The Inflammatory Cell Death in Diabetic Kidney Disease: Integrating Multifactorial Mechanisms into Novel Therapeutics
Abstract
1. Introduction
2. Inflammatory Cell Death: From Molecular Mechanisms to DKD
2.1. Pyroptosis: NLRP3 Inflammasome Activation in Diabetic Kidneys
2.2. Necroptosis: RIPK1/RIPK3/MLKL Signaling and Sterile Inflammation
2.3. Ferroptosis: Metabolic Dysregulation and Lipid Peroxidation in Diabetic Renal Injury
2.4. PANoptosis: An Integrated Cell Death Circuit in Podocyte Demise
2.5. NETosis: Neutrophil Extracellular Traps in Glomerular Damage and Inflammation
2.6. Crosstalk and Therapeutic Implications
3. The Role of Inflammatory Cell Death in Diabetic Kidney Disease
3.1. Inflammatory Cell Death and Tubular Cell Injury
3.2. Inflammatory Cell Death and Glomerular Injury
3.3. Inflammatory Cell Death in Immune Cells
4. Inflammatory Response and the Progression of Diabetic Kidney Disease
4.1. Central Role of Inflammatory Factors and Pyroptosis in DKD
4.2. From Inflammatory Cell Death to Fibrosis
4.3. Amplification of Fibrosis Through Pyroptosis and DAMPs
4.4. Fibroblast Activation by Dying Cell-Derived Signals
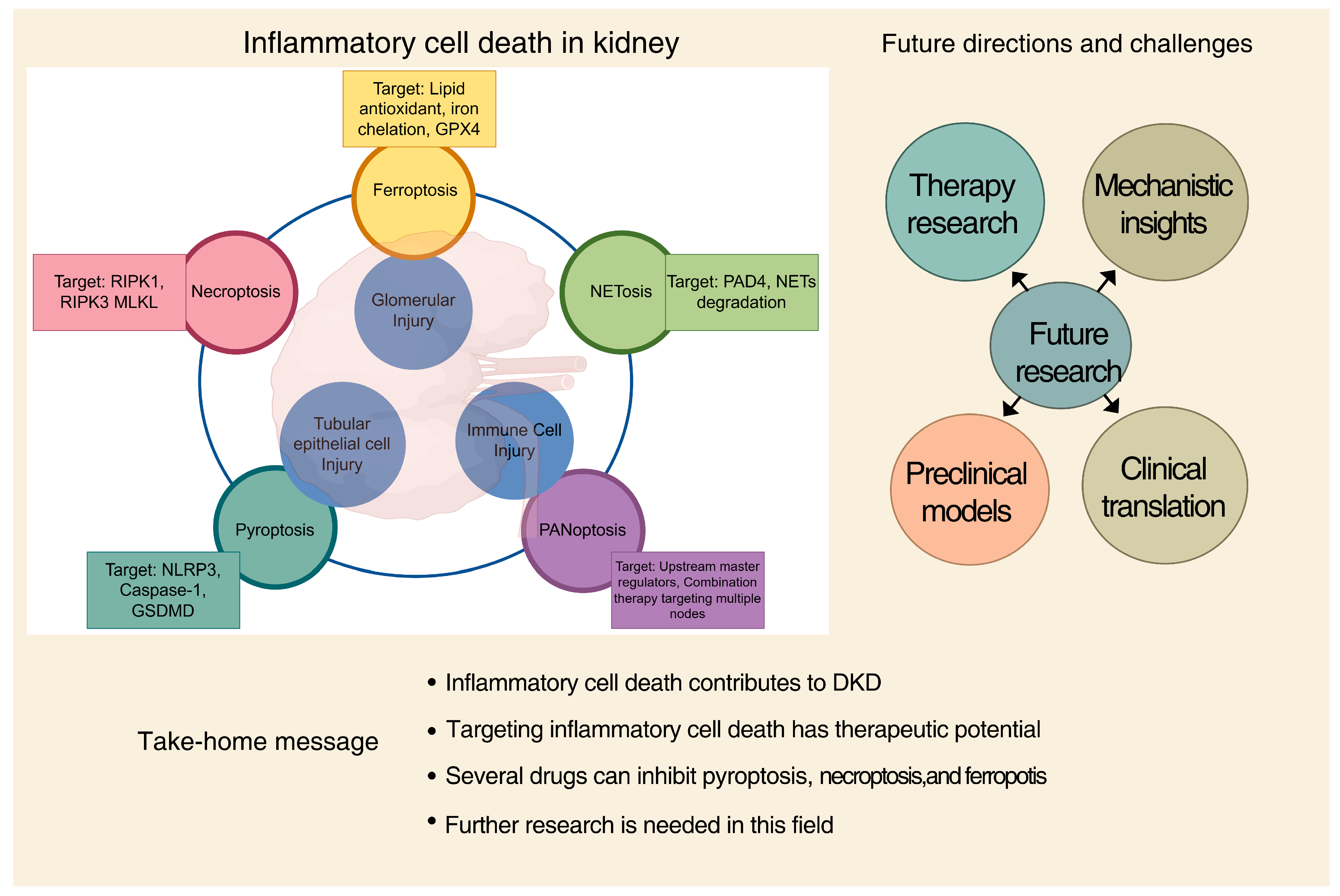
5. Therapeutic Targeting of Inflammatory Cell Death in DKD
5.1. Targeting the NLRP3 Inflammasome and Pyroptosis
5.2. Inhibiting Executioner Caspases
5.3. Targeting Necroptosis: RIPK1, RIPK3, and MLKL
5.4. Ferroptosis Inhibition: Scavenging Lipid Peroxides
5.5. Challenges in Clinical Translation and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSL4 | Acyl-CoA Synthetase Long Chain Family Member 4 |
| AGEs | Advanced Glycation End-Products |
| ASC | Apoptosis associated speck like proteins |
| ATP | Adenosine triphosphate |
| cGAS | cyclic GMP–AMP Synthase |
| CTGF | Connective Tissue Growth Factor |
| DAMPs | Damage-Associated Molecular Patterns |
| DKD | Diabetic Kidney Disease |
| dsDNA | Double-Stranded DNA |
| EMT | Epithelial-to-Mesenchymal Transition |
| EndMT | Endothelial-to-Mesenchymal Transition |
| ESRD | End-Stage Renal Disease |
| Fer-1 | Ferrostatin-1 |
| FTH1 | Ferritin Heavy Chain 1 |
| GBM | Glomerular Basement Membrane |
| GECs | Glomerular Endothelial Cells |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLP-1RA | Glucagon-Like Peptide-1 receptor agonist |
| GPX4 | Glutathione Peroxidase 4 |
| GSDMD | Gasdermin-D |
| HMGB1 | high mobility group box 1 |
| IL-1β | Interleukin 1-beta |
| IL-33 | Interleukin-33 |
| LOX-12 | 12-Lipoxygenase |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| NETs | Neutrophil Extracellular Traps |
| NF-κB | Nuclear Factor kappa B |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| NLRs | NOD-Like Receptors |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| NSA | Necrosulfonamide |
| PAD4 | Peptidyl arginine deiminase 4 |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PDGF | platelet-derived growth factor |
| PRRs | pattern recognition receptors |
| RAS | Renin–Angiotensin System |
| RIPK1 | Receptor-Interacting Protein Kinase 1 |
| RIPK3 | Receptor-Interacting Protein Kinase 3 |
| SGLT2 | Sodium–Glucose Cotransporter 2 Inhibitors |
| SIRT1 | Sirtuin 1 |
| SLC7A11 | Solute Carrier Family 7 Member 11 |
| Smad3 | SMAD Family Member 3 |
| STAT3 | Signal transducer and activator of transcription 3 |
| STING | Stimulator of Interferon Genes |
| System Xc− | acystine/glutamate antiporter system |
| TECs | Tubular Epithelial Cells |
| TFR1 | Transferrin Receptor 1 |
| TGF-β | Transforming growth factor beta |
| TLR2 | Toll like receptor 2 |
| TLR9 | Toll-like receptor 9 |
| TLRs | Toll-Like Receptors |
| TNF | Tumor necrosis factor |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| ZDSD | Zucker Diabetic-Sprague Dawley |
References
- Kalhan, T.A.; Luo, M.; Chai, J.H.; Tai, E.S.; Lim, S.C.; Coffman, T.M.; Venkataraman, K. Health Economic Evaluation of a Risk-Stratified Intervention in Diabetic Kidney Disease. Diabetologia 2025, 68, 2227–2239. [Google Scholar] [CrossRef]
- Cervantes, J.; Kanter, J.E. Salvaged Signals, Scarred Filters: Podocyte NPRC in Diabetic Kidney Disease. Circ. Res. 2025, 137, 548–550. [Google Scholar] [CrossRef]
