Modern Strategies for Osteoporosis Therapy: Current Status and Prospects for Targeted Intervention
Abstract
1. Introduction
2. Clinical Aspects of Osteoporosis
3. Key Mechanisms and Regulatory Pathways in Osteoporosis Pathogenesis
3.1. Osteoclastogenesis
3.2. Osteoblastogenesis
3.3. WNT Pathway Inhibitors
3.4. Genetic Predisposition to Osteoporosis and Epigenetic Regulation
3.5. Osteoimmune Interactions and Regulation: A Delicate Balance
3.6. Oxidative Stress and Cellular Senescence
3.7. The Relationship Between Bone and Adipose Tissue: Regulatory Pathways of Osteogenesis and Adipogenesis in Osteoporosis
4. Therapeutic Approaches Used in Routine Practice
4.1. Anti-Resorptive Agents
4.1.1. Bisphosphonates
4.1.2. Strontium Ranelate
4.1.3. Calcitonin
4.1.4. Estrogen-Progestin Therapy
4.1.5. Selective Estrogen Receptor Modulators (SERMs)
4.1.6. Denosumab
4.2. Anabolic Agents
4.2.1. Teriparatide and Abaloparatide
4.2.2. Romosozumab
5. Prospects for Targeted Therapy
5.1. Agents at the Clinical Trial Stage
5.2. Key Trends in the Development of Novel Approaches for Targeted Osteoporosis Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABL | Abaloparatide |
| ALP | Alkaline phosphatase |
| ASOs | Antisense oligonucleotides |
| ATP | Adenosintriphosphate |
| BMD | Bone mineral density |
| BMSC | Bone marrow-derived MSC |
| BMP | Bone morphogenetic protein |
| BMPR | Receptor for Bone morphogenetic protein |
| BTK | Bruton’s tyrosine kinase |
| CHO | Chinese Hamster Ovary |
| CTX-1 | Carboxy-terminal crosslinked telopeptide of type 1 collagen |
| DKK | Dickkopf |
| DNA | Deoxyribonucleic acid |
| DXA | Dual-energy X-ray absorptiometry |
| EPT | Estrogen-Progestin Therapy |
| ER | Estrogen receptor |
| EVs | Extracellular vesicles |
| FDA | Food and Drug Administration |
| FPPS | Farnesylpyrophosphate synthase |
| FRAX | Fracture Risk Assessment Tool |
| FZD | Frizzled |
| HRT | Hormone replacement therapy |
| IL | Interleukin |
| lncRNAs | Long non-coding RNAs |
| LRP | Low-density lipoprotein receptor-related protein |
| MMP | Matrix metalloproteinase |
| MCP-1 | Monocyte chemotactic protein 1 |
| MCSF-1 | Macrophage colony stimulating factor |
| MCSFR | Receptor for Macrophage colony stimulating factor |
| MPA | Medroxyprogesterone acetate |
| MSC | Mesenchymal stromal cell |
| oCEE | Oral conjugated equine estrogen |
| OP | Osteoporosis |
| OPG | Osteoprotegerin |
| PINP | Procollagen type I N-terminal propeptide |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PTH | Parathyroid hormone |
| RANK | Receptor activator of nuclear factor-κB |
| RANKL | Receptor activator of NF-κB ligand |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenomenon |
| SERMs | Selective estrogen receptor modulators |
| siRNA | Small interfering RNA |
| SOST | Sclerostin |
| TBS | Trabecular Bone Score |
| TGF-b | Transforming growth factor beta |
| TNF | Tumor necrosis factor |
| WHO | World Health Organization |
References
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, Regional Prevalence, and Risk Factors of Osteoporosis According to the World Health Organization Diagnostic Criteria: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Osteoporosis in the World: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R. European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Haddad, Y.K.; Bergen, G.; Florence, C.S. Estimating the Economic Burden Related to Older Adult Falls by State. J. Public Health Manag. Pract. 2019, 25, E17–E24. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden: A Report Prepared in Collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Burns, E.R.; Stevens, J.A.; Lee, R. The Direct Costs of Fatal and Non-Fatal Falls among Older Adults—United States. J. Saf. Res. 2016, 58, 99–103. [Google Scholar] [CrossRef]
- Cosman, F.; Lewiecki, E.M.; Eastell, R.; Ebeling, P.R.; De Beur, S.J.; Langdahl, B.; Rhee, Y.; Fuleihan, G.E.-H.; Kiel, D.P.; Schousboe, J.T.; et al. Goal-Directed Osteoporosis Treatment: ASBMR/BHOF Task Force Position Statement 2024. J. Bone Miner. Res. 2024, 39, 1393–1405. [Google Scholar] [CrossRef]
- Elahmer, N.R.; Wong, S.K.; Mohamed, N.; Alias, E.; Chin, K.-Y.; Muhammad, N. Mechanistic Insights and Therapeutic Strategies in Osteoporosis: A Comprehensive Review. Biomedicines 2024, 12, 1635. [Google Scholar] [CrossRef]
- Khan, M.; Cheung, A.M.; Khan, A.A. Drug-Related Adverse Events of Osteoporosis Therapy. Endocrinol. Metab. Clin. N. Am. 2017, 46, 181–192. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Chen, L.-H.; Ma, W.-J.; You, R.-X. Drug Efficacy and Safety of Denosumab, Teriparatide, Zoledronic Acid, and Ibandronic Acid for the Treatment of Postmenopausal Osteoporosis: A Network Meta-Analysis of Randomized Controlled Trials. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8253–8268. [Google Scholar] [CrossRef]
- McLaughlin, M.B.; Awosika, A.O.; Jialal, I. Calcitonin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jolette, J.; Attalla, B.; Varela, A.; Long, G.G.; Mellal, N.; Trimm, S.; Smith, S.Y.; Ominsky, M.S.; Hattersley, G. Comparing the Incidence of Bone Tumors in Rats Chronically Exposed to the Selective PTH Type 1 Receptor Agonist Abaloparatide or PTH(1–34). Regul. Toxicol. Pharmacol. 2017, 86, 356–365. [Google Scholar] [CrossRef]
- Prather, C.; Adams, E.; Zentgraf, W. Romosozumab: A First-in-Class Sclerostin Inhibitor for Osteoporosis. Am. J. Health Syst. Pharm. 2020, 77, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.N.; Leslie, W.D.; Schousboe, J.T. Osteoporosis: A Review. JAMA 2025, 334, 894. [Google Scholar] [CrossRef] [PubMed]
- Gregson, C.L.; Armstrong, D.J.; Avgerinou, C.; Bowden, J.; Cooper, C.; Douglas, L.; Edwards, J.; Gittoes, N.J.L.; Harvey, N.C.; Kanis, J.A.; et al. The 2024 UK Clinical Guideline for the Prevention and Treatment of Osteoporosis. Arch. Osteoporos. 2025, 20, 119. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Punda, M.; Ali, D.S.; Bazzocchi, A.; Bock, O.; Camacho, P.; Carey, J.J.; Colquhoun, A.; Compston, J.; Engelke, K.; et al. Updated Practice Guideline for Dual-Energy X-Ray Absorptiometry (DXA). Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 539–563. [Google Scholar] [CrossRef]
- Kanis, J.A.; Kanis, J.A. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Synopsis of a WHO Report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-Calibration and Least Significant Change, Spinal Cord Injury, Peri-Prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef]
- Black, D.M.; Bauer, D.C.; Vittinghoff, E.; Lui, L.-Y.; Grauer, A.; Marin, F.; Khosla, S.; De Papp, A.; Mitlak, B.; Cauley, J.A.; et al. Treatment-Related Changes in Bone Mineral Density as a Surrogate Biomarker for Fracture Risk Reduction: Meta-Regression Analyses of Individual Patient Data from Multiple Randomised Controlled Trials. Lancet Diabetes Endocrinol. 2020, 8, 672–682, Correction in Lancet Diabetes Endocrinol. 2020, 8, e5. [Google Scholar] [CrossRef]
