Downregulation of Enteroendocrine Genes Predicts Survival in Colon Cancer: A Bioinformatics-Based Analysis
Abstract
1. Introduction
2. Results
2.1. Transcriptional Profile Analysis Reveals the Conserved Downregulation of EEC Markers in CRC
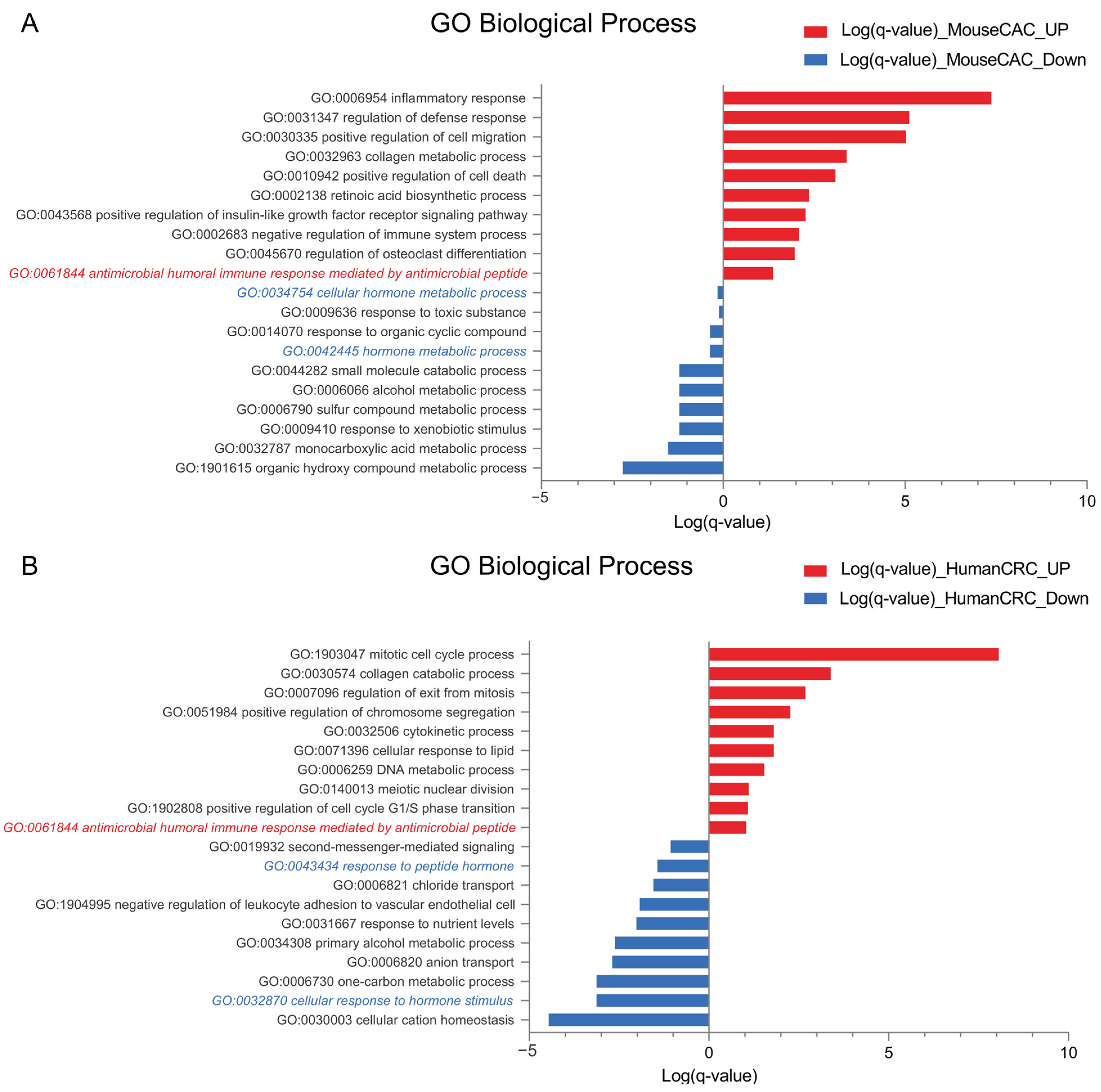
2.2. Antimicrobial Peptide-Related Pathways Are Upregulated While Hormonal Processes Are Downregulated in Colorectal Cancer
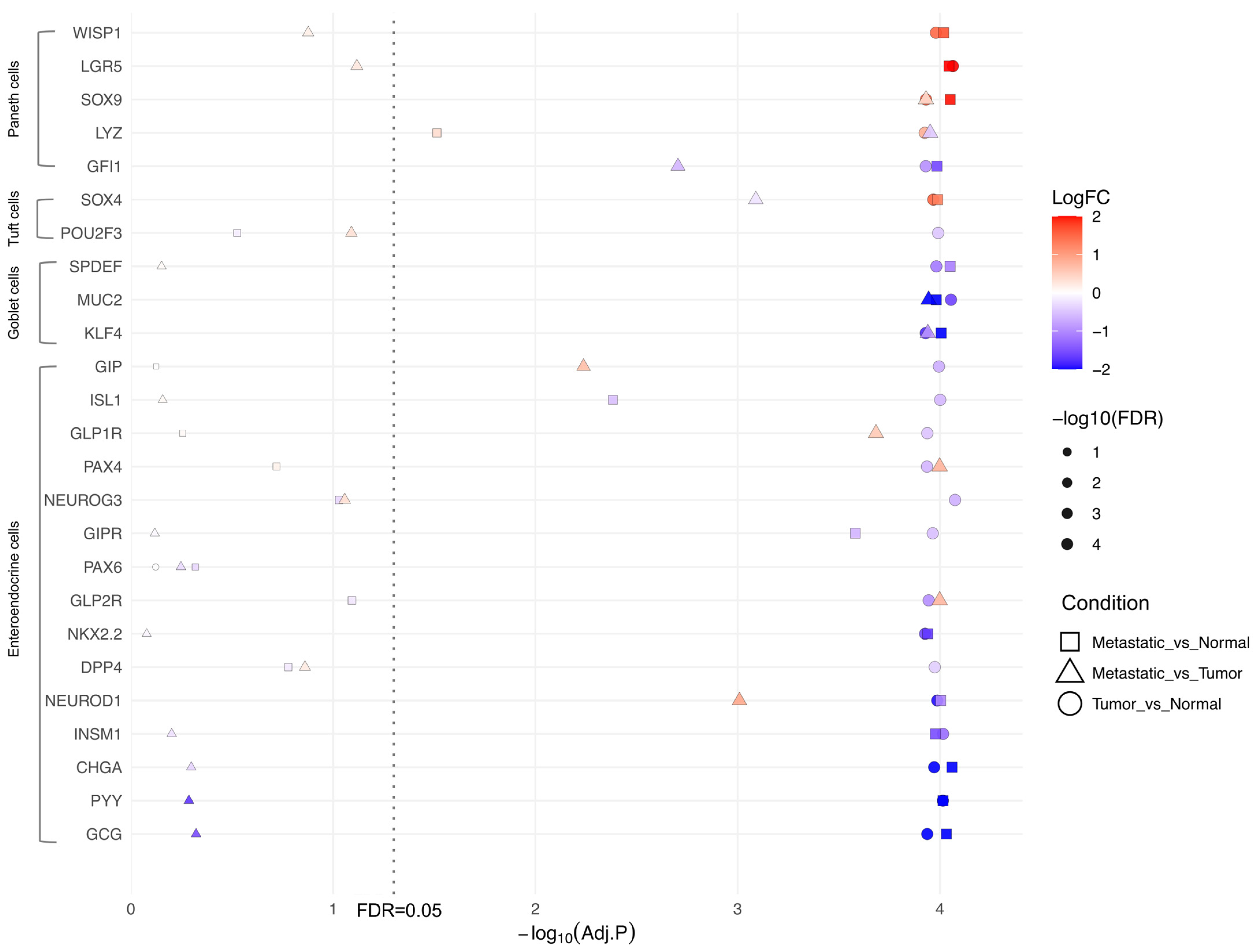
2.3. Upregulation of Paneth Cell Markers and Downregulation of EEC Genes Is Conserved Across Colon Tumor Origin
2.4. RNA-Seq Analysis from TCGA Confirms That EEC Markers Are Among Most Downregulated Genes
2.5. Modulation of EEC and Paneth Cell Markers Occurs Independently of Tumor Stage
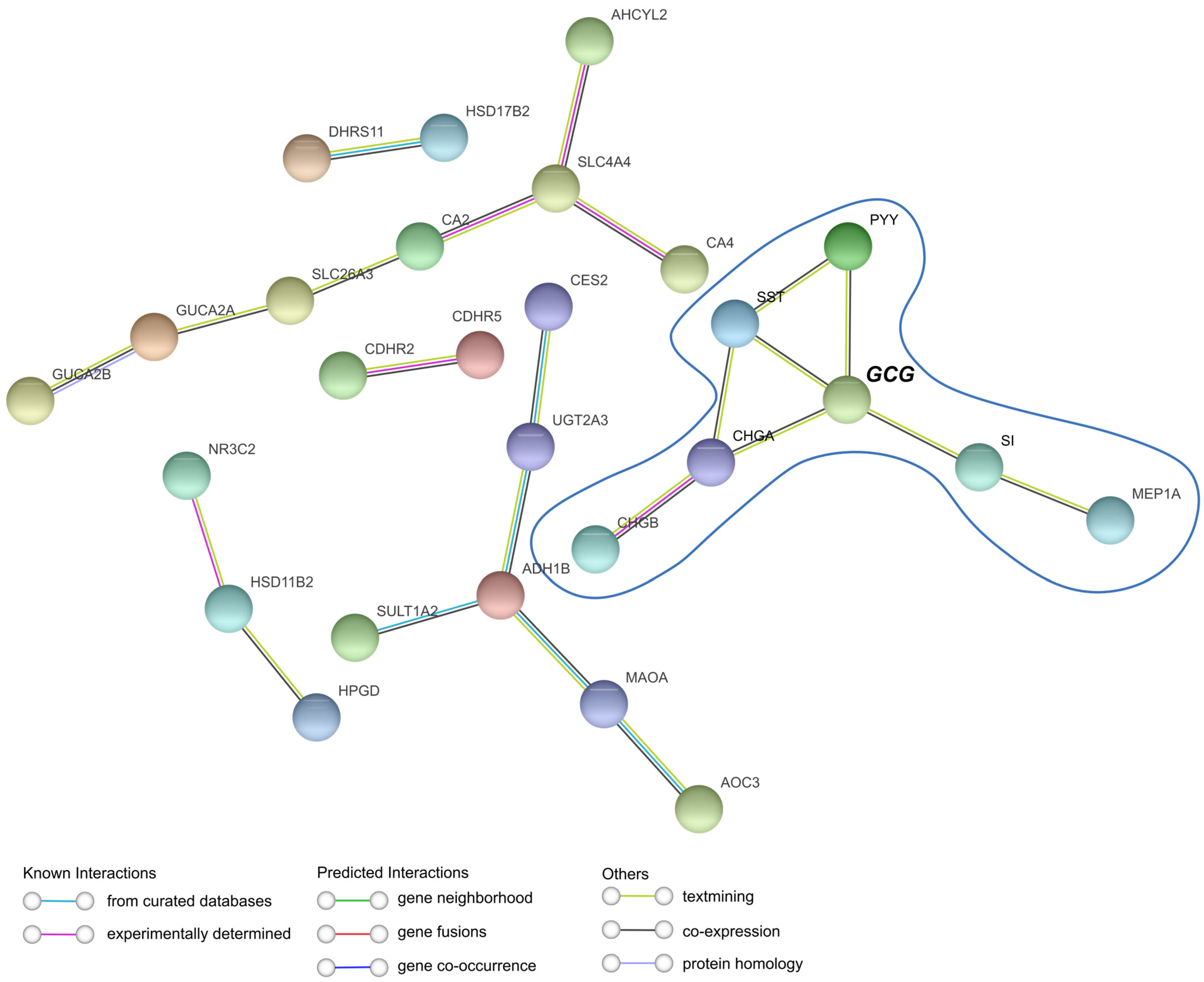
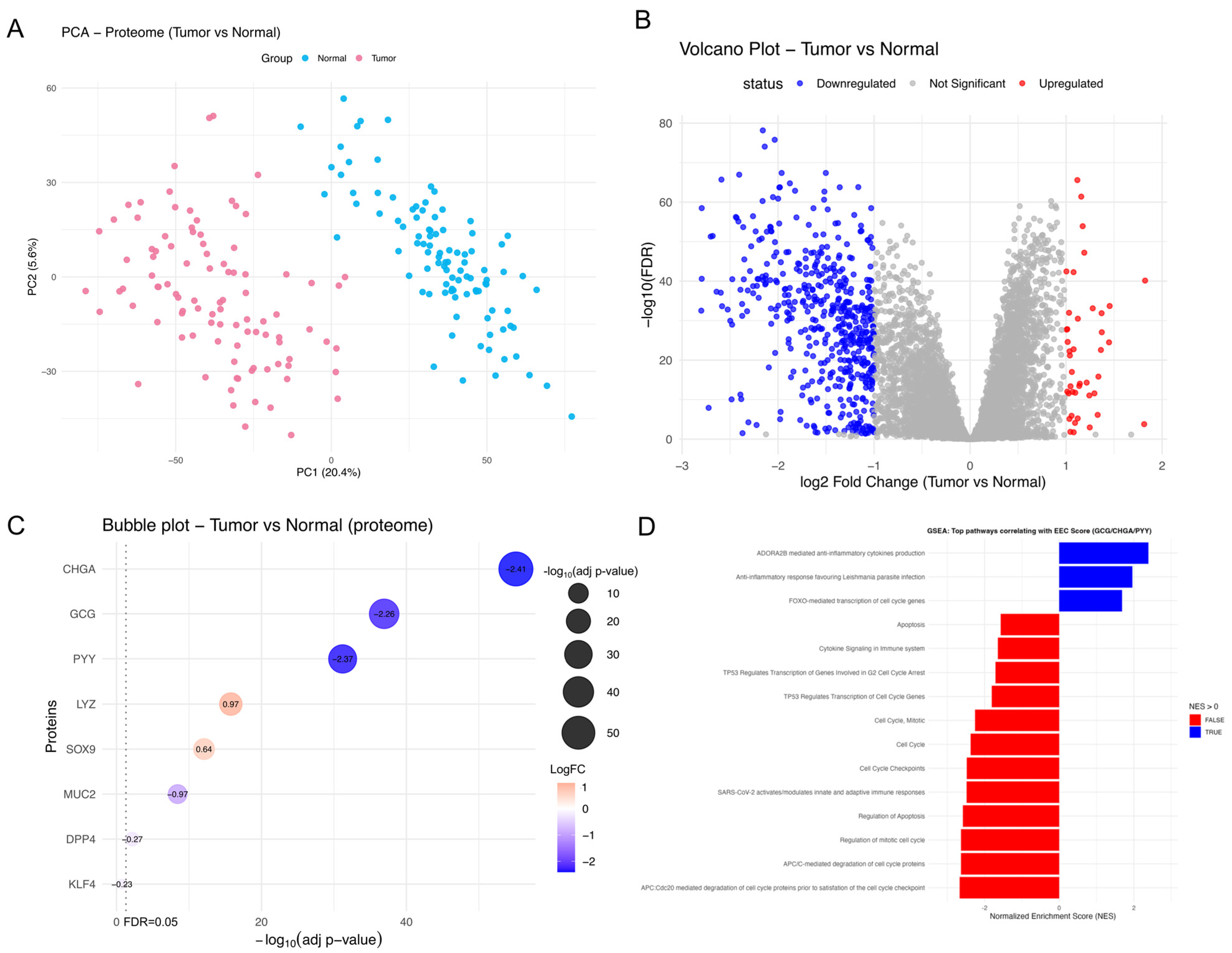
2.6. Protein-Level Evidence Supports EEC/Paneth Cell Marker Balance in CRC
2.7. EEC Modulation in TME Is Linked to Apoptosis and Proliferation and Driven by WNT Signaling
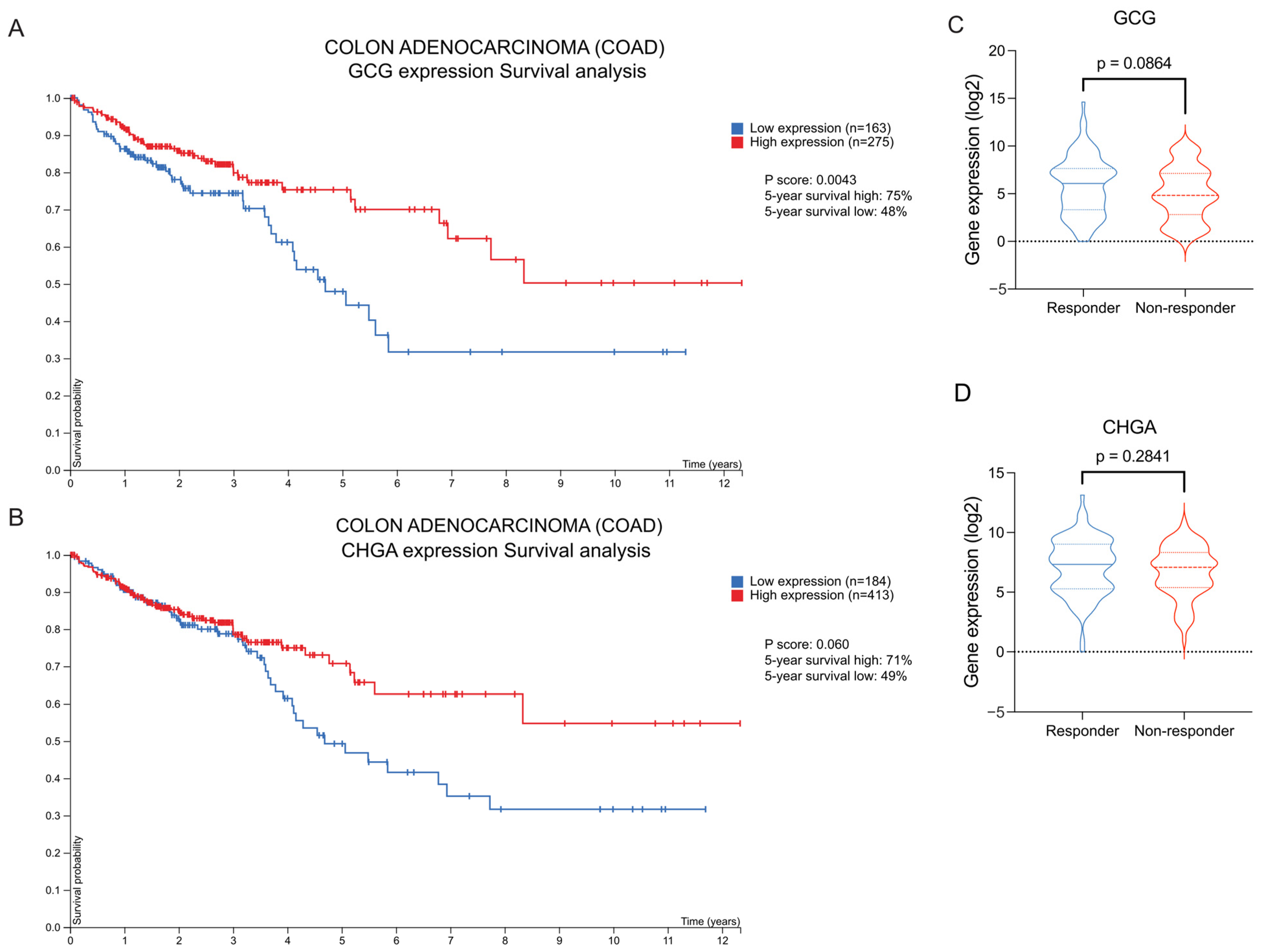
2.8. High GCG and CHGA Expression Correlates with Improved Overall Survival
3. Discussion
4. Materials and Methods
4.1. Database Collection
4.2. Differential Expression Analysis Datasets from NCBI
4.3. RNA-Seq Differential Expression Analysis from TCGA Database
4.4. Proteomics Analysis from CPTAC, Differential Expression, and GSEA
4.5. Mediator Analyses with Proteomics Data
4.6. Survival Analysis and Chemotherapy Responder Correlation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACME | average causal mediation effect |
| ADE | average direct effect |
| AOM | azoxymethane |
| CAC | colitis-associated colorectal cancer |
| COAD | colon adenocarcinoma |
| CPTAC | National Cancer Institute’s Proteomics Tumor Analysis Consortium |
| CRC | colorectal cancer |
| DSS | dextran sulfate sodium |
| EEC | enteroendocrine cell |
| GEO | Gene Expression Omnibus |
| GO | Gene Ontology |
| GSEA | gene set enrichment analysis |
| IEC | intestinal epithelial cell |
| NCBI | National Center for Biotechnology Information |
| PCA | principal component analysis |
| PPI | protein–protein interaction |
| TCGA | The Cancer Genome Atlas Program |
| TME | tumor microenvironment |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and Colon Cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and Cancer: Inflammation Bridges the Two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, J.-S.; Seo, Y.-R.; Sung, M.-K. Effects of Diet-Induced Obesity on Colitis-Associated Colon Tumor Formation in A/J Mice. Int. J. Obes. 2012, 36, 273–280. [Google Scholar] [CrossRef][Green Version]
