Preventive Effects of Eclipta prostrata and Hordeum vulgare Extract Complex on Precocious Puberty in Danazol- and High-Fat Diet-Induced Rat Models
Abstract
1. Introduction
2. Results
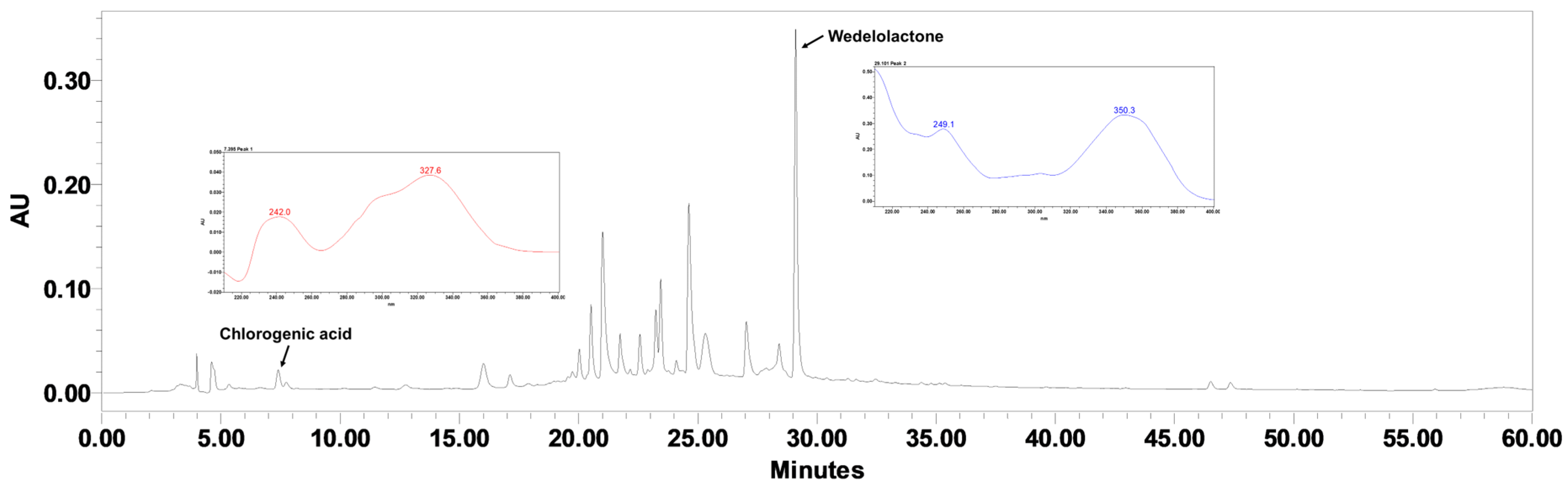
2.1. Quantification of Chlorogenic Acid and Wedelolactone in EHEC
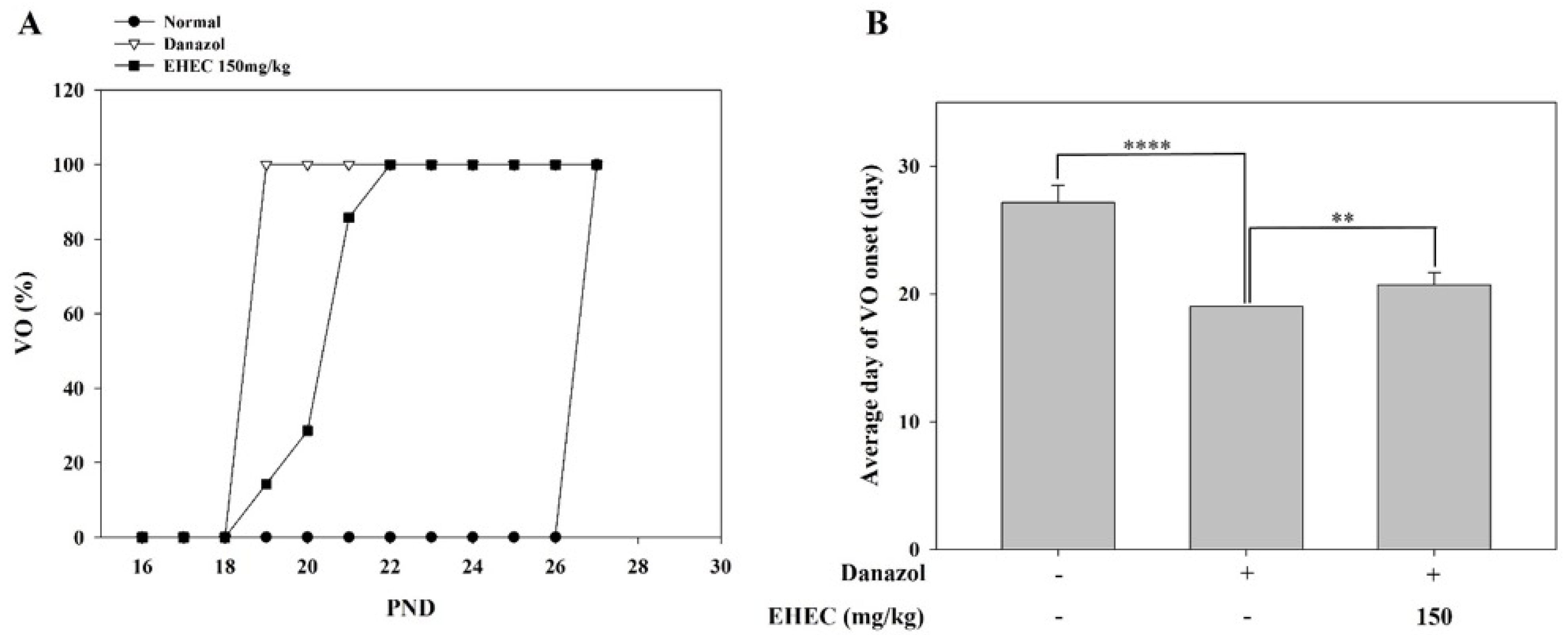
2.2. Effects of EHEC on Vaginal Opening Delay in Danazol-Induced Precocious Puberty Rats
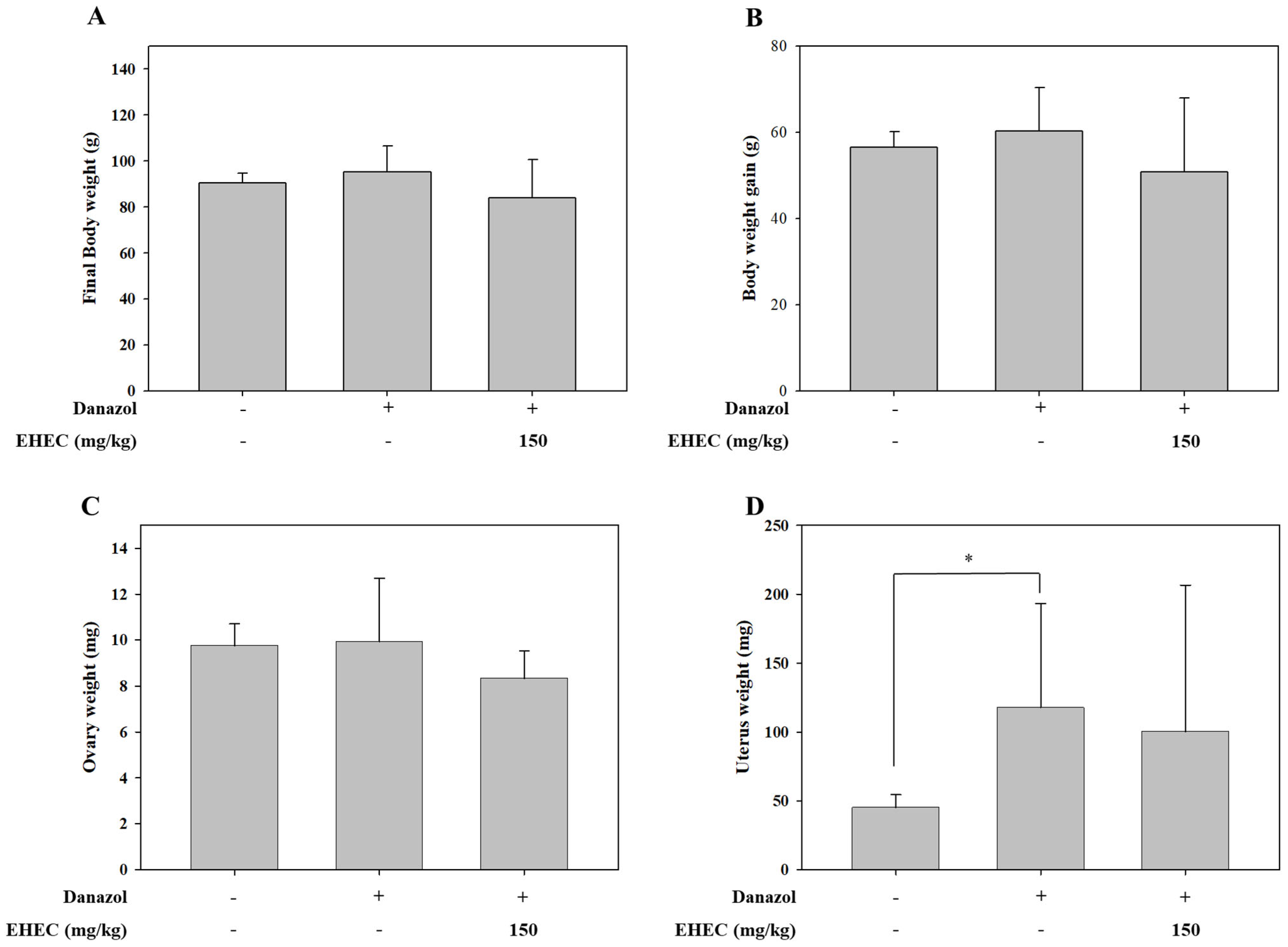
2.3. Effects of EHEC on Body, Uterine, and Ovarian Weights in a Danazol-Induced Precocious Puberty Model
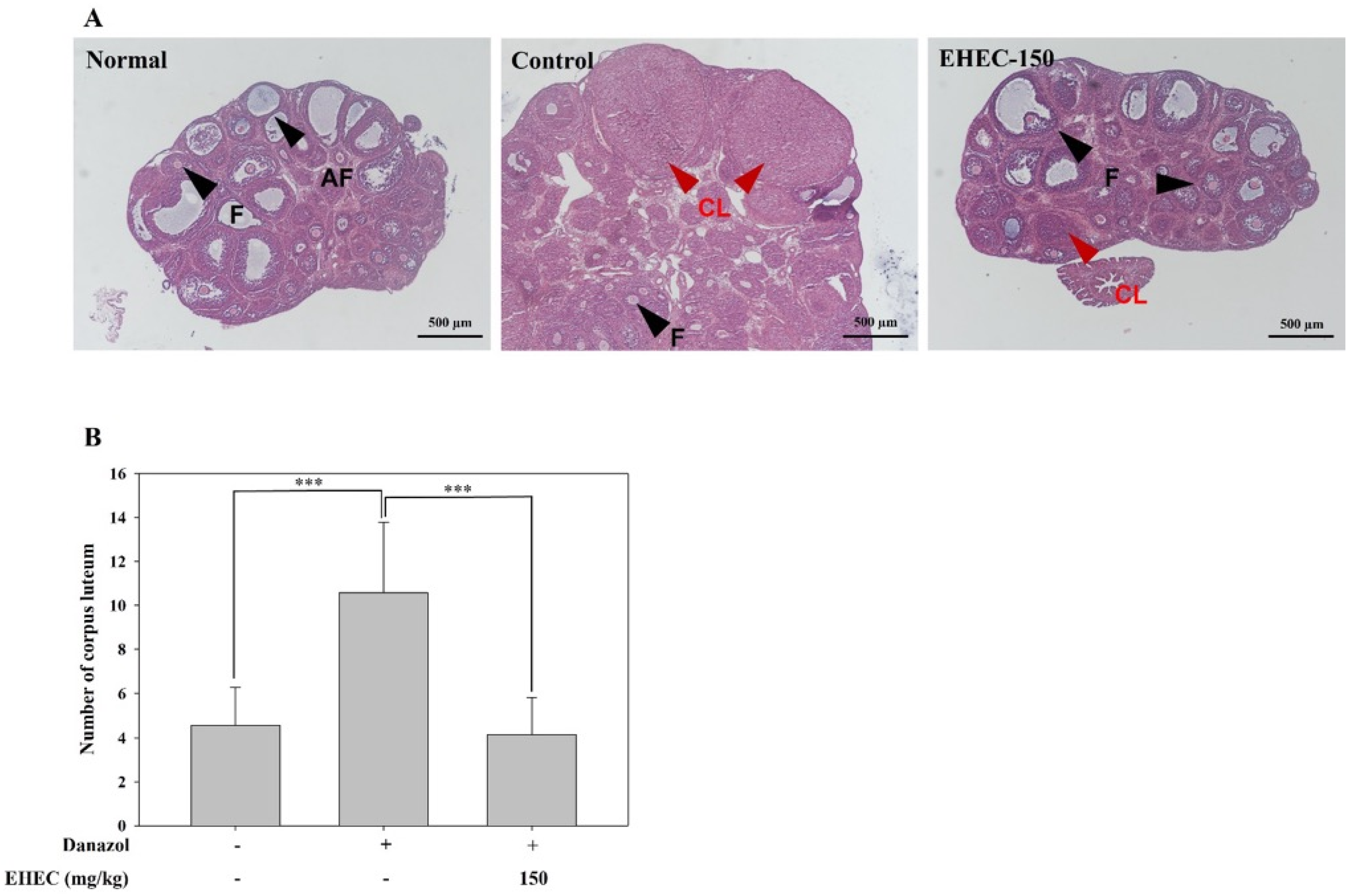
2.4. Influence of EHEC on Ovarian Follicle Formation in the Danazol-Induced Precocious Puberty Model
2.5. Effects of EHEC on GnRH and GnRHR Gene Expression in the Danazol-Induced Precocious Puberty Model
2.6. Effect of EHEC on Delaying Vaginal Opening in Rats with High-Fat Diet-Induced Precocious Puberty
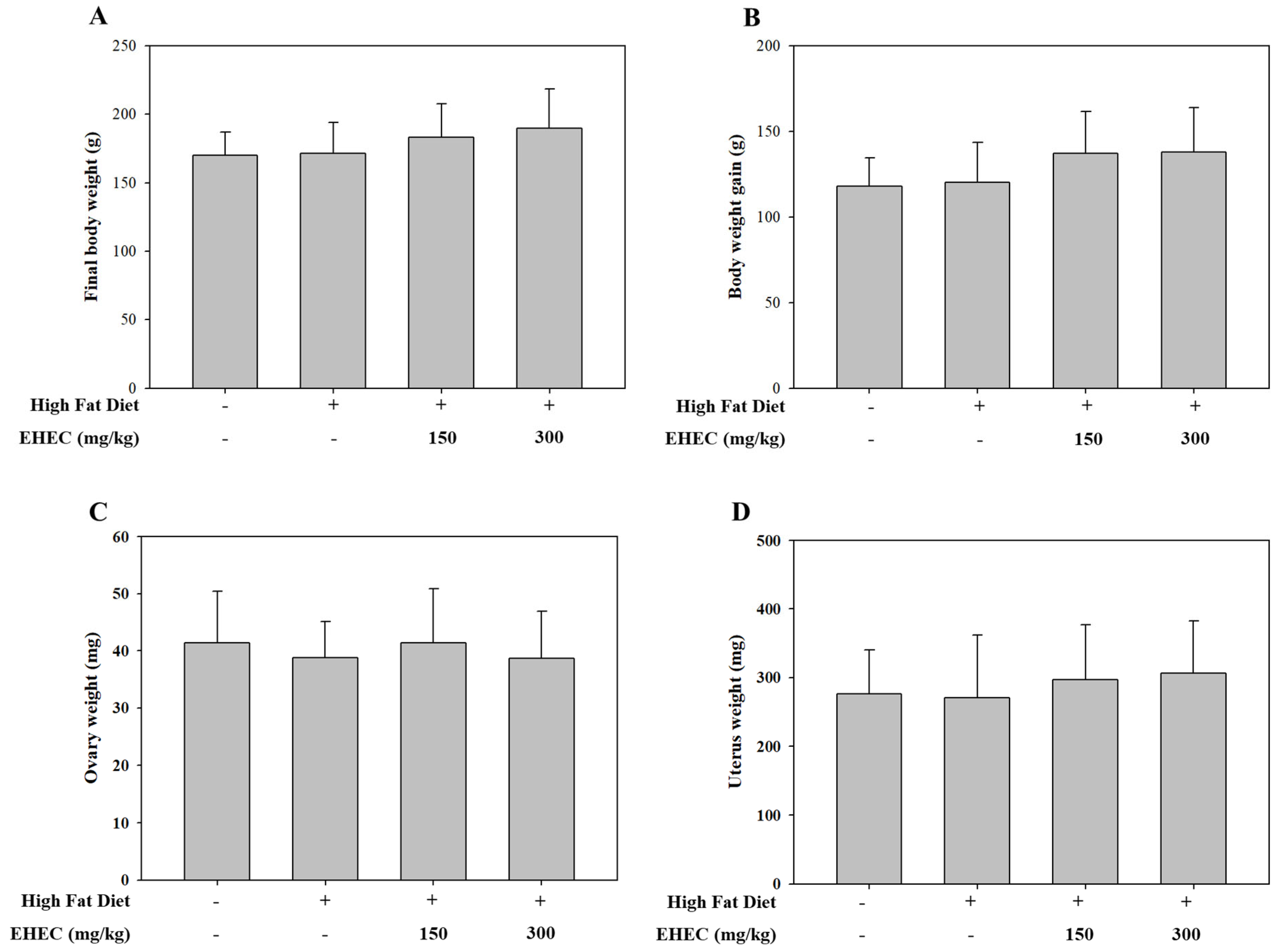
2.7. Effects of EHEC on Body, Uterine, and Ovarian Weights in a HFD-Induced Precocious Puberty Model
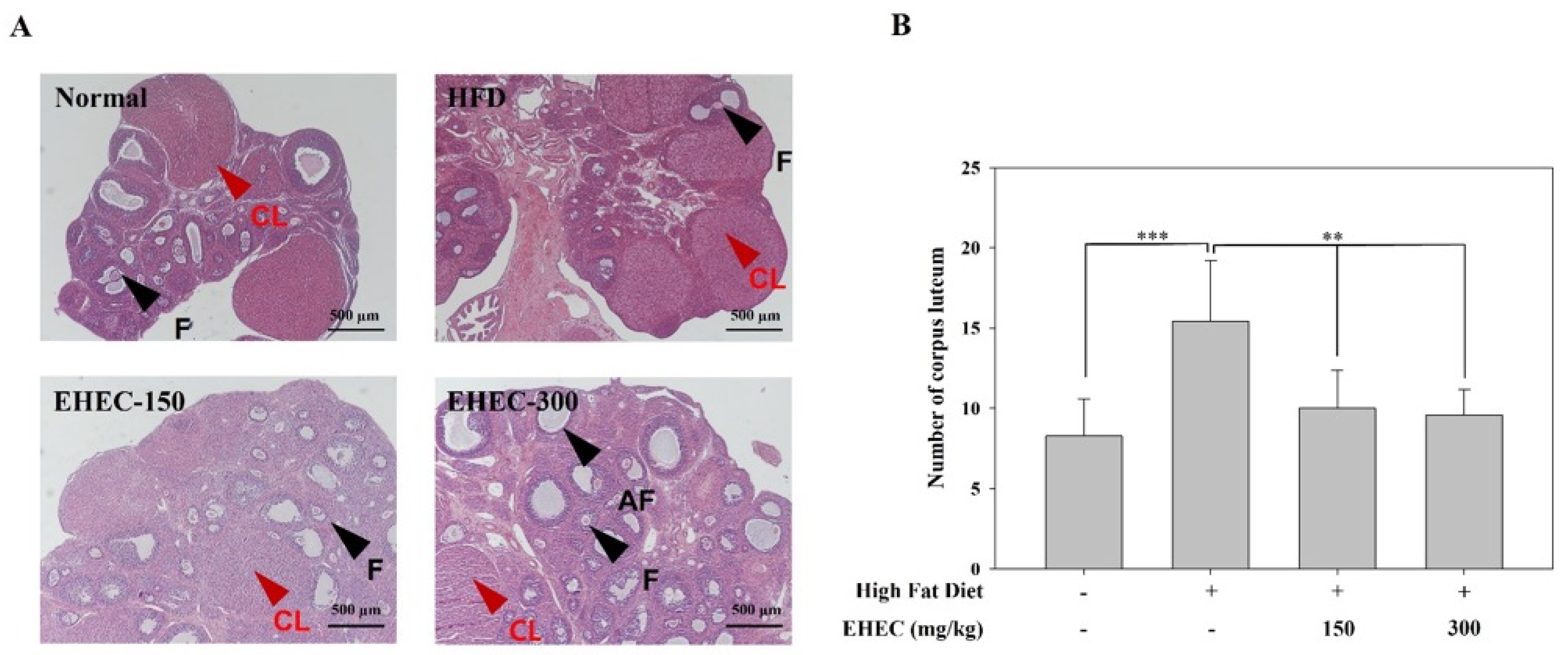
2.8. Influence of EHEC on Ovarian Follicle Formation in the HFD-Induced Precocious Puberty Model
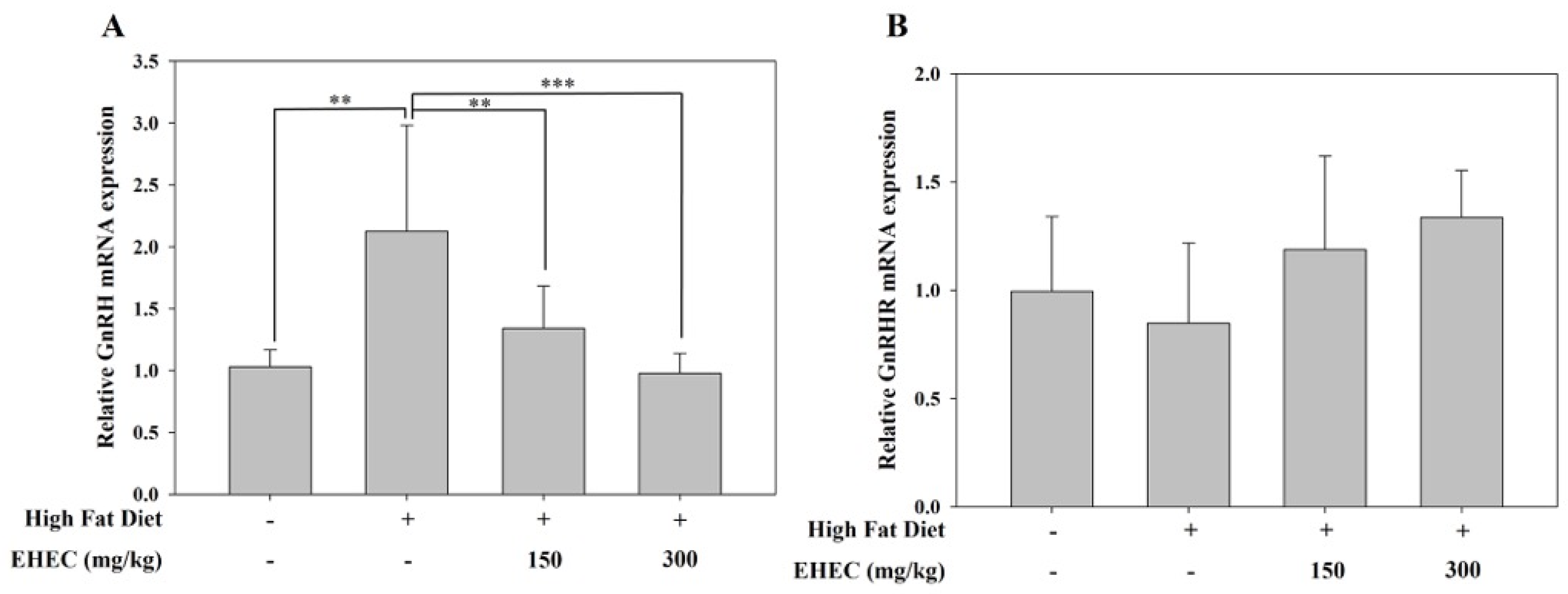
2.9. Influence of EHEC on GnRH and GnRHR Gene Expression in the HF Diet Model
3. Discussion
4. Materials and Methods
4.1. Preparation of E. prostrata and H. vulgare Mixture
4.2. High-Performance Liquid Chromatography (HPLC) Analysis
4.3. Animals, Housing, and Ethical Approval
4.4. Danazol-Induced Precocious Puberty Model Design
Monitoring Vaginal Opening and Estrous Cycle in Danazol-Induced Precocious Puberty Model
4.5. High-Fat Diet-Induced Precocious Puberty Model Design
Monitoring Vaginal Opening and Estrous Cycle in HFD-Induced Precocious Puberty Model
4.6. Euthanasia Procedure
4.7. Organ Collection and Analysis
4.8. Histological Preparation and Staining of Ovarian Tissue
4.9. RNA Extraction and mRNA Expression Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Lenzi, A.; Radicioni, A.F. Hypothalamo-Pituitary axis and puberty. Mol. Cell. Endocrinol. 2021, 520, 111094. [Google Scholar] [CrossRef]