- Cosman, F.; Lewiecki, E.M.; Ebeling, P.R.; Hesse, E.; Napoli, N.; Matsumoto, T.; Crittenden, D.B.; Rojeski, M.; Yang, W.; Libanati, C.; et al. T-Score as an Indicator of Fracture Risk During Treatment with Romosozumab or Alendronate in the ARCH Trial. J. Bone Miner. Res. 2020, 35, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Harvey, N.C.; McCloskey, E.; Bruyère, O.; Veronese, N.; Lorentzon, M.; Cooper, C.; Rizzoli, R.; Adib, G.; Al-Daghri, N.; et al. Algorithm for the Management of Patients at Low, High and Very High Risk of Osteoporotic Fractures. Osteoporos. Int. 2020, 31, 1–12, Correction in Osteoporos. Int. 2020, 31, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Center, J.R.; Bliuc, D.; Nguyen, T.V.; Eisman, J.A. Risk of Subsequent Fracture After Low-Trauma Fracture in Men and Women. JAMA 2007, 297, 387. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-R.; Chen, C.-H. Bone Biomarker for the Clinical Assessment of Osteoporosis: Recent Developments and Future Perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The Effect of Cytokines on Osteoblasts and Osteoclasts in Bone Remodeling in Osteoporosis: A Review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M. Interaction between Bone and Immune Cells: Implications for Postmenopausal Osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef]
- Gómez, M.P.A.; Benavent, C.A.; Simoni, P.; Aparisi, F.; Guglielmi, G.; Bazzocchi, A. Fat and Bone: The Multiperspective Analysis of a Close Relationship. Quant. Imaging Med. Surg. 2020, 10, 1614–1635. [Google Scholar] [CrossRef]
- Ralston, S.H. Genetics of Osteoporosis. Ann. N. Y. Acad. Sci. 2010, 1192, 181–189. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Shi, W.; Xu, K.; Shen, Y.; Dai, B. Oxidative Stress and Inflammation: Roles in Osteoporosis. Front. Immunol. 2025, 16, 1611932. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone Aging, Cellular Senescence, and Osteoporosis. JBMR Plus 2021, 5, e10488. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Horwood, N.J.; Kartsogiannis, V.; Quinn, J.M.W.; Romas, E.; Martin, T.J.; Gillespie, M.T. Activated T Lymphocytes Support Osteoclast Formation in Vitro. Biochem. Biophys. Res. Commun. 1999, 265, 144–150. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.Á.; et al. The RANK–RANKL–OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef]
- Martin, T.J. Historically Significant Events in the Discovery of RANK/RANKL/OPG. World J. Orthop. 2013, 4, 186. [Google Scholar] [CrossRef]
- Bord, S.; Ireland, D.C.; Beavan, S.R.; Compston, J.E. The Effects of Estrogen on Osteoprotegerin, RANKL, and Estrogen Receptor Expression in Human Osteoblasts. Bone 2003, 32, 136–141. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen Prevents Bone Loss via Estrogen Receptor α and Induction of Fas Ligand in Osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Uehara, S.; Koide, M.; Takahashi, N. The Regulation of Osteoclast Differentiation by Wnt Signals. BoneKEy Rep. 2015, 4, 713. [Google Scholar] [CrossRef]
- Huang, W. Signaling and Transcriptional Regulation in Osteoblast Commitment and Differentiation. Front. Biosci. 2007, 12, 3068. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yoon, S.T.; Hutton, W.C. Effect of Bone Morphogenetic Protein-2 (BMP-2) on Matrix Production, Other BMPs, and BMP Receptors in Rat Intervertebral Disc Cells. J. Spinal Disord. Tech. 2004, 17, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, N.; Fu, Z.; Zhang, Q. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules 2023, 13, 483. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT Signaling in Bone Homeostasis and Disease: From Human Mutations to Treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Qin, K.; Yu, M.; Fan, J.; Wang, H.; Zhao, P.; Zhao, G.; Zeng, W.; Chen, C.; Wang, Y.; Wang, A.; et al. Canonical and Noncanonical Wnt Signaling: Multilayered Mediators, Signaling Mechanisms and Major Signaling Crosstalk. Genes. Dis. 2024, 11, 103–134. [Google Scholar] [CrossRef]
- Stamos, J.L.; Weis, W.I. The β-Catenin Destruction Complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef]
- Chae, W.-J.; Bothwell, A.L.M. Canonical and Non-Canonical Wnt Signaling in Immune Cells. Trends Immunol. 2018, 39, 830–847. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Tang, Y.; Xiao, Q. The Involvement of Noncanonical Wnt Signaling in Cancers. Biomed. Pharmacother. 2021, 133, 110946. [Google Scholar] [CrossRef]
- Zhang, R.; Oyajobi, B.O.; Harris, S.E.; Chen, D.; Tsao, C.; Deng, H.-W.; Zhao, M. Wnt/β-Catenin Signaling Activates Bone Morphogenetic Protein 2 Expression in Osteoblasts. Bone 2013, 52, 145–156. [Google Scholar] [CrossRef]
- Dincel, A.S.; Jørgensen, N.R.; on behalf of the IOF-IFCC Joint Committee on Bone Metabolism (C-BM). New Emerging Biomarkers for Bone Disease: Sclerostin and Dickkopf-1 (DKK1). Calcif. Tissue Int. 2022, 112, 243–257. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin Binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Yamamoto, H.; Sato, A. Selective Activation Mechanisms of Wnt Signaling Pathways. Trends Cell Biol. 2009, 19, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xu, X.-J.; Shen, L.; Yang, Y.-P.; Zhu, R.; Shuai, B.; Zhu, X.-W.; Li, C.-G.; Ma, C.; Lv, L. Association of Serum Dkk-1 Levels with β-Catenin in Patients with Postmenopausal Osteoporosis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.Z.; Richards, W.G.; Li, X.; Ominsky, M.S. Sclerostin and Dickkopf-1 as Therapeutic Targets in Bone Diseases. Endocr. Rev. 2012, 33, 747–783. [Google Scholar] [CrossRef]
- Balemans, W. Identification of a 52 Kb Deletion Downstream of the SOST Gene in Patients with van Buchem Disease. J. Med. Genet. 2002, 39, 91–97. [Google Scholar] [CrossRef]
- Li, X.; Ominsky, M.S.; Niu, Q.-T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J.; et al. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation and Bone Strength. J. Bone Miner. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef]
- Li, Z.; Xing, X.; Gomez-Salazar, M.A.; Xu, M.; Negri, S.; Xu, J.; James, A.W. Pharmacological Inhibition of DKK1 Promotes Spine Fusion in an Ovariectomized Rat Model. Bone 2022, 162, 116456. [Google Scholar] [CrossRef]
- Negri, S.; Wang, Y.; Sono, T.; Qin, Q.; Hsu, G.C.-Y.; Cherief, M.; Xu, J.; Lee, S.; Tower, R.J.; Yu, V.; et al. Systemic DKK1 Neutralization Enhances Human Adipose-Derived Stem Cell Mediated Bone Repair. Stem Cells Transl. Med. 2021, 10, 610–622. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Ng, M.Y.; Sham, P.C.; Zintzaras, E.; Lewis, C.M.; Deng, H.-W.; Econs, M.J.; Karasik, D.; Devoto, M.; Kammerer, C.M.; et al. Meta-Analysis of Genome-Wide Scans Provides Evidence for Sex- and Site-Specific Regulation of Bone Mass. J. Bone Miner. Res. 2007, 22, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Halldorsson, B.V.; Gretarsdottir, S.; Gudbjartsson, D.F.; Walters, G.B.; Ingvarsson, T.; Jonsdottir, T.; Saemundsdottir, J.; Center, J.R.; Nguyen, T.V.; et al. Multiple Genetic Loci for Bone Mineral Density and Fractures. N. Engl. J. Med. 2008, 358, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Identification of 613 New Loci Associated with Heel Bone Mineral Density and a Polygenic Risk Score for Bone Mineral Density, Osteoporosis and Fracture. PLoS ONE 2018, 13, e0200785, Correction in PLoS ONE 2019, 14, e0213962. [Google Scholar] [CrossRef] [PubMed]
