IL-34 as a Novel Mediator Linking Vitamin D Deficiency with Osteoporosis and Knee Osteoarthritis
Abstract
1. Introduction
2. Results
2.1. Descriptive Characteristics of the Study Sample
2.2. Group Comparisons by VD Status
2.3. Regression Analyses of VD Status and IL-34 Levels
2.4. Associations Between VD Status, OP, and KOA
2.5. Structural Equation Modeling (SEM)
2.6. X-Ray Images of KOA
3. Discussion
3.1. Main Results of the Study in Relation to Underlying Molecular Mechanisms
3.2. VD Supplementation and Musculoskeletal Diseases
4. Materials and Methods
4.1. Study Sample and Ethics
4.2. Demographic, Anthropometric, and Body Composition Assessment
4.3. Measurement of VD and Cytokine Circulating Levels
4.4. Blood Glucose Levels, Lipid Profile, and Blood Count
4.5. OP and KOA Assessment
4.6. Study Design and Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASMM/WT | Appendicular skeletal muscle mass/weight ratio |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| FM/WT | Fat mass/weight ratio |
| HDL-C | High-density lipoprotein cholesterol |
| Hs-CRP | High-sensitivity C-reactive protein |
| IL-9 | Interleukin-9 |
| IL-34 | Interleukin-34 |
| KOA | Knee osteoarthritis |
| MCP-1 | Monocyte chemoattractant protein-1 |
| OA | Osteoarthritis |
| OP | Osteoporosis |
| SII | Systemic immune-inflammation index |
| TC | Total cholesterol |
| TC/HDL-C | Total cholesterol to HDL-C ratio |
| TG | Triglycerides |
| VD | Vitamin D |
| VDD | Vitamin D deficiency |
| VDS | Vitamin D sufficiency |
| WHR | Waist–hip ratio |
References
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2015, 96, 365–408. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Bakke, D.; Sun, J. Ancient Nuclear Receptor VDR with New Functions: Microbiome and Inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D Storage in Adipose Tissue of Obese and Normal Weight Women. J. Bone Miner. Res. 2017, 32, 237–242. [Google Scholar] [CrossRef]
- Park, C.Y.; Han, S.N. The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation. J. Lipid Atheroscler. 2021, 10, 130–144. [Google Scholar] [CrossRef]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.M.; Buring, J.E.; et al. Association of Body Weight With Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef]
- Orces, C. The Association between Body Mass Index and Vitamin D Supplement Use among Adults in the United States. Cureus 2019, 11, e5721. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H. Vitamin D, Muscle Recovery, Sarcopenia, Cachexia, and Muscle Atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Bollen, S.E.; Bass, J.J.; Fujita, S.; Wilkinson, D.; Hewison, M.; Atherton, P.J. The Vitamin D/Vitamin D Receptor (VDR) Axis in Muscle Atrophy and Sarcopenia. Cell. Signal. 2022, 96, 110355. [Google Scholar] [CrossRef]
- Lepsch, J.; Eshriqui, I.; Farias, D.R.; Vaz, J.S.; Cunha Figueiredo, A.C.; Adegboye, A.R.A.; Brito, A.; Mokhtar, R.; Allen, L.H.; Holick, M.F.; et al. Association between Early Pregnancy Vitamin D Status and Changes in Serum Lipid Profiles throughout Pregnancy. Metabolism 2017, 70, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Peng, M.; Chen, S.; Wu, S.; Zhang, W. Vitamin D Deficiency Is Associated with Dyslipidemia: A Cross-Sectional Study in 3788 Subjects. Curr. Med. Res. Opin. 2019, 35, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Vitezova, A.; Voortman, T.; Zillikens, M.C.; Jansen, P.W.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Kiefte-De Jong, J.C. Bidirectional Associations between Circulating Vitamin D and Cholesterol Levels: The Rotterdam Study. Maturitas 2015, 82, 411–417. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef]