- Moghaddam, A.A.; Woodward, M.; Huxley, R. Obesity and Risk of Colorectal Cancer: A Meta-Analysis of 31 Studies with 70,000 Events. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2533–2547. [Google Scholar] [CrossRef]
- Rustgi, A.K. The Genetics of Hereditary Colon Cancer. Genes. Dev. 2007, 21, 2525–2538. [Google Scholar] [CrossRef]
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine Cells-Sensory Sentinels of the Intestinal Environment and Orchestrators of Mucosal Immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Ray, S.K.; Singh, N.K.; Johnston, B.; Leiter, A.B. Basic Helix-loop-helix Transcription Factors and Enteroendocrine Cell Differentiation. Diabetes Obes. Metab. 2011, 13, 5–12. [Google Scholar] [CrossRef]
- Bastide, P.; Darido, C.; Pannequin, J.; Kist, R.; Robine, S.; Marty-Double, C.; Bibeau, F.; Scherer, G.; Joubert, D.; Hollande, F.; et al. Sox9 Regulates Cell Proliferation and Is Required for Paneth Cell Differentiation in the Intestinal Epithelium. J. Cell Biol. 2007, 178, 635–648. [Google Scholar] [CrossRef]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft Cells and Their Role in Intestinal Diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Koehne de Gonzalez, A.; del Portillo, A. P754 Beyond Paneth Cell Metaplasia: Small Intestinal Metaplasia of the Sigmoid Colon in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2023, 17, i885–i886. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth Cells, Antimicrobial Peptides and Maintenance of Intestinal Homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef]
- Gunawardene, A.R.; Corfe, B.M.; Staton, C.A. Classification and Functions of Enteroendocrine Cells of the Lower Gastrointestinal Tract. Int. J. Exp. Pathol. 2011, 92, 219–231. [Google Scholar] [CrossRef]
- Drucker, D.J. The GLP-1 Journey: From Discovery Science to Therapeutic Impact. J. Clin. Investig. 2024, 134, e175634. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2020, 26, 11–20. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.; Yariwake, V.Y.; Alves, R.W.; de Araujo, D.R.; Andrade-Oliveira, V. Crosstalk between Incretin Hormones, Th17 and Treg Cells in Inflammatory Diseases. Peptides 2022, 155, 170834. [Google Scholar] [CrossRef]
- Okayasu, I.; Ohkusa, T.; Kajiura, K.; Kanno, J.; Sakamoto, S. Promotion of Colorectal Neoplasia in Experimental Murine Ulcerative Colitis. Gut 1996, 39, 87–92. [Google Scholar] [CrossRef]