- Stürznickel, J.; Rolvien, T.; Delsmann, A.; Butscheidt, S.; Barvencik, F.; Mundlos, S.; Schinke, T.; Kornak, U.; Amling, M.; Oheim, R. Clinical Phenotype and Relevance of LRP5 and LRP6 Variants in Patients with Early-Onset Osteoporosis (EOOP). J. Bone Miner. Res. 2020, 36, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H.; Uitterlinden, A.G.; Brandi, M.L.; Balcells, S.; Langdahl, B.L.; Lips, P.; Lorenc, R.; Obermayer-Pietsch, B.; Scollen, S.; Bustamante, M.; et al. Large-Scale Evidence for the Effect of the COLIA1 Sp1 Polymorphism on Osteoporosis Outcomes: The GENOMOS Study. PLoS Med. 2006, 3, e90, Correction in PLoS Med. 2006, 3, e223. [Google Scholar] [CrossRef]
- Kannu, P.; Mahjoub, A.; Babul-Hirji, R.; Carter, M.T.; Harrington, J. PLS3 Mutations in X-Linked Osteoporosis: Clinical and Bone Characteristics of Two Novel Mutations. Horm. Res. Paediatr. 2017, 88, 298–304. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Xue, X.; Ma, H. Comprehensive Analysis of Epigenetics Mechanisms in Osteoporosis. Front. Genet. 2023, 14, 1153585. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Melhus, H.; Ferm, H.; Ahlbom, A.; Pedersen, N.L. Genetic Liability to Fractures in the Elderly. Arch. Intern. Med. 2005, 165, 1825. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Fernández, A.F.; Sainz, J.; Zarrabeitia, M.T.; Sañudo, C.; García-Renedo, R.; Pérez-Núñez, M.I.; García-Ibarbia, C.; Fraga, M.F.; Riancho, J.A. Genome-wide Profiling of Bone Reveals Differentially Methylated Regions in Osteoporosis and Osteoarthritis. Arthritis Rheum. 2013, 65, 197–205. [Google Scholar] [CrossRef]
- Faulkner, B.; Astleford, K.; Mansky, K.C. Regulation of Osteoclast Differentiation and Skeletal Maintenance by Histone Deacetylases. Molecules 2019, 24, 1355. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, Y.; Zhou, X. The Roles of microRNAs in Epigenetic Regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Cianferotti, L.; Brandi, M. Epigenetic Mechanisms in Bone Biology and Osteoporosis: Can They Drive Therapeutic Choices? Int. J. Mol. Sci. 2016, 17, 1329. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yu, D.; Chu, W.; Liu, Z.; Li, H.; Zhai, Z. LncRNA Expression Profiles and the Negative Regulation of lncRNA-NOMMUT037835.2 in Osteoclastogenesis. Bone 2020, 130, 115072. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, D.; Ma, C.; Zhang, Y. microRNA-96 Promotes Osteoblast Differentiation and Bone Formation in Ankylosing Spondylitis Mice through Activating the Wnt Signaling Pathway by Binding to SOST. J. Cell. Biochem. 2019, 120, 15429–15442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, G.; Zhou, C.; Zhao, Q. The Regulatory Roles of Long Noncoding RNAs in Osteoporosis. Am. J. Transl. Res. 2020, 12, 5882–5907. [Google Scholar] [PubMed]
- Arron, J.R.; Choi, Y. Bone versus Immune System. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, P.; Liu, L.; Han, J.; Zeng, H.; Sun, Y. Increased RANKL Expression in Peripheral T Cells Is Associated with Decreased Bone Mineral Density in Patients with COPD. Int. J. Mol. Med. 2016, 38, 585–593. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Regulation of Bone by the Adaptive Immune System in Arthritis. Arthritis Res. Ther. 2011, 13, 219. [Google Scholar] [CrossRef]
- Huang, F.; Wong, P.; Li, J.; Lv, Z.; Xu, L.; Zhu, G.; He, M.; Luo, Y. Osteoimmunology: The Correlation between Osteoclasts and the Th17/Treg Balance in Osteoporosis. J. Cell. Mol. Med. 2022, 26, 3591–3597. [Google Scholar] [CrossRef]
- Francisconi, C.F.; Vieira, A.E.; Azevedo, M.C.S.; Tabanez, A.P.; Fonseca, A.C.; Trombone, A.P.F.; Letra, A.; Silva, R.M.; Sfeir, C.S.; Little, S.R.; et al. RANKL Triggers Treg-Mediated Immunoregulation in Inflammatory Osteolysis. J. Dent. Res. 2018, 97, 917–927. [Google Scholar] [CrossRef]
- Weivoda, M.M.; Bradley, E.W. Macrophages and Bone Remodeling. J. Bone Miner. Res. 2023, 38, 359–369. [Google Scholar] [CrossRef]
- Lang, T.J. Estrogen as an Immunomodulator. Clin. Immunol. 2004, 113, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Osteoporosis and Inflammation. Nutr. Rev. 2008, 65, S147–S151. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tian, T.; Yu, S.; He, N.; Ma, D. Th17 and Treg Cells in Bone Related Diseases. Clin. Dev. Immunol. 2013, 2013, 203705. [Google Scholar] [CrossRef] [PubMed]
- Lencel, P.; Magne, D. Inflammaging: The Driving Force in Osteoporosis? Med. Hypotheses 2011, 76, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-Related Changes in Human Bone Marrow-Derived Mesenchymal Stem Cells: Consequences for Cell Therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, P.; Lin, J.; Zeng, H. The Role of Extracellular Vesicles in Osteoporosis: A Scoping Review. Membranes 2022, 12, 324. [Google Scholar] [CrossRef]
- Kokabu, S.; Lowery, J.W.; Jimi, E. Cell Fate and Differentiation of Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3753581. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Wang, Q.A.; Zhang, F.; Jiang, L.; Ye, R.; An, Y.; Shao, M.; Tao, C.; Gupta, R.K.; Scherer, P.E. Peroxisome Proliferator-Activated Receptor γ and Its Role in Adipocyte Homeostasis and Thiazolidinedione-Mediated Insulin Sensitization. Mol. Cell. Biol. 2018, 38, e00677-17. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M. Marrow Adipocytes Inhibit the Differentiation of Mesenchymal Stem Cells into Osteoblasts via Suppressing BMP-Signaling. J. Biomed. Sci. 2017, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Hiasa, M.; Higa, Y.; Ohnishi, Y.; Endo, I.; Kondo, T.; Takashi, Y.; Tsoumpra, M.; Kainuma, R.; Sawatsubashi, S.; et al. Osteoblast/Osteocyte-Derived Interleukin-11 Regulates Osteogenesis and Systemic Adipogenesis. Nat. Commun. 2022, 13, 7194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate Decision of Mesenchymal Stem Cells: Adipocytes or Osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone Marrow Adipose Tissue Is an Endocrine Organ That Contributes to Increased Circulating Adiponectin during Caloric Restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Schulz, T.J. The Emerging Role of Bone Marrow Adipose Tissue in Bone Health and Dysfunction. J. Mol. Med. 2017, 95, 1291–1301. [Google Scholar] [CrossRef]
- Lewis, J.W.; Edwards, J.R.; Naylor, A.J.; McGettrick, H.M. Adiponectin Signalling in Bone Homeostasis, with Age and in Disease. Bone Res. 2021, 9, 1. [Google Scholar] [CrossRef]
- Liang, H.; Wang, H.; Luo, L.; Fan, S.; Zhou, L.; Liu, Z.; Yao, S.; Zhang, X.; Zhong, K.; Zhao, H.; et al. Toll-like Receptor 4 Promotes High Glucose-Induced Catabolic and Inflammatory Responses in Chondrocytes in an NF-κB-Dependent Manner. Life Sci. 2019, 228, 258–265. [Google Scholar] [CrossRef]
- Furr, N.; Ulmer, A.; Cardon, B. Shoring Up Osteoporosis Management: A Fresh Start? J. Am. Board. Fam. Med. 2024, 37, 490–493. [Google Scholar] [CrossRef]
