound type, dose, and inflammatory stimulus [
31,
32]. In innate immune cells, however, increased IL-8 secretion has a direct functional consequence, as IL-8 is one of the major chemokines responsible for neutrophil recruitment and activation. Even moderate elevations of IL-8 can enhance neutrophil chemotaxis, promote firm adhesion to the endothelium, and prime these cells for oxidative burst and degranulation. Therefore, the IL-8 increase observed in our neutrophil model may reflect an adaptive chemotactic response aimed at improving early immune surveillance rather than a purely pro-inflammatory effect. Such controlled IL-8 upregulation is consistent with a transient, redox-linked signaling shift that prepares neutrophils for environmental stress without inducing excessive inflammatory damage [
33,
34].
In THP-1 macrophages, the tormentil extract and its gut microbiota-derived fractions modulated cytokine secretion in a donor-dependent manner, displaying both inhibitory and stimulatory effects. The ethanolic extract reduced TNF-
α and, to a lesser extent, MCP-1 levels at lower concentrations, which aligns with the suppression of NF-κB-dependent transcription observed for other
Potentilla species [
13,
14,
15]. In contrast, IL-6 secretion remained largely unaffected or was even enhanced at higher concentrations, suggesting a shift from inhibitory to adaptive signaling, possibly linked to moderate redox activation or altered feedback regulation of cytokine expression. Among the microbial metabolite fractions, TRGM_D1_MeOH30 and TRGM_D2_MeOH100 showed the most pronounced inhibitory effects, reducing TNF-
α and, in part, MCP-1 secretion, consistent with their enrichment in catechins, ellagic acid derivatives, and triterpenoid glycosides. In contrast, fractions with lower phenolic content, such as TRGM_D2_MeOH30 and TRGM_D3_MeOH30, tended to enhance IL-6 and MCP-1 release, indicating donor-dependent differences in the metabolic transformation of tormentil constituents. Such divergence likely reflects variation in microbial enzymatic capacity and metabolite composition, as previously observed for ellagitannin-derived urolithins, which exhibit distinct immunomodulatory properties depending on their structure and conjugation state [
16,
17,
18,
19,
20]. In macrophage models, urolithin A has been shown to attenuate TNF-
α and enhance IL-10 via ERK1/2 signaling, whereas its glucuronide conjugates remain inactive [
16]. These findings support the view that microbial transformation of tormentil polyphenols generates low-molecular-weight metabolites capable of fine-tuning macrophage cytokine responses rather than producing uniform suppression.
Taken together, the results obtained from both neutrophil and macrophage models demonstrate that
Tormentillae rhizoma extract and its microbiota-derived metabolites exert complex, concentration- and donor-dependent immunomodulatory effects. The consistent inhibition of oxidative burst and pro-inflammatory cytokines such as IL-1
β, TNF-
α, and MCP-1 reflects the strong antioxidant and anti-inflammatory potential of tormentil constituents, particularly flavan-3-ols and triterpenoid acids [
5,
10,
11,
12,
13,
14,
15]. At the same time, the variable modulation of IL-6 and IL-8 secretion indicates a regulatory rather than purely suppressive mode of action, which may contribute to restoring immune homeostasis under inflammatory stress.
The interplay between extract composition, solvent polarity, and microbial metabolism appears to be a key determinant of the observed bioactivity. The interindividual variability among donors highlights the crucial role of the gut microbiota in shaping the metabolic and immunological outcomes of polyphenol intake [
1,
2,
3]. These findings support the emerging concept that hydrolyzable tannins from tormentil rhizome act as precursors of bioactive postbiotic metabolites capable of fine-tuning innate immune responses rather than exerting uniform suppression. Collectively, the study provides mechanistic evidence that tormentil rhizome and its microbiota-derived metabolites can modulate oxidative and inflammatory pathways in innate immune cells through complementary redox and signaling mechanisms. Such properties underline the potential of tormentil rhizome as a source of functional ingredients for the prevention or management of inflammation-related intestinal and systemic disorders.
