Identification of Novel Metabolic Signatures on Human Gut Microbiota: Ellagic Acid, Naringenin, and Phloroglucinol
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds Selection
2.2. Qualitative and Quantitative Analysis of Phenolic Compounds and Their Metabolites
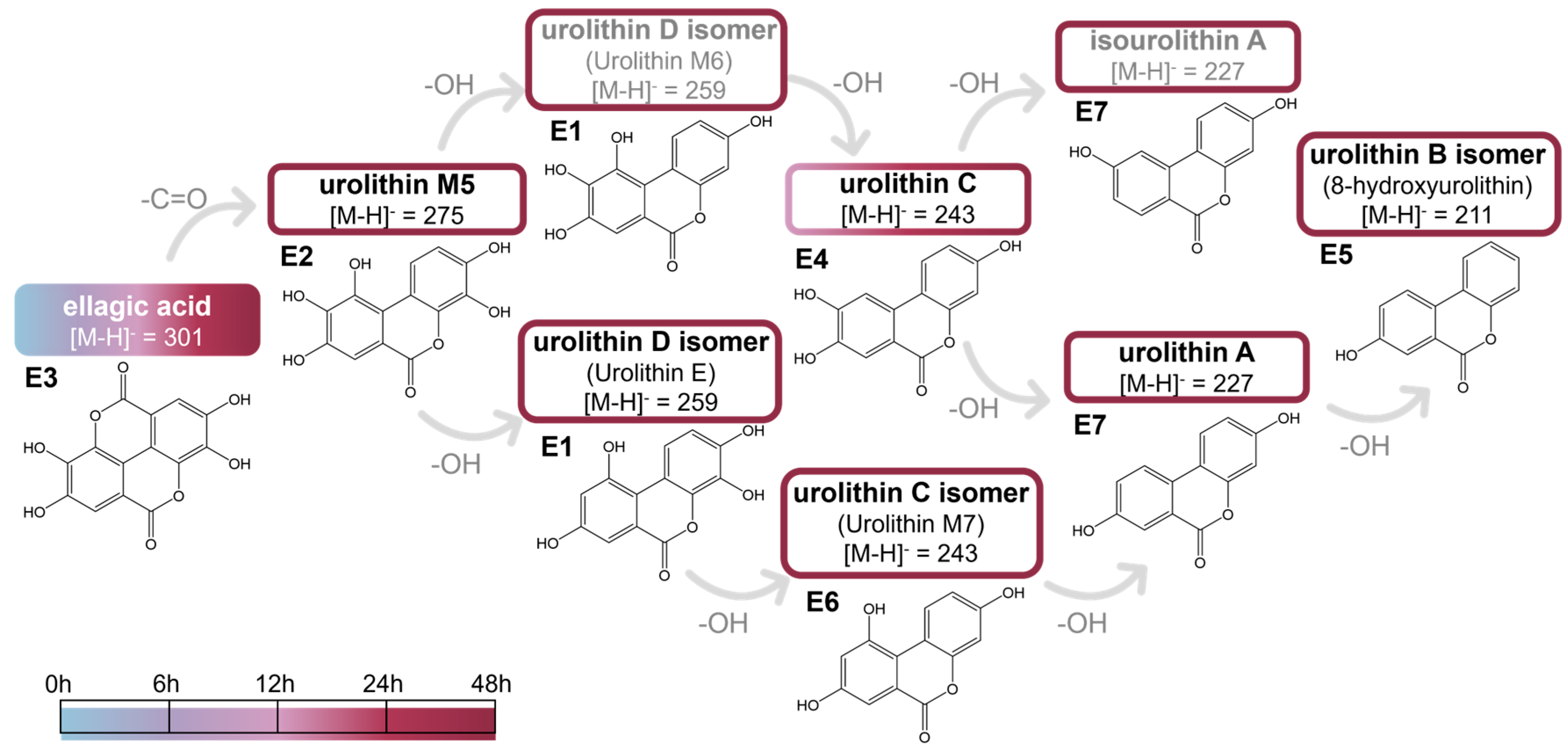
2.2.1. Ellagic Acid and Metabolites from In Vitro Batch Fecal Fermentation
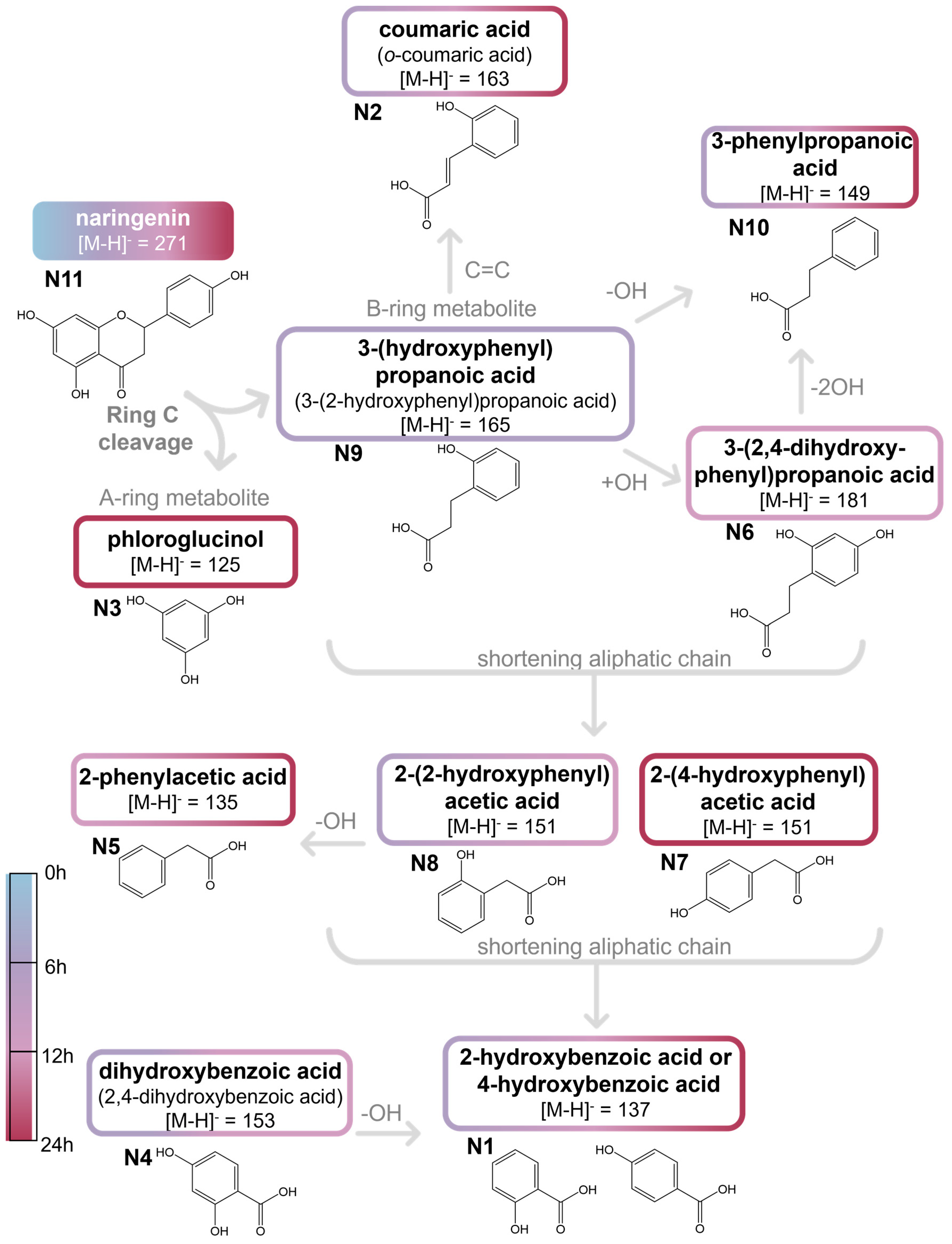
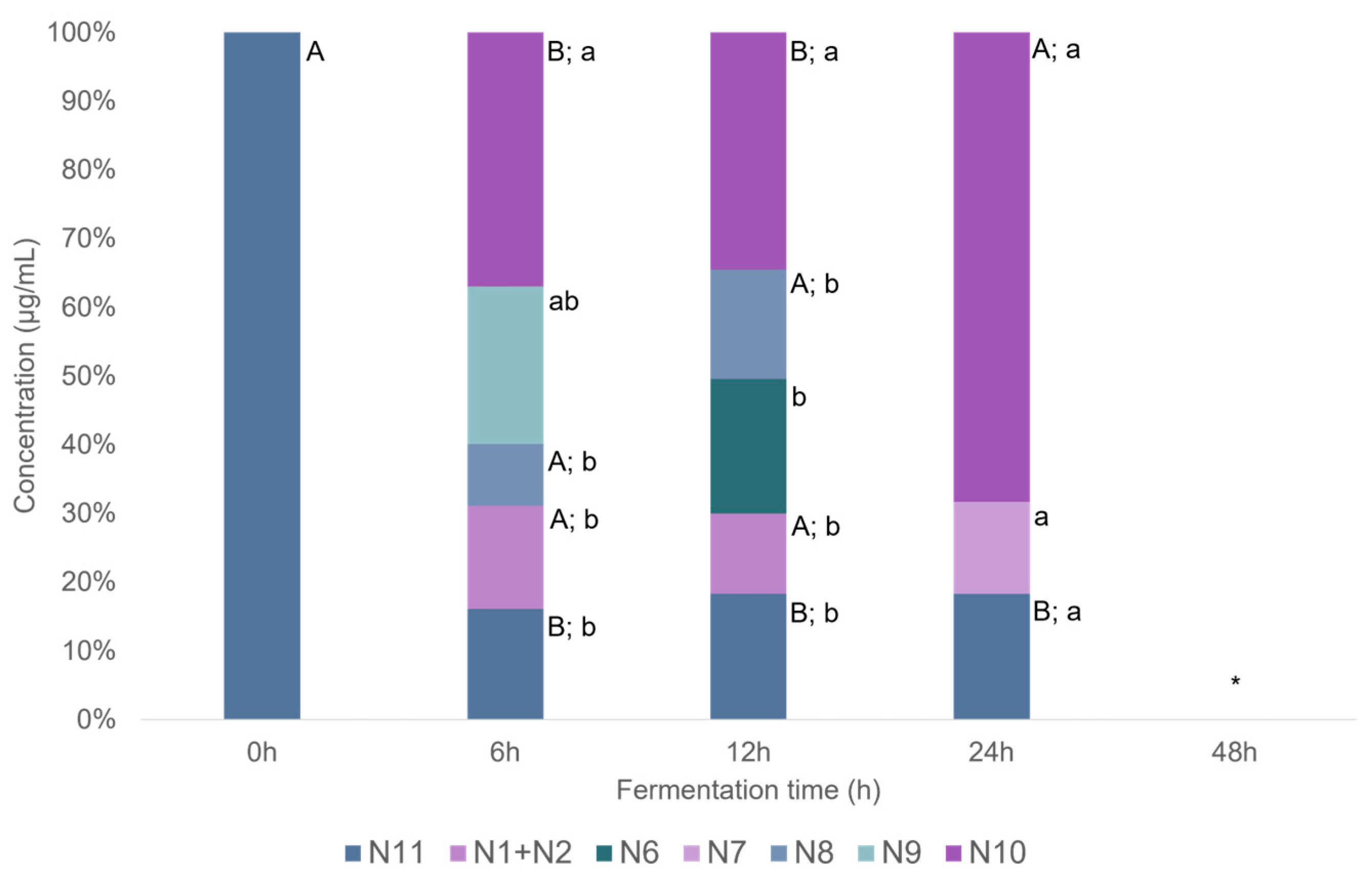
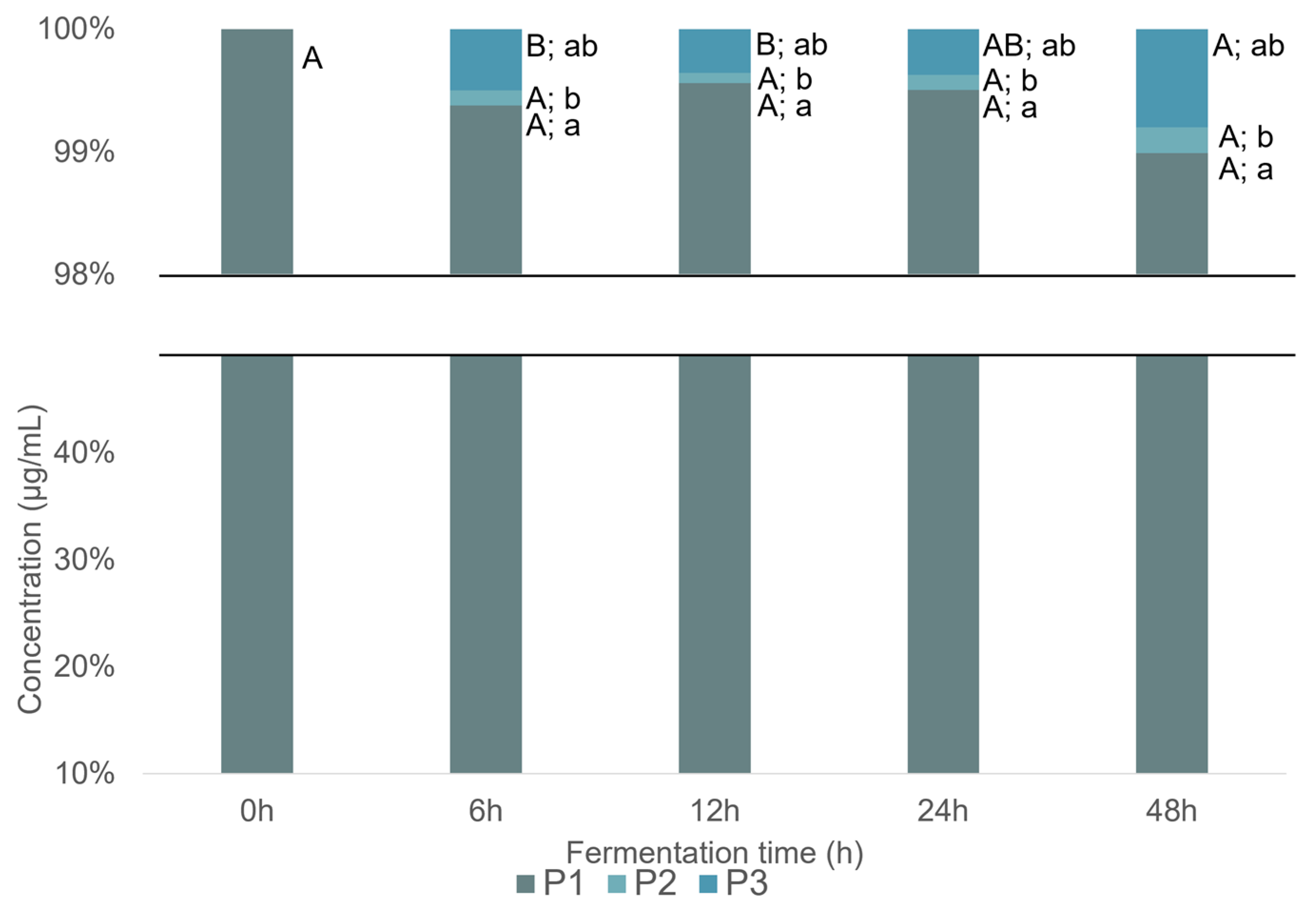
2.2.2. Naringenin and Metabolites from In Vitro Batch Fecal Fermentation
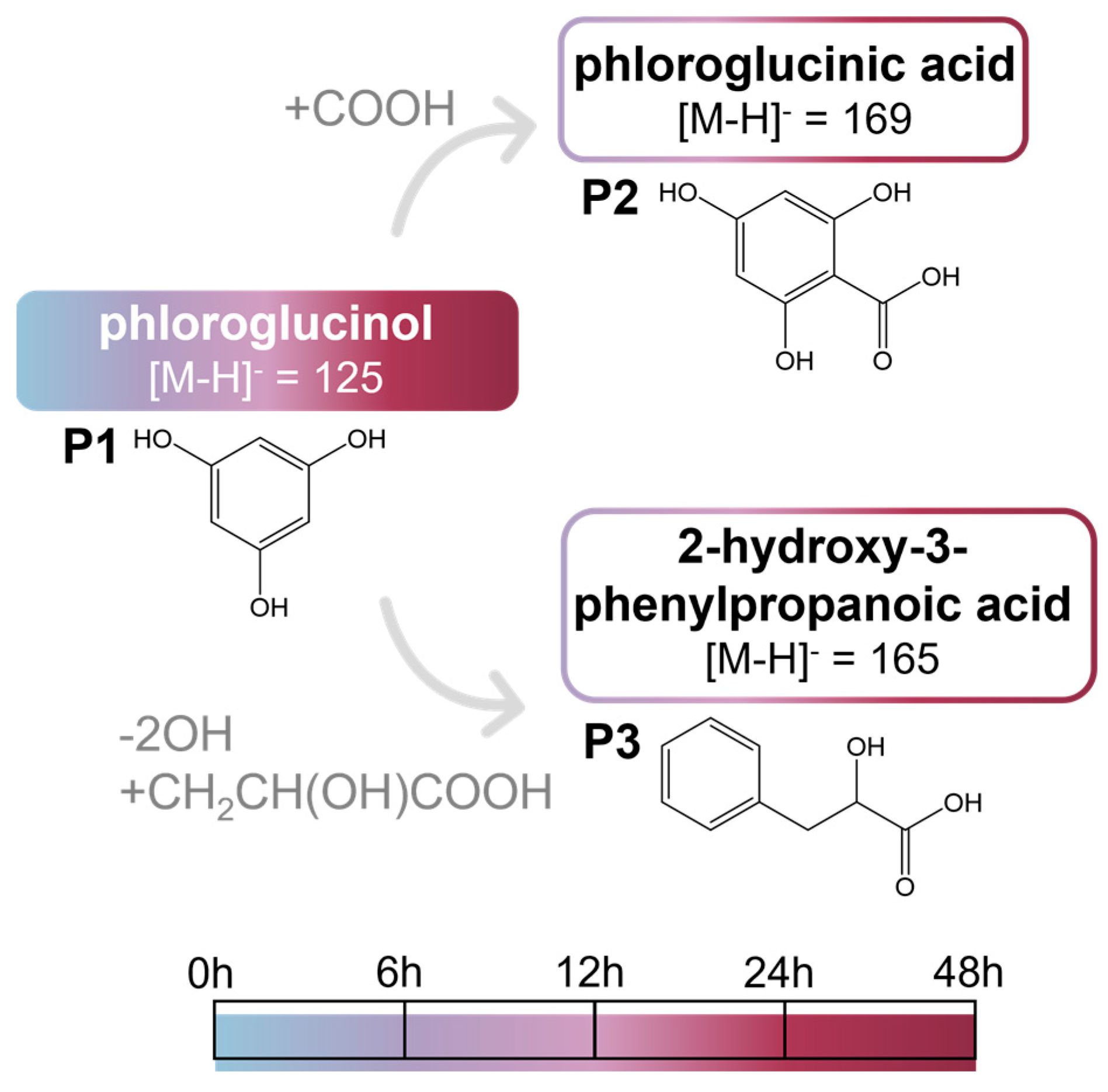
2.2.3. Phloroglucinol and Metabolites from In Vitro Batch Fecal Fermentation
3. Materials and Methods
3.1. Materials
3.2. Preliminary Screening of Antimicrobial and Prebiotic Effects of Phenolic Compounds
3.3. In Vitro Fermentation Assays
3.3.1. Pool of Human Fecal Inoculum
3.3.2. Fermentation Media
3.3.3. In Vitro Fermentation
3.4. Analysis of Phenolic Compounds and Their Metabolites
3.4.1. Qualitative and Quantitative UHPLC-DAD-MSn Analysis
3.4.2. Qualitative GC-MS Analysis
3.4.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UHPLC-DAD-MSn | Ultra-high performance liquid chromatography with diode array detector coupled to an ion trap mass spectrometer |
| GC-MS | Gas chromatography coupled to mass spectrometer |
| MHB | Mueller Hinton broth |
| TMS | Trimethylsilyl |
References
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.-K.; Huber, R. The Relation of Diet and Health: You Are What You Eat. Int. J. Environ. Res. Public Health 2022, 19, 7774. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The Gut Microbiota: A Key Factor in the Therapeutic Effects of (Poly)Phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Bartolomé, B.; Victoria Moreno-Arribas, M. An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A Survey of Modulation of Gut Microbiota by Dietary Polyphenols. Biomed. Res. Int. 2015, 1, 850902. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of Gut Microbiota with Dietary Polyphenols and Consequences to Human Health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Popa, D.E.; Dragoi, C.M.; Arsene, A.L.; Dumitrescu, I.B.; Nicolae, A.C.; Burcea-Dragomiroiu, G.T.A. The Relationship between Phenolic Compounds from Diet and Microbiota. In Phenolic Compounds—Biological Activity; InTech: London, UK, 2017; pp. 25–38. [Google Scholar]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Bacterial Species Involved in the Conversion of Dietary Flavonoids in the Human Gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The Relationship between Phenolic Compounds from Diet and Microbiota: Impact on Human Health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary Polyphenols to Combat the Metabolic Diseases via Altering Gut Microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dicksved, J. Exploring the Human Intestinal Microbiome in Health and Disease. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2008. [Google Scholar]