- Tominaga, O.; Nita, M.E.; Nagawa, H.; Fujii, S.; Tsuruo, T.; Muto, T. Expressions of Cell Cycle Regulators in Human Colorectal Cancer Cell Lines. Jpn. J. Cancer Res. 1997, 88, 855–860. [Google Scholar] [CrossRef]
- Luvhengo, T.; Mabasa, S.; Molepo, E.; Taunyane, I.; Palweni, S.T. Paneth Cell, Gut Microbiota Dysbiosis and Diabetes Mellitus. Appl. Sci. 2023, 13, 6605. [Google Scholar] [CrossRef]
- Wang, L.; Shen, F.; Stroehlein, J.R.; Wei, D. Context-Dependent Functions of KLF4 in Cancers: Could Alternative Splicing Isoforms Be the Key? Cancer Lett. 2018, 438, 10–16. [Google Scholar] [CrossRef]
- Abdullayeva, G.; Liu, H.; Liu, T.-C.; Simmons, A.; Novelli, M.; Huseynova, I.; Lastun, V.L.; Bodmer, W. Goblet Cell Differentiation Subgroups in Colorectal Cancer. Proc. Natl. Acad. Sci. USA 2024, 121, e2414213121. [Google Scholar] [CrossRef]
- Habib, A.M.; Richards, P.; Cairns, L.S.; Rogers, G.J.; Bannon, C.A.M.; Parker, H.E.; Morley, T.C.E.; Yeo, G.S.H.; Reimann, F.; Gribble, F.M. Overlap of Endocrine Hormone Expression in the Mouse Intestine Revealed by Transcriptional Profiling and Flow Cytometry. Endocrinology 2012, 153, 3054–3065. [Google Scholar] [CrossRef]
- Gierl, M.S.; Karoulias, N.; Wende, H.; Strehle, M.; Birchmeier, C. The Zinc-Finger Factor Insm1 (IA-1) Is Essential for the Development of Pancreatic β Cells and Intestinal Endocrine Cells. Genes. Dev. 2006, 20, 2465–2478. [Google Scholar] [CrossRef] [PubMed]
- Naya, F.J.; Huang, H.-P.; Qiu, Y.; Mutoh, H.; DeMayo, F.J.; Leiter, A.B.; Tsai, M.-J. Diabetes, Defective Pancreatic Morphogenesis, and Abnormal Enteroendocrine Differentiation in BETA2/NeuroD-Deficient Mice. Genes. Dev. 1997, 11, 2323–2334. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl Peptidase-4 Inhibitors in the Treatment of Type 2 Diabetes: A Comparative Review. Diabetes Obes. Metab. 2011, 13, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Collombat, P.; Mansouri, A.; Hecksher-Sørensen, J.; Serup, P.; Krull, J.; Gradwohl, G.; Gruss, P. Opposing Actions of Arx and Pax4 in Endocrine Pancreas Development. Genes. Dev. 2003, 17, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Estall, J.L.; Drucker, D.J. Glucagon-Like Peptide-2. Annu. Rev. Nutr. 2006, 26, 391–411. [Google Scholar] [CrossRef]
- Gradwohl, G.; Dierich, A.; LeMeur, M.; Guillemot, F. Neurogenin3 is Required for the Development of the Four Endocrine Cell Lineages of the Pancreas. Proc. Natl. Acad. Sci. USA 2000, 97, 1607–1611. [Google Scholar] [CrossRef]