4. Materials and Methods
4.1. Materials
Plant material, tormetil rhizome, with serial No. 946.2021 was purchased from Kawon (Gostyń, Poland). All solvents, including dimethyl sulfoxide, ethanol and methanol, were purchased from Avantor (Gliwice, Poland). LC-MS grade solvents, such as water and acetonitrile, were purchased from Witko (Łódź, Poland). Ammonium formate and formic acid of LC-MS grade were obtained from Chem-lab (Zedelgem, Belgium). Brain heart infusion (BHI) broth was purchased from bioMerieux SA (Craponne, France). Fetal bovine serum (FBS), phosphate-buffered saline (PBS), RPMI 1640 with stable glutamate and 25 mM of HEPES with and without phenol red, penicillin-streptomycin solution and Hanks’ Balanced Salt Solution (HBSS) without calcium and magnesium were obtained from Biowest (Nuaillé, France). Thiazolyl blue tetrazolium bromide (MTT) was sourced from Acros Organics B.V.B.A. (Geel, Belgium). Lipopolysaccharide (LPS) from E. coli O111:B4 and Triton X-100 (Trt) were purchased from Merck Life Science (Darmstadt, Germany). Dextran from Leuconostoc mesenteroides, dexamethasone (Dex), quercetin (Qr), formyl-methionyl-leucyl-phenylalanine (f-MLP), luminol, phorbol-12-myristate-13-acetate (PMA), roscovitine (Rsc), and propidium iodide (PI) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Annexin V–fluorescein isothiocyanate (Annexin V–FITC), binding buffer, and ELISA sets were purchased from BD (Franklin Lakes, NJ, USA). Pancoll was purchased from Biotech (Aidenbach, Germany). Ultra-pure water was prepared using a Simplicity UV system from Merck Millipore.
4.2. Preparation of Plant Extract
The ethanolic extract of tormentil rhizome was obtained by a three-step extraction using a mixture of ethanol and water (7:3,
v/
v). At each step, the plant material was extracted with 500 mL of the solvent, supported by ultrasonic treatment in a water bath for 30 min at 40 °C. The obtained extract was concentrated using a rotary evaporator (240 mbar, 35 °C water bath, flask rotation speed 120 rpm) to remove ethanol, and subsequently lyophilized to remove water (48 h, vacuum <1 mbar, collector temperature −80 °C). The yield of the dry extract after lyophilization was 13.6%. The extract was stored in a sealed container at −17 °C [
6,
28].
4.3. Quantitative Analysis of the Extract
Quantitative analysis of the ethanolic extract of
Tormentillae rhizoma was performed using a validated UHPLC-DAD-MS/MS method as previously described [
6]. Calibration curves were prepared for representative analytical standards, including (–)-epicatechin, agrimoniin,
α-hederin, and 3,3′-di-
O-methylellagic acid 4′-xylopyranoside. The method demonstrated excellent linearity (
r > 0.997), with limits of detection (LOD) and quantification (LOQ) ranging from 0.05 to 3.13 ng and 0.16 to 939 ng per injection, respectively. Intra- and interday precision values were below 5%. Quantification was based on external calibration and peak area integration at compound-specific wavelengths using DAD and MS data for confirmation. The results were expressed as the percentage contribution of each compound to the total amount of quantified constituents in the extract [
6].