- de Souza, E.L.; de Albuquerque, T.M.R.; dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential Interactions among Phenolic Compounds and Probiotics for Mutual Boosting of Their Health-Promoting Properties and Food Functionalities—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Braune, A.; Gütschow, M.; Engst, W.; Blaut, M. Degradation of Quercetin and Luteolin by Eubacterium ramulus. Appl. Environ. Microbiol. 2001, 67, 5558–5567. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.-P.; Reguant, J.; Ortega, N.; Motilva, M.-J. Metabolic Pathways of the Colonic Metabolism of Flavonoids (Flavonols, Flavones and Flavanones) and Phenolic Acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal Microbial Metabolism of Polyphenols and Its Effects on Human Gut Microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Di Pede, G.; Bresciani, L.; Calani, L.; Petrangolini, G.; Riva, A.; Allegrini, P.; Del Rio, D.; Mena, P. The Human Microbial Metabolism of Quercetin in Different Formulations: An in Vitro Evaluation. Foods 2020, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Sekhon, P.K.; Ambat, A.; Nelson, J.; Jose, D.; Bhat, G.J.; Scaria, J. Screening of Human Gut Bacterial Culture Collection Identifies Species That Biotransform Quercetin into Metabolites with Anticancer Properties. Int. J. Mol. Sci. 2021, 22, 7045. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, L.R.; Bryant, M.P. Eubacterium oxidoreducens Sp. Nov. Requiring H2 or Formate to Degrade Gallate, Pyrogallol, Phloroglucinol and Quercetin. Arch. Microbiol. 1986, 144, 8–14. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Z.; Zhang, N.; Liu, L.; Li, S.; Wei, H. In Vitro Catabolism of Quercetin by Human Fecal Bacteria and the Antioxidant Capacity of Its Catabolites. Food Nutr. Res. 2014, 58, 23406. [Google Scholar] [CrossRef]
- Ulbrich, K.; Reichardt, N.; Braune, A.; Kroh, L.W.; Blaut, M.; Rohn, S. The Microbial Degradation of Onion Flavonol Glucosides and Their Roasting Products by the Human Gut Bacteria Eubacterium ramulus and Flavonifractor plautii. Food Res. Int. 2015, 67, 349–355. [Google Scholar] [CrossRef]
- Jin, J.S.; Hattori, M. Isolation and Characterization of a Human Intestinal Bacterium Eggerthella Sp. CAT-1 Capable of Cleaving the C-Ring of (+)-Catechin and (-)-Epicatechin, Followed by p-Dehydroxylation of the B-Ring. Biol. Pharm. Bull. 2012, 35, 2252–2256. [Google Scholar] [CrossRef]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of Catechin-Converting Human Intestinal Bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Biotransformation of (−)-Epigallocatechin and (−)-Gallocatechin by Intestinal Bacteria Involved in Isoflavone Metabolism. Biol. Pharm. Bull. 2015, 38, 325–330. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Bioconversion of (-)-Epicatechin, (+)-Epicatechin, (-)-Catechin, and (+)-Catechin by (-)-Epigallocatechin-Metabolizing Bacteria. Biol. Pharm. Bull. 2015, 38, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Meselhy, M.R.; Li, Y.; Nakamura, N.; Min, B.-S.; Qin, G.-W.; Hattori, M. The Heterocyclic Ring Fission and Dehydroxylation of Catechins and Related Compounds by Eubacterium Sp. Strain SDG-2, a Human Intestinal Bacterium. Chem. Pharm. Bull. 2001, 49, 1640–1643. [Google Scholar] [CrossRef]
- Meselhy, M.R.; Nakamura, N.; Hattori, M. Biotransformation of (-)-Epicatechin 3-O-Gallate by Human Intestinal Bacteria. Chem. Pharm. Bull. 1997, 45, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Sarnoski, P.; Schneider, K.R.; Song, K.; Khoo, C.; Gu, L. Microbial Catabolism of Procyanidins by Human Gut Microbiota. Mol. Nutr. Food Res. 2014, 58, 2196–2205. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Catabolism of (+)-Catechin and (-)-Epicatechin by Rat Intestinal Microbiota. J. Agric. Food Chem. 2013, 61, 4927–4935. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol Monomer-Induced Changes to the Human Faecal Microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- van’t Slot, G.; Humpf, H.U. Degradation and Metabolism of Catechin, Epigallocatechin-3-Gallate (EGCG), and Related Compounds by the Intestinal Microbiota in the Pig Cecum Model. J. Agric. Food Chem. 2009, 57, 8041–8048. [Google Scholar] [CrossRef]
- Herles, C.; Braune, A.; Blaut, M. First Bacterial Chalcone Isomerase Isolated from Eubacterium ramulus. Arch. Microbiol. 2004, 181, 428–434. [Google Scholar] [CrossRef]
- Schneider, H.; Blaut, M. Anaerobic Degradation of Flavonoids by Eubacterium ramulus. Arch. Microbiol. 2000, 173, 71–75. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic Degradation of Flavonoids by Clostridium orbiscindens. Appl. Env. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef]
- Winter, J.; Moore, L.H.; Dowell, V.R.; Bokkenheuser, V.D. C-Ring Cleavage of Flavonoids by Human Intestinal Bacteria. Appl. Env. Microbiol. 1989, 55, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Schantz, M.; Erk, T.; Richling, E. Metabolism of Green Tea Catechins by the Human Small Intestine. Biotechnol. J. 2010, 5, 1050–1059. [Google Scholar] [CrossRef]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.J.; Edwards And, C.A.; Crozier, A. Green Tea Flavan-3-Ols: Colonic Degradation and Urinary Excretion of Catabolites by Humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]
