The Role of the Organization of Light-Harvesting Complex II in the Drought Sensitivity of Pisum sativum L.
Abstract
1. Introduction
2. Results
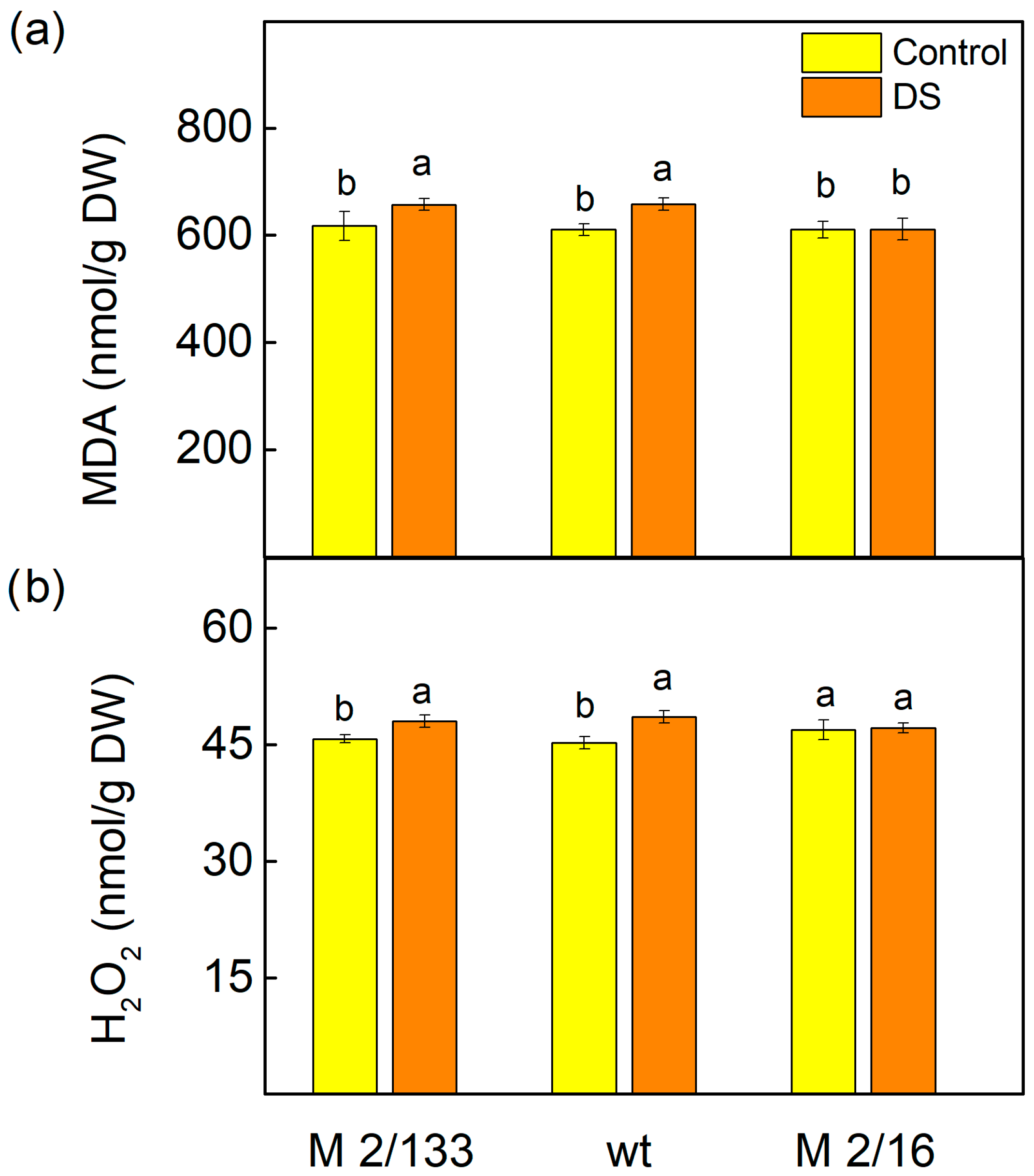
2.1. Stress Markers
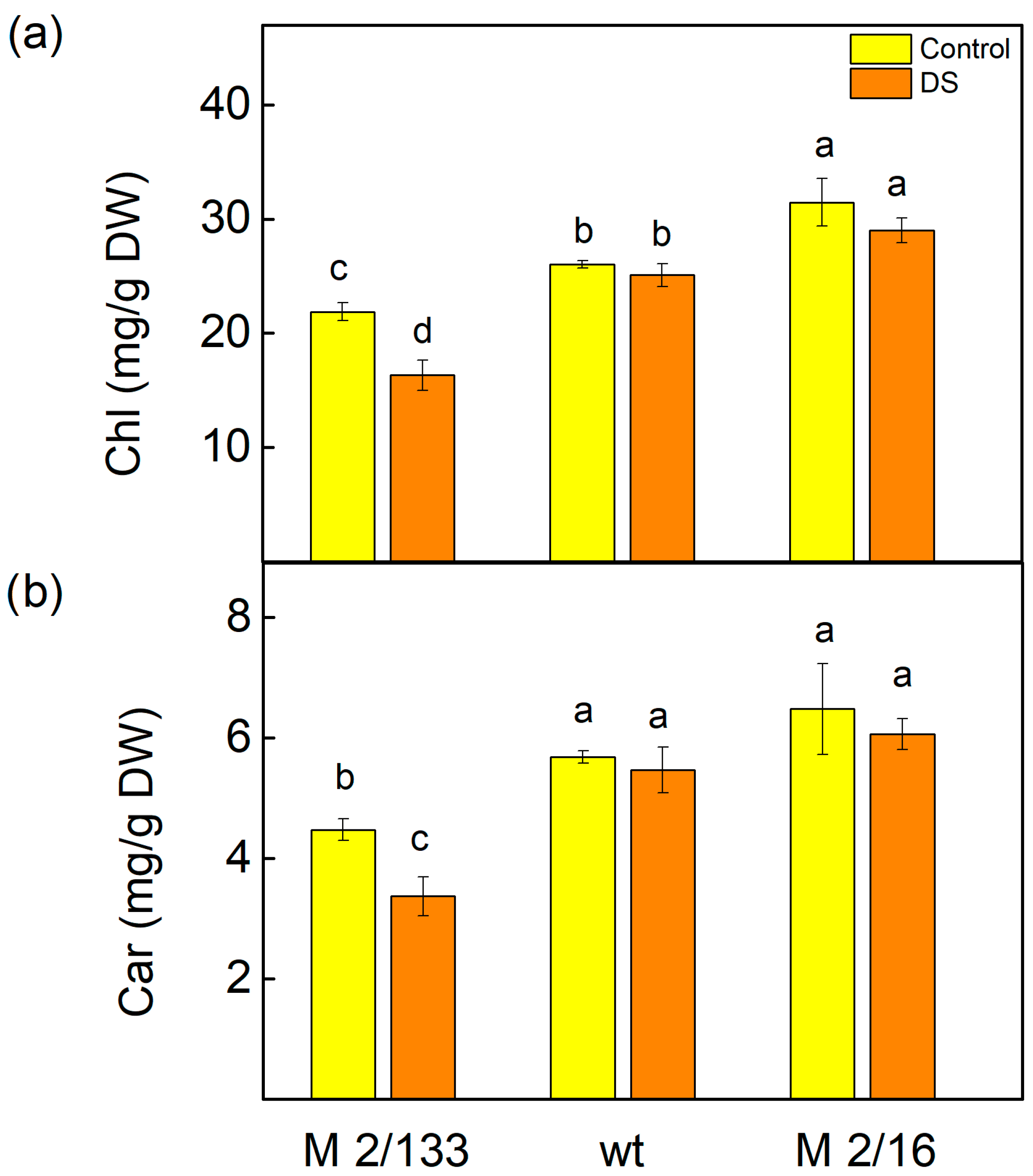
2.2. Pigment Composition
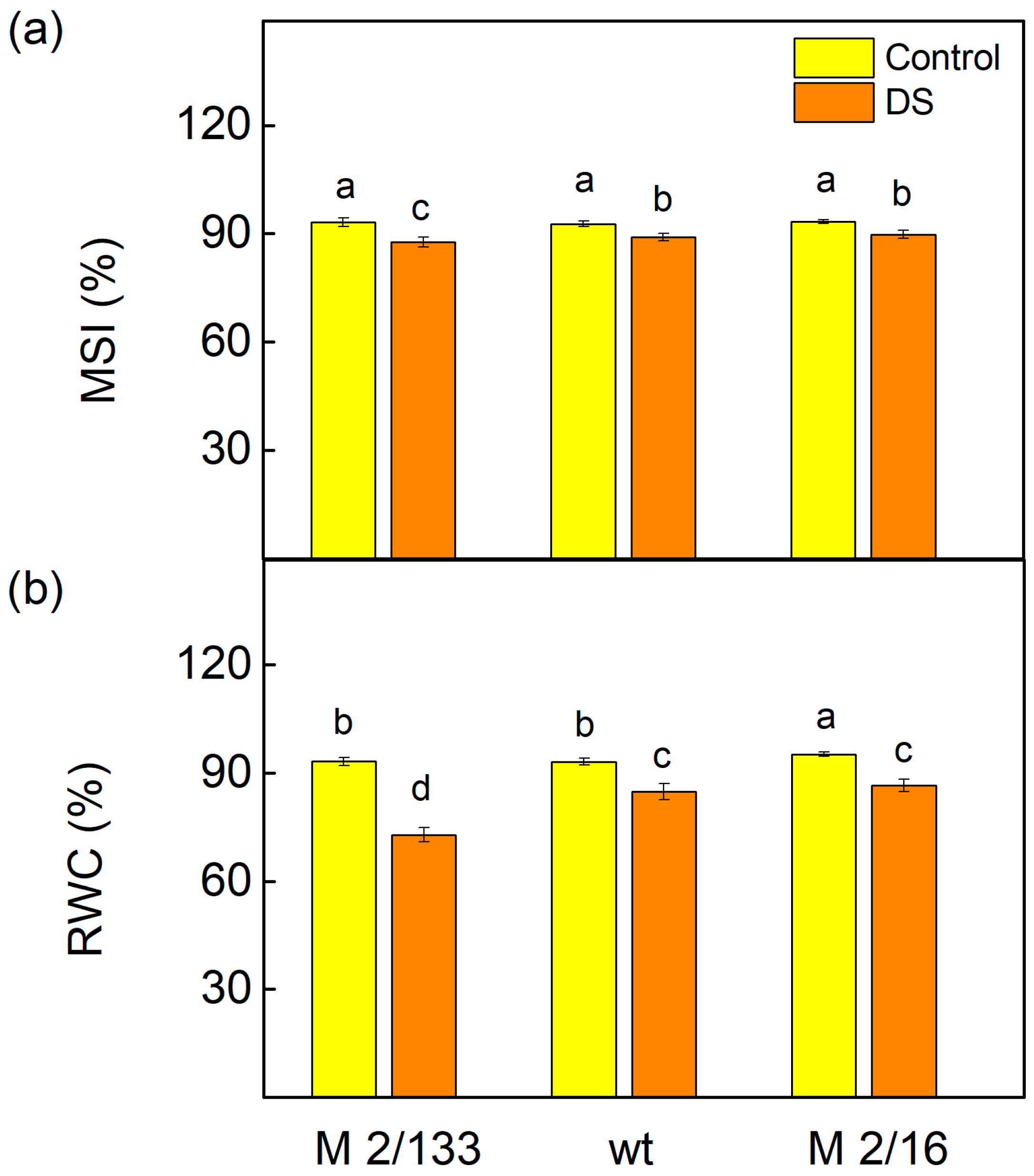
2.3. Membrane Stability Index and Relative Water Content
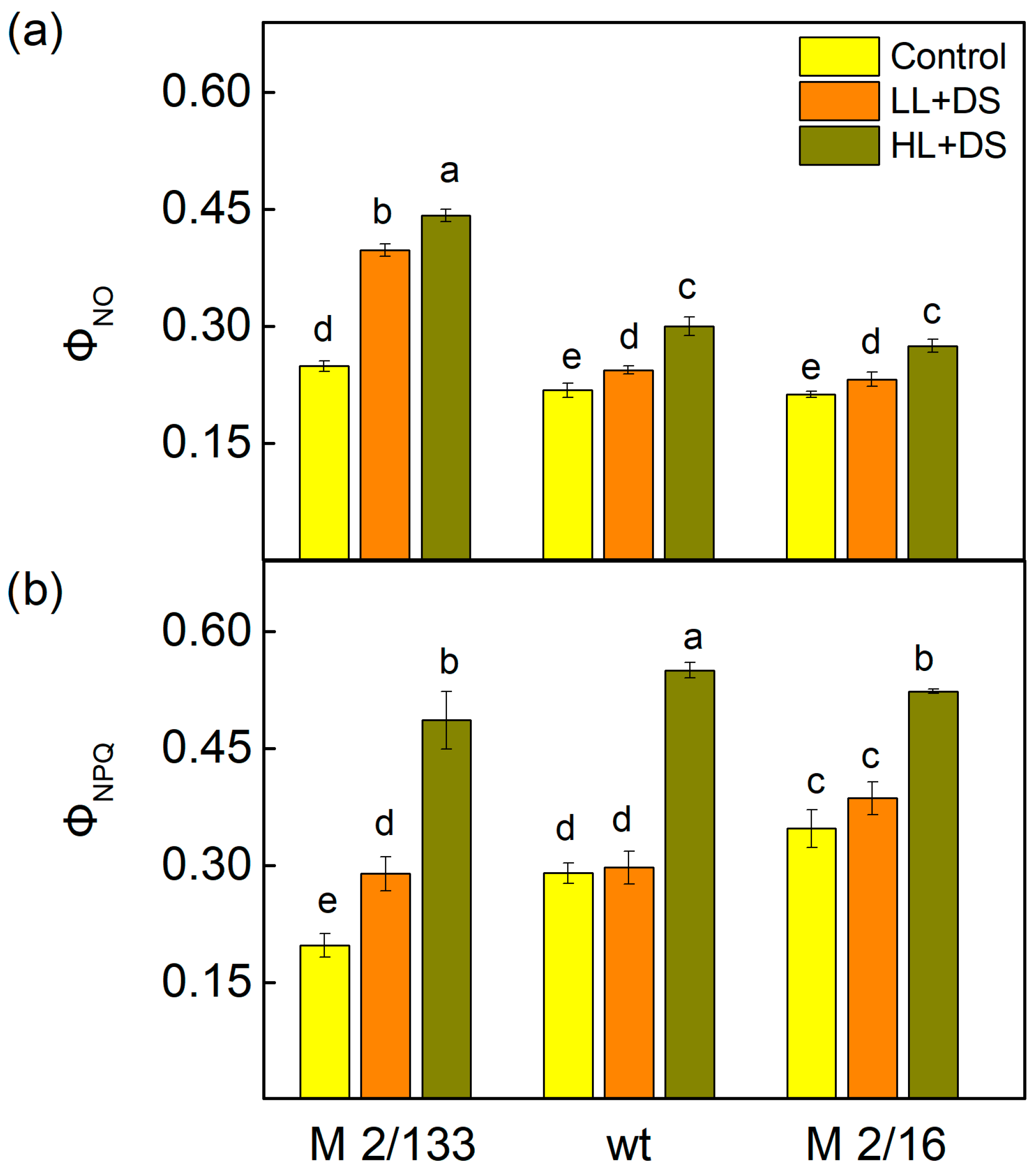
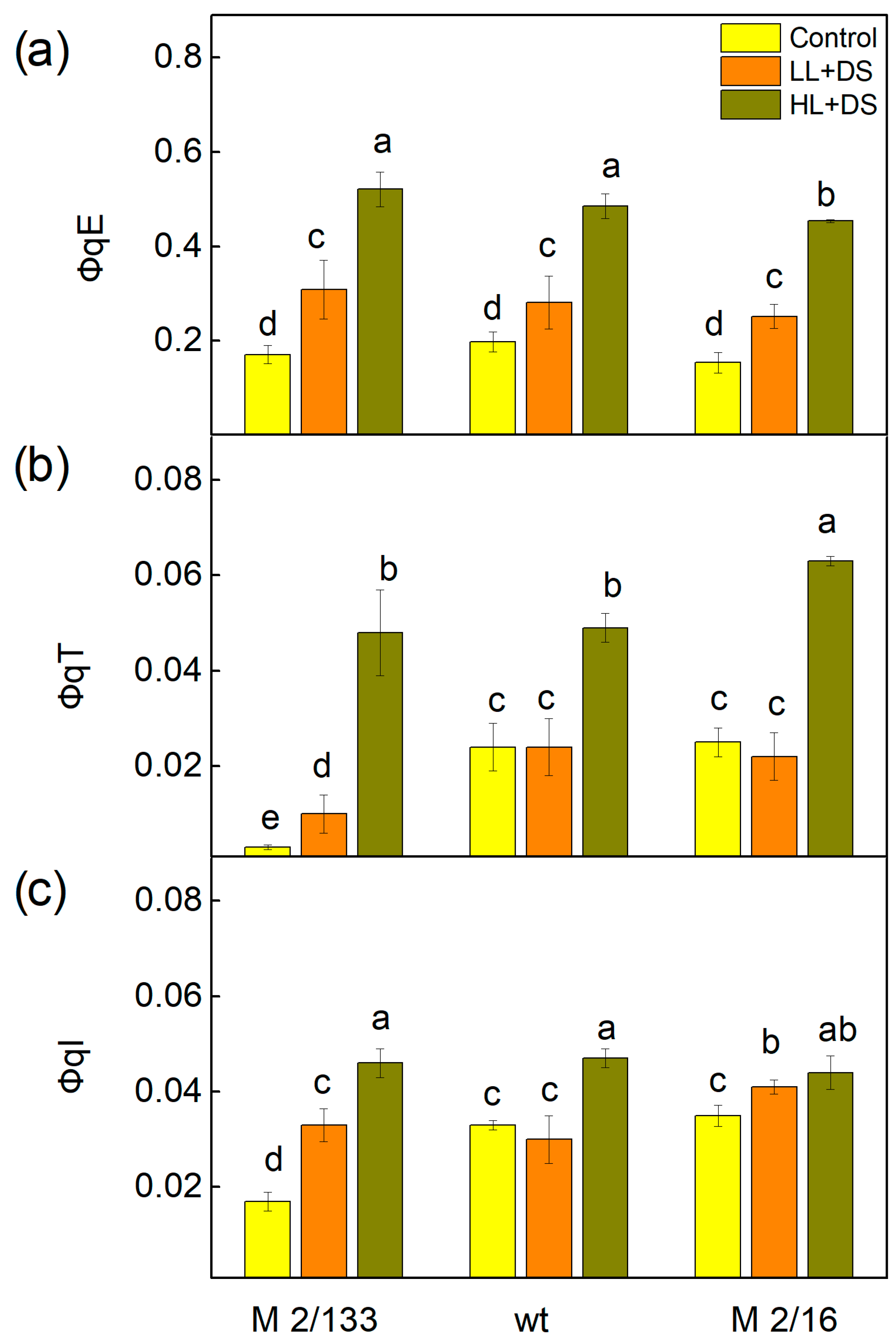
2.4. Pulse Amplitude Modulated Chlorophyll a Fluorescence
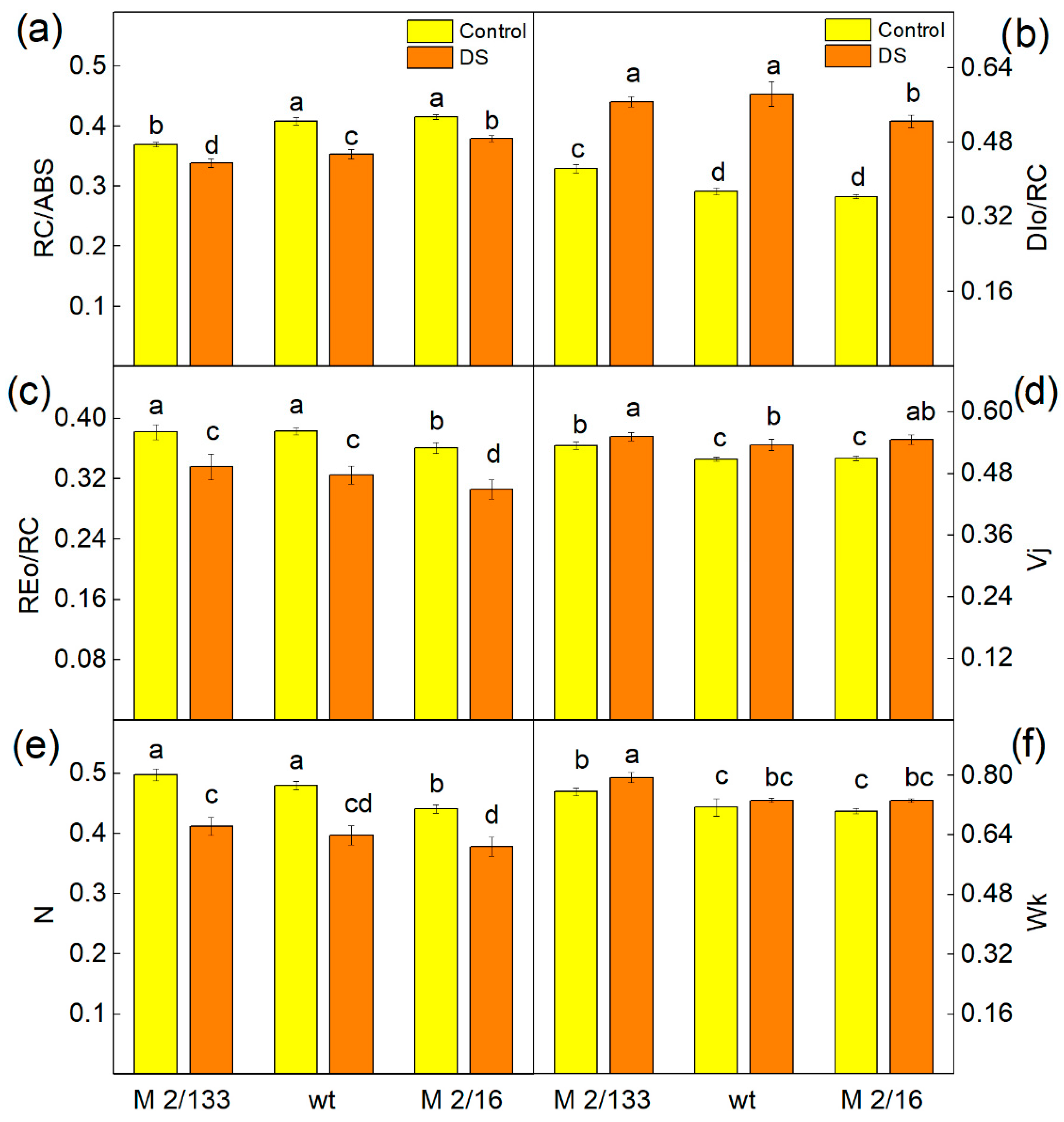
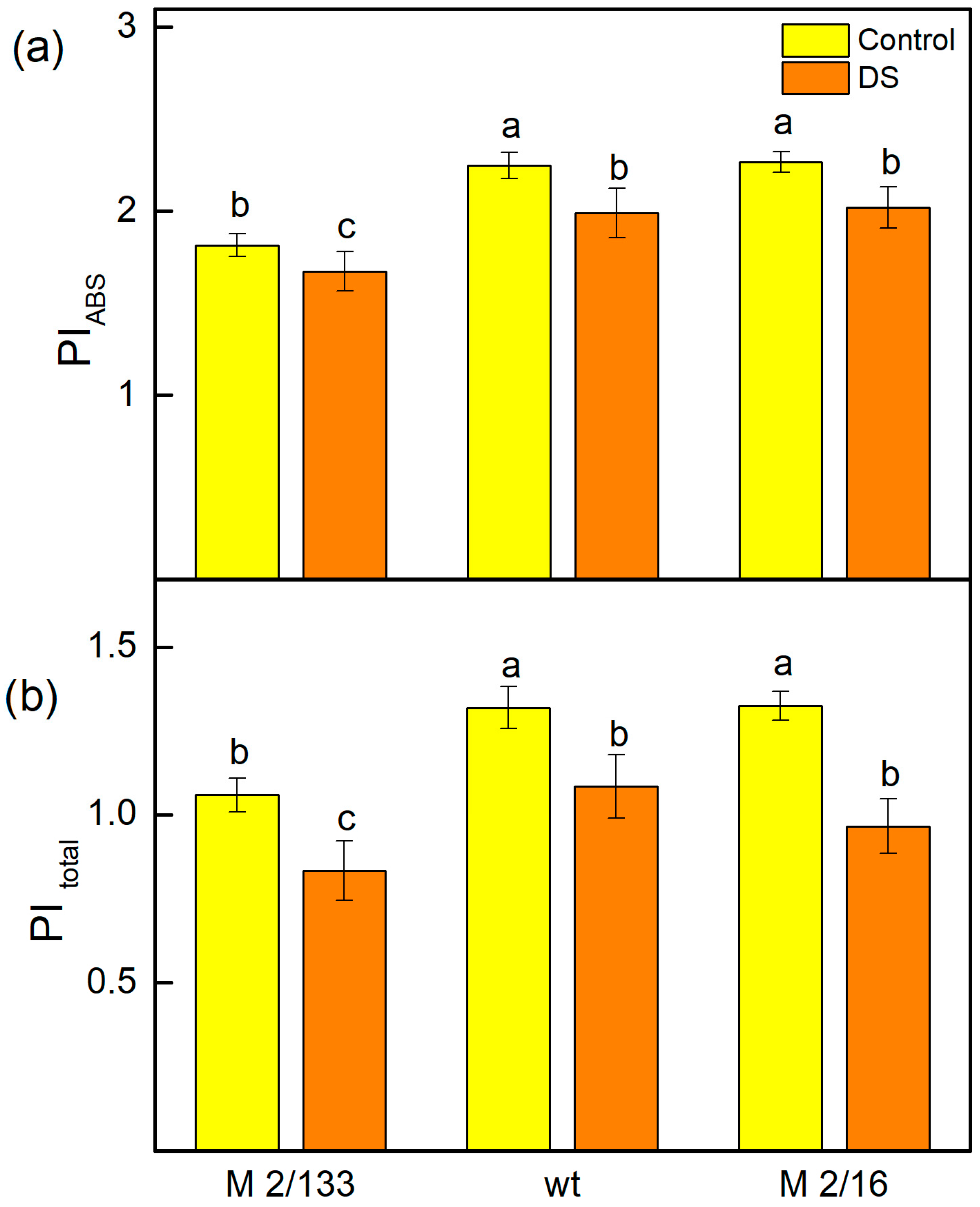
2.5. Fast Chlorophyll a Fluorescence
2.6. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Oxidative Stress Markers
4.3. Determination of Pigment Composition
4.4. Pulse Amplitude Modulated Chlorophyll Fluorescence Measurements
4.5. Fast Chlorophyll a Fluorescence Measurements
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| M 2/133 | Costata 2/133 |
| M 2/16 | Coeruleovireus 2/16 |
| DS | Drought stress |
| HL | High actinic light |
| H2O2 | Hydrogen peroxide |
| PSI | Photosystem I |
| PSII | Photosystem II |
| Chl | Chlorophyll |
| Car | Carotenoids |
| LHCII | Light-harvesting complex of PSII |
| LL | Low actinic light |
| LHCIIm | Monomeric form of LHCII |
| LHCIIo | Oligomeric form of LHCII |
| OEC | oxygen-evolving complex |
| MDA | Malonaldehyde |
| MSI | Membrane stability index |
| wt | Pisum sativum L. Borec |
| RWC | Relative water content |
References
- Bolaji Umar, O.; Amudalat Ranti, L.; Shehu Abdulbaki, A.; Lukman Bola, A.; Khadijat Abdulhamid, A.; Ramat Biola, M.; Oluwagbenga Victor, K. Stresses in Plants: Biotic and Abiotic. In Current Trends in Wheat Research; Ansari, M.-R., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide research on plant defense against Biotic stresses as improvement for sustainable agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef]
- Regassa, M. Plant response to biotic and abiotic stresses. Plant 2025, 13, 43–48. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W.; Chen, Z.; Gu, Y.; Pei, L.; Tian, R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, M.; Rashkov, G.; Borisova, P.; Apostolova, E. Sensitivity of the photosynthetic apparatus in maize and sorghum under different drought levels. Plants 2023, 12, 1863. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Zahra, N.; Hafeez, M.B.; Siddique, K.H.M. Recent advances in plant drought tolerance. J. Plant Growth Regul. 2024, 43, 3337–3369. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Lan, H.; Yu, Q. Enhanced drought tolerance and photosynthetic efficiency in Arabidopsis by overexpressing phosphoenolpyruvate carboxylase from a single-cell C4 halophyte Suaeda aralocaspica. Front. Plant Sci. 2024, 15, 1443691. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Pandey, J.; Devadasu, E.; Saini, D.; Dhokne, K.; Marriboina, S.; Raghavendra, A.S.; Subramanyam, R. Reversible changes in structure and function of photosynthetic apparatus of pea (Pisum sativum) leaves under drought stress. Plant J. 2023, 113, 60–74. [Google Scholar] [CrossRef]
