Endotoxin Pretreatment Mitigates Myocardial Ischemia-Reperfusion Injury Through Preservation of Mitochondrial Respiration: A Combined Assessment of In Vivo, Ex Vivo, and In Vitro Data
Abstract
1. Introduction
2. Results
2.1. Exclusions
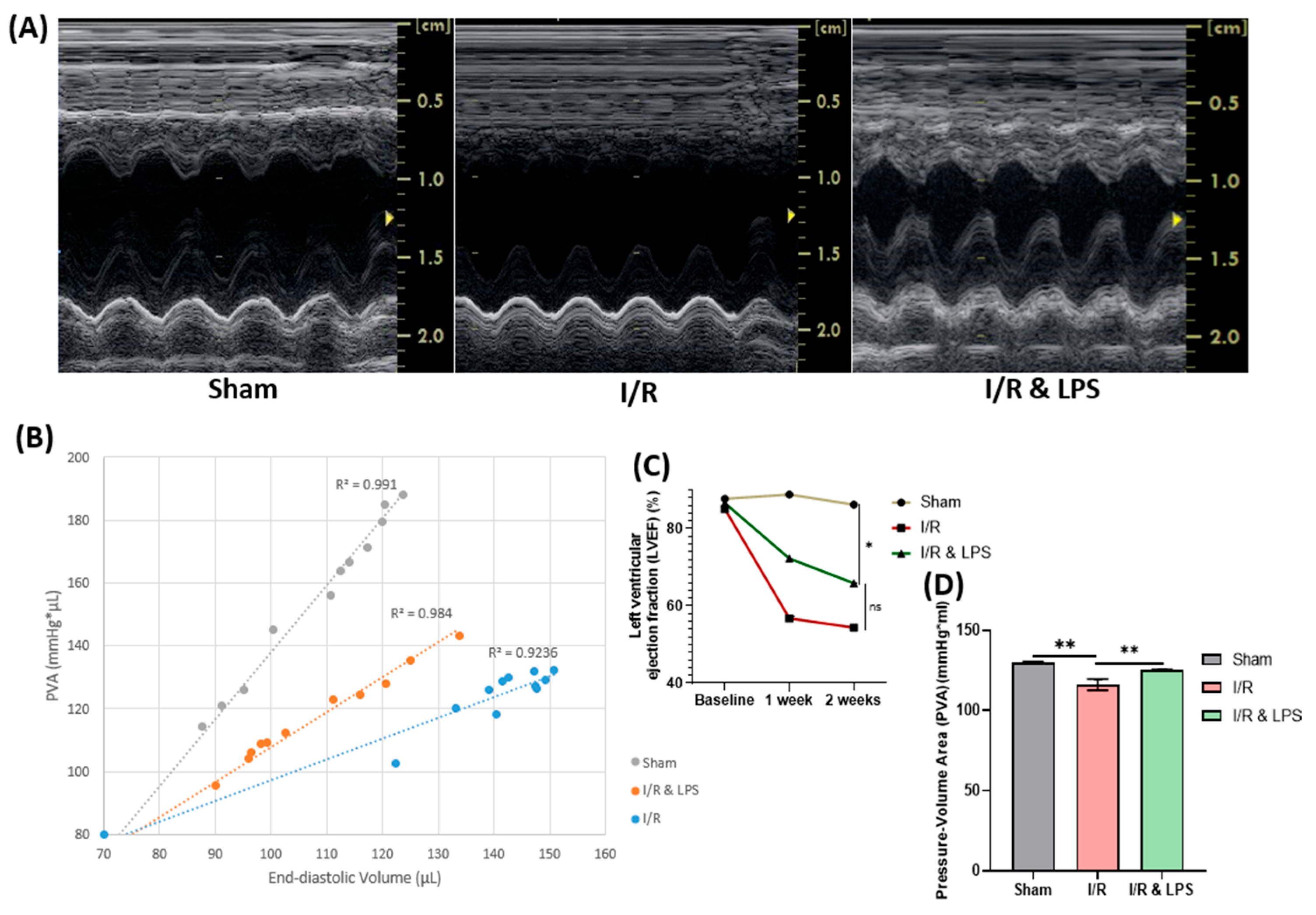
2.2. Endotoxin Pretreatment Preserved Left Ventricular Function and Myocardial Oxygen Consumption Following Ischemia-Reperfusion In Vivo
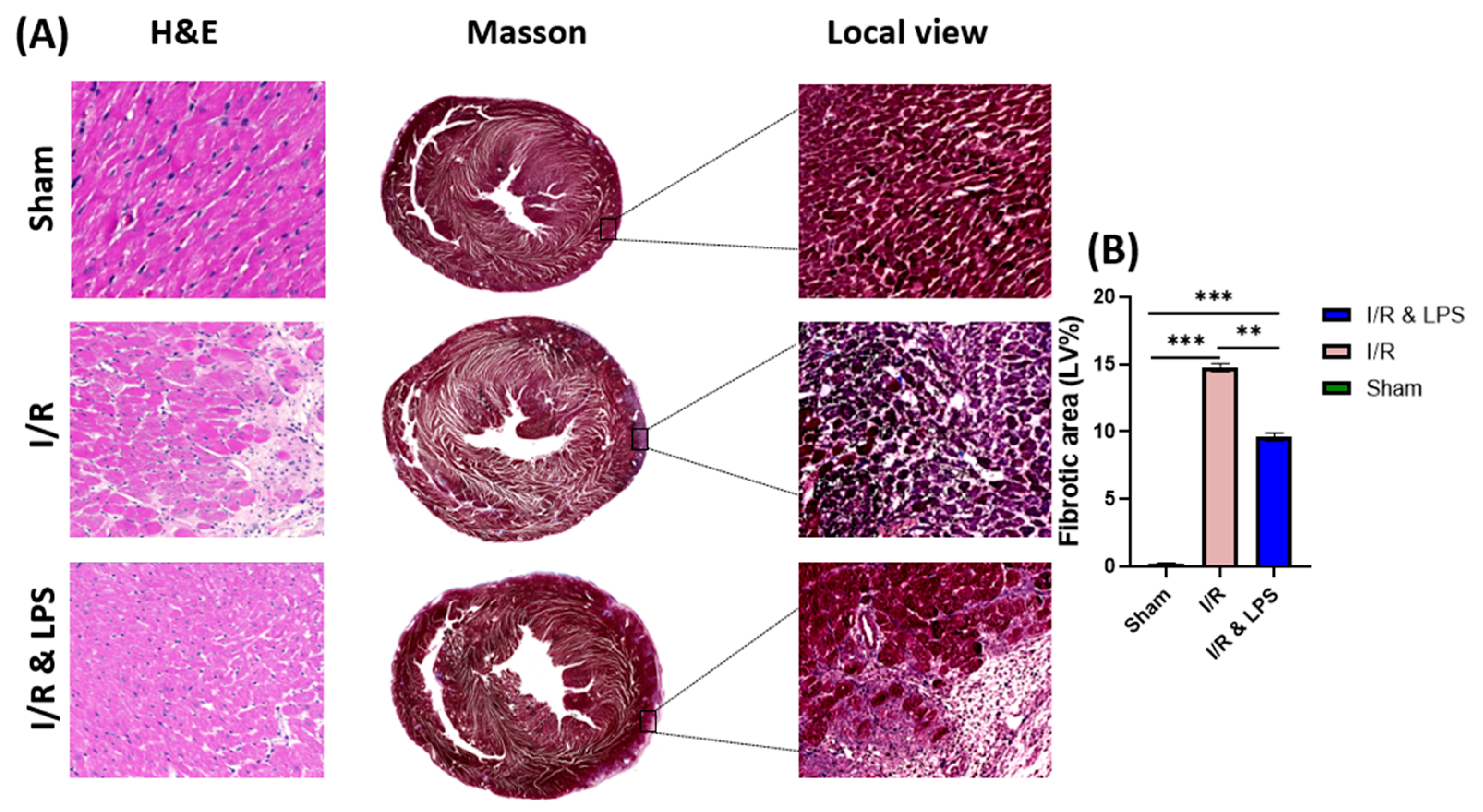
2.3. Endotoxin Pretreatment Reduced Myocardial Fibrosis and Inflammation Following Ischemia–Reperfusion In Vivo
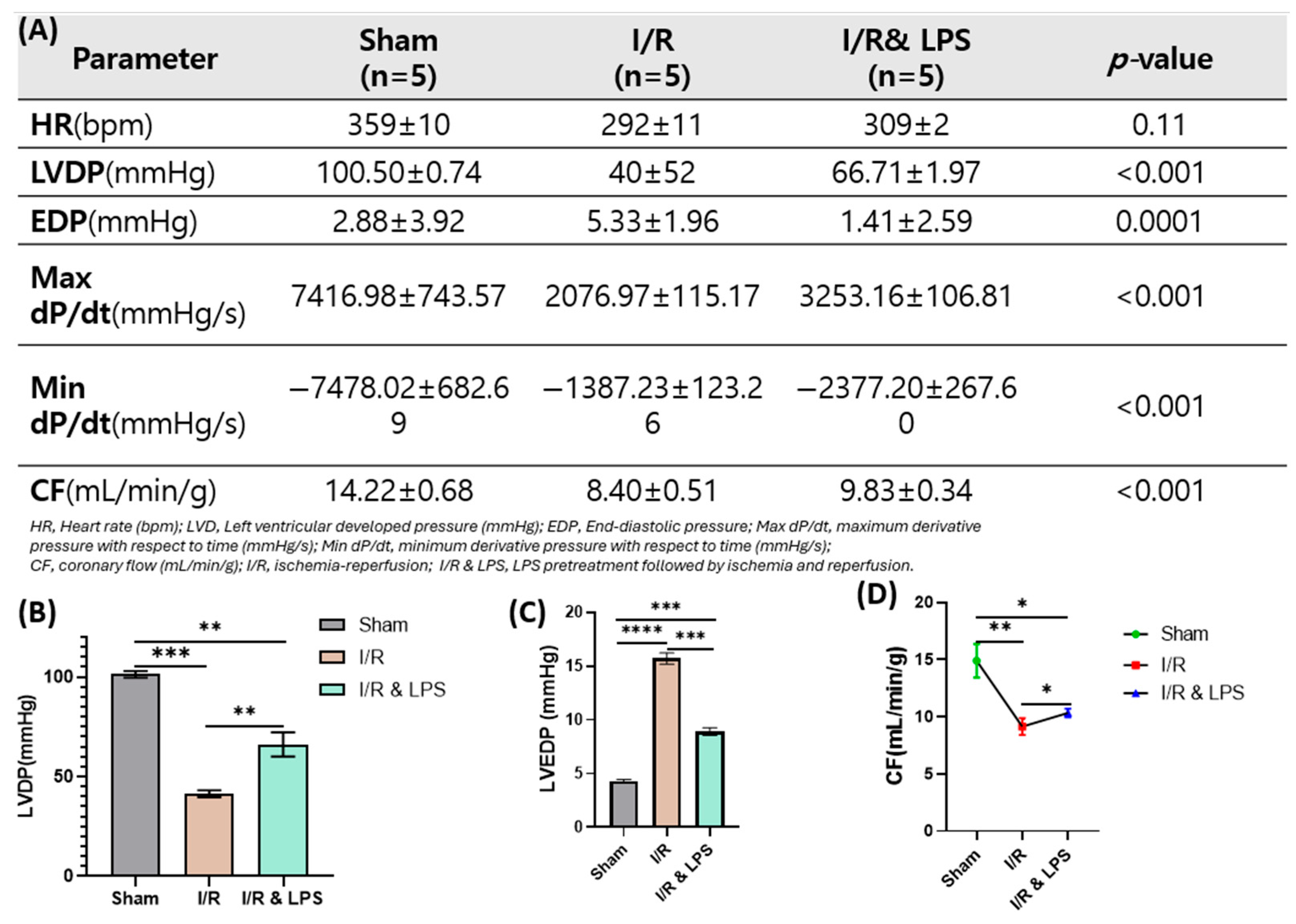
2.4. Endotoxin Pretreatment Preserved Ventricular Function Following Ischemia and Reperfusion in Ex Vivo Normothermic Perfusion
2.5. Identification of Isolated Primary Cardiomyocytes
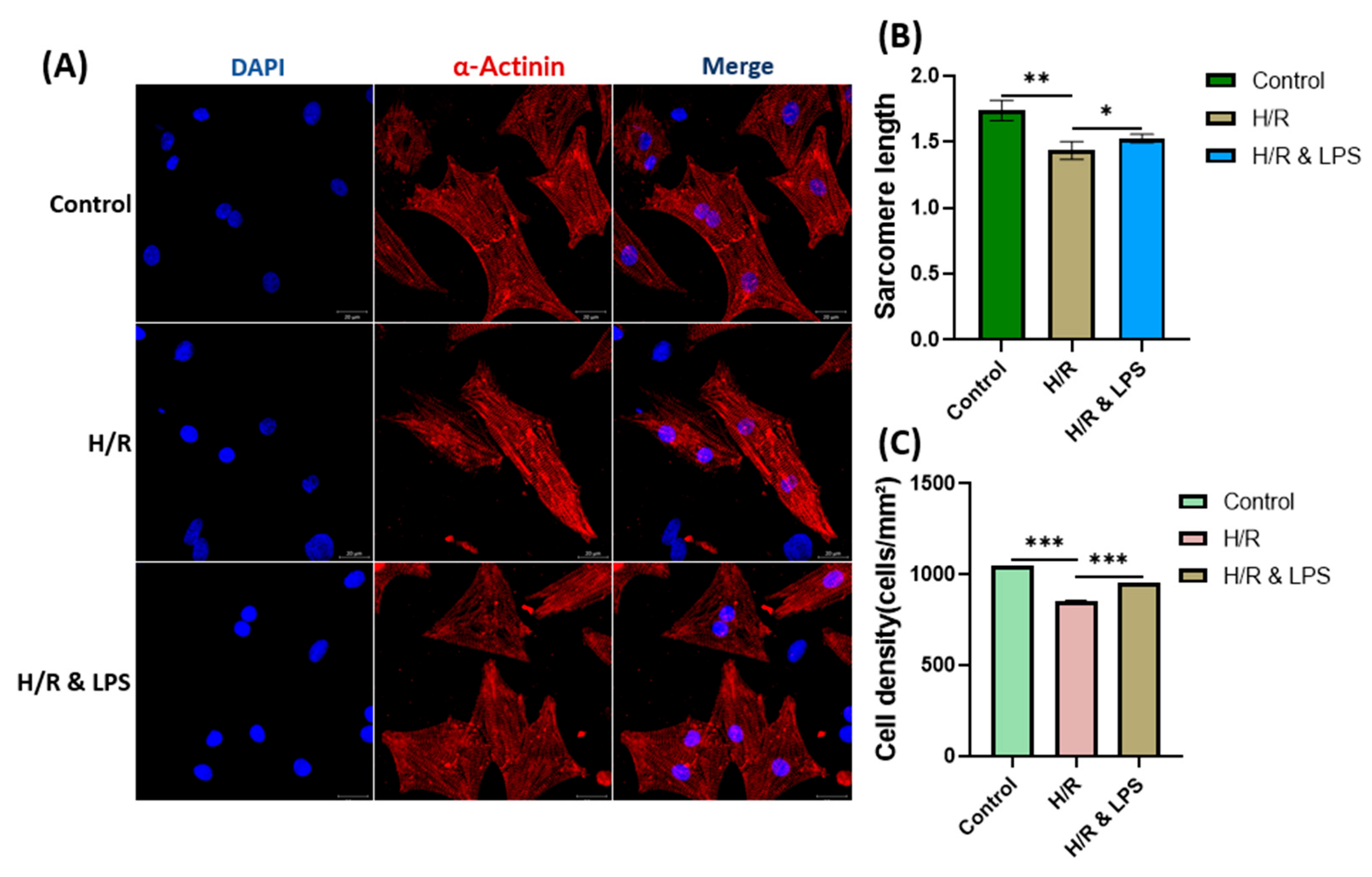
2.6. Endotoxin Pretreatment Preserved Cardiomyocyte Viability After Hypoxia–Reoxygenation
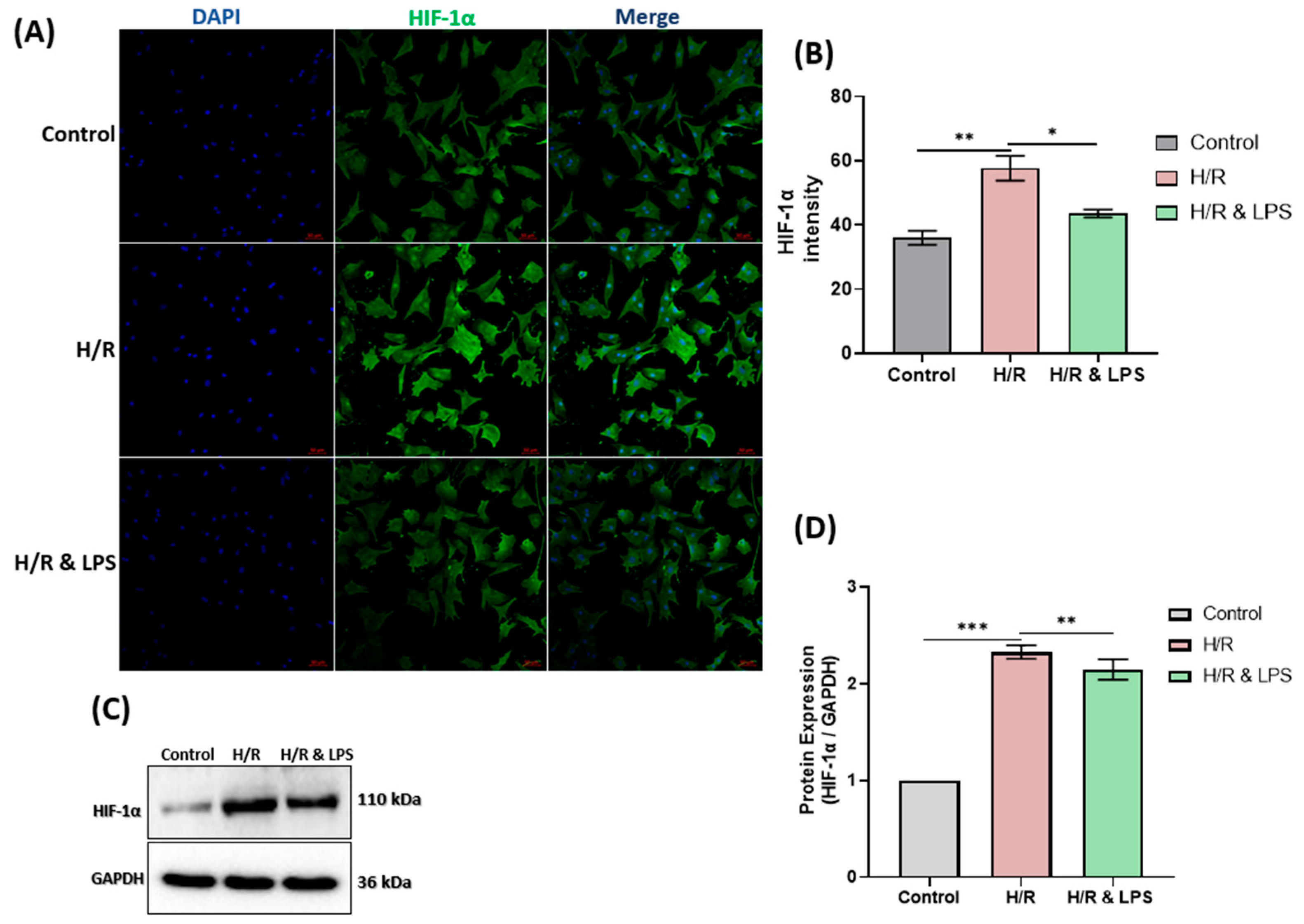
2.7. Endotoxin Pretreatment Reduced Hypoxia in Neonatal Rat Cardiomyocytes
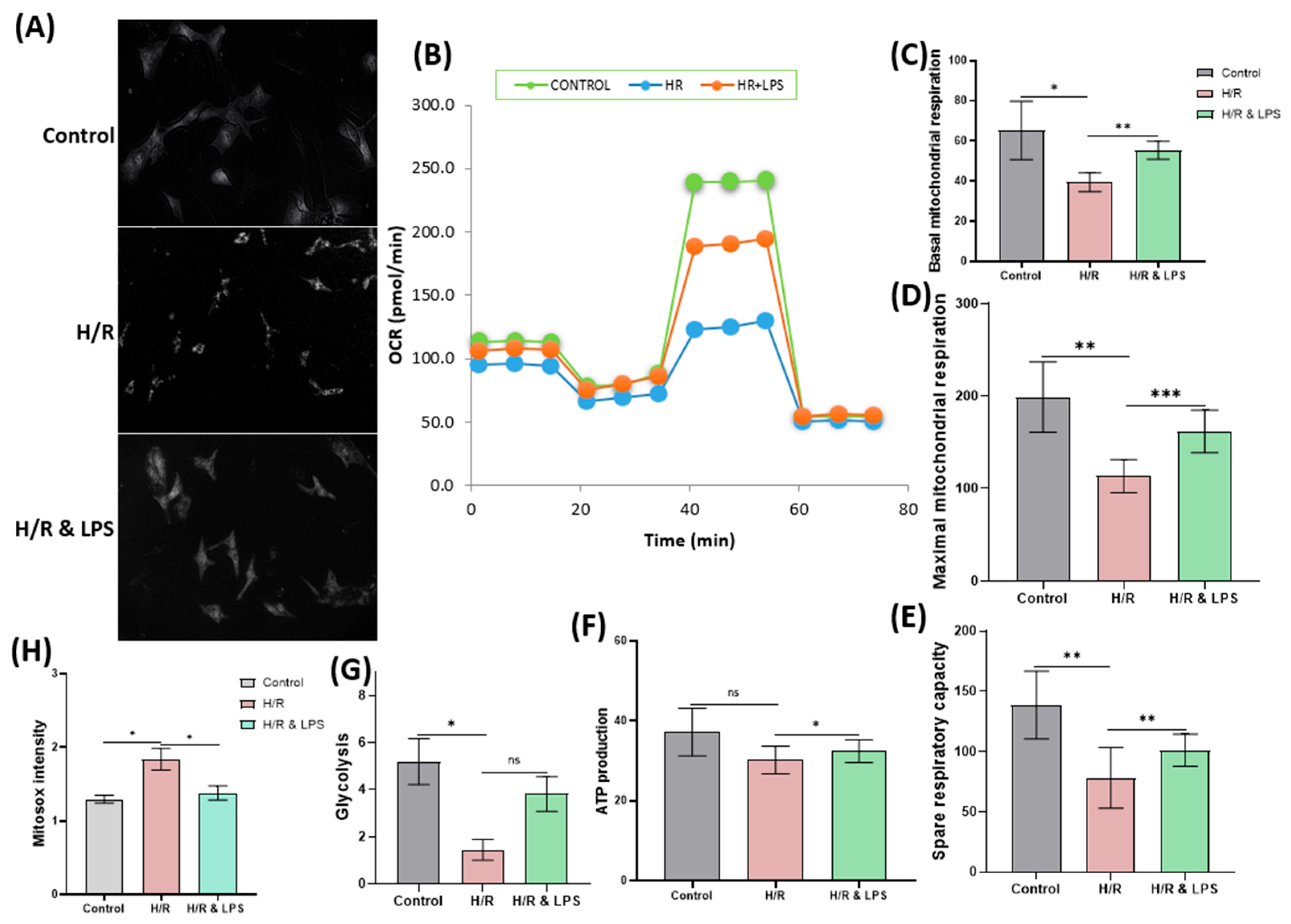
2.8. Endotoxin Pretreatment Reduces Mitochondrial Oxidative Stress and Preserves Cardiac Mitochondrial Respiration Following Hypoxia–Reoxygenation
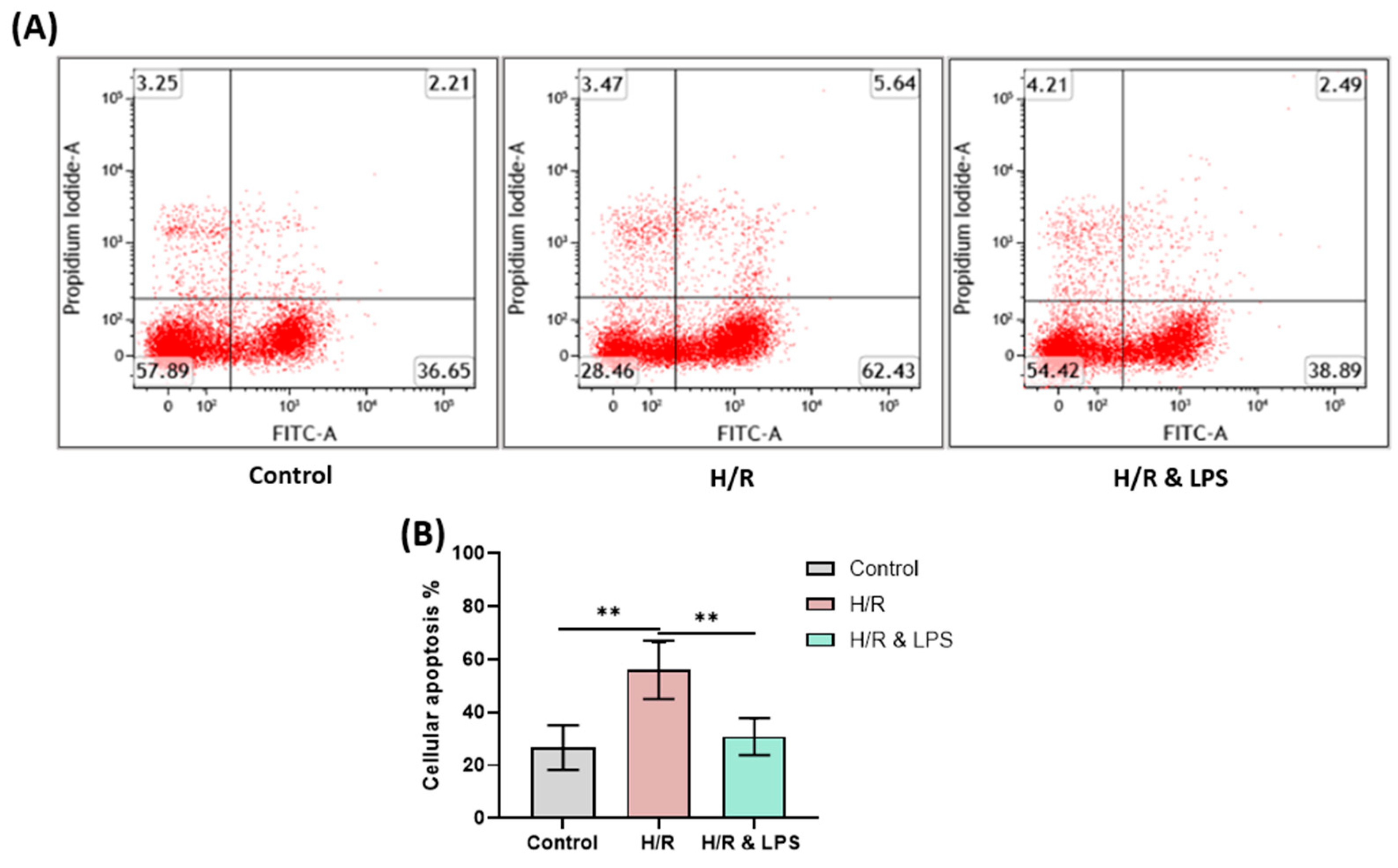
2.9. Endotoxin Pretreatment Reduced Apoptosis Following Hypoxia and Reoxygenation
3. Discussion
Clinical and Translational Implications
4. Materials and Methods
4.1. Animal Preparation
4.2. Experimental Design and Endotoxin Pretreatment
4.3. In Vivo Studies
4.3.1. Ischemia–Reperfusion Surgery
4.3.2. Echocardiography
4.3.3. In Vivo Hemodynamics
4.3.4. Histology
4.4. Ex Vivo Study
Isolated Normothermic Perfused Rat Heart Model
4.5. In Vitro Studies
4.5.1. Primary Culture of Neonatal Rat Cardiomyocytes
4.5.2. Identification of Cultured Cardiomyocytes