- García-Villalba, R.; Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Identification of Novel Urolithin Metabolites in Human Feces and Urine after the Intake of a Pomegranate Extract. J. Agric. Food Chem. 2019, 67, 11099–11107. Erratum in J. Agric. Food Chem. 2019, 68, 3297. [Google Scholar] [CrossRef]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate Ellagitannins Stimulate Growth of Gut Bacteria in Vitro: Implications for Prebiotic and Metabolic Effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In Vitro Gastrointestinal Digestion of Pomegranate Peel (Punica granatum) Flour Obtained from Co-Products: Changes in the Antioxidant Potential and Bioactive Compounds Stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Pais, A.C.S.; Coscueta, E.R.; Pintado, M.M.; Silvestre, A.J.D.; Santos, S.A.O. Exploring the Bioaccessibility and Intestinal Absorption of Major Classes of Pure Phenolic Compounds Using in Vitro Simulated Gastrointestinal Digestion. Heliyon 2024, 10, e28894. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The Inhibitory Effect of Polyphenols on Human Gut Microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar] [PubMed]
- Gwiazdowska, D.; Juś, K.; Jasnowska-Małecka, J.; Kluczyńska, K. The Impact of Polyphenols on Bifidobacterium Growth. Acta Biochim. Pol. 2015, 62, 895–901. [Google Scholar] [CrossRef]
- Krumholz, L.R.; Crawford, R.L.; Hemling, M.E.; Bryant, M.P. Metabolism of Gallate and Phloroglucinol in Eubacterium Oxidoreducens via 3-Hydroxy-5-Oxohexanoate. J. Bacteriol. 1987, 169, 1886–1890. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of Urolithin Production Capacity from Ellagic Acid of Two Human Intestinal Gordonibacter Species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; García-Villalba, R.; Mira, A.; Espín, J.C.; Tomás-Barberán, F.A. Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef]
- García-Villalba, R.; Beltrán, D.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. Time Course Production of Urolithins from Ellagic Acid by Human Gut Microbiota. J. Agric. Food Chem. 2013, 61, 8797–8806. [Google Scholar] [CrossRef]
- Mi, H.; Liu, S.; Hai, Y.; Yang, G.; Lu, J.; He, F.; Zhao, Y.; Xia, M.; Hou, X.; Fang, Y. Lactococcus Garvieae FUA009, a Novel Intestinal Bacterium Capable of Producing the Bioactive Metabolite Urolithin A from Ellagic Acid. Foods 2022, 11, 2621. [Google Scholar] [CrossRef]
- Braune, A.; Engst, W.; Blaut, M. Degradation of Neohesperidin Dihydrochalcone by Human Intestinal Bacteria. J. Agric. Food Chem. 2005, 53, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Li, Q.; Zhou, B. The Tannins from Sanguisorba officinalis l. (Rosaceae): A Systematic Study on the Metabolites of Rats Based on HPLC–LTQ–Orbitrap MS2 Analysis. Molecules 2021, 26, 4053. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Chromatographic and Spectroscopic Characterization of Urolithins for Their Determination in Biological Samples after the Intake of Foods Containing Ellagitannins and Ellagic Acid. J. Chromatogr. A 2016, 1428, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, H.; Li, G.; Lv, N.; Qi, Y. Metabolite Identification of Gut Microflora-cassia Seed Interactions Using UPLC-QTOF/MS. Exp. Ther. Med. 2020, 19, 3305–3315. [Google Scholar] [CrossRef]