- Gross, S.; Garofalo, D.C.; Balderes, D.A.; Mastracci, T.L.; Dias, J.M.; Perlmann, T.; Ericson, J.; Sussel, L. Lmx1a Functions in Intestinal Serotonin-Producing Enterochromaffin Cells Downstream of Nkx2.2. Development 2016, 143, 2616–2625. [Google Scholar] [CrossRef]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Lee, C.S.; Labosky, P.A.; Yang, V.W.; Kaestner, K.H. The Zinc-Finger Transcription Factor Klf4 Is Required for Terminal Differentiation of Goblet Cells in the Colon. Development 2002, 129, 2619–2628. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Noah, T.K.; Kazanjian, A.; Whitsett, J.; Shroyer, N.F. SAM Pointed Domain ETS Factor (SPDEF) Regulates Terminal Differentiation and Maturation of Intestinal Goblet Cells. Exp. Cell Res. 2010, 316, 452–465. [Google Scholar] [CrossRef]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal Epithelial Tuft Cells Initiate Type 2 Mucosal Immunity to Helminth Parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef]
- Gracz, A.D.; Samsa, L.A.; Fordham, M.J.; Trotier, D.C.; Zwarycz, B.; Lo, Y.-H.; Bao, K.; Starmer, J.; Raab, J.R.; Shroyer, N.F.; et al. Sox4 Promotes Atoh1-Independent Intestinal Secretory Differentiation Toward Tuft and Enteroendocrine Fates. Gastroenterology 2018, 155, 1508–1523.e10. [Google Scholar] [CrossRef] [PubMed]
- Shroyer, N.F.; Wallis, D.; Venken, K.J.T.; Bellen, H.J.; Zoghbi, H.Y. Gfi1 Functions Downstream of Math1 to Control Intestinal Secretory Cell Subtype Allocation and Differentiation. Genes. Dev. 2005, 19, 2412–2417. [Google Scholar] [CrossRef]
- Valdez-Miramontes, C.E.; De Haro-Acosta, J.; Aréchiga-Flores, C.F.; Verdiguel-Fernández, L.; Rivas-Santiago, B. Antimicrobial Peptides in Domestic Animals and Their Applications in Veterinary Medicine. Peptides 2021, 142, 170576. [Google Scholar] [CrossRef]
- Yu, S.; Balasubramanian, I.; Laubitz, D.; Tong, K.; Bandyopadhyay, S.; Lin, X.; Flores, J.; Singh, R.; Liu, Y.; Macazana, C.; et al. Paneth Cell-Derived Lysozyme Defines the Composition of Mucolytic Microbiota and the Inflammatory Tone of the Intestine. Immunity 2020, 53, 398–416.e8. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qin, K.; Fan, J.; Zhao, G.; Zhao, P.; Zeng, W.; Chen, C.; Wang, A.; Wang, Y.; Zhong, J.; et al. The Evolving Roles of Wnt Signaling in Stem Cell Proliferation and Differentiation, the Development of Human Diseases, and Therapeutic Opportunities. Genes. Dis. 2024, 11, 101026. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of Stem Cells in Small Intestine and Colon by Marker Gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Ren, J.; Sui, H.; Fang, F.; Li, Q.; Li, B. The Application of ApcMin/+ Mouse Model in Colorectal Tumor Researches. J. Cancer Res. Clin. Oncol. 2019, 145, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Andreu, P.; Peignon, G.; Slomianny, C.; Taketo, M.M.; Colnot, S.; Robine, S.; Lamarque, D.; Laurent-Puig, P.; Perret, C.; Romagnolo, B. A Genetic Study of the Role of the Wnt/β-Catenin Signalling in Paneth Cell Differentiation. Dev. Biol. 2008, 324, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Betge, J.; Schneider, N.I.; Harbaum, L.; Pollheimer, M.J.; Lindtner, R.A.; Kornprat, P.; Ebert, M.P.; Langner, C. MUC1, MUC2, MUC5AC, and MUC6 in Colorectal Cancer: Expression Profiles and Clinical Significance. Virchows Arch. 2016, 469, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, C.B.; Takemoto, Y.; Tanaka, T.; Macchini, M.; Jiang, Z.; Renz, B.W.; Chen, X.; Ormanns, S.; Nagar, K.; Tailor, Y.; et al. Dclk1 Defines Quiescent Pancreatic Progenitors That Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell 2016, 18, 441–455. [Google Scholar] [CrossRef]
- Yamada, Y.; Simon-Keller, K.; Belharazem-Vitacolonnna, D.; Bohnenberger, H.; Kriegsmann, M.; Kriegsmann, K.; Hamilton, G.; Graeter, T.; Preissler, G.; Ott, G.; et al. A Tuft Cell–Like Signature Is Highly Prevalent in Thymic Squamous Cell Carcinoma and Delineates New Molecular Subsets Among the Major Lung Cancer Histotypes. J. Thorac. Oncol. 2021, 16, 1003–1016. [Google Scholar] [CrossRef]
- Li, L.; Ma, M.; Duan, T.; Sui, X. The Critical Roles and Therapeutic Implications of Tuft Cells in Cancer. Front. Pharmacol. 2022, 13, 1047188. [Google Scholar] [CrossRef]
- Koehler, J.A.; Kain, T.; Drucker, D.J. Glucagon-Like Peptide-1 Receptor Activation Inhibits Growth and Augments Apoptosis in Murine CT26 Colon Cancer Cells. Endocrinology 2011, 152, 3362–3372. [Google Scholar] [CrossRef]
- Koehler, J.A.; Baggio, L.L.; Yusta, B.; Longuet, C.; Rowland, K.J.; Cao, X.; Holland, D.; Brubaker, P.L.; Drucker, D.J. GLP-1R Agonists Promote Normal and Neoplastic Intestinal Growth through Mechanisms Requiring Fgf7. Cell Metab. 2015, 21, 379–391. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Shen, B.; Sun, X.-F. Chromogranin-A Expression as a Novel Biomarker for Early Diagnosis of Colon Cancer Patients. Int. J. Mol. Sci. 2019, 20, 2919. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Fan, C.; Hildesjö, C.; Shen, B.; Sun, X.-F. Loss of CHGA Protein as a Potential Biomarker for Colon Cancer Diagnosis: A Study on Biomarker Discovery by Machine Learning and Confirmation by Immunohistochemistry in Colorectal Cancer Tissue Microarrays. Cancers 2022, 14, 2664. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, H.; Ranjbaran, J.; Erfanian, N.; Nomiri, S.; Derakhshani, A.; Gerarduzzi, C.; Miraki Feriz, A.; HosseiniGol, E.; Saghafi, S.; Silvestris, N. Holistic Exploration of CHGA and Hsa-MiR-137 in Colorectal Cancer via Multi-Omic Data Integration. Heliyon 2024, 10, e27046. [Google Scholar] [CrossRef]
- Jing, F.; Liu, G.; Zhang, R.; Xue, W.; Lin, J.; Zhu, H.; Zhu, Y.; Wu, C.; Luo, Y.; Chen, T.; et al. PYY Modulates the Tumorigenesis and Progression of Colorectal Cancer Unveiled by Proteomics. Am. J. Cancer Res. 2022, 12, 5500–5515. [Google Scholar]
- Lei, K.; Li, W.; Huang, C.; Li, Y.; Alfason, L.; Zhao, H.; Miyagishi, M.; Wu, S.; Kasim, V. Neurogenic Differentiation Factor 1 Promotes Colorectal Cancer Cell Proliferation and Tumorigenesis by Suppressing the P53/P21 Axis. Cancer Sci. 2020, 111, 175–185. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Li, Y.; Li, J.; Zhao, H.; Song, G.; Miyagishi, M.; Wu, S.; Kasim, V. NeuroD1 Promotes Tumor Cell Proliferation and Tumorigenesis by Directly Activating the Pentose Phosphate Pathway in Colorectal Carcinoma. Oncogene 2021, 40, 6736–6747. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Li, Y.; Lee, Y.A.; Lu, Y.; George, T.J.; Donahoo, W.T.; Lee, K.P.; Nakshatri, H.; Allen, J.; Guo, Y.; et al. GLP-1 Receptor Agonists and Cancer Risk in Adults With Obesity. JAMA Oncol. 2025, 11, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Park, J.; Lim, J.; Jeong, J.; Dinesh, R.K.; Maher, S.E.; Kim, J.; Park, S.; Hong, J.Y.; Wysolmerski, J.; et al. Metastasis of Colon Cancer Requires Dickkopf-2 to Generate Cancer Cells with Paneth Cell Properties. Elife 2024, 13, 97279. [Google Scholar] [CrossRef]
- Pinto, D.; Gregorieff, A.; Begthel, H.; Clevers, H. Canonical Wnt Signals Are Essential for Homeostasis of the Intestinal Epithelium. Genes. Dev. 2003, 17, 1709–1713. [Google Scholar] [CrossRef]