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Lips, P.; Van Schoor, N.M. The Effect of Vitamin D on Bone and Osteoporosis. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Berthold, H.K. Vitamin D and Vascular Disease. Curr. Vasc. Pharmacol. 2020, 19, 250–268. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Allegra, A.; Sirufo, M.M.; Tonacci, A.; Pioggia, G.; Raggiunti, M.; Ginaldi, L.; Gangemi, S. Vitamin D Deficiency, Osteoporosis and Effect on Autoimmune Diseases and Hematopoiesis: A Review. Int. J. Mol. Sci. 2021, 22, 8855. [Google Scholar] [CrossRef]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int. J. Mol. Sci. 2023, 24, 15485. [Google Scholar] [CrossRef]
- Subarajan, P.; Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: A Review of Latest Guidelines. Endocrinol. Metab. Clin. N. Am. 2024, 53, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, 261–282. [Google Scholar] [CrossRef]
- Livshits, G.; Kalinkovich, A. Targeting Chronic Inflammation as a Potential Adjuvant Therapy for Osteoporosis. Life Sci. 2022, 306, 120847. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, P.; Mechtcheriakova, D.; Meshcheryakova, A.; Föger-Samwald, U.; Ellinger, I. Immunology of Osteoporosis: A Mini-Review. Gerontology 2016, 62, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Yedla, N.; Kim, H.; Sharma, A.; Wang, X. Vitamin D Deficiency and the Presentation of Primary Hyperparathyroidism: A Mini Review. Int. J. Endocrinol. 2023, 2023, 1169249. [Google Scholar] [CrossRef]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; da Silva, D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, Physiological Role, Biokinetics, Deficiency, Therapeutic Use, Toxicity, and Overview of Analytical Methods for Detection of Vitamin D and Its Metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef]
- Sapir-Koren, R.; Livshits, G. Bone Mineralization Is Regulated by Signaling Cross Talk between Molecular Factors of Local and Systemic Origin: The Role of Fibroblast Growth Factor 23. BioFactors 2014, 40, 555–568. [Google Scholar] [CrossRef]
- Vaishya, R.; Vijay, V.; Lama, P.; Agarwal, A. Does Vitamin D Deficiency Influence the Incidence and Progression of Knee Osteoarthritis?—A Literature Review. J. Clin. Orthop. Trauma. 2019, 10, 9–15, Erratum in J. Clin. Orthop. Trauma. 2020, 11, 1175. [Google Scholar] [CrossRef]
- Garfinkel, R.J.; Dilisio, M.F.; Agrawal, D.K. Vitamin D and Its Effects on Articular Cartilage and Osteoarthritis. Orthop. J. Sports Med. 2017, 5, 2325967117711376. [Google Scholar] [CrossRef]
- Kalichman, L.; Kobyliansky, E. Association between Circulatory Levels of Vitamin D and Radiographic Hand Osteoarthritis. Rheumatol. Int. 2012, 32, 253–257. [Google Scholar] [CrossRef]
- Dechsupa, S.; Yingsakmongkol, W.; Limthongkul, W.; Singhatanadgige, W.; Jitjumnong, M.; Honsawek, S. Vitamin D Inadequacy Affects Skeletal Muscle Index and Physical Performance in Lumbar Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 3152. [Google Scholar] [CrossRef]
- Tönük, Ş.B.; Yorgancıoğlu, Z.R.; Ramadan, S.U.; Kocaoğlu, S. Relationship between DXA Measured Systemic Bone Mineral Density and Subchondral Bone Cysts in Postmenopausal Female Patients with Knee Osteoarthritis: A Cross-Sectional Study: Osteoarthritis Cysts and Bone Mineral Density. BMC Musculoskelet. Disord. 2024, 25, 50. [Google Scholar] [CrossRef] [PubMed]