4.5.3. Hypoxia–Reoxygenation Protocol for Cultured Cardiomyocytes
4.5.4. Cell Viability Analysis
4.5.5. Immunocytochemical Evaluation of Hypoxia
4.5.6. Western Blot Analysis of Hypoxia-Related Protein Expression
4.5.7. Evaluation of Mitochondrial Respiration
4.5.8. Measurement of Mitochondrial Reactive Oxygen Species (ROS)
4.5.9. Analysis of Cellular Apoptosis
4.5.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Finegold, J.A.; Asaria, P.; Francis, D.P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013, 168, 934–945. [Google Scholar] [CrossRef]
- Task Force on the Management; Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blomstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef]
- Severino, P.; D'Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Bjorkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef]
- Sagris, M.; Apostolos, A.; Theofilis, P.; Ktenopoulos, N.; Katsaros, O.; Tsalamandris, S.; Tsioufis, K.; Toutouzas, K.; Tousoulis, D. Myocardial Ischemia-Reperfusion Injury: Unraveling Pathophysiology, Clinical Manifestations, and Emerging Prevention Strategies. Biomedicines 2024, 12, 802. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, F.; Luan, F.; Chai, Y.; Li, N.; Wang, Y.W.; Chen, Z.L.; Xu, D.Q.; Tang, Y.P. The pathological mechanisms and potential therapeutic drugs for myocardial ischemia reperfusion injury. Phytomedicine 2024, 129, 155649. [Google Scholar] [CrossRef]
- Mastoor, Y.; Murphy, E.; Roman, B. Mechanisms of postischemic cardiac death and protection following myocardial injury. J. Clin. Investig. 2025, 135, e184134. [Google Scholar] [CrossRef] [PubMed]