- Shao, R.X.; Xin, L.F.; Zheng, H.F.; Li, L.L.; Ran, W.L.; Mao, J.; Yang, Q.H. Changes in chloroplast ultrastructure in leaves of drought-stressed maize inbred lines. Photosynthetica 2016, 54, 74–80. [Google Scholar] [CrossRef]
- Lee, S.; Park, C.-M. Regulation of reactive oxygen species generation under drought conditions in Arabidopsis. Plant Signal. Behav. 2012, 7, 599–601. [Google Scholar] [CrossRef]
- Samanta, S.; Seth, C.S.; Roychoudhury, A. The molecular paradigm of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with different phytohormone signaling pathways during drought stress in plants. Plant Physiol. Biochem. 2024, 206, 108259. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant defense system in plants: Reactive oxygen species production, signaling, and scavenging during abiotic stress-induced oxidative damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Bao, L.; Liu, J.; Mao, T.; Zhao, L.; Wang, D.; Zhai, Y. Nanobiotechnology-mediated regulation of reactive oxygen species homeostasis under heat and drought stress in plants. Front. Plant Sci. 2024, 15, 1418515. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, D.; Pandey, H.; Jatav, R.B.; Singh, V.; Pandey, D. An Overview of Roles of Enzymatic and Nonenzymatic Antioxidants in Plant. In Antioxidant Defense in Plants; Springer Nature: Singapore, 2022; pp. 1–13. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Wang, Y.-F.; Akbar, R.; Alhoqail, W.A. Mechanistic insights and future perspectives of drought stress management in staple crops. Front. Plant Sci. 2025, 16. [Google Scholar] [CrossRef]
- Song, L.; Huang, S.C.; Wise, A.; Castanon, R.; Nery, J.R.; Chen, H.; Watanabe, M.; Thomas, J.; Bar-Joseph, Z.; Ecker, J.R. A transcription factor hierarchy defines an environmental stress response network. Science 2016, 354. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, Z.; Li, L.; Li, Q.; Geng, Z.; Liu, W.; Hou, R.; Zhang, L.; Han, D. MbWRKY50 confers cold and drought tolerance through upregulating antioxidant capacity associated with ROS scavenging. J. Plant Physiol. 2025, 310, 154526. [Google Scholar] [CrossRef]
- Duan, Y.; Han, J.; Guo, B.; Zhao, W.; Zhou, S.; Zhou, C.; Zhang, L.; Li, X.; Han, D. MbICE1 confers drought and cold tolerance through up-regulating antioxidant capacity and stress-resistant genes in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 16072. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, Z.; Song, P.; Wang, Y.; Liu, W.; Zhang, L.; Li, X.; Li, W.; Han, D. Overexpression of a Grape MYB Transcription Factor Gene VhMYB2 Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 10743. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Wei, Y.; Han, J.; Wang, Y.; Li, X.; Zhang, L.; Han, D. Overexpression of a Fragaria vesca NAM, ATAF, and CUC (NAC) Transcription Factor Gene (FvNAC29) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 4088. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, Y.; Xu, M.; Zhao, M.; Chen, H.; Lu, Y.; Pang, S.; Xu, W. Characterization of low-temperature sensitivity and chlorophyll fluorescence in yellow leaf mutants of tomato. Agronomy 2024, 14, 2382. [Google Scholar] [CrossRef]