4.4. Biosynthesis of Tormentil Rhizome Gut Metabolites
The study was approved by the Ethical Committee of the Medical University of Warsaw (approval no. AKBE/151/2021) on 6 September 2021 and conducted in accordance with the Declaration of Helsinki. Fecal samples were collected from three healthy adult donors (one woman and two men, aged 26–36) who met the inclusion criteria, ensuring sample quality and safety, including no history of gastrointestinal or infectious diseases, no antibiotic use within the previous six months, and adherence to a phenolic compound-restricted diet for three days before donation. Fecal samples were processed within 30 min after collection to prepare fecal slurries (FS; 1:10
m/
v in liquid brain heart infusion, 37 °C). For each donor, 3 mL of FS was mixed with 12.5 mL of tormentil rhizome ethanolic extract and 234.5 mL of brain heart infusion medium. Incubations were carried out under anaerobic conditions (Bactron 300, Sheldon Manufacturing, Cornelius, OR, USA) at 37 °C for 24 h. Reactions were terminated at 0 h and 24 h by adding methanol containing 0.1% formic acid (1:1,
v/
v). Controls included an extract without FS and blanks containing only FS in the medium. After incubation, samples were centrifuged and subjected to solid-phase extraction (SPE) using 30% and 100% methanol in water. These solvent proportions were selected based on prior experimental optimization, as they efficiently recover a broad range of metabolites: 30% methanol favors the extraction of polar compounds, whereas 100% methanol extracts less polar, lipophilic constituents. The obtained metabolite fractions were concentrated, freeze-dried, and prior to chromatographic analyses, reconstituted, centrifuged, and filtered. Each fraction was assigned a specific code to indicate its origin and extraction conditions: TRGM_D1–D3_MeOH30 and TRGM_D1–D3_MeOH100, where TRGM refers to Tormentil Rhizome Gut Metabolites, D1–D3 denotes the donor number, and MeOH30 or MeOH100 indicates elution with 30% or 100% methanol during SPE [
28].
4.5. Chromatographic Analysis of Extract
The chemical profiling of tormentil rhizome ethanolic extract was performed on a UHPLC-3000 RS system (Dionex, Leipzig, Germany) coupled to an AmaZon SL ion trap mass spectrometer equipped with an electrospray ionization (ESI) source (Bruker Daltonik GmbH, Bremen, Germany) and a diode array detector (DAD). The system operated in splitless mode. UV spectra were recorded within the 200–450 nm range. The ion source parameters were as follows: nebulizer gas pressure, 40 psi; drying gas (nitrogen) flow rate, 9 L/min; gas temperature, 134 °C; and capillary voltage, 4.5 kV. The instrument scanned ions in the
m/
z range of 70–2200. Chromatographic separation was carried out on a Kinetex XB-C18 column (150 mm × 3.0 mm, 2.6 µm; Phenomenex, Torrance, CA, USA). The mobile phases consisted of water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B), both of analytical or LC-MS grade. The elution was performed with a linear gradient from 5 to 26% B over 0–60 min, followed by 26 to 60% B over 60–90 min, at a constant flow rate of 0.3 mL/min. The column temperature was maintained at 25 °C. Prior to injection, samples were passed through a 0.45 µm syringe filter, and an injection volume of 4 µL was used for all analyses [
6,
28].
4.6. Chromatographic Analysis of Tormentil Gut Metabolites
Metabolite profiling of control and experimental samples was conducted using a Vanquish UHPLC system coupled to an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). Chromatographic separation was achieved on a Kinetex XB-C18 column (150 × 2.1 mm, 1.7 µm particle size). The mobile phases consisted of (A) water and (B) acetonitrile:water (4:1,
v/
v), both containing 0.1% formic acid and 5 mM ammonium formate (NH
4HCOO). The gradient program was as follows: 0–3.5 min, 1% B; 3.5–16.5 min, 1–26% B; 16.5–26.5 min, 26–100% B; 26.5–28.5 min, 100% B. The flow rate was set at 0.3 mL min
−1, and the column temperature was maintained at 45 °C. The mass spectrometer was operated in electrospray ionization (ESI) mode with polarity switching. The spray voltage was 3.5 kV in positive and 2.0 kV in negative mode. The sheath, auxiliary, and sweep gas flows were 48, 11, and 2 arbitrary units, respectively. The ion transfer tube and vaporizer temperatures were set to 320 °C and 280 °C, respectively. Full MS data were acquired at a resolving power of 120,000, followed by data-dependent MS/MS (ddMS
2) scans collected at 15,000 resolution using stepped normalized collision energies (NCE) of 30 and 50%. Four fragmentation scans were triggered per full MS cycle, with a single-charge filter, isotope pattern recognition, and dynamic exclusion enabled. To improve metabolite coverage, experimental samples were analyzed both with and without exclusion lists generated from control runs. All raw data were processed using Compound Discoverer 3.4 (Thermo Fisher Scientific, Austin, TX, USA) for peak alignment, compound annotation, and statistical evaluation [
28].
