Fibroblast Activation Protein Alpha (FAP) Expression Is Associated with Disease Recurrence and Poor Response to Tyrosine Kinase Inhibitors in Advanced Clear Cell Renal Cell Carcinoma
Abstract
1. Introduction
2. Results
2.1. Clinical and Pathological Characteristics of ccRCC Patients and Samples
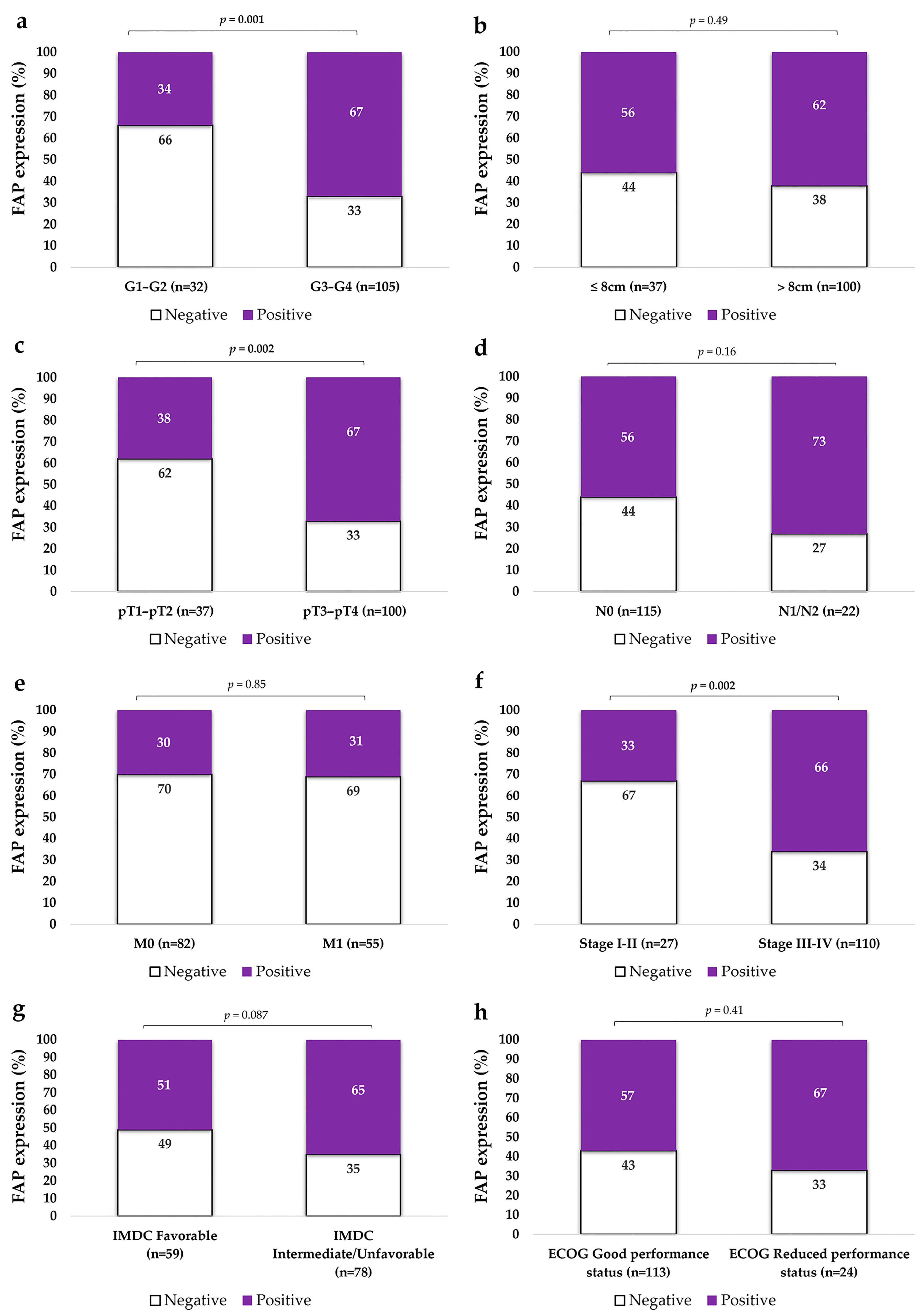
2.2. FAP Expression in Primary ccRCCs in Relation to Aggressive Pathological Features
2.2.1. FAP Expression Is Higher in High-Grade Primary ccRCCs
2.2.2. FAP Expression Is Higher in Non-Organ Confined Tumors
2.2.3. FAP Expression Is Higher in Tumors with Advanced Stage and Metastatic Spread
2.2.4. FAP Expression Does Not Significantly Vary According to IMDC Risk Classification or ECOG Performance Status
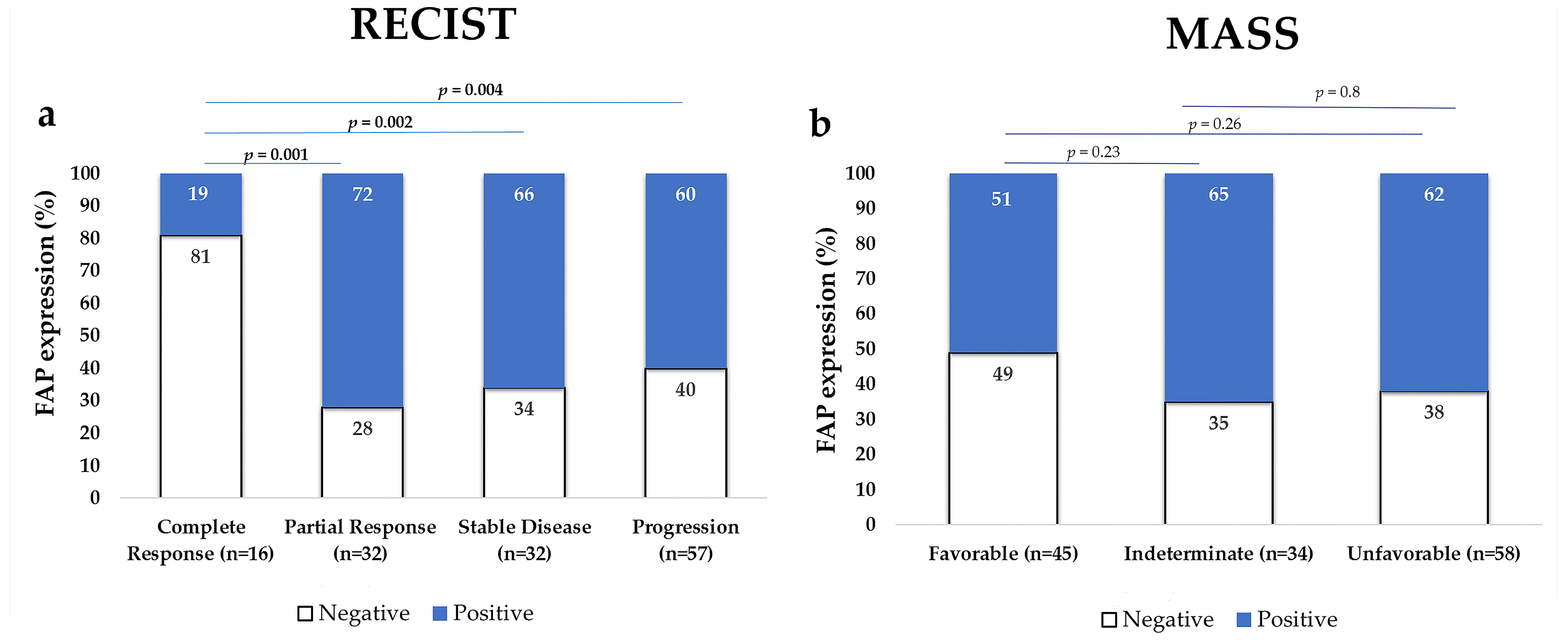
2.3. FAP Expression in the Primary Tumor According to Treatment Response
FAP-Positive Tumors Were Less Responsive to Treatment
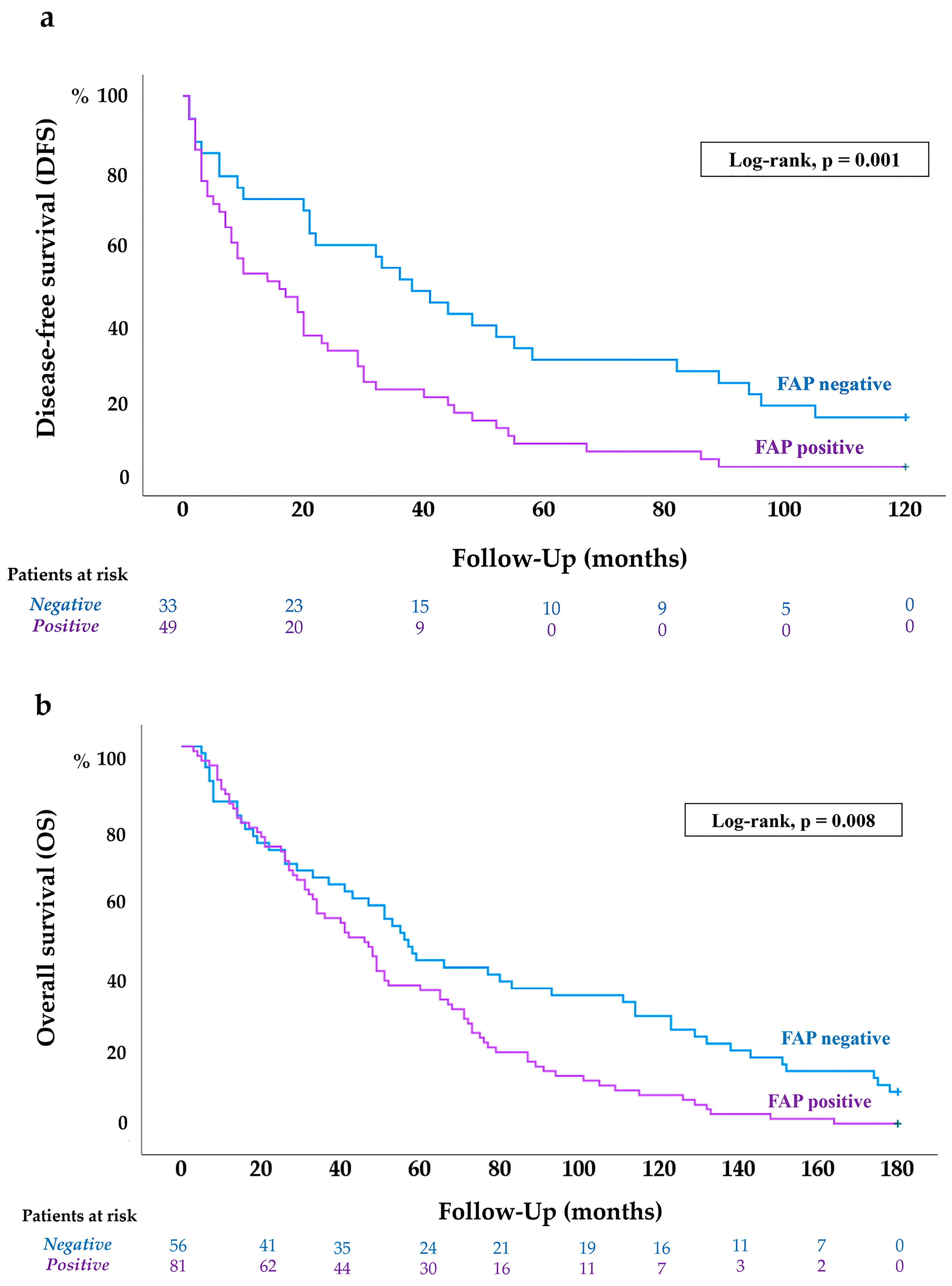
2.4. FAP Expression in ccRCC According to Patients’ Disease-Free (DFS) and Overall Survival (OS)
2.4.1. FAP Expression Is Associated with Shorter DFS in ccRCC Patients
2.4.2. FAP Expression Is Associated with Worse OS of ccRCC Patients
2.5. FAP mRNA Expression in ccRCC (In Silico Analysis)
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemical Detection of FAP in ccRCC Samples
4.3. Statistical and In Silico Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, M.; Jackson-Spence, F.; Beltran, L.; Day, E.; Suarez, C.; Bex, A.; Powles, T.; Szabados, B. Renal cell carcinoma. Lancet 2024, 404, 476–491. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A.; ESMO Guidelines Committee. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef]
- Rinaldi, L.; Senatore, E.; Feliciello, S.; Chiuso, F.; Insabato, L.; Feliciello, A. Kidney cancer: From tumor biology to innovative therapeutics. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189240. [Google Scholar] [CrossRef]
- Singer, E.A.; Rumble, R.B.; Van Veldhuizen, P.J. Management of metastatic clear cell renal cell carcinoma: ASCO guideline Q&A. JCO Oncol. Pract. 2023, 19, 127–131. [Google Scholar] [CrossRef]