- Cao, B.; Guo, Z.; Li, D.-T.; Zhao, L.-Y.; Wang, Z.; Gao, Y.-B.; Wang, Y.-X. The Association between Stress-Induced Hyperglycemia Ratio and Cardiovascular Events as Well as All-Cause Mortality in Patients with Chronic Kidney Disease and Diabetic Nephropathy. Cardiovasc. Diabetol. 2025, 24, 55. [Google Scholar] [CrossRef]
- Pugliese, G.; Penno, G.; Natali, A.; Barutta, F.; Di Paolo, S.; Reboldi, G.; Gesualdo, L.; De Nicola, L.; Italian Diabetes Society and the Italian Society of Nephrology. Diabetic Kidney Disease: New Clinical and Therapeutic Issues. Joint Position Statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The Natural History of Diabetic Kidney Disease and Treatment of Hyperglycemia in Patients with Type 2 Diabetes and Impaired Renal Function”. Nutr. Metab. Cardiovasc. Dis. NMCD 2019, 29, 1127–1150. [Google Scholar] [CrossRef]
- Jung, C.-Y.; Yoo, T.-H. Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease. Diabetes Metab. J. 2022, 46, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Thuy Linh, H.; Nakade, Y.; Wada, T.; Iwata, Y. The Potential Mechanism of D-Amino Acids—Mitochondria Axis in the Progression of Diabetic Kidney Disease. Kidney Int. Rep. 2025, 10, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.-J.; Kuo, C.-W.; Yen, P.-C.; Lin, C.-C.; Tsai, M.-T.; Lu, S.-H.; Chang, Y.-P.; Liu, W.-S.; Tsou, H.-H.; Cheng, H.-W.; et al. Acrolein Plays a Culprit Role in the Pathogenesis of Diabetic Nephropathy in Vitro and in Vivo. Eur. J. Endocrinol. 2022, 187, 579–592. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Z.; Zhang, C.; Shi, Y.; Han, W.; Song, S.; Mu, L.; Du, C.; Shi, Y. Inhibition of NLRP3 Inflammasome Ameliorates Podocyte Damage by Suppressing Lipid Accumulation in Diabetic Nephropathy. Metabolism 2021, 118, 154748. [Google Scholar] [CrossRef]
- Gu, J.; Geng, K.; Guo, M.; Huang, W.; Zhao, T.; Li, X.; Xu, Y.-H.; Xu, Y. Targeting Pyroptosis: New Insights into the Treatment of Diabetic Microvascular Complications. Evid.-Based Complement. Altern. Med. ECAM 2022, 2022, 5277673. [Google Scholar] [CrossRef]
- Zhu, R.; Bai, X.; Xu, C.; Qi, W.; Luo, P.; Wu, M.; Luo, M. Research Progress on Podocyte Pyroptosis in Diabetic Nephropathy. Curr. Med. Chem. 2024, 32, 5772–5789. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated Cell Death Pathways in Kidney Disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef]
- Kolbrink, B.; von Samson-Himmelstjerna, F.A.; Murphy, J.M.; Krautwald, S. Role of Necroptosis in Kidney Health and Disease. Nat. Rev. Nephrol. 2023, 19, 300–314. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, X. Significance of Pyroptosis-Related Genes in the Diagnosis and Classification of Diabetic Kidney Disease. Ren. Fail. 2024, 46, 2409331. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Li, R.; Zhao, X.; Chen, Y.; Cai, Y.; Yang, Y.; Wang, W.; Zheng, S.; Zhang, L.; et al. Podocyte RIPK3 Deletion Improves Diabetic Kidney Disease by Attenuating NF-κB P65 Driven Inflammation. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2025, 12, e03325. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ma, K.; Wang, W.; Han, Z.; Chi, M.; Nasser, M.I.; Liu, C. Research Progress of Pyroptosis in Renal Diseases. Curr. Med. Chem. 2024, 31, 6656–6671. [Google Scholar] [CrossRef] [PubMed]
- Sunilkumar, S.; Subrahmanian, S.M.; Yerlikaya, E.I.; Toro, A.L.; Harhaj, E.W.; Kimball, S.R.; Dennis, M.D. REDD1 Expression in Podocytes Facilitates Renal Inflammation and Pyroptosis in Streptozotocin-Induced Diabetic Nephropathy. Cell Death Dis. 2025, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xing, J.; Li, X.; Tang, X.; Zhang, D. PRDM16 Suppresses Ferroptosis to Protect against Sepsis-Associated Acute Kidney Injury by Targeting the NRF2/GPX4 Axis. Redox Biol. 2024, 78, 103417. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, J.; Su, H.; Yu, Q.; Lang, Y.; Yang, M.; Fan, X.; Liu, Y.; Liu, B.; Zhao, Y.; et al. TRAIL Induces Podocyte PANoptosis via Death Receptor 5 in Diabetic Kidney Disease. Kidney Int. 2025, 107, 317–331. [Google Scholar] [CrossRef]
- Miao, G.; Fortier, T.M.; Liu, H.; Schafer, D.P.; Fitzgerald, K.A.; Mao, J.; Baehrecke, E.H. Microglia Promote Inflammatory Cell Death upon Neuronal Mitochondrial Impairment during Neurodegeneration. Nat. Struct. Mol. Biol. 2025, 32, 2046–2059. [Google Scholar] [CrossRef]