- López-Arribillaga, E.; Yan, B.; Lobo-Jarne, T.; Guillén, Y.; Menéndez, S.; Andreu, M.; Bigas, A.; Iglesias, M.; Espinosa, L. Accumulation of Paneth Cells in Early Colorectal Adenomas Is Associated with Beta-Catenin Signaling and Poor Patient Prognosis. Cells 2021, 10, 2928. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Mao, Y.; Gao, W.; Duan, S. Targeting the P53 Signaling Pathway in Cancers: Molecular Mechanisms and Clinical Studies. MedComm 2023, 4, e288. [Google Scholar] [CrossRef]
- Du, W.; Miao, Y.; Zhang, G.; Luo, G.; Yang, P.; Chen, F.; Zhang, B.; Yang, C.; Li, G.; Chang, J. The Regulatory Role of Neuropeptide Gene Glucagon in Colorectal Cancer: A Comprehensive Bioinformatic Analysis. Dis. Markers 2022, 2022, 4262600. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A Visual Analytics Platform for Comprehensive Gene Expression Profiling and Meta-Analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Cho, M.; Wang, X. OncoDB: An Interactive Online Database for Analysis of Gene Expression and Viral Infection in Cancer. Nucleic Acids Res. 2022, 50, D1334–D1339. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An Update to the Integrated Cancer Data Analysis Platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein–Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science (1979) 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. ROCplot.Org: Validating Predictive Biomarkers of Chemotherapy/Hormonal Therapy/Anti-HER2 Therapy Using Transcriptomic Data of 3,104 Breast Cancer Patients. Int. J. Cancer 2019, 145, 3140–3151. [Google Scholar] [CrossRef]


| Cell Type | Gene | Gene Function/Description | Reference |
|---|---|---|---|
| Enteroendocrine cell | GCG | GLP-1/2 hormone L-cell marker | [24] |
| PYY | Hormone secreted by L cells | ||
| CHGA | Classical marker | [15] | |
| INSM1 | Neuroendocrine transcription factor | [25] | |
| NEUROD1 | Neuroendocrine differentiation | [26] | |
| DPP4 | Incretin degradation (GLP-1, GIP) | [27] | |
| ARX | Regulates the fate of endocrine subtypes | [28] | |
| NKX2.2 | Required for intestinal endocrine differentiation | [24] | |
| GLP2R | GLP-2 receptor | [29] | |
| PAX6 | Regulates EEC subtypes | [24] | |
| GIPR | GIP receptor | ||
| NEUROG3 | EEC master regulator | [30] | |
| PAX4 | Intestinal endocrine development | [24] | |
| LMX1A | Regulates serotonin | [31] | |
| GLP1R | GLP-1 receptor | [24] | |
| ISL1 | Endocrine regulation | ||
| GIP | Hormone produced by K cells | ||
| Goblet cell | KLF4 | Goblet cell differentiation | [32] |
| MUC2 | Major secreted mucin | [33] | |
| SPDEF | Essential for goblet cells | [34] | |
| Tuft cell | POU2F3 | Tuft cell master regulator | [35] |
