Neurodegeneration Through the Lens of Bioinformatics Approaches: Computational Mechanisms of Protein Misfolding
Abstract
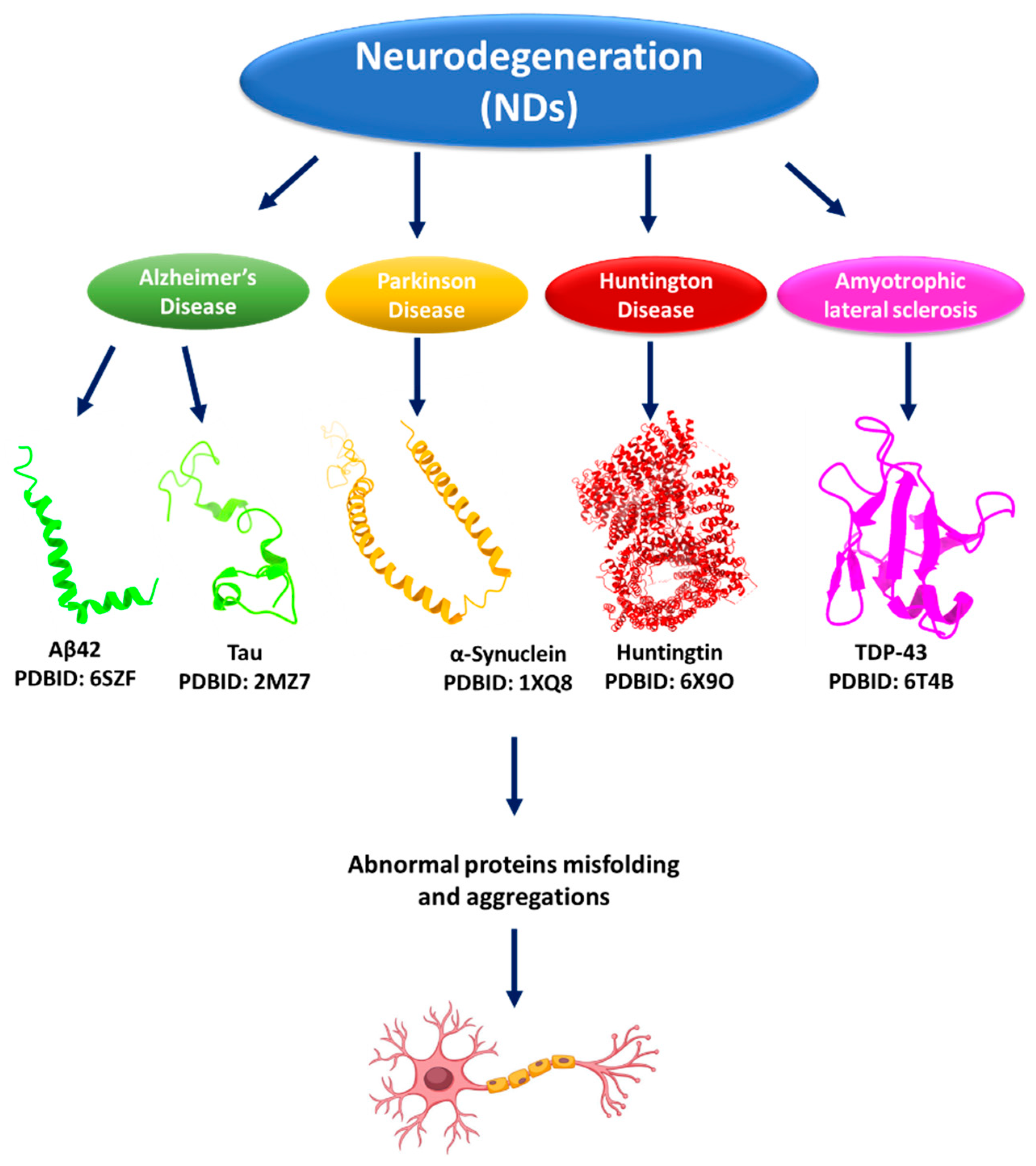
1. Introduction
2. Protein Aggregations and Neurodegenerative Diseases (NDs)
2.1. Protein Aggregation and Alzheimer’s Disease (AD)
2.2. Protein Aggregation and Parkinson’s Disease (PD)
2.3. Protein Aggregation and Huntington’s Disease (HD)
2.4. Protein Aggregation and Amyotrophic Lateral Sclerosis (ALS)
3. Protein Aggregation Resources
| Resources | Classification | Functions | Ref |
|---|---|---|---|
| Fibril_one | Database | Fibril_one database serves as a specialized resource for managing and analyzing data on fibrils, particularly in biological and biochemical research. | [60] |
| ZipperDB | Algorithm (with Database) | ZipperDB employs a novel algorithm that utilizes structural information to predict fibril-forming segments within proteins. | [64] |
| WALTZ-DB 2.0 | Database | WALTZ-DB 2.0 serves as a significant resource for the characterization of short peptides based on their ability to form amyloid fibers | [66] |
| ProADD | Database | The ProADD database focuses on protein aggregation diseases and provides valuable information on the underlying mechanisms of protein aggregation in Alzheimer’s and Parkinson’s diseases. | [67] |
| AmyLoad | Database | AmyLoad is designed for amyloidogenic protein fragments and protein aggregation, with a focus on their significance in Alzheimer’s disease. | [68] |
| AmyPro | Database | AmyPro is an open-access resource specifically designed to collect and analyze proteins with validated amyloidogenic regions. | [71] |
| CPAD 2.0 | Database | CPAD 2.0 focuses on various aspects of protein aggregation, including mechanistic, kinetic, and structural information, which are crucial for understanding protein-related diseases. | [74] |
| AmyloBase | Database | The primary function of the AmyloBase database is to facilitate the organization, retrieval, and analysis of data related to amyloids. | [75] |
| AMYPdb | Database | AMYPdb is a specialized database dedicated to amyloid precursor proteins. | [77] |
| PDB_Amyloid | Database | PDB_Amyloid provides access to a diverse range of amyloid structures, which can be explored for research and educational purposes. | [79] |
| AL-Base | Database | The AL-Base database plays a pivotal role in studying and understanding light chain sequences associated with amyloidosis and related diseases. | [80] |
| A3D | Algorithm (with Database) | A3D is to facilitate the prediction of protein aggregation based on its structural attributes. | [83] |
| CARs-DB | Database | CARs-DB is a pivotal resource for protein chemistry, specifically in understanding the amyloidogenic properties of intrinsically disordered proteins and their links to various diseases. | [85] |
| PASTA 2.0 | Algorithm | PASTA 2.0 serves as a comprehensive tool for researchers studying protein aggregation. Its primary function is to analyze protein sequences and assess their potential for aggregation. | [89] |
4. In-Silico Techniques to Investigate Protein Aggregation
4.1. Protein Sequence and Aggregation
4.2. Protein Aggregation Using Amino Acid Fundamental Characteristics
4.3. Protein Secondary Structure and Aggregation
4.4. Protein Aggregation Based on Amino Acids’ Interactive Profiles
4.5. Structure-Based Techniques
5. Systematic Coarse-Graining Approaches for Protein Aggregation
5.1. Molecular Dynamics Simulations in Protein Aggregation
5.2. Thermodynamic Approaches for Protein Aggregation
5.3. Protein Kinetic Profiles for Aggregation
6. Discussion and Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef]
- Ponomarenko, E.A.; Poverennaya, E.V.; Ilgisonis, E.V.; Pyatnitskiy, M.A.; Kopylov, A.T.; Zgoda, V.G.; Lisitsa, A.V.; Archakov, A.I. The size of the human proteome: The width and depth. Int. J. Anal. Chem. 2016, 2016, 7436849. [Google Scholar] [CrossRef]
- Kundu, D.; Dubey, V.K. Potential alternatives to current cholinesterase inhibitors: An in silico drug repurposing approach. Drug Dev. Ind. Pharm. 2021, 47, 919–930. [Google Scholar] [CrossRef]
- Handa, T.; Kundu, D.; Dubey, V.K. Perspectives on evolutionary and functional importance of intrinsically disordered proteins. Int. J. Biol. Macromol. 2023, 224, 243–255. [Google Scholar] [CrossRef]
- Kundu, D.; Dubey, V.K. Purines and pyrimidines: Metabolism, function and potential as therapeutic options in neurodegenerative diseases. Curr. Protein Pept. Sci. 2021, 22, 170–189. [Google Scholar] [CrossRef]
- Ye, S.; Hsiung, C.-H.; Tang, Y.; Zhang, X. Visualizing the multistep process of protein aggregation in live cells. Acc. Chem. Res. 2022, 55, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.; Yasir, M.; Aftab, B.; Babar, S.; Hassan, M. Exploration of Protein Aggregations in Parkinson’s Disease Through Computational Approaches and Big Data Analytics. In Computer Simulations of Aggregation of Proteins and Peptides; Springer: Berlin/Heidelberg, Germany, 2022; pp. 449–467. [Google Scholar]
- Felice, F.G.D.; Vieira, M.N.; Meirelles, M.N.L.; Morozova-Roche, L.A.; Dobson, C.M.; Ferreira, S.T. Formation of amyloid aggregates from human lysozyme and its disease-associated variants using hydrostatic pressure. FASEB J. 2004, 18, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E.; Bertram, L. Twenty years of the Alzheimer’s disease amyloid hypothesis: A genetic perspective. Cell 2005, 120, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Ramamoorthy, A.; Sahoo, B.R.; Zheng, J.; Faller, P.; Straub, J.E.; Dominguez, L.; Shea, J.-E.; Dokholyan, N.V.; De Simone, A. Amyloid oligomers: A joint experimental/computational perspective on Alzheimer’s disease, Parkinson’s disease, type II diabetes, and amyotrophic lateral sclerosis. Chem. Rev. 2021, 121, 2545–2647. [Google Scholar] [CrossRef]
- Stefani, M. Protein misfolding and aggregation: New examples in medicine and biology of the dark side of the protein world. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2004, 1739, 5–25. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Lombard, J.; Pomara, N. Pathophysiology of Alzheimer’s disease. Neuroimaging Clin. 2005, 15, 727–753. [Google Scholar] [CrossRef]
- Özçelik, R.; van Tilborg, D.; Jiménez-Luna, J.; Grisoni, F. Structure-Based Drug Discovery with Deep Learning. ChemBioChem 2023, 24, e202200776. [Google Scholar] [CrossRef]
- Houben, B.; Rousseau, F.; Schymkowitz, J. Protein structure and aggregation: A marriage of necessity ruled by aggregation gatekeepers. Trends Biochem. Sci. 2022, 47, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, J.; Gao, M.; Zhou, H. How special is the biochemical function of native proteins? F1000Research 2016, 5, F1000 Faculty Rev-207. [Google Scholar] [CrossRef]
