Inflammasomes as Potential Therapeutic Targets to Prevent Chronic Active Viral Myocarditis—Translating Basic Science into Clinical Practice
Abstract
1. Introduction
1.1. Epidemiology of Myocarditis
1.2. Diagnostic Work-Ups
1.3. Natural Course of Myocarditis
1.4. Elimination of Pathogen vs. Progression to Chronic Active Inflammation
2. An Illustrative Case Report
3. Discussion and the Literature Review
3.1. Myocarditis Caused by Human Metapneumovirus (hMPV) and Opportunistic Bacterial Streptococcus pneumoniae Infection: A Brief Literature Review
3.2. Progression from Acute to Chronic Active Myocarditis
3.3. Role of Inflammasomes in Myocarditis
3.3.1. The NLRP3 Pathway
3.3.2. CARD8 Pathway
3.3.3. AIM2 Pathway
3.3.4. Caspase-11 Inflammasome
3.4. Post-Infectious Phase
3.5. Therapeutic Approaches in VMC Based on Pathogen and Disease Phase
3.5.1. Specific Antiviral Therapy in the Acute Phase of Myocarditis
3.5.2. Immunosuppression in Active and Chronic Active Myocarditis
3.6. Targeting Inflammasomes: A Future for Myocarditis
3.6.1. Inhibition of NF-κB Pathway
3.6.2. Direct NLRP3 Inhibitors
3.6.3. Colchicine
3.6.4. Dapansutrile (OLT1177)
3.6.5. INF200
3.6.6. Canakinumab
3.6.7. Anakinra and IL-1 Receptor Accessory Protein Monoclonal Antibody
3.6.8. Monoclonal Antibodies and Drugs Targeting IL-18
| Study | Medication/Comparator | Type of the Study | Study Design | Main Findings | Outcomes | Remarks/Limitations |
|---|---|---|---|---|---|---|
| Inhibitors of the NLRP3 pathway | ||||||
| Chin, CG et al. 2024 [138] | MCC950 vs. placebo | Experimental animal model | Rats with myosin peptide–induced myocarditis (experimental group) were treated with an NLRP3 inhibitor (MCC950; 10 mg/kg, daily for 14 days) or left untreated | Rats treated with MCC950 improved their LV-EF and reduced the frequency of premature ventricular contraction | Changes in heart structure may be mitigated by inhibiting NLRP3 signaling. | A study on animal models |
| Golino M et al. 2024 [139] | Colchicine vs. placebo in a 1:1 proportion | Retrospective multicenter study in the US of patients hospitalized with acute myocarditis | In total, 1137 patients with acute myocarditis treated with colchicine within 14 days of diagnosis vs. those not receiving colchicine | The incidence of the all-cause death was 3.3% vs. 6.6% (HR, 0.48, 95% CI, 0.33–0.71; p < 0.001), ventricular arrhythmias: 6.1% vs. 9.1% (HR, 0.65, 95% CI, 0.48–0.88; p < 0.01), and acute HF: 10.9% vs. 14.7% (HR 0.72, 95% CI, 0.57–0.91; p < 0.01) in patients treated with colchicine or not, respectively | Patients with acute myocarditis treated with colchicine within 14 days of diagnosis have better outcomes at 90 days | Short-term outcomes |
| Collini et al. 2024 [140] | 45.1% of patients were treated with colchicine | Retrospective cohort study | A total of 175 patients with pericarditis and myocarditis, 88.6% with an idiopathic/viral etiology of myocarditis | In multivariable Cox regression analysis, women (HR 1.97, 95% CI 1.04 to 3.73; p = 0.037) and corticosteroid use (HR 2.27, 95% CI 1.15 to 4.47; p = 0.018) were risk factors of recurrence, and colchicine use prevented recurrences (HR 0.39, 95% CI 0.21 to 0.76; p = 0.005) | After a median follow-up of 25.3 (IQR 8.3–45.6) months, colchicine use was associated with a lower incidence of recurrences (respectively, 19.2% vs. 43.8%; p = 0.001) and a longer event-free survival (p = 0.005) | Concomitant pericarditis and myocarditis |
| Pappritz et al. 2022 [142] | Colchicine (5 µmol/kg p.o. daily) vs. vehicle (PBS) | Preclinical, experimental | Murine model of CVB3-induced myocarditis; treatment started 24 h post-infection for 7 days | Colchicine significantly improved LV-EF, reduced viral load, and decreased inflammatory cell infiltration (ASC+, caspase-1+, IL-1β+ cells) in myocardium and spleen. | Reduced fibrosis markers, cardiac troponin I, and lower collagen deposition. | Preclinical only |
| GRECCO-19 trial, 2020 [143] | 52.4% of the total 105 patients were treated with colchicine | RCT phase 3 | to explore the potential of colchicine to attenuate COVID-19–related myocardial injury | Patients who received colchicine had a significantly improved time to clinical deterioration. There were no significant differences in hs-Tn or CRP levels | a significant clinical benefit from colchicine in patients hospitalized with COVID-19 | Cardiac complications of COVID-19 infection |
| NCT05855746 [https://clinicaltrials.gov/study/NCT05855746 accessed on 11 August 2025] | Colchicine vs. placebo | RCT phase 3 | Three hundred adults with acute myocarditis; primary endpoint at 6 months: composite of rehospitalization, recurrent chest pain, arrhythmias, changes in LGE percentage by CMR | No results, as yet | Results expected post 2028 | Results not yet available |
| CMP-MYTHiC NCT06158698 [https://cdek.pharmacy.purdue.edu/trial/NCT06158698/ accessed on 11 August 2025] | Colchicine vs. placebo | RCT phase 3 | In total, 80 adults with chronic inflammatory cardiomyopathy and impaired LV-EF or ventricular arrhythmias were to receive colchicine or placebo for 6 months, with outcomes assessed by imaging, biomarkers, and arrhythmic burden. | No results, as yet | Results expected post 2026 | Chronic myocarditis, small population |
| Wohlford GF et al. 2020 [144] | Dapansutrile (OLT1177) | RCT phase 1B | Patients with HFrEF, dose escalation, single-center, repeat dose safety and pharmacodynamics study of dapansutrile in stable patients with HFrEF | Improvements in LV-EF [from 31.5% (27.5–39) to 36.5% (27.5–45), p = 0.039] and in exercise time [from 570 (399.5–627) to 616 (446.5–688) seconds, p = 0.039] were seen in the dapansutrile 2000 mg cohort | Treatment with dapansutrile was well-tolerated and safe over a 14-day treatment period. | A study for HFrEF, not specifically in myocarditis |
| Wang et al. 2022 [145] | INF200 (1,3,4-oxadiazol-2-one) | preclinical | Experiments on heart stress in rats | INF200 works by inhibiting the NLRP3 inflammasome, which in turn reduces inflammation and its associated detrimental effects on the heart | Reduced cardiac biomarkers and ischemia–reperfusion injury in diet-induced metabolic heart stress in rats | A study on animal models |
| IL-1 receptor antagonists | ||||||
| CANTOS TRIAL [146] | Canakinumab: IL-1 receptor antagonist given s.c. in doses of 50 mg, 150 mg, or 300 mg, vs. placebo | RCT phase 3 | A total of 10,061 patients with prior MI and high hs-CRP (≥2 mg/L) given s.c. canakinumab at 50 mg, 150 mg, or 300 mg every 3 months | The 150 mg dose of canakinumab significantly reduced the incidence of recurrent cardiovascular events compared to placebo | The canakinumab dose of 150 mg was associated with a reduced occurrence of the primary endpoint and a reduction in IL-6 and CRP levels | A study for myocardial infarction, not specifically in myocarditis |
| MRC-ILA [148] | Anakinra given s.c. IL-1 ra vs. placebo, 1:1 allocation | RCT phase 2 | 182 patients with NSTEMI; treatment for 14 days; primary endpoint: 7-day CRP | Significant CRP reduction (≈50% vs. placebo); no difference in 30-day or 3-month MACE | Lower inflammation; no clinical benefit; unexpected rise in CV events at one-year follow-up | A study for myocardial infarction, not specifically in myocarditis |
| VCU-ART3 [149] | Anakinra 100 mg once daily or twice daily vs. placebo | RCT phase 2 | 99 STEMI patients within 12 h of symptom onset; 14-day treatment; primary endpoint: CRP level to day 14; follow-up to 12 months for echocardiographic remodeling and MACE | Not yet fully published, but early phase results showed significant CRP reduction during treatment | Primary: reduced inflammation (CRP AUC); secondary: pending data on LV remodeling and MACE over 12 months. | A study for myocardial infarction, not specifically in myocarditis |
| ARA-MIS Trial (Kerneis et al.) [152] | Anakinra (IL-1 receptor antagonist: IL-1 ra) 100 mg vs. placebo | RCT phase 2 | 120 patients hospitalized with CMR-proven acute myocarditis, without severe hemodynamic instability or cardiogenic shock | No significant difference from myocarditis complications within 28 days. Significant reduction in systemic inflammatory markers such as CRP and IL-6 | Safety confirmed; well-tolerated, but no efficacy signal in low-risk patients | Short course, low-risk population, low incidence of complicated myocarditis in both groups |
| Lema et al. 2024 [153] | IL1RAP monoclonal antibody vs. placebo, or vs. anakinra/IL-1Ra | Preclinical, mice model | Induced CVB3-mediated myocarditis or experimental autoimmune myocarditis in mice, followed by the treatment with anti-mouse IL1RAP monoclonal antibody vs. placebo, or IL-1Ra treatment | IL1RAP blockade with a monoclonal antibody, compared with placebo and IL1Ra, reduced inflammatory monocytes, T cells, neutrophils, and eosinophils in the heart in CVB3-mediated VMC, and preserved heart function on echocardiography in autoimmune myocarditis | The effect on the reduction in inflammation was higher in IL1RAP blockade compared with anti-IL-1Ra treatment alone, and placebo | Study of viral and autoimmune myocarditis |
| Interleukin-18 inhibitors | ||||||
| Li et al. 2021 [154] | Anti–IL-1R7 monoclonal antibody vs. isotype control | Preclinical, in vitro human cells + in vivo mouse models | Assessed suppression of IL-18–mediated signaling in human PBMCs and whole blood; in mice, evaluated protection against LPS- or Candida-induced hyperinflammation | Blockade of IL-1R7 strongly inhibited IL-18–induced NF-κB activation and downstream cytokines (IFNγ, IL-6, TNFα) in mice, significantly reduced systemic inflammation, and protected tissues (lung, liver) from LPS-induced injury | Demonstrated proof-of-concept that IL-1R7 blockade effectively attenuates IL-18–driven inflammation. | No direct myocarditis model was used |
| Jiang L et al. 2024 [155] | anti-human IL-1R7 antibody | Mice model | A novel humanized monoclonal antibody, which specifically blocks the activity of human IL-18 and its inflammatory signaling in human cells and whole blood cultures, was tested in hyperinflammation in the acute lung injury model | In the current study, anti-IL-1R7 suppressed LPS-induced inflammatory cell infiltration in lungs and inhibited subsequent IFN-γ production | An IL-1R7 antibody protects mice from LPS-induced tissue and systemic inflammation | Aimed to combat macrophage activation syndrome and COVID-19 infection |
3.7. Angiotensin Receptor-Neprilysin Inhibitor (ARNI)
3.7.1. Off-Label Use of ARNI
3.7.2. ARNI in Acute Myocardial Infarction: RCT Results
3.7.3. ARNI in Doxorubicin-Induced DCM
3.7.4. Potential of ARNIs in Acute Myocarditis: A Review of the Literature
3.8. Role of Cardiac Fibrosis and Anti-Fibrotic Treatment Approaches
| Study | Medication/Comparator | Type of the Study | Study Design | Main Findings | Outcomes | Remarks/Limitations |
|---|---|---|---|---|---|---|
| ARNI | ||||||
