Hypoxic Training with Calorie Restriction Improves Lipid Profile and Body Composition in Men with Obesity-Related Hypercholesterolemia: A Controlled Intervention Study
Abstract
1. Introduction
2. Results
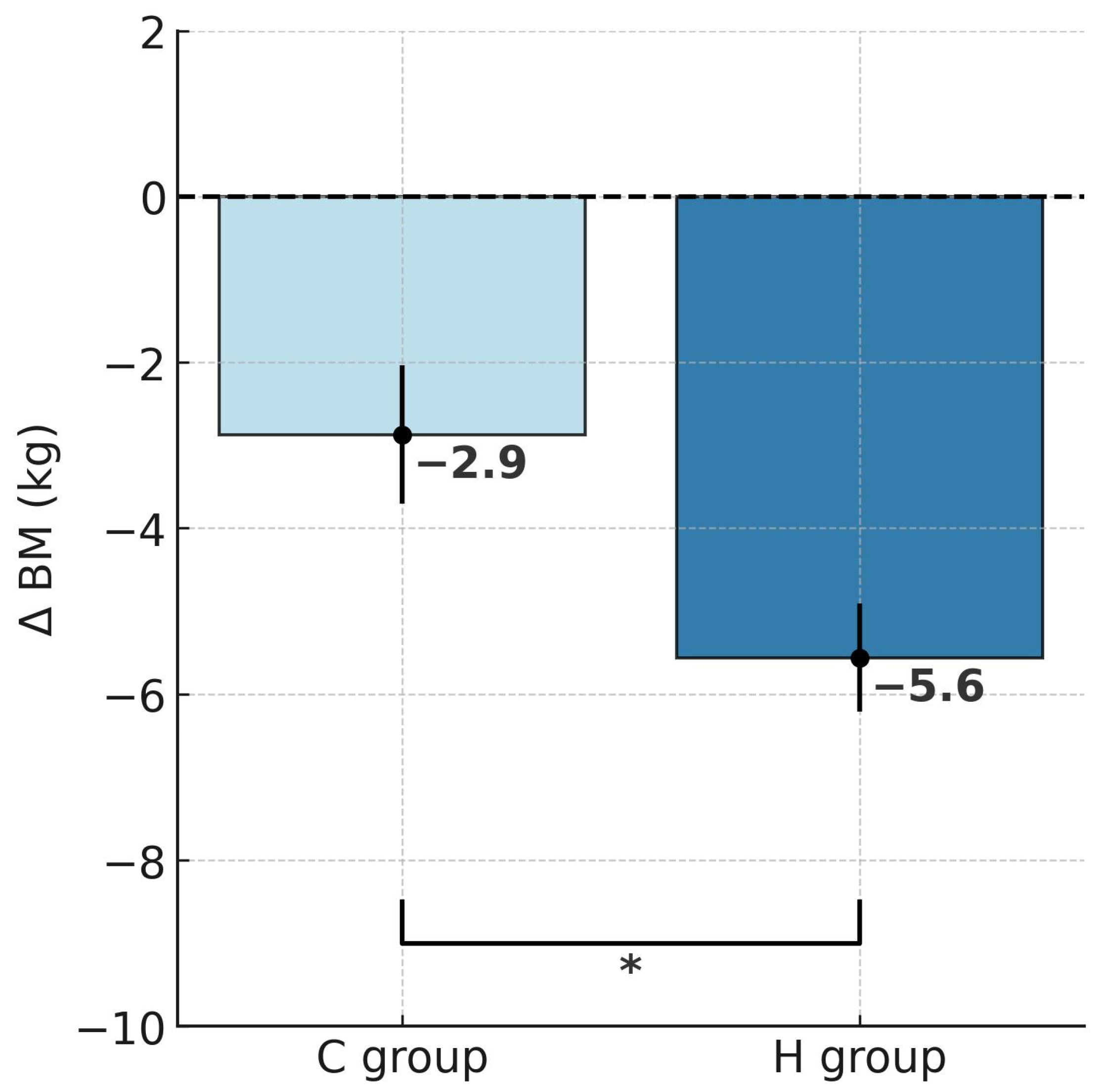
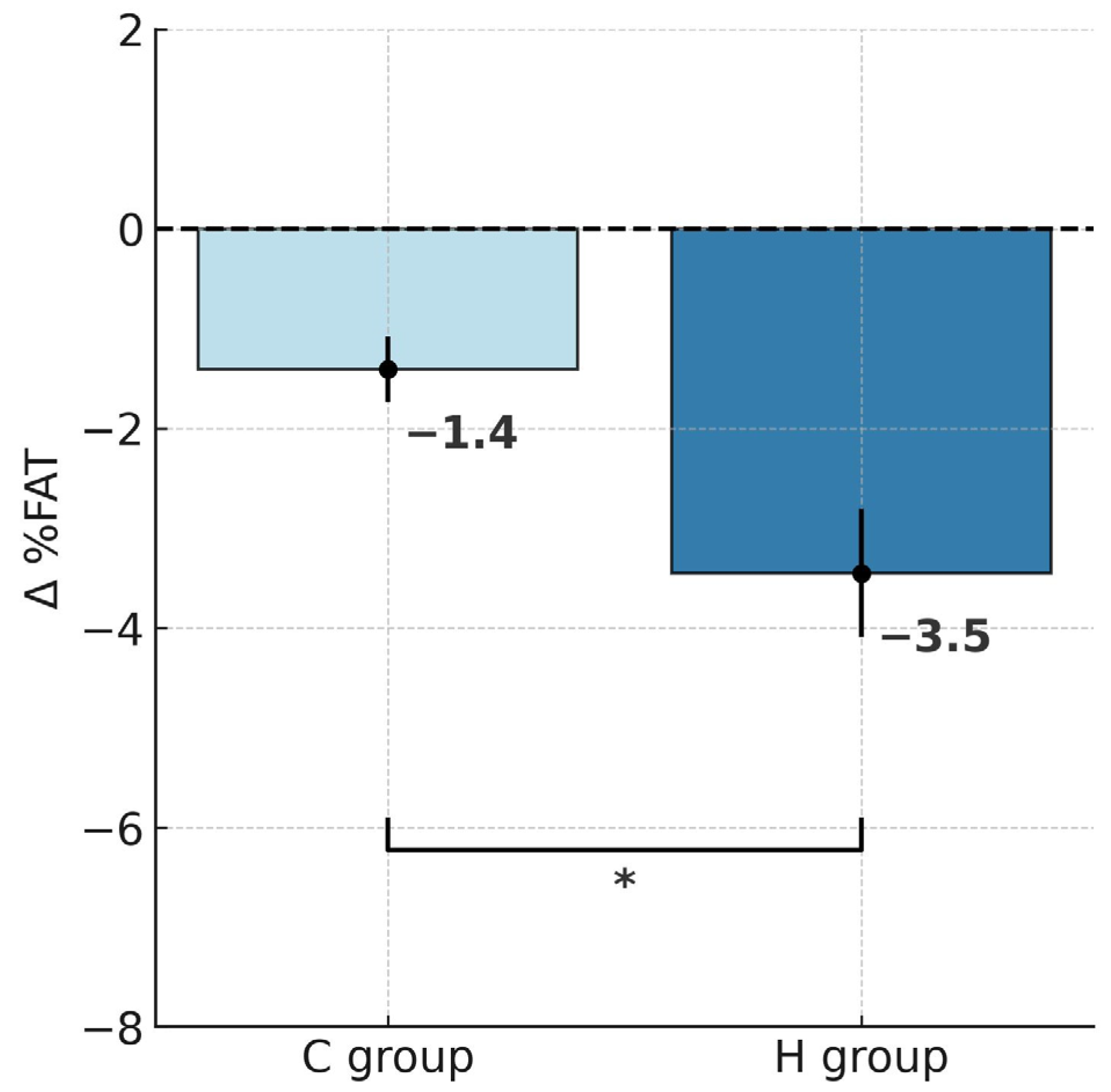
2.1. Changes in Body Mass and Body Composition
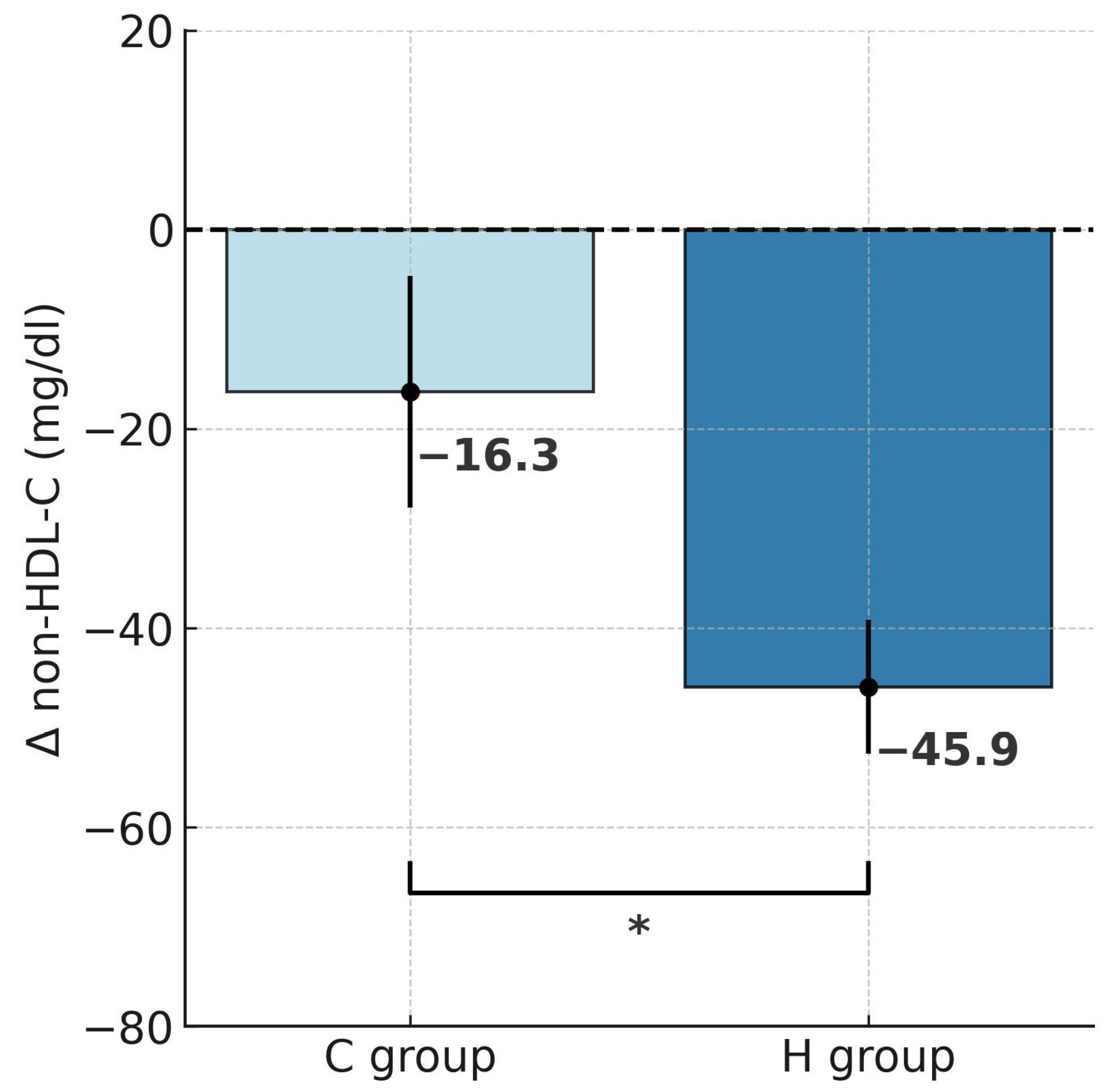
2.2. Changes in Lipid Profile
3. Discussion
Limitations
4. Materials and Methods
4.1. The Participants
4.2. Study Design
4.3. Testing Protocol
- Atherogenic index of plasma (AIP) = log10 (TG/HDL-C);
- Castelli’s risk index I (CRI-I) = TC/HDL-C;
- Castelli’s risk index II (CRI-II) = LDL-C/HDL-C.