- Louros, N.; Schymkowitz, J.; Rousseau, F. Mechanisms and pathology of protein misfolding and aggregation. Nat. Rev. Mol. Cell Biol. 2023, 24, 912–933. [Google Scholar] [CrossRef]
- Trivedi, R.; Nagarajaram, H.A. Intrinsically disordered proteins: An overview. Int. J. Mol. Sci. 2022, 23, 14050. [Google Scholar] [CrossRef] [PubMed]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef]
- Ayyadevara, S.; Ganne, A.; Balasubramaniam, M.; Shmookler Reis, R.J. Intrinsically disordered proteins identified in the aggregate proteome serve as biomarkers of neurodegeneration. Metab. Brain Dis. 2022, 37, 147–152. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically disordered proteins and their (disordered) proteomes in neurodegenerative disorders. Front. Aging Neurosci. 2015, 7, 18. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Randolph, T.W.; Jiskoot, W.; Crommelin, D.J.; Middaugh, C.R.; Winter, G.; Fan, Y.-X.; Kirshner, S.; Verthelyi, D.; Kozlowski, S. Overlooking subvisible particles in therapeutic protein products: Gaps that may compromise product quality. J. Pharm. Sci. 2009, 98, 1201–1205. [Google Scholar] [CrossRef]
- Pham, N.B.; Meng, W.S. Protein aggregation and immunogenicity of biotherapeutics. Int. J. Pharm. 2020, 585, 119523. [Google Scholar] [CrossRef]
- Lundahl, M.L.; Fogli, S.; Colavita, P.E.; Scanlan, E.M. Aggregation of protein therapeutics enhances their immunogenicity: Causes and mitigation strategies. RSC Chem. Biol. 2021, 2, 1004–1020. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, Y.; Liu, S.; Liu, B.; Jin, X.; Cao, X. Targeting Protein Aggregates with Natural Products: An Optional Strategy for Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 11275. [Google Scholar] [CrossRef]
- Wells, C.; Brennan, S.; Keon, M.; Ooi, L. The role of amyloid oligomers in neurodegenerative pathologies. Int. J. Biol. Macromol. 2021, 181, 582–604. [Google Scholar] [CrossRef] [PubMed]
- Berrill, A.; Biddlecombe, J.; Bracewell, D. Product quality during manufacture and supply. In Peptide and Protein Delivery; Elsevier: Amsterdam, The Netherlands, 2011; pp. 313–339. [Google Scholar]
- Kumar, V.; Barwal, A.; Sharma, N.; Mir, D.S.; Kumar, P.; Kumar, V. Therapeutic proteins: Developments, progress, challenges, and future perspectives. 3 Biotech 2024, 14, 112. [Google Scholar] [CrossRef]
- Rahban, M.; Ahmad, F.; Piatyszek, M.A.; Haertlé, T.; Saso, L.; Saboury, A.A. Stabilization challenges and aggregation in protein-based therapeutics in the pharmaceutical industry. RSC Adv. 2023, 13, 35947–35963. [Google Scholar] [CrossRef] [PubMed]
- Kalita, P.; Tripathi, T.; Padhi, A.K. Computational Protein Design for COVID-19 Research and Emerging Therapeutics. ACS Cent. Sci. 2023, 9, 602–613. [Google Scholar] [CrossRef]
- Blanco, M.A. Computational models for studying physical instabilities in high concentration biotherapeutic formulations. mAbs 2022, 14, 2044744. [Google Scholar] [CrossRef] [PubMed]
- Candelise, N.; Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C.; Manganelli, V.; Garofalo, T.; Sorice, M.; Misasi, R. Protein aggregation landscape in neurodegenerative diseases: Clinical relevance and future applications. Int. J. Mol. Sci. 2021, 22, 6016. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Herrup, K.; Daly, T. Finding the falsification threshold of the toxic proteinopathy hypothesis in neurodegeneration. Handb. Clin. Neurol. 2023, 192, 143–154. [Google Scholar]
- Gerasimavicius, L.; Livesey, B.J.; Marsh, J.A. Loss-of-function, gain-of-function and dominant-negative mutations have profoundly different effects on protein structure. Nat. Commun. 2022, 13, 3895. [Google Scholar] [CrossRef]
- Lippi, A.; Krisko, A. Protein aggregation: A detrimental symptom or an adaptation mechanism? J. Neurochem. 2024, 168, 1426–1441. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Orobets, K.S.; Karamyshev, A.L. Amyloid Precursor Protein and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14794. [Google Scholar] [CrossRef]
- Fisher, R.A.; Miners, J.S.; Love, S. Pathological changes within the cerebral vasculature in Alzheimer’s disease: New perspectives. Brain Pathol. 2022, 32, e13061. [Google Scholar] [CrossRef] [PubMed]
- Iliyasu, M.O.; Musa, S.A.; Oladele, S.B.; Iliya, A.I. Amyloid-beta aggregation implicates multiple pathways in Alzheimer’s disease: Understanding the mechanisms. Front. Neurosci. 2023, 17, 1081938. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 206. [Google Scholar] [CrossRef]
- Jiménez, J.S. Macromolecular structures and proteins interacting with the microtubule associated tau protein. Neuroscience 2023, 518, 70–82. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated tau in Alzheimer’s disease and other tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef] [PubMed]
- Tabeshmehr, P.; Eftekharpour, E. Tau; one protein, so many diseases. Biology 2023, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Thirumalai, D.; Palaniappan, B. Role of tau protein in Alzheimer’s disease: The prime pathological player. Int. J. Biol. Macromol. 2020, 163, 1599–1617. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P. Alpha-synuclein aggregation in Parkinson’s disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef]
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein aggregation pathway in Parkinson’s disease: Current status and novel therapeutic approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef]
- Kayed, R.; Dettmer, U.; Lesné, S.E. Soluble endogenous oligomeric α-synuclein species in neurodegenerative diseases: Expression, spreading, and cross-talk. J. Park. Dis. 2020, 10, 791–818. [Google Scholar] [CrossRef]
- Bridi, J.C.; Hirth, F. Mechanisms of α-synuclein induced synaptopathy in Parkinson’s disease. Front. Neurosci. 2018, 12, 338212. [Google Scholar] [CrossRef]
- Prabakaran, R.; Rawat, P.; Kumar, S.; Gromiha, M.M. Deciphering the modulatory role of mutations in protein aggregation through in silico methods. In PROTEIN MUTATIONS: Consequences on Structure, Functions, and Diseases; World Scientific: Singapore, 2025; pp. 3–38. [Google Scholar]
- Molero, A.; Mehler, M.F. Huntington’s disease. In Neuroscience in the 21st Century: From Basic to Clinical; Springer: Berlin/Heidelberg, Germany, 2022; pp. 4293–4322. [Google Scholar]
- Jarosińska, O.D.; Rüdiger, S.G. Molecular strategies to target protein aggregation in Huntington’s disease. Front. Mol. Biosci. 2021, 8, 769184. [Google Scholar] [CrossRef]
- Daldin, M.; Fodale, V.; Cariulo, C.; Azzollini, L.; Verani, M.; Martufi, P.; Spiezia, M.C.; Deguire, S.M.; Cherubini, M.; Macdonald, D. Polyglutamine expansion affects huntingtin conformation in multiple Huntington’s disease models. Sci. Rep. 2017, 7, 5070. [Google Scholar] [CrossRef]