| PARADIGM-HF [161] | Sac/Val vs. enalapril | RCT phase 3 | In total, 8442 patients with chronic HF, NYHA class II–IV symptoms, an elevated plasma BNP or NT-proBNP level, and an LVEF of ≤35% | The primary outcome of CVD or hospitalization for HF was significantly lower in the ARNI arm compared with the enalapril arm (21.8% vs. 26.5%; HR, 0.80; 95% CI, 0.73 to 0.87; p < 0.001). | ARNI use reduced the risk of CVD by 16%, hospitalization for HF by 21% and decreased the symptoms and physical limitations of HF. | Study terminated earlier due to high benefits from ARNI use. Only 0.7% of patients in the NYHA functional class IV had symptoms |
| OUT-STEP [162] | Sac/Val vs. enalapril | Observation-al study | A total of 621 ambulatory patients with stable symptomatic HFrEF were randomized 1:1 to Sac/Val (n = 310) or enalapril (n = 311) | The study found no difference between the effect of Sac/Val vs. enalapril on 6-min walk test (6MWT) distance, non-sedentary daytime physical activity, and HF symptoms | No significant benefit of Sac/Val compared with enalapril on either 6MWT or daytime physical activity after 12 weeks | Only 0.7% of patients in the NYHA functional class IV had symptoms |
| PIONEER-HF trial [163] | Sac/Val vs. enalapril | RCT phase 3 | A total of 736 hospitalized patients were treated for acute decompensated HF with HFrEF after stabilization. | A greater reduction in the NTproBNP, hs-TnT, and a lower rate of rehospitalization for HF in Sac/Val treatment compared to enalapril treatment was observed at 4 and 8 weeks | Fewer hospital admissions for HF in the Sac/Val arm | Patients hospitalized for acute HF after stabilization, irrespective of HF background |
| LIFE trial [164] | Sac/Val vs. valsartan | RCT phase 3 | In total, 335 patients with HFrEF and recently advanced HF (NYHA class IV) | Compared to valsartan, treatment with Sac/Val has not improved the clinical composite of number of days alive, out of hospital, and free from HF events (p = 0.450). | The results showed that Sac/Val was not superior to valsartan and had a 29% discontinuation rate during the 24 weeks of the trial | Not enrolled, a predefined group of 400 patients; it was terminated prematurely due to the COVID-19 pandemic |
| She et al. 2021 [171] | ARNI, ACEI, and ARB groups | Propensity score of patients included in the Hospital of Xi’an Jiaotong University database | A total of 646 eligible patients with AMI were assigned to the ARNI, ACEI, and ARB groups, respectively. | Patients receiving ARNI had significantly lower rates of the composite cardiovascular outcome of CVD, MI, HF hospitalization, and IS than ACEI [HR, 0.51, 95% CI, 0.27–0.95, p = 0.02], and ARB users [HR 0.47, 95% CI, 0.24–0.90, p = 0.02]. Patients receiving ARNI showed lower rates of CVD than ACEI [HR, 0.37, 95% CI, 0.18–0.79, p = 0.01] and ARB users [HR, 0.41, 95% CI, 0.18–0.95, p = 0.04]. | Subgroup analysis indicated that patients with LVEF < 40% benefit more from ARNI as compared with ACEI [HR 0.30, (95%CI, 0.11–0.86), p = 0.01] or ARB [HR 0.21, (95%CI, 0.04–1.1), p = 0.05]. Patients aged <60 years exhibited reduced composite endpoints [HR for ARNI vs. ARB: 0.11, (95%CI, 0.03–0.46), p = 0.002]. | Not randomized, not specific for myocarditis |
| PARADISE-MI [172] | Sac/Val vs. ramipril | RCT phase 3 | 5661 post-MI patients with reduced LV-EF (≤40%) ± pulmonary congestion, randomized within 0.5–7 days post-infarction, followed for ~23 months | No significant difference in the primary endpoint (CV death, first HF hospitalization, or outpatient HF: 11.9% vs. 13.2%, p = 0.17) | Numerically fewer total HF events and coronary events with Sac/Val; higher incidence of hypotension (28% vs. 22%). | A study for myocardial infarction, not specifically in myocarditis |
| Doxorubicine-induced DCM | ||||||
| Boutagy et al. 2020 [177] | Sac/Val vs. valsartan vs. placebo | Preclinical experimental study | DOX-induced cardiotoxicity in rats. The study aimed to compare the cardioprotective effect of ARNI, valsartan, vs. placebo | The treatment with ARNI caused lower LV-EF reduction compared with valsartan alone and placebo (p < 0.05). Cardiac fibrosis was similar in rats treated with valsartan alone, compared with Sac/Val, and significantly lower compared to DOX alone | Preservation of LV-EF in the group with ARNI. Sac/Val occurred more cardioprotective than Val in a rodent model of progressive DOX-induced cardiotoxicity. Val therapy alone only attenuated DOX-induced toxicity and fibrosis at the cellular level, whereas ARNI therapy preserved LV-EF and inhibited myocardial MMP activation. | A study on animal models |
| Dindas et al. 2021 [180] | Sac/Val pretreatment vs. doxorubicin | Preclinical experimental study | Four groups in mice (control; DOX only; Sac/Val only; Sac/Val pretreatment + DOX); Sac/Val given 80 mg/kg from day 1 before DOX (20 mg/kg at day 5) | Pretreatment with Sac/Val significantly attenuated DOX-induced ECG changes, oxidative stress, and inflammation, compared with DOX alone (p < 0.001). NT-proBNP levels were lower in the Sac/Val+DOX group compared with the DOX group, along with less caspase-3 apoptosis. | Sac/Val protects the cardiac electrophysiology, reducing biochemical and histologic markers of injury during DOX therapy. | A study on animal models |
| Kim et al. 2022 [181] | Low-dose ARNI + SGLT2i vs. monotherapy or placebo | Preclinical experimental study | Mouse model of doxorubicin-induced cardiotoxicity | Low-dose ARNI + SGLT2i improved survival, cardiac function, and reduced myocardial damage more than monotherapies or full-dose combo. | Additive value of low-dose ARNI + SGLT2i | A study on animal models |
| Myocarditis | ||||||
| Nesukay et al. 2024 [187] | Sac/Val vs. enalapril | Prospective observational | Patients with acute myocarditis and HFrEF ≤ 40% treated with either enalapril (n = 48) or Sac/Val (n = 42), followed for 12 months with echocardiography and CMR | The Sac/Val group showed greater improvement in EF, myocardial strain, and functional capacity than the enalapril group | Improved cardiac function and exercise tolerance with Sac/Val | Non-randomized design and longer-term safety data are needed |
| Liang et al. 2022 [188] | Sac/Val vs. valsartan alone | Preclinical, experimental animal study | Mice induced with experimental autoimmune myocarditis; treated with Sac/Val or valsartan during the disease course. | Sac/Val significantly reduced myocardial inflammation, decreased Th17 cell differentiation, and lowered IL-1β and IL-6 expression | Reduced inflammatory cell infiltration, improved histology. | A study on animal models |
| Anti-fibrotic treatment | ||||||
| Liu et al. 2024 [206] | Sac/Val vs. placebo | In vitro | In total, 30 patients were diagnosed with AMC (Autoimmune acute myocarditis) | Sac/Val alleviated myocardial inflammation while augmenting circulating CNP levels rather than BNP and ANP, accompanied by reductions in intracardial M1 macrophage infiltration and expression of inflammatory cytokines IL-1β, TNF-α, and IL-6 | Sac/Val exerts a protective effect in myocarditis by increasing CNP concentration and inhibiting M1 macrophage polarization | C-type natriuretic peptide (CNP) |
| Wang et al. 2023 [208] | Tranilast | Experimental cell culture | An H/R model of H9c2 cardiomyocytes was established to simulate I/R-induced cardiomyocyte injury | Tranilast increased the viability of H9c2 cells, while decreasing I/R injury-induced cardiomyocyte apoptosis through reducing the expression levels of the Nrf2/HO-1/NF-κB signaling pathway | Tranilast decreased apoptosis, oxidative stress, and inflammatory response in H/R-induced H9c2 cells by activating Nrf2/HO-1/NF-κB signaling | |
| Huang et al. 2014 [209] | Tranilast | Experimental (mice) | Three subgroups of mice with CVB3-induced myocarditis, receiving tranilast (n = 24), placebo (n = 24), and 24 controls | The mRNA and protein expression of TGF-β1 and OPN was lower in the tranilast group than in the other groups | Tranilast reduced myocardial fibrosis by decreasing the number of mast cells and inhibiting the expression of TGF-β1 and OPN | |
| Levis et al. 2021 (PIROUETTE, NCT02932566) [210] | Pirfenidone vs. placebo | RCT phase 2 | A total of 94 patients with stable symptomatic HFpEF (≥45%), elevated levels of natriuretic peptides, and CMR documented myocardial fibrosis (ECV ≥ 27%), randomized 1:1 to oral pirfenidone (n = 47) or placebo (n = 47) for 52 weeks | The primary outcome of the change in myocardial extracellular volume, from baseline to 52 weeks, was higher in the pirfenidone receiving group compared with the placebo (between-group difference, −1.21%; 95%CI, −2.12 to −0.31; p = 0.009) | In comparison to the placebo, pirfenidone significantly reduced myocardial extracellular volume | 26% in the pirfenidone group and 30% in the placebo group experienced serious adverse events (nausea, rash, insomnia) |
3.9. MicroRNAs in VMC
| Study | Pathogen | microRNA | Down vs. Upregulated | Rationale for Use of Individual microRNA | Therapeutic Approach |
|---|---|---|---|---|---|
| Goldberg et al. 2018 [219] | enteroviral, adenoviral, or parvoviral B19 myocarditis | miR-208a, miR-208b, miR-499, and miR-21 | Up or Down | upward or downward dynamics depending on the phase of infection | No data |
| Gong et al. 2023 [224] | miR-21 | Down | miR-21 downregulation protects myocardial cells against LPS-induced apoptosis and inflammation through Rcan1 signaling | No data | |
| Li et al. 2022 [225] | miR-21 | Down | miR-21 downregulation protects myocardial cells against LPS-induced apoptosis and inflammation by targeting Bcl-2 and CDK6 | No data | |
| Yang et al. 2018 [226] | miR-21 | Down | miR-21 deficiency promoted inflammatory cytokine production and worsened cardiac function in cardiac ischemia through targeting KBTBD7 | No data | |
| Bao et al. 2014 [229] | Coxsackie B3 myocarditis | miR-155, miR-148 | Up | miR-155 and miR-148a were shown to reduce cardiac injury during the acute phase in humans by inhibiting the NF-κB pathway | miR-155 reduced cardiac myoblast cytokine expression. Increased survival in miR-155-treated mice |
| Corsten MF et al. 2012 [230] | CVB3 myocarditis in humans and susceptible mice | miR-155, miR-146b, miR-21 | Up | Inhibition of miR-155 by a systemically delivered LNA-anti-miR attenuated cardiac infiltration by monocyte-macrophages, decreased T lymphocyte activation, and reduced myocardial damage during acute myocarditis in mice | LNA-anti-miR-155 may reduce inflammation activity in mice with CVB3 |
| Zhang Y et al. 2016 [231] | CVB3 myocarditis | miR-155 | Up | miR-155 is upregulated in CVB3 myocarditis, and localized primarily in heart-infiltrating macrophages and CD4+ T lymphocytes, promoting macrophage polarization to pro-inflammatory M1. Silencing miR-155 led to increased levels of alternatively activated macrophages (anti-inflammatory M2) | miR-155 may be a potential therapeutic target for VMC |
| Liu et al. 2013 [232] | CVB3 myocarditis | miR-146b | Up | miR-146b was highly expressed in mice with CVB3. Its inhibition reduced inflammatory lesions and suppressed Th17 cells differentiation | Inhibiting miR-146b may lead to a reduction in the severity of myocarditis |
| Blanco-Domínguez et al. [234] | A murine model of viral/autoimmune myocarditis in mice | miR-721 | Up | Increased expression levels of miR-721 in a murine model of viral/autoimmune myocarditis. miR-721, synthesized by Th17 cells, was detectable in the plasma of mice with myocarditis but absent in infarcted mice. A murine model of viral/autoimmune myocarditis in mice | Antagomir miR-721: potential to silence Th17 cells and thus suppress inflammatory pathways in VMC |