- Min, R.; Bai, Y.; Wang, N.-R.; Liu, X. Gasdermins in Pyroptosis, Inflammation, and Cancer. Trends Mol. Med. 2025, 31, 860–875. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Zhang, Y. Dying to Survive: Harnessing Inflammatory Cell Death for Better Immunotherapy. Trends Cancer 2025, 11, 376–402. [Google Scholar] [CrossRef]
- Montalban-Bravo, G.; Class, C.A.; Ganan-Gomez, I.; Kanagal-Shamanna, R.; Sasaki, K.; Richard-Carpentier, G.; Naqvi, K.; Wei, Y.; Yang, H.; Soltysiak, K.A.; et al. Transcriptomic Analysis Implicates Necroptosis in Disease Progression and Prognosis in Myelodysplastic Syndromes. Leukemia 2020, 34, 872–881. [Google Scholar] [CrossRef]
- Zheng, Z.; Deng, W.; Bai, Y.; Miao, R.; Mei, S.; Zhang, Z.; Pan, Y.; Wang, Y.; Min, R.; Deng, F.; et al. The Lysosomal Rag-Ragulator Complex Licenses RIPK1 and Caspase-8-Mediated Pyroptosis by Yersinia. Science 2021, 372, eabg0269. [Google Scholar] [CrossRef]
- Khan, S.R.; Alli, A.A. Apoptosis, Ferroptosis, Necrosis, Necroptosis and Pyroptosis in the Formation of Calcium Oxalate Kidney Stones. Urolithiasis 2025, 53, 153. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, C.; Chen, Y.; Wang, D.; Yao, D. Nanomedicine-Induced Pyroptosis for Anti-Tumor Immunotherapy: Mechanism Analysis and Application Prospects. Acta Pharm. Sin. B 2025, 15, 3487–3510. [Google Scholar] [CrossRef]
- Liu, F.; Yang, Z.; Li, J.; Wu, T.; Li, X.; Zhao, L.; Wang, W.; Yu, W.; Zhang, G.; Xu, Y. Targeting Programmed Cell Death in Diabetic Kidney Disease: From Molecular Mechanisms to Pharmacotherapy. Mol. Med. Camb. Mass 2024, 30, 265. [Google Scholar] [CrossRef]
- Jin, Q.; Li, L.; Qu, P.; Ma, F.; Li, P.; Qiao, Y.; Zhang, Y.; Ran, S.; Li, X.; Liu, T.; et al. Mitigation of Renal Tubular Injury by SIRT6 May Improve Individual Outcomes in Diabetic Kidney Disease-Potential Mechanisms Involving Epigenetic Repression of Inflammatory Responses. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xue, S.-J.; Yang, F.; Xiao, M.; Tang, Y.-B.; Wu, Y. LncRNA SNHG7/miR-181b-5p/TLR4 Activates Inflammation and Promotes Pyroptosis Through NF-κB Signaling in Diabetic Nephropathy. Inflammation 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Chen, Y.; Cheng, Y.; Wang, Y.; Xu, N.; Wang, H.; Wang, L.; Chi, Y.; Ye, X.; et al. Canagliflozin Attenuates Podocyte Inflammatory Injury through Suppressing the TXNIP/NLRP3 Signaling Pathway in Diabetic Kidney Disease Mice. Inflammation 2025, 48, 3180–3193. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wang, M.; Wang, X.; Liu, Q.; Lv, S.; Nie, H.; Liu, G. Epigallocatechin-3-Gallate Ameliorates Diabetic Kidney Disease by Inhibiting the TXNIP/NLRP3/IL-1β Signaling Pathway. Food Sci. Nutr. 2024, 12, 10800–10815. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Cho, N.-J.; Lee, S.W.; Lee, J.G.; Lee, J.-H.; Yi, J.; Choi, M.S.; Park, S.; Gil, H.-W.; Oh, J.C.; et al. RIPK3 Causes Mitochondrial Dysfunction and Albuminuria in Diabetic Podocytopathy through PGAM5-Drp1 Signaling. Metabolism 2024, 159, 155982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, X.; Li, Y.; Niu, S.; Li, J.; Shi, H.; Wang, G.; Wang, L. Targeting Inflammation and Necroptosis in Diabetic Kidney Disease: A Novel Approach via PPARα Modulation. Int. Immunopharmacol. 2025, 154, 114562. [Google Scholar] [CrossRef]
- Yang, H.; Sun, J.; Sun, A.; Wei, Y.; Xie, W.; Xie, P.; Zhang, L.; Zhao, L.; Huang, Y. Podocyte Programmed Cell Death in Diabetic Kidney Disease: Molecular Mechanisms and Therapeutic Prospects. Biomed. Pharmacother. 2024, 177, 117140. [Google Scholar] [CrossRef]
- Tovey Crutchfield, E.C.; Garnish, S.E.; Day, J.; Anderton, H.; Chiou, S.; Hempel, A.; Hall, C.; Patel, K.M.; Gangatirkar, P.; Martin, K.R.; et al. MLKL Deficiency Protects against Low-Grade, Sterile Inflammation in Aged Mice. Cell Death Differ. 2023, 30, 1059–1071. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Y.; Huang, Y.; Zhang, L.; Zhang, C.; Gao, H.; Yan, Q. Inhibition of Tubular Epithelial Cells Ferroptosis Alleviates Renal Interstitial Fibrosis by Reducing Lipid Hydroperoxides and TGF-β/Smad Signaling. Cell Commun. Signal. CCS 2025, 23, 81. [Google Scholar] [CrossRef]