- Rummens, J.; Khalil, B.; Yıldırım, G.; Silva, P.; Zorzini, V.; Peredo, N.; Wojno, M.; Ramakers, M.; Van Den Bosch, L.; Van Damme, P. TDP-43 seeding induces cytoplasmic aggregation heterogeneity and nuclear loss of function of TDP-43. Neuron 2025, 113, 1597–1613.e8. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.M.; Shaban, M.; Mahmood, F.; Ganguly, P.; Todeschini, L.; Van Vactor, D.; Artavanis-Tsakonas, S. cAMP/PKA signaling regulates TDP-43 aggregation and mislocalization. Proc. Natl. Acad. Sci. USA 2024, 121, e2400732121. [Google Scholar] [CrossRef]
- Oiwa, K.; Watanabe, S.; Onodera, K.; Iguchi, Y.; Kinoshita, Y.; Komine, O.; Sobue, A.; Okada, Y.; Katsuno, M.; Yamanaka, K. Monomerization of TDP-43 is a key determinant for inducing TDP-43 pathology in amyotrophic lateral sclerosis. Sci. Adv. 2023, 9, eadf6895. [Google Scholar] [CrossRef]
- Tsekrekou, M.; Giannakou, M.; Papanikolopoulou, K.; Skretas, G. Protein aggregation and therapeutic strategies in SOD1-and TDP-43-linked ALS. Front. Mol. Biosci. 2024, 11, 1383453. [Google Scholar] [CrossRef]
- Revesz, P. Introduction to Databases; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Hassan, M.; Shahzadi, S.; Li, M.S.; Kloczkowski, A. Prediction and Evaluation of Protein Aggregation with Computational Methods. In Prediction of Protein Secondary Structure; Springer: Berlin/Heidelberg, Germany, 2024; pp. 299–314. [Google Scholar]
- Siepen, J.A.; Westhead, D.R. The fibril_one online database: Mutations, experimental conditions, and trends associated with amyloid fibril formation. Protein Sci. 2002, 11, 1862–1866. [Google Scholar] [CrossRef]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Rapp, B.A.; Wheeler, D.L. GenBank. Nucleic Acids Res. 2000, 28, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000, 28, 45–48. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Sievers, S.A.; Karanicolas, J.; Ivanova, M.I.; Baker, D.; Eisenberg, D. The 3D profile method for identifying fibril-forming segments of proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 4074–4078. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompson, M.J.; Balbirnie, M.; Wiltzius, J.J.; McFarlane, H.T. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef]
- Louros, N.; Konstantoulea, K.; De Vleeschouwer, M.; Ramakers, M.; Schymkowitz, J.; Rousseau, F. WALTZ-DB 2.0: An updated database containing structural information of experimentally determined amyloid-forming peptides. Nucleic Acids Res. 2020, 48, D389–D393. [Google Scholar] [CrossRef]
- Shobana, R.; Pandaranayaka, E.P. ProADD: A database on protein aggregation diseases. Bioinformation 2014, 10, 390. [Google Scholar] [CrossRef]
- Wozniak, P.P.; Kotulska, M. AmyLoad: Website dedicated to amyloidogenic protein fragments. Bioinformatics 2015, 31, 3395–3397. [Google Scholar] [CrossRef]
- Rizzo, G.; Whittington, A.; Hesterman, J.; Gunn, R.N. AmyloidIQ: An advanced analytical algorithm to quantify amyloid-PET [18F] NAV4694 scans: Neuroimaging/New imaging methods. Alzheimer’s Dement. 2020, 16, e043823. [Google Scholar] [CrossRef]
- Giorgetti, S.; Greco, C.; Tortora, P.; Aprile, F.A. Targeting amyloid aggregation: An overview of strategies and mechanisms. Int. J. Mol. Sci. 2018, 19, 2677. [Google Scholar] [CrossRef]
- Varadi, M.; De Baets, G.; Vranken, W.F.; Tompa, P.; Pancsa, R. AmyPro: A database of proteins with validated amyloidogenic regions. Nucleic Acids Res. 2018, 46, D387–D392. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Biswas, A.; Radhakrishna, M. Advanced computational approaches to understand protein aggregation. Biophys. Rev. 2024, 5, 021302. [Google Scholar] [CrossRef]
- Thangakani, A.M.; Nagarajan, R.; Kumar, S.; Sakthivel, R.; Velmurugan, D.; Gromiha, M.M. CPAD, curated protein aggregation database: A repository of manually curated experimental data on protein and peptide aggregation. PLoS ONE 2016, 11, e0152949. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Prabakaran, R.; Sakthivel, R.; Mary Thangakani, A.; Kumar, S.; Gromiha, M.M. CPAD 2.0: A repository of curated experimental data on aggregating proteins and peptides. Amyloid 2020, 27, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Ramazzotti, M.; Chiti, F. Prediction of amyloid aggregation in vivo. EMBO Rep. 2011, 12, 657–663. [Google Scholar] [CrossRef]
- Morgan, G.J.; Nau, A.N.; Wong, S.; Spencer, B.H.; Shen, Y.; Hua, A.; Bullard, M.J.; Sanchorawala, V.; Prokaeva, T. An updated AL-Base reveals ranked enrichment of immunoglobulin light chain variable genes in AL amyloidosis. Amyloid 2024, 32, 129–138. [Google Scholar] [CrossRef]
- Pawlicki, S.; Le Béchec, A.; Delamarche, C. AMYPdb: A database dedicated to amyloid precursor proteins. BMC Bioinform. 2008, 9, 273. [Google Scholar] [CrossRef]
- Zibaee, S.; Makin, O.S.; Goedert, M.; Serpell, L.C. A simple algorithm locates β-strands in the amyloid fibril core of α-synuclein, Aβ, and tau using the amino acid sequence alone. Protein Sci. 2007, 16, 906–918. [Google Scholar] [CrossRef]
- Takács, K.; Varga, B.; Grolmusz, V. PDB_Amyloid: An extended live amyloid structure list from the PDB. FEBS Open Bio 2019, 9, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Bodi, K.; Prokaeva, T.; Spencer, B.; Eberhard, M.; Connors, L.H.; Seldin, D.C. AL-Base: A visual platform analysis tool for the study of amyloidogenic immunoglobulin light chain sequences. Amyloid 2009, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuriata, A.; Iglesias, V.; Pujols, J.; Kurcinski, M.; Kmiecik, S.; Ventura, S. Aggrescan3D (A3D) 2.0: Prediction and engineering of protein solubility. Nucleic Acids Res. 2019, 47, W300–W307. [Google Scholar] [CrossRef]
- Pujols, J.; Peña-Díaz, S.; Ventura, S. AGGRESCAN3D: Toward the prediction of the aggregation propensities of protein structures. Comput. Drug Discov. Des. 2018, 1762, 427–443. [Google Scholar]
- Badaczewska-Dawid, A.E.; Garcia-Pardo, J.; Kuriata, A.; Pujols, J.; Ventura, S.; Kmiecik, S. A3D database: Structure-based predictions of protein aggregation for the human proteome. Bioinformatics 2022, 38, 3121–3123. [Google Scholar] [CrossRef]
- Badaczewska-Dawid, A.E.; Kuriata, A.; Pintado-Grima, C.; Garcia-Pardo, J.; Burdukiewicz, M.; Iglesias, V.; Kmiecik, S.; Ventura, S. A3D model organism database (A3D-MODB): A database for proteome aggregation predictions in model organisms. Nucleic Acids Res. 2024, 52, D360–D367. [Google Scholar] [CrossRef]
- Pintado-Grima, C.; Bárcenas, O.; Manglano-Artuñedo, Z.; Vilaça, R.; Macedo-Ribeiro, S.; Pallares, I.; Santos, J.; Ventura, S. CARs-DB: A database of cryptic amyloidogenic regions in intrinsically disordered proteins. Front. Mol. Biosci. 2022, 9, 882160. [Google Scholar] [CrossRef]