- Li, W.; Wei, Y.; Zhang, L.; Wang, Y.; Song, P.; Li, X.; Han, D. FvMYB44, a strawberry R2R3-MYB transcription factor, improved salt and cold stress tolerance in transgenic Arabidopsis. Agronomy 2023, 13, 1051. [Google Scholar] [CrossRef]
- Pawłowicz, I.; Kosmala, A.; Rapacz, M. Expression pattern of the psbO gene and its involvement in acclimation of the photosynthetic apparatus during abiotic stresses in Festuca arundinacea and F. pratensis. Acta Physiol. Plant. 2012, 34, 1915–1924. [Google Scholar] [CrossRef]
- Chaves, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Quartacci, M.F.; Pinzino, C.; Rascio, N.; Vazzana, C.; Sgherri, C.L.M. Protein dynamics in thylakoids of the desiccation-tolerant plant Boea Hygroscopica during dehydration and rehydration. Plant Physiol. 2000, 124, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.K. Modulation of photosynthesis and other proteins during water–stress. Mol. Biol. Rep. 2021, 48, 3681–3693. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Sperdouli, I.; İşgören, S.; Şaş, B.; Moustakas, M. Deciphering the mechanism of melatonin-induced enhancement of photosystem II function in moderate drought-stressed oregano plants. Plants 2024, 13, 2590. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Differential response of photosystem II photochemistry in young and mature leaves of Arabidopsis thaliana to the onset of drought stress. Acta Physiol. Plant. 2012, 34, 1267–1276. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Su, Y.; Cui, J.; Zhang, Z.; Yuan, M.; Zhang, H.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant. 2016, 158, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Sapeta, H.; Costa, J.M.; Lourenço, T.; Maroco, J.; van der Linde, P.; Oliveira, M.M. Drought stress response in Jatropha curcas: Growth and physiology. Environ. Exp. Bot. 2013, 85, 76–84. [Google Scholar] [CrossRef]
- Huseynova, I.M.; Rustamova, S.M.; Suleymanov, S.Y.; Aliyeva, D.R.; Mammadov, A.C.; Aliyev, J.A. Drought-induced changes in photosynthetic apparatus and antioxidant components of wheat (Triticum durum Desf.) varieties. Photosynth. Res. 2016, 130, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Elias, E.; Nawrocki, W.J.; Croce, R. Drought affects both photosystems in Arabidopsis thaliana. New Phytol. 2023, 240, 663–675. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.-E.; Zhao, Y.-Q.; Ding, C.-B.; Liao, J.-Q.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Giardi, M.T.; Cona, A.; Geiken, B.; Kučera, T.; Masojídek, J.; Mattoo, A.K. Long-term drought stress induces structural and functional reorganization of photosystem II. Planta 1996, 199, 118–125. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.Y.; Bai, Y.W.; Li, H.J.; Xue, J.Q.; Zhang, R.H. Response of Photosynthesis in Maize to Drought and Re-Watering. Russ. J. Plant Physiol. 2019, 66, 424–432. [Google Scholar] [CrossRef]