- Liu, M.; Kong, X.-Y.; Yao, Y.; Wang, X.-A.; Yang, W.; Wu, H.; Li, S.; Ding, J.-W.; Yang, J. The Critical Role and Molecular Mechanisms of Ferroptosis in Antioxidant Systems: A Narrative Review. Ann. Transl. Med. 2022, 10, 368. [Google Scholar] [CrossRef]
- Nakamura, T.; Hipp, C.; Santos Dias Mourão, A.; Borggräfe, J.; Aldrovandi, M.; Henkelmann, B.; Wanninger, J.; Mishima, E.; Lytton, E.; Emler, D.; et al. Phase Separation of FSP1 Promotes Ferroptosis. Nature 2023, 619, 371–377. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-Mediated Ferroptosis Defense Is a Targetable Vulnerability in Cancer. Nature 2021, 593, 586–590, Correction in Nature 2021, 596, E13. https://doi.org/10.1038/s41586-021-03820-9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, H.; Yang, Y.; Gong, X. Crosstalk between Ferroptosis and Innate Immune in Diabetic Kidney Disease: Mechanisms and Therapeutic Implications. Front. Immunol. 2025, 16, 1505794. [Google Scholar] [CrossRef]
- Zhu, S.; Kang, Z.; Zhang, F. Tanshinone IIA Suppresses Ferroptosis to Attenuate Renal Podocyte Injury in Diabetic Nephropathy through the Embryonic Lethal Abnormal Visual-like Protein 1 and Acyl-Coenzyme A Synthetase Long-Chain Family Member 4 Signaling Pathway. J. Diabetes Investig. 2024, 15, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.I.; Zhang, X.; Xiao, Y.; Zhuang, Z.; Yang, X.; Li, X. Acupuncture Improve Proteinuria in Diabetic Kidney Disease Rats by Inhibiting Ferroptosis and Epithelial–Mesenchymal Transition. Heliyon 2024, 10, e33675. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yu, X.; Shi, C.; Fang, Y.; Dai, C.; Zhou, Y. ACSL4 Predicts Rapid Kidney Function Decline in Patients with Diabetic Kidney Disease. Front. Endocrinol. 2025, 16, 1499555. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ma, F.; Chen, X.; Lu, X.; Lu, Y.; Jiang, Y. F-Box Only Protein 10 Protects against Kidney Tubulointerstitial Fibrosis by Inhibiting ACSL4-Mediated Lipid Peroxidation and Ferroptosis. Cell. Signal. 2025, 132, 111841. [Google Scholar] [CrossRef]
- Yuan, C.; Chang, F.; Zhou, Q.; Chen, F.; Gao, X.; Yusufu, A.; Chen, J.; Liao, Z.; Wu, X.; Ni, L. S1R Mediates NRF2 Dependent Ferroptosis of Renal Tubular Epithelial Cells to Promote Renal Fibrosis in Diabetic Nephropathy. Int. J. Med. Sci. 2025, 22, 955–970. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.-N.; Yang, L.-T.; Liang, Y.; Guo, F.; Fu, P.; Ma, L. Fisetin Ameliorates Fibrotic Kidney Disease in Mice via Inhibiting ACSL4-Mediated Tubular Ferroptosis. Acta Pharmacol. Sin. 2024, 45, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Upmanyu, K.; Upadhyay, S. Wiring and Rewiring PANoptosis: Molecular Vulnerabilities for Targeting Inflammatory Cell Death in Human Disease. Cytokine Growth Factor Rev. 2025, 86, 1–16. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Qiao, Y.; Xing, Y.; Fang, Y.; Zhao, Y.; Yang, H.; Chen, Y.; Yang, B. Targeting Panoptosis: A Narrative Review of Its Therapeutic Potential in Kidney Disease. BMC Nephrol. 2025, 26, 545. [Google Scholar] [CrossRef]
- Hughes, E.; Wang, X.X.; Sabol, L.; Barton, K.; Hegde, S.; Myakala, K.; Krawczyk, E.; Rosenberg, A.; Levi, M. Role of Nuclear Receptors, Lipid Metabolism, and Mitochondrial Function in the Pathogenesis of Diabetic Kidney Disease. Am. J. Physiol. Renal Physiol. 2025, 329, F510–F547. [Google Scholar] [CrossRef]
- Zheng, F.; Ma, L.; Li, X.; Wang, Z.; Gao, R.; Peng, C.; Kang, B.; Wang, Y.; Luo, T.; Wu, J.; et al. Neutrophil Extracellular Traps Induce Glomerular Endothelial Cell Dysfunction and Pyroptosis in Diabetic Kidney Disease. Diabetes 2022, 71, 2739–2750. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, K.; Fatima, S.; Ambreen, S.; Zimmermann, S.; Younis, R.; Krishnan, S.; Rana, R.; Gadi, I.; Schwab, C.; et al. Neutrophil Extracellular Traps Promote NLRP3 Inflammasome Activation and Glomerular Endothelial Dysfunction in Diabetic Kidney Disease. Nutrients 2022, 14, 2965, Erratum in Nutrients 2023, 15, 2429. https://doi.org/10.3390/nu15112429. [Google Scholar] [CrossRef]
- Ye, Y.; Huang, A.; Huang, X.; Jin, Q.; Gu, H.; Liu, L.; Yu, B.; Zheng, L.; Chen, W.; Guo, Z. IL-33, a Neutrophil Extracellular Trap-Related Gene Involved in the Progression of Diabetic Kidney Disease. Inflamm. Res. 2025, 74, 15. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Liu, Y.; Fu, W.; He, X.; Liu, S.; Xiao, D.; Tao, Y. Mechanisms and Cross-Talk of Regulated Cell Death and Their Epigenetic Modifications in Tumor Progression. Mol. Cancer 2024, 23, 267. [Google Scholar] [CrossRef]