4.7. Ultrafiltration of Extract and Its Gut Metabolites
To remove high-molecular-weight pyrogens, all samples underwent ultrafiltration using Microsep™ Advance Centrifugal Devices (Pall Corporation, Port Washington, NY, USA). The extracts were centrifuged sequentially through filters with 100 kDa and 30 kDa molecular weight cut-offs. Subsequently, the filtrates were sterilized by passage through 0.22 µm syringe filters to ensure sterility prior to further assays [
30,
35].
4.8. Isolation of Human Neutrophils
Human neutrophils were obtained from buffy coats provided by the Warsaw Blood Donation Centre. Samples were collected from three healthy male donors under 35 years of age who declared that they were non-smokers and were not taking any medication. Only anonymized buffy coats from donors who had given consent for scientific use of their blood components were used. All donors underwent routine laboratory testing, and only samples with parameters within normal physiological ranges were included. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Warsaw (AKBE/31/2023) on 16 January 2023. Neutrophils were separated by dextran sedimentation followed by centrifugation using a Pancoll density gradient (1.077 g/mL; 1500 rpm, 4 °C). Residual erythrocytes were removed by hypotonic lysis. The final neutrophil preparations showed a purity exceeding 97%. Cells were suspended in RPMI 1640 medium with phenol red, stable glutamine, 25 mM HEPES, and supplemented 10% FBS and 1% penicillin–streptomycin medium and stored at 4 °C until further use [
36,
37].
4.9. Incubation of Neutrophils with Plant Extracts
The cell suspension from point 4.6 (2 × 10
