Catecholaminergic Adaptation to Extreme Military Stress: Norepinephrine and Dopamine Responses During and After SERE Training
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
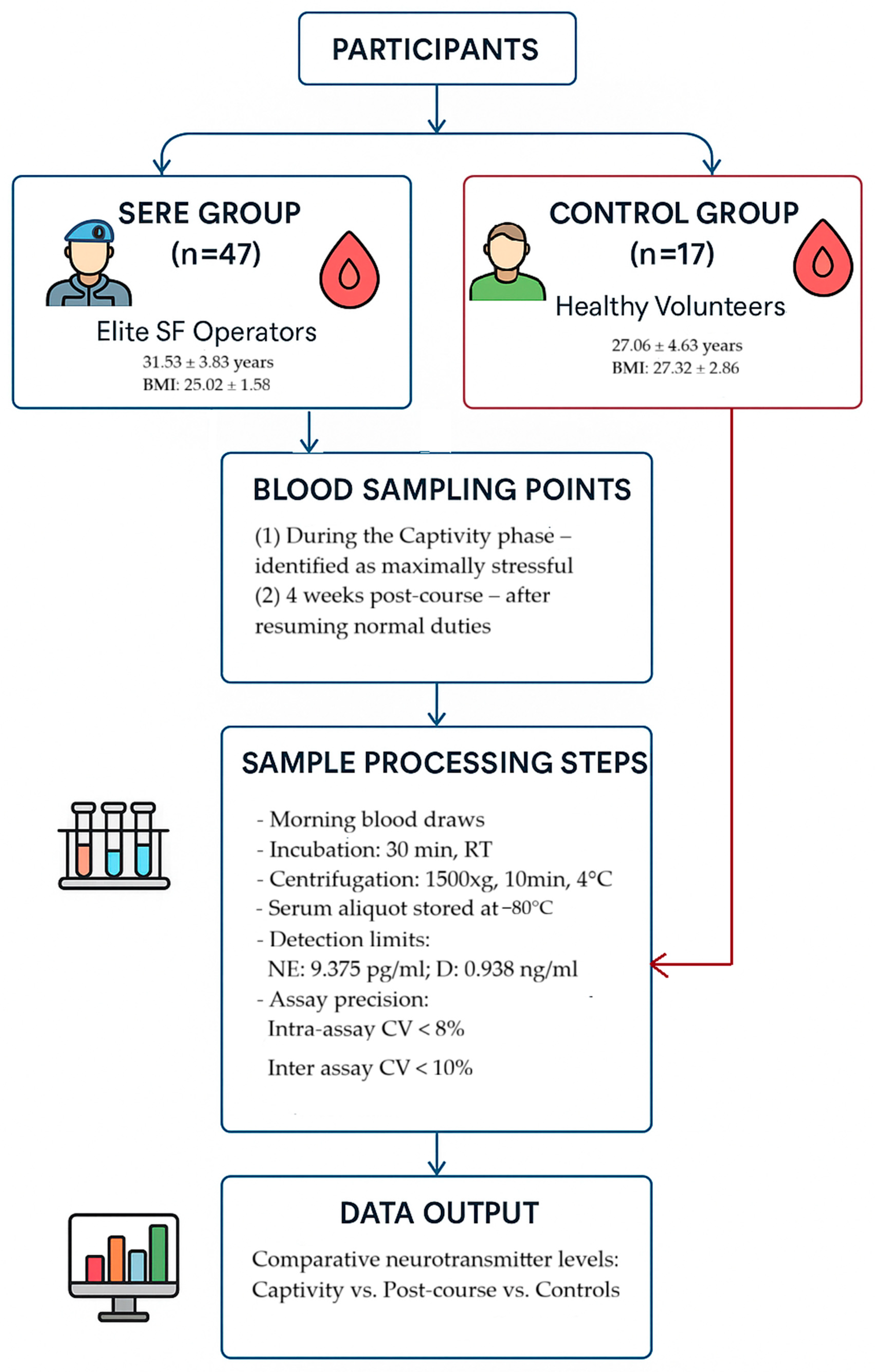
4.1. Participants
4.2. SERE Training Protocol and Stress Exposure
4.3. Blood Sampling and Biochemical Assays
4.4. Statistical Analysis
4.5. Ethical Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López, J.F.; Akil, H.; Watson, S.J. Neural Circuits Mediating Stress. Biol. Psychiatry 1999, 46, 1461–1471. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural Regulation of Endocrine and Autonomic Stress Responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Sara, S.J. The Locus Coeruleus and Noradrenergic Modulation of Cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The Locus Coeruleus–Noradrenergic System: Modulation of Behavioral State and State-Dependent Cognitive Processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Schultz, W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol. Rev. 2015, 95, 853–951. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Wingfield, J.C. The Concept of Allostasis in Biology and Biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Juster, R.-P.; McEwen, B.S.; Lupien, S.J. Allostatic Load Biomarkers of Chronic Stress and Impact on Health and Cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Southwick, S.M.; Bremner, J.D.; Rasmusson, A.; Morgan, C.A.; Arnsten, A.; Charney, D.S. Role of Norepinephrine in the Pathophysiology and Treatment of Posttraumatic Stress Disorder. Biol. Psychiatry 1999, 46, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A. Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model. Annu. Rev. Clin. Psychol. 2014, 10, 393–423. [Google Scholar] [CrossRef]
- Herman, J.P. Neural Control of Chronic Stress Adaptation. Front. Behav. Neurosci. 2013, 7, 61. [Google Scholar] [CrossRef]
- Grissom, N.; Bhatnagar, S. Habituation to Repeated Stress: Get Used to It. Neurobiol. Learn. Mem. 2009, 92, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Douma, E.H.; de Kloet, E.R. Stress-Induced Plasticity and Functioning of Ventral Tegmental Dopamine Neurons. Neurosci. Biobehav. Rev. 2020, 108, 48–77. [Google Scholar] [CrossRef]
- Morgan, C.A.; Doran, A.; Steffian, G.; Hazlett, G.; Southwick, S.M. Stress-Induced Deficits in Working Memory and Visuo-Constructive Abilities in Special Operations Soldiers. Biol. Psychiatry 2006, 60, 722–729. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Bathalon, G.P.; Falco, C.M.; Kramer, F.M.; Morgan, C.A.; Niro, P. Severe Decrements in Cognition Function and Mood Induced by Sleep Loss, Heat, Dehydration, and Undernutrition during Simulated Combat. Biol. Psychiatry 2005, 57, 422–429. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sausen, K.P.; Mujica-Parodi, L.R.; Potterat, E.G.; Yanagi, M.A.; Kim, H. Neurophysiologic Methods to Measure Stress during Survival, Evasion, Resistance, and Escape Training. Aviat. Space Env. Med. 2007, 78, B224–B230. [Google Scholar]