| Li, J. et al. 2021 [235] | CVB3-infected mice | miR-425-3p | Up | Reduction in IL-6, IL-12, and TNF-α in VMC mice treated with miR-425-3p compared to non-treated VMC mice | miR-425-3p inhibits myocardial inflammation and cardiomyocyte apoptosis in CVB3 myocarditis |
| Li W et al. 2020 [236] | CVB3-infected mice | miR-1/133a | Up | miR-1/133 mimics upregulated the expression of miR-1 and miR-133, the potassium channel genes Kcnd2 and Kcnj2, as well as Bcl-2, and downregulated the expression of the potassium channel suppressor gene Irx5, L-type calcium channel subunit gene a1c (Cacna1c), Bax, and caspase-9 in the myocardium of VMC mice. miR-1/133 also upregulated the protein levels of Kv4.2 and Kir2.1, and downregulated the expression of CaV1.2 | miR-1/133 mimics attenuate cardiomyocyte apoptosis and electrical remodeling in mice with VMC |
| Deng et al. 2014 [238] | hMPV infection | 142 miRs upregulated, 32 miRs downregulated let-7f | Up Down Up | let-7f was significantly upregulated and exhibited antiviral effects: its inhibitors increased viral replication | Let-7f mimics reduced viral replication |
| Wu et al. 2020 [239] | hMPV infection | miR-16, miR-30a | Up | miR-16 regulation depended on type I IFN signaling, whereas miR-30a was IFN independent, suggesting potential therapeutic targets | No data |
| Martínez-Espinoza et al. 2023 [59] | hMPV infection | miR-4634 | Up | hsa-miR-4634 enhances viral immune evasion by inhibiting type I IFN responses and IFN-stimulated genes, increasing viral replication in macrophages and epithelial cells | No data |
| Srivastava et al. 2023 [240] | SARS-CoV-2 | miR-335-3p | Up | miR-335-3p expression level predicted COVID-19 infection severity | No data |
| Salvado et al. 2025 [241] | Parvovirus B19 | miR-4799-5p, miR-5690, miR-335-3p, miR-193b-5p, and miR-6771-3p were highly expressed in the B19V transcripts | Up | promising biomarkers of infection progression | No data |
| Eilam-Frenkel et al. 2018 [242] | Respiratory syncytial virus (RSV) | miR-146a let-7c, miR-345, miR-221 | Up Down | miR-146a-5p were upregulated, whereas let-7c, miR-345-5p, and miR-221 were downregulated by prolonged RSV infection | No data |
| Zhang Y et al. 2016 [243] | Respiratory syncytial virus (RSV) | miR-29a | Up | Respiratory syncytial virus non-structural protein 1 facilitates virus replication through miR-29a-mediated inhibition of IFN-alpha receptor | No data |
| Chen et al. 2022 [244] | Fulminant myocarditis | miR-29b, miR-125b | Up | miR-29b demonstrated higher sensitivity and specificity for the fulminant myocarditis. Upregulation of miR-29b and miR-125b in the plasma of patients with fulminant myocarditis positively correlated with the area of myocardial edema and was negatively correlated with the LVEF | No data |
| Taubel et al. 2021 [247] | HF | miR-132 | Up | Randomized, phase 1b controlled trial evaluating the impact of CDR132L (antagomir-miR-132) on cardiac function over 3 months in a chronic HF model. Treatment with CDR132L significantly improved systolic and diastolic cardiac function, reduced cardiac fibrosis, and attenuated adverse remodeling | Demonstrated the therapeutic potential of targeting microRNA-132 for HF management |
| HF-REVERT, 2025 [248] | HF after myocardial infarction | miR-132 | Up | Randomized, phase 2, controlled study in patients with HFrEE following myocardial infarction showed the safety of antagomir-132 treatment, without an effect on LV remodeling | Demonstrated safety of the treatment with antagomir-132 |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammirati, E.; Moslehi, J.J. Diagnosis and Treatment of Acute Myocarditis. JAMA 2023, 329, 1098. [Google Scholar] [CrossRef] [PubMed]
- Golpour, A.; Patriki, D.; Hanson, P.J.; McManus, B.; Heidecker, B. Epidemiological Impact of Myocarditis. J. Clin. Med. 2021, 10, 603. [Google Scholar] [CrossRef]
- Brociek, E.; Tymińska, A.; Giordani, A.S.; Caforio, A.L.; Wojnicz, R.; Grabowski, M.; Ozierański, K. Myocarditis: Etiology, Pathogenesis, and Their Implications in Clinical Practice. Biology 2023, 12, 874. [Google Scholar] [CrossRef]
- Pollack, A.; Kontorovich, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis—Diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636d–2648d. [Google Scholar] [CrossRef]
- Albakri, A. Bacterial cardiomyopathy: A review of clinical status and meta-analysis of diagnosis and clinical management. Trends Res. 2019, 2, 2516–7138. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Giordani, A.S.; Simone, T.; Baritussio, A.; Vicenzetto, C.; Scognamiglio, F.; Donato, F.; Licchelli, L.; Cacciavillani, L.; Fraccaro, C.; Tarantini, G.; et al. Hydrocarbon Exposure in Myocarditis: Rare Toxic Cause or Trigger? Insights from a Biopsy-Proven Fulminant Viral Case and a Systematic Literature Review. Int. J. Mol. Sci. 2025, 26, 4006. [Google Scholar] [CrossRef] [PubMed]
- Blauwet, L.A.; Cooper, L.T. Antimicrobial agents for myocarditis: Target the pathway, not the pathogen. Heart 2009, 96, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, Z.; Mo, P.; Li, X.; Ma, Z.; Song, S.; Chen, X.; Luo, M.; Liang, K.; Gao, S.; et al. Clinical Characteristics and Outcomes of Older Patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China: A Single-Centered, Retrospective Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1788–1795. [Google Scholar] [CrossRef]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Schmidt, M.; Hekimian, G.; Peretto, G.; Bochaton, T.; et al. Prevalence, characteristics, and outcomes of COVID-19 associated acute myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Porcari, A.; Merlo, M.; Roncon, L.; Sinagra, G. One-Year Risk of Myocarditis After COVID-19 Infection: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2023, 39, 839–844. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Toth, K.; Alexy, T. Myocarditis after COVID-19 and influenza infections: Insights from a large data set. Open Heart 2024, 11, e002973. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, B.; Prasad, V. COVID-19 vaccine induced myocarditis in young males: A systematic review. Eur. J. Clin. Investig. 2023, 53, e13947. [Google Scholar] [CrossRef]
- Yao, Z.; Liang, M.; Zhu, S. Infectious factors in myocarditis: A comprehensive review of common and rare pathogens. Egypt. Heart J. 2024, 76, 64. [Google Scholar] [CrossRef]
- Dion, C.F.; Ashurst, J.V. Streptococcus pneumoniae. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Brown, A.O.; Mann, B.; Gao, G.; Hankins, J.S.; Humann, J.; Giardina, J.; Faverio, P.; Restrepo, M.I.; Halade, G.V.; Mortensen, E.M.; et al. Streptococcus pneumoniae Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function. PLoS Pathog. 2014, 10, e1004383. [Google Scholar] [CrossRef]
- Shenoy, A.T.; Orihuela, C.J. Anatomical site-specific contributions of pneumococcal virulence determinants. Pneumonia 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Kruckow, K.L.; D’Mello, A.; Ganaie, F.; Martinez, E.; Luck, J.N.; Cichos, K.H.; Riegler, A.N.; Song, X.; Ghanem, E.; et al. Anatomical Site-Specific Carbohydrate Availability Impacts Streptococcus pneumoniae Virulence and Fitness during Colonization and Disease. Infect. Immun. 2022, 90, e0045121. [Google Scholar] [CrossRef]
- Piazza, I.; Ferrero, P.; Marra, A.; Cosentini, R. Early Diagnosis of Acute Myocarditis in the ED: Proposal of a New ECG-Based Protocol. Diagnostics 2022, 12, 481. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Bozkurt, B.; Cooper, L.T.; Aggarwal, N.R.; Basso, C.; Bhave, N.M.; Caforio, A.L.P.; Ferreira, V.M.; Heidecker, B.; Kontorovich, A.R.; et al. 2024 ACC Expert Consensus Decision Pathway on Strategies and Criteria for the Diagnosis and Management of Myocarditis: A Report of the American College of Cardiology Solution Set Oversight Committee. JACC 2025, 85, 391–431. [Google Scholar] [CrossRef] [PubMed]
- Ramantauskaitė, G.; Okeke, K.A.; Mizarienė, V. Myocarditis: Differences in Clinical Expression between Patients with ST-Segment Elevation in Electrocardiogram vs. Patients without ST-Segment Elevation. J. Pers. Med. 2024, 14, 1057. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Collini, V.; Gröschel, J.; Adler, Y.; Brucato, A.; Christian, V.; Ferreira, V.M.; Gandjbakhch, E.; Heidecker, B.; Kerneis, M.; et al. 2025 ESC Guidelines for the management of myocarditis and pericarditis. Eur. Heart J. 2025, 46, 3952–4041. [Google Scholar] [CrossRef]
- Khanna, S.; Li, C.; Amarasekera, A.T.; Bhat, A.; Chen, H.H.L.; Gan, G.C.H.; Tan, T.C. Echocardiographic parameters of cardiac structure and function in the diagnosis of acute myocarditis in adult patients: A systematic review and meta-analysis. Echocardiography 2024, 41, e15760. [Google Scholar] [CrossRef]
- Supeł, K.; Wieczorkiewicz, P.; Przybylak, K.; Zielińska, M. 2D Strain Analysis in Myocarditis—Can We Be Any Closer to Diagnose the Acute Phase of the Disease? J. Clin. Med. 2023, 12, 2777. [Google Scholar] [CrossRef]
- Joudar, I.; Aichouni, N.; Nasri, S.; Kamaoui, I.; Skiker, I. Diagnostic criteria for myocarditis on cardiac magnetic resonance imaging: An educational review. Ann. Med. Surg. 2023, 85, 3960–3964. [Google Scholar] [CrossRef]
- Domínguez, F.; Uribarri, A.; Larrañaga-Moreira, J.M.; Ruiz-Guerrero, L.; Pastor-Pueyo, P.; Gayán-Ordás, J.; Fernández-González, B.; Esteban-Fernández, A.; Barreiro, M.; López-Fernández, S.; et al. Diagnosis and treatment of myocarditis and inflammatory cardiomyopathy. Consensus document of the SEC-Working Group on Myocarditis. Rev. Española Cardiol. 2024, 77, 667–679. [Google Scholar] [CrossRef]
- Ammirati, E.; Buono, A.; Moroni, F.; Gigli, L.; Power, J.R.; Ciabatti, M.; Garascia, A.; Adler, E.D.; Pieroni, M. State-of-the-Art of Endomyocardial Biopsy on Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Curr. Cardiol. Rep. 2022, 24, 597–609. [Google Scholar] [CrossRef]
- Hutt, E.; Kaur, S.; Jaber, W.A. Modern tools in cardiac imaging to assess myocardial inflammation and infection. Eur. Heart J. Open 2023, 3, oead019. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yi, W.; Gao, C.; Qi, T.; Li, L.; Xie, K.; Zhao, W.; Chen, W. Cardiac magnetic resonance feature tracking myocardial strain analysis in suspected acute myocarditis: Diagnostic value and association with severity of myocardial injury. BMC Cardiovasc. Disord. 2023, 23, 162. [Google Scholar] [CrossRef]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O.; et al. Recognition and Initial Management of Fulminant Myocarditis. Circulation 2020, 141, E69–E92. [Google Scholar] [CrossRef]
- Lasica, R.; Djukanovic, L.; Savic, L.; Krljanac, G.; Zdravkovic, M.; Ristic, M.; Lasica, A.; Asanin, M.; Ristic, A. Update on Myocarditis: From Etiology and Clinical Picture to Modern Diagnostics and Methods of Treatment. Diagnostics 2023, 13, 3073. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Xie, R.; Kirklin, J.K.; Cowger, J.; Oliveira, G.H.; Krabatsch, T.; Nakatani, T.; Schueler, S.; Leet, A.; Golstein, D.; et al. Outcomes of Durable Mechanical Circulatory Support in Myocarditis: Analysis of the International Society for Heart and Lung Transplantation Registry for Mechanically Assisted Circulatory Support Registry. ASAIO J. 2022, 68, 190–196. [Google Scholar] [CrossRef]