- Arellano, J.B. Non-photochemical quenching of photosystem I as an adaptive response to prolonged drought. J. Exp. Bot. 2023, 74, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.; Paunov, M.; Stoichev, S.; Todinova, S.; Taneva, S.G.; Goltsev, V.; Krumova, S. Thylakoid membrane reorganization, induced by growth light intensity, affects the plants susceptibility to drought stress. Photosynthetica 2020, 58, 369–378. [Google Scholar] [CrossRef]
- Lehretz, G.G.; Schneider, A.; Leister, D.; Sonnewald, U. High non-photochemical quenching of VPZ transgenic potato plants limits CO 2 assimilation under high light conditions and reduces tuber yield under fluctuating light. J. Integr. Plant Biol. 2022, 64, 1821–1832. [Google Scholar] [CrossRef]
- Grinzato, A.; Albanese, P.; Marotta, R.; Swuec, P.; Saracco, G.; Bolognesi, M.; Zanotti, G.; Pagliano, C. High-light versus low-light: Effects on paired photosystem ii supercomplex structural rearrangement in pea plants. Int. J. Mol. Sci. 2020, 21, 8643. [Google Scholar] [CrossRef]
- Janusauskaite, D. Comparison of physiological characteristics of pea (Pisum sativum L.) varieties under different nutritional conditions and their relationship with meteorological parameters. Plants 2025, 14, 2020. [Google Scholar] [CrossRef]
- Martin, R.J.; Wilson, D.R.; Riddle, M.U.; Gillespie, R.N. Response of pea seed yield to water deficit. Agron. N. Z. 2006, 36, 36–43. [Google Scholar]
- Kachout, S.S.; Benyoussef, S.; Zoghlami, A.; Chakroun, M. Effect of water deficit during germination and flowering period of grass pea (Lathyrus sativus L.). Int. J. Plant Breed. Genet. 2018, 13, 12–18. [Google Scholar] [CrossRef]
- Gutiérrez-Villamil, D.A.; Alvarado-Sanabria, O.H.; Álvarez-Herrera, J.G. Water deficit during pod development affects eco-physiological traits, growth, and yield in pea varieties under greenhouse conditions in tropical highlands. Crops 2025, 5, 65. [Google Scholar] [CrossRef]
- Alvarado-Sanabria, O.; Arias-Aguirre, D.M.; Alvarez-Herrera, J.; Melgarejo, L.M. Effect of water deficit on photosynthesis and yield in pea plants (Pisum sativum L.): A systematic review. Agron. Colomb. 2025, 43, e118788. [Google Scholar] [CrossRef]
- Bagheri, M.; Santos, C.S.; Rubiales, D.; Vasconcelos, M.W. Challenges in pea breeding for tolerance to drought: Status and prospects. Ann. Appl. Biol. 2023, 183, 108–120. [Google Scholar] [CrossRef]
- Dankov, K.G.; Dobrikova, A.G.; Ughy, B.; Bogos, B.; Gombos, Z.; Apostolova, E.L. LHCII organization and thylakoid lipids affect the sensitivity of the photosynthetic apparatus to high-light treatment. Plant Physiol. Biochem. 2011, 49, 629–635. [Google Scholar] [CrossRef]
- Ivanova, P.I.; Dobrikova, A.G.; Taneva, S.G.; Apostolova, E.L. Sensitivity of the photosynthetic apparatus to UV-A radiation: Role of light-harvesting complex II–photosystem II supercomplex organization. Radiat. Environ. Biophys. 2008, 47, 169–177. [Google Scholar] [CrossRef]