- Mbuyi, M.K.; Kavangh, H.S.; Grubišić, F.; Vajdić, I.D.; Grazio, S. Is Vitamin D Associated with Disease Activity in Patients with Axial or Peripheral Spondyloarthritis? A Real-Life Study. Rheumatol. Int. 2024, 44, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, B.; Wang, X.; Chen, C.; Zhan, E.; Lv, Z.; He, Q.; Hu, Y.; Zhang, F. Vitamin D Deficiency Promotes Intervertebral Disc Degeneration via P38/NCoR2-Mediated Extracellular Matrix Degradation. Eur. J. Nutr. 2025, 64, 163. [Google Scholar] [CrossRef]
- Wang, X.; Hunter, D.; Xu, J.; Ding, C. Metabolic Triggered Inflammation in Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 22–30. [Google Scholar] [CrossRef]
- Courties, A.; Gualillo, O.; Berenbaum, F.; Sellam, J. Metabolic Stress-Induced Joint Inflammation and Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Kalinkovich, A. Hierarchical, Imbalanced pro-Inflammatory Cytokine Networks Govern the Pathogenesis of Chronic Arthropathies. Osteoarthr. Cartil. 2018, 26, 7–17. [Google Scholar] [CrossRef]
- De Roover, A.; Escribano-Núñez, A.; Monteagudo, S.; Lories, R. Fundamentals of Osteoarthritis: Inflammatory Mediators in Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1303–1311. [Google Scholar] [CrossRef]
- Kruit, A.; Zanen, P. The Association between Vitamin D and C-Reactive Protein Levels in Patients with Inflammatory and Non-Inflammatory Diseases. Clin. Biochem. 2016, 49, 534–537. [Google Scholar] [CrossRef]
- Chan, F.; Cui, C.; Peng, Y.; Liu, Z. The Associations among Serum Vitamin D Concentration, Systemic Immune-Inflammation Index, and Lifestyle Factors in Chinese Adults: A Cross-Sectional Analysis. Front. Nutr. 2025, 12, 1543925. [Google Scholar] [CrossRef] [PubMed]
- Pajulas, A.; Zhang, J.; Kaplan, M.H. The World According to IL-9. J. Immunol. 2023, 211, 7–14. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Chang, C.; Lu, L.; Lau, C.S.; Lu, Q. Th9 Cells and IL-9 in Autoimmune Disorders: Pathogenesis and Therapeutic Potentials. Hum. Immunol. 2017, 78, 120–128. [Google Scholar] [CrossRef]
- Sapra, L.; Saini, C.; Sharma, S.; Nanda, D.; Nilakhe, A.; Chattopadhyay, N.; Meena, A.S.; Mishra, P.K.; Gupta, S.; Garg, B.; et al. Targeting the Osteoclastogenic Cytokine IL-9 as a Novel Immunotherapeutic Strategy in Mitigating Inflammatory Bone Loss in Post-Menopausal Osteoporosis. JBMR Plus 2024, 8, ziae120. [Google Scholar] [CrossRef]
- Qi, C.; Shan, Y.; Wang, J.; Ding, F.; Zhao, D.; Yang, T.; Jiang, Y. Circulating T Helper 9 Cells and Increased Serum Interleukin-9 Levels in Patients with Knee Osteoarthritis. Clin. Exp. Pharmacol. Physiol. 2016, 43, 528–534. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ninomiya, K.; Sonoda, K.H.; Miyauchi, Y.; Hoshi, H.; Iwasaki, R.; Miyamoto, H.; Yoshida, S.; Sato, Y.; Morioka, H.; et al. MCP-1 Expressed by Osteoclasts Stimulates Osteoclastogenesis in an Autocrine/Paracrine Manner. Biochem. Biophys. Res. Commun. 2009, 383, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Wang, X.S.; Cheng, F.B.; Wang, F.; Wan, L.; Wang, F.; Huang, H.X. Elevated CCL2/MCP-1 Levels Are Related to Disease Severity in Postmenopausal Osteoporotic Patients. Clin. Lab. 2016, 62, 2173–2181. [Google Scholar] [CrossRef]