- Kim, M.J.; Jung, H.O.; Kim, H.; Bae, Y.; Lee, S.Y.; Jeo, D.S. 10-year survival outcome after clinically suspected acute myocarditis in adults: A nationwide study in dunish the pre-COVID-19 era. PLoS ONE 2023, 18, e0281296. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- Hashmani, S.; Manla, Y.; Al Matrooshi, N.; Bader, F. Red Flags in Acute Myocarditis. Card. Fail. Rev. 2024, 10, e02. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Katsuki, M.; Amemiya, K.; Takahashi, A.; Oyama-Manabe, N.; Ohta-Ogo, K.; Imanaka-Yoshida, K.; Ishibashi-Ueda, H.; Anzai, T. Chronic Active Myocarditis and Inflammatory Cardiomyopathy—Challenges in Diagnosis and Treatment. Circ. J. 2025; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Ammirati, E.; Ponnaiah, M.; Montero, S.; Raimbault, V.; Abrams, D.; Lebreton, G.; Pellegrino, V.; Ihle, J.; Bottiroli, M.; et al. Fulminant myocarditis proven by early biopsy and outcomes. Eur. Heart J. 2023, 44, 5110–5124. [Google Scholar] [CrossRef] [PubMed]
- Ghanizada, M.; Kristensen, S.L.; Bundgaard, H.; Rossing, K.; Sigvardt, F.; Madelaire, C.; Gislason, G.H.; Schou, M.; Hansen, M.L.; Gustafsson, F. Long-term prognosis following hospitalization for acute myocarditis—A matched nationwide cohort study. Scand. Cardiovasc. J. 2021, 55, 264–269. [Google Scholar] [CrossRef]
- Martens, C.R.; Accornero, F. Viruses in the Heart: Direct and Indirect Routes to Myocarditis and Heart Failure. Viruses 2021, 13, 1924. [Google Scholar] [CrossRef]
- Falleti, J.; Orabona, P.; Municinò, M.; Castellaro, G.; Fusco, G.; Mansueto, G. An Update on Myocarditis in Forensic Pathology. Diagnostics 2024, 14, 760. [Google Scholar] [CrossRef]
- Van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef]
- Uddin, S.; Thomas, M. Human Metapneumovirus. [Updated 17 July 2023]. In StatPearls; StatPearls Publishing: Treasure Island FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560910/ (accessed on 11 August 2025).
- Hennawi, H.A.; Khan, A.; Shpilman, A.; Mazzoni, J.A. Acute myocarditis secondary to human metapneumovirus: A case series and mini-review. Glob. Cardiol. Sci. Pract. 2024, 2024, e202452. [Google Scholar] [CrossRef]
- Makhlouf, A.; Peipoch, L.; Duport, P.; Darrieux, E.; Reguerre, Y.; Ramful, D.; Alessandri, J.L.; Levy, Y. First Case of Acute Myocarditis Caused by Metapneumovirus in an Immunocompromised 14-year-old Girl. Indian J. Crit. Care Med. 2022, 26, 745–747. [Google Scholar] [CrossRef]
- Wang, S.H.; Lee, M.H.; Lee, Y.J.; Liu, Y.C. Metapneumovirus-Induced Myocarditis Complicated by Klebsiella pneumoniae Co-Infection: A Case Report. Am. J. Case Rep. 2024, 25, e946119. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xie, Z.; Xu, L. Receptors and host factors: Key players in human metapneumovirus infection. Front. Cell. Infect. Microbiol. 2025, 15, 1557880. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef]
- De Pasquale, V.; Quiccione, M.S.; Tafuri, S.; Avallone, L.; Pavone, L.M. Heparan sulfate proteoglycans in viral infection and treatment: A special focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 6574. [Google Scholar] [CrossRef]
- Ballegeer, M.; Saelens, X. Cell-Mediated Responses to Human Metapneumovirus Infection. Viruses 2020, 12, 542. [Google Scholar] [CrossRef]
- Bao, X.; Liu, T.; Shan, Y.; Li, K.; Garofalo, R.P.; Casola, A. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog. 2008, 4, e1000077. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Fuentes, S.M.; Wang, P.; Taddeo, E.C.; Klatt, A.; Henderson, A.J.; He, B. Function of small hydrophobic proteins of paramyxovirus. J. Virol. 2006, 80, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Kolli, D.; Liu, T.; Shan, Y.; Garofalo, R.P.; Casola, A. Human metapneumovirus small hydrophobic protein inhibits NF-kappaB transcriptional activity. J. Virol. 2008, 82, 8224–8229. [Google Scholar] [CrossRef]
- Lê, V.B.; Dubois, J.; Couture, C.; Cavanagh, M.H.; Uyar, O.; Pizzorno, A.; Rosa-Calatrava, M.; Hamelin, M.È.; Boivin, G. Human metapneumovirus activates NOD-like receptor protein 3 inflammasome via its small hydrophobic protein which plays a detrimental role during infection in mice. PLoS Pathog. 2019, 15, e1007689. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wu, S.; Cai, J.; Yang, Y.; Ren, X.; Feng, Y.; Chen, L.; Qin, B.; Xu, C.; Yang, H.; et al. The H7N9 influenza A virus infection results in lethal inflammation in the mammalian host via the NLRP3-caspase-1 inflammasome. Sci. Rep. 2017, 7, 7625. [Google Scholar] [CrossRef]
- Shrivastava, G.; Leo’n-Jua’rez, M.; Garcı’a-Cordero, J.; Meza-Sa’nchez, D.E.; Cedillo-Barro’n, L. Inflammasomes and its importance in viral infections. Immunol. Res. 2016, 64, 1101–1117. [Google Scholar] [CrossRef]
- Guo, L.; Li, L.; Liu, L.; Zhang, T.; Sun, M. Neutralising antibodies against human metapneumovirus. Lancet Microbe 2023, 4, e732–e744. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Espinoza, I.; Bungwon, A.D.; Guerrero-Plata, A. Human metapneumovirus-induced host microRNA expression impairs the interferon response in macrophages and epithelial cells. Viruses 2023, 15, 2272. [Google Scholar] [CrossRef] [PubMed]
- Baños-Lara Mdel, R.; Ghosh, A.; Guerrero-Plata, A. Critical role of MDA5 in the interferon response induced by human metapneumovirus infection in dendritic cells and in vivo. J. Virol. 2013, 87, 1242–1251. [Google Scholar] [CrossRef]
- McMichael, T.M.; Zhang, Y.; Kenney, A.D.; Zhang, L.; Zani, A.; Lu, M.; Chemudupati, M.; Li, J.; Yount, J.S. IFITM3 restricts human metapneumovirus infection. J. Infect. Dis. 2018, 218, 1582–1591. [Google Scholar] [CrossRef]
- Lê, V.B.; Riteau, B.; Alessi, M.C.; Couture, C.; Jandrot-Perrus, M.; Rhéaume, C.; Hamelin, M.È.; Boivin, G. Protease-activated receptor 1 inhibition protects mice against thrombindependent respiratory syncytial virus and human metapneumovirus infections. Br. J. Pharmacol. 2018, 175, 388–403. [Google Scholar] [CrossRef]
- Shirogane, Y.; Takeda, M.; Iwasaki, M.; Ishiguro, N.; Takeuchi, H.; Nakatsu, Y.; Tahara, M.; Kikuta, H.; Yanagi, Y. Efficient multiplication of human metapneumovirus in Vero cells expressing the transmembrane serine protease TMPRSS2. J. Virol. 2008, 82, 8942–8946. [Google Scholar] [CrossRef]
- Ogonczyk Makowska, D.; Hamelin, M.E.; Boivin, G. Engineering of live chimeric vaccines against human metapneumovirus. Pathogens 2020, 9, 135. [Google Scholar] [CrossRef]
- August, A.; Shaw, C.A.; Lee, H.; Knightly, C.; Kalidindia, S.; Chu, L.; Essink, B.J.; Seger, W.; Zaks, T.; Smolenov, I.; et al. Safety and immunogenicity of an mRNA-based human metapneumovirus and parainfluenza virus type 3 combined vaccine in healthy adults. Open Forum Infect. Dis. 2022, 9, ofac206. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, F.; Xu, Y.; Wen, H.; Rao, M.; Zhang, P.; Peng, W.; Cui, Y.; Yang, H.; Tan, C.; et al. Development of a novel multi-epitope mRNA vaccine candidate to combat HMPV virus. Hum. Vaccines Immunother. 2023, 20, 2293300. [Google Scholar] [CrossRef]
- Pereira, J.M.; Xu, S.; Leong, J.M.; Sousa, S. The Yin and Yang of Pneumolysin During Pneumococcal Infection. Front. Immunol. 2022, 13, 878244. [Google Scholar] [CrossRef]
- Fillon, S.; Soulis, K.; Rajasekaran, S.; Benedict-Hamilton, H.; Radin, J.N.; Orihuela, C.J.; El Kasmi, K.C.; Murti, G.; Kaushal, D.; Gaber, M.W.; et al. Platelet-activating factor receptor and innate immunity: Uptake of Gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J. Immunol. 2006, 177, 6182–6191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, S.; Zhang, S.; Zhang, S.; Yu, Y.; Yao, H.; Liu, Y.; Zhang, W.; Liu, G. Development and evaluation of a multi-epitope subunit vaccine against group B Streptococcus infection. Emerg. Microbes Infect. 2022, 11, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 130. [Google Scholar] [CrossRef]
- Hu, M.; Deng, F.; Song, X.; Zhao, H.; Yan, F. The crosstalk between immune cells and tumor pyroptosis: Advancing cancer immunotherapy strategies. J. Exp. Clin. Cancer Res. 2024, 43, 190. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Z.; Zheng, Y.; Yang, S.; Huang, K.; Li, H. Roles of inflammasomes in viral myocarditis. Front. Cell. Infect. Microbiol. 2023, 13, 1149911. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xu, S.; Jiang, R.; Yu, Y.; Bian, J.; Zou, Z. The gasdermin family: Emerging therapeutic targets in diseases. Signal Transduct. Target. Ther. 2024, 9, 87. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, H.; Wang, J.J.; Zhang, J.S.; Shen, J.; An, X.B.; Zhang, C.C.; Wu, J.M.; Song, Y.; Wang, X.Y.; et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur. Heart J. 2018, 39, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, D.; Yin, D. Pathophysiological Effects of Various Interleukins on Primary Cell Types in Common Heart Disease. Int. J. Mol. Sci. 2023, 24, 6497. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001, 12, 53–72. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Shyam, S.; Das, S.; Nandi, D. Global transcriptome analysis identifies nicotinamide metabolism to play key roles in IFN-γ and nitric oxide modulated responses. Cytokine 2025, 195, 157015. [Google Scholar] [CrossRef]
- Camilli, G.; Blagojevic, M.; Naglik, J.R.; Richardson, J.P. Programmed Cell Death: Central Player in Fungal Infections. Trends Cell Biol. 2021, 31, 179–196. [Google Scholar] [CrossRef]
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Olsen, M.B.; Gregersen, I.; Sandanger, Ø.; Yang, K.; Sokolova, M.; Halvorsen, B.E.; Gullestad, L.; Broch, K.; Aukrust, P.; Louwe, M.C. Targeting the Inflammasome in Cardiovascular Disease. JACC Basic Transl. Sci. 2021, 7, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y.; Liu, W.; Liu, W.; Dong, J.; Liu, Q.; Hao, H.; Ren, H. Research progress on the activation mechanism of NLRP3 inflammasome in septic cardiomyopathy. Immun. Inflamm. Dis. 2023, 11, e1039. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, W.; Lv, Y.; Li, H.; Li, J.; Zhong, M.; Pu, D.; Jian, F.; Song, J.; Zhang, Y. NLRP3-dependent pyroptosis exacerbates coxsackievirus A16 and coxsackievirus A10-induced inflammatory response and viral replication in SH-SY5Y cells. Virus Res. 2024, 345, 199386. [Google Scholar] [CrossRef]
- El Gendy, A.; Abo Ali, F.H.; Baioumy, S.A.; Taha, S.I.; El-Bassiouny, M.; Abdel Latif, O.M. NOD-like receptor family pyrin domain containing 3 (rs10754558) gene polymorphism in chronic spontaneous urticaria: A pilot case-control study. Immunobiology 2025, 230, 152868. [Google Scholar] [CrossRef]
- Remnitz, A.D.; Hadad, R.; Keane, R.W.; Dietrich, W.D.; de Rivero Vaccari, J.P. Comparison of Methods of Detecting IL-1β in the Blood of Alzheimer’s Disease Subjects. Int. J. Mol. Sci. 2025, 26, 831. [Google Scholar] [CrossRef]
- Badacz, R.; Przewłocki, T.; Legutko, J.; Żmudka, K.; Kabłak-Ziembicka, A. microRNAs Associated with Carotid Plaque Development and Vulnerability: The Clinician’s Perspective. Int. J. Mol. Sci. 2022, 23, 15645. [Google Scholar] [CrossRef]