6 cells/mL) was distributed into 96-well plates, and the tested EtTR extract and its gut metabolites were added to achieve final concentrations of 31.125, 62.5, 125, or 250 µg/mL. The concentration range was selected based on previous studies using structurally similar polyphenol-rich plant extracts, in which higher doses frequently caused non-specific cytotoxic effects in immune cell models [
30,
37]. The medium containing DMSO was added to the stimulated and non-stimulated controls (final concentration in well 5%). After 1 h preincubation with the tested samples, neutrophils were stimulated with LPS (100 ng/mL). The cells were then incubated for 24 h at 37 °C in a humidified atmosphere containing 5% CO
2 [
36,
37].
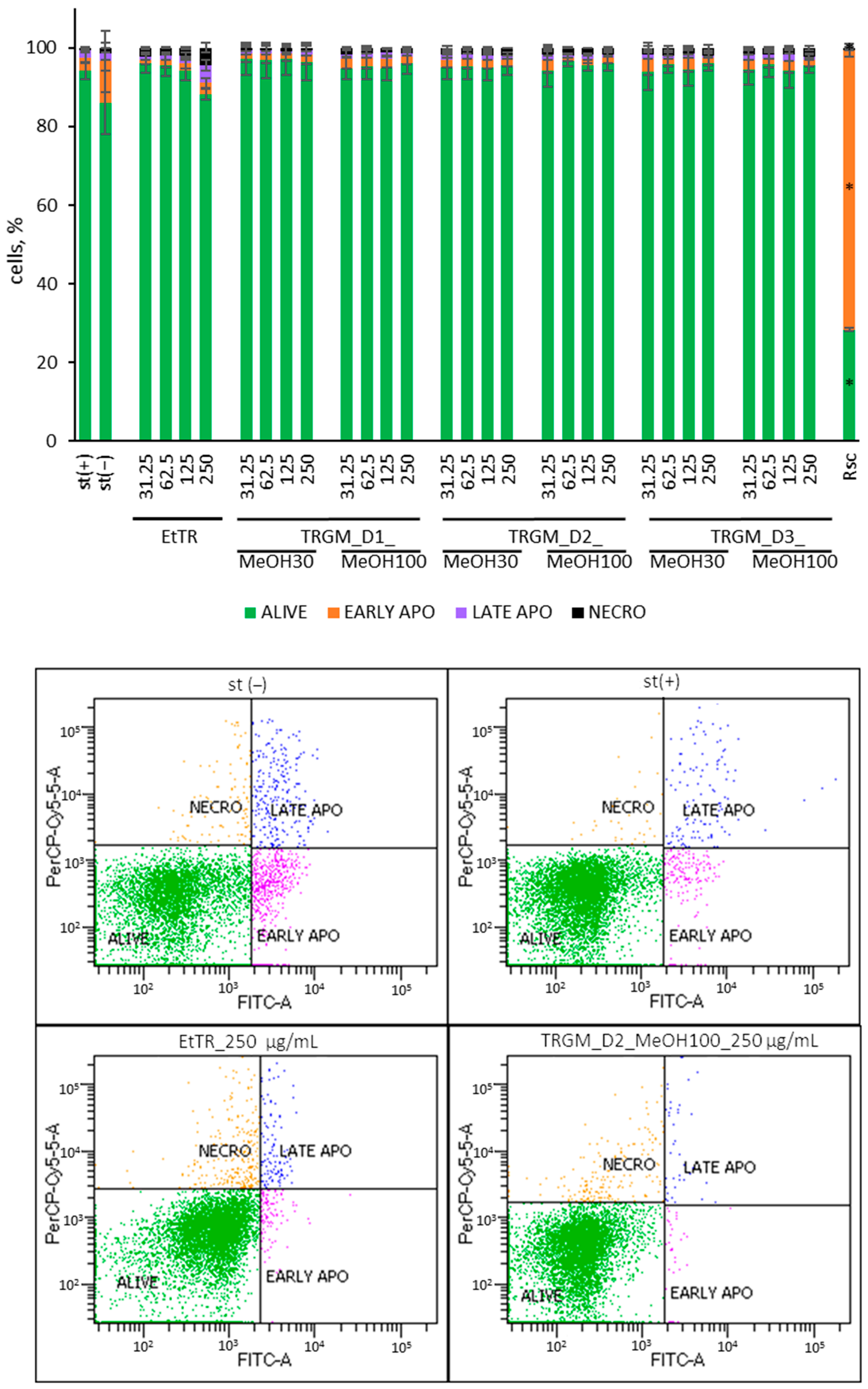
4.10. Neutrophil Viability, Apoptosis and Necrosis Assay
Cell viability, apoptosis and necrosis were evaluated using Annexin V-FITC and propidium iodide staining. Neutrophils from point 4.6 after 24 h of incubation, cells were centrifuged (2000 rpm, 10 min, 4 °C), supernatants were collected, and the pellets were washed twice with PBS. Neutrophils were then resuspended in binding buffer and transferred to a new 96-well plate at a concentration of 0.5 × 10
6 cells/well (4-fold dilution). A staining solution containing Annexin V-FITC (5 µL/mL) and PI (0.5 µg/mL) in binding buffer was added to each well. After 15 min of incubation in the dark at room temperature, the cells were analyzed by flow cytometry (BD FACSCelesta, Becton Dickinson, Franklin Lakes, NJ, USA) with laser settings: FSC: 550 V, SSC: 284 V, FITC: 421 V, Per CP-Cy5-5: 601 V. Data were collected from 10,000 gated events. Instrument compensation and quadrant settings were adjusted before each measurement. Roscovitine (50 µM) was added to cells at the same time as the tested samples served as positive controls for apoptosis induction. The cytotoxicity effect of the extract and its metabolites on human neutrophils was expressed as a percentage relative to the stimulated control (100%) [
30].