- Apostolova, E.; Dobrikova, A. Role of the LHCII Organization for the Sensitivity of the Photosyntheticapparatus to Temperature and High Light Intensity. In Handbook of Photosynthesis; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 243–256. [Google Scholar] [CrossRef]
- Dobrikova, A.; Morgan, R.M.; Ivanov, A.G.; Apostolova, E.; Petkanchin, I.; Huner, N.P.A.; Taneva, S.G. Electric properties of thylakoid membranes from pea mutants with modified carotenoid and chlorophyll-protein complex composition. Photosynth. Res. 2000, 65, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.L.; Dobrikova, A.G.; Ivanova, P.I.; Petkanchin, I.B.; Taneva, S.G. Relationship between the organization of the PSII supercomplex and the functions of the photosynthetic apparatus. J. Photochem. Photobiol. B Biol. 2006, 83, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Rashkov, G.D.; Stefanov, M.A.; Misra, A.N.; Apostolova, E.L. The role of light-harvesting complex II organization in the efficiency of light-dependent reactions in the photosynthetic apparatus of Pisum sativum L. Plants 2025, 14, 1846. [Google Scholar] [CrossRef]
- de Faria, A.P.; Lemos-Filho, J.P.; Modolo, L.V.; França, M.G.C. Electrolyte leakage and chlorophyll a fluorescence among castor bean cultivars under induced water deficit. Acta Physiol. Plant. 2013, 35, 119–128. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Samson, G.; Carpentier, R. Nonphotosynthetic reduction of the intersystem electron transport chain of chloroplasts following heat stress. The pool size of stromal reductants. Photochem. Photobiol. 2001, 74, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Shirao, M.; Kuroki, S.; Kaneko, K.; Kinjo, Y.; Tsuyama, M.; Förster, B.; Takahashi, S.; Badger, M.R. Gymnosperms have increased capacity for electron leakage to oxygen (Mehler and PTOX reactions) in photosynthesis compared with angiosperms. Plant Cell Physiol. 2013, 54, 1152–1163. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee; Bosa, K.; Kościelniak, J.; Zuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Giorio, P.; Sellami, M.H. Polyphasic okjip chlorophyll a fluorescence transient in a landrace and a commercial cultivar of sweet pepper (Capsicum annuum, L.) under long-term salt stress. Plants 2021, 10, 887. [Google Scholar] [CrossRef]
- Bussotti, F.; Desotgiu, R.; Pollastrini, M.; Cascio, C. The JIP test: A tool to screen the capacity of plant adaptation to climate change. Scand. J. For. Res. 2010, 25, 43–50. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop Breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef]
- Mihaljević, I.; Viljevac Vuletić, M.; Šimić, D.; Tomaš, V.; Horvat, D.; Josipović, M.; Zdunić, Z.; Dugalić, K.; Vuković, D. Comparative Study of Drought Stress Effects on Traditional and Modern Apple Cultivars. Plants 2021, 10, 561. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Monteoliva, M.I.; Guzzo, C.; Posada, G.A. Breeding for drought tolerance by monitoring chlorophyll content. Gene Technol. 2021, 10, 165. [Google Scholar]