- Rogers, M.J.; Mönkkönen, J.; Munoz, M.A. Molecular Mechanisms of Action of Bisphosphonates and New Insights into Their Effects Outside the Skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O.; Peter, J.-U. Interventions for Managing Medication-Related Osteonecrosis of the Jaw. Cochrane Database Syst. Rev. 2022, 2022, CD012432. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Berencsi, K.; Marinier, K.; Deltour, N.; Perez-Guthann, S.; Pedersen, L.; Rijnbeek, P.; Lapi, F.; Simonetti, M.; Reyes, C.; et al. Comparative Cardiovascular Safety of Strontium Ranelate and Bisphosphonates: A Multi-Database Study in 5 EU Countries by the EU-ADR Alliance. Osteoporos. Int. 2020, 31, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Naot, D.; Musson, D.S.; Cornish, J. The Activity of Peptides of the Calcitonin Family in Bone. Physiol. Rev. 2019, 99, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Ghani, A.; Arfee, S. Role of Calcitonin and Strontium Ranelate in Osteoporosis. Indian J. Orthop. 2023, 57, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, E.; Dagistan, Y.; Kukner, A.; Yilmaz, B.; Agus, S.; Soyler, G.; Tore, F. Salmon Calcitonin Ameliorates Migraine Pain through Modulation of CGRP Release and Dural Mast Cell Degranulation in Rats. Clin. Exp. Pharmacol. Physiol. 2018, 45, 536–546. [Google Scholar] [CrossRef]
- Tan, L.; Sheng, B.; Deng, S. The Safety and Efficacy of Long-Term Use of Calcitonin Analogs in the Treatment of Osteoporosis in the Elderly: A Pharmacovigilance and RCT Meta-Analysis. Front. Pharmacol. 2025, 16, 1514387. [Google Scholar] [CrossRef]
- Henriksen, K.; Byrjalsen, I.; Andersen, J.R.; Bihlet, A.R.; Russo, L.A.; Alexandersen, P.; Valter, I.; Qvist, P.; Lau, E.; Riis, B.J.; et al. A Randomized, Double-Blind, Multicenter, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Oral Salmon Calcitonin in the Treatment of Osteoporosis in Postmenopausal Women Taking Calcium and Vitamin D. Bone 2016, 91, 122–129. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef]
- Mehta, J.; Kling, J.M.; Manson, J.E. Risks, Benefits, and Treatment Modalities of Menopausal Hormone Therapy: Current Concepts. Front. Endocrinol. 2021, 12, 564781. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Wang, H.; Li, F.; Yu, F.; Ye, L. Mechanistic Advances in Osteoporosis and Anti-osteoporosis Therapies. MedComm 2023, 4, e244. [Google Scholar] [CrossRef] [PubMed]
- Anish, R.J.; Nair, A. Osteoporosis Management-Current and Future Perspectives—A Systemic Review. J. Orthop. 2024, 53, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla Rodriguez, B.S.; Correa, R. Raloxifene. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shogry, F.F.; Hayes, K.N.; Kim, S.; Burden, A.M.; Tadrous, M.; Aggarwal, S.; Cadarette, S.M. The Rise and Fall of Raloxifene Use for Osteoporosis, 1999–2022. Osteoporos. Int. 2025, 36, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M. Monoclonal Antibodies for the Treatment of Osteoporosis. Expert. Opin. Biol. Ther. 2013, 13, 183–196. [Google Scholar] [CrossRef]
- Diédhiou, D.; Cuny, T.; Sarr, A.; Norou Diop, S.; Klein, M.; Weryha, G. Efficacy and Safety of Denosumab for the Treatment of Osteoporosis: A Systematic Review. Ann. d’Endocrinologie 2015, 76, 650–657. [Google Scholar] [CrossRef]
- Aditya, S.; Rattan, A. Sclerostin Inhibition: A Novel Target for the Treatment of Postmenopausal Osteoporosis. J. Mid Life Health 2021, 12, 267–275. [Google Scholar] [CrossRef]
- Tsourdi, E.; Zillikens, M.C.; Meier, C.; Body, J.-J.; Gonzalez, E.R.; Anastasilakis, A.D.; Abrahamsen, B.; McCloskey, E.; Hofbauer, L.C.; Guañabens, N.; et al. Fracture Risk and Management of Discontinuation of Denosumab Therapy: A Systematic Review and Position Statement by ECTS. J. Clin. Endocrinol. Metab. 2021, 106, 264–281. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Oursler, M.J.; Khosla, S. Cathepsin K Inhibitors for Osteoporosis: Biology, Potential Clinical Utility, and Lessons Learned. Endocr. Rev. 2017, 38, 325–350. [Google Scholar] [CrossRef]
- Moon, D.O. Review of Cathepsin K Inhibitor Development and the Potential Role of Phytochemicals. Molecules 2024, 30, 91. [Google Scholar] [CrossRef]
- McClung, M.R.; O’Donoghue, M.L.; Papapoulos, S.E.; Bone, H.; Langdahl, B.; Saag, K.G.; Reid, I.R.; Kiel, D.P.; Cavallari, I.; Bonaca, M.P.; et al. Odanacatib for the Treatment of Postmenopausal Osteoporosis: Results of the LOFT Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial and LOFT Extension Study. Lancet Diabetes Endocrinol. 2019, 7, 899–911. [Google Scholar] [CrossRef]
- Wang, Y.; Grainger, D.W. siRNA Knock-Down of RANK Signaling to Control Osteoclast-Mediated Bone Resorption. Pharm. Res. 2010, 27, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Ariza, Y.; Murata, M.; Ueda, Y.; Yoshizawa, T. Bruton’s Tyrosine Kinase (Btk) Inhibitor Tirabrutinib Suppresses Osteoclastic Bone Resorption. Bone Rep. 2019, 10, 100201. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wei, Z.; Dong, X.; Xing, J.; Meng, X.; Qiu, Y.; Zhou, H.; Zheng, W.; Xu, Z.; Huang, S.; et al. A P38 MAP Kinase Inhibitor Suppresses Osteoclastogenesis and Alleviates Ovariectomy-Induced Bone Loss through the Inhibition of Bone Turnover. Biochem. Pharmacol. 2024, 226, 116391. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, G.; Dean, T.; Corbin, B.A.; Bahar, H.; Gardella, T.J. Binding Selectivity of Abaloparatide for PTH-Type-1-Receptor Conformations and Effects on Downstream Signaling. Endocrinology 2016, 157, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.-L.; Aboishava, L.; Mannstadt, M. Advances in Parathyroid Hormone-Based Medicines. J. Bone Miner. Res. 2025, 40, 1195–1206. [Google Scholar] [CrossRef]
- Boyce, E.G.; Mai, Y.; Pham, C. Abaloparatide: Review of a Next-Generation Parathyroid Hormone Agonist. Ann. Pharmacother. 2018, 52, 462–472. [Google Scholar] [CrossRef]
- McColm, J.; Hu, L.; Womack, T.; Tang, C.C.; Chiang, A.Y. Single- and Multiple-Dose Randomized Studies of Blosozumab, a Monoclonal Antibody Against Sclerostin, in Healthy Postmenopausal Women. J. Bone Miner. Res. 2014, 29, 935–943. [Google Scholar] [CrossRef]
- Su, Y.; Wang, W.; Liu, F.; Cai, Y.; Li, N.; Li, H.; Li, G.; Ma, L. Blosozumab in the Treatment of Postmenopausal Women with Osteoporosis: A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2022, 11, 3203–3212. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lin, C.Y.; Chen, Y.-C.M.; Tian, W.-T.; Chu, H.-M.; Chang, T.W. Bispecific Antibody Binding to RANKL and Osteonectin with Enhanced Localization to the Bone. Mol. Pharm. 2017, 14, 4113–4120. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Z.; Yu, Y.; Chu, H.Y.; Yu, S.; Yao, S.; Zhang, G.; Zhang, B.-T. Drug Discovery of DKK1 Inhibitors. Front. Pharmacol. 2022, 13, 847387. [Google Scholar] [CrossRef]
