Targeting Multidrug Resistance in Cancer: Impact of Retinoids, Rexinoids, and Carotenoids on ABC Transporters
Abstract
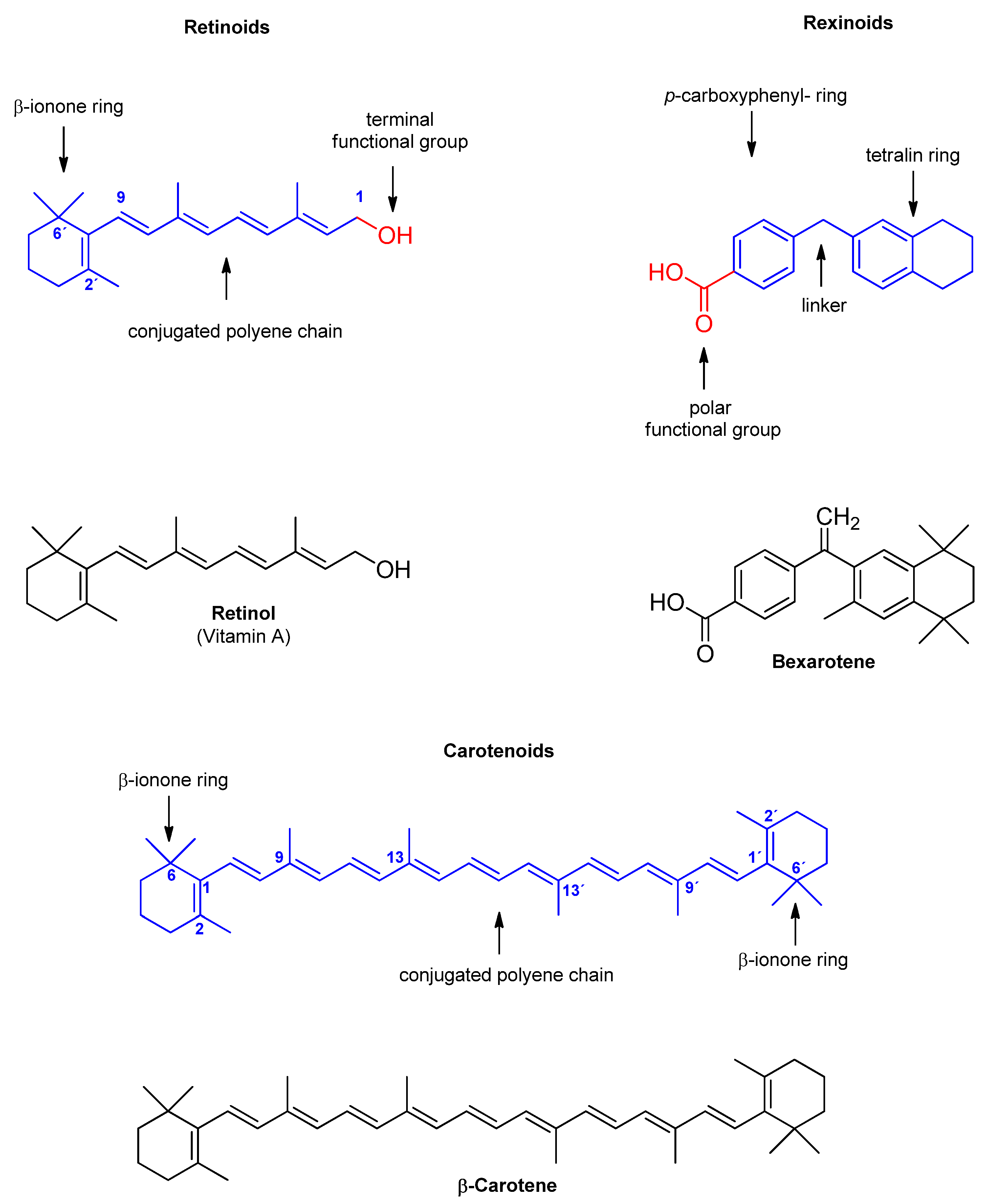
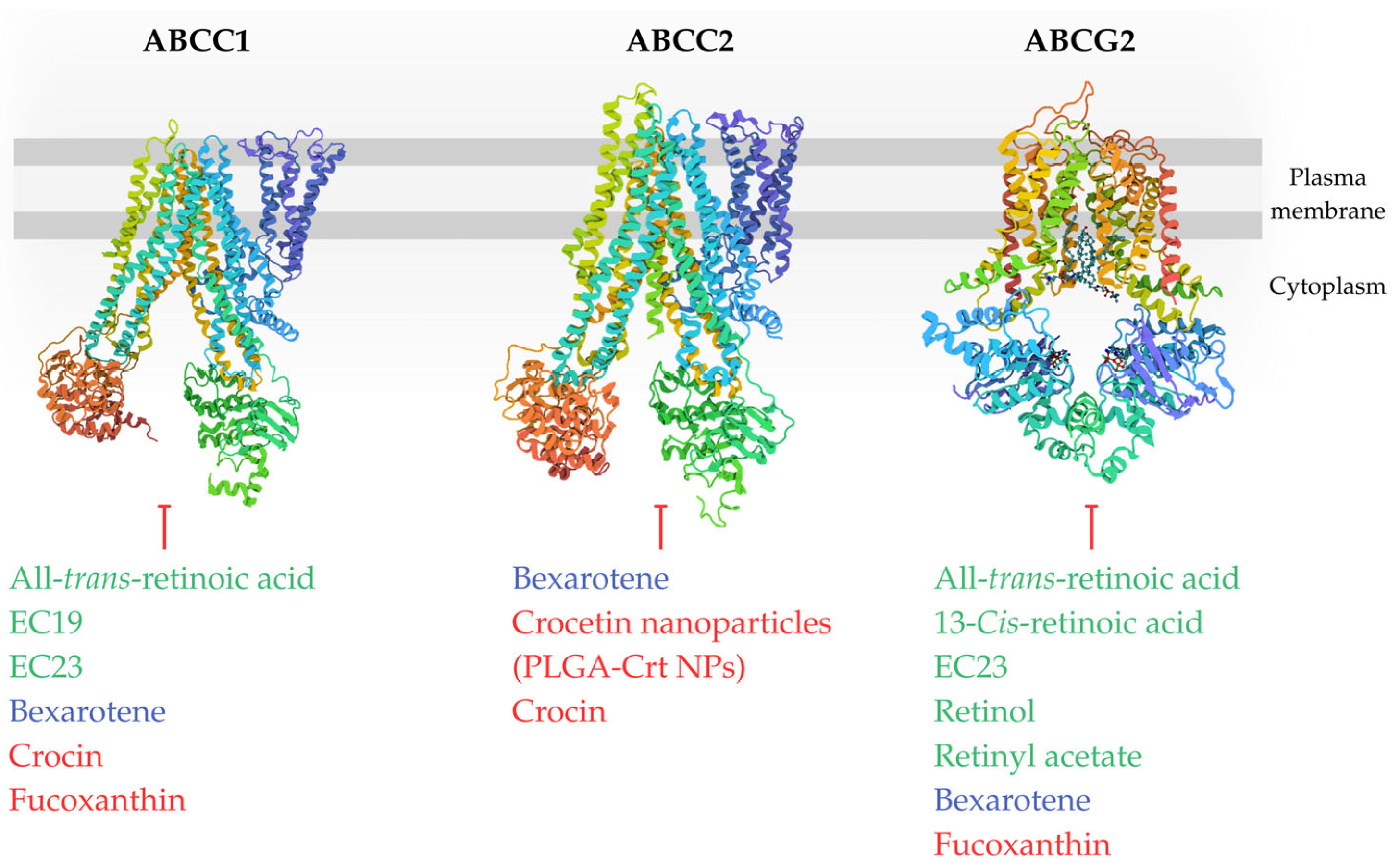
1. Introduction
2. Methodology and Main Results
3. Retinoids and ABC Transporters
3.1. Retinoids and ABC Transporters in Hematological Malignancies
3.2. Retinoids and ABC Transporters in Gastrointestinal Tumors
3.3. Retinoids and ABC Transporters in Malignancies of the Urogenital Tract
3.4. Retinoids and ABC Transporters in Other Cells or Cell Models
4. Rexinoids and ABC Transporters
5. Carotenoids and ABC Transporters
5.1. Carotenoids and ABC Transporters in Hematologic Malignancies
5.2. Carotenoids and ABC Transporters in Gastrointestinal Malignancies
5.3. Carotenoids and ABC Transporters in Malignancies of the Urogenital Tract
5.4. Carotenoids and ABC Transporters in Other Malignancies or Other Cell Models
6. Summary of Potential Mechanisms Involved in Regulation of ABC Transporters
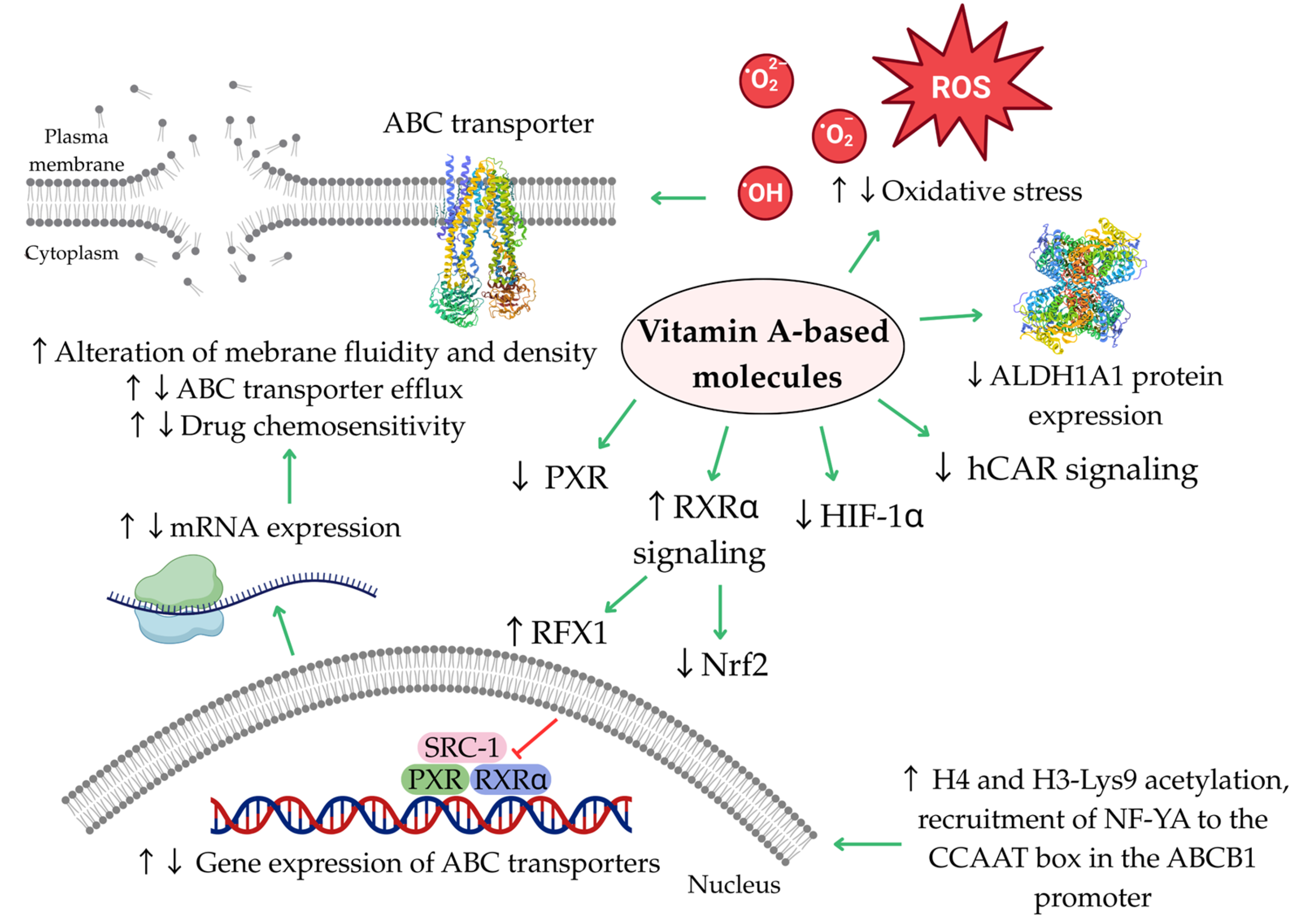
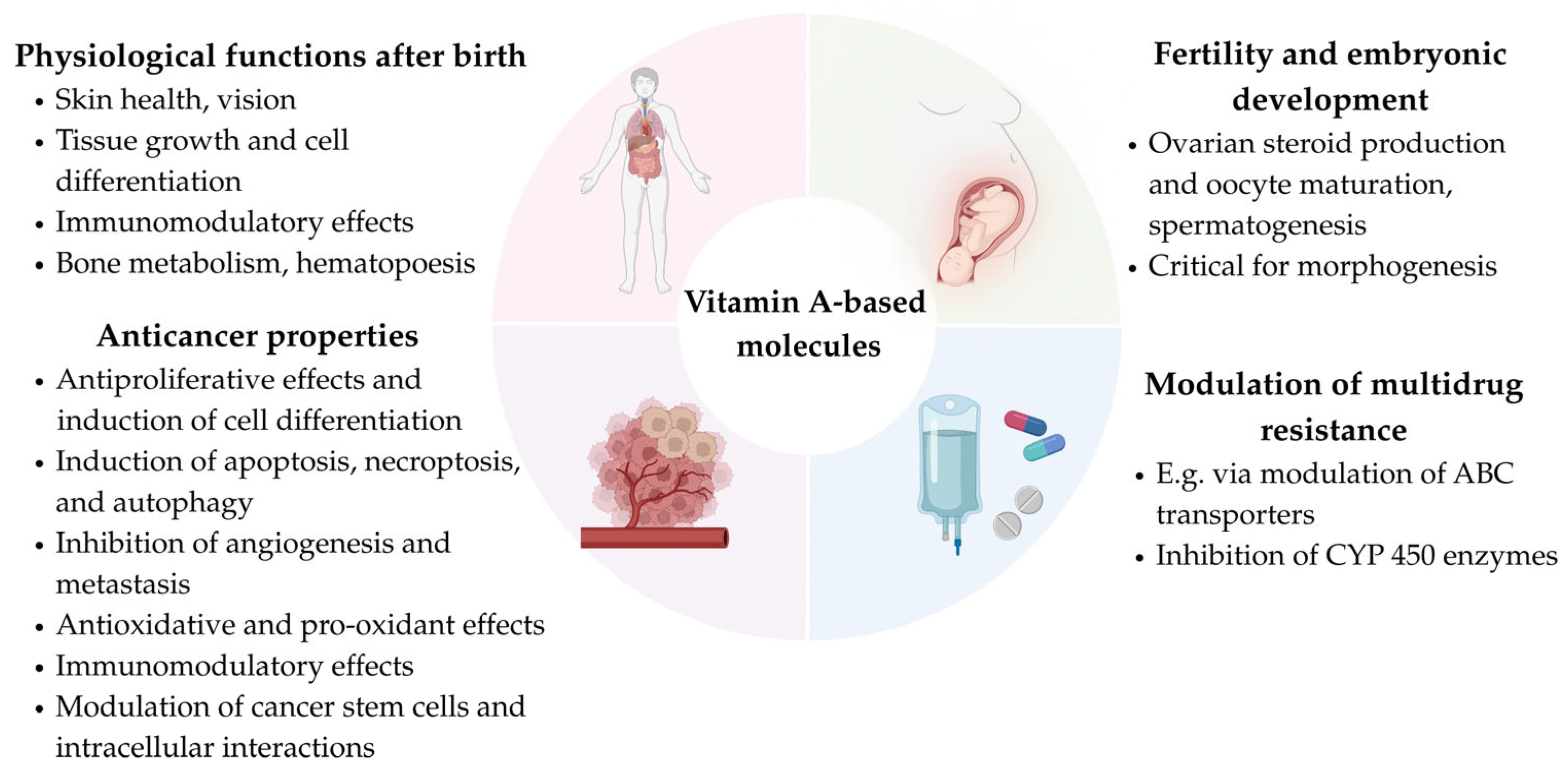
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ABCB1 | ATP-binding cassette subfamily b member 1 (P-glycoprotein) |
| ABCB1/Flp-In TM-293 | Flipase recombinase-compatible human embryonic kidney 293 with transfected human ABCB1 |
| ABCC1 | ATP-binding cassette subfamily c member 1 (multidrug resistance-associated protein 1) |
| ABCC1/Flp-In TM-293 | Flipase recombinase-compatible human embryonic kidney 293 with transfected human ABCC1 |
| ABCC2 | ATP-binding cassette subfamily c member 2 (multidrug resistance-associated protein 2) |
| ABCC3 | ATP-binding cassette subfamily c member 3 (multidrug resistance-associated protein 3) |
| ABCG2 | ATP-binding cassette subfamily g member 2 (breast cancer resistance protein) |
| ABCG2/Flp-In TM-293 | Flipase recombinase-compatible human embryonic kidney 293 with transfected human ABCG2 |
| ABCT | ATP-binding cassette transporter |
| AC261066 | RARβ-2 agonist |
| ALDH1 | Aldehyde dehydrogenase 1 |
| ALDH1A1 | Aldehyde dehydrogenase 1 family member 1A |
| AML | Acute myeloid leukemia |
| APL | Acute promyelocytic leukemia |
| ARE | Antioxidant response element |
| ATO | Arsenic trioxide |
| ATP | Adenosine triphosphate |
| ATPase | Adenosine triphosphatase |
| ATRA | All-trans-retinoic acid |
| A2780/RCIS | Human ovarian cancer, cisplatin-resistant subline |
| BCRP | Breast cancer resistance protein |
| Caco-2 | Human colon cancer |
| CAR | Constitutive androstane receptor |
| CARET | Carotene and Retinol Efficacy Trial |
| CD437 | RAR-γ selective agonist |
| CD44 | Cluster of Differentiation 44 |
| CEM/ADR5000 | Human acute lymphoblastic leukemia (T-cell type), doxorubicin-resistant subline |
| Colo 320 | Human colon cancer overexpressing ABCB1 and lung resistant protein (LRP) |
| CSC | Cancer stem cell |
| CYP3A4 | Cytochrome P450 3A4 |
| CYP450 | Cytochrome P450 |
| EC19 | 3-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-ylethynyl)benzoic acid |
| EC23 | 4-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-ylethynyl)benzoic acid |
| EPG85-257 | Human gastric carcinoma |
| EPG85-257RDB | Human gastric carcinoma, doxorubicin-resistant subline |
| Egr1 | Early growth response 1 gene |
| FAB | French-American-British classification system of AML |
| FAR | Fluorescence activity ratio |
| FDA | Food and Drug Administration |
| FK228 | Histone deacetylase inhibitor (HDACI), Romidepsin (depsipeptide) |
| FOXP1 | Forkhead box protein P1 |
| 5-FU | 5-Fluorouracil |
| GST | Glutathione S-transferase |
| hCAR | Human constitutive androstane receptor |
| HeLaS3 | Human cervical carcinoma |
| HepG-2 | Human hepatocellular carcinoma |
| HepG-2/Dox | Human hepatocellular carcinoma, doxorubicin-resistant subline |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HL-60/RS | Multidrug-resistant human acute promyelocytic leukemia cells overexpressing ABCB1, ABCC1, and ABCG2 |
| HT29 | Human colon cancer |
| H3-Lys9 | Histone H3 at lysine 9 |
| H9 | Human acute T-cell leukemia |
| H9/RAR | RARα transfected human acute T-cell leukemia |
| IC50 | Half-maximal inhibitory concentration |
| IIF | 6-OH-11-O-hydroxyphenanthrene |
| Kasumi-1 | Human acute myeloid leukemia with t(8:21) karyotype |
| Kasumi-6 | Human acute myeloid leukemia |
| KB-vin | Human cervical carcinoma, vincristine-resistant subline |
| Keap1 | Kelch-like ECH-associated protein 1 |
| KG-1 | Human acute myeloid leukemia |
| KG-1/RAR | RARα transfected human acute myeloid leukemia |
| K562 | Human cell line from the patient in blast crisis of chronic myeloid leukemia |
| K562/RAR | RARα transfected human cell line from the patient in blast crisis of chronic myeloid leukemia |
| LoVo/MDR | Human colorectal cancer, doxorubicin-resistant subline |
| LRP | Lung resistant protein |
| LS174T | Human colorectal carcinoma |
| L1210/R | Mouse lymphocytic leukemia, subline with ABCB1-overexpression induced by vincristine |
| L1210/S | Mouse lymphocytic leukemia/without detectable ABCB1 expression and efflux activity |
| L1210/T | Mouse lymphocytic leukemia, subline with ABCB1 overexpression induced by transfection |
| L1210/VCR | Mouse lymphocytic leukemia, vincristine-resistant subline |
| L5178Y (MDR1/A) | Mouse T-cell lymphoma cells transfected by human ABCB1 gene |
| MAF | Multidrug Activity Factor |
| MCF-7 | Human breast cancer |
| MCF-7/Doc | Human breast cancer, docetaxel-resistant subline |
| MCF-7/Dox | Human breast cancer, doxorubicin-resistant subline |
| MCF-7/Pac | Human breast cancer, paclitaxel-resistant subline |
| MCF-7/Vinc | Human breast cancer, vincristine-resistant subline |
| MDA-MB-231 | Human triple-negative breast cancer |
| MDA-MB-468 | Human triple-negative breast cancer |
| MDCK ABCG2 | Madine-Darby canine kidney cell line overexpressing ABCG2 |
| MDR | Multidrug resistance |
| MDR1 | Multidrug resistance protein 1 |
| mdr1 | Multidrug resistance 1 gene (the rodent equivalent of human ABCB1) |
| mdr2 | Multidrug resistance 2 gene |
| mdr3 | Multidrug resistance 3 gene |
| MM CS-like cells | Cancer stem cells derived from spheroids of human A375 melanoma cells |
| MRP1 | Multidrug resistance-associated protein 1 (ABCC1) |
| MRP2 | Multidrug resistance-associated protein 2 (ABCC2) |
| MRP3 | Multidrug resistance-associated protein 3 (ABCC3) |
| MTT | Methyltiazoltetrazolium assay |
| MVP | Major vault protein/Lung resistant protein |
| M1 | Acute myeloblastic leukemia without maturation |
| M2 | Acute myeloblastic leukemia with maturation with t(8;21) karyotype |
| M3 | Acute promyelocytic leukemia |
| M4 | Acute myelomonocytic leukemia |
| NB4 | Human acute promyelocytic leukemia |
| NB4/RAR | RARα transfected human acute promyelocytic leukemia |
| NCI-H460 | Human non-small-cell lung carcinoma |
| NCI-H460/MX20 | Human non-small-cell lung carcinoma, mitoxantrone-resistant subline |
| NF-YA | Nuclear transcription factor Y alpha |
| NF-κB | Nuclear factor kappa-B |
| NIH 3T3 MDR1 | Mouse fibroblast cell line overexpressing ABCB1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NT2 | Human pluripotent embryonal carcinoma |
| PARPi | Poly ADP-ribose polymerase inhibitor |
| PI3K/Akt/mTOR | Phosphoinositide 3-kinase/protein kinase B/mechanistic target of rapamycin signaling pathway |
| PXR | Pregnane X receptor |
| RAR | Retinoic acid receptor |
| RFX1 | Regulatory factor X1 |
| Rh-123 | Rhodamine 123 |
| ROS | Reactive oxygen species |
| RT-PCR | Real-time quantitative polymerase chain reaction |
| RXR | Retinoid X receptor |
| SKOV-3 | Human ovarian adenocarcinoma |
| SKOV-3/Dox | Human ovarian adenocarcinoma, doxorubicin-resistant subline |
| SOX9 | SRY-box transcription factor 9 |
| SOX10 | SRY-box transcription factor 10 |
| SRC-1 | Steroid receptor coactivator-1 |
| SW620 | Human colon cancer |
| VER | Verapamil |
| WIPO | World Intellectual Property Organization |
| WNT/β-catenin | Wingless-related integration site/β-catenin |
| WT1 | Wilms’ tumor suppressor gene |
| W1PR | Human ovarian cancer, paclitaxel-resistant subline (primary cell line) |
| W1TR | Human ovarian cancer, topotecan-resistant subline (primary cell line) |
References
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef]
- El-Awady, R.; Saleh, E.; Hashim, A.; Soliman, N.; Dallah, A.; Elrasheed, A.; Elakraa, G. The Role of Eukaryotic and Prokaryotic ABC Transporter Family in Failure of Chemotherapy. Front. Pharmacol. 2017, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Silva, A.M. Natural Products as a Tool to Modulate the Activity and Expression of Multidrug Resistance Proteins of Intestinal Barrier. J. Xenobiotics 2023, 13, 172–192. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.; Kumar, G. Cancer Multidrug-Resistance Reversal by ABCB1 Inhibition: A Recent Update. Eur. J. Med. Chem. 2022, 239, 114542. [Google Scholar] [CrossRef]