- Romeo, R.D. Pubertal maturation and programming of hypothalamic–pituitary–adrenal reactivity. Front. Neuroendocrinol. 2010, 31, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.P.; Kaiser, U.B. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016, 4, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Delemarre-van de Waal, H.A. Regulation of puberty. Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Meethal, S.V.; Atwood, C.S. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell. Mol. Life Sci. 2005, 62, 257–270. [Google Scholar]
- Terasawa, E.; Fernandez, D.L. Neurobiological mechanisms of the onset of puberty in primates. Endocr. Rev. 2001, 22, 111–151. [Google Scholar]
- Radovick, S.; Levine, J.E.; Wolfe, A. Estrogenic regulation of the GnRH neuron. Front. Endocrinol. 2012, 3, 52. [Google Scholar] [CrossRef]
- Pawson, A.J.; McNeilly, A.S. The pituitary effects of GnRH. Anim. Reprod. Sci. 2005, 88, 75–94. [Google Scholar] [CrossRef]
- Cheuiche, A.V.; da Silveira, L.G.; de Paula, L.C.P.; Lucena, I.R.S.; Silveiro, S.P. Diagnosis and management of precocious sexual maturation: An updated review. Eur. J. Pediatr. 2021, 180, 3073–3087. [Google Scholar] [CrossRef]
- Carel, J.-C.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Palmert, M.R.; Antoniazzi, F.; Berenbaum, S.; Bourguignon, J.-P.; Chrousos, G.P.; Coste, J.; et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef]
- Parent, A.-S.; Teilmann, G.; Juul, A.; Skakkebaek, N.E.; Toppari, J.; Bourguignon, J.-P. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr. Rev. 2003, 24, 668–693. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Moscogiuri, L.A.; Chiarito, M.; De Santis, S.; Giordano, P. Genetic, epigenetic and enviromental influencing factors on the regulation of precocious and delayed puberty. Front. Endocrinol. 2022, 13, 1019468. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, Y.; Yang, Z.; Gong, Z.; Zhang, S.; Liu, X.; Chen, Y.; Ye, C.; Chen, L.; Wang, T. Overweight/obesity in childhood and the risk of early puberty: A systematic review and meta-analysis. Front. Pediatr. 2022, 10, 795596. [Google Scholar] [CrossRef]