- Pagliaro, P.; Penna, C. Inhibitors of NLRP3 Inflammasome in Ischemic Heart Disease: Focus on Functional and Redox Aspects. Antioxidants 2023, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, X.; Yang, X.; Xiao, S.; Ouyang, S.; Hu, Z.; Zhou, Z.; Jiang, Z.E. Coli Nissle 1917 improves gut microbiota composition and serum metabolites to counteract atherosclerosis via the homocitrulline/Caspase 1/NLRP3/GSDMD axis. Int. J. Med. Microbiol. 2025, 318, 151642. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Shao, F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr. Opin. Immunol. 2015, 32, 78–83. [Google Scholar] [CrossRef]
- Fernández-Duran, I.; Quintanilla, A.; Tarrats, N.; Birch, J.; Hari, P.; Millar, F.R.; Lagnado, A.B.; Smer-Barreto, V.; Muir, M.; Brunton, V.G.; et al. Cytoplasmic innate immune sensing by the caspase-4 non-canonical inflammasome promotes cellular senescence. Cell Death Differ. 2022, 29, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Lupino, E.; Ramondetti, C.; Piccinini, M. IκB kinase β is required for activation of NF-κB and AP-1 in CD3/CD28-stimulated primary CD4(+) T cells. J. Immunol. 2012, 188, 2545–2555. [Google Scholar] [CrossRef]
- Abdrabou, A.M. The Yin and Yang of IκB Kinases in Cancer. Kinases Phosphatases 2024, 2, 9–27. [Google Scholar] [CrossRef]
- Sánchez, K.E.; Bhaskar, K.; Rosenberg, G.A. Apoptosis-associated speck-like protein containing a CARD-mediated release of matrix metalloproteinase 10 stimulates a change in microglia phenotype. Front. Mol. Neurosci. 2022, 15, 976108. [Google Scholar] [CrossRef]
- Bazzone, L.E.; King, M.; MacKay, C.R.; Kyawe, P.P.; Meraner, P.; Lindstrom, D.; Rojas-Quintero, J.; Owen, C.A.; Wang, J.P.; Brass, A.L.; et al. A Disintegrin and Metalloproteinase 9 Domain (ADAM9) Is a Major Susceptibility Factor in the Early Stages of Encephalomyocarditis Virus Infection. MBio 2019, 10, e02734-e18. [Google Scholar] [CrossRef]
- Spector, L.; Subramanian, N. Revealing the dance of NLRP3: Spatiotemporal patterns in inflammasome activation. Immunometabolism 2025, 7, e00053. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.M.; Ma, X.; Abdullah, S.W.; Zheng, H. Activation and Inhibition of the NLRP3 Inflammasome by RNA Viruses. J. Inflamm. Res. 2021, 14, 1145–1163. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Tsioufis, C.; Oikonomou, E.; Antoniades, C.; Crea, F.; Kaski, J.C.; Tousoulis, D. Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives. Int. J. Mol. Sci. 2021, 22, 6607. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y. Pyroptosis and respiratory diseases: A review of current knowledge. Front. Immunol. 2022, 13, 920464. [Google Scholar] [CrossRef]
- Ingram, T.; Evbuomwan, M.O.; Jyothidasan, A. A case report of sepsis-induced dilated cardiomyopathy secondary to human metapneumovirus infection. Cureus 2024, 16, e57951. [Google Scholar] [CrossRef]
- Cox, R.G.; Mainou, B.A.; Johnson, M.; Hastings, A.K.; Schuster, J.E.; Dermody, T.S.; Williams, J.V. Human metapneumovirus is capable of entering cells by fusion with endosomal membranes. PLoS Pathog. 2015, 11, e1005303. [Google Scholar] [CrossRef]
- Soto, J.A.; Galvez, N.M.S.; Benavente, F.M.; Pizarro-Ortega, M.S.; Lay, M.K.; Riedel, C.; Bueno, S.M.; Gonzalez, P.A.; Kalergis, A.M. Human metapneumovirus: Mechanisms and molecular targets used by the virus to avoid the immune system. Front. Immunol. 2018, 9, 2466. [Google Scholar] [CrossRef]
- Van Den Bergh, A.; Bailly, B.; Guillon, P.; von Itzstein, M.; Dirr, L. Antiviral strategies against human metapneumovirus: Targeting the fusion protein. Antivir. Res. 2022, 207, 105405. [Google Scholar] [CrossRef]
- Tsigkou, V.; Oikonomou, E.; Anastasiou, A.; Lampsas, S.; Zakynthinos, G.E.; Kalogeras, K.; Katsioupa, M.; Kapsali, M.; Kourampi, I.; Pesiridis, T.; et al. Molecular Mechanisms and Therapeutic Implications of Endothelial Dysfunction in Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 4321. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Y.; Wang, H.; Chen, H.; Hou, H.; Hu, Q. Dual Regulation of Nicotine on NLRP3 Inflammasome in Macrophages with the Involvement of Lysosomal Destabilization, ROS and α7nAChR. Inflammation 2025, 48, 61–74. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Moura, A.K.; Hu, J.Z.; Wang, Y.-T.; Zhang, Y. Lysosome Functions in Atherosclerosis: A Potential Therapeutic Target. Cells 2025, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, A.M.; Ganesan, K.; Ramkumar, K.M. A Co-Culture System for Studying Cellular Interactions in Vascular Disease. Bioengineering 2024, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Casella, M.; Gasperetti, A.; Compagnucci, P.; Narducci, M.L.; Pelargonio, G.; Catto, V.; Carbucicchio, C.; Bencardino, G.; Conte, E.; Sschicci, N.; et al. Different Phases of Disease in Lymphocytic Myocarditis: Clinical and Electrophysiological Characteristics. JACC Clin. Electrophysiol. 2023, 9, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; Hirsch, E.; Anker, S.D.; Aukrust, P.; Balligand, J.L.; Cohen-Tervaert, J.W.; Drexler, H.; Filippatos, G.; Felix, S.B.; Gullestad, L.; et al. Inflammation as a therapeutic target in heart failure? Cardiovasc. Res. 2019, 115, 1189–1201. [Google Scholar] [CrossRef]
- Ying, C. Viral Myocarditis. Yale J. Biol. Med. 2024, 97, 515–520. [Google Scholar] [CrossRef]
- Vicenzetto, C.; Giordani, A.S.; Menghi, C.; Baritussio, A.; Scognamiglio, F.; Pontara, E.; Bison, E.; Peloso-Cattini, M.G.; Marcolongo, R.; Caforio, A.L.P. Cellular Immunology of Myocarditis: Lights and Shades-A Literature Review. Cells 2024, 13, 2082. [Google Scholar] [CrossRef]
- Badrinath, A.; Bhatta, S.; Kloc, A. Persistent viral infections and their role in heart disease. Front. Microbiol. 2022, 13, 1030440. [Google Scholar] [CrossRef]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911–915. [Google Scholar] [CrossRef]
- Kühl, U.; Pauschinger, M.; Schwimmbeck, P.L.; Seeberg, B.; Lober, C.; Noutsias, M.; Poller, W.; Schultheiss, H.P. Interferon-β treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 2003, 107, 2793–2798. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Piper, C.; Sowade, O.; Waagstein, F.; Kapp, J.F.; Wegscheider, K.; Groetzbach, G.; Pauschinger, M.; Escher, F.; Arbustini, E.; et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon-β treatment in patients with chronic viral cardiomyopathy. Clin. Res. Cardiol. 2016, 105, 763–773. [Google Scholar] [CrossRef]
- Hang, W.; Chen, C.; Seubert, J.M.; Wang, D.W. Fulminant myocarditis: A comprehensive review from etiology to treatments and outcomes. Signal Transduct. Target. Ther. 2020, 5, 287. [Google Scholar] [CrossRef]
- Hafezi-Moghadam, A.; Simoncini, T.; Yang, Z.; Limbourg, F.P.; Plumier, J.C.; Rebsamen, M.C.; Hsieh, C.M.; Chui, D.S.; Thomas, K.L.; Prorock, A.J.; et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 2002, 8, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.W.; O’Connell, J.B.; Herskowitz, A.; Rose, N.R.; McManus, B.M.; Billingham, M.E.; Moon, T.E. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 1995, 333, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Russo, M.A.; Frustaci, A. Immunosuppressive therapy in virus-negative inflammatory cardiomyopathy: 20-year follow-up of the TIMIC trial. Eur. Heart J. 2022, 43, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, R.; Nowalany-Kozielska, E.; Wojciechowska, C.; Glanowska, G.; Wilczewski, P.; Niklewski, T.; Zembala, M.; Polonski, L.; Rozek, M.M.; Wodniecki, J. Randomized, placebocontrolled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: Two-year follow-up results. Circulation 2001, 104, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Chimenti, C.; Calabrese, F.; Pieroni, M.; Thiene, G.; Maseri, A. Immunosuppressive therapy for active lymphocytic myocarditis: Virological and immunologic profile of responders versus nonresponders. Circulation 2003, 107, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Randomized study on the efficacy of immunosuppressive therapy in patients with virusnegative inflammatory cardiomyopathy: The TIMIC study. Eur. Heart J. 2009, 30, 1995–2002. [Google Scholar] [CrossRef]
- Merken, J.; Hazebroek, M.; Van Paassen, P.; Verdonschot, J.; Van Empel, V.; Knackstedt, C.; Abdul Hamid, M.; Seiler, M.; Kolb, J.; Hoermann, P.; et al. Immunosuppressive therapy improves both short- and long-term prognosis in patients with virus-negative nonfulminant inflammatory cardiomyopathy. Circ. Heart Fail. 2018, 11, e004228. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Giordani, A.S.; Baritussio, A.; Marcolongo, D.; Vicenzetto, C.; Tarantini, G.; Napodano, M.; Toscano, G.; Gregori, D.; Brigiari, G.; et al. Long-term efficacy and safety of tailored immunosuppressive therapy in immune-mediated biopsy-proven myocarditis: A propensity-weighted study. Eur. J. Heart Fail. 2024, 26, 1175–1185. [Google Scholar] [CrossRef]
- Hahn, E.A.; Hartz, V.L.; Moon, T.E.; O’Connell, J.B.; Herskowitz, A.; McManus, B.M.; Mason, J.W. The Myocarditis Treatment Trial: Design, methods and patients enrollment. Eur. Heart J. 1995, 16 (Suppl. O), 162–167. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.H.C.N.; Fraga, C.A.M. NF-κB-IKKβ Pathway as a Target for Drug Development: Realities, Challenges and Perspectives. Curr. Drug Targets 2018, 19, 1933. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Makris, N.; Laina, A.; Theodorakakou, F.; Briasoulis, A.; Trougakos, I.P.; Dimopoulos, M.A.; Kastritis, E.; Stamatelopoulos, K. Cardiovascular Toxicity of Proteasome Inhibitors: Underlying Mechanisms and Management Strategies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2023, 5, 1–21. [Google Scholar] [CrossRef]
- Chin, C.G.; Chen, Y.C.; Lin, F.J.; Lin, Y.K.; Lu, Y.Y.; Cheng, T.Y.; Chen, S.A.; Chen, Y.J. Targeting NLRP3 signaling reduces myocarditis-induced arrhythmogenesis and cardiac remodeling. J. Biomed. Sci. 2024, 31, 42. [Google Scholar] [CrossRef]
- Golino, M.; Coe, A.; Aljabi, A.; Talasaz, A.H.; Van Tassell, B.; Abbate, A.; Markley, R. Effect of colchicine on 90-day outcomes in patients with acute myocarditis: A real-world analysis. Am. Heart J. Plus. 2024, 47, 100478. [Google Scholar] [CrossRef]
- Collini, V.; De Martino, M.; Andreis, A.; De Biasio, M.; Gaspard, F.; Paneva, E.; Tomat, M.; Deferrari, G.M.; Isola, M.; Imazio, M. Efficacy and safety of colchicine for the treatment of myopericarditis. Heart 2024, 110, 735–739. [Google Scholar] [CrossRef]
- Kavgacı, A.; Incedere, F.; Terlemez, S.; Kula, S. Successful treatment of two cases of acute myocarditis with colchicine. Cardiol. Young 2023, 33, 1741–1742. [Google Scholar] [CrossRef]
- Pappritz, K.; Lin, J.; El-Shafeey, M.; Fechner, H.; Kühl, U.; Alogna, A.; Spillmann, F.; Elsanhoury, A.; Schulz, R.; Tschöpe, C.; et al. Colchicine prevents disease progression in viral myocarditis via modulating the NLRP3 inflammasome in the cardiosplenic axis. ESC Heart Fail. 2022, 9, 925–941. [Google Scholar] [CrossRef]
- Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.; Baltagiannis, S.; Panagopoulos, P.; et al. Effect of Colchicine vs. Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2013136. [Google Scholar] [CrossRef] [PubMed]