- Skinner, K.T.; Palkar, A.M.; Hong, A.L. Genetics of ABCB1 in Cancer. Cancers 2023, 15, 4236. [Google Scholar] [CrossRef]
- Kunická, T.; Souček, P. Importance of ABCC1 for Cancer Therapy and Prognosis. Drug Metab. Rev. 2014, 46, 325–342. [Google Scholar] [CrossRef]
- Mandić, D.; Nežić, L.; Amdžić, L.; Vojinović, N.; Gajanin, R.; Popović, M.; Đeri, J.; Balint, M.T.; Dumanović, J.; Milovanović, Z.; et al. Overexpression of MRP1/ABCC1, Survivin and BCRP/ABCC2 Predicts the Resistance of Diffuse Large B-Cell Lymphoma to R-CHOP Treatment. Cancers 2023, 15, 4106. [Google Scholar] [CrossRef]
- Álvarez-Fernández, L.; Millán-García, A.; Merino, G.; Blanco-Paniagua, E. ABCG2 Transporter: From Structure to Function—Current Insights and Open Questions. Int. J. Mol. Sci. 2025, 26, 6119. [Google Scholar] [CrossRef]
- Jedlitschky, G.; Hoffmann, U.; Kroemer, H.K. Structure and Function of the MRP2 (ABCC2) Protein and Its Role in Drug Disposition. Expert. Opin. Drug Metab. Toxicol. 2006, 2, 351–366. [Google Scholar] [CrossRef]
- Shibayama, Y.; Nakano, K.; Maeda, H.; Taguchi, M.; Ikeda, R.; Sugawara, M.; Iseki, K.; Takeda, Y.; Yamada, K. Multidrug Resistance Protein 2 Implicates Anticancer Drug-Resistance to Sorafenib. Biol. Pharm. Bull. 2011, 34, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Rigalli, J.P.; Gagliardi, A.; Diester, K.; Bajraktari-Sylejmani, G.; Blank, A.; Burhenne, J.; Lenard, A.; Werntz, L.; Huppertz, A.; Münch, L.; et al. Extracellular Vesicles as Surrogates for the Regulation of the Drug Transporters ABCC2 (MRP2) and ABCG2 (BCRP). Int. J. Mol. Sci. 2024, 25, 4118. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.V.L.; Ruginsk, B.E.; Prado, L.d.O.; de Lima, D.E.; Daniel, I.W.; Moure, V.R.; Valdameri, G. The Association of ABC Proteins with Multidrug Resistance in Cancer. Biochim. Biophys. Acta Mol. Cell. Res. 2025, 1872, 119878. [Google Scholar] [CrossRef] [PubMed]
- Begicevic, R.R.; Falasca, M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int. J. Mol. Sci. 2017, 18, 2362. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, M.J.; Yu, A.M. Efflux ABC Transporters in Drug Disposition and Their Posttranscriptional Gene Regulation by MicroRNAs. Front. Pharmacol. 2024, 15, 1423416. [Google Scholar] [CrossRef]
- Nagampalli, R.S.K.; Vadla, G.P.; Nadendla, E.K. Emerging Strategies to Overcome Chemoresistance: Structural Insights and Therapeutic Targeting of Multidrug Resistance-Linked ATP-Binding Cassette Transporters. Int. J. Transl. Med. 2025, 5, 6. [Google Scholar] [CrossRef]
- Fan, W.; Shao, K.; Luo, M. Structural View of Cryo-Electron Microscopy-Determined ATP-Binding Cassette Transporters in Human Multidrug Resistance. Biomolecules 2024, 14, 231. [Google Scholar] [CrossRef]
- Chai, A.B.; Callaghan, R.; Gelissen, I.C. Regulation of P-Glycoprotein in the Brain. Int. J. Mol. Sci. 2022, 23, 14667. [Google Scholar] [CrossRef]
- Schulz, J.A.; Hartz, A.M.S.; Bauer, B. ABCB1 and ABCG2 Regulation at the Blood-Brain Barrier: Potential New Targets to Improve Brain Drug Delivery. Pharmacol. Rev. 2023, 75, 815–853. [Google Scholar] [CrossRef]
- Shchulkin, A.V.; Abalenikhina, Y.V.; Kosmachevskaya, O.V.; Topunov, A.F.; Yakusheva, E.N. Regulation of P-Glycoprotein during Oxidative Stress. Antioxidants 2024, 13, 215. [Google Scholar] [CrossRef]
- Álvarez-Carrasco, P.; Morales-Villamil, F.; Maldonado-Bernal, C. P-Glycoprotein as a Therapeutic Target in Hematological Malignancies: A Challenge to Overcome. Int. J. Mol. Sci. 2025, 26, 4701. [Google Scholar] [CrossRef]
- Goebel, J.; Chmielewski, J.; Hrycyna, C.A. The Roles of the Human ATP-Binding Cassette Transporters P-Glycoprotein and ABCG2 in Multidrug Resistance in Cancer and at Endogenous Sites: Future Opportunities for Structure-Based Drug Design of Inhibitors. Cancer Drug Resist. 2021, 4, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Li, Y.C.; Murakami, M.; Hsiao, S.H.; Lee, Y.C.; Huang, Y.H.; Chang, Y.T.; Hung, T.H.; Wu, Y.S.; Ambudkar, S.V. Furmonertinib, a Third-Generation EGFR Tyrosine Kinase Inhibitor, Overcomes Multidrug Resistance through Inhibiting ABCB1 and ABCG2 in Cancer Cells. Int. J. Mol. Sci. 2023, 24, 13972. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Joung, J.Y.; Cho, J.H.; Son, C.G.; Lee, N. Overcoming P-Glycoprotein-Mediated Multidrug Resistance in Colorectal Cancer: Potential Reversal Agents among Herbal Medicines. Evid. Based Complement. Alternat. Med. 2018, 2018, 3412074. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, P.; Yadav, P.; Sajid, A.; Ambudkar, S.V.; Prasad, N.R. Natural Medicinal Compounds Target Signal Transduction Pathways to Overcome ABC Drug Efflux Transporter-Mediated Multidrug Resistance in Cancer. Drug Resist. Updat. 2023, 71, 101004. [Google Scholar] [CrossRef]
- Chen, T.; Xiao, Z.; Liu, X.; Wang, T.; Wang, Y.; Ye, F.; Su, J.; Yao, X.; Xiong, L.; Yang, D.H. Natural Products for Combating Multidrug Resistance in Cancer. Pharmacol. Res. 2024, 202, 107099. [Google Scholar] [CrossRef]
- Alhazza, A.; Oyegbesan, A.; Bousoik, E.; Montazeri Aliabadi, H. Multidrug Resistance: Are We Still Afraid of the Big Bad Wolf. Pharmaceuticals 2025, 18, 895. [Google Scholar] [CrossRef]
- Choi, C.Y.; Lim, S.C.; Lee, T.B.; Han, S.I. Molecular Basis of Resveratrol-Induced Resensitization of Acquired Drug-Resistant Cancer Cells. Nutrients 2022, 14, 699. [Google Scholar] [CrossRef]
- Warias, P.; Plewa, P.; Poniewierska-Baran, A. Resveratrol, Piceatannol, Curcumin, and Quercetin as Therapeutic Targets in Gastric Cancer-Mechanisms and Clinical Implications for Natural Products. Molecules 2024, 30, 3. [Google Scholar] [CrossRef]
- Liu, M.; Yin, H.; Qian, X.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Hops, Inhibits the Viability and Stemness of Doxorubicin-Resistant MCF-7/ADR Cells. Molecules 2016, 22, 36. [Google Scholar] [CrossRef]
- Čižmáriková, M.; Takáč, P.; Spengler, G.; Kincses, A.; Nové, M.; Vilková, M.; Mojžiš, J. New Chalcone Derivative Inhibits ABCB1 in Multidrug Resistant T-Cell Lymphoma and Colon Adenocarcinoma Cells. Anticancer Res. 2019, 39, 6499–6505. [Google Scholar] [CrossRef] [PubMed]
- Franko, O.; Čižmáriková, M.; Kello, M.; Michalková, R.; Wesołowska, O.; Środa-Pomianek, K.; Marques, S.M.; Bednář, D.; Háziková, V.; Liška, T.J.; et al. Acridine-Based Chalcone 1C and ABC Transporters. Int. J. Mol. Sci. 2025, 26, 4138. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural Alkaloids as P-Gp Inhibitors for Multidrug Resistance Reversal in Cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Ye, C.; Bai, E.H.; Zhang, L.L.; Huo, S.J.; Yu, H.H.; Xiang, S.Y.; Yu, S.Q. Co-Delivery Nanoparticles of Doxorubicin and Chloroquine for Improving the Anti-Cancer Effect in Vitro. Nanotechnology 2019, 30, 085101. [Google Scholar] [CrossRef]
- Lopez, D.; Martinez-Luis, S. Marine Natural Products with P-Glycoprotein Inhibitor Properties. Mar. Drugs 2014, 12, 525–546. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Wei, X.; Yang, M.; Ma, W.; Yu, R.; Liu, M.; Jiang, T. Design, Synthesis, and Biological Evaluation of Marine Lissodendrins B Analogues as Modulators of ABCB1-Mediated Multidrug Resistance. Mar. Drugs 2023, 21, 314. [Google Scholar] [CrossRef]
- Liao, D.; Zhang, W.; Gupta, P.; Lei, Z.N.; Wang, J.Q.; Cai, C.Y.; De Vera, A.A.; Zhang, L.; Chen, Z.S.; Yang, D.H. Tetrandrine Interaction with ABCB1 Reverses Multidrug Resistance in Cancer Cells Through Competition with Anti-Cancer Drugs Followed by Downregulation of ABCB1 Expression. Molecules 2019, 24, 4383. [Google Scholar] [CrossRef]
- Otarigho, B.; Duffin, P.M.; Falade, M.O. Potential Natural Inhibitors of MRSA ABC Transporters and MecA Identified Through In Silico Approaches. Microorganisms 2025, 13, 1431. [Google Scholar] [CrossRef]
- Oh, S.J.; Han, H.K.; Kang, K.W.; Lee, Y.J.; Lee, M.Y. Menadione Serves as a Substrate for P-Glycoprotein: Implication in Chemosensitizing Activity. Arch. Pharm. Res. 2013, 36, 509–516. [Google Scholar] [CrossRef]
- Ding, Y.; Peng, Y.; Deng, L.; Fan, J.; Huang, B. Gamma-Tocotrienol Reverses Multidrug Resistance of Breast Cancer Cells with a Mechanism Distinct from That of Atorvastatin. J. Steroid Biochem. Mol. Biol. 2017, 167, 67–77. [Google Scholar] [CrossRef]
- Tan, K.W.; Sampson, A.; Osa-Andrews, B.; Iram, S.H. Calcitriol and Calcipotriol Modulate Transport Activity of ABC Transporters and Exhibit Selective Cytotoxicity in MRP1-Overexpressing Cells. Drug Metab. Dispos. 2018, 46, 1856–1866. [Google Scholar] [CrossRef]