- Ni, F.; Zhang, Y.; Peng, X.; Li, J. Correlation between Osteoarthritis and Monocyte Chemotactic Protein-1 Expression: A Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 516. [Google Scholar] [CrossRef]
- Xu, Y.K.; Ke, Y.; Wang, B.; Lin, J.H. The Role of MCP-1-CCR2 Ligand-Receptor Axis in Chondrocyte Degradation and Disease Progress in Knee Osteoarthritis. Biol. Res. 2015, 48, 64. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y.M. Immunomodulation of Interleukin-34 and Its Potential Significance as a Disease Biomarker and Therapeutic Target. Int. J. Biol. Sci. 2019, 15, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Udomsinprasert, W.; Panon, K.; Preechanukul, S.; Jittikoon, J.; Jinawath, A.; Honsawek, S. Diagnostic Value of Interleukin-34 as a Novel Biomarker for Severity of Knee Osteoarthritis. Cartilage 2021, 13, 1174S–1184S. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Jittikoon, J.; Honsawek, S. Interleukin-34 as a Promising Clinical Biomarker and Therapeutic Target for Inflammatory Arthritis. Cytokine Growth Factor Rev. 2019, 47, 43–53. [Google Scholar] [CrossRef]
- Guruprasad, C.N.; Pradeep, A.R. Interleukin-34 Levels in Gingival Crevicular Fluid and Plasma in Periodontal Health and Disease with and without Type-2 Diabetes Mellitus. J. Investig. Clin. Dent. 2018, 9, e12317. [Google Scholar] [CrossRef]
- Tarabeih, N.; Shalata, A.; Kalinkovich, A.; Higla, O.; Livshits, G. Elevated Circulating Levels of IL-34 Are Strongly Associated with Osteoporosis. Arch. Osteoporos. 2023, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Recent Insights into Vitamin D, Adipocyte, and Adipose Tissue Biology. Obes. Rev. 2022, 23, e13453. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic Obesity or Obese Sarcopenia: A Cross Talk between Age-Associated Adipose Tissue and Skeletal Muscle Inflammation as a Main Mechanism of the Pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Varra, F.N.; Varras, M.; Varra, V.K.; Theodosis-Nobelos, P. Molecular and Pathophysiological Relationship between Obesity and Chronic Inflammation in the Manifestation of Metabolic Dysfunctions and Their Inflammation-mediating Treatment Options (Review). Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Thomopoulos, K.; Mouzaki, A.; Triantos, C. Vitamin D-VDR Novel Anti-Inflammatory Molecules-New Insights into Their Effects on Liver Diseases. Int. J. Mol. Sci. 2022, 23, 8465. [Google Scholar] [CrossRef]
- Baghdadi, M.; Umeyama, Y.; Hama, N.; Kobayashi, T.; Han, N.; Wada, H.; Seino, K. Ichiro Interleukin-34, a Comprehensive Review. J. Leukoc. Biol. 2018, 104, 931–951. [Google Scholar] [CrossRef]
- Kausz, M. Distribution of Neurons in the Lateral Pontine Tegmentum Projecting to Thoracic, Lumbar and Sacral Spinal Segments in the Cat. J. Hirnforsch. 1986, 27, 485–493. [Google Scholar]
- Wang, Q.; Zhou, X.; Zhang, P.; Zhao, P.; Nie, L.; Ji, N.; Ding, Y.; Wang, Q. 25-Hydroxyvitamin D3 Positively Regulates Periodontal Inflammaging via SOCS3/STAT Signaling in Diabetic Mice. Steroids 2020, 156, 108570. [Google Scholar] [CrossRef]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 Signaling in Immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, S.; Wang, Y.; Tu, S.; Wang, Z.; Chen, Z. Interleukin 34 Upregulation Contributes to the Increment of MicroRNA 21 Expression through STAT3 Activation Associated with Disease Activity in Rheumatoid Arthritis. J. Rheumatol. 2016, 43, 1312–1319. [Google Scholar] [CrossRef]