- Barragan-Carrillo, R.; Saad, E.; Saliby, R.M.; Sun, M.; Albiges, L.; Bex, A.; Heng, D.; Mejean, A.; Motzer, R.J.; Plimack, E.R.; et al. First and second-line treatments in metastatic renal cell carcinoma. Eur. Urol. 2025, 87, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; MacKenzie, M.; Wood, L.; Donskov, F.; Tan, M.-H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.; Calderon, L.; Khaleel, S.; Hakimi, A.A. Current and future biomarkers in the management of renal cell carcinoma. Urol. Clin. N. Am. 2023, 50, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Marsh, T.; Pietras, K.; McAllister, S. Fibroblasts as architects of cancer pathogenesis. Biochim. Biophys. Acta 2013, 1832, 1070–1078. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Lin, S.; Liu, B.; Wu, P.; Li, L. Fibroblast diversity and plasticity in the tumor microenvironment: Roles in immunity and relevant therapies. Cell Commun. Signal. 2023, 21, 234. [Google Scholar] [CrossRef]
- Zhang, Z.; Tao, J.; Qiu, J.; Cao, Z.; Huang, H.; Xiao, J.; Zhang, T. From basic research to clinical application: Targeting fibroblast activation protein for cancer diagnosis and treatment. Cell Oncol. 2024, 47, 361–381. [Google Scholar] [CrossRef]
- Liu, F.; Qi, L.; Liu, B.; Liu, J.; Zhang, H.; Che, D. Fibroblast activation protein overexpression and clinical implications in solid tumours: A meta-analysis. PLoS ONE 2015, 10, e0116683. [Google Scholar] [CrossRef]
- Peltier, A.; Seban, R.D.; Buvat, I.; Bidard, F.-C.; Mechta-Grigoriou, F. Fibroblast heterogeneity in solid tumors: From single cell analysis to whole-body imaging. Semin. Cancer Biol. 2022, 86, 262–272. [Google Scholar] [CrossRef]
- Hagens, M.J.; van Leeuwen, P.J.; Wondergem, M.; Boellaard, T.N.; Sanguedolce, F.; Oprea-Lager, D.E.; Bex, A.; Vis, A.N.; van der Poel, H.G.; Mertens, L.S.; et al. A systematic review on the diagnostic value of fibroblast activation protein inhibitor PET/CT in genitourinary cancers. J. Nucl. Med. 2024, 65, 888–896. [Google Scholar] [CrossRef]