4.11. Determination of ROS Production
The production of reactive oxygen species (ROS) was evaluated in isolated human neutrophils stimulated with f-MLP. A volume of 50 μL of the extract or its gut metabolites, dissolved in HBSS at concentrations of 31.25, 62.5, 125, and 250 μg/mL, was placed in white 96-well plates. Subsequently, 70 μL of the neutrophil suspension (3 × 10
6 cells/mL) was added to each well, followed by 50 μL of luminol (0.4 mg/mL). The reaction was initiated by adding 30 μL of f-MLP (1.5 μg/mL) to the mixture of cells with the tested samples and the stimulated control (st(+)), whereas 30 μL of HBSS was added to the non-stimulated control (st(−)). All samples and reagents were prepared in HBSS without calcium and magnesium ions. Luminescence was recorded immediately after stimulation for 30 min at 2 min intervals. The maximum chemiluminescence value (observed between 10 and 20 min) was used for calculations. The inhibitory effect of the extract and its metabolites on ROS production was expressed as a percentage relative to the stimulated control (100%) [
38].
4.12. THP-1 Cell Line Culture
THP-1 human monocytic cells (DSMZ, Braunschweig, Germany) were cultured in 75 cm
2 culture flasks in RPMI 1640 medium without phenol red, containing stable glutamine, 25 mM HEPES, and supplemented with 10% (
v/
v) FBS, and 1% (
v/
v) penicillin–streptomycin solution. The culture medium was replaced with fresh medium three times per week. Cell confluency was monitored microscopically, and passaging was performed at 90–100% confluency. All cultures and experiments were maintained at 37 °C in a humidified atmosphere with 5% CO
2 [
39].
4.13. Incubation of THP-1 Macrophages with Plant Extracts
Before performing the viability and anti-inflammatory assays, monocytes were differentiated into THP-1-derived macrophages. THP-1 monocytes were seeded in 24-well plates at a density of 4 × 10
5 cells per well and cultured at 37 °C in a humidified atmosphere containing 5% CO
2. The culture medium consisted of RPMI 1640 without phenol red, supplemented with 10% FBS and 2 mM glutamine. To induce differentiation into macrophages, cells were treated with 25 ng/mL PMA for 48 h, followed by medium replacement and an additional 24 h resting period. Successful differentiation was confirmed by characteristic macrophage features, including cell adherence, morphological changes, and responsiveness to bacterial LPS stimulation. After the resting period, the tested tormentil rhizome extract and its gut metabolites were added to the cells to obtain final concentrations of 31.25, 62.5, 125, and 250 µg/mL. The same concentration range was used as in neutrophil assays, as it had previously been shown to be non-cytotoxic for polyphenol-rich plant extracts [
30,
37]. For both the stimulated and non-stimulated controls, culture medium containing DMSO was added to reach a final DMSO concentration of 0.5%. Following the addition of the test samples, the cells were incubated for 1 h, after which LPS (100 ng/mL) was added to the appropriate wells to induce inflammation [
39].
4.14. Determination of Cytokine Secretion
Supernatants from points were collected after 24 h incubation from point 4.6 and were analyzed for cytokine production (IL-8, TNF-α, and IL-1β) using commercial Enzyme-Linked Immunosorbent Assay (ELISA) kits following the manufacturer’s instructions. Absorbance was measured at 450 nm with background correction at 570 nm using a BioTek microplate reader. Dexamethasone (20 µM) added to cells at the same time as the tested samples was used as a positive control. The modulatory effect of the extract and its metabolites on cytokine production was expressed as a percentage relative to the stimulated control (100%).