- Aghaee, S.; Deardorff, J.; Quesenberry, C.P.; Greenspan, L.C.; Kushi, L.H.; Kubo, A. Associations between childhood obesity and pubertal timing stratified by sex and race/ethnicity. Am. J. Epidemiol. 2022, 191, 2026–2036. [Google Scholar] [CrossRef]
- Alghamdi, A.; Alghamdi, A.H. Precocious puberty: Types, pathogenesis and updated management. Cureus 2023, 15, e47485. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, J.S. Treatment and outcomes of precocious puberty: An update. J. Clin. Endocrinol. Metab. 2013, 98, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Christine, L.; Pierre, B. Central and peripheral isosexual precocious puberty. Curr. Opin. Endocrinol. Diabetes 2003, 18, 17–22. [Google Scholar]
- Zevin, E.L.; Eugster, E.A. Central precocious puberty: A review of diagnosis, treatment, and outcomes. Lancet Child Adolesc. Health 2023, 7, 886–896. [Google Scholar] [CrossRef]
- Guaraldi, F.; Beccuti, G.; Gori, D.; Ghizzoni, L. Management of endocrine disease: Long-term outcomes of the treatment of central precocious puberty. Eur. J. Endocrinol. 2016, 174, R79–R87. [Google Scholar] [CrossRef]
- Soriano-Guillén, L.; Argente, J. Central precocious puberty, functional and tumor-related. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101262. [Google Scholar] [CrossRef]
- Laube, C.; Fuhrmann, D. Is early good or bad? Early puberty onset and its consequences for learning. Curr. Opin. Behav. Sci. 2020, 36, 150–156. [Google Scholar] [CrossRef]
- Mul, D.; Hughes, I. The use of GnRH agonists in precocious puberty. Eur. J. Endocrinol. 2008, 159 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef]
- Soliman, A.; Alaaraj, N.; De Sanctis, V.; Alyafei, F.; Ahmed, S.; Hamed, N. Long-term health consequences of central precocious/early puberty (CPP) and treatment with Gn-RH analogue: A short update. Acta Biomed. 2023, 94, e2023222. [Google Scholar]
- Lee, Y.B.; Lee, J.A.; Lee, H.L. Herbal medicine for idiopathic central precocious puberty: A systematic review and meta-analysis. J. Altern. Complement. Med. 2020, 26, 976–999. [Google Scholar] [CrossRef]
- Lee, H.L.; Lee, Y.B.; Choi, J.-Y.; Lee, J.A. Herbal medicine for idiopathic central precocious puberty: A protocol for a systematic review of controlled trials. Medicine 2018, 97, e0267. [Google Scholar] [CrossRef]