- Walshe, C.M.; Laffey, J.G.; Kevin, L.; O'Toole, D. Sepsis protects the myocardium and other organs from subsequent ischaemic/reperfusion injury via a MAPK-dependent mechanism. Intensive Care Med. Exp. 2015, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Sato, C.; Mizoguchi, K.; Yamashita, Y.; Oe, M.; Maeta, H. Lipopolysaccharide triggers late preconditioning against myocardial infarction via inducible nitric oxide synthase. Cardiovasc. Res. 2002, 56, 33–42. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, F.; Wang, L.; Zhang, G.; Wang, Z.; Chen, J.; Gao, X. Lipopolysaccharide preconditioning enhances the efficacy of mesenchymal stem cells transplantation in a rat model of acute myocardial infarction. J. Biomed. Sci. 2009, 16, 74. [Google Scholar] [CrossRef]
- Chao, W.; Shen, Y.; Zhu, X.; Zhao, H.; Novikov, M.; Schmidt, U.; Rosenzweig, A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J. Biol. Chem. 2005, 280, 21997–22005. [Google Scholar] [CrossRef]
- Chao, W. Toll-like receptor signaling: A critical modulator of cell survival and ischemic injury in the heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1–H12. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhang, W.; He, J.; Xu, B.; Lei, B.; Wang, Z.; Cates, C.; Rousselle, T.; Li, J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-kappaB pathway. Metabolism 2018, 83, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Hausenloy, D.J.; Andreadou, I.; Horman, S.; Bertrand, L.; Beauloye, C. AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic. Biol. Med. 2021, 166, 238–254. [Google Scholar] [CrossRef]
- Pohjoismaki, J.L.; Goffart, S. The role of mitochondria in cardiac development and protection. Free Radic. Biol. Med. 2017, 106, 345–354. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Sun, Q.; Long, J.; Liu, J.; Ding, J. Mitochondria regulate cardiac contraction through ATP-dependent and independent mechanisms. Free Radic. Res. 2018, 52, 1256–1265. [Google Scholar] [CrossRef]