- Solano-Iturri, J.D.; Errarte, P.; Etxezarraga, M.C.; Echevarria, E.; Angulo, J.C.; López, J.I.; Larrinaga, G. Altered tissue and plasma levels of fibroblast activation protein-α (FAP) in renal tumours. Cancers 2020, 12, 3393. [Google Scholar] [CrossRef]
- López, J.I.; Errarte, P.; Erramuzpe, A.; Guarch, R.; Cortés, J.M.; Angulo, J.C.; Pulido, R.; Irazusta, J.; Llarena, R.; Larrinaga, G. Fibroblast activation protein predicts prognosis in clear cell renal cell carcinoma. Hum. Pathol. 2016, 54, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Errarte, P.; Guarch, R.; Pulido, R.; Blanco, L.; Nunes-Xavier, C.E.; Beitia, M.; Gil, J.; Angulo, J.C.; López, J.I.; Larrinaga, G. The expression of fibroblast activation protein in clear cell renal cell carcinomas is associated with synchronous lymph node metastases. PLoS ONE 2016, 11, e0169105. [Google Scholar] [CrossRef]
- Ambrosetti, D.; Coutts, M.; Paoli, C.; Durand, M.; Borchielli, D.; Montemagno, C.; Rastoin, O.; Borderie, A.; Grepin, R.; Rioux-Leclercq, N.; et al. Cancer-associated fibroblasts in renal cell carcinoma: Implication in prognosis and resistance to anti-angiogenic therapy. BJU Int. 2022, 129, 80–92. [Google Scholar] [CrossRef]
- Davidson, G.; Helleux, A.; Vano, Y.A.; Lindner, V.; Fattori, A.; Cerciat, M.; Elaidi, R.T.; Verkarre, V.; Sun, C.-M.; Chevreau, C.; et al. Mesenchymal-like tumor cells and myofibroblastic cancer-associated fibroblasts are associated with progression and immunotherapy response of clear cell renal cell carcinoma. Cancer Res. 2023, 83, 2952–2969. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.C.; Larrinaga, G.; Lecumberri, D.; Iturregui, A.M.; Solano-Iturri, J.D.; Lawrie, C.H.; Armesto, M.; Dorado, J.F.; Nunes-Xavier, C.E.; Pulido, R.; et al. Predicting survival of metastatic clear cell renal cell cancer treated with VEGFR-TKI-based sequential therapy. Cancers 2024, 16, 2786. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Assayag, J.; Kim, C.; Chu, H.; Webster, J. The prognostic value of Eastern Cooperative Oncology Group performance status on overall survival among patients with metastatic prostate cancer: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1194718. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, K.M.; Nishino, M.; Ramaiya, N.H.; Choueiri, T.K. RECIST 1.1 compared with RECIST 1.0 in patients with ad-vanced renal cell carcinoma receiving vascular endothelial growth factor-targeted therapy. AJR Am. J. Roentgenol. 2015, 204, W282–W288. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Shah, S.N.; Rini, B.I.; Lieber, M.L.; Remer, E.M. Morphology, attenuation, size, and structure (MASS) criteria: Assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am. J. Roentgenol. 2010, 194, 1470–1478. [Google Scholar] [CrossRef]
- Robinson, A.G.; Booth, C.M.; Eisenhauer, E.A. Disease-free survival as an end-point in the treatment of solid tumours:perspectives from clinical trials and clinical practice. Eur. J. Cancer 2014, 50, 2298–2302. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Wang, L.; Wang, R.; Yang, X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: A meta-analysis. Medicine 2019, 98, e13788. [Google Scholar] [CrossRef]
- Larrinaga, G.; Redrado, M.; Loizaga-Iriarte, A.; Pérez-Fernández, A.; Santos-Martín, A.; Angulo, J.C.; Fernández, J.A.; Calvo, A.; López, J.I. Spatial expression of fibroblast activation protein-α in clear cell renal cell carcinomas revealed by multiplex immunoprofiling analysis of the tumor microenvironment. Cancer Immunol. Immunother. 2025, 74, 53. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Y.; Xu, K.; Liu, C.; Zhong, H.; Wu, Y.; Zhang, K.; Wei, S. Cancer-associated fibroblasts in clear cell renal cell carcinoma: Functional heterogeneity, tumor microenvironment crosstalk, and therapeutic opportunities. Front. Immunol. 2025, 16, 1617968. [Google Scholar] [CrossRef]
- Hajiran, A.; Chakiryan, N.; Aydin, A.M.; Zemp, L.; Nguyen, J.; Laborde, J.M.; Chahoud, J.; Spiess, P.E.; Zaman, S.; Falasiri, S.; et al. Reconnaissance of tumor immune microenvironment spatial heterogeneity in metastatic renal cell carcinoma and correlation with immunotherapy response. Clin. Exp. Immunol. 2021, 204, 96–106. [Google Scholar] [CrossRef]