- Son, H.-E.; Kim, Y.-S.; Kim, Y.; Na, S.; Kim, H. Review of non-clinical experimental studies on precocious puberty using herbal medicine. Herb. Formula Sci. 2023, 31, 373–388. [Google Scholar]
- Bai, G.-L.; Hu, K.-L.; Huan, Y.; Wang, X.; Lei, L.; Zhang, M.; Guo, C.-Y.; Chang, H.-S.; Zhao, L.-B.; Liu, J. The traditional Chinese medicine fuyou formula alleviates precocious puberty by inhibiting GPR54/GnRH in the hypothalamus. Front. Pharmacol. 2021, 11, 596525. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Trinh, T.A.; Lee, W.-Y.; Baek, J.Y.; Lee, S.; Choi, K.; Ha, J.; Kim, C.-E.; Kang, K.S.; Lee, H.L. Effects of estrogen inhibition formula herbal mixture for danazol-induced precocious puberty in female rats: An experimental study with network pharmacology. Integr. Med. Res. 2021, 10, 100708. [Google Scholar] [CrossRef]
- Yin, W.; Li, S.; Zhang, K.; Xu, Y.; Ma, D.; Shi, T.; Xiong, L.; Xia, J. The Therapeutic Effect of Shugan Xiehuo Formula in the Female Rat Model with Central Precocious Puberty. Evid.-Based Complement. Altern. Med. 2020, 2020, 5916168. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Han, X.; Sun, W.; Yu, J.; Tamadon, A. Precocious puberty and the Lin28/Let7 pathway: The therapeutic effect of the nourishing “Yin” and purging “Fire” traditional chinese medicine mixture in a rat model. Evid.-Based Complement. Altern. Med. 2018, 2018, 4868045. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, S.C.; Oh, J.; Kim, C.-E.; Kang, K.S.; Yoo, H.S.; Lee, H.L. Preventive effect and safety of a follicle stimulating hormone inhibitory formulation containing a mixture of Coicis semen and Artemisia capillaris for precocious puberty: A preliminary experimental study using female rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 2906014. [Google Scholar] [CrossRef]
- Sun, Y.; Perry, G.N.; Yu, J.; Chen, B.; Tian, Z. Effect of nourishing “Yin”-removing “Fire” Chinese herbal mixture on hypothalamic kisspeptin expression in female precocious rats. J. Ethnopharmacol. 2010, 127, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhai, Y.-Y.; Xu, J.; Yao, W.-F.; Cao, Y.-D.; Cheng, F.-F.; Bao, B.-H.; Zhang, L. A review on traditional uses, phytochemistry and pharmacology of Eclipta prostrata (L.) L. J. Ethnopharmacol. 2019, 245, 112109. [Google Scholar] [CrossRef] [PubMed]