- Attia, Y.M.; El-Kersh, D.M.; Ammar, R.A.; Adel, A.; Khalil, A.; Walid, H.; Eskander, K.; Hamdy, M.; Reda, N.; Mohsen, N.E.; et al. Inhibition of Aldehyde Dehydrogenase-1 and p-Glycoprotein-Mediated Multidrug Resistance by Curcumin and Vitamin D3 Increases Sensitivity to Paclitaxel in Breast Cancer. Chem. Biol. Interact. 2020, 315, 108865. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Alvarez, S.; de Lera, A.R. Natural and Structure-Based RXR Ligand Scaffolds and Their Functions. Curr. Top. Med. Chem. 2017, 17, 631–662. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, O.V.; Klyuyeva, A.V.; Vyas, A.; Berger, W.K.; Halasz, L.; Yu, J.; Atigadda, V.R.; Slay, A.; Goggans, K.R.; Renfrow, M.B.; et al. The Retinoid X Receptor Has a Critical Role in Synthetic Rexinoid-Induced Increase in Cellular All-Trans-Retinoic Acid. PLoS ONE 2024, 19, e0301447. [Google Scholar] [CrossRef] [PubMed]
- Kam, R.K.T.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic Acid Synthesis and Functions in Early Embryonic Development. Cell. Biosci. 2012, 2, 11. [Google Scholar] [CrossRef]
- Liu, C.L.; Lim, Y.P.; Hu, M.L. Fucoxanthin Attenuates Rifampin-Induced Cytochrome P450 3A4 (CYP3A4) and Multiple Drug Resistance 1 (MDR1) Gene Expression Through Pregnane X Receptor (PXR)-Mediated Pathways in Human Hepatoma HepG2 and Colon Adenocarcinoma LS174T Cells. Mar. Drugs 2012, 10, 242–257. [Google Scholar] [CrossRef]
- Best, M.W.; Wu, J.; Pauli, S.A.; Kane, M.A.; Pierzchalski, K.; Session, D.R.; Woods, D.C.; Shang, W.; Taylor, R.N.; Sidell, N. A Role for Retinoids in Human Oocyte Fertilization: Regulation of Connexin 43 by Retinoic Acid in Cumulus Granulosa Cells. Mol. Hum. Reprod. 2015, 21, 527–534. [Google Scholar] [CrossRef]
- El-Abaseri, T.B.; El-Metwally, T.H.; Iversen, P.L.; Adrian, T.E. Inhibition of Cytochrome P450 and Multidrug Resistance Proteins Potentiates the Efficacy of All-Trans Retinoic Acid in Pancreatic Cancer In Vitro and In Vivo. J. Clin. Exp. Oncol. 2015, 4, 138. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S.; Daglia, M.; Rengasamy, K.R. Dietary Carotenoids in Cancer Chemoprevention and Chemotherapy: A Review of Emerging Evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Sajovic, J.; Meglič, A.; Glavač, D.; Markelj, Š.; Hawlina, M.; Fakin, A. The Role of Vitamin A in Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 1014. [Google Scholar] [CrossRef]
- Rozanowska, M.; Edge, R.; Land, E.J.; Navaratnam, S.; Sarna, T.; Truscott, T.G. Scavenging of Cation Radicals of the Visual Cycle Retinoids by Lutein, Zeaxanthin, Taurine, and Melanin. Int. J. Mol. Sci. 2023, 25, 506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, S.; Li, C.; Cao, J.; Wu, A.; Chen, M.; Ma, X.; Wu, S.; Lian, Z. The Role of Retinoic Acid in Spermatogenesis and Its Application in Male Reproduction. Cells 2024, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Adamantidi, T.; Lafara, M.P.; Venetikidou, M.; Likartsi, E.; Toganidou, I.; Tsoupras, A. Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties. Appl. Sci. 2025, 15, 1657. [Google Scholar] [CrossRef]
- Medina-García, M.; Baeza-Morales, A.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; María Martínez-Espinosa, R.; Sempere-Ortells, J.M. Carotenoids and Their Interaction with the Immune System. Antioxidants 2025, 14, 1111. [Google Scholar] [CrossRef]
- Vašková, J.; Stupák, M.; Vidová Ugurbaş, M.; Židzik, J.; Mičková, H. Therapeutic Uses of Retinol and Retinoid-Related Antioxidants. Molecules 2025, 30, 2191. [Google Scholar] [CrossRef]
- Smirnov, V.M.; Wilmet, B.; Nassisi, M.; Condroyer, C.; Antonio, A.; Andrieu, C.; Devisme, C.; Sancho, S.; Sahel, J.A.; Zeitz, C.; et al. Large Benefit from Simple Things: High-Dose Vitamin A Improves RBP4-Related Retinal Dystrophy. Int. J. Mol. Sci. 2022, 23, 6590. [Google Scholar] [CrossRef]
- Sui, J.; Guo, J.; Pan, D.; Wang, Y.; Xu, Y.; Sun, G.; Xia, H. The Efficacy of Dietary Intake, Supplementation, and Blood Concentrations of Carotenoids in Cancer Prevention: Insights from an Umbrella Meta-Analysis. Foods 2024, 13, 1321. [Google Scholar] [CrossRef]
- Crespi, O.; Rosset, F.; Pala, V.; Sarda, C.; Accorinti, M.; Quaglino, P.; Ribero, S. Cosmeceuticals for Anti-Aging: Mechanisms, Clinical Evidence, and Regulatory Insights—A Comprehensive Review. Cosmetics 2025, 12, 209. [Google Scholar] [CrossRef]
- Shanaida, M.; Mykhailenko, O.; Lysiuk, R.; Hudz, N.; Balwierz, R.; Shulhai, A.; Shapovalova, N.; Shanaida, V.; Bjørklund, G. Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals 2025, 18, 403. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Esquivel, P.; Meléndez-Martínez, A.J.; Cilla, A.; Cassano, A.; Barreira, J.C.M.; Kumar, S.R.; Rodriguez-Amaya, D.B.; Esquivel, P.; Meléndez-Martínez, A.J. Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry. Foods 2023, 12, 4080. [Google Scholar] [CrossRef]
- Vega, E.N.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Barros, L.; Morales, P. Natural Sources of Food Colorants as Potential Substitutes for Artificial Additives. Foods 2023, 12, 4102. [Google Scholar] [CrossRef] [PubMed]
- Yen, W.C.; Lamph, W.W. The Selective Retinoid X Receptor Agonist Bexarotene (LGD1069, Targretin) Prevents and Overcomes Multidrug Resistance in Advanced Breast Carcinoma. Mol. Cancer Ther. 2005, 4, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Hessel, S.; Lampen, A. All-Trans Retinoic Acid Enhances the Transport of Phase II Metabolites of Benzo[a]Pyrene by Inducing the Breast Cancer Resistance Protein Expression in Caco-2 Cells. Toxicol. Lett. 2010, 197, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Januchowski, R.; Wojtowicz, K.; Sterzyńska, K.; Sosińska, P.; Andrzejewska, M.; Zawierucha, P.; Nowicki, M.; Zabel, M. Inhibition of ALDH1A1 Activity Decreases Expression of Drug Transporters and Reduces Chemotherapy Resistance in Ovarian Cancer Cell Lines. Int. J. Bioch. Cell Biol. 2016, 78, 248–259. [Google Scholar] [CrossRef]
- Mahdizadeh, S.; Karimi, G.; Behravan, J.; Arabzadeh, S.; Lage, H.; Kalalinia, F. Crocin Suppresses Multidrug Resistance in MRP Overexpressing Ovarian Cancer Cell Line. Daru 2016, 24, 17. [Google Scholar] [CrossRef]
- Teng, Y.N.; Sheu, M.J.; Hsieh, Y.W.; Wang, R.Y.; Chiang, Y.C.; Hung, C.C. β-Carotene Reverses Multidrug Resistant Cancer Cells by Selectively Modulating Human P-Glycoprotein Function. Phytomedicine 2016, 23, 316–323. [Google Scholar] [CrossRef]
- Tarapcsák, S.; Szalóki, G.; Telbisz, Á.; Gyöngy, Z.; Matúz, K.; Csosz, É.; Nagy, P.; Holb, I.J.; Rühl, R.; Nagy, L.; et al. Interactions of Retinoids with the ABC Transporters P-Glycoprotein and Breast Cancer Resistance Protein. Sci. Rep. 2017, 7, 41376. [Google Scholar] [CrossRef]
- Neyshaburinezhad, N.; Kalalinia, F.; Hashemi, M. Encapsulation of Crocetin into Poly (Lactic-Co-Glycolic Acid) Nanoparticles Overcomes Drug Resistance in Human Ovarian Cisplatin-Resistant Carcinoma Cell Line (A2780-RCIS). Mol. Biol. Rep. 2019, 46, 6525–6532. [Google Scholar] [CrossRef]
- Eid, S.Y.; Althubiti, M.A.; Abdallah, M.E.; Wink, M.; El-Readi, M.Z. The Carotenoid Fucoxanthin Can Sensitize Multidrug Resistant Cancer Cells to Doxorubicin via Induction of Apoptosis, Inhibition of Multidrug Resistance Proteins and Metabolic Enzymes. Phytomedicine 2020, 77, 153280. [Google Scholar] [CrossRef]
- Abdelaal, M.R.; Ibrahim, E.; Elnagar, M.R.; Soror, S.H.; Haffez, H. Augmented Therapeutic Potential of EC-Synthetic Retinoids in Caco-2 Cancer Cells Using an In Vitro Approach. Int. J. Mol. Sci. 2022, 23, 9442. [Google Scholar] [CrossRef]
- Issac, J.; Raveendran, P.S.; Kunnummal, M.; Angelin, M.; Ravindran, S.; Basu, B.; Das, A.V. RXR Agonist, Bexarotene, Effectively Reduces Drug Resistance via Regulation of RFX1 in Embryonic Carcinoma Cells. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119510. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Shinagawa, A.; Ota, M.; Virgona, N.; Yano, T. Resveratrol Can Differentiate Human Melanoma Stem-like Cells from Spheroids Treated With All-Trans Retinoic Acid. Anticancer Res. 2024, 44, 5283–5292. [Google Scholar] [CrossRef] [PubMed]
- Üstün, C.; Beksac, M.; Dalva, K.; Koc, H.; Konuk, N.; Ilhan, O.; Özcan, M.; Topcuoglu, P.; Sertkaya, D.; Hayran, M. In Vivo Use of All-Trans Retinoic Acid Prior to Induction Chemotherapy Improves Complete Remission Rate and Increases Rhodamine 123 Uptake in Patients with de Novo Acute Myeloid Leukemia. Med. Oncol. 2002, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Shikami, M.; Miwa, H.; Watarai, M.; Sugamura, K.; Wakabayashi, M.; Satoh, A.; Imamura, A.; Mihara, H.; Katoh, Y.; et al. Augmented Expression of P-Gp/Multi-Drug Resistance Gene by All-Trans Retinoic Acid in Monocytic Leukemic Cells. Leuk. Res. 2002, 26, 29–36. [Google Scholar] [CrossRef]
- Stromskaya, T.P.; Rybalkina, E.Y.; Zabotina, T.N.; Shishkin, A.A.; Stavrovskaya, A.A. Influence of RARα Gene on MDR1 Expression and P-Glycoprotein Function in Human Leukemic Cells. Cancer Cell Int. 2005, 5, 1–9. [Google Scholar] [CrossRef][Green Version]