4.4. Training Program
4.5. Diets During the Experiment
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| %FAT | Body fat percentage |
| AIP | Atherogenic index of plasma |
| BH | Body height |
| BM | Body mass |
| BMI | Body mass index |
| CRI-I | Castelli Risk Index I |
| CRI-II | Castelli Risk Index II |
| CVD | Cardiovascular disease |
| FFM | Fat-free mass |
| FM | Fat mass |
| HDL-C | High-density lipoprotein cholesterol |
| HIF-1 | Hypoxia-inducible factor |
| HRLT | Heart rate at lactate threshold |
| IHE | Intermittent hypoxic exposure |
| IHT | Intermittent hypoxic training |
| LDL-C | Low-density lipoprotein cholesterol |
| LH–TL | Live high—train low |
| PGC-1α | Peroxisome proliferator-activated receptor-gamma coactivator 1α |
| RMR | Resting metabolic rate |
| SD | Standard deviations |
| TC | Total cholesterol |
| TG | Triglycerides |
| WHO | World Health Organization |
| WHR | Waist-to-hip ratio |
References
- Park, H.Y.; Kim, J.; Park, M.Y.; Chung, N.; Hwang, H.; Nam, S.S.; Lim, K. Exposure and exercise training in hypoxic conditions as a new obesity therapeutic modality: A mini review. J. Obes. Metab. Syndr. 2018, 27, 93–101. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 September 2025).
- Cannon, C.P. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin. Cornerstone 2007, 8, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Adiels, M.; Taskinen, M.R.; Borén, J. Fatty liver, insulin resistance, and dyslipidemia. Curr. Diabetes Rep. 2008, 8, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Wauters, M.A.; de Leeuw, I.H. The beneficial effects of modest weight loss on cardiovascular risk factors. Int. J. Obes. Relat. Metab. Disord. 1997, 21, S5–S9. [Google Scholar]
- Poobalan, A.; Aucott, L.; Smith, W.C.; Avenell, A.; Jung, R.; Broom, J.; Grant, A.M. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes—A systematic review. Obes. Rev. 2004, 5, 43–50. [Google Scholar] [CrossRef]
- Niemaszyk, A.; Płoszczyca, K.; Czuba, M. The use of intermittent hypoxic training in rehabilitation, prevention, and treatment of non-communicable diseases (NCDs): A narrative review. Biomed. Hum. Kinet. 2025, 17, 173–185. [Google Scholar] [CrossRef]
- Millet, G.P.; Debevec, T.; Brocherie, F.; Malatesta, D.; Girard, O. Therapeutic Use of Exercising in Hypoxia: Promises and Limitations. Front. Physiol. 2016, 7, 224. [Google Scholar] [CrossRef]
- Debevec, T.; Simpson, E.J.; Macdonald, I.A.; Eiken, O.; Mekjavic, I.B. Exercise training during normobaric hypoxic confinement does not alter hormonal appetite regulation. PLoS ONE 2014, 9, e98874. [Google Scholar] [CrossRef]
- He, Z.; Qiang, L.; Liu, Y.; Gao, W.; Feng, T.; Li, Y.; Yan, B.; Girard, O. Effect of Hypoxia Conditioning on Body Composition in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis. Sports Med. Open 2023, 9, 89. [Google Scholar] [CrossRef]
- Debevec, T. Hypoxia-related hormonal appetite modulation in humans during rest and exercise: Mini review. Front. Physiol. 2017, 8, 366. [Google Scholar] [CrossRef]
- Lippl, F.J.; Neubauer, S.; Schipfer, S.; Lichter, N.; Tufman, A.; Otto, B.; Fischer, R. Hypobaric hypoxia causes body weight reduction in obese subjects. Science 2010, 18, 675–681. [Google Scholar] [CrossRef]
- Urdampilleta, A.; González-Muniesa, P.; Portillo, M.P.; Martínez, J.A. Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J. Physiol. Biochem. 2012, 68, 289–304. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Girard, O.; Pérez, A.; Rubio-Arias, J. Additive stress of normobaric hypoxic conditioning to improve body mass loss and cardiometabolic markers in individuals with overweight or obesity: A systematic review and meta-analysis. Physiol. Behav. 2019, 207, 28–40. [Google Scholar] [CrossRef]