- Timalsina, D.; Devkota, H.P. Eclipta prostrata (L.) L.(Asteraceae): Ethnomedicinal uses, chemical constituents, and biological activities. Biomolecules 2021, 11, 1738. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Singh, J.P.; Tripathi, A.; Srivastava, S.; Chaurasia, V.K.; Kumar, R.; Tiwari, S.; Pandey, S. A Review on the Pharmacological, Biological, Chemical and Therapeutic Value of Eclipta Prostrate (Bhringraj Plant). Biochem. Cell. Arch. 2024, 24. [Google Scholar] [CrossRef]
- Kaur, A.; Purewal, S.S.; Phimolsiripol, Y.; Punia Bangar, S. Unraveling the hidden potential of Barley (Hordeum vulgare): An important review. Plants 2024, 13, 2421. [Google Scholar] [CrossRef]
- Boanta, E.-A.; Muntean, L.; Russu, F.; Ona, A.D.; Porumb, I.; Filip, E. Barley (Hordeum vulgare L.): Medicinal and therapeutic uses-review. Hop Med. Plants 2019, 2019, 87–95. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, J.K.; Kumar, S.; Chopra, H.; Kumar, S.; Chanchal, D.K.; Singh, T.; Chaudhary, R.; Garg, A.; Saha, S. Pharmacological and therapeutic potential of Hordeum vulgare. Pharmacol. Res.-Mod. Chin. Med. 2023, 8, 100300. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Ma, Y.; Huang, M.; Qiu, T.; Bian, H.; Han, N.; Wang, J. Function, mechanism, and application of plant melatonin: An update with a focus on the cereal crop, barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634. [Google Scholar] [CrossRef]
- Şoica, C.; Voicu, M.; Ghiulai, R.; Dehelean, C.; Racoviceanu, R.; Trandafirescu, C.; Roșca, O.-J.; Nistor, G.; Mioc, M.; Mioc, A. Natural compounds in sex hormone-dependent cancers: The role of triterpenes as therapeutic agents. Front. Endocrinol. 2021, 11, 612396. [Google Scholar] [CrossRef]
- Wahman, L.F.; Abd Rabo, M.M.; Elgoly, A.H.M.; Yousef, M.H. Potential role of plants Hordeum vulgare L. and Panax ginseng L. in resolving the fertility disorders and stress-induced oxidative stress arises from hypothyroidism in adult female rats. In Plant Stress Physiology; IntechOpen: London, UK, 2020. [Google Scholar]