- Basha, G.; Ordobadi, M.; Scott, W.R.; Cottle, A.; Liu, Y.; Wang, H.; Cullis, P.R. Lipid Nanoparticle Delivery of siRNA to Osteocytes Leads to Effective Silencing of SOST and Inhibition of Sclerostin In Vivo. Mol. Ther. Nucleic Acids 2016, 5, e363. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-T.; Yang, Y.-S.; Xie, J.; Ma, H.; Kim, J.-M.; Park, K.-H.; Oh, D.S.; Park-Min, K.-H.; Greenblatt, M.B.; Gao, G.; et al. WNT-Modulating Gene Silencers as a Gene Therapy for Osteoporosis, Bone Fracture, and Critical-Sized Bone Defects. Mol. Ther. 2023, 31, 435–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-S.; Ko, J.-Y.; Lin, C.-L.; Wu, H.-L.; Ke, H.-J.; Tai, P.-J. Knocking down Dickkopf-1 Alleviates Estrogen Deficiency Induction of Bone Loss. A Histomorphological Study in Ovariectomized Rats. Bone 2007, 40, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Ni, S.; Li, D.; Liu, J.; Chu, H.Y.; Zhang, N.; Sun, M.; Li, N.; Ren, Q.; et al. Targeting Loop3 of Sclerostin Preserves Its Cardiovascular Protective Action and Promotes Bone Formation. Nat. Commun. 2022, 13, 4241. [Google Scholar] [CrossRef]
- Kawai, M.; Mödder, U.I.; Khosla, S.; Rosen, C.J. Emerging Therapeutic Opportunities for Skeletal Restoration. Nat. Rev. Drug Discov. 2011, 10, 141–156. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the Future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; McClung, M.R.; Roux, C.; Recker, R.R.; Eisman, J.A.; Verbruggen, N.; Hustad, C.M.; DaSilva, C.; Santora, A.C.; Ince, B.A. Odanacatib, a Cathepsin-K Inhibitor for Osteoporosis: A Two-Year Study in Postmenopausal Women with Low Bone Density. J. Bone Miner. Res. 2010, 25, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Santora, A.C.; Black, D.M.; Russell, R.G.G. History of Alendronate. Bone 2020, 137, 115411. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Diab, D.L. Long-Term Use of Bisphosphonates in Osteoporosis. J. Clin. Endocrinol. Metab. 2010, 95, 1555–1565. [Google Scholar] [CrossRef]
- Billington, E.; Aghajafari, F.; Skulsky, E.; Kline, G.A. Bisphosphonates. BMJ 2024, 386, e076898. [Google Scholar] [CrossRef]
- Allen, M.R.; Reinwald, S.; Burr, D.B. Alendronate Reduces Bone Toughness of Ribs without Significantly Increasing Microdamage Accumulation in Dogs Following 3 Years of Daily Treatment. Calcif. Tissue Int. 2008, 82, 354–360. [Google Scholar] [CrossRef]
- Allen, M.R.; Burr, D.B. Three Years of Alendronate Treatment Results in Similar Levels of Vertebral Microdamage as After One Year of Treatment. J. Bone Miner. Res. 2007, 22, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Mashiba, T.; Hirano, T.; Turner, C.H.; Forwood, M.R.; Johnston, C.C.; Burr, D.B. Suppressed Bone Turnover by Bisphosphonates Increases Microdamage Accumulation and Reduces Some Biomechanical Properties in Dog Rib. J. Bone Miner. Res. 2000, 15, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Begkas, D.; Pastroudis, A.; Touzopoulos, P.; Markeas, N.G.; Chatzopoulos, S.-T. The Effects of Long-Term Use of Nitrogen-Containing Bisphosphonates on Fracture Healing. Cureus 2019, 11, e4307. [Google Scholar] [CrossRef] [PubMed]
- Gorter, E.A.; Reinders, C.R.; Krijnen, P.; Appelman-Dijkstra, N.M.; Schipper, I.B. The Effect of Osteoporosis and Its Treatment on Fracture Healing a Systematic Review of Animal and Clinical Studies. Bone Rep. 2021, 15, 101117. [Google Scholar] [CrossRef] [PubMed]
- Pors Nielsen, S. The Biological Role of Strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Guañabens, N.; Casado, E.; Blanch-Rubió, J.; Gómez-Alonso, C.; Díaz-Guerra, G.M.; Del Pino-Montes, J.; De Lamadrid, C.V.D.; Peris, P.; Muñoz-Torres, M.; SEIOMM Working Group. The next Step after Anti-Osteoporotic Drug Discontinuation: An up-to-Date Review of Sequential Treatment. Endocrine 2019, 64, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2016. Endocr. Pract. 2016, 22, 1–42. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Chen, S.; Lu, L.-L.; Gong, W.; Zhang, A.-H. Raloxifene in the Treatment of Osteoporosis in Postmenopausal Women with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2021, 53, 730–737. [Google Scholar] [CrossRef]
- Motlani, G.; Motlani, V.; Acharya, N.; Dave, A.; Pamnani, S.; Somyani, D.; Agrawal, S. Novel Advances in the Role of Selective Estrogen Receptor Modulators in Hormonal Replacement Therapy: A Paradigm Shift. Cureus 2023, 15, e49079. [Google Scholar] [CrossRef] [PubMed]
- Kawate, H.; Takayanagi, R. Efficacy and Safety of Bazedoxifene for Postmenopausal Osteoporosis. Clin. Interv. Aging 2011, 6, 151–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guan, G.; Du, Y.; Tang, W.; Chen, M.; Yu, W.; Li, H.; Cheng, Q. Impacts of Prior Anti-Osteoporosis Treatments on Sequential Denosumab Responses in BMD Changes Among Postmenopausal Osteoporosis Women in East China: Real-World Data Analysis. Clin. Interv. Aging 2025, 20, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hou, S.; Deng, X.; Hu, L.; Wang, J.; Hou, D. Sequential Treatment from Bisphosphonate to Denosumab Improves Lumbar Spine Bone Mineral Density in Postmenopausal Osteoporosis Patients: A Meta-Analysis of Randomized Controlled Trials. Medicine 2024, 103, e40594. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Sessa, C.; Morale, W.; Zanoli, L.; Catalano, A.; Silipigni, S.; Soraci, L.; Corsonello, A.; Princiotto, M.; Lomonte, C.; et al. Fracture Risk in Chronic Kidney Disease: Addressing an Overlooked Complication. Metabolites 2025, 15, 460. [Google Scholar] [CrossRef]
- Tutaworn, T.; Nieves, J.W.; Wang, Z.; Levin, J.E.; Yoo, J.E.; Lane, J.M. Bone Loss after Denosumab Discontinuation Is Prevented by Alendronate and Zoledronic Acid but Not Risedronate: A Retrospective Study. Osteoporos. Int. 2023, 34, 573–584. [Google Scholar] [CrossRef]
- Laursen, N.; Sølling, A.S.; Harsløf, T.; Langdahl, B. Clinical Experience with Denosumab Discontinuation. Osteoporos. Int. 2025, 36, 435–446. [Google Scholar] [CrossRef]
- Grassi, G.; Ghielmetti, A.; Zampogna, M.; Chiodini, I.; Arosio, M.; Mantovani, G.; Eller-Vainicher, C. Zoledronate After Denosumab Discontinuation: Is Repeated Administrations More Effective Than Single Infusion? J. Clin. Endocrinol. Metab. 2024, 109, e1817–e1826, Correction in J. Clin. Endocrinol. Metab. 2024, 109, e1986. [Google Scholar] [CrossRef]
- Reid, I.R.; Billington, E.O. Drug Therapy for Osteoporosis in Older Adults. Lancet 2022, 399, 1080–1092, Correction in Lancet 2022, 400, 732. [Google Scholar] [CrossRef]
- Hoong, C.W.S.; Saul, D.; Khosla, S.; Sfeir, J.G. Advances in the Management of Osteoporosis. BMJ 2025, 390, e081250. [Google Scholar] [CrossRef]
- Cosman, F.; Oates, M.; Betah, D.; Timoshanko, J.; Wang, Z.; Ferrari, S.; McClung, M.R. Romosozumab Followed by Denosumab versus Denosumab Only: A Post Hoc Analysis of FRAME and FRAME Extension. J. Bone Miner. Res. 2024, 39, 1268–1277. [Google Scholar] [CrossRef]
- Cosman, F.; Langdahl, B.; Leder, B.Z. Treatment Sequence for Osteoporosis. Endocr. Pract. 2024, 30, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ebina, K.; Etani, Y.; Noguchi, T.; Nakata, K.; Okada, S. Clinical Effects of Teriparatide, Abaloparatide, and Romosozumab in Postmenopausal Osteoporosis. J. Bone Miner. Metab. 2025, 43, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Casado, E.; Martínez-Díaz-Guerra, G.; Caeiro, J.R. Los análogos de la PTH/PTHrP como tratamiento osteoanabólico de los pacientes con osteoporosis. Med. Clin. 2025, 165, 107076. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.F.; Hu, M.; Harris, A.G.; et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis: A Randomized Clinical Trial. JAMA 2016, 316, 722. [Google Scholar] [CrossRef]