- Haufe, S.; Wiesner, S.; Engeli, S.; Luft, F.; Jordan, J. Influences of Normobaric Hypoxia Training on Metabolic Risk Markers in Human Subjects. Med. Sci. Sports Exerc. 2008, 40, 1939–1944. [Google Scholar] [CrossRef]
- Costalat, G.; Lemaitre, F.; Tobin, B.; Renshaw, G. Intermittent hypoxia revisited: A promising non-pharmaceutical strategy to reduce cardio-metabolic risk factors? Sleep Breath 2018, 22, 267–271. [Google Scholar] [CrossRef]
- Wang, R.; Guo, S.; Tian, H.; Huang, Y.; Yang, Q.; Zhao, K.; Kuo, C.H.; Hong, S.; Chen, P.; Liu, T. Hypoxic Training in Obese Mice Improves Metabolic Disorder. Front. Endocrinol. 2019, 10, 527. [Google Scholar] [CrossRef]
- Płoszczyca, K.; Czuba, M.; Langfort, J.; Baranowski, M. Exposure to Normobaric Hypoxia Combined with a Mixed Diet Contributes to Improvement in Lipid Profile in Trained Cyclists. Nutrients 2021, 13, 3481. [Google Scholar] [CrossRef]
- Ghaith, A.; Chacaroun, S.; Borowik, A.; Chatel, L.; Doutreleau, S.; Wuyam, B.; Tamisier, R.; Pépin, J.L.; Flore, P.; Verges, S. Hypoxic high-intensity interval training in individuals with overweight and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R700–R709. [Google Scholar] [CrossRef]
- Fernández Menéndez, A.; Saudan, G.; Sperisen, L.; Hans, D.; Saubade, M.; Millet, G.P.; Malatesta, D. Effects of Short-Term Normobaric Hypoxic Walking Training on Energetics and Mechanics of Gait in Adults with Obesity. Obesity 2018, 26, 819–827. [Google Scholar] [CrossRef]
- Klug, L.; Mähler, A.; Rakova, N.; Mai, K.; Schulz-Menger, J.; Rahn, G.; Busjahn, A.; Jordan, J.; Boschmann, M.; Luft, F.C. Normobaric hypoxic conditioning in men with metabolic syndrome. Physiol. Rep. 2018, 6, e13949. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: An overview of 12 systematic reviews and 149 studies. Obes. Rev. 2021, 22, e13256. [Google Scholar] [CrossRef]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar]
- Degoutte, F.; Jouanel, P.; Bègue, R.J.; Colombier, M.; Lac, G.; Pequignot, J.M.; Filaire, E. Food restriction, performance, biochemical, psychological, and endocrine changes in judo athletes. Int. J. Sports Med. 2006, 27, 9–18. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Kłapcińska, B.; Podgórski, T.; Szade, B.; Tyl, K.; Hadzik, A. Effects of supplementation with acai (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defence capacity and lipid profile in junior hurdlers. A pilot study. Biol. Sport 2015, 32, 161–168. [Google Scholar] [CrossRef]
- Creighton, B.C.; Hyde, P.N.; Maresh, C.M.; Kraemer, W.J.; Phinney, S.D.; Volek, J.S. Paradox of hypercholesterolaemia in highly trained, keto-adapted athletes. BMJ Open Sport Exerc. Med. 2018, 4, e000429. [Google Scholar] [CrossRef]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef]
- Burtscher, M. Effects of living at higher altitudes on mortality: A narrative review. Aging Dis. 2013, 5, 274–280. [Google Scholar] [CrossRef]
- Sharma, S. Clinical, biochemical, electrocardiographic and noninvasive hemodynamic assessment of cardiovascular status in natives at high to extreme altitudes (3000m-5500m) of the Himalayan region. Indian Heart J. 1990, 42, 375–379. [Google Scholar]
- Dominguez Coello, S.; Cabrera De León, A.; Bosa Ojeda, F.; Pérez Méndez, L.I.; Díaz González, L.; Aguirre-Jaime, A.J. High density lipoprotein cholesterol increases with living altitude. Int. J. Epidemiol. 2020, 29, 65–70. [Google Scholar] [CrossRef]