- González-Barrio, R.; Truchado, P.; Ito, H.; Espín, J.C.; Tomás-Barberán, F.A. UV and MS Identification of Urolithins and Nasutins, the Bioavailable Metabolites of Ellagitannins and Ellagic Acid in Different Mammals. J. Agric. Food Chem. 2011, 59, 1152–1162. [Google Scholar] [CrossRef]
- Pfundstein, B.; Haubner, R.; Würtele, G.; Gehres, N.; Ulrich, C.M.; Owen, R.W. Pilot Walnut Intervention Study of Urolithin Bioavailability in Human Volunteers. J. Agric. Food Chem. 2014, 62, 10264–10273. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the Rescue of “Old” Metabolites to Understand a “New” Concept: Metabotypes as a Nexus among Phenolic Metabolism, Microbiota Dysbiosis, and Host Health Status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Selma, M.V.; Tomás-Barberán, F.A.; Beltrán, D.; García-Villalba, R.; Espín, J.C. Gordonibacter urolithinfaciens Sp. Nov., a Urolithin-Producing Bacterium Isolated from the Human Gut. Int. J. Syst. Evol. Microbiol. 2014, 64, 2346–2352. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Álvarez, I.; Landete, J.M. Bifidobacterium pseudocatenulatum INIA P815: The First Bacterium Able to Produce Urolithins A and B from Ellagic Acid. J. Funct. Foods 2018, 45, 95–99. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Tresserra-Rimbau, A.; Miliarakis, E.; Arancibia-Riveros, C.; Jáuregui, O.; Ruiz-León, A.M.; Castro-Baquero, S.; et al. Identification and Quantification of Urinary Microbial Phenolic Metabolites by HPLC-ESI-LTQ-Orbitrap-HRMS and Their Relationship with Dietary Polyphenols in Adolescents. Antioxidants 2022, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jung, E.-A.; Sohng, I.-S.; Han, J.-A.; Kim, T.-H.; Han, M.J. Intestinal Bacterial Metabolism of Flavonoids and Its Relation to Some Biological Activities. Arch. Pharm. Res. 1998, 21, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huélamo, M.; Tulipani, S.; Jáuregui, O.; Valderas-Martinez, P.; Vallverdú-Queralt, A.; Estruch, R.; Torrado, X.; Lamuela-Raventós, R. Sensitive and Rapid UHPLC-MS/MS for the Analysis of Tomato Phenolics in Human Biological Samples. Molecules 2015, 20, 20409–20425. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, X.; Zhao, C.; Yin, J.; Li, X.; Zhang, X.; Wang, J.; Wang, S. Distinctive Anti-Inflammatory Effects of Resveratrol, Dihydroresveratrol, and 3-(4-Hydroxyphenyl)-Propionic Acid on DSS-Induced Colitis in Pseudo-Germ-Free Mice. Food Chem. 2023, 400, 133904. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic Metabolism of Dietary Polyphenols: Influence of Structure on Microbial Fermentation Products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Chen, T.; Su, W.; Yan, Z.; Wu, H.; Zeng, X.; Peng, W.; Gan, L.; Zhang, Y.; Yao, H. Identification of Naringin Metabolites Mediated by Human Intestinal Microbes with Stable Isotope-Labeling Method and UFLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2018, 161, 262–272. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial Metabolism of Caffeic Acid and Its Esters Chlorogenic and Caftaric Acids by Human Faecal Microbiota in Vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Marçal, C.; Bonifácio-Lopes, T.; Campos, D.; Mateus, N.; Silva, A.M.S.; Pintado, M.M.; Cardoso, S.M. Impact of Phlorotannin Extracts from Fucus Vesiculosus on Human Gut Microbiota. Mar. Drugs 2021, 19, 375. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, B.; Santos-Zea, L.; Heredia-Olea, E.; Acevedo-Pacheco, L.; Santacruz, A.; Gutiérrez-Uribe, J.A.; Cruz-Suárez, L.E. Effects of Phlorotannin and Polysaccharide Fractions of Brown Seaweed Silvetia Compressa on Human Gut Microbiota Composition Using an in Vitro Colonic Model. J. Funct. Foods 2021, 84, 104596. [Google Scholar] [CrossRef]
- Schneider, H.; Schwiertz, A.; Collins, M.D.; Blaut, M. Anaerobic Transformation of Quercetin-3-Glucoside by Bacteria from the Human Intestinal Tract. Arch. Microbiol. 1999, 171, 81–91. [Google Scholar] [CrossRef]
- Bordiga, M.; Meudec, E.; Williams, P.; Montella, R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D.; Doco, T. The Impact of Distillation Process on the Chemical Composition and Potential Prebiotic Activity of Different Oligosaccharidic Fractions Extracted from Grape Seeds. Food Chem. 2019, 285, 423–430. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Coscueta, E.R.; Pereira, M.F.; Cunha, S.A.; Almeida, A.; Rosa, A.; Martins, R.; Pereira, C.D.; Pintado, M.E. Membrane Fractionation of Cynara cardunculus Swine Blood Hydrolysate: Ingredients of High Nutritional and Nutraceutical Value. Food Res. Int. 2022, 158, 111549. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Ferreira, I.C.F.R.; Martins, A.; Pintado, M. Antimicrobial Activity of Wild Mushroom Extracts against Clinical Isolates Resistant to Different Antibiotics. J. Appl. Microbiol. 2012, 113, 466–475. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Oliveira, D.L.; Dib Saleh, M.A.; Pintado, M.; Madureira, A.R. Preservation of Human Gut Microbiota Inoculums for in Vitro Fermentations Studies. Fermentation 2021, 7, 14. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Vilas-Boas, A.A.; Silva, S.; Teixeira, J.A.; Pastrana, L.M.; Pintado, M.M. Impact of Functional Flours from Pineapple By-Products on Human Intestinal Microbiota. J. Funct. Foods 2020, 67, 103830. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Applied to the Identification of Valuable Phenolic Compounds from Eucalyptus Wood. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 938, 65–74. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Oliveira, C.S.D.; Trindade, S.S.; Abreu, M.H.; Rocha, S.S.M.; Silvestre, A.J.D. Bioprospecting for Lipophilic-like Components of Five Phaeophyta Macroalgae from the Portuguese Coast. J. Appl. Phycol. 2016, 28, 3151–3158. [Google Scholar] [CrossRef]