- Schwarzer, R.; Laurien, L.; Pasparakis, M. New Insights into the Regulation of Apoptosis, Necroptosis, and Pyroptosis by Receptor Interacting Protein Kinase 1 and Caspase-8. Curr. Opin. Cell Biol. 2020, 63, 186–193. [Google Scholar] [CrossRef]
- Lei, C.; Chen, K.; Gu, Y.; Li, Y.; Wang, L.; Zhu, X.; Deng, Q. HMGB1/TLR4 Axis Promotes Pyroptosis after ICH by Activating the NLRP3 Inflammasome. J. Neuroimmunol. 2024, 393, 578401. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Pan, J.; Gan, L.; Xue, J. Ferroptosis, Necroptosis, and Pyroptosis in Cancer: Crucial Cell Death Types in Radiotherapy and Post-Radiotherapy Immune Activation. Radiother. Oncol. 2023, 184, 109689. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Sun, Y.; Sun, W.; Kang, X.; Zhao, X.; Jiang, L.; Gao, Q.; An, X.; Ji, H.; Lian, F. Programmed Cell Death in Diabetic Kidney Disease: Mechanisms and Therapeutic Targeting. J. Inflamm. Res. 2025, 18, 13001–13037. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Fu, Y.; Xie, L.; Kong, Y.; Yang, X. Inflammation, Apoptosis, and Fibrosis in Diabetic Nephropathy: Molecular Crosstalk in Proximal Tubular Epithelial Cells and Therapeutic Implications. Curr. Issues Mol. Biol. 2025, 47, 885. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Xiong, H.; Yuan, F. Induction of Pyroptosis in Renal Tubular Epithelial Cells Using High Glucose. Front. Med. 2022, 9, 874916. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, J.; Wu, Y.; Yang, Q.; Zhou, Y.; Yang, J.; Lang, Y.; Cai, L.; Ju, X.; Liu, F. Mechanism of TGR5 in Ferroptosis of the Renal Tubular Epithelial Cells in Diabetes Mellitus and the Effect of Notoginsenoside Ft1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2025, 39, e70686. [Google Scholar] [CrossRef]
- Henedak, N.T.; El-Abhar, H.S.; Soubh, A.A.; Abdallah, D.M. NLRP3 Inflammasome: A Central Player in Renal Pathologies and Nephropathy. Life Sci. 2024, 351, 122813. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Xiao, H.; Kumar, V.; Lan, X.; Malhotra, A.; Singhal, P.C.; Chen, J. The Molecular Mechanism of Renal Tubulointerstitial Inflammation Promoting Diabetic Nephropathy. Int. J. Nephrol. Renov. Dis. 2023, 16, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, Y.; Zhao, Y.; Huang, S.; Xin, X.; Jiang, L.; Wang, H.; Wu, W.; Qu, L.; Xiang, C.; et al. Nephropathy Is Aggravated by Fatty Acids in Diabetic Kidney Disease through Tubular Epithelial Cell Necroptosis and Is Alleviated by an RIPK-1 Inhibitor. Kidney Dis. 2023, 9, 408–423. [Google Scholar] [CrossRef]
- Das, A.K.; Ghosh, S.; Sil, P.C. Determination of Beneficial Effects of Cuminaldehyde on Hyperglycemia Associated Kidney Malfunctions. Naunyn. Schmiedebergs Arch. Pharmacol. 2025, 398, 3049–3065. [Google Scholar] [CrossRef]
- Wang, H.; Yu, X.; Liu, D.; Qiao, Y.; Huo, J.; Pan, S.; Zhou, L.; Wang, R.; Feng, Q.; Liu, Z. VDR Activation Attenuates Renal Tubular Epithelial Cell Ferroptosis by Regulating Nrf2/HO-1 Signaling Pathway in Diabetic Nephropathy. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2024, 11, e2305563. [Google Scholar] [CrossRef]
- Yuan, Q.; Tang, B.; Xie, Y.; Xie, Y.; Zhu, Y.; Su, H.; Liu, Y.; Zhang, C. PRDM16 Deficiency Promotes Podocyte Injury by Impairing Insulin Receptor Signaling. Cell Death Differ. 2025, 32, 1536–1554. [Google Scholar] [CrossRef]
- Ding, S.; Xu, J.-L.; Tong, J.-Y.; Cheng, Y.-Y.; Shi, L.-F.; Wei, W.; Zhang, L.-M.; Zhang, J.-J.; Meng, B.-Y.; Peng, X.-Y.; et al. Brown Adipose Tissue Alleviates Podocyte Apoptosis through NRG4 in a Male Mouse Model of Diabetic Kidney Disease. Diabetologia 2025, 68, 1057–1075. [Google Scholar] [CrossRef]
- Fu, R.; Guo, C.; Wang, S.; Huang, Y.; Jin, O.; Hu, H.; Chen, J.; Xu, B.; Zhou, M.; Zhao, J.; et al. Podocyte Activation of NLRP3 Inflammasomes Contributes to the Development of Proteinuria in Lupus Nephritis. Arthritis Rheumatol. 2017, 69, 1636–1646. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Hu, J.-E.; Zhang, M. Solasonine Alleviates High Glucose-Induced Podocyte Injury through Increasing Nrf2-Medicated Inhibition of NLRP3 Activation. Drug Dev. Res. 2022, 83, 1697–1706. [Google Scholar] [CrossRef]