- Aspromonte, M.C.; Nugnes, M.V.; Quaglia, F.; Bouharoua, A.; Tosatto, S.C.; Piovesan, D. DisProt in 2024: Improving function annotation of intrinsically disordered proteins. Nucleic Acids Res. 2024, 52, D434–D441, Correction in Nucleic Acids Res. 2025, 53, gkaf228. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, F.; Mészáros, B.; Salladini, E.; Hatos, A.; Pancsa, R.; Chemes, L.B.; Pajkos, M.; Lazar, T.; Peña-Díaz, S.; Santos, J. DisProt in 2022: Improved quality and accessibility of protein intrinsic disorder annotation. Nucleic Acids Res. 2022, 50, D480–D487. [Google Scholar] [CrossRef]
- Trovato, A.; Seno, F.; Tosatto, S.C. The PASTA server for protein aggregation prediction. Protein Eng. Des. Sel. 2007, 20, 521–523. [Google Scholar] [CrossRef]
- Walsh, I.; Seno, F.; Tosatto, S.C.; Trovato, A. PASTA 2.0: An improved server for protein aggregation prediction. Nucleic Acids Res. 2014, 42, W301–W307. [Google Scholar] [CrossRef]
- Prabakaran, R.; Rawat, P.; Thangakani, A.M.; Kumar, S.; Gromiha, M.M. Protein aggregation: In silico algorithms and applications. Biophys. Rev. 2021, 13, 71–89. [Google Scholar] [CrossRef]
- Housmans, J.A.; Wu, G.; Schymkowitz, J.; Rousseau, F. A guide to studying protein aggregation. FEBS J. 2023, 290, 554–583. [Google Scholar] [CrossRef]
- Ventura, S. Sequence determinants of protein aggregation: Tools to increase protein solubility. Microb. Cell Factories 2005, 4, 11. [Google Scholar] [CrossRef]
- Gsponer, J.; Vendruscolo, M. Theoretical approaches to protein aggregation. Protein Pept. Lett. 2006, 13, 287–293. [Google Scholar] [CrossRef] [PubMed]
- López de la Paz, M.; Serrano, L. Sequence determinants of amyloid fibril formation. Proc. Natl. Acad. Sci. USA 2004, 101, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Pujols, J.; Pallarès, I.; Iglesias, V.; Ventura, S. Computational prediction of protein aggregation: Advances in proteomics, conformation-specific algorithms and biotechnological applications. Comput. Struct. Biotechnol. J. 2020, 18, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Pallarès, I.; Ventura, S. Understanding and predicting protein misfolding and aggregation: Insights from proteomics. Proteomics 2016, 16, 2570–2581. [Google Scholar] [CrossRef]
- Pallarès, I.; Ventura, S. Advances in the prediction of protein aggregation propensity. Curr. Med. Chem. 2019, 26, 3911–3920. [Google Scholar] [CrossRef]
- Conchillo-Solé, O.; de Groot, N.S.; Avilés, F.X.; Vendrell, J.; Daura, X.; Ventura, S. AGGRESCAN: A server for the prediction and evaluation of “hot spots” of aggregation in polypeptides. BMC Bioinform. 2007, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Dovidchenko, N.V.; Galzitskaya, O.V. Computational approaches to identification of aggregation sites and the mechanism of amyloid growth. Lipids Protein Misfolding 2015, 855, 213–239. [Google Scholar]
- Bhattacharya, D.; Kleeblatt, D.C.; Statt, A.; Reinhart, W.F. Predicting aggregate morphology of sequence-defined macromolecules with recurrent neural networks. Soft Matter 2022, 18, 5037–5051. [Google Scholar] [CrossRef]
- Família, C.; Dennison, S.R.; Quintas, A.; Phoenix, D.A. Prediction of peptide and protein propensity for amyloid formation. PLoS ONE 2015, 10, e0134679. [Google Scholar] [CrossRef]
- Kim, C.; Choi, J.; Lee, S.J.; Welsh, W.J.; Yoon, S. NetCSSP: Web application for predicting chameleon sequences and amyloid fibril formation. Nucleic Acids Res. 2009, 37, W469–W473. [Google Scholar] [CrossRef]
- Tsolis, A.C.; Papandreou, N.C.; Iconomidou, V.A.; Hamodrakas, S.J. A consensus method for the prediction of ‘aggregation-prone’peptides in globular proteins. PLoS ONE 2013, 8, e54175. [Google Scholar] [CrossRef] [PubMed]
- Emily, M.; Talvas, A.; Delamarche, C. MetAmyl: A METa-predictor for AMYLoid proteins. PLoS ONE 2013, 8, e79722. [Google Scholar] [CrossRef]
- Biro, J. Amino acid size, charge, hydropathy indices and matrices for protein structure analysis. Theor. Biol. Med. Model. 2006, 3, 15. [Google Scholar] [CrossRef]
- Qing, R.; Hao, S.; Smorodina, E.; Jin, D.; Zalevsky, A.; Zhang, S. Protein design: From the aspect of water solubility and stability. Chem. Rev. 2022, 122, 14085–14179. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.G.; Vendruscolo, M. The Zyggregator method for predicting protein aggregation propensities. Chem. Soc. Rev. 2008, 37, 1395–1401. [Google Scholar] [CrossRef]
- Oliveberg, M. Waltz, an exciting new move in amyloid prediction. Nat. Methods 2010, 7, 187–188. [Google Scholar] [CrossRef]
- Prabakaran, R.; Rawat, P.; Kumar, S.; Gromiha, M.M. ANuPP: A versatile tool to predict aggregation nucleating regions in peptides and proteins. J. Mol. Biol. 2021, 433, 166707. [Google Scholar] [CrossRef]
- Rudnev, V.R.; Kulikova, L.I.; Nikolsky, K.S.; Malsagova, K.A.; Kopylov, A.T.; Kaysheva, A.L. Current approaches in supersecondary structures investigation. Int. J. Mol. Sci. 2021, 22, 11879. [Google Scholar] [CrossRef]
- Ono, K.; Watanabe-Nakayama, T. Aggregation and structure of amyloid β-protein. Neurochem. Int. 2021, 151, 105208. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Rehman, M.; Agarwal, V.; Kaushik, A.S.; Mishra, V. Protein Aggregation in Neurodegenerative Diseases. In Neurodegenerative Diseases: Multifactorial Degenerative Processes, Biomarkers and Therapeutic Approaches; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 26–58. [Google Scholar]
- Fernandez-Escamilla, A.-M.; Rousseau, F.; Schymkowitz, J.; Serrano, L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 2004, 22, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Hamodrakas, S.J. A protein secondary structure prediction scheme for the IBM PC and compatibles. Bioinformatics 1988, 4, 473–477. [Google Scholar] [CrossRef]
- Thu, T.T.M.; Co, N.T.; Tu, L.A.; Li, M.S. Aggregation rate of amyloid beta peptide is controlled by beta-content in monomeric state. J. Chem. Phys. 2019, 150, 225101. [Google Scholar] [CrossRef] [PubMed]
- Thangakani, A.M.; Kumar, S.; Nagarajan, R.; Velmurugan, D.; Gromiha, M.M. GAP: Towards almost 100 percent prediction for β-strand-mediated aggregating peptides with distinct morphologies. Bioinformatics 2014, 30, 1983–1990. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, S.; Dong, H.; Liu, Y.; Liu, C.; Zhang, X. Advanced techniques for detecting protein misfolding and aggregation in cellular environments. Chem. Rev. 2023, 123, 12254–12311. [Google Scholar] [CrossRef]
- Iglesias Mas, V. Bioinformatic Analysis on the Determinants of Protein Aggregation and Conformational Conversion. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2021. [Google Scholar]