- Hinton, A., Jr.; Claypool, S.M.; Neikirk, K.; Senoo, N.; Wanjalla, C.N.; Kirabo, A.; Williams, C.R. Mitochondrial Structure and Function in Human Heart Failure. Circ. Res. 2024, 135, 372–396. [Google Scholar] [CrossRef]
- de Carvalho, A.; Bassaneze, V.; Forni, M.F.; Keusseyan, A.A.; Kowaltowski, A.J.; Krieger, J.E. Early Postnatal Cardiomyocyte Proliferation Requires High Oxidative Energy Metabolism. Sci. Rep. 2017, 7, 15434. [Google Scholar] [CrossRef]
- Garcia-Nino, W.R.; Zazueta, C.; Buelna-Chontal, M.; Silva-Palacios, A. Mitochondrial Quality Control in Cardiac-Conditioning Strategies against Ischemia-Reperfusion Injury. Life 2021, 11, 1123. [Google Scholar] [CrossRef]
- Murphy, E.; Liu, J.C. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc. Res. 2023, 119, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Rozich, E.; Ozkurede, U.; Pakkiriswami, S.; Gemilere, R.; Azarin, S.M.; Liu, J.C. Mitochondrial oxidative stress, calcium and dynamics in cardiac ischaemia-reperfusion injury. J. Physiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Grosso, M.A.; Terada, L.S.; Whitman, G.J.; Banerjee, A.; White, C.W.; Harken, A.H.; Repine, J.E. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc. Natl. Acad. Sci. USA 1989, 86, 2516–2520. [Google Scholar] [CrossRef]
- Rowland, R.T.; Meng, X.; Cleveland, J.C., Jr.; Meldrum, D.R.; Harken, A.H.; Brown, J.M. LPS-induced delayed myocardial adaptation enhances acute preconditioning to optimize postischemic cardiac function. Am. J. Physiol. 1997, 272 Pt 2, H2708–H2715. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Y.; Peng, H.Q.; Wen, Z.M.; Ying, Z.Y.; Geng, C.; Wu, J.; Lv, H.Y.; Xu, B. LPS preconditioning of MSC-CM improves protection against hypoxia/reoxygenation-induced damage in H9c2 cells partly via HMGB1/Bach1 signalling. Clin. Exp. Pharmacol. Physiol. 2022, 49, 1319–1333. [Google Scholar] [CrossRef]