- Elias, E.; Liguori, N.; Croce, R. At the origin of the selectivity of the chlorophyll-binding sites in Light Harvesting Complex II (LHCII). Int. J. Biol. Macromol. 2023, 243, 125069. [Google Scholar] [CrossRef]
- Li, R.; Guo, P.; Baum, M.; Grand, S.; Ceccarelli, S. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Guo, P.; Baum, M.; Varshney, R.K.; Graner, A.; Grando, S.; Ceccarelli, S. QTLs for chlorophyll and chlorophyll fluorescence parameters in barley under post-flowering drought. Euphytica 2008, 163, 203–214. [Google Scholar] [CrossRef]
- Batra, N.G.; Sharma, V.; Kumari, N. Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J. Plant Interact. 2014, 9, 712–721. [Google Scholar] [CrossRef]
- Caferri, R.; Guardini, Z.; Bassi, R.; Dall’Osto, L. Assessing photoprotective functions of carotenoids in photosynthetic systems of plants and green algae. Methods Enzymol. 2022, 674, 53–84. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Formaggio, E.; Cinque, G.; Bassi, R. Functional architecture of the major light-harvesting complex from higher plants. J. Mol. Biol. 2001, 314, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Trebst, A. Function of β-carotene and tocopherol in photosystem II. Zeitschrift für Naturforsch. C 2003, 58, 609–620. [Google Scholar] [CrossRef]
- Zhou, R.; Hyldgaard, B.; Yu, X.; Rosenqvist, E.; Ugarte, R.M.; Yu, S.; Wu, Z.; Ottosen, C.O.; Zhao, T. Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 2018, 214, 68. [Google Scholar] [CrossRef]
- Badr, A.; Brüggemann, W. Comparative analysis of drought stress response of maize genotypes using chlorophyll fluorescence measurements and leaf relative water content. Photosynthetica 2020, 58, 638–645. [Google Scholar] [CrossRef]
- Xia, Q.; Tang, H.; Fu, L.; Tan, J.; Govindjee, G.; Guo, Y. Determination of F/F from Chlorophyll a Fluorescence without Dark Adaptation by an LSSVM Model. Plant Phenomics 2023, 5, 0034. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Special issue in honour of Prof. Reto J. Strasser-Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Dobrikova, A.; Apostolova, E.; Adamakis, I.-D.S.; Hanć, A.; Sperdouli, I.; Moustakas, M. Combined impact of excess zinc and cadmium on elemental uptake, leaf anatomy and pigments, antioxidant capacity, and function of photosynthetic apparatus in clary sage (Salvia sclarea L.). Plants 2022, 11, 2407. [Google Scholar] [CrossRef]
- Deák, Z.; Sass, L.; Kiss, É.; Vass, I. Characterization of wave phenomena in the relaxation of flash-induced chlorophyll fluorescence yield in cyanobacteria. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, M.A.; Rashkov, G.D.; Yotsova, E.K.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. Different sensitivity levels of the photosynthetic apparatus in Zea mays L. and Sorghum bicolor L. under salt stress. Plants 2021, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, M.; Yotsova, E.; Markovska, Y.; Apostolova, E.L. Effect of high light intensity on the photosynthetic apparatus of two hybrid lines of Paulownia grown on soils with different salinity. Photosynthetica 2018, 56, 832–840. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Rashkov, G.D.; Ivanova, K.; Markovska, Y.; Apostolova, E.L. Effects of salinity on the photosynthetic apparatus of two Paulownia lines. Plant Physiol. Biochem. 2016, 101, 54–59. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Gesheva, E.; Dimitrova, V.; Markovska, Y.; Doncheva, S.; Apostolova, E.L. Role of flavonoids and proline in the protection of photosynthetic apparatus in Paulownia under salt stress. S. Afr. J. Bot. 2021, 139, 246–253. [Google Scholar] [CrossRef]
- Stefanov, M.A.; Rashkov, G.D.; Apostolova, E.L. Assessment of the photosynthetic apparatus functions by chlorophyll fluorescence and P700 absorbance in C3 and C4 plants under physiological conditions and under salt stress. Int. J. Mol. Sci. 2022, 23, 3768. [Google Scholar] [CrossRef]
- Jursinic, P. Govindjee Effects of hydroxylamine and silicomolybdate on the decay in delayed light emission in the 6?100 ?s range after a single 10 ns flash in pea thylakoids. Photosynth. Res. 1982, 3, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams III, W.W.; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Heber, U.; Wiese, C.; Shuvalov, V.A. Energy dissipation in photosynthesis: Does the quenching of chlorophyll fluorescence originate from antenna complexes of photosystem II or from the reaction center? Planta 2001, 212, 749–758. [Google Scholar] [CrossRef]
- Zuo, G. Non-photochemical quenching (NPQ) in photoprotection: Insights into NPQ levels required to avoid photoinactivation and photoinhibition. New Phytol. 2025, 246, 1967–1974. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta-Bioenerg. 2015, 1847, 468–485. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P. Dynamics of higher plant photosystem cross-section associated with state transitions. Photosynth. Res. 2009, 99, 173–183. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Yunus, M., Pathre, E., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. ISBN 0748408215. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; Arnon, D.I., Ed.; Circular 3; College of Agriculture, University of California: Berkeley, CA, USA, 1950. [Google Scholar]
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Yotsova, E.K.; Dobrikova, A.G.; Stefanov, M.A.; Kouzmanova, M.; Apostolova, E.L. Improvement of the rice photosynthetic apparatus defence under cadmium stress modulated by salicylic acid supply to roots. Theor. Exp. Plant Physiol. 2018, 30, 57–70. [Google Scholar] [CrossRef]
- Beadle, C.L.; Ludlow, M.M.; Honeysett, J.L. Water relations. In Techniques in Bioproductivity and Photosynthesis; Coombs, J., Hall, D.O., Long, S.P., Scurlock, J.M.O., Eds.; Elsevier: Oxford, UK, 1985; pp. 50–61. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Na, Y.-W.; Jeong, H.J.; Lee, S.-Y.; Choi, H.G.; Kim, S.-H.; Rho, I.R. Chlorophyll fluorescence as a diagnostic tool for abiotic stress tolerance in wild and cultivated strawberry species. Hortic. Environ. Biotechnol. 2014, 55, 280–286. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Guo, P. Chlorophyll Fluorescence: A Useful Tool in Barley Plant Breeding Programs. In Photochemistry Research Progress; Sánchez, A., Gutierrez, S.J., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 447–471. ISBN 9781604562323. [Google Scholar]