- Compston, J.E. Skeletal Actions of Intermittent Parathyroid Hormone: Effects on Bone Remodelling and Structure. Bone 2007, 40, 1447–1452. [Google Scholar] [CrossRef]
- Kobayakawa, T.; Nakamura, Y. Verifying the Effectiveness of Romosozumab Re-Administration on Bone Mineral Density. J. Bone Miner. Res. 2025, 40, 201–210. [Google Scholar] [CrossRef]
- Masuda, S.; Fukasawa, T.; Matsuda, S.; Yoshida, S.; Kawakami, K. Comparative Effectiveness and Cardiovascular Safety of Romosozumab versus Teriparatide in Patients with Osteoporosis: A Population-Based Cohort Study. Osteoporos. Int. 2024, 35, 2165–2174. [Google Scholar] [CrossRef]
- Di Filippo, L.; Rosen, C.J. Latest on Anabolic Agents for Osteoporosis Treatment. Endocrinol. Metab. Clin. N. Am. 2024, 53, 513–523. [Google Scholar] [CrossRef]
- Song, I.-W.; Nagamani, S.C.S.; Nguyen, D.; Grafe, I.; Sutton, V.R.; Gannon, F.H.; Munivez, E.; Jiang, M.-M.; Tran, A.; Wallace, M.; et al. Targeting TGF-β for Treatment of Osteogenesis Imperfecta. J. Clin. Investig. 2022, 132, e152571. [Google Scholar] [CrossRef]
- June, R.R.; Olsen, N.J. Room for More IL-6 Blockade? Sarilumab for the Treatment of Rheumatoid Arthritis. Expert. Opin. Biol. Ther. 2016, 16, 1303–1309. [Google Scholar] [CrossRef]
- Boyapati, A.; Msihid, J.; Fiore, S.; Van Adelsberg, J.; Graham, N.M.H.; Hamilton, J.D. Sarilumab plus Methotrexate Suppresses Circulating Biomarkers of Bone Resorption and Synovial Damage in Patients with Rheumatoid Arthritis and Inadequate Response to Methotrexate: A Biomarker Study of MOBILITY. Arthritis Res. Ther. 2016, 18, 225. [Google Scholar] [CrossRef]
- Kelly, J.J.; Garapati, S.S. Combination Therapies in the Treatment of Osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Rocho, F.R.; Bonatto, V.; Lameiro, R.F.; Lameira, J.; Leitão, A.; Montanari, C.A. A Patent Review on Cathepsin K Inhibitors to Treat Osteoporosis (2011–2021). Expert. Opin. Ther. Pat. 2022, 32, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Enitan, S.S.; Adejumo, E.N.; Imaralu, J.O.; Adelakun, A.A.; Ladipo, O.A.; Enitan, C.B. Personalized Medicine Approach to Osteoporosis Management in Women: Integrating Genetics, Pharmacogenomics, and Precision Treatments. Clin. Res. Commun. 2023, 6, 18. [Google Scholar] [CrossRef]
- Aboul-Ella, H.; Gohar, A.; Ali, A.A.; Ismail, L.M.; Mahmoud, A.E.E.-R.; Elkhatib, W.F.; Aboul-Ella, H. Monoclonal Antibodies: From Magic Bullet to Precision Weapon. Mol. Biomed. 2024, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Shah, A.; Shoback, D. Sclerostin Inhibition: A Novel Therapeutic Approach in the Treatment of Osteoporosis. Int. J. Women Health 2015, 7, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, D.; Zhang, N.; Ni, S.; Sun, M.; Wang, L.; Xiao, H.; Liu, D.; Liu, J.; Yu, Y.; et al. Drug Discovery of Sclerostin Inhibitors. Acta Pharm. Sin. B 2022, 12, 2150–2170. [Google Scholar] [CrossRef] [PubMed]
- Klavdianou, K.; Liossis, S.-N.; Daoussis, D. Dkk1: A Key Molecule in Joint Remodelling and Fibrosis. Mediterr. J. Rheumatol. 2017, 28, 174–182. [Google Scholar] [CrossRef]
- McCarthy, H.S.; Marshall, M.J. Dickkopf-1 as a Potential Therapeutic Target in Paget’s Disease of Bone. Expert. Opin. Ther. Targets 2010, 14, 221–230. [Google Scholar] [CrossRef]
- Florio, M.; Gunasekaran, K.; Stolina, M.; Li, X.; Liu, L.; Tipton, B.; Salimi-Moosavi, H.; Asuncion, F.J.; Li, C.; Sun, B.; et al. A Bispecific Antibody Targeting Sclerostin and DKK-1 Promotes Bone Mass Accrual and Fracture Repair. Nat. Commun. 2016, 7, 11505. [Google Scholar] [CrossRef] [PubMed]
- Heiland, G.R.; Zwerina, K.; Baum, W.; Kireva, T.; Distler, J.H.; Grisanti, M.; Asuncion, F.; Li, X.; Ominsky, M.; Richards, W.; et al. Neutralisation of Dkk-1 Protects from Systemic Bone Loss during Inflammation and Reduces Sclerostin Expression. Ann. Rheum. Dis. 2010, 69, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Kroupova, K.; Palicka, V.; Rosa, J. Monoclonal Antibodies for Treatment of Osteoporosis. Drugs Today 2023, 59, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.; Greene, R.; Ardawi, M.; Qari, M.; Mousa, S. Pharmacogenomics in Osteoporosis: Steps toward Personalized Medicine. Pharmacogenom. Pers. Med. 2009, 2, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Usategui-Martín, R.; De Luis-Román, D.-A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.-L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Jawiarczyk-Przybyłowska, A.; Halupczok-Żyła, J.; Kolačkov, K.; Gojny, Ł.; Zembska, A.; Bolanowski, M. Association of Vitamin D Receptor Polymorphisms with Activity of Acromegaly, Vitamin D Status and Risk of Osteoporotic Fractures in Acromegaly Patients. Front. Endocrinol. 2019, 10, 643. [Google Scholar] [CrossRef]
- Mann, V.; Hobson, E.E.; Li, B.; Stewart, T.L.; Grant, S.F.A.; Robins, S.P.; Aspden, R.M.; Ralston, S.H. A COL1A1 Sp1 Binding Site Polymorphism Predisposes to Osteoporotic Fracture by Affecting Bone Density and Quality. J. Clin. Investig. 2001, 107, 899–907. [Google Scholar] [CrossRef]
- Littman, J.; Yang, W.; Olansen, J.; Phornphutkul, C.; Aaron, R.K. LRP5, Bone Mass Polymorphisms and Skeletal Disorders. Genes 2023, 14, 1846. [Google Scholar] [CrossRef]
- Van Velsen, E.F.S.; Wijnen, M.; Muradin, G.S.R.; Zillikens, M.C. Incident Vertebral Fractures During Romosozumab Treatment in a Patient with a Pathogenic LRP5 Variant. JCEM Case Rep. 2024, 3, luae238. [Google Scholar] [CrossRef]
- Stringer, F.; Sims, N.A.; Sachithanandan, N.; Aleksova, J. Severe Osteoporosis with Pathogenic LRP5 Variant. JCEM Case Rep. 2024, 2, luae021. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-de-Souza, N.C.; Da Silva, I.D.C.G.; De Carvalho, C.V.; Pulchinelli, A.; Haidar, M.A.; Baracat, E.C.; Massad-Costa, A.M. Effect of Estrogen Receptor-Alpha (ESR1) Gene Polymorphism on High Density Lipoprotein Levels in Response to Hormone Replacement Therapy. Braz. J. Med. Biol. Res. 2009, 42, 1138–1142. [Google Scholar] [CrossRef]
- Zavratnik, A.; Žegura, B.; Marc, J.; Preželj, J.; Pfeifer, M. XbaI Polymorphism of the Estrogen Receptor Alpha Gene Influences the Effect of Raloxifene on the Endothelial Function. Maturitas 2010, 67, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Uzar, I.; Bogacz, A.; Sowińska-Przepiera, E.; Kotrych, K.; Wolek, M.; Sulikowski, T.; Kamiński, A. The Influence of ESR1 Polymorphisms on Selected Hormonal, Metabolic and Mineral Balance Markers in Women with Hyperandrogenism. Sci. Rep. 2022, 12, 19712. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L. The Genetics of Response to Estrogen Treatment. Clin. Cases Miner. Bone Metab. 2009, 6, 44–49. [Google Scholar] [PubMed]
- Nicholson, T.A.; Sagmeister, M.; Wijesinghe, S.N.; Farah, H.; Hardy, R.S.; Jones, S.W. Oligonucleotide Therapeutics for Age-Related Musculoskeletal Disorders: Successes and Challenges. Pharmaceutics 2023, 15, 237. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.; Singh, B.; Sharma, K.; Kumar, N.; Singh, D.; Klair, H.S.; Mastana, S. Implications of siRNA Therapy in Bone Health: Silencing Communicates. Biomedicines 2024, 12, 90. [Google Scholar] [CrossRef]