- Tabe, Y.; Konopleva, M.; Contractor, R.; Munsell, M.; Schober, W.D.; Jin, L.; Tsutsumi-Ishii, Y.; Nagaoka, I.; Igari, J.; Andreeff, M. Up-Regulation of MDR1 and Induction of Doxorubicin Resistance by Histone Deacetylase Inhibitor Depsipeptide (FK228) and ATRA in Acute Promyelocytic Leukemia Cells. Blood 2006, 107, 1546–1554. [Google Scholar] [CrossRef]
- Sulová, Z.; Macejová, D.; Šereš, M.; Sedlák, J.; Brtko, J.; Breier, A. Combined Treatment of P-Gp-Positive L1210/VCR Cells by Verapamil and All-Trans Retinoic Acid Induces down-Regulation of P-Glycoprotein Expression and Transport Activity. Toxicol. In Vitro 2008, 22, 96–105. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids Reverse Multidrug Resistance in Cancer Cells by Interfering with ABC-Transporters. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef]
- Breier, A.; Stetka, J.; Bohacova, V.; Macejova, D.; Brtko, J.; Sulova, Z. Effect of 9-Cis Retinoic Acid and All-Trans Retinoic Acid in Combination with Verapamil on P-Glycoprotein Expression in L1210 Cells. Neoplasma 2014, 61, 553–565. [Google Scholar] [CrossRef]
- Molnár, J.; Gyémánt, N.; Mucsi, I.; Molnár, A.; Szabó, M.; Körtvélyesi, T.; Varga, A.; Molnár, P.; Tóth, G. Modulation of Multidrug Resistance and Apoptosis of Cancer Cells by Selected Carotenoids. In Vivo 2004, 18, 237–244. [Google Scholar] [PubMed]
- Gyémánt, N.; Tanaka, M.; Molnár, P.; Deli, J.; Mándoky, L.; Molnár, J. Reversal of Multidrug Resistance of Cancer Cells In Vitro: Modification of Drug Resistance by Selected Carotenoids. Anticancer Res. 2006, 26, 367–374. [Google Scholar] [PubMed]
- Ugocsai, K.; Varga, A.; Molnár, P.; Antus, S.; Molnár, J. Effects of Selected Flavonoids and Carotenoids on Drug Accumulation and Apoptosis Induction in Multidrug-Resistant Colon Cancer Cells Expressing MDR1/LRP. In Vivo 2005, 19, 433–438. [Google Scholar]
- Bartolini, G.; Orlandi, M.; Papi, A.; Ammar, K.; Guerra, F.; Ferreri, A.M.; Rocchi, P. A Search for Multidrug Resistance Modulators: The Effects of Retinoids in Human Colon Carcinoma Cells. In Vivo 2006, 20, 729–733. [Google Scholar]
- Klamt, F.; Passos, D.T.; Castro, M.A.A.; Gelain, D.P.; Grivicich, I.; Moreira, J.C.F. Inhibition of MDR1 Expression by Retinol Treatment Increases Sensitivity to Etoposide (VP16) in Human Neoplasic Cell Line. Toxicol. In Vitro 2008, 22, 873–878. [Google Scholar] [CrossRef]
- Razavi, S.M.S.; Vaziri, R.M.; Karimi, G.; Arabzadeh, S.; Keyvani, V.; Behravan, J.; Kalalinia, F. Crocin Increases Gastric Cancer Cells’ Sensitivity to Doxorubicin. Asian Pac. J. Cancer Prev. 2020, 21, 1959–1967. [Google Scholar] [CrossRef]
- Kars, M.D.; Işeri, O.D.; Gunduz, U.; Molnar, J. Reversal of Multidrug Resistance by Synthetic and Natural Compounds in Drug-Resistant MCF-7 Cell Lines. Chemotherapy 2008, 54, 194–200. [Google Scholar] [CrossRef]
- Croker, A.K.; Allan, A.L. Inhibition of Aldehyde Dehydrogenase (ALDH) Activity Reduces Chemotherapy and Radiation Resistance of Stem-like ALDH HiCD44 + Human Breast Cancer Cells. Breast Cancer Res. Treat. 2012, 133, 75–87. [Google Scholar] [CrossRef]
- Conte da Frota, M.L.; Klamt, F.; Dal-Pizzol, F.; Schiengold, M.; Fonseca Moreira, J.C. Retinol-Induced Mdr1 and Mdr3 Modulation in Cultured Rat Sertoli Cells Is Attenuated by Free Radical Scavengers. Redox Rep. 2004, 9, 161–165. [Google Scholar] [CrossRef]
- Menezes, M.S.S.; Almeida, C.M.M. Structural, Functional, Nutritional and Clinical Aspects of Vitamin A: A Review. PharmaNutrition 2024, 27, 100383. [Google Scholar] [CrossRef]
- Jin, Y.; Teh, S.S.; Lau, H.L.N.; Xiao, J.; Mah, S.H. Retinoids as Anti-Cancer Agents and Their Mechanisms of Action. Am. J. Cancer Res. 2022, 12, 938. [Google Scholar] [PubMed]
- Kawczak, P.; Feszak, I.; Brzeziński, P.; Bączek, T. Structure-Activity Relationships and Therapeutic Applications of Retinoids in View of Potential Benefits from Drug Repurposing Process. Biomedicines 2024, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Conneely, S.E. Management of Acute Promyelocytic Leukemia at Extremes of Age. Cancers 2023, 15, 3637. [Google Scholar] [CrossRef]
- Htet, K.Z.; Waul, M.A.; Leslie, K.S. Topical Treatments for Kaposi Sarcoma: A Systematic Review. Skin Health Dis. 2022, 2, e107. [Google Scholar] [CrossRef]
- Ramchatesingh, B.; Martínez Villarreal, A.; Arcuri, D.; Lagacé, F.; Setah, S.A.; Touma, F.; Al-Badarin, F.; Litvinov, I.V. The Use of Retinoids for the Prevention and Treatment of Skin Cancers: An Updated Review. Int. J. Mol. Sci. 2022, 23, 12622. [Google Scholar] [CrossRef]
- Makimoto, A.; Fujisaki, H.; Matsumoto, K.; Takahashi, Y.; Cho, Y.; Morikawa, Y.; Yuza, Y.; Tajiri, T.; Iehara, T. Retinoid Therapy for Neuroblastoma: Historical Overview, Regulatory Challenges, and Prospects. Cancers 2024, 16, 544. [Google Scholar] [CrossRef]
- Bouriez, D.; Giraud, J.; Gronnier, C.; Varon, C. Efficiency of All-Trans Retinoic Acid on Gastric Cancer: A Narrative Literature Review. Int. J. Mol. Sci. 2018, 19, 3388. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Sharoni, Y.; Hałubiec, P.; Łazarczyk, A.; Szafrański, O.; McCubrey, J.A.; Gąsiorkiewicz, B.; Laidler, P.; Bohn, T. Recent Progress in Discovering the Role of Carotenoids and Their Metabolites in Prostatic Physiology and Pathology with a Focus on Prostate Cancer—A Review—Part I: Molecular Mechanisms of Carotenoid Action. Antioxidants 2021, 10, 585. [Google Scholar] [CrossRef]
- Hałubiec, P.; Łazarczyk, A.; Szafrański, O.; Bohn, T.; Dulińska-Litewka, J. Synthetic Retinoids as Potential Therapeutics in Prostate Cancer—An Update of the Last Decade of Research: A Review. Int. J. Mol. Sci. 2021, 22, 10537. [Google Scholar] [CrossRef]
- Boulos, J.C.; Chatterjee, M.; Shan, L.; Efferth, T. In Silico, In Vitro, and In Vivo Investigations on Adapalene as Repurposed Third Generation Retinoid against Multiple Myeloma and Leukemia. Cancers 2023, 15, 4136. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a Combination of Beta Carotene and Vitamin A on Lung Cancer and Cardiovascular Disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Bates, S.E.; Mickley, L.A.; Chen, Y.-N.; Richert, N.; Rudick, J.; Biedler, J.L.; Fojo, A.T. Expression of a Drug Resistance Gene in Human Neuroblastoma Cell Lines: Modulation by Retinoic Acid-Induced Differentiation. Mol. Cell. Biol. 1989, 9, 4337–4344. [Google Scholar] [CrossRef]
- Flynn, P.; Miller, W.; Weisdorf, D.; Arthur, D.; Brunning, R.; Branda, R. Retinoic Acid Treatment of Acute Promyelocytic Leukemia In Vitro and In Vivo Observations. Blood 1983, 62, 1211–1217. [Google Scholar] [CrossRef]
- Nagai, Y.; Ambinder, A.J. The Promise of Retinoids in the Treatment of Cancer: Neither Burnt Out Nor Fading Away. Cancers 2023, 15, 3535. [Google Scholar] [CrossRef] [PubMed]
- Bidikian, A.; Bewersdorf, J.P.; Kewan, T.; Stahl, M.; Zeidan, A.M. Acute Promyelocytic Leukemia in the Real World: Understanding Outcome Differences and How We Can Improve Them. Cancers 2024, 16, 4092. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Qiao, G.; Liu, Y.; Tian, L.; Hui, N.; Li, J.; Ma, Y.; Li, H.; Zhao, Q.; Cao, W.; et al. Overview of All-Trans-Retinoic Acid (ATRA) and Its Analogues: Structures, Activities, and Mechanisms in Acute Promyelocytic Leukaemia. Eur. J. Med. Chem. 2021, 220, 113451. [Google Scholar] [CrossRef] [PubMed]
- Orfali, N.; O’Donovan, T.R.; Nyhan, M.J.; Britschgi, A.; Tschan, M.P.; Cahill, M.R.; Mongan, N.P.; Gudas, L.J.; McKenna, S.L. Induction of Autophagy Is a Key Component of All-Trans-Retinoic Acid-Induced Differentiation in Leukemia Cells and a Potential Target for Pharmacologic Modulation. Exp. Hematol. 2015, 43, 781–793. [Google Scholar] [CrossRef]
- Tang, D.; Wang, H.; Jiang, Y.; Chen, M.; Zhang, G.; Wu, S.; Wang, Y. ATRA-Induced NEAT1 Upregulation Promotes Autophagy during APL Cell Granulocytic Differentiation. PLoS ONE 2024, 19, e0316109. [Google Scholar] [CrossRef]
- Chen, J.; Wei, H.; Cheng, J.; Xie, B.; Wang, B.; Yi, J.; Tian, B.; Liu, Z.; Wang, F.; Zhang, Z. Characteristics of Doxorubicin-Selected Multidrug-Resistant Human Leukemia HL-60 Cells with Tolerance to Arsenic Trioxide and Contribution of Leukemia Stem Cells. Oncol. Lett. 2018, 15, 1255–1262. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Galski, H.; Fojo, A.; Willingham, M.; Lai, S.L.; Gazdar, A.; Pirker, R.; Green, A.; Crist, W.; Brodeur, G.M.; et al. Expression of Multidrug Resistance Gene in Human Cancers. J. Natl. Cancer Inst. 1989, 81, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Leith, C.P.; Kopecky, K.J.; Godwin, J.; McConnell, T.; Slovak, M.L.; Chen, I.M.; Head, D.R.; Appelbaum, F.R.; Willman, C.L. Acute Myeloid Leukemia in the Elderly: Assessment of Multidrug Resistance (MDR1) and Cytogenetics Distinguishes Biologic Subgroups With Remarkably Distinct Responses to Standard Chemotherapy. A Southwest Oncology Group Study. Blood 1997, 89, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, T.D.; Evangelista, F.C.G.; Sabino, A.d.P. Acute Promyelocytic Leukemia (APL): A Review of the Classic and Emerging Target Therapies towards Molecular Heterogeneity. Future Pharmacol. 2023, 3, 162–179. [Google Scholar] [CrossRef]