- Hwang, S.J.; Choi, B.; Kang, S.S.; Chang, J.H.; Kim, Y.G.; Chung, Y.H.; Sohn, D.H.; So, M.W.; Lee, C.K.; Robinson, W.H.; et al. Interleukin-34 Produced by Human Fibroblast-like Synovial Cells in Rheumatoid Arthritis Supports Osteoclastogenesis. Arthritis Res. Ther. 2012, 14, R14. [Google Scholar] [CrossRef] [PubMed]
- Baud’Huin, M.; Renault, R.; Charrier, C.; Riet, A.; Moreau, A.; Brion, R.; Gouin, F.; Duplomb, L.; Heymann, D. Interleukin-34 Is Expressed by Giant Cell Tumours of Bone and Plays a Key Role in RANKL-Induced Osteoclastogenesis. J. Pathol. 2010, 221, 77–86. [Google Scholar] [CrossRef]
- Chen, Z.; Buki, K.; Vääräniemi, J.; Gu, G.; Väänänen, H.K. The Critical Role of IL-34 in Osteoclastogenesis. PLoS ONE 2011, 6, e18689. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, Y.; Mizoguchi, T.; Arai, A.; Kobayashi, Y.; Sato, M.; Penninger, J.M.; Yasuda, H.; Kato, S.; DeLuca, H.F.; Suda, T.; et al. Spleen Serves as a Reservoir of Osteoclast Precursors through Vitamin D-Induced IL-34 Expression in Osteopetrotic Op/Op Mice. Proc. Natl. Acad. Sci. USA 2012, 109, 10006–10011. [Google Scholar] [CrossRef]
- Zhou, R.P.; Wu, X.S.; Xie, Y.Y.; Dai, B.B.; Hu, W.; Ge, J.F.; Chen, F.H. Functions of Interleukin-34 and Its Emerging Association with Rheumatoid Arthritis. Immunology 2016, 149, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Zhang, R.; Hu, K.Z.; Li, M.Q.; Li, Z.C. Interleukin-34 Synovial Fluid Was Associated with Knee Osteoarthritis Severity: A Cross-Sectional Study in Knee Osteoarthritis Patients in Different Radiographic Stages. Dis. Markers 2018, 2018, 2095480. [Google Scholar] [CrossRef]
- Lelios, I.; Cansever, D.; Utz, S.G.; Mildenberger, W.; Stifter, S.A.; Greter, M. Emerging Roles of IL-34 in Health and Disease. J. Exp. Med. 2020, 217, e20190290. [Google Scholar] [CrossRef] [PubMed]
- Chemel, M.; Le Goff, B.; Brion, R.; Cozic, C.; Berreur, M.; Amiaud, J.; Bougras, G.; Touchais, S.; Blanchard, F.; Heymann, M.F.; et al. Interleukin-34 Expression Is Associated with Synovitis Severity in Rheumatoid Arthritis Patients. Ann. Rheum. Dis. 2012, 71, 150–154. [Google Scholar] [CrossRef]
- Chang, E.J.; Lee, S.K.; Song, Y.S.; Jang, Y.J.; Park, H.S.; Hong, J.P.; Ko, A.R.; Kim, D.Y.; Kim, J.H.; Lee, Y.J.; et al. IL-34 Is Associated with Obesity, Chronic Inflammation, and Insulin Resistance. J. Clin. Endocrinol. Metab. 2014, 99, E1263–E1271. [Google Scholar] [CrossRef]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine Vitamin D Signaling Switches off Pro-Inflammatory Programs of TH1 Cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar] [CrossRef]
- Fenercioglu, A.K. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr. Issues Mol. Biol. 2024, 46, 13514–13525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, M.; Dong, Y.; Zhang, X.; Liu, X.; Chen, Z.; Zhu, Y.; Wang, H.; Liu, X.; Zhu, J.; et al. 1α,25-Dihydroxyvitamin D3 up-Regulates IL-34 Expression in SH-SY5Y Neural Cells. Innate Immun. 2017, 23, 584–591. [Google Scholar] [CrossRef]
- Appel, L.J.; Michos, E.D.; Mitchell, C.M.; Blackford, A.L.; Sternberg, A.L.; Miller, E.R.; Juraschek, S.P.; Schrack, J.A.; Szanton, S.L.; Charleston, J.; et al. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults a Response-Adaptive, Randomized Clinical Trial. Ann. Intern. Med. 2021, 174, 145–156. [Google Scholar] [CrossRef]