- Weng, L.; Wang, C.; Ko, J.; Sun, Y.; Wang, F. Control of Dkk-1 Ameliorates Chondrocyte Apoptosis, Cartilage Destruction, and Subchondral Bone Deterioration in Osteoarthritic Knees. Arthritis Rheum. 2010, 62, 1393–1402. [Google Scholar] [CrossRef]
- Nakamura, A.; Ali, S.A.; Kapoor, M. Antisense Oligonucleotide-Based Therapies for the Treatment of Osteoarthritis: Opportunities and Roadblocks. Bone 2020, 138, 115461. [Google Scholar] [CrossRef]
- Santarpia, G.; Carnes, E. Therapeutic Applications of Aptamers. Int. J. Mol. Sci. 2024, 25, 6742. [Google Scholar] [CrossRef]
- Cesarini, V.; Appleton, S.L.; De Franciscis, V.; Catalucci, D. The Recent Blooming of Therapeutic Aptamers. Mol. Asp. Med. 2025, 102, 101350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Z.-K.; Yu, Y.; Zhuo, Z.; Zhang, G.; Zhang, B.-T. Pros and Cons of Denosumab Treatment for Osteoporosis and Implication for RANKL Aptamer Therapy. Front. Cell Dev. Biol. 2020, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, Y.; Ni, S.; Li, D.; Liu, J.; Xie, D.; Chu, H.Y.; Ren, Q.; Zhong, C.; Zhang, N.; et al. Therapeutic Aptamer Targeting Sclerostin Loop3 for Promoting Bone Formation without Increasing Cardiovascular Risk in Osteogenesis Imperfecta Mice. Theranostics 2022, 12, 5645–5674. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Hu, J.; Yu, Y.; Sun, L.; Wang, Y.; Zhang, Q.; Jiang, Y.; Wang, O.; Xing, X.; Xia, W.; et al. Novel Aptamers Targeting Sclerostin Loop3 Improve Skeletal and Muscle Properties Without Adverse Cardiovascular Effects in Orchiectomized Mice. J. Cachexia Sarcopenia Muscle 2025, 16, e13831. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of Nucleic Acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, B.; Tang, Y.; Shen, A.; Lin, Y.; Zhao, X.; Li, J.; Monteiro, M.J.; Gu, W. Bone Targeted Nano-Drug and Nano-Delivery. Bone Res. 2024, 12, 51. [Google Scholar] [CrossRef]
- Tonk, C.H.; Shoushrah, S.H.; Babczyk, P.; El Khaldi-Hansen, B.; Schulze, M.; Herten, M.; Tobiasch, E. Therapeutic Treatments for Osteoporosis—Which Combination of Pills Is the Best among the Bad? Int. J. Mol. Sci. 2022, 23, 1393. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhong, Y.-H.; Chen, L.-M.; Zhuo, X.-L.; Zhao, L.-J.; Wang, Y.-T. Recent Advance of Small-Molecule Drugs for Clinical Treatment of Osteoporosis: A Review. Eur. J. Med. Chem. 2023, 259, 115654. [Google Scholar] [CrossRef]
- Boonen, S.; Rosenberg, E.; Claessens, F.; Vanderschueren, D.; Papapoulos, S. Inhibition of Cathepsin K for Treatment of Osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 73–79. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Xia, M.; Tian, Z.; Zhou, J.; Berger, J.M.; Zhang, X.H.-F.; Xiao, H. Engineering Small-Molecule and Protein Drugs for Targeting Bone Tumors. Mol. Ther. 2024, 32, 1219–1237. [Google Scholar] [CrossRef]
- Wang, S.; Yin, J.; Chen, D.; Nie, F.; Song, X.; Fei, C.; Miao, H.; Jing, C.; Ma, W.; Wang, L.; et al. Small-Molecule Modulation of Wnt Signaling via Modulating the Axin-LRP5/6 Interaction. Nat. Chem. Biol. 2013, 9, 579–585. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Li, J.; Jiang, Y.; Xu, K.; Su, J. Bone-Targeted Nanoparticle Drug Delivery System: An Emerging Strategy for Bone-Related Disease. Front. Pharmacol. 2022, 13, 909408. [Google Scholar] [CrossRef]
- Yin, P.; Dong, S.; Yu, J.; Zhao, Z.; Hu, Y. The Emerging Roles of Nano Drug Delivery Systems in Treatment of Osteoporosis-Current Knowledge, Challenges and Future Perspectives. Int. J. Nanomed. 2025, 20, 11061–11079. [Google Scholar] [CrossRef]
- Cai, R.; Jiang, Y.; Sun, H.; Du, F.; Zhu, L.; Tao, J.; Xiao, L.; Wang, Z.; Shi, H. Advances in Functionalized Nanoparticles for Osteoporosis Treatment. Int. J. Nanomed. 2025, 20, 7869–7891. [Google Scholar] [CrossRef]
- Gharat, S.A.; Momin, M.M.; Khan, T. Pharmacokinetics and Pharmacodynamics of Nanocarriers and Novel Drug Delivery Systems. In Pharmacokinetics and Pharmacodynamics of Novel Drug Delivery Systems: From Basic Concepts to Applications; Gharat, S.A., Momin, M.M., Khan, T., Eds.; Springer Nature: Singapore, 2024; pp. 179–274. ISBN 978-981-9978-57-1. [Google Scholar]
- Gao, W.; Liang, C.; Zhao, K.; Hou, M.; Wen, Y. Multifunctional Gold Nanoparticles for Osteoporosis: Synthesis, Mechanism and Therapeutic Applications. J. Transl. Med. 2023, 21, 889. [Google Scholar] [CrossRef]
- Gu, W.; Wu, C.; Chen, J.; Xiao, Y. Nanotechnology in the Targeted Drug Delivery for Bone Diseases and Bone Regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
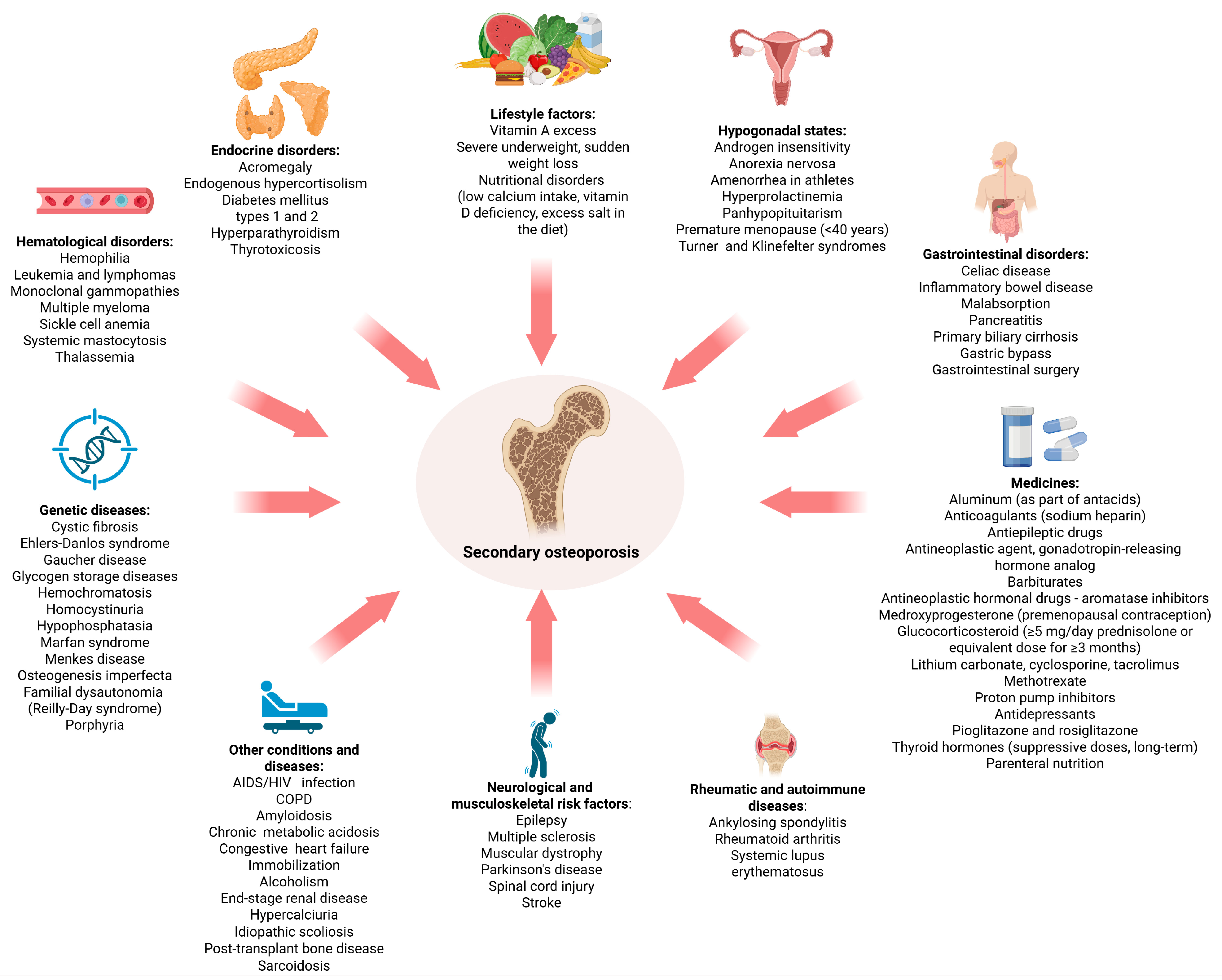

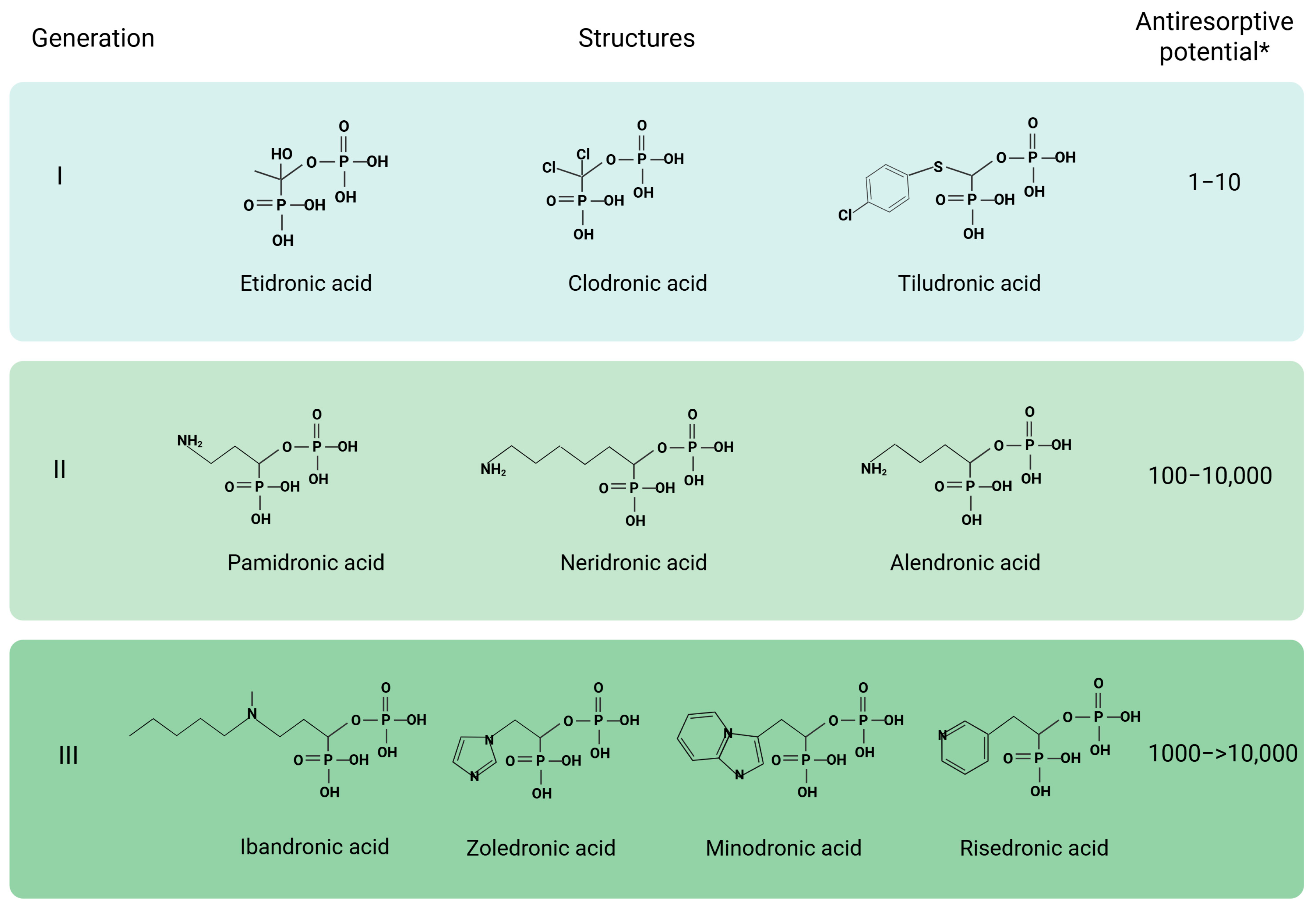
| Class of Anti-Osteoporotic Drug (International Nonproprietary Names) | Mechanism | Efficacy | Side Effects | Clinical Status |
|---|---|---|---|---|
| Anti-resorptive drugs | ||||
| Bisphosphonates (zoledronate, alendronate, ibandronate, risedronate) | Inhibit hydroxyapatite crystals destruction. Suppress osteoclasts (through cell death due to toxic ATP analogues (nitrogen-free bisphosphonates)/promote osteoclast apoptosis by inhibiting FPPS (nitrogen-containing bisphosphonates) [101]. | Increase BMD values and reducing the risk of osteoporotic fractures. First-line drugs. | Oral administration: heartburn, indigestion, esophageal erosion, and ulcers. Parenteral administration: flu-like symptoms (fever, muscle pain, and arthralgia), hypocalcemia. Rare: excessive bone fragility, jaw osteonecrosis [11,102] Potential teratogenicity/placental permeability. | Phase 4 (post-market) |
| Strontium ranelate | Increases osteoblasts proliferation and differentiation. Incorporates into bone cells, increasing their density. Suppresses the formation and differentiation of osteoclasts and promotes their apoptosis [103]. | Significant bone mass increasing. Useful as a component of modified biomaterials [103]. Second-line drug. | Severe skin reactions (toxic epidermal necrolysis), cardiovascular disease, thromboembolic complications, hypocalcemia [103,104]. | Phase 4 (post-market) |
| Calcitonin | Causes osteoclasts’ outflow from areas of active bone resorption. Inhibits the differentiation and proliferation of osteoclasts [13,105]. | The resorption of bone matrix is diminished. Reduces solely the risk of vertebral fractures and only when administered nasally Has analgesic effect [13,106,107]. Second-line drug. | Nasal discomfort, abnormal product odor, nausea, loss of appetite, diarrhea, vomiting, abdominal pain, hot flushes, hypocalcemia, allergic reactions [108,109]. | Phase 4 (post-market) |
| Estrogen- Progestin Therapy | Compensates for the hormone deficiency and reduces bone loss by suppressing osteoclast activity [110]. | Reduces estrogen deficiency-mediated increased bone turnover and prevent further bone loss. Significantly reduces the risks of hip and vertebral fractures [111,112]. Second-line drug. | High risk of cardiovascular disease, stroke, venous thromboembolism, invasive breast cancer [113]. | Phase 4 (post-market) |