- Waibl, F.; Fernández-Quintero, M.L.; Wedl, F.S.; Kettenberger, H.; Georges, G.; Liedl, K.R. Comparison of hydrophobicity scales for predicting biophysical properties of antibodies. Front. Mol. Biosci. 2022, 9, 960194. [Google Scholar] [CrossRef]
- Montesinos Estrada, J. Optimització Computacional de Proteïnes Dissenyades de Novo. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2022. [Google Scholar]
- Zalewski, M.; Iglesias, V.; Bárcenas, O.; Ventura, S.; Kmiecik, S. Aggrescan4D: A comprehensive tool for pH-dependent analysis and engineering of protein aggregation propensity. Protein Sci. 2024, 33, e5180. [Google Scholar] [CrossRef] [PubMed]
- Bárcenas, O.; Kuriata, A.; Zalewski, M.; Iglesias, V.; Pintado-Grima, C.; Firlik, G.; Burdukiewicz, M.; Kmiecik, S.; Ventura, S. Aggrescan4D: Structure-informed analysis of pH-dependent protein aggregation. Nucleic Acids Res. 2024, 52, W170–W175. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.; Krystek, S.R., Jr.; Carl, S.M.; Day, T.; Maier, J.K. AggScore: Prediction of aggregation-prone regions in proteins based on the distribution of surface patches. Proteins Struct. Funct. Bioinform. 2018, 86, 1147–1156. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y. A comprehensive assessment of sequence-based and template-based methods for protein contact prediction. Bioinformatics 2008, 24, 924–931. [Google Scholar] [CrossRef]
- Wang, W.; Shuai, Y.; Zeng, M.; Fan, W.; Li, M. DPFunc: Accurately predicting protein function via deep learning with domain-guided structure information. Nat. Commun. 2025, 16, 70. [Google Scholar] [CrossRef]
- Zheng, L.; Shi, S.; Lu, M.; Fang, P.; Pan, Z.; Zhang, H.; Zhou, Z.; Zhang, H.; Mou, M.; Huang, S. AnnoPRO: A strategy for protein function annotation based on multi-scale protein representation and a hybrid deep learning of dual-path encoding. Genome Biol. 2024, 25, 41. [Google Scholar] [CrossRef]
- Sormanni, P.; Aprile, F.A.; Vendruscolo, M. The CamSol method of rational design of protein mutants with enhanced solubility. J. Mol. Biol. 2015, 427, 478–490. [Google Scholar] [CrossRef]
- Camilloni, C.; Sala, B.M.; Sormanni, P.; Porcari, R.; Corazza, A.; De Rosa, M.; Zanini, S.; Barbiroli, A.; Esposito, G.; Bolognesi, M. Rational design of mutations that change the aggregation rate of a protein while maintaining its native structure and stability. Sci. Rep. 2016, 6, 25559. [Google Scholar] [CrossRef]
- Oeller, M.; Kang, R.J.; Bolt, H.L.; Gomes dos Santos, A.L.; Weinmann, A.L.; Nikitidis, A.; Zlatoidsky, P.; Su, W.; Czechtizky, W.; De Maria, L. Sequence-based prediction of the intrinsic solubility of peptides containing non-natural amino acids. Nat. Commun. 2023, 14, 7475. [Google Scholar] [CrossRef] [PubMed]
- Pintado, C.; Santos, J.; Iglesias, V.; Ventura, S. SolupHred: A server to predict the pH-dependent aggregation of intrinsically disordered proteins. Bioinformatics 2021, 37, 1602–1603. [Google Scholar] [CrossRef]
- Gokcan, H.; Isayev, O. Prediction of Protein p K a with Representation Learning. Chem. Sci. 2022, 13, 2462–2474. [Google Scholar] [CrossRef]
- Pintado-Grima, C.; Bárcenas, O.; Bartolomé-Nafría, A.; Fornt-Suñé, M.; Iglesias, V.; Garcia-Pardo, J.; Ventura, S. A review of fifteen years developing computational tools to study protein aggregation. Biophysica 2023, 3, 1–20. [Google Scholar] [CrossRef]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33 (Suppl. S2), W382–W388. [Google Scholar] [CrossRef]
- Kuriata, A.; Gierut, A.M.; Oleniecki, T.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. CABS-flex 2.0: A web server for fast simulations of flexibility of protein structures. Nucleic Acids Res. 2018, 46, W338–W343. [Google Scholar] [CrossRef]
- Koszła, O.; Sołek, P. Misfolding and aggregation in neurodegenerative diseases: Protein quality control machinery as potential therapeutic clearance pathways. Cell Commun. Signal. 2024, 22, 421. [Google Scholar] [CrossRef]
- Bondarev, S.A.; Uspenskaya, M.V.; Leclercq, J.; Falgarone, T.; Zhouravleva, G.A.; Kajava, A.V. AmyloComp: A bioinformatic tool for prediction of amyloid co-aggregation. J. Mol. Biol. 2024, 436, 168437. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, J.; Kim, J.; Kang, S.; Park, E.; Seo, S.-w.; Min, K. Enhancing protein aggregation prediction: A unified analysis leveraging graph convolutional networks and active learning. RSC Adv. 2024, 14, 31439–31450. [Google Scholar] [CrossRef] [PubMed]
- Eisele, Y.S.; Monteiro, C.; Fearns, C.; Encalada, S.E.; Wiseman, R.L.; Powers, E.T.; Kelly, J.W. Targeting protein aggregation for the treatment of degenerative diseases. Nat. Rev. Drug Discov. 2015, 14, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Abbasbeigi, S. A Brief Look at the Enigma of Protein Aggregation: Unraveling Mechanisms, Exploring Implications, and Proposing Therapeutic Strategies. Int. J. Med. Rev. 2024, 11, 704–709. [Google Scholar]
- Kotulska, M.; Unold, O. On the amyloid datasets used for training PAFIG how (not) to extend the experimental dataset of hexapeptides. BMC Bioinform. 2013, 14, 351. [Google Scholar] [CrossRef]
- Tartaglia, G.G.; Cavalli, A.; Pellarin, R.; Caflisch, A. The role of aromaticity, exposed surface, and dipole moment in determining protein aggregation rates. Protein Sci. 2004, 13, 1939–1941. [Google Scholar] [CrossRef]
- Tartaglia, G.G.; Cavalli, A.; Pellarin, R.; Caflisch, A. Prediction of aggregation rate and aggregation-prone segments in polypeptide sequences. Protein Sci. 2005, 14, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.; Tung, C.-W.; Ho, S.-Y. Prediction and analysis of antibody amyloidogenesis from sequences. PLoS ONE 2013, 8, e53235. [Google Scholar] [CrossRef] [PubMed]
- Garbuzynskiy, S.O.; Lobanov, M.Y.; Galzitskaya, O.V. FoldAmyloid: A method of prediction of amyloidogenic regions from protein sequence. Bioinformatics 2010, 26, 326–332. [Google Scholar] [CrossRef]
- Burdukiewicz, M.; Sobczyk, P.; Rödiger, S.; Duda-Madej, A.; Mackiewicz, P.; Kotulska, M. Amyloidogenic motifs revealed by n-gram analysis. Sci. Rep. 2017, 7, 12961. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.B.; Znassi, N.; Château, M.-T.; Kajava, A.V. A structure-based approach to predict predisposition to amyloidosis. Alzheimer’s Dement. 2015, 11, 681–690. [Google Scholar] [CrossRef]
- Bondarev, S.A.; Bondareva, O.V.; Zhouravleva, G.A.; Kajava, A.V. BetaSerpentine: A bioinformatics tool for reconstruction of amyloid structures. Bioinformatics 2018, 34, 599–608. [Google Scholar] [CrossRef]
- Bryan Jr, A.W.; Menke, M.; Cowen, L.J.; Lindquist, S.L.; Berger, B. BETASCAN: Probable β-amyloids identified by pairwise probabilistic analysis. PLoS Comput. Biol. 2009, 5, e1000333. [Google Scholar] [CrossRef]