- Tanaka, K.; Ao, M.; Tamaru, J.; Kuwabara, A. Vitamin D Insufficiency and Disease Risk in the Elderly. J. Clin. Biochem. Nutr. 2024, 74, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of Vitamin D Supplementation on Musculoskeletal Health: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, B.; Cristea, A.E.; Oprea, D.; Lupu, A.A.; Stanciu, L.E.; Borgazi, E.; Caraban, B.M.; Ciortea, V.M.; Irsay, L.; Iliescu, M.G. Current Evidence on and Clinical Implications of Vitamin D Levels in Pain and Functional Management of Knee Osteoarthritis: A Systematic Review. Clin. Pract. 2024, 14, 1997–2012. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jin, X.; Cicuttini, F.; Wang, X.; Zhu, Z.; Wluka, A.; Han, W.; Winzenberg, T.; Antony, B.; Aitken, D.; et al. Maintaining Vitamin D Sufficiency Is Associated with Improved Structural and Symptomatic Outcomes in Knee Osteoarthritis. Am. J. Med. 2017, 130, 1211–1218. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Z.; Pan, F.; Zheng, S.; Parameswaran, V.; Blizzard, L.; Ding, C.; Antony, B. Long-Term Effects of Vitamin D Supplementation and Maintaining Sufficient Vitamin D on Knee Osteoarthritis over 5 Years. Arthritis Res. Ther. 2023, 25, 178. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y. Vitamin D in the Prevention and Treatment of Osteoarthritis: From Clinical Interventions to Cellular Evidence. Nutrients 2019, 11, 243. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, B.; Han, W.; Zhu, Z.; Wang, X.; Jin, X.; Antony, B.; Cicuttini, F.; Wluka, A.; Winzenberg, T.; et al. Vitamin D Supplementation and Inflammatory and Metabolic Biomarkers in Patients with Knee Osteoarthritis: Post Hoc Analysis of a Randomised Controlled Trial. Br. J. Nutr. 2018, 120, 41–48, Corrigendum to Br. J. Nutr. 2019, 121, 118–119. [Google Scholar] [CrossRef]
- McAlindon, T.; LaValley, M.; Schneider, E.; Nuite, M.; Lee, J.Y.; Price, L.L.; Lo, G.; Dawson-Hughes, B. Effect of Vitamin D Supplementation on Progression of Knee Pain and Cartilage Volume Loss in Patients with Symptomatic Osteoarthritis: A Randomized Controlled Trial. JAMA 2013, 309, 155–162. [Google Scholar] [CrossRef]
- Sha, L.; Zhang, L.; Zhao, X.; Xiang, R.; Wu, X.; Zhu, J.; Hou, J.; Deng, Q.; Qin, C.; Xiao, C.; et al. Shared Genetic Architecture and Causal Relationship Between Serum 25-Hydroxyvitamin D and Bone Mineral Density. J. Clin. Endocrinol. Metab. 2025, 110, 1605–1616. [Google Scholar] [CrossRef]
- Zafeiris, E.P.; Babis, G.C.; Zafeiris, C.P.; Chronopoulos, E. Association of Vitamin D, BMD and Knee Osteoarthritis in Postmenopausal Women. J. Musculoskelet. Neuronal Interact. 2021, 21, 509–516. [Google Scholar]
- Amirkhizi, F.; Asoudeh, F.; Hamedi-Shahraki, S.; Asghari, S. Vitamin D Status Is Associated with Inflammatory Biomarkers and Clinical Symptoms in Patients with Knee Osteoarthritis. Knee 2022, 36, 44–52. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Wira, J.F.; Nababan, S.P.; Oey, E.R.C.; AL-Gunaid, S.T.; Buana, A.C.; Gusti, N.; Habiburrahman, M.; Mulyana, R.M. Global Prevalence of Vitamin D Deficiency among Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Nutr. Health 2025, 21, 260106025136600. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kiel, D.P.; Kraft, P. The Genetics of Vitamin D. Bone 2019, 126, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Hyppönen, E. Determinants of Vitamin D Status: Focus on Genetic Variations. Curr. Opin. Nephrol. Hypertens. 2011, 20, 331–336. [Google Scholar] [CrossRef]