- Wohlford, G.F.; Van Tassell, B.W.; Billingsley, H.E.; Kadariya, D.; Canada, J.M.; Carbone, S.; Mihalick, V.L.; Bonaventura, A.; Vecchié, A.; Chiabrando, J.G.; et al. Phase 1B, Randomized, Double-Blinded, Dose Escalation, Single-Center, Repeat Dose Safety and Pharmacodynamics Study of the Oral NLRP3 Inhibitor Dapansutrile in Subjects With NYHA II-III Systolic Heart Failure. J. Cardiovasc. Pharmacol. 2020, 77, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Wang, Y.; Jiang, X.; Guo, M.; Yang, Z. NLRP3 inflammasome as a novel therapeutic target for heart failure. Anatol. J. Cardiol. 2022, 26, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. NEJM 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Silvis, M.J.M.; Demkes, E.J.; Fiolet, A.T.L.; Dekker, M.; Bosch, L.; van Hout, G.P.J.; Timmers, L.; de Kleijn, D.P.V. Immunomodulation of the NLRP3 Inflammasome in Atherosclerosis, Coronary Artery Disease, and Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2021, 14, 23–34. [Google Scholar] [CrossRef]
- Morton, A.C.; Rothman, A.M.; Greenwood, J.P.; Gunn, J.; Chase, A.; Clarke, B.; Hall, A.S.; Fox, K.; Foley, C.; Banya, W.; et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: The MRC-ILA Heart Study. Eur. Heart J. 2015, 36, 377–384. [Google Scholar] [CrossRef]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Cavalli, G.; Foppoli, M.; Cabrini, L.; Dinarello, C.A.; Tresoldi, M.; Dagna, L. Interleukin-1 Receptor Blockade Rescues Myocarditis-Associated End-Stage Heart Failure. Front. Immunol. 2017, 8, 131. [Google Scholar] [CrossRef]
- Ma, P.; Jimenez, J.; Villanueva, A.; Wu, G.; Bender, D.; Hector-Greene, M.; Lin, C.Y.; Lavine, K. IL-1 Signaling Blockade in Human Lymphocytic Myocarditis. Circ. Res. 2025, 137, 576–579. [Google Scholar] [CrossRef]
- Kerneis, M.; Cohen, F.; Combes, A.; Amoura, Z.; Pare, C.; Brugier, D.; Puymirat, E.; Abtan, J.; Lattuca, B.; Dillinger, J.-G.; et al. ACTION Study Group, Rationale and design of the ARAMIS trial: Anakinra versus placebo, a double blind randomized controlled trial for the treatment of acute myocarditis. Arch. Cardiovasc. Dis. 2023, 116, 460–466. [Google Scholar] [CrossRef]
- Lema, D.A.; Jakobsson, G.; Daoud, A.; Elias, D.; Talor, M.V.; Rattik, S.; Grönberg, C.; Kalinoski, H.; Jaensson Gyllenbäck, E.; Wang, N.; et al. IL1RAP Blockade With a Monoclonal Antibody Reduces Cardiac Inflammation and Preserves Heart Function in Viral and Autoimmune Myocarditis. Circ. Heart Fail. 2024, 17, e011729. [Google Scholar] [CrossRef]
- Li, S.; Jiang, L.; Beckmann, K.; Højen, J.F.; Pessara, U.; Powers, N.E.; de Graaf, D.M.; Azam, T.; Lindenberger, J.; Eisenmesser, E.Z.; et al. A novel anti-human IL-1R7 antibody reduces IL-18-mediated inflammatory signaling. J. Biol. Chem. 2021, 296, 100630. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lunding, L.P.; Webber, W.S.; Beckmann, K.; Azam, T.; Højen, J.F.; Amo-Aparicio, J.; Dinarello, A.; Nguyen, T.T.; Pessara, U.; et al. An antibody to IL-1 receptor 7 protects mice from LPS-induced tissue and systemic inflammation. Front. Immunol. 2024, 15, 1427100. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Nariai, Y.; Obayashi, E.; Tajima, Y.; Koga, T.; Kawakami, A.; Urano, T.; Kamino, H. Generation of antagonistic monoclonal antibodies against the neoepitope of active mouse interleukin (IL)-18 cleaved by inflammatory caspases. Arch. Biochem. Biophys. 2022, 727, 109322. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Khan, Z. Sacubitril/Valsartan in the Treatment of Heart Failure with Reduced Ejection Fraction Focusing on the Impact on the Quality of Life: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Cureus 2023, 15, e48674. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: Rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur. J. Heart Fail. 2013, 15, 1062–1073. [Google Scholar]
- Song, Y.; Zhao, Z.; Zhang, J.; Zhao, F.; Jin, P. Effects of sacubitril/valsartan on life quality in chronic heart failure: A systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 922721. [Google Scholar] [CrossRef]
- Tsutsui, H.; Momomura, S.I.; Saito, Y.; Ito, H.; Yamamoto, K.; Sakata, Y.; Desai, A.S.; Ohishi, T.; Iimori, T.; Kitamura, T.; et al. Efficacy and safety of sacubitril/valsartan in Japanese patients with chronic heart failure and reduced ejection fraction- results from the PARALLEL-HF study. Circ. J. 2021, 85, 584–594. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hussain, R.I.; Comin-Colet, J.; Dosantos, R.; Ferber, P.; Jaarsma, T.; Edelmann, F. OUTSTEP-HF: Randomised controlled trial comparing short-term effects of sacubitril/valsartan versus enalapril on daily physical activity in patients with chronic heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2021, 23, 127–135. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E.; PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548, Correction in N. Engl. J. Med. 2019, 380, 1090. https://doi.org/10.1056/NEJMx190009. [Google Scholar] [CrossRef]
- Mann, D.L.; Greene, S.J.; Givertz, M.M.; Vader, J.M.; Starling, R.C.; Ambrosy, A.P.; Shah, P.; McNulty, S.E.; Mahr, C.; Gupta, D.; et al. Sacubitril/Valsartan in Advanced Heart Failure With Reduced Ejection Fraction Rationale and Design of the LIFE Trial. J. Am. Coll. Cardiol. Heart Fail. 2020, 8, 789–799. [Google Scholar] [CrossRef]
- Mann, D.L.; Givertz, M.M.; Vader, J.M.; Starling, R.C.; Shah, P.; McNulty, S.E.; Anstrom, K.J.; Margulies, K.B.; Kiernan, M.S.; Mahr, C.; et al. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: A randomized clinical trial. JAMA Cardiol. 2022, 7, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Manzi, L.; Buongiorno, F.; Narciso, V.; Florimonte, D.; Forzano, I.; Castiello, D.S.; Sperandeo, L.; Paolillo, R.; Verde, N.; Spinelli, A.; et al. Acute Heart Failure and Non-Ischemic Cardiomyopathies: A Comprehensive Review and Critical Appraisal. Diagnostics 2025, 15, 540. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.K.; Polhemus, D.J.; Li, Z.; Yoo, D.; Koiwaya, H.; Scarborough, A.; Goodchild, T.T.; Lefer, D.J. Combined angiotensin receptor-neprilysin inhibitors improve cardiac and vascular function via increased NO bioavailability in heart failure. J. Am. Heart Assoc. 2018, 7, 8268. [Google Scholar] [CrossRef]
- Shi, Y.J.; Yang, C.G.; Qiao, W.B.; Liu, Y.C.; Dong, G.J. Sacubitril/valsartan attenuates myocardial inflammation, hypertrophy, and fibrosis in rats with heart failure with preserved ejection fraction. Eur. J. Pharmacol. 2023, 961, 176170. [Google Scholar] [CrossRef]
- Volpe, M.; Rubattu, S.; Battistoni, A. ARNi: A Novel Approach to Counteract Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 2092. [Google Scholar] [CrossRef]
- Caobelli, F.; Cabrero, J.B.; Galea, N.; Haaf, P.; Loewe, C.; Luetkens, J.A.; Muscogiuri, G.; Francone, M. Cardiovascular magnetic resonance (CMR) and positron emission tomography (PET) imaging in the diagnosis and follow-up of patients with acute myocarditis and chronic inflammatory cardiomyopathy: A review paper with practical recommendations on behalf of the European Society of Cardiovascular Radiology (ESCR). Int. J. Cardiovasc. Imaging 2023, 39, 2221–2235. [Google Scholar] [CrossRef]
- She, J.; Lou, B.; Liu, H.; Zhou, B.; Jiang, G.T.; Luo, Y.; Wu, H.; Wang, C.; Yuan, Z. ARNI versus ACEI/ARB in Reducing Cardiovascular Outcomes after Myocardial Infarction. ESC Heart Fail. 2021, 8, 4607–4616. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Lewis, E.F.; Granger, C.B.; Køber, L.; Maggioni, A.P.; Mann, D.L.; McMurray, J.J.V.; Rouleau, J.L.; Solomon, S.D.; et al. Angiotensin Receptor-Neprilysin Inhibition in Acute Myocardial Infarction. N. Engl. J. Med. 2021, 385, 1845–1855, Erratum in N. Engl. J. Med. 2021, 385, 2592. https://doi.org/10.1056/NEJMx210024. [Google Scholar] [CrossRef]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Hahn, V.S.; Sharma, K. Angiotensin Receptor-Neprilysin Inhibition for Doxorubicin-Mediated Cardiotoxicity: Time for a Paradigm Shift. JACC CardioOncol. 2020, 2, 788–790. [Google Scholar] [CrossRef]
- Wei, S.; Ma, W.; Li, X.; Jiang, C.; Sun, T.; Li, Y.; Zhang, B.; Li, W. Involvement of ROS/NLRP3 Inflammasome Signaling Pathway in Doxorubicin-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2020, 20, 507–519. [Google Scholar] [CrossRef]
- Polegato, B.F.; Minicucci, M.F.; Azevedo, P.S.; Carvalho, R.F.; Chiuso-Minicucci, F.; Pereira, E.J.; Paiva, S.A.; Zornoff, L.A.; Okoshi, M.P.; Matsubara, B.B.; et al. Acute doxorubicin-induced cardiotoxicity is associated with matrix metalloproteinase-2 alterations in rats. Cell Physiol. Biochem. 2015, 35, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; Feher, A.; Pfau, D. Dual angiotensin receptor neprilysin inhibition with sacubitril/valsartan attenuates systolic dysfunction in experimental doxorubicin-induced cardiotoxicity. J. Am. Coll. Cardiol. CardioOncol. 2020, 2, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Desuki, A.; Karbach, S.; Göbel, S. Successful treatment of doxorubicin-induced cardiomyopathy with low-dose sacubitril/valsartan: A case report. Eur. Heart J. Case Rep. 2022, 6, ytac396. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Yan, S.; Lin, L.; Qiu, X.; Lin, X.; Wang, W. Sacubitril/valsartan attenuated myocardial inflammation, fibrosis, apoptosis and promoted autophagy in doxorubicin-induced cardiotoxicity mice via regulating the AMPKα–mTORC1 signaling pathway. Mol. Cell Biochem. 2025, 480, 1891–1908. [Google Scholar] [CrossRef]
- Dindas, F.; Turkey, U.; Gungor, H.; Ekici, M.; Akokay, P.; Erhan, F.; Dogdus, M.; Yilmaz, M.B. Angiotensin receptor-neprilysin inhibition by sacubitril/valsartan attenuates doxorubicin-induced cardiotoxicity in a pretreatment mice model by interfering with oxidative stress, inflammation, and Caspase 3 apoptotic pathway. Anatol. J. Cardiol. 2021, 25, 821. [Google Scholar] [CrossRef]
- Kim, D.; Jang, G.; Hwang, J.; Wei, X.; Kim, H.; Son, J.; Rhee, S.-J.; Yun, K.-H.; Oh, S.-K.; Oh, C.-M.; et al. Combined Therapy of Low-Dose Angiotensin Receptor–Neprilysin Inhibitor and Sodium–Glucose Cotransporter-2 Inhibitor Prevents Doxorubicin-Induced Cardiac Dysfunction in Rodent Model with Minimal Adverse Effects. Pharmaceutics 2022, 14, 2629. [Google Scholar] [CrossRef]
- Quagliariello, V.; Di Mauro, A.; Ferrara, G.; Bruzzese, F.; Palma, G.; Luciano, A.; Canale, M.L.; Bisceglia, I.; Iovine, M.; Cadeddu Dessalvi, C.; et al. Cardio-Renal and Systemic Effects of SGLT2i Dapagliflozin on Short-Term Anthracycline and HER-2-Blocking Agent Therapy-Induced Cardiotoxicity. Antioxidants 2025, 14, 612. [Google Scholar] [CrossRef]
- Lo, S.H.; Liu, Y.C.; Dai, Z.K.; Chen, I.C.; Wu, Y.H.; Hsu, J.H. Case Report: Low Dose of Valsartan/Sacubitril Leads to Successful Reversal of Acute Heart Failure in Chemotherapy-Induced Cardiomyopathy. Front. Pediatr. 2021, 9, 639551. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Pasupuleti, V.; Scarpelli, N.; Malespini, J.; Banach, M.; Bielecka-Dabrowa, A.M. Efficacy and safety of sacubitril/valsartan in heart failure compared to renin–angiotensin–aldosterone system inhibitors: A systematic review and meta-analysis of randomised controlled trials. Arch. Med. Sci. 2023, 3, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Cemin, R.; Casablanca, S.; Foco, L.; Schoepf, E.; Erlicher, A.; Di Gaetano, R.; Ermacora, D. Reverse Remodeling and Functional Improvement of Left Ventricle in Patients with Chronic Heart Failure Treated with Sacubitril/Valsartan: Comparison between Non-Ischemic and Ischemic Etiology. J. Clin. Med. 2023, 12, 621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zou, Y.; Li, Y.; Wang, H.; Sun, W.; Liu, B. The history and mystery of sacubitril/valsartan: From clinical trial to the real world. Front. Cardiovasc. Med. 2023, 10, 1102521. [Google Scholar] [CrossRef] [PubMed]