- Rabah, S.; Kouachi, K.; Ramos, P.A.B.; Gomes, A.P.; Almeida, A.; Haddadi-Guemghar, H.; Khodir, M.; Silvestre, A.J.D.; Santos, S.A.O. Unveiling the Bioactivity Potential of Allium triquetrum L. Lipophilic Fraction: Chemical Characterization and in Vitro Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus. Food Funct. 2020, 11, 5257–5265. [Google Scholar] [CrossRef]
- Bayle, M.; Roques, C.; Marion, B.; Audran, M.; Oiry, C.; Bressolle-Gomeni, F.M.; Cros, G. Development and validation of a liquid chromatography-electrospray ionization-tandem mass spectrometry method for the determination of urolithin C in rat plasma and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2016, 131, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lech, K.; Fornal, E. A mass spectrometry-based approach for characterization of red, blue, and purple natural dyes. Molecules 2020, 25, 3223. [Google Scholar] [CrossRef] [PubMed]
- Ibdah, M.; Gang, D.R. Use of coupled ion mobility spectrometry-time of flight mass spectrometry to analyze saturated and unsaturated phenylpropanoic acids and chalcones. Chem. Cent. J. 2014, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]

| Reagent Name | Supplier |
|---|---|
| 2-(2-Hydroxyphenyl)acetic acid (99% purity) | Sigma-Aldrich (Madrid, Spain) |
| 2-(4-Hydroxyphenyl)acetic acid (>98% purity) | Sigma-Aldrich (Madrid, Spain) |
| 3-(2,4-Dihydroxyphenyl)propanoic acid (95% purity) | Sigma-Aldrich (Madrid, Spain) |
| 3-(4-Hydroxyphenylpropanoic acid (98% purity) | Sigma-Aldrich (Madrid, Spain) |
| 3,4-Dihydroxybenzoic acid (97% purity) | Thermo-scientific (Slovakia) |
| Acetonitrile (HPLC grade) | Honeywell (Villepinte, France) |
| Bile salts (48305) | Sigma-Aldrich (St. Louis, MO, USA) |
| Calcium chloride (102378) | Merck (New Jersey, USA) |
| D-(+)-Glucose (G8270) | Sigma-Aldrich (St. Louis, MO, USA) |
| D-(+)-Maltose monohydrate (63419) | Sigma-Aldrich (St. Louis, MO, USA) |
| Ellagic acid (96% purity) | Sigma-Aldrich (Madrid, Spain) |
| Heme chloride (A11165) | Alfa Aesar (Stoughton, MA, USA) |
| L-Cysteine hydrochloride (1.02839.0100) | Merck (Rahway, NJ, USA) |
| Lysozyme (P00698) | Sigma-Aldrich (St. Louis, MO, USA) |
| Magnesium sulfate hexahydrate (459337) | Carlo Erba (Emmendingen, Deutschland) |
| N,O-bis(trimethylsilyl)trifluoroacetamide (99% purity) | Merck (Madrid, Spain) |
| Naringenin (98% purity) | Biosynth® CarboSynth (Bratislava, Slovakia) |
| Naringin (95% purity) | Biosynth® CarboSynth (Bratislava, Slovakia) |