- Mohanna, S.; Baracco, R.; Seclen, S. Lipid profile, waist circumference, and body mass index in a high altitude population. High Alt. Med. Biol. 2006, 7, 245–255. [Google Scholar] [CrossRef]
- Férézou, J.; Richalet, J.P.; Coste, T.; Rathat, C. Changes in plasma lipids and lipoprotein cholesterol during a high altitude mountaineering expedition (4800 m). Eur. J. Appl. Physiol. Occup. Physiol. 1988, 57, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Verratti, V.; Falone, S.; Doria, C.; Pietrangelo, T.; Di Giulio, C. Kilimanjaro Abruzzo expedition: Effects of high-altitude trekking on anthropometric, cardiovascular and blood biochemical parameters. Sport Sci. Health 2015, 11, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Gutwenger, I.; Hofer, G.; Gutwenger, A.K.; Sandri, M.; Wiedermann, C.J. Pilot study on the effects of a 2-week hiking vacation at moderate versus low altitude on plasma parameters of carbohydrate and lipid metabolism in patients with metabolic syndrome. BMC Res. Notes 2015, 28, 103. [Google Scholar] [CrossRef] [PubMed]
- Minvaleev, R.S. Comparison of the rates of changes in the lipid spectrum of human blood serum at moderate altitudes. Hum. Physiol. 2011, 37, 355–360. [Google Scholar] [CrossRef]
- Greie, S.; Humpeler, E.; Gunga, H.C.; Koralewski, E.; Klingler, A.; Mittermayr, M.; Fries, D.; Lechleitner, M.; Hoertnagl, H.; Hoffmann, G.; et al. Improvement of metabolic syndrome markers through altitude specific hiking vacations. J. Endocrinol. Investig. 2006, 29, 497–504. [Google Scholar] [CrossRef]
- Matei, D.; Buculei, I.; Luca, C.; Corciova, C.-P.; Andritoi, D.; Fuior, R.; Iordan, D.-A.; Onu, I. Impact of Non-Pharmacological Interventions on the Mechanisms of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 9097. [Google Scholar] [CrossRef]
- Caruso, L.; Zauli, E.; Vaccarezza, M. Physical Exercise and Appetite Regulation: New Insights. Biomolecules 2023, 13, 1170. [Google Scholar] [CrossRef]
- Thackray, A.E.; Stensel, D.J. The impact of acute exercise on appetite control: Current insights and future perspectives. Appetite 2023, 186, 106557. [Google Scholar] [CrossRef]
- Kong, Z.; Shi, Q.; Nie, J.; Tong, T.K.; Song, L.; Yi, L.; Hu, Y. High-Intensity Interval Training in Normobaric Hypoxia Improves Cardiorespiratory Fitness in Overweight Chinese Young Women. Front. Physiol. 2017, 8, 175. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1 and cardiovascular disease. Annu. Rev. Physiol. 2014, 76, 39–56. [Google Scholar] [CrossRef]
- Zoll, J.; Ponsot, E.; Dufour, S.; Doutreleau, S.; Ventura-Clapier, R.; Vogt, M.; Hoppeler, H.; Richard, R.; Flück, M. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J. Appl. Physiol. 2006, 100, 1258–1266. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Atakan, M.M.; Kuang, J.; Hu, Y.; Bishop, D.J.; Yan, X. The Molecular Adaptive Responses of Skeletal Muscle to High-Intensity Exercise/Training and Hypoxia. Antioxidants 2020, 9, 656. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Guo, W.; Gao, B.H. Hypoxic training upregulates mitochondrial turnover and angiogenesis of skeletal muscle in mice. Life Sci. 2022, 291, 119340. [Google Scholar] [CrossRef]
- Cheng, C.F.; Ku, H.C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef]
- Shen, G.M.; Zhao, Y.Z.; Chen, M.T.; Zhang, F.L.; Liu, X.L.; Wang, Y.; Liu, C.Z.; Yu, J.; Zhang, J.W. Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake through inducing VLDLR under hypoxia. Biochem. J. 2012, 441, 675–683. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Du, X.; Girard, O.; Fan, R.Y.; Ma, F. Effects of Active and Passive Hypoxic Conditioning for 6 Weeks at Different Altitudes on Blood Lipids, Leptin, and Weight in Rats. High Alt. Med. Biol. 2020, 21, 243–248. [Google Scholar] [CrossRef]