- Chakiryan, N.H.; Kim, Y.; Berglund, A.; Chang, A.; Kimmel, G.J.; Hajiran, A.; Nguyen, J.; Moran-Segura, C.; Saeed-Vafa, D.; Katende, E.N.; et al. Geospatial characterization of immune cell distributions and dynamics across the microenvironment in clear cell renal cell carcinoma. J. Immunother. Cancer 2023, 11, e006195. [Google Scholar] [CrossRef] [PubMed]

| Variables | n = 137 |
|---|---|
| Age | |
| ≤60 years | 76 |
| >60 years | 61 |
| Gender | |
| Male | 97 |
| Female | 40 |
| Fuhrman grade | |
| Low (G1–G2) | 32 |
| High (G3–G4) | 105 |
| Diameter | |
| ≤8 cm | 71 |
| >8 cm | 66 |
| Local invasion (pT) | |
| Organ-confined (pT1–pT2) | 37 |
| Not confined (pT3–pT4) | 100 |
| Lymph node invasion (N) | |
| No | 115 |
| Yes | 22 |
| Metastasis (M) | |
| Metachronous | 82 |
| Synchronous | 55 |
| NCCN 2010 Stage | |
| Stage I–II | 27 |
| Stage III–IV | 110 |
| ECOG (*) | |
| Preserved function (ECOG 0) | 113 |
| Reduced function (ECOG 1–2) | 24 |
| IMDC (*) | |
| Favorable | 59 |
| Intermediate/Unfavorable | 78 |
| Cox Regression Model | 10-Year Disease-Free Survival | 15-Year Overall Survival | |||||
|---|---|---|---|---|---|---|---|
| Variables | p | HR | 95% CI | p | HR | 95% CI | |
| Univariate analysis | Sex | 0.7 | 1.11 | 0.52–1.16 | 0.42 | 0.86 | 0.59–1.25 |
| Age | 0.049 | 1.58 | 1.03–2.5 | 0.002 | 1.77 | 1.24–2.51 | |
| Grade | <0.001 | 2.34 | 1.42–3.86 | <0.001 | 2.1 | 1.38–3.11 | |
| pT | 0.001 | 2.32 | 1. 38–3.89 | <0.001 | 2.07 | 1.38–3.08 | |
| N | 0.003 | 3.12 | 1.46–6.69 | <0.001 | 2.86 | 1.79–4.57 | |
| M | - | - | - | <0.001 | 3.06 | 2.13–4.38 | |
| ECOG | - | - | - | 0.01 | 1.78 | 1.15–2.75 | |
| IMDC | - | - | - | <0.001 | 1.81 | 1.28–2.56 | |
| MASS | - | - | - | <0.001 | 1.98 | 1.37–2.85 | |
| RECIST | - | - | - | <0.001 | 3.1 | 1.7–5.66 | |
| FAP | 0.002 | 2.16 | 1.33–3.51 | 0.009 | 1.62 | 1.13–2.34 | |
| Multivariate analysis Final step of Wald method | Age | 0.002 | 2.21 | 1.34–3.65 | 0.008 | 1.65 | 1.14–2.39 |
| Grade | 0.002 | 2.4 | 1.39–4.15 | - | - | - | |