- Sapir-Koren, R.; Livshits, G.; Kobyliansky, E. Genetic Effects of Estrogen Receptor α and Collagen IA1 Genes on the Relationships of Parathyroid Hormone and 25 Hydroxyvitamin D with Bone Mineral Density in Caucasian Women. Metabolism 2003, 52, 1129–1135. [Google Scholar] [CrossRef]
- Polzonetti, V.; Pucciarelli, S.; Vincenzetti, S.; Polidori, P. Dietary Intake of Vitamin D from Dairy Products Reduces the Risk of Osteoporosis. Nutrients 2020, 12, 1743. [Google Scholar] [CrossRef]
- Tarabeih, N.; Kalinkovich, A.; Shalata, A.; Livshits, G. Circulating Levels of Visceral Adipose Tissue-Derived Serine Protease Inhibitor (Vaspin) Appear as a Marker of Musculoskeletal Pain Disability. Diagnostics 2020, 10, 797. [Google Scholar] [CrossRef]
- Tarabeih, N.; Kalinkovich, A.; Shalata, A.; Higla, O.; Livshits, G. Pro-Inflammatory Biomarkers Combined with Body Composition Display a Strong Association with Knee Osteoarthritis in a Community-Based Study. Biomolecules 2023, 13, 1315. [Google Scholar] [CrossRef] [PubMed]
- Tarabeih, N.; Kalinkovich, A.; Shalata, A.; Cherny, S.S.; Livshits, G. Deciphering the Causal Relationships Between Low Back Pain Complications, Metabolic Factors, and Comorbidities. J. Pain. Res. 2022, 15, 215–227. [Google Scholar] [CrossRef]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of Body Composition Assessment by DXA and BIA According to the Body Mass Index: A Retrospective Study on 3655 Measures. PloS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Bioelectrical Impedance Analysis in Body Composition Measurement: National Institutes of Health Technology Assessment Conference Statement. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [CrossRef]
- Merchant, R.A.; Seetharaman, S.; Au, L.; Wong, M.W.K.; Wong, B.L.L.; Tan, L.F.; Chen, M.Z.; Ng, S.E.; Soong, J.T.Y.; Hui, R.J.Y.; et al. Relationship of Fat Mass Index and Fat Free Mass Index With Body Mass Index and Association With Function, Cognition and Sarcopenia in Pre-Frail Older Adults. Front. Endocrinol. 2021, 12, 765415. [Google Scholar] [CrossRef]
- Saliba, W.; Rennert, H.S.; Kershenbaum, A.; Rennert, G. Serum 25(OH)D Concentrations in Sunny Israel. Osteoporos. Int. 2012, 23, 687–694. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C.; Brustad, M.; Meyer, H.E.; Steingrimsdottir, L. Vitamin D—A Systematic Literature Review for the 5th Edition of the Nordic Nutrition Recommendations. Food Nutr. Res. 2013, 57, 22671. [Google Scholar] [CrossRef] [PubMed]
- Arinzon, Z.; Peisakh, A.; Schrire, S.; Berner, Y. C-Reactive Protein (CRP): An Important Diagnostic and Prognostic Tool in Nursing-Home-Associated Pneumonia. Arch. Gerontol. Geriatr. 2011, 53, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Regev, A.; Mordel, I.; Berliner, S.; Rimon, A. Estimated C-Reactive Protein (CRP) Velocity for Rapidly Distinguishing Bacterial from Other Etiologies in Children Presenting to Emergency Department with Remarkably Elevated CRP Levels. Eur. J. Pediatr. 2024, 183, 1925–1933. [Google Scholar] [CrossRef]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International 2014, 25, 2359–2381, Erratum in Osteoporosis International 2015, 26, 2045–2047. [Google Scholar] [CrossRef]