- Wu, K.; Zha, H.; Wu, T.; Liu, H.; Peng, R.; Lin, Z.; Lv, D.; Liao, X.; Sun, Y.; Zhang, Z. Cytosolic Hmgb1 Accumulation in Mesangial Cells Aggravates Diabetic Kidney Disease Progression via NFκB Signaling Pathway. Cell. Mol. Life Sci. CMLS 2024, 81, 408. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Ara Mimi, A.; Wu, Y.; Zaeem, M.; Abdul Aziz, M.; Aktar Suchi, S.; Alyafeai, E.; Munir, F.; Xiao, J. Pyroptosis in Diabetic Nephropathy. Clin. Chim. Acta 2021, 523, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Casalena, G.; Shi, S.; Yu, L.; Ebefors, K.; Sun, Y.; Zhang, W.; D’Agati, V.; Schlondorff, D.; Haraldsson, B.; et al. Glomerular Endothelial Mitochondrial Dysfunction Is Essential and Characteristic of Diabetic Kidney Disease Susceptibility. Diabetes 2017, 66, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.Y.; Xie, L.X.; Wang, C. Promoting critical care system and capacity building in pulmonary and critical care medicine subspecialties. Zhonghua Yi Xue Za Zhi 2023, 103, 3149–3151. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, D.; Tang, C.; Lu, Y.; Huang, S.; Peng, C.; Hu, X. Pyroptosis in Diabetes and Diabetic Nephropathy. Clin. Chim. Acta 2022, 531, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Prasad, M.; Natarajan, S.R.; Krishnamoorthy, R.; Alshuniaber, M.A.; Gatasheh, M.K.; Veeraraghavan, V.P.; Rajagopal, P.; Palanisamy, C.P. Molecular Mechanisms Underlying the Effects of Beta-Sitosterol on TGF-Β1/Nrf2/SIRT1/P53-Mediated Signaling in the Kidney of a High-Fat Diet and Sucrose-Induced Type-2 Diabetic Rat. Chem. Biol. Interact. 2025, 411, 111443. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Yang, L.; Feng, L.; Zhang, S.; An, J.; Li, J.; Gao, Y.; Pan, Z.; Xu, Y.; et al. Syringaresinol Protects against Diabetic Nephropathy by Inhibiting Pyroptosis via NRF2-Mediated Antioxidant Pathway. Cell Biol. Toxicol. 2023, 39, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Zhao, N.; Chen, Q.; Wang, M. FOXC1 Aggravates the Ischemia—Reperfusion Induced Injury in Renal Tubular Epithelial Cells by Activating NF-κB/NLRP3 Signaling. J. Biochem. Mol. Toxicol. 2025, 39, e70301. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Ren, J.; Wang, Y.; Perwaiz, I.; Su, H.; Li, J.; Qu, P. Pharmacological Inhibition of the NLRP3 Inflammasome Attenuates Kidney Apoptosis, Fibrosis, and Injury in Dahl Salt-Sensitive Rats. Clin. Exp. Nephrol. 2025, 29, 113–122. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Wang, X.; Sun, N.; Gong, Y.-H. Pathway Network of Pyroptosis and Its Potential Inhibitors in Acute Kidney Injury. Pharmacol. Res. 2022, 175, 106033. [Google Scholar] [CrossRef]
- Elsayed, M.S.; Abu-Elsaad, N.M.; Nader, M.A. The NLRP3 Inhibitor Dapansutrile Attenuates Folic Acid Induced Nephrotoxicity via Inhibiting Inflammasome/Caspase-1/IL Axis and Regulating Autophagy/Proliferation. Life Sci. 2021, 285, 119974. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Pan, Y.; Liu, Y.; Zheng, S.; Ding, K.; Mu, K.; Yuan, Y.; Li, Z.; Song, H.; et al. Novel Role for Tranilast in Regulating NLRP3 Ubiquitination, Vascular Inflammation, and Atherosclerosis. J. Am. Heart Assoc. 2020, 9, e015513. [Google Scholar] [CrossRef]
- Xu, X.; Huang, X.; Zhang, L.; Huang, X.; Qin, Z.; Hua, F. Adiponectin Protects Obesity-Related Glomerulopathy by Inhibiting ROS/NF-κB/NLRP3 Inflammation Pathway. BMC Nephrol. 2021, 22, 218. [Google Scholar] [CrossRef]
- Naeem, A.; Prakash, R.; Kumari, N.; Ali Khan, M.; Quaiyoom Khan, A.; Uddin, S.; Verma, S.; Ab Robertson, A.; Boltze, J.; Shadab Raza, S. MCC950 Reduces Autophagy and Improves Cognitive Function by Inhibiting NLRP3-Dependent Neuroinflammation in a Rat Model of Alzheimer’s Disease. Brain. Behav. Immun. 2024, 116, 70–84. [Google Scholar] [CrossRef]
- Oizumi, T.; Mayanagi, T.; Toya, Y.; Sugai, T.; Matsumoto, T.; Sobue, K. NLRP3 Inflammasome Inhibitor OLT1177 Suppresses Onset of Inflammation in Mice with Dextran Sulfate Sodium-Induced Colitis. Dig. Dis. Sci. 2022, 67, 2912–2921. [Google Scholar] [CrossRef]
- Miyajima, A.; Asano, T.; Asano, T.; Yoshimura, I.; Seta, K.; Hayakawa, M. Tranilast Ameliorates Renal Tubular Damage in Unilateral Ureteral Obstruction. J. Urol. 2001, 165, 1714–1718. [Google Scholar] [CrossRef]