- Guadagno, C.R.; Virzo De Santo, A.; D’Ambrosio, N. A revised energy partitioning approach to assess the yields of non-photochemical quenching components. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 525–530. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Jinhang, S. The Analysis and Research of Clustering Algorithm Based on PCA. In Proceedings of the 2017 13th IEEE International Conference on Electronic Measurement & Instruments (ICEMI), Yangzhou, China, 20–22 October 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 361–365. [Google Scholar]



| Variants | t1 (s) | t2 (s) | A2/A1 |
|---|---|---|---|
| Control | |||
| M 2/133 | 0.577 ± 0.045 b | 13.054 ± 0.732 c | 0.236 ± 0.012 b |
| wt | 0.626 ± 0.054 b | 16.046 ± 0.946 b | 0.206 ± 0.010 c |
| M 2/16 | 0.621 ± 0.065 b | 16.811 ± 0.952 b | 0.205 ± 0.011 c |
| +20% PEG | |||
| M 2/133 | 0.732 ± 0.058 a | 18.870 ± 1.229 a | 0.301 ± 0.024 a |
| wt | 0.707 ± 0.043 a | 18.132 ± 1.229 a | 0.218 ± 0.013 c |
| M 2/16 | 0.702 ± 0.045 a | 18.865 ± 1.235 a | 0.216 ± 0.011 c |
| Variant | γRC/(1 − γRC) | φPo/(1 − φPo) | ψEo/(1 − ψEo) | δREo/(1 − δREo) |
|---|---|---|---|---|
| M 2/133 | 0.354 ± 0.007 | 5.005 ± 0.094 | 0.946 ± 0.062 | 0.494 ± 0.032 |
| wt | 0.398 ± 0.008 * | 5.086 ± 0.207 | 0.981 ± 0.051 | 0.511 ± 0.027 |
| M 2/16 | 0.399 ± 0.005 * | 5.018 ± 0.155 | 1.005 ± 0.034 | 0.475 ± 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashkov, G.D.; Stefanov, M.A.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. The Role of the Organization of Light-Harvesting Complex II in the Drought Sensitivity of Pisum sativum L. Int. J. Mol. Sci. 2025, 26, 11078. https://doi.org/10.3390/ijms262211078
Rashkov GD, Stefanov MA, Borisova PB, Dobrikova AG, Apostolova EL. The Role of the Organization of Light-Harvesting Complex II in the Drought Sensitivity of Pisum sativum L. International Journal of Molecular Sciences. 2025; 26(22):11078. https://doi.org/10.3390/ijms262211078
Chicago/Turabian StyleRashkov, Georgi D., Martin A. Stefanov, Preslava B. Borisova, Anelia G. Dobrikova, and Emilia L. Apostolova. 2025. "The Role of the Organization of Light-Harvesting Complex II in the Drought Sensitivity of Pisum sativum L." International Journal of Molecular Sciences 26, no. 22: 11078. https://doi.org/10.3390/ijms262211078
APA StyleRashkov, G. D., Stefanov, M. A., Borisova, P. B., Dobrikova, A. G., & Apostolova, E. L. (2025). The Role of the Organization of Light-Harvesting Complex II in the Drought Sensitivity of Pisum sativum L. International Journal of Molecular Sciences, 26(22), 11078. https://doi.org/10.3390/ijms262211078