- Cueva, J.H.; Castillo, D.; Espinós-Morató, H.; Durán, D.; Díaz, P.; Lakshminarayanan, V. Detection and Classification of Knee Osteoarthritis. Diagnostics 2022, 12, 2362. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of Criteria for the Classification and Reporting of Osteoarthritis: Classification of Osteoarthritis of the Knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to Use the Bonferroni Correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- van Zyl, L.E.; ten Klooster, P.M. Exploratory Structural Equation Modeling: Practical Guidelines and Tutorial With a Convenient Online Tool for Mplus. Front. Psychiatry 2022, 12, 795672. [Google Scholar] [CrossRef] [PubMed]
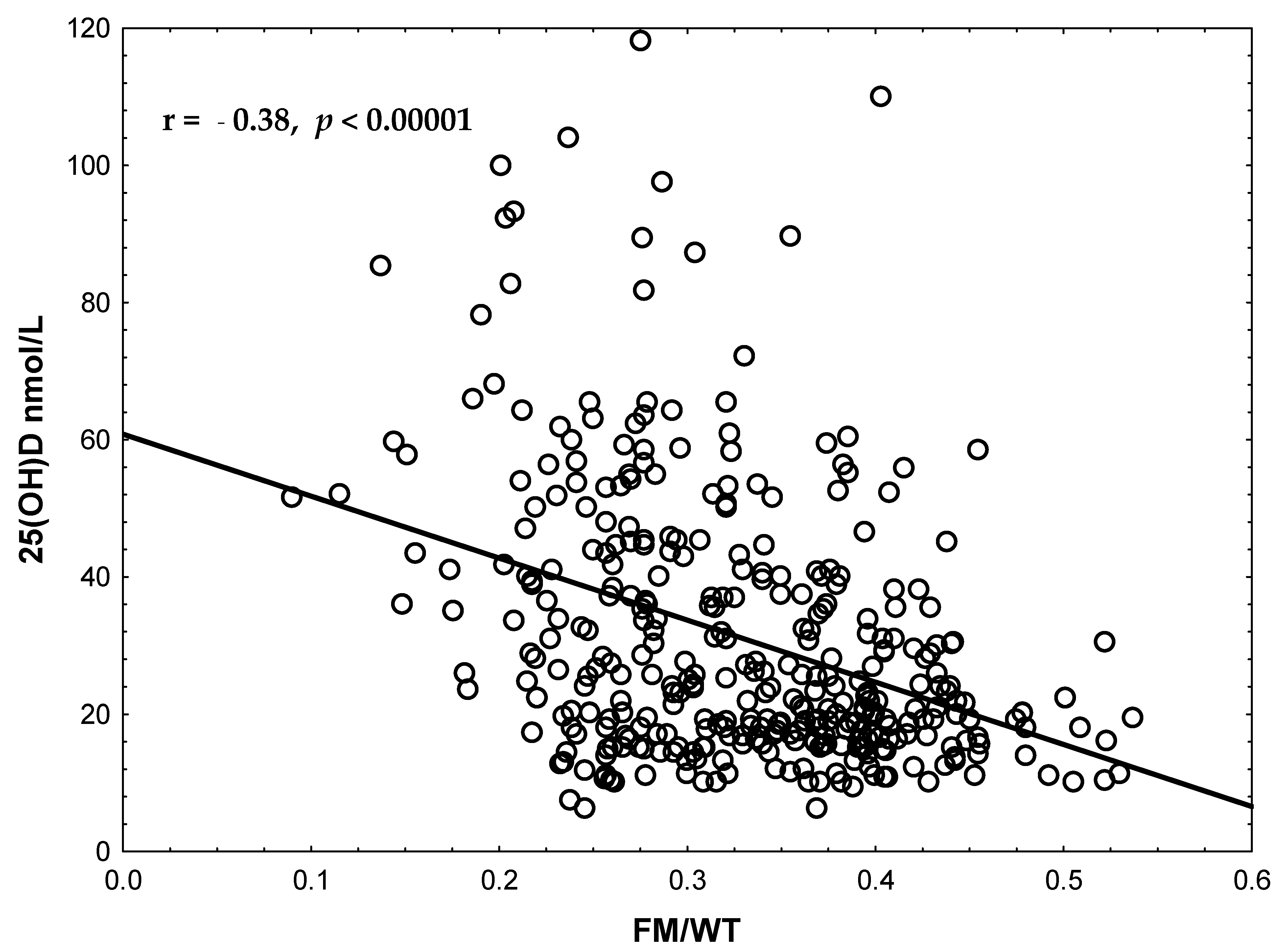
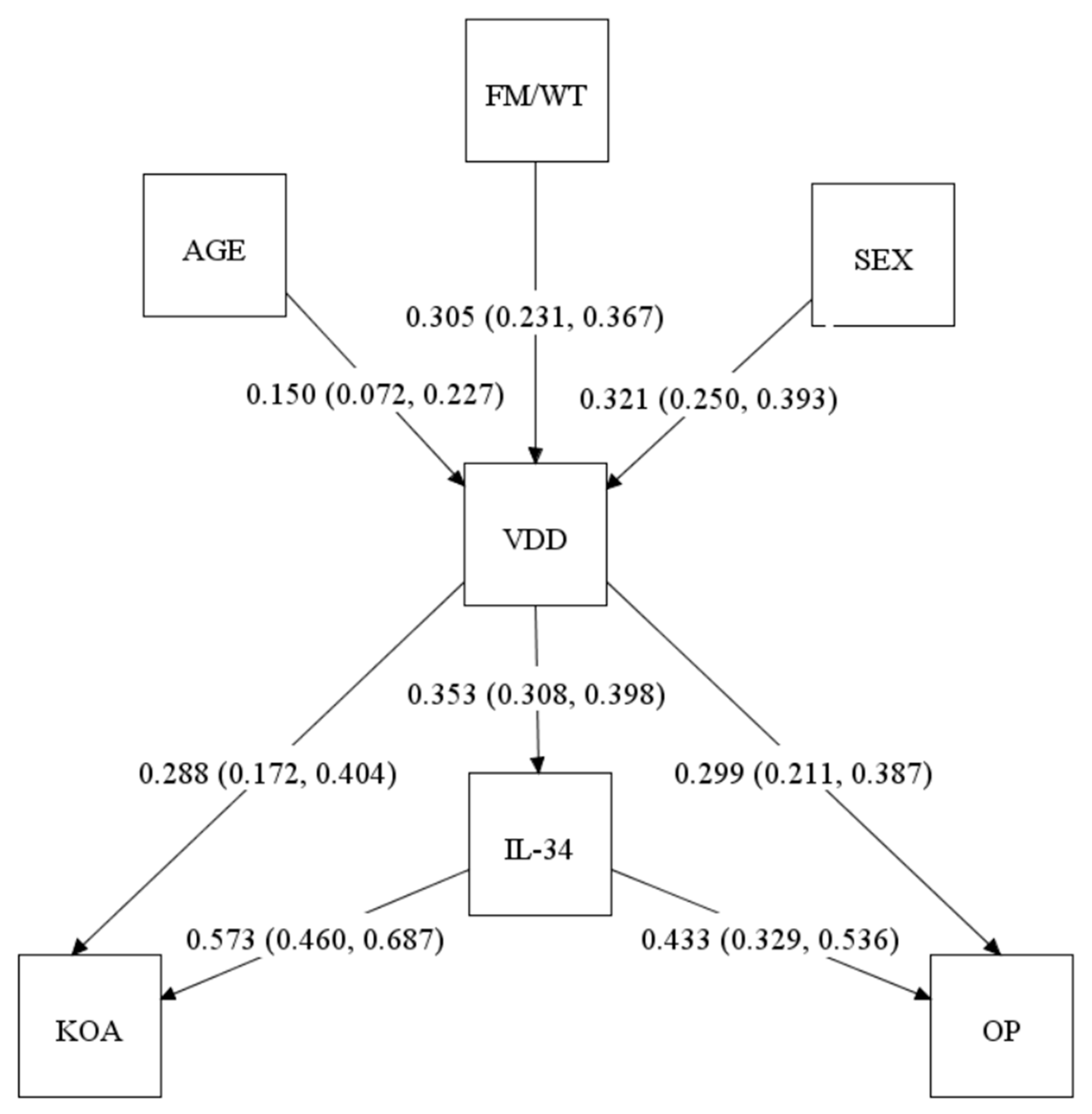
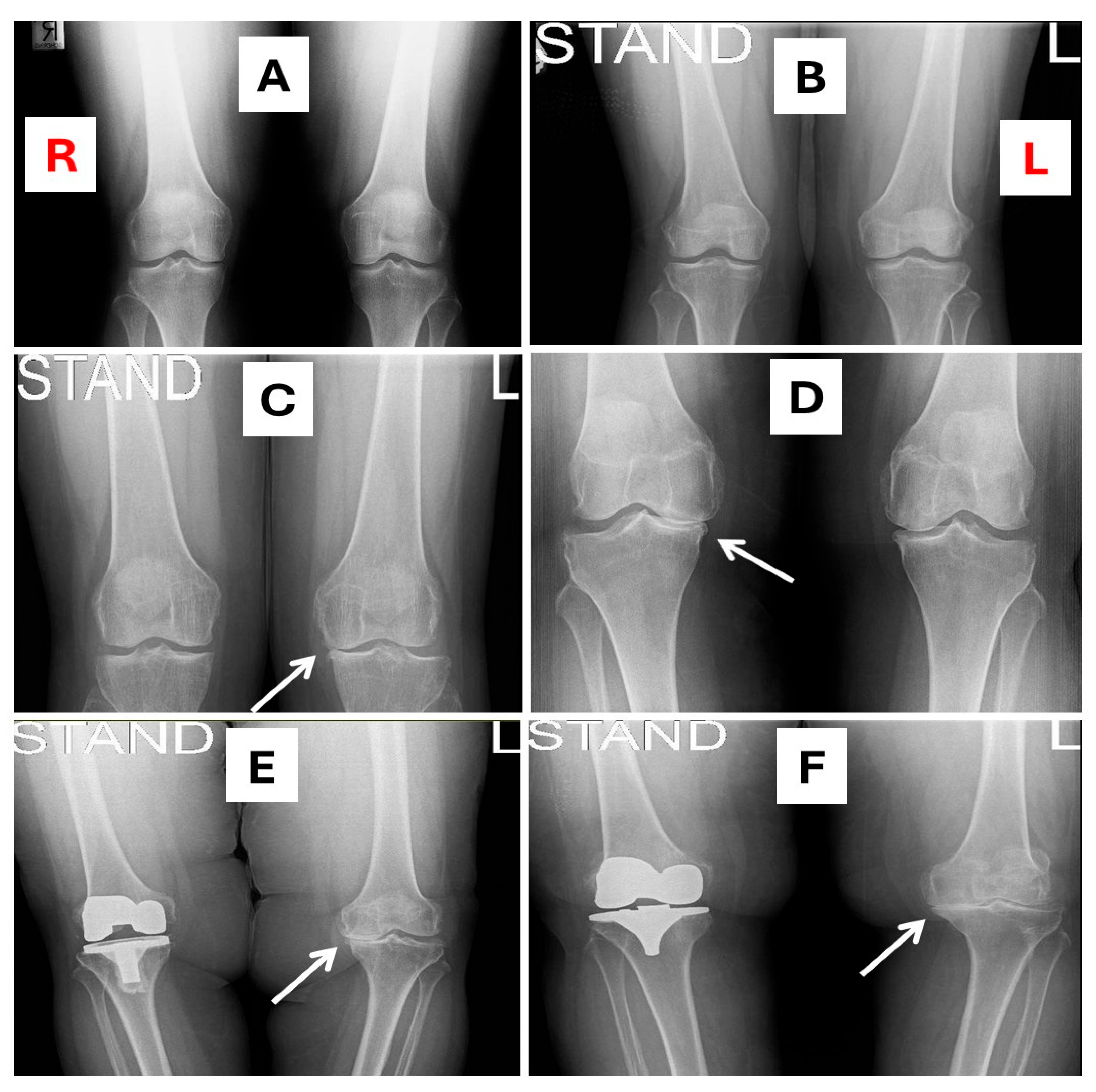
| Study Group/ Variable | VDS (n = 771) (VD Levels ≥ 25 nmol/L) | VDD (n = 304) (VD Levels < 25 nmol/L) | p-Value |
|---|---|---|---|
| Age (y) | 42.008 ± 0.498 | 45.718 ± 0.762 | 0.00006 # |
| BMI (kg/m2) | 27.851 ± 0.182 | 29.214 ± 0.349 | NS |
| WHR | 0.905 ± 0.003 | 0.904 ± 0.005 | NS |
| FM/WT | 0.299 ± 0.002 | 0.362 ± 0.004 | 0.001 # |
| ASMM/WT | 0.271 ± 0.001 | 0.246 ± 0.002 | NS |
| Glucose (mg/dL) | 96.657 ± 1.054 | 98.154 ± 1.423 | NS |
| TG (mg/dL) | 126.100 ± 3.267 | 125.926 ± 4.455 | NS |
| TC (mg/dL) | 177.263 ± 1.353 | 180.816 ± 2.180 | NS |
| TC/HDL-C | 4.109 ± 0.049 | 3.996 ± 0.071 | 0.01 |
| IL-9 (pg/mL) | 205.367 ± 61.127 | 204.221 ± 89.217 | NS |
| IL-34 (pg/mL) | 72.585 ± 5.439 | 567.698 ± 68.486 | 0.0000001 # |
| MCP-1 (pg/mL) | 3.823 ± 0.023 | 3.893 ± 0.039 | NS |
| hs-CRP (mg/L) | 0.925 ± 0.127 | 1.948 ± 0.305 | 0.004 |
| SII | 513.944 ± 16.170 | 516.601 ± 19.966 | NS |
| OP, % | 1.7% (13) | 9.8% (30) | <0.00001 # |
| KOA, % | 4.3% (33) | 15.7% (48) | <0.00001 # |
| VD Status (771 VDS vs. 304 VDD) | |||
|---|---|---|---|
| Independent Covariate | OR (95% CI) | β (SE) | p-Value |
| Sex (females vs. males) | 4.11 (2.56–6.59) | 1.41 (0.24) | 4.39 × 10−8 |
| Age (years) | 1.38 (1.14–1.66) | 0.32 (0.09) | 0.0005 |
| FM/WT | 1.37 (1.09–1.72) | 0.31 (0.11) | 0.006 |
| Dependent Variable: Serum IL-34 | |||
|---|---|---|---|
| Independent Variables | Standardized β | SE of β | p-Value |
| Sex (females vs. males) | −0.006 | 0.049 | NS |
| Age (years) | −0.090 | 0.048 | NS |
| VD | 0.529 | 0.049 | 6.99 × 10−23 |
| Pair | χ2 | Phi Coefficient | p-Value |
|---|---|---|---|
| VD × OP | 38.11 | 0.19 | <0.00001 ** |
| VD × KOA | 43.15 | 0.20 | <0.00001 ** |
| OP × KOA | 21.07 | 0.14 | <0.00001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarabeih, N.; Sleiman, A.; Kalinkovich, A.; Ashkenazi, S.; Shalata, A.; Livshits, G. IL-34 as a Novel Mediator Linking Vitamin D Deficiency with Osteoporosis and Knee Osteoarthritis. Int. J. Mol. Sci. 2025, 26, 11090. https://doi.org/10.3390/ijms262211090
Tarabeih N, Sleiman A, Kalinkovich A, Ashkenazi S, Shalata A, Livshits G. IL-34 as a Novel Mediator Linking Vitamin D Deficiency with Osteoporosis and Knee Osteoarthritis. International Journal of Molecular Sciences. 2025; 26(22):11090. https://doi.org/10.3390/ijms262211090
Chicago/Turabian StyleTarabeih, Nader, Ali Sleiman, Alexander Kalinkovich, Shai Ashkenazi, Adel Shalata, and Gregory Livshits. 2025. "IL-34 as a Novel Mediator Linking Vitamin D Deficiency with Osteoporosis and Knee Osteoarthritis" International Journal of Molecular Sciences 26, no. 22: 11090. https://doi.org/10.3390/ijms262211090
APA StyleTarabeih, N., Sleiman, A., Kalinkovich, A., Ashkenazi, S., Shalata, A., & Livshits, G. (2025). IL-34 as a Novel Mediator Linking Vitamin D Deficiency with Osteoporosis and Knee Osteoarthritis. International Journal of Molecular Sciences, 26(22), 11090. https://doi.org/10.3390/ijms262211090