- Carel, J.-C.; Léger, J. Precocious puberty. N. Engl. J. Med. 2008, 358, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Eckert-Lind, C.; Busch, A.S.; Petersen, J.H.; Biro, F.M.; Butler, G.; Bräuner, E.V.; Juul, A. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: A systematic review and meta-analysis. JAMA Pediatr. 2020, 174, e195881. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, M.J.; Kim, J.M.; Yuk, J.-S.; Kim, S.-H. Ongoing increasing trends in central precocious puberty incidence among Korean boys and girls from 2008 to 2020. PLoS ONE 2023, 18, e0283510. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-X.; Zhao, F.-Y.; Gu, K.-R.; Wang, G.-P.; Zhang, J.; Tao, R.; Yuan, J.; Gu, J.; Yu, J.-Q. Development of precocious puberty in children: Surmised medicinal plant treatment. Biomed. Pharmacother. 2022, 156, 113907. [Google Scholar] [CrossRef]
- Smith, C.; Harris, F. The role of danazol in the management of precocious puberty. Postgrad. Med. J. 1979, 55, 81–86. [Google Scholar]
- Morishita, H.; Takemoto, M.; Kondo, H.; Higuchi, K.; Aono, T. Induction of true precocious puberty by neonatal treatment with danazol in female rats. Neurosci. Lett. 1993, 157, 33–36. [Google Scholar] [CrossRef]
- Zha, Z.; Lei, E.; Wu, X.; Bai, S.; Huang, T.; Lu, T.; Wang, Z.; Cai, Y.; Li, H.; Chen, Y. Therapeutic potential of luteolin in central precocious puberty: Insights from a danazol-induced rat model. Front. Endocrinol. 2025, 16, 1666932. [Google Scholar] [CrossRef]
- Kim, K.R.; Trinh, T.A.; Baek, J.Y.; Lee, D.; Lim, S.; Kim, J.; Lee, W.-Y.; Kim, C.-E.; Kang, K.S.; Lee, H.L. Preventive effect of Anemarrhenae rhizome and Phellodendri cortex on Danazol-induced in precocious puberty in female rats and network pharmacological analysis of active compounds. Plants 2021, 11, 23. [Google Scholar] [CrossRef]
- Nieuwenhuis, D.; Pujol--Gualdo, N.; Arnoldussen, I.A.; Kiliaan, A.J. Adipokines: A gear shift in puberty. Obes. Rev. 2020, 21, e13005. [Google Scholar] [CrossRef]
- Lee, H.S.; Yoon, J.S.; Hwang, J.S. Luteinizing hormone secretion during gonadotropin-releasing hormone stimulation tests in obese girls with central precocious puberty. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 392. [Google Scholar] [CrossRef]