- Aliperti, V.; Sgueglia, G.; Aniello, F.; Vitale, E.; Fucci, L.; Donizetti, A. Identification, Characterization, and Regulatory Mechanisms of a Novel EGR1 Splicing Isoform. Int. J. Mol. Sci. 2019, 20, 1548. [Google Scholar] [CrossRef]
- Niktoreh, N.; Weber, L.; Walter, C.; Karimifard, M.; Hoffmeister, L.M.; Breiter, H.; Thivakaran, A.; Soldierer, M.; Drexler, H.G.; Schaal, H.; et al. Understanding WT1 Alterations and Expression Profiles in Hematological Malignancies. Cancers 2023, 15, 3491. [Google Scholar] [CrossRef]
- Tao, W.; Shi, J.F.; Zhang, Q.; Xue, B.; Sun, Y.J.; Li, C.J. Egr-1 Enhances Drug Resistance of Breast Cancer by Modulating MDR1 Expression in a GGPPS-Independent Manner. Biomed. Pharmacother. 2013, 67, 197–202. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, F.; Wei, D.; Chen, F.; Jiang, H.; Qin, S. EGR1 Mediates MDR1 Transcriptional Activity Regulating Gemcitabine Resistance in Pancreatic Cancer. BMC Cancer 2024, 24, 268. [Google Scholar] [CrossRef]
- McCoy, C.; McGee, S.B.; Cornwell, M.M. The Wilms’ Tumor Suppressor, WT1, Inhibits 12-O-Tetradecanoylphorbol-13-Acetate Activation of the Multidrug Resistance-1 Promoter. Cell Growth Differ. 1999, 10, 377–386. [Google Scholar]
- Galimberti, S.; Guerrini, F.; Carulli, G.; Fazzi, R.; Palumbo, G.A.; Morabito, F.; Petrini, M. Significant Co-Expression of WT1 and MDR1 Genes in Acute Myeloid Leukemia Patients at Diagnosis. Eur. J. Haematol. 2004, 72, 45–51. [Google Scholar] [CrossRef]
- Rondoni, M.; Marconi, G.; Nicoletti, A.; Giannini, B.; Zuffa, E.; Giannini, M.B.; Mianulli, A.; Norata, M.; Monaco, F.; Zaccheo, I.; et al. Low WT1 Expression Identifies a Subset of Acute Myeloid Leukemia with a Distinct Genotype. Cancers 2025, 17, 1213. [Google Scholar] [CrossRef]
- Goel, H.; Pandey, A.K.; Kumar, R.; Kumar, R.; Ningombam, S.S.; Naz, F.; Makkar, H.; Singh, J.; Ali, S.; Chopra, A.; et al. RNA Sequencing Identifies WT1 Overexpression as a Predictor of Poor Outcomes in Acute Myeloid Leukemia. Cancers 2025, 17, 1818. [Google Scholar] [CrossRef]
- Hu, T.; Li, Z.; Gao, C.Y.; Cho, C.H. Mechanisms of Drug Resistance in Colon Cancer and Its Therapeutic Strategies. World J. Gastroenterol. 2016, 22, 6876–6889. [Google Scholar] [CrossRef]
- Marin, J.J.G.; Monte, M.J.; Macias, R.I.R.; Romero, M.R.; Herraez, E.; Asensio, M.; Ortiz-Rivero, S.; Cives-Losada, C.; Di Giacomo, S.; Gonzalez-Gallego, J.; et al. Expression of Chemoresistance-Associated ABC Proteins in Hepatobiliary, Pancreatic and Gastrointestinal Cancers. Cancers 2022, 14, 3524. [Google Scholar] [CrossRef]
- Wu, Z.X.; Yang, Y.; Zeng, L.; Patel, H.; Bo, L.; Lin, L.; Chen, Z.S. Establishment and Characterization of an Irinotecan-Resistant Human Colon Cancer Cell Line. Front. Oncol. 2021, 10, 624954. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-P.; Hung, C.-Y.; Hsieh, Y.-J.; Murakami, M.; Huang, Y.-H.; Su, T.-Y.; Hung, T.-H.; Yu, J.-S.; Wu, Y.-S.; Ambudkar, S.V. ABCB1 and ABCG2 Overexpression Mediates Resistance to the Phosphatidylinositol 3-Kinase Inhibitor HS-173 in Cancer Cell Lines. Cells 2023, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.; Di Leo, L.; Bechmann, M.B.; Nawabi, M.; Ambjørner, S.; Ardeshir-Larijani, D.; Colstrup, L.T.; Borchert, S.V.; Saaby, L.; Brodin, B.; et al. Inhibition of ABCG2 by SCO-101 Enhances Chemotherapy Efficacy in Cancer. Int. J. Mol. Sci. 2025, 26, 3790. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, A.; Shinjo, K.; Naito, K.; Ohnishi, K.; Sugimoto, Y.; Yamakawa, Y.; Tanimoto, M.; Kitamura, K.; Naoe, T.; Ohno, R. Role of P-Glycoprotein in All-Trans Retinoic Acid (ATRA) Resistance in Acute Promyelocytic Leukaemia Cells: Analysis of Intracellular Concentration of ATRA. Br. J. Haematol. 2000, 108, 90–92. [Google Scholar] [CrossRef]
- Haffez, H.; Chisholm, D.R.; Valentine, R.; Pohl, E.; Redfern, C.; Whiting, A. The Molecular Basis of the Interactions between Synthetic Retinoic Acid Analogues and the Retinoic Acid Receptors. MedChemComm 2017, 8, 578–592. [Google Scholar] [CrossRef]
- Yue, H.; Hu, Z.; Hu, R.; Guo, Z.; Zheng, Y.; Wang, Y.; Zhou, Y. ALDH1A1 in Cancers: Bidirectional Function, Drug Resistance, and Regulatory Mechanism. Front. Oncol. 2022, 12, 918778. [Google Scholar] [CrossRef]
- Xanthis, V.; Mantso, T.; Dimtsi, A.; Pappa, A.; Fadouloglou, V.E. Human Aldehyde Dehydrogenases: A Superfamily of Similar Yet Different Proteins Highly Related to Cancer. Cancers 2023, 15, 4419. [Google Scholar] [CrossRef]
- Mei, B.; Li, J.; Wang, D.; Feng, L.; Huang, J.; Zhang, G. All-Trans Retinoic Acid Sensitizes Epithelial Ovarian Cancer to PARP Inhibition after Exposure to Cisplatin. Mol. Cancer Ther. 2025, 24, 453–463. [Google Scholar] [CrossRef]
- Saw, Y.T.; Yang, J.; Ng, S.K.; Liu, S.; Singh, S.; Singh, M.; Welch, W.R.; Tsuda, H.; Fong, W.P.; Thompson, D.; et al. Characterization of Aldehyde Dehydrogenase Isozymes in Ovarian Cancer Tissues and Sphere Cultures. BMC Cancer 2012, 12, 329. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yo, Y.T.; Lee, H.Y.; Liao, Y.P.; Chao, T.K.; Su, P.H.; Lai, H.C. ALDH1-Bright Epithelial Ovarian Cancer Cells Are Associated with CD44 Expression, Drug Resistance, and Poor Clinical Outcome. Am. J. Pathol. 2012, 180, 1159–1169. [Google Scholar] [CrossRef]
- Brown, G. Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells. Int. J. Mol. Sci. 2023, 24, 2373. [Google Scholar] [CrossRef]
- Lavudi, K.; Banerjee, A.; Li, N.; Yang, Y.; Cai, S.; Bai, X.; Zhang, X.; Li, A.; Wani, E.; Yang, S.M.; et al. ALDH1A1 Promotes PARP Inhibitor Resistance by Enhancing Retinoic Acid Receptor-Mediated DNA Polymerase θ Expression. NPJ Precis. Oncol. 2023, 7, 66. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Z.; Wang, T.; Zhao, P.; Liu, M.; Wang, Y.; Zhao, W.; Yuan, Y.; Li, S. Resensitizing Paclitaxel-Resistant Ovarian Cancer via Targeting Lipid Metabolism Key Enzymes CPT1A, SCD and FASN. Int. J. Mol. Sci. 2023, 24, 16503. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Wu, S.; Li, F.; Xu, Q.; Wu, M.; Chen, G.; Liao, G.; Wang, S.; Zhou, J.; Lu, Y.; et al. Breast Cancer Resistance Protein-Mediated Topotecan Resistance in Ovarian Cancer Cells. Int. J. Gynecol. Cancer 2005, 15, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; McArthur, C.; Jaffe, R.B. Ovarian Cancer Stem-like Side-Population Cells Are Tumourigenic and Chemoresistant. Br. J. Cancer 2010, 102, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Seo, E.J.; Kim, D.K.; Lee, S.I.; Kwon, Y.W.; Jang, I.H.; Kim, K.H.; Suh, D.S.; Kim, J.H. FOXP1 Functions as an Oncogene in Promoting Cancer Stem Cell-like Characteristics in Ovarian Cancer Cells. Oncotarget 2016, 7, 3506–3519. [Google Scholar] [CrossRef]
- Brayboy, L.M.; Knapik, L.O.; Long, S.; Westrick, M.; Wessel, G.M. Ovarian Hormones Modulate Multidrug Resistance Transporters in the Ovary. Contracept. Reprod. Med. 2018, 3, 26. [Google Scholar] [CrossRef]
- Ween, M.P.; Armstrong, M.A.; Oehler, M.K.; Ricciardelli, C. The Role of ABC Transporters in Ovarian Cancer Progression and Chemoresistance. Crit. Rev. Oncol. Hematol. 2015, 96, 220–256. [Google Scholar] [CrossRef] [PubMed]
- Eoss, A.C. Cellular Metabolism and Activation of Retinoids: Roles of Cellular Retinoid-Binding Proteins. FASEB J. 1993, 7, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.; Zhi, X.; Ding, W.; Xiong, J.; Tao, T.; Yang, Y.; Zhang, H.; Zi, X.; Zhou, W.; et al. SOX9 Enhances Sorafenib Resistance through Upregulating ABCG2 Expression in Hepatocellular Carcinoma. Biomed. Pharmacother. 2020, 129, 110315. [Google Scholar] [CrossRef] [PubMed]
- Atigadda, V.R.; Xia, G.; Deshpande, A.; Wu, L.; Kedishvili, N.; Smith, C.D.; Krontiras, H.; Bland, K.I.; Grubbs, C.J.; Brouillette, W.J.; et al. Conformationally Defined Rexinoids and Their Efficacy in the Prevention of Mammary Cancers. J. Med. Chem. 2015, 58, 7763–7774. [Google Scholar] [CrossRef]
- Uray, I.P.; Dmitrovsky, E.; Brown, P.H. Retinoids and Rexinoids in Cancer Prevention: From Laboratory to Clinic. Semin. Oncol. 2016, 43, 49–64. [Google Scholar] [CrossRef]
- Mahajan, A.; Singh, L.; Singh, G.; Dhawan, R.K.; Kaur, M.; Malhi, P.K.; Thakur, K.; Kaur, L. An Evidence-Based Review on Bexarotene. Tumor Discov. 2023, 2, 0436. [Google Scholar] [CrossRef]
- Song, J.I.; Lango, M.N.; Hwang, J.D.; Drenning, S.D.; Zeng, Q.; Grandis, J.R.; Grandis, J.R.; Lamph, W.W.; Lamph, W.W. Abrogation of Transforming Growth Factor-α/Epidermal Growth Factor Receptor Autocrine Signaling by an RXR-Selective Retinoid (LGD1069, Targretin) in Head and Neck Cancer Cell Lines. Cancer Res. 2001, 61, 5919–5925. [Google Scholar]