- Merry, H.E.; Wolf, P.S.; Fitzsullivan, E.; Keech, J.C.; Mulligan, M.S. Lipopolysaccharide pre-conditioning is protective in lung ischemia-reperfusion injury. J. Heart Lung Transplant. 2010, 29, 471–478. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47 (Suppl. S8), C7–C12. [Google Scholar] [CrossRef]
- Mallat, Z.; Binder, C.J. The why and how of adaptive immune responses in ischemic cardiovascular disease. Nat. Cardiovasc. Res. 2022, 1, 431–444. [Google Scholar] [CrossRef]
- Weissman, D.; Maack, C. Mitochondrial function in macrophages controls cardiac repair after myocardial infarction. J. Clin. Investig. 2023, 133, e167079. [Google Scholar] [CrossRef]
- Wang, Y.; Abarbanell, A.M.; Herrmann, J.L.; Weil, B.R.; Poynter, J.; Manukyan, M.C.; Crisostomo, P.R.; Meldrum, D.R. Toll-like receptor signaling pathways and the evidence linking toll-like receptor signaling to cardiac ischemia/reperfusion injury. Shock 2010, 34, 548–557. [Google Scholar] [CrossRef]
- Ha, T.; Liu, L.; Kelley, J.; Kao, R.; Williams, D.; Li, C. Toll-like receptors: New players in myocardial ischemia/reperfusion injury. Antioxid. Redox Signal 2011, 15, 1875–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Amrute, J.; Kovacs, A.; Diwan, A.; Williams, D.L.; Mann, D.L. Lipopolysaccharide Induces Trained Innate Immune Tolerance in the Heart Through Interferon Signaling in a Model of Stress-Induced Cardiomyopathy. bioRxiv 2024. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Senderovic, A.; Galijasevic, S. The Role of Inducible Nitric Oxide Synthase in Assessing the Functional Level of Coronary Artery Lesions in Chronic Coronary Syndrome. Cardiol. Res. 2024, 15, 330–339. [Google Scholar] [CrossRef]