- O’Donnell, C.W.; Waldispühl, J.; Lis, M.; Halfmann, R.; Devadas, S.; Lindquist, S.; Berger, B. A method for probing the mutational landscape of amyloid structure. Bioinformatics 2011, 27, i34–i42. [Google Scholar] [CrossRef]
- Bryan Jr, A.W.; O’Donnell, C.W.; Menke, M.; Cowen, L.J.; Lindquist, S.; Berger, B. STITCHER: Dynamic assembly of likely amyloid and prion β-structures from secondary structure predictions. Proteins Struct. Funct. Bioinform. 2012, 80, 410–420. [Google Scholar] [CrossRef]
- Gasior, P.; Kotulska, M. FISH Amyloid–A new method for finding amyloidogenic segments in proteins based on site specific co-occurence of aminoacids. BMC Bioinform. 2014, 15, 54. [Google Scholar] [CrossRef]
- Orlando, G.; Silva, A.; Macedo-Ribeiro, S.; Raimondi, D.; Vranken, W. Accurate prediction of protein beta-aggregation with generalized statistical potentials. Bioinformatics 2020, 36, 2076–2081. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Lai, L. Identification of amyloid fibril-forming segments based on structure and residue-based statistical potential. Bioinformatics 2007, 23, 2218–2225. [Google Scholar] [CrossRef]
- Louros, N.; Orlando, G.; De Vleeschouwer, M.; Rousseau, F.; Schymkowitz, J. Structure-based machine-guided mapping of amyloid sequence space reveals uncharted sequence clusters with higher solubilities. Nat. Commun. 2020, 11, 3314. [Google Scholar] [CrossRef]
- Wojciechowski, J.W.; Kotulska, M. Path-prediction of amyloidogenicity by threading and machine learning. Sci. Rep. 2020, 10, 7721. [Google Scholar] [CrossRef] [PubMed]
- Lauer, T.M.; Agrawal, N.J.; Chennamsetty, N.; Egodage, K.; Helk, B.; Trout, B.L. Developability index: A rapid in silico tool for the screening of antibody aggregation propensity. J. Pharm. Sci. 2012, 101, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Klimov, D.; Straub, J.; Thirumalai, D. Probing the mechanisms of fibril formation using lattice models. J. Chem. Phys. 2008, 129, 175101. [Google Scholar] [CrossRef] [PubMed]
- Vácha, R.; Frenkel, D. Relation between molecular shape and the morphology of self-assembling aggregates: A simulation study. Biophys. J. 2011, 101, 1432–1439. [Google Scholar] [CrossRef]
- Li, M.S.; Reddy, G.; Hu, C.-K.; Straub, J.; Thirumalai, D. Factors governing fibrillogenesis of polypeptide chains revealed by lattice models. Phys. Rev. Lett. 2010, 105, 218101. [Google Scholar] [CrossRef]
- Šarić, A.; Chebaro, Y.C.; Knowles, T.P.; Frenkel, D. Crucial role of nonspecific interactions in amyloid nucleation. Proc. Natl. Acad. Sci. USA 2014, 111, 17869–17874. [Google Scholar] [CrossRef]
- Shell, M.S. The relative entropy is fundamental to multiscale and inverse thermodynamic problems. J. Chem. Phys. 2008, 129, 144108. [Google Scholar] [CrossRef]
- Izvekov, S.; Voth, G.A. A multiscale coarse-graining method for biomolecular systems. J. Phys. Chem. B 2005, 109, 2469–2473. [Google Scholar] [CrossRef]
- Reith, D.; Pütz, M.; Müller-Plathe, F. Deriving effective mesoscale potentials from atomistic simulations. J. Comput. Chem. 2003, 24, 1624–1636. [Google Scholar] [CrossRef]
- Bezkorovaynaya, O.; Lukyanov, A.; Kremer, K.; Peter, C. Multiscale simulation of small peptides: Consistent conformational sampling in atomistic and coarse-grained models. J. Comput. Chem. 2012, 33, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Voth, G.A. Molecular dynamics simulations of polyglutamine aggregation using solvent-free multiscale coarse-grained models. J. Phys. Chem. B 2010, 114, 8735–8743. [Google Scholar] [CrossRef]
- Simunovic, M.; Mim, C.; Marlovits, T.C.; Resch, G.; Unger, V.M.; Voth, G.A. Protein-mediated transformation of lipid vesicles into tubular networks. Biophys. J. 2013, 105, 711–719. [Google Scholar] [CrossRef]
- Larini, L.; Shea, J.-E. Coarse-grained modeling of simple molecules at different resolutions in the absence of good sampling. J. Phys. Chem. B 2012, 116, 8337–8349. [Google Scholar] [CrossRef] [PubMed]
- Davtyan, A.; Schafer, N.P.; Zheng, W.; Clementi, C.; Wolynes, P.G.; Papoian, G.A. AWSEM-MD: Protein structure prediction using coarse-grained physical potentials and bioinformatically based local structure biasing. J. Phys. Chem. B 2012, 116, 8494–8503. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wolynes, P.G.; Papoian, G.A. AWSEM-IDP: A coarse-grained force field for intrinsically disordered proteins. J. Phys. Chem. B 2018, 122, 11115–11125. [Google Scholar] [CrossRef]
- Bereau, T.; Deserno, M. Generic coarse-grained model for protein folding and aggregation. Biophys. J. 2009, 96, 405a. [Google Scholar] [CrossRef]
- Sterpone, F.; Derreumaux, P.; Melchionna, S. Protein simulations in fluids: Coupling the OPEP coarse-grained force field with hydrodynamics. J. Chem. Theory Comput. 2015, 11, 1843–1853. [Google Scholar] [CrossRef]
- Kmiecik, S.; Gront, D.; Kolinski, M.; Wieteska, L.; Dawid, A.E.; Kolinski, A. Coarse-grained protein models and their applications. Chem. Rev. 2016, 116, 7898–7936. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Tam, B.; Wang, S.M. Applications of molecular dynamics simulation in protein study. Membranes 2022, 12, 844. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.L.; Drechsel, N.J.D.; Alcántara, R.; Villa-Freixa, J. Multiscale molecular dynamics of protein aggregation. Curr. Protein Pept. Sci. 2011, 12, 221–234. [Google Scholar] [CrossRef]
- Samantray, S.; Schumann, W.; Illig, A.-M.; Carballo-Pacheco, M.; Paul, A.; Barz, B.; Strodel, B. Molecular dynamics simulations of protein aggregation: Protocols for simulation setup and analysis with Markov state models and transition networks. In Computer Simulations of Aggregation of Proteins and Peptides; Springer: Berlin/Heidelberg, Germany, 2022; pp. 235–279. [Google Scholar]
- Euston, S.R. Molecular dynamics simulation of protein adsorption at fluid interfaces: A comparison of all-atom and coarse-grained models. Biomacromolecules 2010, 11, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Pacheco, M.; Strodel, B. Advances in the simulation of protein aggregation at the atomistic scale. J. Phys. Chem. B 2016, 120, 2991–2999. [Google Scholar] [CrossRef]
- Dror, R.O.; Dirks, R.M.; Grossman, J.; Xu, H.; Shaw, D.E. Biomolecular simulation: A computational microscope for molecular biology. Annu. Rev. Biophys. 2012, 41, 429–452. [Google Scholar] [CrossRef]
- Dignon, G.L.; Zheng, W.; Kim, Y.C.; Best, R.B.; Mittal, J. Sequence determinants of protein phase behavior from a coarse-grained model. PLoS Comput. Biol. 2018, 14, e1005941. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, R.; Souza, P.C.; Thallmair, S.; Melo, M.N.; De Vries, A.H.; Marrink, S.J. Pitfalls of the Martini model. J. Chem. Theory Comput. 2019, 15, 5448–5460. [Google Scholar] [CrossRef]