| SERMs (raloxifene, bazedoxifene) | Induces osteoclast apoptosis. Act as estrogenic agonists in bone [112]. | Inhibits accelerated bone resorption in both the short- and long-term, increases BMD, and bone strength. Prevents bone loss and reduces the risk of spinal fractures, but not fractures in other locations [114]. Second-line drug. | Risk of deep vein thrombosis, pulmonary embolism, vaginal bleeding, stroke, cardiovascular disease, hot flashes, leg cramps [11,115]. | Phase 4 (post-market) |
| Anti- RANKL agent (denosumab) | Blocks RANKL, an essential factor regulating osteoclast activity [116]. | Reduction in the risk of hip and vertebral fractures with long-term use [117]. First-line drugs. | “Rebound effect” (increase in bone turnover overriding pre-treatment status, a rapid bone loss in the majority and multiple vertebral fracture), hypocalcemia, cellulitis, musculoskeletal pain, jaw osteonecrosis [11,102,118,119]. | Phase 4 (post-market) |
| Cathepsin K inhibitors (odanacatib, balicatib) | Limits osteoclast activity without suppression of osteoblast function [120]. | Reduces the risk of hip and spine fractures due to increasing in BMD and improving bone strength at the spine and hip [121]. | High risk of stroke, probable pycnodystosis [121,122]. | Phase 3 |
| RANKL- suppressing siRNA [123] | Reduction in osteoclast formation. | Reduction in bone loss, BMD increase. | No data available. | Pre-clinical research |
| BTK inhibitor [124] | Inhibits the M-CSF and RANKL-driven osteoclast differentiation. | Suppresses bone loss in mice. | No data available. | Pre-clinical research |
| p38 MAP kinase inhibitor [125] | Represses RANKL-induced osteoclast differentiation. | Prevents bone loss in ovariectomized mouse mode by inhibiting both bone resorption and bone formation in vivo. | No data available. | Pre-clinical research |
| Anabolic drugs | ||||
| PTH analogues (teriparatide, abaloparatide) | Increases osteoblastic activity through binding with the parathyroid hormone receptor 1 (PTHR1) [126]. | Stimulates bone formation. Prevents non-vertebral fractures and improving spine BMD [12,127]. | Probable osteosarcoma risk, cephalgia, dizziness, limb cramps, nausea, hypercalcemia [10,11,12,128]. | Phase 4 (post-market) |
| Anti-sclerostin agent (romosozumab) | Inhibits the binding of sclerostin to the LRP 5/6-frizzled receptor complex, thereby activating the Wnt signaling pathway [118]. | Rapid increases in bone mineral density. Significant reductions in the risk of fractures Loss of anabolic effect after stopping treatment [118]. | Arthralgia, nasopharyngitis, injection-site reactions, headache, cataracts, risk of cardiac events, hypocalcemia, osteonecrosis of the jaw, atypical femur fracture, serious infections for elderly patients [118]. | Phase 4 (post-market) |
| Anti-sclerostin agent (blosozumab) | Binds directly to sclerostin, which is an inhibitor of the Wnt signaling pathway [129]. | Improves lumbar bone mass in postmenopausal women [130]. | Arthralgia, back pain, fatigue, headache, injection site reactions, nasal congestion, nausea, upper respiratory tract infection, and vomiting [118,129,130]. | Phase 2 |
| Bispecific antibodies blocking RANKL and sclerostin [131] | Allows for both the suppression of osteoclastic activity and stimulation of osteoblastic function. | No data available. | No data available. | Pre-clinical research |
| siRNA targeting WNT antagonists (DKK-1, SOST) [132,133] | Activation of the Wnt pathway (increase in β-catenin level). Increased mineralization of osteoblasts. | Increase BMD, mineralization, trabecular bone, decrease osteolysis in mice. Provide reduction in fracture frequency. | No data available. | Pre-clinical research |
| miRNA [134] | Suppress the expression of the SHN3 and SOST genes, thus activate the Wnt pathway. | Increases in trabecular bone mass in mice. | No data available. | Pre-clinical research |
| Antisense oligonucleotides targeting DKK-1 [135] | Activation of the Wnt pathway. Stimulate osteoblast activity. Reduce ovariectomy promotion of ex vivo osteoclast differentiation of primary M-CSF-dependent bone marrow macrophages. Increase osteoblast number. | Reduce bone loss and improves the biomechanical properties of bone in mice. | No data available. | Pre-clinical research |
| Aptamers targeting sclerostin [136] | Attenuate inhibitory effect of SOST on bone formation. | Promote bone formation in mice and ovariectomy-induced osteoporotic rats. | No data available. | Pre-clinical research |
| Model | Target | siRNA | Key Findings and Evaluation Methods | References |
|---|---|---|---|---|
| Human (TNFSF11) | RANKL | siRANKL-1, Sense: 5′-GCAUGAAGACUCCAGAACAdTdT-3′ siRANKL-2, Sense: 5′-CCUGGAAACUGCUGAAAUAdTdT-3′ | Molecular level: suppression of RANKL mRNA by 60–80% (qPCR); reduction of RANKL protein level (Western blot/ELISA/IHC). Functional/macro level:—Reduction in osteoclast formation by 50–70%; reduction in bone loss, increase in BMD by 20–30%; reduction in the number of TRAP+ osteoclasts. | [123] |
| Human (NM_012242) | DKK1 | siDKK1-1: Sense: 5′-GCAUGUACUGUGGAUCAUAdTdT-3′ siDKK1-2: Sense: 5′-CCACCAAGUGUACAUCUAUdTdT-3′ | Molecular level: suppression of DKK1 mRNA and protein levels by 60–80%; activation of the Wnt pathway (increase in β-catenin level). Functional/macro level:—Increased mineralization of osteoblasts; increase in bone mass by 25–30% (micro-CT); reduction in resorption markers (CTX-1). | [132] |
| Human (NM_025237) | SOST | siSOST-1: Sense: 5′-GCAUGAAGCUCCUGAAACAdTdT-3′ siSOST-2: Sense: 5′-CCUGGAACUGCCAGAAGAUdTdT-3′ | Molecular level:—Suppression of SOST mRNA by 70–90%.—Increased Wnt/β-catenin activity. Functional/macro level:—30% increase in BMD.—Increased mineralization, decreased osteolysis.—Increase in trabecular bone, reduction in fracture frequency. | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omelchenko, V.; Koval, V.; Slazhneva, N.; Bondarenko, N.; Shatunova, E.; Vorobyeva, M.; Korolev, M. Modern Strategies for Osteoporosis Therapy: Current Status and Prospects for Targeted Intervention. Int. J. Mol. Sci. 2025, 26, 11092. https://doi.org/10.3390/ijms262211092
Omelchenko V, Koval V, Slazhneva N, Bondarenko N, Shatunova E, Vorobyeva M, Korolev M. Modern Strategies for Osteoporosis Therapy: Current Status and Prospects for Targeted Intervention. International Journal of Molecular Sciences. 2025; 26(22):11092. https://doi.org/10.3390/ijms262211092
Chicago/Turabian StyleOmelchenko, Vitalii, Vladimir Koval, Natalya Slazhneva, Natalya Bondarenko, Elizaveta Shatunova, Mariya Vorobyeva, and Maxim Korolev. 2025. "Modern Strategies for Osteoporosis Therapy: Current Status and Prospects for Targeted Intervention" International Journal of Molecular Sciences 26, no. 22: 11092. https://doi.org/10.3390/ijms262211092
APA StyleOmelchenko, V., Koval, V., Slazhneva, N., Bondarenko, N., Shatunova, E., Vorobyeva, M., & Korolev, M. (2025). Modern Strategies for Osteoporosis Therapy: Current Status and Prospects for Targeted Intervention. International Journal of Molecular Sciences, 26(22), 11092. https://doi.org/10.3390/ijms262211092