| SOX4 | Tuft development | [36] | |
| Paneth cell | GFI1 | Regulation of Paneth cells | [37] |
| DEFA1 | Antimicrobial peptide produced by Paneth cells | [38] | |
| LYZ | Lysozyme classic marker | [39] | |
| SOX9 | Essential transcription factor | [10] | |
| WISP1 | Maintains niche and Paneth differentiation | [40] | |
| Stem cell | LGR5 | Intestinal stem cell marker | [41] |
| Mediator | Outcome | ACME_ Estimate | ACME_p | ADE_ Estimate | ADE_P Value | Summary |
|---|---|---|---|---|---|---|
| SOX9 | CHGA | −0.39 | 0.27 | −1.15 | 0.02 | Direct effect of wnt_score on CHGA, independent of SOX9 |
| SOX9 | GCG | −0.10 | 0.82 | −1.12 | 0.03 | Direct effect of wnt_score on GCG, independent of SOX9 |
| SOX9 | PYY | −0.09 | 0.81 | −1.37 | 0.01 | Direct effect of wnt_score on PYY, independent of SOX9 |
| LYZ | CHGA | −0.18 | 0.18 | −1.36 | 0.0001 | Direct effect of wnt_score on CHGA, independent of LYZ |
| LYZ | GCG | −0.17 | 0.17 | −1.05 | 0.0001 | Direct effect of wnt_score on GCG, independent of LYZ |
| LYZ | PYY | −0.19 | 0.21 | −1.27 | 0.0001 | Direct effect of wnt_score on PYY, independent of LYZ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, E.M.; Cipelli, M.; do Amaral, M.A.; Pacheco-Silva, A.; Câmara, N.O.S.; Andrade-Oliveira, V. Downregulation of Enteroendocrine Genes Predicts Survival in Colon Cancer: A Bioinformatics-Based Analysis. Int. J. Mol. Sci. 2025, 26, 11127. https://doi.org/10.3390/ijms262211127
da Silva EM, Cipelli M, do Amaral MA, Pacheco-Silva A, Câmara NOS, Andrade-Oliveira V. Downregulation of Enteroendocrine Genes Predicts Survival in Colon Cancer: A Bioinformatics-Based Analysis. International Journal of Molecular Sciences. 2025; 26(22):11127. https://doi.org/10.3390/ijms262211127
Chicago/Turabian Styleda Silva, Eloisa Martins, Marcella Cipelli, Mariana Aamaral do Amaral, Alvaro Pacheco-Silva, Niels O. S. Câmara, and Vinicius Andrade-Oliveira. 2025. "Downregulation of Enteroendocrine Genes Predicts Survival in Colon Cancer: A Bioinformatics-Based Analysis" International Journal of Molecular Sciences 26, no. 22: 11127. https://doi.org/10.3390/ijms262211127
APA Styleda Silva, E. M., Cipelli, M., do Amaral, M. A., Pacheco-Silva, A., Câmara, N. O. S., & Andrade-Oliveira, V. (2025). Downregulation of Enteroendocrine Genes Predicts Survival in Colon Cancer: A Bioinformatics-Based Analysis. International Journal of Molecular Sciences, 26(22), 11127. https://doi.org/10.3390/ijms262211127