- Kobayashi, T.; Mitsuhashi, A.; Hongying, P.; Shioya, M.; Kojima, K.; Nishikimi, K.; Yahiro, K.; Shozu, M. Bexarotene-Induced Cell Death in Ovarian Cancer Cells through Caspase-4-Gasdermin E Mediated Pyroptosis. Sci. Rep. 2022, 12, 11123. [Google Scholar] [CrossRef]
- Yen, W.C.; Corpuz, M.R.; Prudente, R.Y.; Cooke, T.A.; Bissonnette, R.P.; Negro-Vilar, A.; Lamph, W.W. A Selective Retinoid X Receptor Agonist Bexarotene (Targretin) Prevents and Overcomes Acquired Paclitaxel (Taxol) Resistance in Human Non–Small Cell Lung Cancer. Clin. Cancer Res. 2004, 10, 8656–8664. [Google Scholar] [CrossRef]
- Tooker, P.; Yen, W.C.; Ng, S.C.; Negro-Vilar, A.; Hermann, T.W. Bexarotene (LGD1069, Targretin), a Selective Retinoid X Receptor Agonist, Prevents and Reverses Gemcitabine Resistance in NSCLC Cells by Modulating Gene Amplification. Cancer Res. 2007, 67, 4425–4433. [Google Scholar] [CrossRef]
- Yen, W.C.; Lamph, W.W. A Selective Retinoid X Receptor Agonist Bexarotene (LGD1069, Targretin) Prevents and Overcomes Multidrug Resistance in Advanced Prostate Cancer. Prostate 2006, 66, 305–316. [Google Scholar] [CrossRef]
- Yen, W.C.; Prudente, R.Y.; Lamph, W.W. Synergistic Effect of a Retinoid X Receptor-Selective Ligand Bexarotene (LGD1069, Targretin) and Paclitaxel (Taxol) in Mammary Carcinoma. Breast Cancer Res. Treat. 2004, 88, 141–148. [Google Scholar] [CrossRef]
- Issac, J.; Raveendran, P.S.; Das, A.V. RFX1: A Promising Therapeutic Arsenal against Cancer. Cancer Cell. Int. 2021, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Averitt, D.L.; Maier, C.; Basu, A. The Effects of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (Nrf2) Activation in Preclinical Models of Peripheral Neuropathic Pain. Antioxidants 2022, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, F.; Soozangar, N.; Sadeghi, M.R.; Somi, M.H.; Shirmohamadi, M.; Eftekhar-Sadat, A.T.; Samadi, N. Nrf2 Overexpression Is Associated with P-Glycoprotein Upregulation in Gastric Cancer. Biomed. Pharmacother. 2018, 97, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Abalenikhina, Y.V.; Shchulkin, A.V.; Mylnikov, P.Y.; Rokunov, E.D.; Yakusheva, E.N. Mechanisms of P-Glycoprotein Regulation Under Exogenous and Endogenous Oxidative Stress In Vitro. Acta Naturae 2022, 14, 69–78. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Widjaja-Adhi, M.A.K.; Golczak, M. The Molecular Aspects of Absorption and Metabolism of Carotenoids and Retinoids in Vertebrates. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158571. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Focsan, A.L.; Gao, Y.; Kispert, L.D. The Endless World of Carotenoids-Structural, Chemical and Biological Aspects of Some Rare Carotenoids. Int. J. Mol. Sci. 2023, 24, 9885. [Google Scholar] [CrossRef]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid Biotechnology in Plants for Nutritionally Improved Foods. Physiol. Plant 2006, 126, 369–381. [Google Scholar] [CrossRef]
- Ashenafi, E.L.; Nyman, M.C.; Shelley, J.T.; Mattson, N.S. Spectral Properties and Stability of Selected Carotenoid and Chlorophyll Compounds in Different Solvent Systems. Food Chem. Adv. 2023, 2, 100178. [Google Scholar] [CrossRef]
- Baeza-Morales, A.; Medina-García, M.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; López-Jaén, A.B.; Martínez-Espinosa, R.M.; Sempere-Ortells, J.M. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants 2024, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kageyama, H.; Hibino, T.; Zhang, Y.; Diono, W.; Kanda, H.; Yamaguchi, R.; Takemura, R.; Fukaya, T.; Goto, M. Improved Carotenoid Processing with Sustainable Solvents Utilizing Z-Isomerization-Induced Alteration in Physicochemical Properties: A Review and Future Directions. Molecules 2019, 24, 2149. [Google Scholar] [CrossRef]
- Abramczyk, H.; Kopeć, M.; Surmacki, J. The Triangle: Carotenoids–Retinoids–Cytochromes Govern Essential Functions for Development and Progression of Cancer. Spectrosc. J. 2025, 3, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Na, X.; Zhao, A. Association between β-Carotene Supplementation and Risk of Cancer: A Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2023, 81, 1118–1130. [Google Scholar] [CrossRef]
- Socaciu, C.; Bojarski, P.; Aberle, L.; Diehl, H.A. Different Ways to Insert Carotenoids into Liposomes Affect Structure and Dynamics of the Bilayer Differently. Biophys. Chem. 2002, 99, 1–15. [Google Scholar] [CrossRef]
- Socaciu, C.; Jessel, R.; Diehl, H.A. Competitive Carotenoid and Cholesterol Incorporation into Liposomes: Effects on Membrane Phase Transition, Fluidity, Polarity and Anisotropy. Chem. Phys. Lipids 2000, 106, 79–88. [Google Scholar] [CrossRef]
- Hendrich, A.; Michalak, K. Lipids as a Target for Drugs Modulating Multidrug Resistance of Cancer Cells. Curr. Drug Targets 2003, 4, 23–30. [Google Scholar] [CrossRef]
- Du, H.F.; Jiang, J.M.; Wu, S.H.; Shi, Y.F.; Liu, H.T.; Hua, Z.H.; Wang, C.S.; Qian, G.Y.; Ding, H.M. Fucoxanthin Inhibits the Proliferation and Metastasis of Human Pharyngeal Squamous Cell Carcinoma by Regulating the PI3K/Akt/MTOR Signaling Pathway. Molecules 2024, 29, 3603. [Google Scholar] [CrossRef]
- Goodwin, B.; Hodgson, E.; Liddle, C. The Orphan Human Pregnane X Receptor Mediates the Transcriptional Activation of CYP3A4 by Rifampicin through a Distal Enhancer Module. Mol. Pharmacol. 1999, 56, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Martinec, O.; Biel, C.; de Graaf, I.A.M.; Huliciak, M.; de Jong, K.P.; Staud, F.; Cecka, F.; Olinga, P.; Vokral, I.; Cerveny, L. Rifampicin Induces Gene, Protein, and Activity of P-Glycoprotein (ABCB1) in Human Precision-Cut Intestinal Slices. Front. Pharmacol. 2021, 12, 684156. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Kurian, R.; Wang, H. Clinical Relevance of the Constitutive Androstane Receptor. Drug Metab. Dispos. 2022, 50, 1010. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Luo, Y.Y.; Ren, H.W.; Li, C.J.; Xiang, Z.X.; Luan, Z.L. The Role of Pregnane X Receptor (PXR) in Substance Metabolism. Front. Endocrinol. 2022, 13, 959902. [Google Scholar] [CrossRef]
- Wiśniewska, A.; Draus, J.; Subczynski, W.K. Is a Fluid-Mosaic Model of Biological Membranes Fully Relevant? Studies on Lipid Organization in Model and Biological Membranes. Cell Mol. Biol. Lett. 2003, 8, 147–159. [Google Scholar]
- Pavek, P. Pregnane X Receptor (PXR)-Mediated Gene Repression and Cross-Talk of PXR with Other Nuclear Receptors via Coactivator Interactions. Front. Pharmacol. 2016, 7, 227770. [Google Scholar] [CrossRef]
- Borst, P.; Evers, R.; Kool, M.; Wijnholds, J. A Family of Drug Transporters: The Multidrug Resistance-Associated Proteins. J. Natl. Cancer Inst. 2000, 92, 1295–1302. [Google Scholar] [CrossRef]
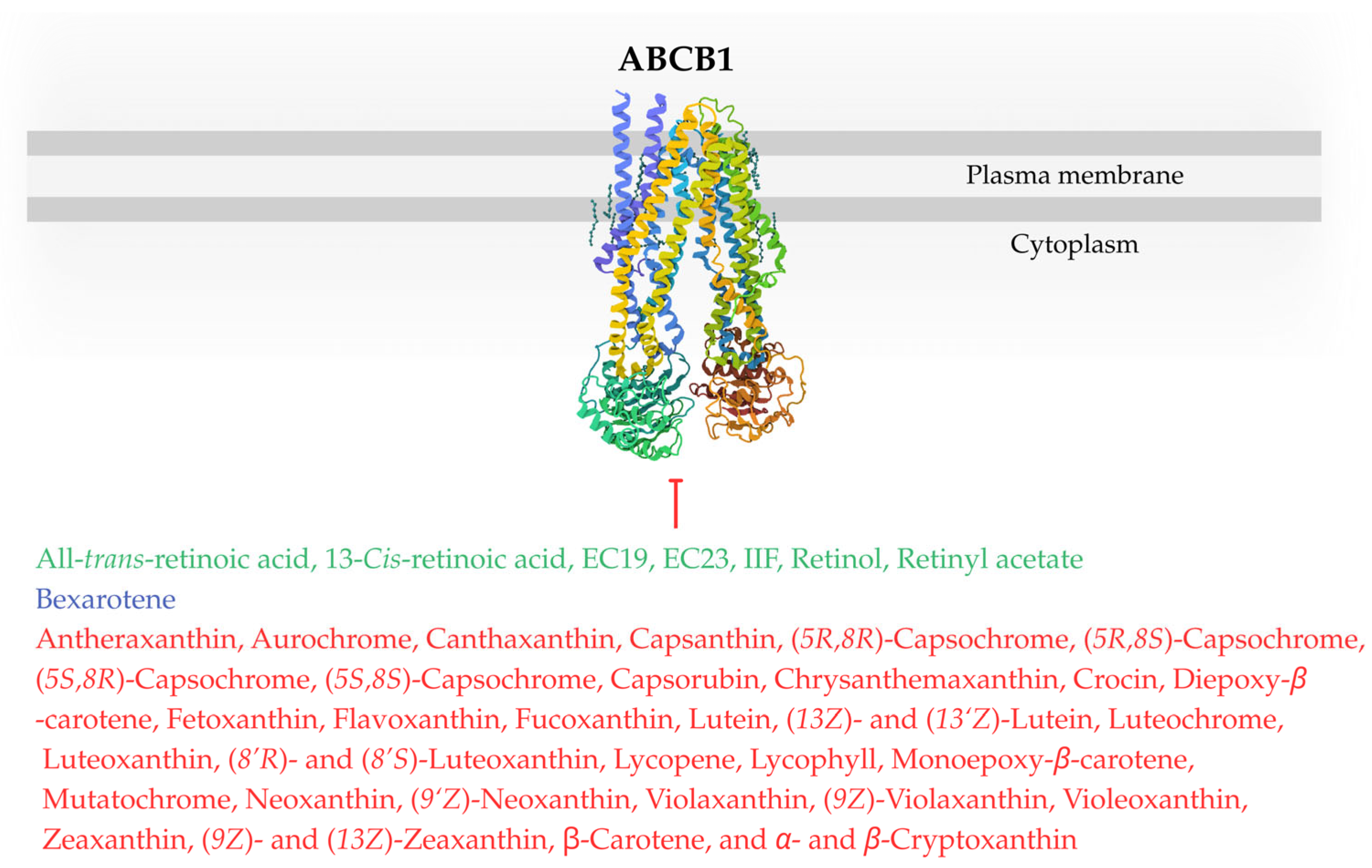
| Drug | Drug Origin | Cell Line/Organism | Effects on ABC Transporters and Chemosensitivity (Drug Concentration; Incubation Time) | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| All-trans-retinoic acid (ATRA, Tretinoin) | U | AML cells from human individuals | Variable effect on ABCB1 mRNA and ABCB1 substrate accumulation (0.1 μM; 72 h) | Possible inverse effect of Egr1 mRNA on ABCB1 expression or positive effect on ABCB1 substrate accumulation in some cells and some incubation periods; no regulatory effect of WT1 | [75] |
| U | AML cells from human individuals | ↑ABCB1 substrate accumulation (45 mg/m2/d; 72 h prior to the standard treatment) | U | [74] | |
| C | Caco-2 | ↑ABCG2 mRNA (0.01, 0.1, 1, 10 and 25 μM; 8 h), (1 μM; 6, 12 and 24 h); ↑ABCG2 protein expression (0.01, 0.1, 1, 10 and 25 μM; 2 days), ↑efflux of B[a]P-3-sulfate (0.1, 1, 5 and 10 μM; 48 h) | RAR/RXR signaling | [64] | |
| C | Caco-2 | ↑ABCB1 substrate accumulation (1–250 µM; 30 min); synergism with cisplatin, DOX, ETO, 5-FU and VINB, antagonism with PAC (40 μM; 24 h); no decrease in ABCB1 mRNA (40 μM; 48 h) | U | [79] | |
| C | Caco-2 | ↓mRNA of ABCC1 and ABCG2, no change in ABCB1 mRNA (IC50 = 97.70 ± 9.0, 24 h); ↓protein expression of ABCB1 and ABCC1 (IC50, 24 h); no impact on calcium-independent ATPase and no synergism with AC261066 and CD437 (IC50; 24 h) | U | [71] | |
| C | CEM/ADR5000 | ↑ABCB1 substrate accumulation (10, 20, 50 and 100 µM; 30 min pretreatment) | U | [79] | |
| C | H9 | ↑ABCB1 mRNA, no impact on ABCB1 substrate accumulation (5 μM; 48 h) | U | [76] | |
| C | H9/RAR | ↑ABCB1 mRNA; ↓ABCB1 substrate accumulation (5 μM; 48 h) | U | [76] | |
| C | Kasumi-1, Kasumi-6 | ↑ABCB1 mRNA (1 μM; U) alone and in combination with FK228 | U | [77] | |
| U | Kasumi-1 | ↑ABCB1 mRNA and ↓ABCB1 substrate accumulation (0.1 μM; 1–72 h) | No regulatory effect of Egr1 and WT1 | [75] | |
| C | KG-1 | ↑ABCB1 mRNA; ↓ABCB1 substrate accumulation (5 μM; 48 h) | U | [76] | |
| U | KG-1 | Maintenance of basal ABCB1 mRNA and ABCB1 substrate accumulation (0.1 μM; 1–72 h) | No regulatory effect of Egr1 and WT1 | [75] | |