| NZY Tissue gDNA kit for DNA extraction (MB13502) | NZYTech (Lisbon, Portugal) |
| p-Coumaric acid (≥98% purity) | Sigma-Aldrich (Madrid, Spain) |
| Phloroglucinol (99% purity) | Sigma-Aldrich (Madrid, Spain) |
| Potassium dihydrogen phosphate (1.04871.1000) | Merck (Rahway, NJ, USA) |
| Pyridine (≥ 99.5% purity) | Merck (Madrid, Spain) |
| Quercetin (95% purity) | Sigma-Aldrich (Madrid, Spain) |
| Resazurin sodium salt (199303) | Sigma-Aldrich (St. Louis, MO, USA) |
| Sodium bicarbonate (1.06329.1000) | Merck (Rahway, NJ, USA) |
| Sodium chloride (31434) | Honeywell (Charlotte, NC, USA) |
| Solvent Filtration Apparatus 58061 | Supelco (Bellefonte, PA, USA) |
| Soya peptone (84616.0500) | VWR Chemicals (Radnor, PA, USA) |
| Tetracosane (99% purity) | Merck (Madrid, Spain) |
| Trimethylchlorosilane (99% purity) | Merck (Madrid, Spain) |
| Tris-EDTA buffer (TE, 10× concentrate, PPB010) | Sigma-Aldrich (St. Louis, MO, USA) |
| Tryptone (A1401 HA) | Biokar Diagnostics (Cedex, France) |
| Tween 80 (28830,291) | VWR Chemicals (Radnor, PA, USA) |
| Urolithin B (≥98% purity) | Cayman Chemical company (Ann Arbor, MI, USA) |
| Urolithin C (>97% purity) | Sigma-Aldrich (Madrid, Spain) |
| Urolithin D (95% purity) | Sigma-Aldrich (Madrid, Spain) |
| Vitamin K1 (V3501) | Sigma-Aldrich (St. Louis, MO, USA) |
| Water (HPLC grade) | Merck (Darmstadt, Germany) |
| Yeast extract (A1202) | Biokar Diagnostics (Paris La Défense Cedex, France) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pais, A.C.S.; Ribeiro, T.B.; Coscueta, E.R.; Pintado, M.M.; Silvestre, A.J.D.; Santos, S.A.O. Identification of Novel Metabolic Signatures on Human Gut Microbiota: Ellagic Acid, Naringenin, and Phloroglucinol. Int. J. Mol. Sci. 2025, 26, 11009. https://doi.org/10.3390/ijms262211009
Pais ACS, Ribeiro TB, Coscueta ER, Pintado MM, Silvestre AJD, Santos SAO. Identification of Novel Metabolic Signatures on Human Gut Microbiota: Ellagic Acid, Naringenin, and Phloroglucinol. International Journal of Molecular Sciences. 2025; 26(22):11009. https://doi.org/10.3390/ijms262211009
Chicago/Turabian StylePais, Adriana C. S., Tânia B. Ribeiro, Ezequiel R. Coscueta, Maria Manuela Pintado, Armando J. D. Silvestre, and Sónia A. O. Santos. 2025. "Identification of Novel Metabolic Signatures on Human Gut Microbiota: Ellagic Acid, Naringenin, and Phloroglucinol" International Journal of Molecular Sciences 26, no. 22: 11009. https://doi.org/10.3390/ijms262211009
APA StylePais, A. C. S., Ribeiro, T. B., Coscueta, E. R., Pintado, M. M., Silvestre, A. J. D., & Santos, S. A. O. (2025). Identification of Novel Metabolic Signatures on Human Gut Microbiota: Ellagic Acid, Naringenin, and Phloroglucinol. International Journal of Molecular Sciences, 26(22), 11009. https://doi.org/10.3390/ijms262211009