- Dudek, M.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Sassek, M.; Ziarniak, K.; Nowak, K.; Sliwowska, J. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic–pituitary–gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides 2016, 56, 41–49. [Google Scholar] [CrossRef]
- Ullah, R.; Su, Y.; Shen, Y.; Li, C.; Xu, X.; Zhang, J.; Huang, K.; Rauf, N.; He, Y.; Cheng, J. Postnatal feeding with high-fat diet induces obesity and precocious puberty in C57BL/6J mouse pups: A novel model of obesity and puberty. Front. Med. 2017, 11, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, N.; Zhang, M.; Ding, Q.; Wang, Q.; Liang, Y.; He, H.; Yang, Y.; Guo, C. Effects of Fuyou formula on GnRH secretion and related gene expression in treating precocious puberty. Front. Pharmacol. 2022, 13, 852550. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, X.; Xie, L.; Yao, X.; Qian, L.; Yu, Z.; Shen, X. Green tea catechin EGCG could prevent obesity-related precocious puberty through NKB/NK3R signaling pathway. J. Nutr. Biochem. 2022, 108, 109085. [Google Scholar] [CrossRef] [PubMed]
- Janjic, M.M.; Stojilkovic, S.S.; Bjelobaba, I. Intrinsic and regulated gonadotropin-releasing hormone receptor gene transcription in mammalian pituitary gonadotrophs. Front. Endocrinol. 2017, 8, 221. [Google Scholar] [CrossRef]
- Herbison, A.E. The gonadotropin-releasing hormone pulse generator. Endocrinology 2018, 159, 3723–3736. [Google Scholar] [CrossRef]
- Howles, C.M. Role of LH and FSH in ovarian function. Mol. Cell. Endocrinol. 2000, 161, 25–30. [Google Scholar] [CrossRef]
- Kang, H.; Chen, R. Hydroxytyrosol mitigates high-fat diet-induced precocious puberty in rats through gut microbiome remodeling. Endocr. Abstr. 2025, 110, OC10.6. [Google Scholar] [CrossRef]
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, H.; Shang, Y. The effectiveness and safety of Chinese herbal medicine in infertile women with luteal phase deficiency: A systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 2492502. [Google Scholar] [CrossRef]
- Cai, H.; Wu, Y.; Zhang, X. A comprehensive review on wedelolactone: Natural sources, total synthesis, and pharmacological activities. Chin. J. Nat. Med. 2025, 23, 169–181. [Google Scholar] [CrossRef]