| C | KG-1/RAR | ↑ABCB1 mRNA; ↓ABCB1 substrate accumulation (5 μM; 48 h) | U | [76] | |
| C | K562 | ↑ABCB1 mRNA; ↓ABCB1 substrate accumulation (5 μM; 48 h) | U | [76] | |
| C | K562/RAR | ↑ABCB1 mRNA; ↓ABCB1 substrate accumulation (5 μM; 48 h) | U | [76] | |
| C | LoVo/MDR | ↓ABCB1 protein expression (20 μM; 48 h) | U | [84] | |
| U | L1210/S | No significant change in ABCB1 mRNA and protein expression (3.3 μM; U) | Different effects in particular cells, probably altered gene transcription | [80] | |
| U | L1210/R | No effect in monotherapy, in combination with VER: ↓ABCB1 mRNA and protein expression, ↑ABCB1 substrate accumulation, ↑chemosensitivity to VINC (3.3 μM; U) | Different effects in particular cells; probably altered gene transcription | [80] | |
| U | L1210/T | ↑ABCB1 mRNA and protein expression in monotherapy and with VER, no effect on ABCB1 substrate accumulation, ↑chemosensitivity to VINC (3.3 μM; U) | Different effects in particular cells; probably altered gene transcription | [80] | |
| C | L1210/VCR | No effect in monotherapy, in combination with VER: ↓protein expression and ↑ABCB1 substrate accumulation (3.3 μM; 20 h) | Likely through VER-mediated CYP450 inhibition of retinoid metabolism | [78] | |
| C | MDA-MB-231, MDA-MB-468 | No significant change in ABCB1 protein expression (5 μM; 7 days) | U | [88] | |
| C | MDCK ABCG2 | No effect on ABCG2 substrate accumulation (10, 25, 50, 100 μM; 20 min) | No effect on membrane density/fluidity | [68] | |
| C | MM cancer stem-like cells | ↓ABCG2 mRNA (20 μM; 24 h) and ↑chemosensitivity to DOC when ATRA (20 μM; 24 h + 24 h) used with RES | U | [73] | |
| C | NB4 | ↑ABCB1 mRNA; undetermined impact on efflux activity (5 μM; U) | U | [76] | |
| C | NB4 | ↑ABCB1 mRNA and protein expression (1 μM; 72 h), ↑ABCB1 substrate efflux (1 μM; U), especially in combination with FK228; ↓cytotoxicity of DOX in ATRA/FK228 pretreatment (1 μM; 24 h); ↑cytotoxicity of DOX in ATRA/FK228 posttreatment (1 μM; 48 h) | ↑ H4 and H3-Lys9 acetylation, recruitment of NF-YA to the CCAAT box in the ABCB1 promoter | [77] | |
| C | NB4/RAR | ↑ABCB1 mRNA; undetermined impact on efflux activity (5 μM; U) | U | [76] | |
| C | NIH 3T3 MDR1 | No effect on ABCB1 substrate accumulation (10, 25, 50 μM; 20 min) | No effect on membrane properties | [68] | |
| U | W1PR | No change in ABCB1 mRNA (5 μM; 1–4 d); ↓ABCB1 protein expression (5 μM; 1–4 days); ↑chemosensitivity to paclitaxel (5 μM; 48 + 72 h) | ↓ALDH1A1 protein expression | [65] | |
| U | W1TR | Transiently ↑ABCG2 mRNA (5 μM; 1–2 d); ↓ABCG2 protein expression (5 μM; 3–4 d); ↑chemosensitivity to topotecan (5 μM; 48 + 72 h) | ↓ALDH1A1 protein expression | [65] | |
| 9-Cis-retinoic acid (Alitretinoin) | U | L1210/S | No significant change in ABCB1 mRNA and protein expression (3.3 μM; U) | Different effects in particular cells, probably altered gene transcription | [80] |
| U | L1210/R | ↑ABCB1 mRNA in monotherapy and with VER, ↑ABCB1 protein expression in combination with VER, no effect on ABCB1 substrate accumulation, ↑vincristine chemosensitivity (3.3 μM; U) | Different effects in particular cells, probably altered gene transcription | [80] | |
| U | L1210/T | ↑ABCB1 mRNA in combination with VER, ↑ABCB1 protein level in monotherapy and with VER, no effect on ABCB1 substrate accumulation, ↑vincristine chemosensitivity (3.3 μM; U) | Different effects in particular cells, probably altered gene transcription | [80] | |
| C | MDCK ABCG2 | No effect on ABCG2 substrate accumulation (10, 25, 50, 100 μM; 20 min) | No effect on membrane properties | [68] | |
| C | NIH 3T3 MDR1 | No effect on ABCB1 substrate accumulation (10, 25, 50 μM; 20 min) | No effect on membrane properties | [68] | |
| 13-Cis-retinoic acid (Isotretinoin) | C | MDCK ABCG2 | ↑ABCG2 substrate accumulation (10–100 μM; 20 min) | Alteration of membrane properties | [68] |
| C | NIH 3T3 MDR1 | ↑ABCB1 substrate accumulation (25 μM; 20 min) | Alteration of membrane fluidity and density | [68] | |
| EC19 | C | Caco-2 | ↓mRNA of ABCB1 and ABCC1, no change in ABCG2 mRNA (IC50 = 27.20 ± 1.8; 24 h); ↓protein expression of ABCB1 and ABCC1 (IC50; 24 h); ↓activity of calcium-independent ATPase and synergism with AC261066 and CD437 (IC50; 24 h) | U | [71] |
| EC23 | C | Caco-2 | ↓mRNA of ABCC1 and ABCG2, no change in ABCB1 mRNA (IC50 = 23.00 ± 1.2, 24 h); ↓protein expression of ABCB1 and ABCC1 (IC50, 24 h); ↓activity of calcium-independent ATPase and synergism with AC261066 and CD437 (IC50; 24 h) | U | [71] |
| IIF | Synthetic | LoVo/MDR | ↓ABCB1 protein expression (20 μM; 48 h) | U | [84] |
| Retinol (vitamin A) | U | HT29 | No effect on ABCB1 mRNA expression (7 μM; 24 h) | U | [85] |
| C | MDCK ABCG2 | ↑ABCG2 substrate accumulation (50–100 μM; 20 min) | Alteration of membrane fluidity and density | [68] | |
| C | NIH 3T3 MDR1 | ↑ABCB1 substrate accumulation (50 μM; 20 min) | Alteration of membrane fluidity and density | [68] | |
| U | SW620 | ↓ABCB1 mRNA (7 μM; 24 h) and ↑chemosensitivity to etoposide after pretreatment (7 μM; 24 h) | ↑oxidative state | [85] | |
| Retinyl acetate | C | MDCK ABCG2 | ↑ABCG2 substrate accumulation (100 μM; 20 min) | Alteration of membrane fluidity and density | [68] |
| C | NIH 3T3 MDR1 | ↑ABCB1 substrate accumulation (50 μM; 20 min) | Alteration of membrane fluidity and density | [68] | |
| Retinyl palmitate | C | MDCK ABCG2 | No effect on ABCG2 substrate accumulation (10, 25, 50, 100 μM; 20 min) | No effect on membrane properties | [68] |
| C | NIH 3T3 MDR1 | No effect on ABCB1 substrate accumulation (10, 25, 50 μM; 20 min) | No effect on membrane properties | [68] | |
| Retinyl propionate | C | MDCK ABCG2 | No effect on ABCG2 substrate accumulation (10, 25, 50, 100 μM; 20 min) | No effect on membrane properties | [68] |
| C | NIH 3T3 MDR1 | No effect on ABCB1 substrate accumulation (10, 25, 50 μM; 20 min) | No effect on membrane properties | [68] |
| Drug | Drug Origin | Cell Line/Organism | Effects on ABC Transporters and Chemosensitivity (Drug Concentration; Incubation Time) | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Bexarotene | C | MDA-MB-231 (resistant variants) | ↓ABCB1 mRNA and ↑ABCB1 substrate accumulation in PAC-resistant cells (1 µM, U); ↑chemosensitivity to CIS, DOX, and PAC (1 µM, 1–90 days) | U | [63] |
| U | NT2 | ↓mRNA of ABCB1, ABCC1, ABCC2, and ABCG2 (25 µM; U); ↑chemosensitivity to CIS (10 µM; 48 h) | ↑RXRα signaling → ↑RFX1; ↓Nrf2; ↓HIF-1α | [72] |
| Drug | Drug Origin | Cell Line/Organism | Effects on ABC Transporters and Chemosensitivity (Drug Concentration; Incubation Time) | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Antheraxanthin | U | Colo 320 | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [83] |
| Viola tricolor, yellow flowers | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] | |
| Aurochrome | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | Slightly ↓ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| Canthaxanthin | C | Caco-2 | ↑ABCB1 substrate accumulation (1–250 µM; 30 min); synergistic effect with CIS, DOX, ETO, 5-FU, PAC, and VINB (40 µM; 24 h); ↓ABCB1 mRNA (40 µM; 48 h) | U | [79] |
| C | CEM/ADR5000 | ↑ABCB1 substrate accumulation (10, 20, 50 and 100 µM; 90 min) | U | [79] | |
| Capsanthin | Capsicum annuum, red paprika | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] |
| U | MCF-7/Doc | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min); additive effect with DOC (U; 72 h) | U | [87] | |
| U | MCF-7/Dox | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min); additive effect with DOX (U; 72 h) | U | [87] | |
| U | MCF-7/Pac | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min); additive effect with PAC (U; 72 h) | U | [87] | |
| U | MCF-7/Vinc | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min); indifferent effect with VINC (U; 72 h) | U | [87] | |
| Capsorubin | Capsicum annuum, red paprika | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] |
| (5R,8R)-Capsochrome | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| (5R,8S)-Capsochrome | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| (5S,8R)-Capsochrome | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| (5S,8S)-Capsochrome | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); synergism with EPI (U; 72 h) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); antagonism with EPI (U; 72 h) | U | [82] | |
| Chrysanthemaxanthin + flavoxanthin | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min) | U | [82] | |
| Crocetin nanoparticles (PLGA-Crt NPs) | Crocus sativus L., saffron | A2780/RCIS | ↓ABCC2 mRNA and no decrease in ABCC1 mRNA (25, 50, 100 and 200 µM; 48 h); ↓efflux of DOX (25, 50, 100 and 200 µM; 48 h) | U | [69] |