- Nesukay, E.G.; Kovalenko, V.M.; Cherniuk, S.V.; Kyrychenko, R.M.; Titov, I.Y.; Hiresh, I.I.; Dmytrychenko, O.V.; Slyvna, A.B. Choice of RAAS Blocker in the treatment of Heart Failure in Acute Myocarditis. Ukr. J. Cardiol. 2024, 31, 32–40. [Google Scholar] [CrossRef]
- Liang, W.; Xie, B.K.; Ding, P.W.; Wang, M.; Yuan, J.; Cheng, X.; Liao, Y.H.; Yu, M. Sacubitril/Valsartan Alleviates Experimental Autoimmune Myocarditis by Inhibiting Th17 Cell Differentiation Independently of the NLRP3 Inflammasome Pathway. Front. Pharmacol. 2021, 12, 727838. [Google Scholar] [CrossRef]
- Bellis, A.; Mauro, C.; Barbato, E.; Trimarco, B.; Morisco, C. The Rationale for Angiotensin Receptor Neprilysin Inhibitors in a Multi-Targeted Therapeutic Approach to COVID-19. Int. J. Mol. Sci. 2020, 21, 8612. [Google Scholar] [CrossRef]
- Zile, M.R.; O’Meara, E.; Claggett, B.; Prescott, M.F.; Solomon, S.D.; Swedberg, K.; Packer, M.; McMurray, J.J.V.; Shi, V.; Lefkowitz, M.; et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J. Am. Coll. Cardiol. 2019, 73, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Li, D.; Zhang, Q.; Liu, T.; Gu, Z.; Huang, L.; Dai, J.; Wang, J.; Hou, X. Complex regulation of cardiac fibrosis: Insights from immune cells and signaling pathways. J. Transl. Med. 2025, 23, 242. [Google Scholar] [CrossRef]
- Thomas, T.P.; Grisanti, L.A. The Dynamic Interplay Between Cardiac Inflammation and Fibrosis. Front. Physiol. 2020, 11, 529075. [Google Scholar] [CrossRef]
- Dziewięcka, E.; Banyś, R.; Wiśniowska-Śmiałek, S.; Winiarczyk, M.; Urbańczyk-Zawadzka, M.; Krupiński, M.; Mielnik, M.; Lisiecka, M.; Gąsiorek, J.; Kyslyi, V.; et al. Prevalence and prognostic implications of the longitudinal changes of right ventricular systolic function on cardiac magnetic resonance in dilated cardiomyopathy. Kardiol. Pol. 2025, 83, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Bayes-Genis, A.; Bodegård, J.; Mullin, K.; Gustafsson, S.; Rosano, G.M.C.; Sundström, J. Suspected de novo heart failure in outpatient care: The REVOLUTION HF study. Eur. Heart J. 2025, 46, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Podolec, J.; Baran, J.; Siedlinski, M.; Urbanczyk, M.; Krupinski, M.; Bartus, K.; Niewiara, L.; Podolec, M.; Guzik, T.; Tomkiewicz-Pajak, L.; et al. Serum rantes, transforming growth factor-1 and interleukin-6 levels correlate with cardiac muscle fibrosis in patients with aortic valve stenosis. J. Physiol. Pharmacol. 2018, 69, 615–623. [Google Scholar]
- Kablak-Ziembicka, A.; Badacz, R.; Okarski, M.; Wawak, M.; Przewłocki, T.; Podolec, J. Cardiac microRNAs: Diagnostic and therapeutic potential. Arch. Med. Sci. 2023, 19, 1360–1381. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032, Correction in Circulation 2022, 145, e1033. https://doi.org/10.1161/CIR.0000000000001073. [Google Scholar] [CrossRef]
- Huang, S.; Chen, B.; Su, Y.; Alex, L.; Humeres, C.; Shinde, A.V.; Conway, S.J.; Frangogiannis, N.G. Distinct roles of myofibroblast-specific Smad2 and Smad3 signaling in repair and remodeling of the infarcted heart. J. Mol. Cell Cardiol. 2019, 132, 84–97. [Google Scholar] [CrossRef]
- Nevers, T.; Salvador, A.M.; Velazquez, F.; Ngwenyama, N.; Carrillo-Salinas, F.J.; Aronovitz, M.; Blanton, R.M.; Alcaide, P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J. Exp. Med. 2017, 214, 3311–3329. [Google Scholar] [CrossRef]
- Duangrat, R.; Parichatikanond, W.; Mangmool, S. Dual Blockade of TGF-β Receptor and Endothelin Receptor Synergistically Inhibits Angiotensin II-Induced Myofibroblast Differentiation: Role of AT1R/Gαq-Mediated TGF-β1 and ET-1 Signaling. Int. J. Mol. Sci. 2023, 24, 6972. [Google Scholar] [CrossRef]
- Zhao, X.; Kwan, J.Y.Y.; Yip, K.; Liu, P.P.; Liu, F.F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2020, 19, 57–75. [Google Scholar] [CrossRef]
- Anguita-Sánchez, M.; Castillo-Domínguez, J.C.; Mesa-Rubio, D.; Ruiz-Ortiz, M.; López-Granados, A.; Suárez de Lezo, J. Should Angiotensin-converting enzyme inhibitors be continued over the long term in patients whose left ventricular ejection fraction normalizes after an episode of acute myocarditis? Rev. Esp. Cardiol. 2006, 59, 1199–1201. [Google Scholar] [CrossRef]
- Kayyale, A.A.; Timms, P.; Xiao, H.B. ACE inhibitors reduce the risk of myocardial fibrosis post-cardiac injury: A systematic review. Br. J. Cardiol. 2025, 32, 72–76. [Google Scholar] [CrossRef]
- Seko, Y. Effect of the angiotensin II receptor blocker olmesartan on the development of murine acute myocarditis caused by coxsackievirus B3. Clin. Sci. 2006, 110, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Lighthouse, J.K.; Mickelsen, D.M.; Small, E.M. Sacubitril/Valsartan Decreases Cardiac Fibrosis in Left Ventricle Pressure Overload by Restoring PKG Signaling in Cardiac Fibroblasts. Circ. Heart Fail 2019, 12, e005565. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Long, Q.; Yang, H.; Yang, H.; Tang, Y.; Liu, B.; Zhou, Z.; Yuan, J. Sacubitril/Valsartan inhibits M1 type macrophages polarization in acute myocarditis by targeting C-type natriuretic peptide. Biomed. Pharmacother. 2024, 174, 116535. [Google Scholar] [CrossRef]
- Ding, N.; Wei, B.; Fu, X.; Wang, C.; Wu, Y. Natural Products that Target the NLRP3 Inflammasome to Treat Fibrosis. Front. Pharmacol. 2020, 11, 591393. [Google Scholar] [CrossRef]
- Wang, W.; Shen, Q. Tranilast reduces cardiomyocyte injury induced by ischemia-reperfusion via Nrf2/HO-1/NF-κB signaling. Exp. Ther. Med. 2023, 25, 160. [Google Scholar] [CrossRef]
- Huang, L.F.; Wen, C.; Xie, G.; Chen, C.Y. Effect of tranilast on myocardial fibrosis in mice with viral myocarditis. Zhongguo Dang Dai Er Ke Za Zhi 2014, 16, 1154–1161. [Google Scholar]
- Lewis, G.A.; Dodd, S.; Clayton, D.; Bedson, E.; Eccleson, H.; Schelbert, E.B.; Naish, J.H.; Jimenez, B.D.; Williams, S.G.; Cunnington, C.; et al. Pirfenidone in heart failure with preserved ejection fraction: A randomized phase 2 trial. Nat. Med. 2021, 27, 1477–1482. [Google Scholar] [CrossRef]
- AbdelMassih, A.; Agha, H.; El-Saiedi, S.; El-Sisi, A.; El Shershaby, M.; Gaber, H.; Ismail, H.A.; El-Husseiny, N.; Amin, A.R.; ElBoraie, A.; et al. The role of miRNAs in viral myocarditis, and its possible implication in induction of mRNA-based COVID-19 vaccines-induced myocarditis. Bull. Natl. Res. Cent. 2022, 46, 267. [Google Scholar] [CrossRef]
- Młynarska, E.; Badura, K.; Kurcinski, S.; Sinkowska, J.; Jakubowska, P.; Rysz, J.; Franczyk, B. The Role of MicroRNA in the Pathophysiology and Diagnosis of Viral Myocarditis. Int. J. Mol. Sci. 2024, 25, 10933. [Google Scholar] [CrossRef]
- Kabłak-Ziembicka, A.; Badacz, R.; Przewłocki, T. Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease. J. Clin. Med. 2022, 11, 6849. [Google Scholar] [CrossRef]
- Grodzka, O.; Procyk, G.; Gąsecka, A. The Role of MicroRNAs in Myocarditis—What Can We Learn from Clinical Trials? Int. J. Mol. Sci. 2022, 23, 16022. [Google Scholar] [CrossRef]
- Singh, D. Next-generation miRNA therapeutics: From computational design to translational engineering. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 21, 8612. [Google Scholar] [CrossRef]
- Bruscella, P.; Bottini, S.; Baudesson, C.; Pawlotsky, J.M.; Feray, C.; Trabucchi, M. Viruses and MiRNAs: More Friends than Foes. Front. Microbiol. 2017, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Pasławska, M.; Grodzka, A.; Peczyńska, J.; Sawicka, B.; Bossowski, A.T. Role of miRNA in Cardiovascular Diseases in Children—Systematic Review. Int. J. Mol. Sci. 2024, 25, 956. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, B. Dysregulated CD4+ T Cells and microRNAs in Myocarditis. Front. Immunol. 2020, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.; Tirosh-Wagner, T.; Vardi, A.; Abbas, H.; Pillar, N.; Shomron, N.; Nevo-Caspi, Y.; Paret, G. Circulating MicroRNAs: A Potential Biomarker for Cardiac Damage, Inflammatory Response, and Left Ventricular Function Recovery in Pediatric Viral Myocarditis. J. Cardiovasc. Transl. Res. 2018, 11, 319–328. [Google Scholar] [CrossRef]
- Abdou, D.M.; Aziz, E.E.; Kazem, Y.S.; Elgabrty, S.; Taha, H.S. The diagnostic value of circulating microRNA-499 versus high-sensitivity cardiac troponin T in early diagnosis of ST segment elevation myocardial infarction. Comp. Clin. Pathol. 2021, 30, 565–570. [Google Scholar] [CrossRef]
- Gacoń, J.; Badacz, R.; Stępień, E.; Karch, I.; Enguita, F.J.; Żmudka, K.; Przewłocki, T.; Kabłak-Ziembicka, A. Diagnostic and prognostic micro-RNAs in ischaemic stroke due to carotid artery stenosis and in acute coronary syndrome: A four-year prospective study. Kardiol. Pol. 2018, 76, 362–369. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Tian, L.; Sun, Q. Circulating microRNA-208 family as early diagnostic biomarkers for acute myocardial infarction: A meta-analysis. Medicine 2021, 100, e27779. [Google Scholar] [CrossRef]
- Badacz, R.; Przewlocki, T.; Gacoń, J.; Stępień, E.; Enguita, F.J.; Karch, I.; Żmudka, K.; Kabłak-Ziembicka, A. Circulating miRNA levels differ with respect to carotid plaque characteristics and symptom occurrence in patients with carotid artery stenosis and provide information on future cardiovascular events. Adv. Interv. Cardiol. 2018, 14, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Tao, L.; Li, X. MicroRNA-21-3p/Rcan1 Signaling Axis Affects Apoptosis of Cardiomyocytes of Sepsis Rats. Gen. Physiol. Biophys. 2023, 42, 217–227. [Google Scholar] [CrossRef]
- Li, Y.; Sun, G.; Wang, L. MiR-21 Participates in LPS-Induced Myocardial Injury by Targeting Bcl-2 and CDK6. Inflamm. Res. 2022, 71, 205–214. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 Prevents Excessive Inflammation and Cardiac Dysfunction after Myocardial Infarction through Targeting KBTBD7. Cell Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, L.; Yang, Z. Role of non-coding RNAs in the pathogenesis of viral myocarditis. Virulence 2025, 16, 2466480. [Google Scholar] [CrossRef]
- Procyk, G.; Grodzka, O.; Procyk, M.; Gąsecka, A.; Głuszek, K.; Wrzosek, M. MicroRNAs in Myocarditis—Review of the preclinical In Vivo Trials. Biomedicines 2023, 11, 2723. [Google Scholar] [CrossRef]
- Bao, J.L.; Lin, L. MiR-155 and miR-148a reduce cardiac injury by inhibiting NF-κB pathway during acute viral myocarditis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2349–2356. [Google Scholar] [PubMed]