- Szivak, T.K.; Lee, E.C.; Saenz, C.; Flanagan, S.D.; Focht, B.C.; Volek, J.S.; Maresh, C.M.; Kraemer, W.J. Adrenal Stress and Physical Performance During Military Survival Training. Aerosp. Med. Hum. Perform. 2018, 89, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Farina, E.K.; Caldwell, J.; Williams, K.W.; Thompson, L.A.; Niro, P.J.; Grohmann, K.A.; McClung, J.P. Cognitive Function, Stress Hormones, Heart Rate and Nutritional Status during Simulated Captivity in Military Survival Training. Physiol. Behav. 2016, 165, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; McClung, H.L.; Sepowitz, J.J.; Gaffney-Stomberg, E.; Ferrando, A.A.; McClung, J.P.; Pasiakos, S.M. Testosterone Status Following Short-term, Severe Energy Deficit Is Associated with Fat-free Mass Loss in U.S. Marines. Physiol. Rep. 2022, 10, e15461. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Thompson, L.A.; Caruso, C.M.; Niro, P.J.; Mahoney, C.R.; McClung, J.P.; Caron, G.R. The Catecholamine Neurotransmitter Precursor Tyrosine Increases Anger during Exposure to Severe Psychological Stress. Psychopharmacology 2015, 232, 943–951. [Google Scholar] [CrossRef]
- Ojanen, T.; Pihlainen, K.; Yli-Renko, J.; Vaara, J.P.; Nykänen, T.; Heikkinen, R.; Kyröläinen, H. Effects of 36-Hour Recovery on Marksmanship and Hormone Concentrations during Strenuous Winter Military Survival Training. BMC Sports Sci. Med. Rehabil. 2023, 15, 105. [Google Scholar] [CrossRef]
- Varanoske, A.N.; McClung, H.L.; Sepowitz, J.J.; Halagarda, C.J.; Farina, E.K.; Berryman, C.E.; Lieberman, H.R.; McClung, J.P.; Pasiakos, S.M.; Philip Karl, J. Stress and the Gut-Brain Axis: Cognitive Performance, Mood State, and Biomarkers of Blood-Brain Barrier and Intestinal Permeability Following Severe Physical and Psychological Stress. Brain Behav. Immun. 2022, 101, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Cabib, S.; Puglisi-Allegra, S. The Mesoaccumbens Dopamine in Coping with Stress. Neurosci. Biobehav. Rev. 2012, 36, 79–89. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the Dopamine System in the Pathophysiology of Schizophrenia and Depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress Signalling Pathways That Impair Prefrontal Cortex Structure and Function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Champagne, F.; Meaney, M.J.; Dagher, A. Dopamine Release in Response to a Psychological Stress in Humans and Its Relationship to Early Life Maternal Care: A Positron Emission Tomography Study Using [11C]Raclopride. J. Neurosci. 2004, 24, 2825–2831. [Google Scholar] [CrossRef]
- Lataster, J.; Collip, D.; Ceccarini, J.; Haas, D.; Booij, L.; van Os, J.; Pruessner, J.; Van Laere, K.; Myin-Germeys, I. Psychosocial Stress Is Associated with in Vivo Dopamine Release in Human Ventromedial Prefrontal Cortex: A Positron Emission Tomography Study Using [18F]Fallypride. Neuroimage 2011, 58, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Holly, E.N.; Miczek, K.A. Ventral Tegmental Area Dopamine Revisited: Effects of Acute and Repeated Stress. Psychopharmacology 2016, 233, 163–186. [Google Scholar] [CrossRef]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.-C.; Finkelstein, J.; Kim, S.-Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine Neurons Modulate Neural Encoding and Expression of Depression-Related Behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; D’Esposito, M. Inverted-U-Shaped Dopamine Actions on Human Working Memory and Cognitive Control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef]