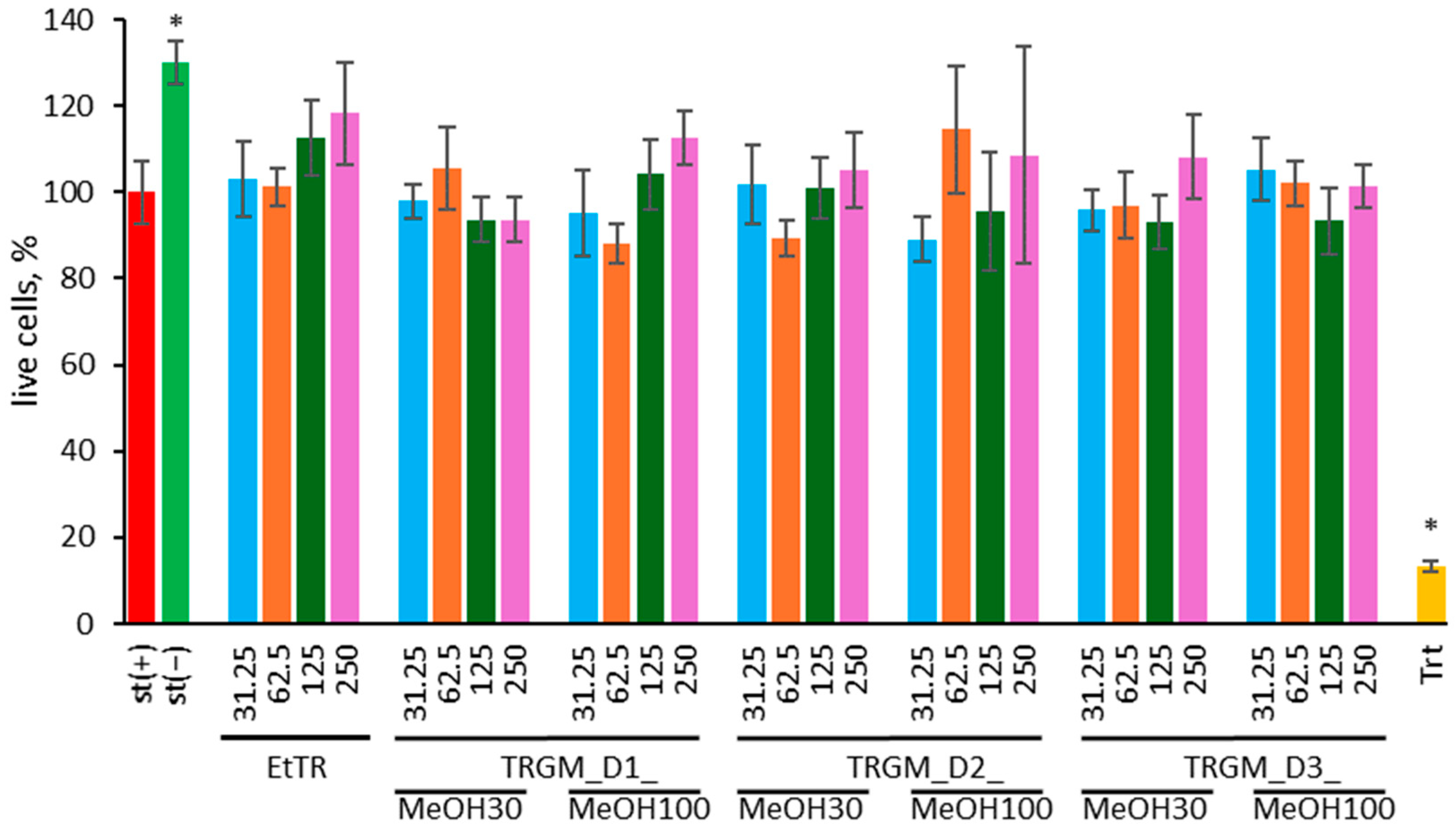
4.15. MTT Viability Assay
Cell viability was evaluated using the MTT assay. THP-1-derived macrophages stimulated with LPS, after 24 h incubation with the tested samples described in
Section 4.12, were washed twice with warm PBS. After washing, MTT solution (0.5 mg/mL in culture medium) was added to each well. Following 4 h of incubation, the medium containing MTT was removed, and the resulting formazan crystals were dissolved in DMSO. A 0.1% (
v/
v) Triton X-100 solution in culture medium was used as a positive control. Absorbance at λ = 570 nm with the correction to 630 nm was measured using a microplate reader (Synergy 4, BioTek, Winooski, VT, USA) [
6].
4.16. Statistical Analysis
Data processing and statistical evaluations were carried out using Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA) and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). The results are expressed as mean values ± standard deviation (SD) obtained from at least three independent experiments. Differences between groups were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. A p-value below 0.05 was considered statistically significant.