- Gilde, A.J.; Van Bilsen, M. Peroxisome proliferator-activated receptors (PPARS): Regulators of gene expression in heart and skeletal muscle. Acta Physiol. Scand. 2003, 178, 425–434. [Google Scholar] [CrossRef]
- Tin’kov, A.N.; Aksenov, V.A. Effects of intermittent hypobaric hypoxia on blood lipid concentrations in male coronary heart disease patients. High Alt. Med. Biol. 2002, 3, 277–282. [Google Scholar] [CrossRef]
- Lizamore, C.A.; Hamlin, M.J. The Use of Simulated Altitude Techniques for Beneficial Cardiovascular Health Outcomes in Nonathletic, Sedentary, and Clinical Populations: A Literature Review. High Alt. Med. Biol. 2017, 18, 305–321. [Google Scholar] [CrossRef]
- Gao, H.; Xu, J.; Zhang, L.; Lu, Y.; Gao, B.; Feng, L. Effects of Living High-Training Low and High on Body Composition and Metabolic Risk Markers in Overweight and Obese Females. BioMed Res. Int. 2020, 2020, 3279710. [Google Scholar] [CrossRef]
- Thompson, P.D.; Jeffery, R.W.; Wing, R.R.; Wood, P.D. Unexpected decrease in plasma high density lipoprotein cholesterol with weight loss. Am. J. Clin. Nutr. 1979, 32, 2016–2021. [Google Scholar] [CrossRef]
- Brinton, E.A.; Eisenberg, S.; Breslow, J.L. A low-fat diet decreases high density lipoprotein (HDL) cholesterol levels by decreasing HDL apolipoprotein transport rates. J. Clin. Investig. 1990, 85, 144–151. [Google Scholar] [CrossRef]
- Englert, I.; Bosy-Westphal, A.; Bischoff, S.C.; Kohlenberg-Müller, K. Impact of Protein Intake during Weight Loss on Preservation of Fat-Free Mass, Resting Energy Expenditure, and Physical Function in Overweight Postmenopausal Women: A Randomized Controlled Trial. Obes. Facts 2021, 14, 259–270. [Google Scholar] [CrossRef]
- Bergström, H.; Ekström, L.; Warnqvist, A.; Bergman, P.; Björkhem-Bergman, L. Variations in biomarkers of dyslipidemia and dysbiosis during the menstrual cycle: A pilot study in healthy volunteers. BMC Women’s Health 2021, 21, 166. [Google Scholar] [CrossRef]
- Vogler, A.J.; Rice, A.J.; Gore, C.J. Validity and reliability of the cortex MetaMax3B portable metabolic system. J. Sports Sci. 2010, 28, 733–742. [Google Scholar] [CrossRef]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Iannelli, A.; Barbarisi, A. Fat mass, fat-free mass, and resting metabolic rate in weight-stable sleeve gastrectomy patients compared with weight-stable nonoperated patients. Surg. Obes. Relat. Dis. 2017, 13, 1692–1699. [Google Scholar] [CrossRef]
- Almajwal, A.M.; Abulmeaty, M.J. New predictive equations for resting energy expenditure in normal to overweight and obese population. Int. J. Endocrinol. 2019, 2019, 5727496. [Google Scholar] [CrossRef]
- Gao Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Castelli, W.P.; Abbott, R.D.; McNamara, P.M. Summary estimates of cholesterol used to predict coronary heart disease. Circulation 1983, 67, 730–734. [Google Scholar] [CrossRef]
- Dobiášová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FERHDL). Clin. Biochem. 2001, 34, 583–588. [Google Scholar]
- Cheng, B.; Kuipers, H.; Snyder, A.C.; Keizer, H.A.; Jeukendrup, A.; Hesselink, M.A. New Approach for the determination of ventilatory and lactate thresholds. Int. J. Sports Med. 1992, 13, 518–522. [Google Scholar] [CrossRef]