- Wang, F.; Liang, Q.; Ma, Y.; Sun, M.; Li, T.; Lin, L.; Sun, Z.; Duan, J. Silica Nanoparticles Induce Pyroptosis and Cardiac Hypertrophy via ROS/NLRP3/Caspase-1 Pathway. Free Radic. Biol. Med. 2022, 182, 171–181. [Google Scholar] [CrossRef]
- Su, L.; Lu, H.; Zhang, D.; Zhu, X.; Li, J.; Zong, Y.; Zhao, Y.; He, Z.; Chen, W.; Du, R. Total Paeony Glycoside Relieves Neuroinflammation to Exert Antidepressant Effect via the Interplay between NLRP3 Inflammasome, Pyroptosis and Autophagy. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 128, 155519. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Peng, Z.; Zhong, S.; Zhou, P.; Huang, H.; Li, J.; He, Z. VX-765 Ameliorates CKD VSMC Calcification by Regulating STAT3 Activation. Eur. J. Pharmacol. 2023, 945, 175610. [Google Scholar] [CrossRef]
- Wen, S.; Deng, F.; Li, L.; Xu, L.; Li, X.; Fan, Q. VX-765 Ameliorates Renal Injury and Fibrosis in Diabetes by Regulating Caspase-1-Mediated Pyroptosis and Inflammation. J. Diabetes Investig. 2022, 13, 22–33. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Xiao, Y.; Liu, J.; Chen, Y.; Ruan, X.; Zhao, X.; Liu, Y.; Shi, Y.; Tian, J.; et al. MLKL Triggers NLRP3 Activation in Sodium Arsenite-Induced Myocardial Necroinflammation. Toxicology 2025, 515, 154132. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Zheng, M.; An, S.; Park, I.G.; Song, L.; Noh, M.; Sung, J.-H. The Involvement of RIPK1 in Alopecia Areata. Int. J. Mol. Sci. 2025, 26, 1565. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Du, C.; Yan, Z.; Shi, Y.; Duan, H.; Ren, Y. Inhibition of Necroptosis Attenuates Kidney Inflammation and Interstitial Fibrosis Induced By Unilateral Ureteral Obstruction. Am. J. Nephrol. 2017, 46, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-H.; Wang, J.-N.; Suo, X.-G.; Ji, M.-L.; He, X.-Y.; Chen, X.; Zhu, S.; He, Y.; Xie, S.-S.; Li, C.; et al. RIPK3 Inhibitor-AZD5423 Alleviates Acute Kidney Injury by Inhibiting Necroptosis and Inflammation. Int. Immunopharmacol. 2022, 112, 109262. [Google Scholar] [CrossRef]
- Pefanis, A.; Bongoni, A.K.; McRae, J.L.; Salvaris, E.J.; Fisicaro, N.; Murphy, J.M.; Ierino, F.L.; Cowan, P.J. Inhibition of RIPK1 or RIPK3 Kinase Activity Post Ischemia—Reperfusion Reduces the Development of Chronic Kidney Injury. Biochem. J. 2025, 482, 73–86. [Google Scholar] [CrossRef]
- Prasad Panda, S.; Kesharwani, A.; Prasanna Mallick, S.; Prasanth, D.; Kumar Pasala, P.; Bharadwaj Tatipamula, V. Viral-Induced Neuronal Necroptosis: Detrimental to Brain Function and Regulation by Necroptosis Inhibitors. Biochem. Pharmacol. 2023, 213, 115591. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, L.; Zhang, B.; Hou, A. Cell Death in Acute Lung Injury: Caspase-Regulated Apoptosis, Pyroptosis, Necroptosis, and PANoptosis. Front. Pharmacol. 2025, 16, 1559659. [Google Scholar] [CrossRef]
- Wang, M.-Z.; Cai, Y.-F.; Fang, Q.-J.; Liu, Y.-L.; Wang, J.; Chen, J.-X.; Fu, Y.; Wan, B.-Y.; Tu, Y.; Wu, W.; et al. Inhibition of Ferroptosis of Renal Tubular Cells with Total Flavones of Abelmoschus Manihot Alleviates Diabetic Tubulopathy. Anat. Rec. 2023, 306, 3199–3213, Erratum in Anat. Rec. 2023, 308, 2275–2277. https://doi.org/10.1002/ar.25617. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Bae, J.Y.; Yoo, S.; Kim, H.W.; Lee, S.A.; Kim, E.T.; Koh, G. 2-Deoxy-d-Ribose Induces Ferroptosis in Renal Tubular Epithelial Cells via Ubiquitin—Proteasome System-Mediated xCT Protein Degradation. Free Radic. Biol. Med. 2023, 208, 384–393. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Ru, F.; Gan, Y.; Li, B.; Xia, W.; Dai, G.; He, Y.; Chen, Z. Liproxstatin-1 Attenuates Unilateral Ureteral Obstruction-Induced Renal Fibrosis by Inhibiting Renal Tubular Epithelial Cells Ferroptosis. Cell Death Dis. 2021, 12, 843. [Google Scholar] [CrossRef]
- Wang, X.; Pan, W.; Zhang, M.; Li, Y.; Wu, M.; Tian, M.; Guo, D. Diabetic Retinopathy from the Perspective of Programmed Cell Death: Focusing on Apoptosis, Pyroptosis, and Necroptosis. Biochem. Biophys. Res. Commun. 2025, 788, 152829. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, Q.; Zhou, Z.; Li, M. Regulated Cell Death in Cancer: Mechanisms, Crosstalk, and Opportunities for Therapy. Cancer Lett. 2025, 635, 218077. [Google Scholar] [CrossRef]