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.; Hazebroek, M.; van Leeuwen, R.; et al. MicroRNA Profiling Identifies MicroRNA-155 as an Adverse Mediator of Cardiac Injury and Dysfunction During Acute Viral Myocarditis. Circ. Res. 2012, 111, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Li, X.; Tang, Z.; Wang, X.; Zhong, M.; Suo, Q.; Zhang, Y.; Lv, K. Silencing MicroRNA-155 Attenuates Cardiac Injury and Dysfunction in Viral Myocarditis via Promotion of M2 Phenotype Polarization of Macrophages. Sci. Rep. 2016, 6, 22613. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wu, W.F.; Xue, Y.; Gao, M.; Yan, Y.; Kong, Q.; Pang, Y.; Yang, F. MicroRNA-21 and -146b Are Involved in the Pathogenesis of Murine Viral Myocarditis by Regulating TH-17 Differentiation. Arch. Virol. 2013, 158, 1953–1963. [Google Scholar] [CrossRef]
- Xu, H.F.; Ding, Y.J.; Zhang, Z.X.; Wang, Z.F.; Luo, C.L.; Li, B.X.; Shen, Y.W.; Tao, L.Y.; Zhao, Z.Q. MicroRNA-21 regulation of the progression of viral myocarditis to dilated cardiomyopathy. Mol. Med. Rep. 2014, 10, 161–168. [Google Scholar] [CrossRef]
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; de la Fuente, H. A novel circulating MicroRNA for the detection of acute myocarditis. N. Engl. J. Med. 2021, 384, 2014–2027. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, L.; Duan, X.; Liu, K.; Yingnan, Y.; Ma, M.; Han, B. MiR-425-5p intervenes in autoimmune myocarditis by regulating Treg cell differentiation through NRAS. Front. Cell Dev. Biol. 2025, 13, 1600103. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Zhao, C.; Chen, C.; Kong, Q.; Cai, Z.; Li, D. MiR-1/133 attenuates cardiomyocyte apoptosis and electrical remodeling in mice with viral myocarditis. Cardiol. J. 2020, 27, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Baños-Lara, M.D.R.; Zabaleta, J.; Garai, J.; Baddoo, M.; Guerrero-Plata, A. Comparative analysis of miRNA profile in human dendritic cells infected with respiratory syncytial virus and human metapneumovirus. BMC Res. Notes 2018, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ptashkin, R.N.; Wang, Q.; Liu, G.; Zhang, G.; Lee, I.; Lee, Y.S.; Bao, X. Human metapneumovirus infection induces significant changes in small noncoding RNA expression in airway epithelial cells. Mol. Ther. Nucleic Acids 2014, 3, e163. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Choi, E.-J.; Lee, I.; Lee, Y.S.; Bao, X. Non-Coding RNAs and Their Role in Respiratory Syncytial Virus (RSV) and Human Metapneumovirus (hMPV) Infections. Viruses 2020, 12, 345. [Google Scholar] [CrossRef]
- Srivastava, S.; Garg, I.; Singh, Y.; Meena, R.; Ghosh, N.; Kumari, B.; Kumar, V.; Eslavath, M.R.; Singh, S.; Dogra, V.; et al. Evaluation of Altered MiRNA Expression Pattern to Predict COVID-19 Severity. Heliyon 2023, 9, e13388. [Google Scholar] [CrossRef] [PubMed]
- Salvado, V.d.A.; Alves, A.D.R.; Coelho, W.L.d.C.N.P.; Costa, M.A.; Guterres, A.; Amado, L.A. Screening out microRNAs and Their Molecular Pathways with a Potential Role in the Regulation of Parvovirus B19 Infection Through In Silico Analysis. Int. J. Mol. Sci. 2025, 26, 5038. [Google Scholar] [CrossRef]
- Eilam-Frenkel, B.; Naaman, H.; Brkic, G.; Veksler-Lublinsky, I.; Rall, G.; Shemer-Avni, Y.; Gopas, J. MicroRNA 146–5p, miR-let-7c-5p, miR-221 and miR-345–5p are differentially expressed in Respiratory Syncytial Virus (RSV) persistently infected HEp-2 cells. Virus Res. 2018, 251, 34–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Wang, H.; Zhang, G.; Sun, X. Respiratory syncytial virus non-structural protein 1 facilitates virus replication through miR-29a-mediated inhibition of interferon-alpha receptor. Biochem. Biophys. Res. Commun. 2016, 478, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; He, J.; Zhou, R.; Zheng, N. Expression and Significance of Circulating microRNA-29b in Adult Fulminant Myocarditis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2022, 44, 102–109. [Google Scholar] [PubMed]
- Maruyama, K.; Imanaka-Yoshida, K. The Pathogenesis of Cardiac Fibrosis: A Review of Recent Progress. Int. J. Mol. Sci. 2022, 23, 2617. [Google Scholar] [CrossRef] [PubMed]
- Foinquinos, A.; Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Gyöngyösi, M.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat. Commun. 2020, 11, 633. [Google Scholar] [CrossRef]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef]
- Bauersachs, J.; Solomon, S.D.; Anker, S.D.; Antorrena-Miranda, I.; Batkai, S.; Viereck, J.; Rump, S.; Filippatos, G.; Granzer, U.; Ponikowski, P.; et al. Efficacy and safety of CDR132L in patients with reduced left ventricular ejection fraction after myocardial infarction: Rationale and design of the HF-REVERT trial. Eur. J. Heart Fail. 2024, 26, 674–682. [Google Scholar] [CrossRef]
- Kawai, C. From Myocarditis to Cardiomyopathy: Mechanisms of Inflammation and Cell Death Learning From the Past for the Future. Circulation 1999, 99, 1091–1100. [Google Scholar] [CrossRef]
- Martens, P.; Cooper, L.T.; Tang, W.H.W. Diagnostic Approach for Suspected Acute Myocarditis: Considerations for Standardization and Broadening Clinical Spectrum. J. Am. Heart Assoc. 2023, 12, e031454. [Google Scholar] [CrossRef]
- Shinge, S.A.U.; Zhang, B.; Zheng, B.; Qiang, Y.; Ali, H.M.; Melchiade, Y.T.V.; Zhang, L.; Gao, M.; Feng, G.; Zeng, K.; et al. Unveiling the Future of Infective Endocarditis Diagnosis: The Transformative Role of Metagenomic Next-Generation Sequencing in Culture-Negative Cases. J. Epidemiol. Glob. Health 2025, 15, 108. [Google Scholar] [CrossRef]
- Uccello, G.; Bonacchi, G.; Rossi, V.A.; Montrasio, G.; Beltrami, M. Myocarditis and Chronic Inflammatory Cardiomyopathy, from Acute Inflammation to Chronic Inflammatory Damage: An Update on Pathophysiology and Diagnosis. J. Clin. Med. 2024, 13, 150. [Google Scholar] [CrossRef]
- Crisci, G.; Bobbio, E.; Gentile, P.; Bromage, D.I.; Bollano, E.; Ferone, E.; Israr, M.Z.; Heaney, L.M.; Polte, C.L.; Cannatà, A.; et al. Biomarkers in Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Updated Review of the Literature. J. Clin. Med. 2023, 12, 7214. [Google Scholar] [CrossRef]
- Estepa, M.; Niehues, M.H.; Vakhrusheva, O.; Haritonow, N.; Ladilov, Y.; Barcena, M.L.; Regitz-Zagrosek, V. Sex Differences in Expression of Pro-Inflammatory Markers and miRNAs in a Mouse Model of CVB3 Myocarditis. Int. J. Mol. Sci. 2024, 25, 9666. [Google Scholar] [CrossRef]
- Podolec, J.; Przewłocki, T.; Kabłak-Ziembicka, A. Optimization of Cardiovascular Care: Beyond the Guidelines. J. Clin. Med. 2025, 14, 2406. [Google Scholar] [CrossRef]
- Fiore, G.; Sanvito, F.; Fragasso, G.; Spoladore, R. Case report of cardiogenic shock in COVID-19 myocarditis: Peculiarities on diagnosis, histology, and treatment. Eur. Heart J. Case Rep. 2021, 5, ytab357. [Google Scholar] [CrossRef]
- Nagai, T.; Inomata, T.; Kohno, T.; Sato, T.; Tada, A.; Kubo, T.; Nakamura, K.; Oyama-Manabe, N.; Ikeda, Y.; Fujino, T.; et al. JCS 2023 Guideline on the Diagnosis and Treatment of Myocarditis. Circ. J. 2023, 87, 674–754. [Google Scholar] [CrossRef]

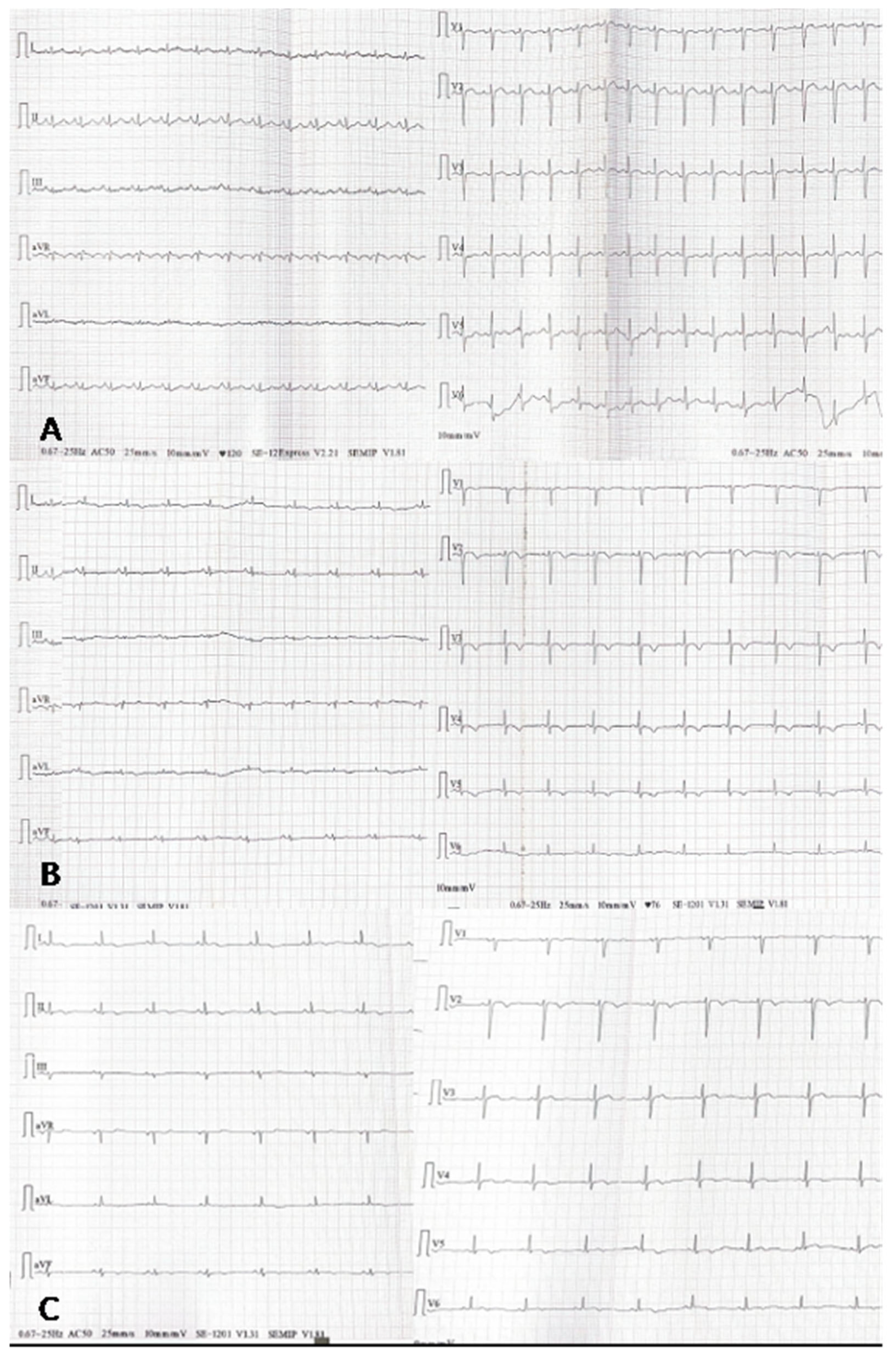
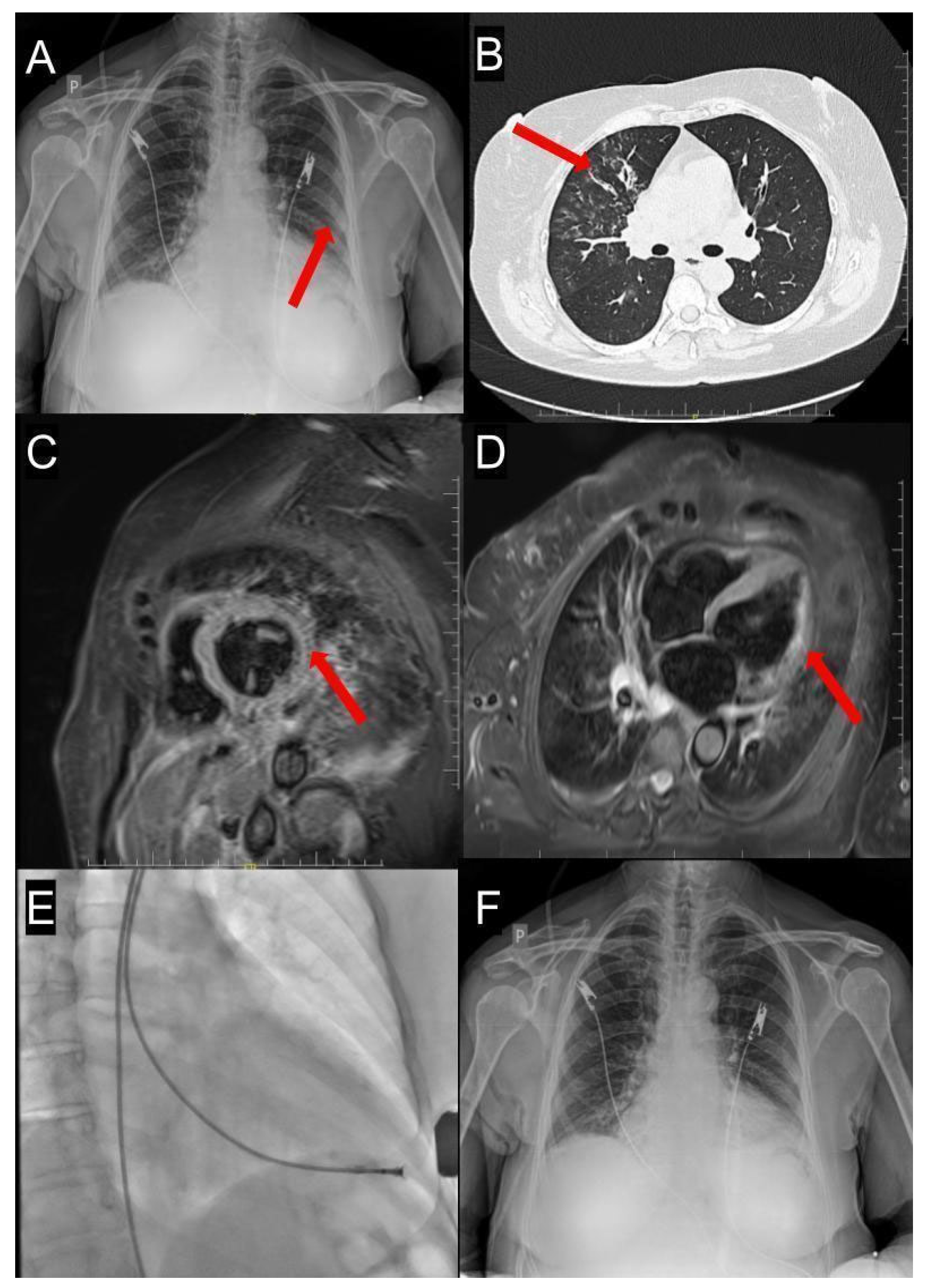
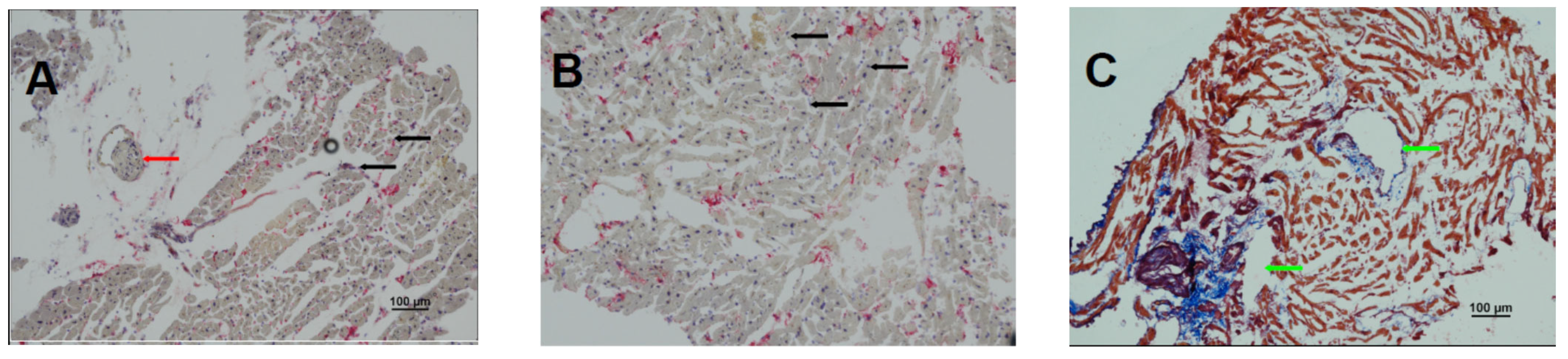
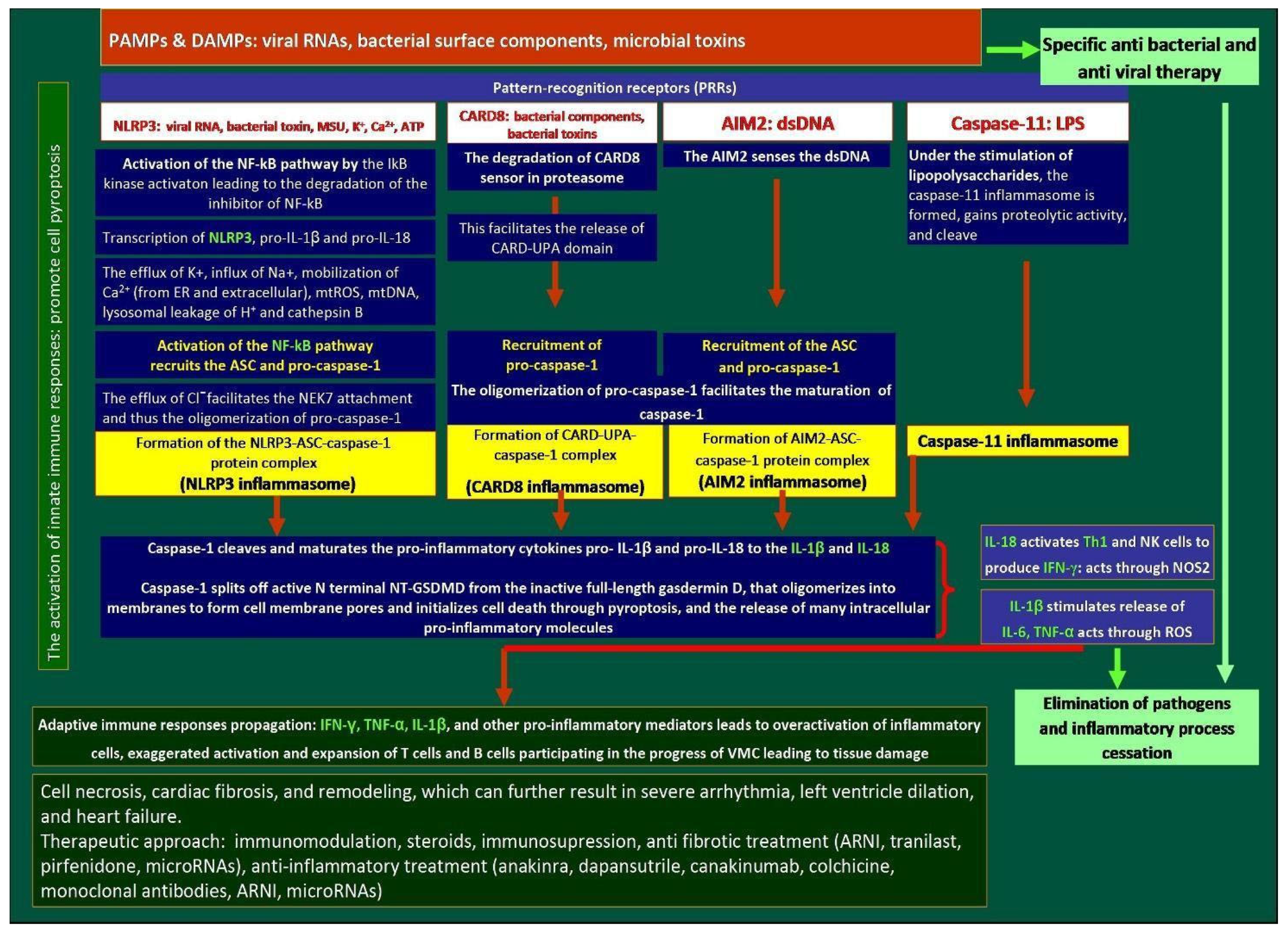
| Time | Course of Myocarditis: From Symptom Onset Until 6 Months Follow-Up Visit |
|---|---|
| Day 1 | First symptoms: sore throat, chest pain during coughing, productive cough, chills, muscle joint pain/stiffness, fever related to acute upper respiratory tract infection |
| Day 5 | Initial presentation: The patient presented to the emergency department with chest pain and signs of rapidly deteriorating symptoms of HF. Admission laboratory findings: PCT 1.27 ng/mL, CRP 98.7 mg/L, IL-6 19.2 pg/mL, hs-TnT 786 ng/L, NT-proBNP 6978 pg/mL, CK 343 U/L, CK-MB 20 U/L. TTE findings: reduced systolic function with LV-EF of 45% without segmental contractile abnormalities. Initial management: infection symptoms and HF treatment initiated, primarily with ACEi and diuretics, non-steroidal anti-inflammatory agents, and proton pump inhibitor |
| Day 6 | Positive hMPV result |
| Day 8 and Day 9 | Further decline in LV-EF to 35%, and then to 25% on TTE, change from ACEi to ARNI, decision to perform EBM. Follow-up laboratory results: PCT 0.43 ng/mL, CRP 45 mg/L, IL-6 59.7 pg/mL, hs-TnT 435 ng/L, NT-proBNP 3794 pg/mL, CK 247 U/L, CK-MB 11 U/L |
| Day 10 | EMB performed. Post-procedurally, the patient presented with cardiogenic shock and pericardial effusion and required continuous infusion of noradrenaline and dobutamine. I.V. ARNI was discontinued. |
| Day 11–14 | Treatment with inotropics continued. Steady reduction in pericardial effusion, and improvement in LV-EF to 45%. Reduction in inflammatory markers: PCT < 0.05 ng/mL, CRP 17 mg/L, IL-6 4.3 pg/mL, hs-TnT 39 ng/L, NT-proBNP 1876 pg/mL, CK 65 U/L, CK-MB 12 U/L |
| Day 14 | Clinical stabilization: Complete weaning off inotropic support |
| Day 15 | Recurrence of infection symptoms: fever, cough, dyspnoea. Laboratory findings: new increase in inflammatory markers: PCT 0.11 ng/mL, CRP 275 mg/L, IL-6 156 pg/mL, hs-TnT 115 ng/L, NT-proBNP 2731 pg/mL, CK 146 U/L, CK-MB 13 U/L. Identified co-infection with Streptococcus pneumoniae. Initiated azithromycin. Started prednisone as a result of EBM findings. |
| Day 15–19 | TEE: Steady improvement in LV-EF up to 60%. Steady reduction in inflammatory parameters, as well as the improvement of the patient’s symptoms. |
| Day 20 | Patient’s assessment on discharge: laboratory results: PCT < 0.05 ng/mL, CRP 10 mg/L, IL-6 9 pg/mL, hs-TnT 21 ng/L, NT-proBNP 3017 pg/mL, CK 15 U/L, CK-MB 9 U/L. Optimal HF therapy |
| 3 months F-U | Follow-up visit: no resting dyspnoea, symptom in NYHA class II, ARNI titrated to intermediate dose. Laboratory findings: PCT < 0.05 ng/mL, CRP 5.0 mg/L, IL-6 1.6 pg/mL, hs-TnT 6 ng/L, NT-proBNP 1850 pg/mL, CK-MB 6 U/L |
| 6 months F-U | Follow-up visit: no resting dyspnoea, symptom in NYHA class II, ARNI titrated to intermediate dose. Laboratory findings: PCT < 0.05 ng/mL, CRP 3.5 mg/L, IL-6 0.58 pg/mL, hs-TnT 4 ng/L, NT-proBNP 1240 pg/mL, CK-MB 3 U/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przytuła, N.; Podolec, J.; Przewłocki, T.; Podolec, P.; Kabłak-Ziembicka, A. Inflammasomes as Potential Therapeutic Targets to Prevent Chronic Active Viral Myocarditis—Translating Basic Science into Clinical Practice. Int. J. Mol. Sci. 2025, 26, 11003. https://doi.org/10.3390/ijms262211003
Przytuła N, Podolec J, Przewłocki T, Podolec P, Kabłak-Ziembicka A. Inflammasomes as Potential Therapeutic Targets to Prevent Chronic Active Viral Myocarditis—Translating Basic Science into Clinical Practice. International Journal of Molecular Sciences. 2025; 26(22):11003. https://doi.org/10.3390/ijms262211003
Chicago/Turabian StylePrzytuła, Natalia, Jakub Podolec, Tadeusz Przewłocki, Piotr Podolec, and Anna Kabłak-Ziembicka. 2025. "Inflammasomes as Potential Therapeutic Targets to Prevent Chronic Active Viral Myocarditis—Translating Basic Science into Clinical Practice" International Journal of Molecular Sciences 26, no. 22: 11003. https://doi.org/10.3390/ijms262211003
APA StylePrzytuła, N., Podolec, J., Przewłocki, T., Podolec, P., & Kabłak-Ziembicka, A. (2025). Inflammasomes as Potential Therapeutic Targets to Prevent Chronic Active Viral Myocarditis—Translating Basic Science into Clinical Practice. International Journal of Molecular Sciences, 26(22), 11003. https://doi.org/10.3390/ijms262211003