- San Mauro, M.I.; Garicano, V.E.; Romo Orozco, D.A.; Mendive Dubourdieu, P.; Paredes Barato, V.; Rincón Barrado, M.; Valente, A.; Bentancor, F.; Morales Hurtado, A.D.; Garagarza, C. Hydration Status: Influence of Exercise and Diet Quality. Am. J. Lifestyle Med. 2017, 13, 414–423. [Google Scholar] [CrossRef]




| Variables | H Group | C Group | ||
|---|---|---|---|---|
| Before (S1) | After (S2) | Before (S1) | After (S2) | |
| BM (kg) | 103.1 ± 11.8 | 97.5 ± 11.3 *** | 99.5 ± 7.8 | 96.7 ± 7.8 ** |
| %FAT | 33.6 ± 7.7 | 30.2 ± 8.1 *** | 32.9 ± 4.9 | 31.5 ± 4.1 # |
| FM (kg) | 35.3 ± 10.7 | 30.1 ± 10.1 *** | 32.8 ± 5.8 | 30.5 ± 4.8 ** |
| FFM (kg) | 67.8 ± 5.5 | 67.5 ± 6.1 | 66.7 ± 6.3 | 66.1 ± 6.2 |
| RMR (kcal/day) | 2395.0 ± 215.3 | 2411.5 ± 205.6 | 2337.9 ± 238.5 | 2310.8 ± 135.8 |
| Variables | H Group | C Group | ||
|---|---|---|---|---|
| Before (S1) | After (S2) | Before (S1) | After (S2) | |
| TC (mg/dL) | 220.2 ± 26.9 | 170.4 ±19.7 *** | 230.4 ± 24.1 | 211.0 ± 31.1 ### |
| HDL-C (mg/dL) | 46.9 ± 7.3 | 41.9 ±6.4 | 46.1 ±14.2 | 43.3 ± 10.3 |
| LDL-C (mg/dL) | 149.1 ± 22.3 | 110.5 ± 15.8 *** | 141.9 ± 24.8 | 129.0 ± 16.5 |
| non-HDL-C (mg/dL) | 173.1 ± 26.7 | 127.2 ± 22.5 *** | 183.9 ± 26.3 | 167.6 ± 33.7 # |
| TG (mg/dL) | 139.8 ± 51.1 | 95.9 ± 34.2 ** | 236.5 ± 164.9 | 210.3 ± 150.9 |
| Variables | H Group | C Group | ||
|---|---|---|---|---|
| Before (S1) | After (S2) | Before (S1) | After (S2) | |
| AIP | 0.45 ± 0.18 | 0.34 ± 0.18 * | 0.64 ± 0.35 | 0.61 ± 0.36 |
| CRI-I | 4.78 ± 0.94 | 4.1 ± 0.82 | 5.38 ± 1.57 | 5.17 ± 1.81 |
| CRI-II | 3.26 ± 0.82 | 2.7 ± 0.66 | 3.32 ± 1.07 | 3.13 ± 0.87 |
| Variables | H Group | C Group |
|---|---|---|
| Age (years) | 37.3 ± 5.8 | 33.3 ± 4.4 |
| Body height (cm) | 178.7 ± 5.2 | 177.5 ± 5.3 |
| Body mass (kg) | 103.1 ± 11.8 | 99.5 ± 7.9 |
| Body fat (%) | 33.6 ± 7.7 | 32.9 ± 4.9 |
| Systolic blood pressure (mmHg) | 137.1 ± 6.1 | 135.0 ± 12.5 |
| Diastolic blood pressure (mmHg) | 81.1 ± 9.2 | 84.8 ± 12.7 |
| Protein (g) | Fat (g) | Carbohydrates (g) | Caloric Intake (kcal) |
|---|---|---|---|
| 191.2 ± 16.4 | 65.6 ± 8.4 | 287.3 ± 26.8 | 2515.7 ± 40.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejewski, E.; Czuba, M.; Niemaszyk, A.; Płoszczyca, K.; Kaczmarczyk, K.; Langfort, J.; Gajda, R. Hypoxic Training with Calorie Restriction Improves Lipid Profile and Body Composition in Men with Obesity-Related Hypercholesterolemia: A Controlled Intervention Study. Int. J. Mol. Sci. 2025, 26, 11048. https://doi.org/10.3390/ijms262211048
Jędrzejewski E, Czuba M, Niemaszyk A, Płoszczyca K, Kaczmarczyk K, Langfort J, Gajda R. Hypoxic Training with Calorie Restriction Improves Lipid Profile and Body Composition in Men with Obesity-Related Hypercholesterolemia: A Controlled Intervention Study. International Journal of Molecular Sciences. 2025; 26(22):11048. https://doi.org/10.3390/ijms262211048
Chicago/Turabian StyleJędrzejewski, Emil, Miłosz Czuba, Adam Niemaszyk, Kamila Płoszczyca, Katarzyna Kaczmarczyk, Józef Langfort, and Robert Gajda. 2025. "Hypoxic Training with Calorie Restriction Improves Lipid Profile and Body Composition in Men with Obesity-Related Hypercholesterolemia: A Controlled Intervention Study" International Journal of Molecular Sciences 26, no. 22: 11048. https://doi.org/10.3390/ijms262211048
APA StyleJędrzejewski, E., Czuba, M., Niemaszyk, A., Płoszczyca, K., Kaczmarczyk, K., Langfort, J., & Gajda, R. (2025). Hypoxic Training with Calorie Restriction Improves Lipid Profile and Body Composition in Men with Obesity-Related Hypercholesterolemia: A Controlled Intervention Study. International Journal of Molecular Sciences, 26(22), 11048. https://doi.org/10.3390/ijms262211048