- Chen, E.; Chen, C.; Niu, Z.; Gan, L.; Wang, Q.; Li, M.; Cai, X.; Gao, R.; Katakam, S.; Chen, H.; et al. Poly(I:C) preconditioning protects the heart against myocardial ischemia/reperfusion injury through TLR3/PI3K/Akt-dependent pathway. Signal Transduct. Target. Ther. 2020, 5, 216. [Google Scholar] [CrossRef]
- Deng, R.M.; Zhou, J. The role of PI3K/AKT signaling pathway in myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 2023, 123, 110714. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Richardson, A.P. The mitochondrial permeability transition: A current perspective on its identity and role in ischaemia/reperfusion injury. J. Mol. Cell Cardiol. 2015, 78, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef] [PubMed]
- Morciano, G.; Pinton, P. Modulation of mitochondrial permeability transition pores in reperfusion injury: Mechanisms and therapeutic approaches. Eur. J. Clin. Investig. 2025, 55, e14331. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Bolli, R.; Canty, J.M., Jr.; Du, X.J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef]
- Hou, Y. Improvements in the Establishment of a Rat Myocardial Infarction Model. J. Int. Med. Res. 2011, 3.9, 1284–1292. [Google Scholar] [CrossRef]
- Cave, A.C.; Hearse, D.J. Ischemic preconditioning and contractile function_studies with normothermic and hypothermic global ischemia. J. Mol. Cell. Cardiol. 1992, 24, 1113–1123. [Google Scholar] [CrossRef]
- Nader, N.D.; Asgeri, M.; Davari-Farid, S.; Pourafkari, L.; Ahmadpour, F.; Porhomayon, J.; Javadzadeghan, H.; Negargar, S.; Knight, P.R., 3rd. The Effect of Lipopolysaccharide on Ischemic-Reperfusion Injury of Heart: A Double Hit Model of Myocardial Ischemia and Endotoxemia. J. Cardiovasc. Thorac. Res. 2015, 7, 81–86. [Google Scholar] [CrossRef]
- Qiu, Z.; He, Y.; Ming, H.; Lei, S.; Leng, Y.; Xia, Z.Y. Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes. J. Diabetes Res. 2019, 2019, 8151836. [Google Scholar] [CrossRef]