- Majewski, M.; Pérez, A.; Thölke, P.; Doerr, S.; Charron, N.E.; Giorgino, T.; Husic, B.E.; Clementi, C.; Noé, F.; De Fabritiis, G. Machine learning coarse-grained potentials of protein thermodynamics. Nat. Commun. 2023, 14, 5739. [Google Scholar] [CrossRef]
- Noid, W.G. Perspective: Advances, challenges, and insight for predictive coarse-grained models. J. Phys. Chem. B 2023, 127, 4174–4207. [Google Scholar] [CrossRef]
- Brini, E.; Algaer, E.A.; Ganguly, P.; Li, C.; Rodríguez-Ropero, F.; van der Vegt, N.F. Systematic coarse-graining methods for soft matter simulations–A review. Soft Matter 2013, 9, 2108–2119. [Google Scholar] [CrossRef]
- Praprotnik, M.; Site, L.D.; Kremer, K. Multiscale simulation of soft matter: From scale bridging to adaptive resolution. Annu. Rev. Phys. Chem. 2008, 59, 545–571. [Google Scholar] [CrossRef]
- Souza, P.C.; Alessandri, R.; Barnoud, J.; Thallmair, S.; Faustino, I.; Grünewald, F.; Patmanidis, I.; Abdizadeh, H.; Bruininks, B.M.; Wassenaar, T.A. Martini 3: A general purpose force field for coarse-grained molecular dynamics. Nat. Methods 2021, 18, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Bueno, C.; Schafer, N.P.; Moller, J.; Jin, S.; Chen, X.; Chen, M.; Gu, X.; Davtyan, A.; de Pablo, J.J. OpenAWSEM with Open3SPN2: A fast, flexible, and accessible framework for large-scale coarse-grained biomolecular simulations. PLoS Comput. Biol. 2021, 17, e1008308. [Google Scholar] [CrossRef] [PubMed]
- Papoian, G.A.; Wolynes, P.G. AWSEM-MD: From neural networks to protein structure prediction and functional dynamics of complex biomolecular assemblies. In Coarse-Grained Modeling of Biomolecules; CRC Press: Boca Raton, FL, USA, 2017; pp. 121–190. [Google Scholar]
- Strodel, B. Energy landscapes of protein aggregation and conformation switching in intrinsically disordered proteins. J. Mol. Biol. 2021, 433, 167182. [Google Scholar] [CrossRef] [PubMed]
- Laio, A.; Parrinello, M. Escaping free-energy minima. Proc. Natl. Acad. Sci. USA 2002, 99, 12562–12566. [Google Scholar] [CrossRef]
- Torrie, G.M.; Valleau, J.P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: Umbrella sampling. J. Comput. Phys. 1977, 23, 187–199. [Google Scholar] [CrossRef]
- Sugita, Y.; Okamoto, Y. Replica-exchange molecular dynamics method for protein folding. Chem. Phys. Lett. 1999, 314, 141–151. [Google Scholar] [CrossRef]
- Swope, W.C.; Pitera, J.W.; Suits, F. Describing protein folding kinetics by molecular dynamics simulations. 1. Theory. J. Phys. Chem. B 2004, 108, 6571–6581. [Google Scholar] [CrossRef]
- Singhal, N.; Snow, C.D.; Pande, V.S. Using path sampling to build better Markovian state models: Predicting the folding rate and mechanism of a tryptophan zipper beta hairpin. J. Chem. Phys. 2004, 121, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Redler, R.L.; Shirvanyants, D.; Dagliyan, O.; Ding, F.; Kim, D.N.; Kota, P.; Proctor, E.A.; Ramachandran, S.; Tandon, A.; Dokholyan, N.V. Computational approaches to understanding protein aggregation in neurodegeneration. J. Mol. Cell Biol. 2014, 6, 104–115. [Google Scholar] [CrossRef] [PubMed]
| Resource Name | Disease Focus | Description | URL |
|---|---|---|---|
| AlzData | AD | Integrates high-throughput omics data for Alzheimer’s Disease, including transcriptomics and exome sequencing. | http://www.alzdata.org |
| AlzBiomarker | AD | Interactive database of fluid biomarkers for Alzheimer’s Disease, including curated measurements and meta-analyses. | https://www.alzforum.org |
| NIAGADS | AD | Genomic data sharing platform for Alzheimer’s and related dementias, supporting large-scale genetic studies. | https://www.niagads.org |
| AMP-PD | PD | Longitudinal clinical and omics data relevant to α-synuclein aggregation in Parkinson’s Disease. | https://www.amp-pd.org |
| PDGene Database | PD | Catalogs genetic associations and variants linked to PD, including those affecting aggregation pathways. | https://www.parkinson.org/PDGENEration |
| HDinHD | HD | Transcriptomic and proteomic data from Huntington’s Disease models, useful for studying HTT protein aggregation. | https://www.hdinhd.org |
| CHDI Foundation Resources | HD | Offers datasets and tools focused on HTT aggregation and therapeutic screening. | https://www.chdifoundation.org |
| Target ALS Data Portal | ALS | Multi-omic datasets including transcriptomics, proteomics, and imaging data from ALS patient samples and models. | https://www.targetals.org |
| ALSoD (ALS Online Database) | ALS | Genetic and clinical data related to ALS, including mutations in aggregation-prone proteins like TDP-43 and SOD1. | https://www.alsod.ac.uk |
| Methods | Features | Performance Metrics | System Suitability | Ref |
|---|---|---|---|---|
| Amyloidogenic pattern | Pattern derived from positional scanning mutagenesis experiments on amyloidogenic peptide STVIIE | Qualitative pattern-based detection | Short amyloidogenic motifs | [94] |
| AGGRESCAN | Aggregation propensity scale for amino acids derived from in vivo experiments on amyloidogenic proteins | Sensitivity ~85%, Specificity ~80% | Globular proteins, therapeutic design | [98] |
| Zyggregator | Amino acid scales for α-helix and β-sheet formation, hydrophobicity and charge, hydrophobic pattern, and presence of Gatekeeper residues | Balanced accuracy ~80% | Proteome-wide aggregation screening | [107] |
| Pafig | 41 physicochemical properties of amino acid | Accuracy ~82% | Sequence-based aggregation prediction | [140] |
| PAGE | Aromaticity, β-sheet propensity, charge, polar-nonpolar surfaces, and solubility | Not benchmarked | Peptide-level aggregation analysis | [141,142] |
| WALTZ | PSSM, physicochemical properties, position-specific pseudo energy terms | Specificity ~90%, Sensitivity ~70% | Short peptide amyloid prediction | [108] |
| AbAmyloid | Amino acid composition, dipeptide composition, and physicochemical properties | Accuracy ~85% | General amyloidogenic region detection | [143] |
| FoldAmyloid | Packing density and hydrogen bond probabilities obtained from protein structures | MCC ~0.72, Accuracy ~83% | Amyloid-forming proteins and peptides | [144] |
| SALSA β-Strand Contiguity (β-SC) | β-strand propensity | Not benchmarked | β-sheet-rich amyloid structures | [78] |
| APPNN | 7 amino acid physicochemical and biochemical properties | Accuracy ~87% | Sequence-based prediction | [101,103] |
| Amylogram | 17 amino acid properties such as size of residues, hydrophobicity, solvent accessible surface area, frequency of β-sheets, contactivity, and contact site propensities | Accuracy ~84% | Peptide-level amyloid prediction | [145] |