| pT | 0.002 | 2.45 | 1.41–4.26 | <0.001 | 2.26 | 1.44–3.56 | |
| N | - | - | - | <0.001 | 2.57 | 1.57–4.23 | |
| M | - | - | - | <0.001 | 2.01 | 1.37–2.95 | |
| MASS | - | - | - | <0.001 | 2.21 | 1.49–3.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaza Montes, M.; Suárez, B.; Solano-Iturri, J.D.; Lecumberri, D.; Iturregui, A.M.; Lawrie, C.H.; Armesto, M.; Nunes-Xavier, C.E.; Pulido, R.; López, J.I.; et al. Fibroblast Activation Protein Alpha (FAP) Expression Is Associated with Disease Recurrence and Poor Response to Tyrosine Kinase Inhibitors in Advanced Clear Cell Renal Cell Carcinoma. Int. J. Mol. Sci. 2025, 26, 11112. https://doi.org/10.3390/ijms262211112
Riaza Montes M, Suárez B, Solano-Iturri JD, Lecumberri D, Iturregui AM, Lawrie CH, Armesto M, Nunes-Xavier CE, Pulido R, López JI, et al. Fibroblast Activation Protein Alpha (FAP) Expression Is Associated with Disease Recurrence and Poor Response to Tyrosine Kinase Inhibitors in Advanced Clear Cell Renal Cell Carcinoma. International Journal of Molecular Sciences. 2025; 26(22):11112. https://doi.org/10.3390/ijms262211112
Chicago/Turabian StyleRiaza Montes, María, Beatriz Suárez, Jon Danel Solano-Iturri, David Lecumberri, Ane Miren Iturregui, Charles H. Lawrie, María Armesto, Caroline E. Nunes-Xavier, Rafael Pulido, José I. López, and et al. 2025. "Fibroblast Activation Protein Alpha (FAP) Expression Is Associated with Disease Recurrence and Poor Response to Tyrosine Kinase Inhibitors in Advanced Clear Cell Renal Cell Carcinoma" International Journal of Molecular Sciences 26, no. 22: 11112. https://doi.org/10.3390/ijms262211112
APA StyleRiaza Montes, M., Suárez, B., Solano-Iturri, J. D., Lecumberri, D., Iturregui, A. M., Lawrie, C. H., Armesto, M., Nunes-Xavier, C. E., Pulido, R., López, J. I., Angulo, J. C., & Larrinaga, G. (2025). Fibroblast Activation Protein Alpha (FAP) Expression Is Associated with Disease Recurrence and Poor Response to Tyrosine Kinase Inhibitors in Advanced Clear Cell Renal Cell Carcinoma. International Journal of Molecular Sciences, 26(22), 11112. https://doi.org/10.3390/ijms262211112