- Annie, S.; Prabhu, R.; Malini, S. Activity of Wedelia calendulacea Less. in post-menopausal osteoporosis. Phytomedicine 2006, 13, 43–48. [Google Scholar] [CrossRef]
- Eom, T.; Kim, I.-H.; Kim, H.-J.; Choi, Y.; Nam, T.-J. Calystegia soldanella extract exerts anti-oxidative and anti-inflammatory effects via the regulation of the NF-κB/Nrf-2 pathways in mouse macrophages. Antioxidants 2021, 10, 1639. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, C.; Wang, Z. Therapeutic effects and molecular mechanism of chlorogenic acid on polycystic ovarian syndrome: Role of HIF-1alpha. Nutrients 2023, 15, 2833. [Google Scholar] [CrossRef]
- Abedpour, N.; Javanmard, M.Z.; Karimipour, M.; Liqvan, A.P. Effect of chlorogenic acid on follicular development, hormonal status and biomarkers of oxidative stress in rats with polycystic ovary syndrome. Vet. Res. Forum 2022, 12, 513–520. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Eom, T.; Kim, Y.; Rhee, J.; Kim, H. Preventive Effects of Eclipta prostrata and Hordeum vulgare Extract Complex on Precocious Puberty in Danazol- and High-Fat Diet-Induced Rat Models. Int. J. Mol. Sci. 2025, 26, 11158. https://doi.org/10.3390/ijms262211158
Kim Y-S, Eom T, Kim Y, Rhee J, Kim H. Preventive Effects of Eclipta prostrata and Hordeum vulgare Extract Complex on Precocious Puberty in Danazol- and High-Fat Diet-Induced Rat Models. International Journal of Molecular Sciences. 2025; 26(22):11158. https://doi.org/10.3390/ijms262211158
Chicago/Turabian StyleKim, Young-Sik, Taekil Eom, Yongbin Kim, Jinhui Rhee, and Hongjun Kim. 2025. "Preventive Effects of Eclipta prostrata and Hordeum vulgare Extract Complex on Precocious Puberty in Danazol- and High-Fat Diet-Induced Rat Models" International Journal of Molecular Sciences 26, no. 22: 11158. https://doi.org/10.3390/ijms262211158
APA StyleKim, Y.-S., Eom, T., Kim, Y., Rhee, J., & Kim, H. (2025). Preventive Effects of Eclipta prostrata and Hordeum vulgare Extract Complex on Precocious Puberty in Danazol- and High-Fat Diet-Induced Rat Models. International Journal of Molecular Sciences, 26(22), 11158. https://doi.org/10.3390/ijms262211158