- Qiao, S.; Xie, H.; Wang, C.; Wu, X.; Liu, H.; Liu, C. Delayed anesthetic preconditioning protects against myocardial infarction via activation of nuclear factor-kappaB and upregulation of autophagy. J. Anesth. 2013, 27, 251–260. [Google Scholar] [CrossRef]
- Zhang, S.Z. Isoflurane reduces endotoxin-induced oxidative, inflammatory, and apoptotic responses in H9c2 cardiomyocytes. Eur. Rev. Med. Pharmacol Sci. 2018, 22, 3976–3987. [Google Scholar] [PubMed]
- McDonald, T.E.; Grinman, M.N.; Carthy, C.M.; Walley, K.R. Endotoxin infusion in rats induces apoptotic and survival pathways in hearts. Am. J. Physiol. Heart. Circ. Physiol. 2000, 279, H2053–H2061. [Google Scholar] [CrossRef]
- Chagnon, F.; Metz, C.N.; Bucala, R.; Lesur, O. Endotoxin-induced myocardial dysfunction: Effects of macrophage migration inhibitory factor neutralization. Circ. Res. 2005, 96, 1095–1102. [Google Scholar] [CrossRef]
- McDonough, K.H.; Virag, J.I. Sepsis-induced myocardial dysfunction and myocardial protection from ischemia/reperfusion injury. Front. Biosci. 2006, 11, 23–32. [Google Scholar] [CrossRef]
- Balija, T.M.; Lowry, S.F. Lipopolysaccharide and sepsis-associated myocardial dysfunction. Curr. Opin. Infect. Dis. 2011, 24, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour, A.; Azar, A.D.; Tavakoli, F.; Moladoust, H.; Zare, A. Echocardiographic evaluation of cardiac function in ischemic rats: Value of m-mode echocardiography. Res. Cardiovasc. Med. 2014, 3, e22941. [Google Scholar] [CrossRef]
- Tachibana, S. Analyzing Oxygen Consumption Rate in Primary Cultured Mouse Neonatal Cardiomyocytes Using an Extracellular Flux Analyzer. J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Cho, H.J.; Hong, H.; Kim, D.W.; Lee, K.S.; Han, H.S.; Kim, G.H.; Choi, K.S.; Kim, Y.S.; Kayumov, M.; Ki, K.K.; et al. Viability of Mesenchymal Stem Cells in an Ex Vivo Circulation System. ASAIO J. 2020, 66, 433–440. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habimana, R.; Seong, J.; Jo, Y.; Kim, R.-H.; Kim, H.-J.; Choi, K.S.; Kayumov, M.; Obiweluozor, F.O.; Kim, W.-I.; Cho, H.J.; et al. Endotoxin Pretreatment Mitigates Myocardial Ischemia-Reperfusion Injury Through Preservation of Mitochondrial Respiration: A Combined Assessment of In Vivo, Ex Vivo, and In Vitro Data. Int. J. Mol. Sci. 2025, 26, 11162. https://doi.org/10.3390/ijms262211162
Habimana R, Seong J, Jo Y, Kim R-H, Kim H-J, Choi KS, Kayumov M, Obiweluozor FO, Kim W-I, Cho HJ, et al. Endotoxin Pretreatment Mitigates Myocardial Ischemia-Reperfusion Injury Through Preservation of Mitochondrial Respiration: A Combined Assessment of In Vivo, Ex Vivo, and In Vitro Data. International Journal of Molecular Sciences. 2025; 26(22):11162. https://doi.org/10.3390/ijms262211162
Chicago/Turabian StyleHabimana, Reverien, Jiae Seong, YeongEun Jo, Ryul-Hee Kim, Hyo-Jung Kim, Kyung Soon Choi, Mukhammad Kayumov, Francis O. Obiweluozor, Wang-In Kim, Hwa Jin Cho, and et al. 2025. "Endotoxin Pretreatment Mitigates Myocardial Ischemia-Reperfusion Injury Through Preservation of Mitochondrial Respiration: A Combined Assessment of In Vivo, Ex Vivo, and In Vitro Data" International Journal of Molecular Sciences 26, no. 22: 11162. https://doi.org/10.3390/ijms262211162
APA StyleHabimana, R., Seong, J., Jo, Y., Kim, R.-H., Kim, H.-J., Choi, K. S., Kayumov, M., Obiweluozor, F. O., Kim, W.-I., Cho, H. J., Kim, D., Na, K. J., & Jeong, I. (2025). Endotoxin Pretreatment Mitigates Myocardial Ischemia-Reperfusion Injury Through Preservation of Mitochondrial Respiration: A Combined Assessment of In Vivo, Ex Vivo, and In Vitro Data. International Journal of Molecular Sciences, 26(22), 11162. https://doi.org/10.3390/ijms262211162