- Morgan, C.A.; Wang, S.; Southwick, S.M.; Rasmusson, A.; Hazlett, G.; Hauger, R.L.; Charney, D.S. Plasma Neuropeptide-Y Concentrations in Humans Exposed to Military Survival Training. Biol. Psychiatry 2000, 47, 902–909. [Google Scholar] [CrossRef]
- Morgan, C.A.; Southwick, S.; Hazlett, G.; Rasmusson, A.; Hoyt, G.; Zimolo, Z.; Charney, D. Relationships among Plasma Dehydroepiandrosterone Sulfate and Cortisol Levels, Symptoms of Dissociation, and Objective Performance in Humans Exposed to Acute Stress. Arch. Gen. Psychiatry 2004, 61, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M.; Pu, Z.; Wiegert, O.; Oitzl, M.S.; Krugers, H.J. Learning under Stress: How Does It Work? Trends Cogn. Sci. 2006, 10, 152–158. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Sterling, P. Allostasis: A Model of Predictive Regulation. Physiol. Behav. 2012, 106, 5–15. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; McEwen, B.S. Psychobiological Allostasis: Resistance, Resilience and Vulnerability. Trends Cogn. Sci. 2011, 15, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. The Molecular Neurobiology of Depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.-C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid Regulation of Depression-Related Behaviors by Control of Midbrain Dopamine Neurons. Nature 2013, 493, 532–536. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzesik-Pietrasiewicz, M.; Łach, K.; Przednowek, K.; Podgórski, R. Catecholaminergic Adaptation to Extreme Military Stress: Norepinephrine and Dopamine Responses During and After SERE Training. Int. J. Mol. Sci. 2025, 26, 11012. https://doi.org/10.3390/ijms262211012
Grzesik-Pietrasiewicz M, Łach K, Przednowek K, Podgórski R. Catecholaminergic Adaptation to Extreme Military Stress: Norepinephrine and Dopamine Responses During and After SERE Training. International Journal of Molecular Sciences. 2025; 26(22):11012. https://doi.org/10.3390/ijms262211012
Chicago/Turabian StyleGrzesik-Pietrasiewicz, Michalina, Kornelia Łach, Krzysztof Przednowek, and Rafał Podgórski. 2025. "Catecholaminergic Adaptation to Extreme Military Stress: Norepinephrine and Dopamine Responses During and After SERE Training" International Journal of Molecular Sciences 26, no. 22: 11012. https://doi.org/10.3390/ijms262211012
APA StyleGrzesik-Pietrasiewicz, M., Łach, K., Przednowek, K., & Podgórski, R. (2025). Catecholaminergic Adaptation to Extreme Military Stress: Norepinephrine and Dopamine Responses During and After SERE Training. International Journal of Molecular Sciences, 26(22), 11012. https://doi.org/10.3390/ijms262211012