| ANuPP | Atom compositions of peptides and protein segments | Not benchmarked | Structural fragment analysis | [109] |
| TANGO | Segmental β-sheet probability derived from empirical and statistically derived energy functions | AUC ~0.82, Precision ~78% | Intrinsically disordered proteins | [113] |
| SecStr | Secondary structure preferences | Not benchmarked | Structural motif analysis | [114] |
| NetCSSP | Residue interactions and solvation energies computed using AMBER force-field. | Included in AMYLPRED2 ensemble | Sequence-based aggregation prediction | [102] |
| Archcandy | Scoring function derived for steric tension, electrostatic interactions, packing, and hydrogen bond formation | Not benchmarked | Structural amyloid motif detection | [146] |
| BetaSerpentine | β-arches (β-strand-loop-β-strand motif from Archcandy), compatibility of β-arches, compactness | Not benchmarked | β-arch motif analysis | [147] |
| BETASCAN | Pairwise probability tables to identify hydrogen bond-forming residues in strand pairs | Not benchmarked | Strand-pair amyloid prediction | [148] |
| AmyloidMutants | Potential energy scoring function derived from observed residue/residue interactions in PDB | Included in AMYLPRED2 ensemble | Mutation impact on aggregation | [149] |
| STITCHER | Scoring function addressing enthalpic and entropic changes in protofibril formation and BETASCAN strand pair predictions | Not benchmarked | Protofibril formation modeling | [150] |
| PASTA 2 | Hydrogen-bonding energy functions for residue pairs derived from β-strand structures | AUC ~0.85, F1-score ~0.81 | Amyloidogenic sequence screening | [89] |
| GAP | Residue pair potentials derived from hexapeptide sequences | Not benchmarked | Short peptide aggregation analysis | [116] |
| FISH Amyloid | Residue cooccurrence matrix derived from amyloidogenic and non-amyloidogenic peptides of length (4–10) | Accuracy ~83% | Peptide-level aggregation prediction | [151] |
| AgMata | Statistical potentials derived for residue position, secondary structure probabilities, and interaction energies | Accuracy ~86% | Sequence and structure-based prediction | [152] |
| 3D PROFILE (ZipperDB) | Microcrystal structure of the NNQQNY peptide and atomic-level potential ROSETTADESIGN | Qualitative scoring | β-sheet segment prediction | [64] |
| Pre-Amyl | Template ensemble obtained from microcrystal structures of the NNQQNY peptide and KBP, atomic distance-dependent knowledge-based pairwise residue potentials | Not benchmarked | Template-based amyloid prediction | [153] |
| CORDAX | Thermodynamic stability calculated by threading over 140 amyloid fibril cores | Not benchmarked | Fibril core stability modeling | [154] |
| PATH | Modeller Dope score and Rosetta (REF15) energy values from homology models of 7 template structures | Not benchmarked | Homology-based aggregation modeling | [155] |
| AMYLPRED2 | Consensus predictor includes outputs from AGGRESCAN, NetCSSP, AmyloidMutants, Pafig, Amyloidogenic Pattern, SecStr, Average Packing Density, TANGO, Beta-strand contiguity, WALTZ, Hexapeptide Conformational Energy. | Accuracy ~88%, Sensitivity ~85% | Broad-spectrum amyloid prediction | [103] |
| MetAmyl | Consensus predictor that includes PAFIG, SALSA, WALTZ, and FoldAmyloid | Accuracy ~86% | Ensemble-based prediction | [104] |
| SAP | Residue hydrophobicity, solvent accessible area over time obtained from MD | Not benchmarked | MD-based aggregation risk assessment | [104] |
| Developability Index | SAP and PROPKA values | Not benchmarked | Biotherapeutic developability screening | [156] |
| AggScore | Hydrophobic and hydrophilic patches obtained by using atom partial charges and logP values | Not benchmarked | Surface aggregation risk in biologics | [123] |
| AGGRESCAN3D 2.0 | AGGRESCAN residue score, exposed surface area, FoldX energy-minimized protein structure, or Ensemble from CABS-flex simulations | AUC ~0.85, Precision ~82% | Folded proteins, therapeutic protein design | [81] |
| Simulation Tool | Resolution | Core Features | Aggregation Suitability |
|---|---|---|---|
| GROMACS | All-atom | High-performance MD engine; supports multiple force fields (e.g., AMBER, CHARMM) | Early aggregation events, folding pathways, and solvent interactions |
| NAMD | All-atom | Scalable parallel simulations; long timescale modeling | Amyloid fibril growth, protein-protein interactions |
| Desmond | All-atom | Optimized for speed; integrated with Schrödinger suite | Drug-protein aggregation, therapeutic screening |
| LAMMPS | Atomistic/CG | Highly customizable; supports hybrid simulations | Aggregation in complex or heterogeneous environments |
| Martini 3 | Coarse-grained | Refined mapping; improved protein-lipid and protein-protein interactions | Large-scale aggregation, phase separation, and membrane systems |
| AWSEM | Coarse-grained | Physics-based energy terms; folding and aggregation modeling | Intrinsically disordered proteins, conformational transitions |
| OpenAWSEM | Hybrid CG/Atomistic | GPU-accelerated; multiscale modeling capability | Aggregation with structural transitions |
| CABS-flex | Coarse-grained | Ensemble generation; flexibility modeling | Aggregation-prone regions, conformational sampling |
| CHARMM | All-atom | Versatile force field; detailed protein and solvent modeling | Mutation effects, aggregation kinetics |
| AMBER | All-atom | Accurate protein dynamics; widely used in folding and binding studies | Early-stage aggregation, residue-level interactions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.; Shahzadi, S.; Moustafa, A.A.; Kloczkowski, A. Neurodegeneration Through the Lens of Bioinformatics Approaches: Computational Mechanisms of Protein Misfolding. Int. J. Mol. Sci. 2025, 26, 11021. https://doi.org/10.3390/ijms262211021
Hassan M, Shahzadi S, Moustafa AA, Kloczkowski A. Neurodegeneration Through the Lens of Bioinformatics Approaches: Computational Mechanisms of Protein Misfolding. International Journal of Molecular Sciences. 2025; 26(22):11021. https://doi.org/10.3390/ijms262211021
Chicago/Turabian StyleHassan, Mubashir, Saba Shahzadi, Ahmed A. Moustafa, and Andrzej Kloczkowski. 2025. "Neurodegeneration Through the Lens of Bioinformatics Approaches: Computational Mechanisms of Protein Misfolding" International Journal of Molecular Sciences 26, no. 22: 11021. https://doi.org/10.3390/ijms262211021
APA StyleHassan, M., Shahzadi, S., Moustafa, A. A., & Kloczkowski, A. (2025). Neurodegeneration Through the Lens of Bioinformatics Approaches: Computational Mechanisms of Protein Misfolding. International Journal of Molecular Sciences, 26(22), 11021. https://doi.org/10.3390/ijms262211021