| Crocin | Crocus sativus L., saffron | A2780/RCIS | ↓ABCC1 mRNA (25 and 100 µM; 48 h) and ABCC2 mRNA (25, 50 and 100 µM; 48 h); ↑chemosensitivity to DOX (25, 50, and 100 µM; 24, 48 and 72 h) | U | [66] |
| C | Caco-2 | ↑ABCB1 substrate accumulation (1–250 µM; 30 min); synergistic effect with CIS, DOX, and VINB (40 µM; 24 h), antagonism with ETO, 5-FU and PAC (40 µM; 24 h); ↓ABCB1 mRNA (40 µM; 48 h) | U | [79] | |
| C | CEM/ADR5000 | ↑ABCB1 substrate accumulation (10, 20, 50 and 100 µM; 30 min) | U | [79] | |
| Crocus sativus L., saffron | EPG85-257 | No decrease in ABCB1 mRNA (25, 50, and 100 µM; 48 h); ↑chemosensitivity to DOX (25, 50, and 100 µM; 24, 48, and 72 h) | U | [86] | |
| Crocus sativus L., saffron | EPG85-257RDB | No decrease in ABCB1 mRNA (25, 50, and 100 µM; 48 h); ↑chemosensitivity to DOX (25, 50, and 100 µM; 24, 48, and 72 h) | U | [86] | |
| 15,15′-Dehydrodiepoxy-β-carotene | Lab internal collection | L5178Y (MDR1/A) | ↓ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↓ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| Diepoxy-β-carotene | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| Fetoxanthin | Isolated from apple peel | Colo 320 | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [83] |
| Fucoxanthin | C | Caco-2 | ↑ABCB1 substrates accumulation (1–250 µM; 30 min); synergism with CIS, DOX, ETO, 5-FU, PAC, and VINB (40 µM; 24 h), ↓ABCB1 mRNA (40 µM; 48 h) | U | [79] |
| C | CEM/ADR5000 | ↑ABCB1 substrates accumulation (10, 20, 50 and 100 µM; 90 min) | U | [79] | |
| Undaria pinnatifida, wakame | HepG-2 | ↓ABCB1 mRNA (1, 5, and 10 µM; 24 h) | ↓PXR signaling via inhibition of interaction with SRC-1 coactivator and ↓hCAR | [46] | |
| C | HepG-2/Dox | ↑ABCB1 substrate accumulation (20 µM; 24 h); ↑DOX accumulation and synergism with DOX (20 µM; 30 min) | U | [70] | |
| Undaria pinnatifida, wakame | LS174T | ↓ABCB1 mRNA (5 and 10 µM; 24 h) | ↓PXR signaling via inhibition of interaction with SRC-1 coactivator and ↓hCAR | [46] | |
| C | MCF-7/Dox | ↑ABCB1 substrate accumulation (20 µM; 24 h); ↑DOX accumulation and synergism with DOX (20 µM; 30 min); ↓mRNA levels of ABCB1, ABCC1, and ABCG2 (U; 24 h) | ↓PXR mRNA | [70] | |
| C | SKOV-3/Dox | ↑ABCB1 substrate accumulation (20 µM; 24 h); ↑DOX accumulation and synergism with DOX (20 µM; 30 min) | U | [70] | |
| Lutein | U | Colo 320 | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [83] |
| Caltha palustris, marsh marigold | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] | |
| (13Z) + (13′Z)-Lutein | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| Luteochrome | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| Luteoxanthin | U | Colo 320 | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [83] |
| (8′R)-Luteoxanthin | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| (8′S)-Luteoxanthin | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); synergism with EPI (U; 72 h) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); additive effect with EPI (U; 72 h) | U | [82] | |
| Lycopene | Lycopersicon esculentum, tomato | L5178Y (MDR1/A) | No effect on ABCB1 substrate accumulation (2 µg/mL; 10 min); ↑ABCB1 substrate accumulation (20 µg/mL; 10 min) | U | [81] |
| Lycophyll | Solanum dulcamara, bittersweet nightshade | L5178Y (MDR1/A) | No effect on ABCB1 substrate accumulation (2 µg/mL; 10 min); ↑ABCB1 substrate accumulation (20 µg/mL; 10 min) | U | [81] |
| Monoepoxy-α-carotene | Lab internal collection | L5178Y (MDR1/A) | ↓ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↓ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| Monoepoxy-β-carotene | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); additive effect with EPI (U; 72 h) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); indifferent effect with EPI (U; 72 h) | U | [82] | |
| Mutatochrome | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| Neoxanthin | U | Colo 320 | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min) | U | [83] |
| (9′Z)-Neoxanthin | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↓ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [82] | |
| Violaxanthin | U | Colo 320 | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [83] |
| Viola tricolor, yellow flowers | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] | |
| (9Z)-Violaxanthin | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); additive effect with EPI (U; 72 h) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); synergism with EPI (U; 72 h) | U | [82] | |
| Violeoxanthin | U | Colo 320 | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [83] |
| Zeaxanthin | Lycium halimifolium | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] |
| U | MCF-7/Doc | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min); additive effect with DOC (U; 72 h) | U | [87] | |
| U | MCF-7/Dox | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min); synergism with DOX (U; 72 h) | U | [87] | |
| U | MCF-7/Pac | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min); additive effect with PAC (U; 72 h) | U | [87] | |
| U | MCF-7/Vinc | ↑ABCB1 substrate accumulation (40 µg/mL; 10 min); additive effect with VINC (U; 72 h) | U | [87] | |
| (9Z)-Zeaxanthin | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); synergism with EPI (U; 72 h) | U | [82] |
| Lab internal collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); additive effect with EPI (U; 72 h) | U | [82] | |
| (13Z)-Zeaxanthin | Lab internal collection | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); synergism with EPI (U; 72 h) | U | [82] |
| Lab collection | MCF-7 (DOX-resistant) | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min); synergism with EPI (U; 72 h) | U | [82] | |
| α-Carotene | Daucus Carotta, carrot | L5178Y (MDR1/A) | No effect on ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] |
| β-Carotene | C | ABCB1/Flp-In TM-293 | ↑Calcein accumulation (10, 25, 50, and 100 µM; 30 min); ↑Rh-123 accumulation (IC50 = 25.72 ± 0.2 μM; 30 min); ↑DOX accumulation (IC50 = 16.81 ± 0.43 μM; 3 h); ↑ABCB1 ATP-ase activity (10–100 μM; U); slight conformational change of ABCB1 protein (100 μM; U); ↑chemosensitivity to DOX (10 and 20 µM; 72 h); no impact on ABCB1 mRNA (100 µM; 48 h) | U | [67] |
| C | ABCC1/Flp-In TM-293 | No impact on calcein accumulation (10, 25, 50, and 100 µM; 30 min) | U | [67] | |
| C | ABCG2/Flp-In TM-293 | ↑Mitoxantrone accumulation (10, 25, 50, and 100 µM; 30 min) | U | [67] | |
| C | Caco-2 | ↑ABCB1 substrates accumulation (1–250 µM; 30 min); synergism with CIS, DOX, ETO, 5-FU, and VINB (40 µM; 24 h), antagonism with PAC; ↓ABCB1 mRNA (40 µM; 48 h) | U | [79] | |
| C | CEM/ADR5000 | ↑ABCB1 substrates accumulation (10, 20, 50 and 100 µM; 90 min) | U | [79] | |
| C | KB-vin | ↑Chemosensitivity to PAC, DOX and 5-FU, ↓chemosensitivity to ETO (50 µM, 72 h); ↑ABCB1 mRNA (100 µM; 72 h) | U | [67] | |
| Daucus carotta, carrot | L5178Y (MDR1/A) | No effect on ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] | |
| C | NCI-H460/MX20 | ↑Chemosensitivity to mitoxantrone (50 µM; 72 h) | U | [67] | |
| α-Cryptoxanthin | Yellow paprika, Valencia orange peels | L5178Y (MDR1/A) | No effect on ABCB1 substrate accumulation (2 µg/mL; 10 min); ↑ABCB1 substrate accumulation (20 µg/mL; 10 min) | U | [81] |
| β-Cryptoxanthin | U | Colo 320 | ↑ABCB1 substrate accumulation (4/40 µg/mL; 10 min) | U | [83] |
| Yellow paprika, Valencia orange peels | L5178Y (MDR1/A) | ↑ABCB1 substrate accumulation (2/20 µg/mL; 10 min) | U | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čižmáriková, M.; Háziková, V.; Michalková, R.; Franko, O.; Lešková, B.; Homolya, A.D.; Gabzdilová, J.; Takáč, P., Jr. Targeting Multidrug Resistance in Cancer: Impact of Retinoids, Rexinoids, and Carotenoids on ABC Transporters. Int. J. Mol. Sci. 2025, 26, 11157. https://doi.org/10.3390/ijms262211157
Čižmáriková M, Háziková V, Michalková R, Franko O, Lešková B, Homolya AD, Gabzdilová J, Takáč P Jr. Targeting Multidrug Resistance in Cancer: Impact of Retinoids, Rexinoids, and Carotenoids on ABC Transporters. International Journal of Molecular Sciences. 2025; 26(22):11157. https://doi.org/10.3390/ijms262211157
Chicago/Turabian StyleČižmáriková, Martina, Viktória Háziková, Radka Michalková, Ondrej Franko, Beáta Lešková, Atila David Homolya, Juliana Gabzdilová, and Peter Takáč, Jr. 2025. "Targeting Multidrug Resistance in Cancer: Impact of Retinoids, Rexinoids, and Carotenoids on ABC Transporters" International Journal of Molecular Sciences 26, no. 22: 11157. https://doi.org/10.3390/ijms262211157
APA StyleČižmáriková, M., Háziková, V., Michalková, R., Franko, O., Lešková, B., Homolya, A. D., Gabzdilová, J., & Takáč, P., Jr. (2025). Targeting Multidrug Resistance in Cancer: Impact of Retinoids, Rexinoids, and Carotenoids on ABC Transporters. International Journal of Molecular Sciences, 26(22), 11157. https://doi.org/10.3390/ijms262211157