- Ji, Y.; Hua, H.; Jia, Z.; Zhang, A.; Ding, G. Therapy Targeted to the NLRP3 Inflammasome in Chronic Kidney Disease. Kidney Dis. 2024, 10, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Lea, S.T.; Chen, C.-H.; Wei, J.; William, I.; Lopez Del Castillo, I.; Curran, M.A. NLRP3 Inflammasome Activation Expands the Immunosuppressive Myeloid Stroma and Antagonizes the Therapeutic Benefit of STING Activation in Glioblastoma. Cancer Res. Commun. 2025, 5, 960–972. [Google Scholar] [CrossRef] [PubMed]
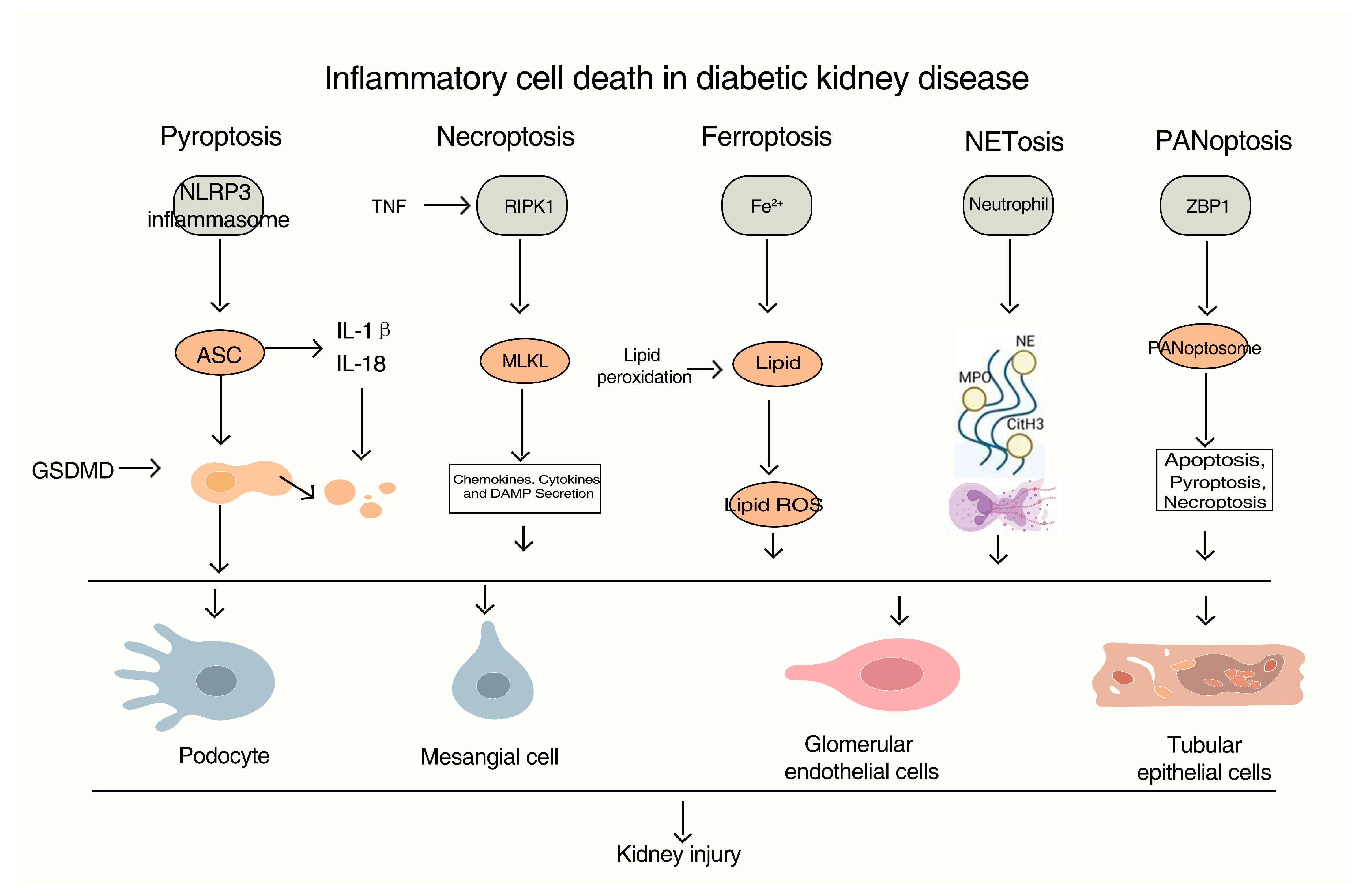
| Cell Death Type | Core Molecular Mediators | Key Effectors/DAMPs Released | Primary Renal Cellular Targets in DKD | Potential Biomarkers |
|---|---|---|---|---|
| Pyroptosis | NLRP3 inflammasome, Caspase-1/4/5/11, GSDMD | IL-1β, IL-18, GSDMD pores | Podocytes, Tubular epithelial cells, Macrophages | Plasma IL-18, Cleaved GSDMD (tissue) |
| Necroptosis | RIPK1, RIPK3, MLKL | HMGB1, ATP | Tubular epithelial cells, Podocytes | Urinary HMGB1, p-MLKL (tissue) |
| Ferroptosis | GPX4, FSP1, System Xc−, ACSL4 | Lipid peroxides (e.g., 4-HNE) | Tubular epithelial cells | Plasma/Sermal lipid peroxides, 4-HNE (tissue) |
| NETosis | PAD4, Neutrophil Elastase, MPO | Citrullinated Histones, NETs (DNA fibers) | Glomerular endothelial cells | Circulating cf-DNA, MPO-DNA complexes |
| PANoptosis | Integrated molecular complex from Pyroptosis, Apoptosis, and Necroptosis | Combination of all above | Podocytes | Multianalyte panels (e.g., IL-18 + HMGB1) |
| Target | Representative Inhibitor/Agent | Mechanism of Action | Key Effects in DKD Models |
|---|---|---|---|
| NLRP3 | MCC950 | Selective NLRP3 inflammasome inhibitor | IL-1β/IL-18; renal fibrosis; proteinuria; preserves podocytes |
| OLT1177 | NLRP3 inflammasome suppressor | Tubular injury; inflammation; attenuates glomerulosclerosis | |
| Tranilast | NLRP3 pathway inhibitor | Oxidative stress; macrophage infiltration; improves renal function | |
| Caspase-1 | VX-765 | Caspase-1 inhibitor | GSDMD cleavage; pyroptosis; IL-1β; reduces podocyte injury |
| GSDMD | Necrosulfonamide | Blocks GSDMD pore formation | Pyroptosis-induced inflammation; protects tubular cells |
| RIPK1 | Necrostatin-1 (Nec-1) | RIPK1 kinase inhibitor | Necroptosis; TNF-α-driven inflammation; fibrosis; podocyte loss |
| RIPK3 | GSK872 | RIPK3 kinase inhibitor | MLKL phosphorylation; necroinflammation; improves renal function |
| MLKL | Necrosulfonamide (NSA) | Covalently modifies MLKL to prevent oligomerization | Blocks necroptosis; reduces renal cell death |
| Ferroptosis (GPX4/ACSL4/SLC7A11) | Ferrostatin-1 (Fer-1) | Radical-trapping antioxidant | Lipid peroxidation; rescues tubular cell death; fibrosis |
| Liproxstatin-1 (Lip-1) | Inhibits lipid peroxidation | Ferroptosis markers; improves glomerular filtration | |
| Multi-target | Solasonine | Modulates Nrf2/NLRP3 axis | Pyroptosis in podocytes; oxidative stress |
| β-Sitosterol | Suppresses NLRP3 activation | Renal inflammation; GSDMD cleavage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, B.; Huang, W.; Du, S.; Hao, Y.; He, F.; Zhang, C. The Inflammatory Cell Death in Diabetic Kidney Disease: Integrating Multifactorial Mechanisms into Novel Therapeutics. Int. J. Mol. Sci. 2025, 26, 11033. https://doi.org/10.3390/ijms262211033
Fang B, Huang W, Du S, Hao Y, He F, Zhang C. The Inflammatory Cell Death in Diabetic Kidney Disease: Integrating Multifactorial Mechanisms into Novel Therapeutics. International Journal of Molecular Sciences. 2025; 26(22):11033. https://doi.org/10.3390/ijms262211033
Chicago/Turabian StyleFang, Bin, Wei Huang, Sijia Du, Yu Hao, Fangfang He, and Chun Zhang. 2025. "The Inflammatory Cell Death in Diabetic Kidney Disease: Integrating Multifactorial Mechanisms into Novel Therapeutics" International Journal of Molecular Sciences 26, no. 22: 11033. https://doi.org/10.3390/ijms262211033
APA StyleFang, B., Huang, W., Du, S., Hao, Y., He, F., & Zhang, C. (2025). The Inflammatory Cell Death in Diabetic Kidney Disease: Integrating Multifactorial Mechanisms into Novel Therapeutics. International Journal of Molecular Sciences, 26(22), 11033. https://doi.org/10.3390/ijms262211033







