Influence of Agronomic Practices on the Bioactive Compound Production in Cannabis sativa L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Cultivar, Planting Density, and Flowering Time
2.1.1. Cannabinoids

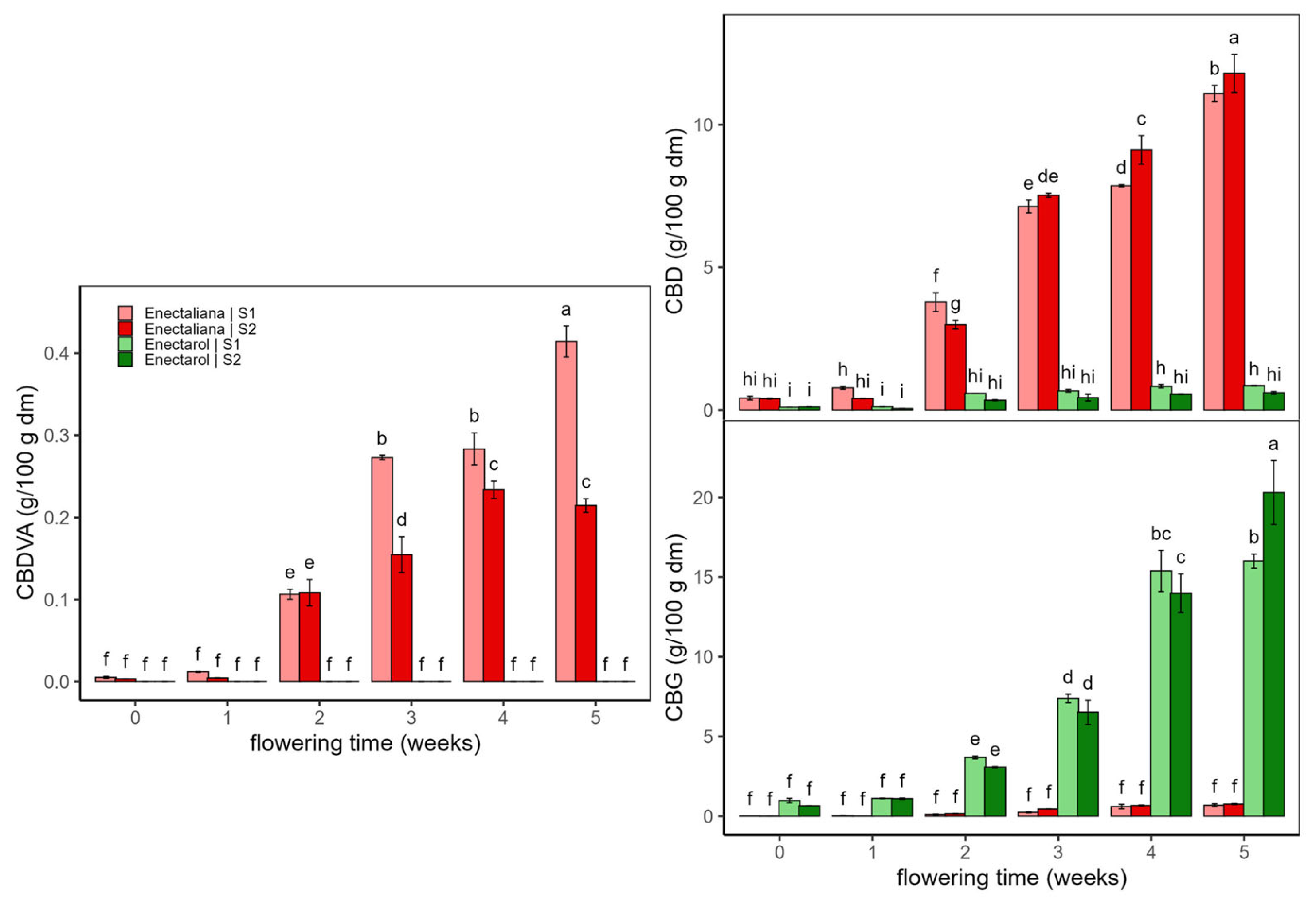
| F (D × T×C) | p (D × T × C) | η2p (D × T × C) | Two-Way Interaction | F (2-v) | p (2-v) | η2p (2-v) | Main Effect | F (Main) | p (Main) | η2p (Main) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| THCVA | 17.7 | 7 × 10−10 | 0.65 | T × C | 161 | <2 × 10−16 | 0.94 | T | 1236 | <2 × 10−16 | 0.99 |
| THC | 10.8 | 5 × 10−7 | 0.53 | T × C | 912 | <2 × 10−16 | 0.99 | T | 1760 | <2 × 10−16 | 0.99 |
| CBC | 12.2 | 1 × 10−7 | 0.56 | T × C | 60 | <2 × 10−16 | 0.86 | C | 1453 | <2 × 10−16 | 0.97 |
| CBN | 1.8 | 0.1 | 0.16 | T × C | 192 | 1.7 × 10−30 | 0.95 | C | 1727 | 2.7 × 10−39 | 0.97 |
| CBDVA | 69.8 | <2 × 10−16 | 0.88 | T × C | 727 | <2 × 10−16 | 0.99 | C | 5783 | <2 × 10−16 | 0.99 |
| CBD | 11.6 | 2 × 10−7 | 0.55 | T × C | 1300 | <2 × 10−16 | 0.99 | C | 10,117 | <2 × 10−16 | 1 |
| CBG | 9.6 | 2 × 10−6 | 0.50 | T × C | 438 | <2 × 10−16 | 0.98 | C | 2769 | <2 × 10−16 | 0.98 |
| α-Pinene | 7.6 | 2.7 × 10−5 | 0.44 | T × C | 60.4 | <2 × 10−16 | 0.86 | C | 1114 | <2 × 10−16 | 0.96 |
| Myrcene | 11.3 | 3.1 × 10−7 | 0.54 | T × C | 24.4 | 3.8 × 10−12 | 0.72 | T | 148 | <2 × 10−16 | 0.94 |
| β-Caryophyllene | 50.8 | <2 × 10−16 | 0.84 | T × C | 95.1 | <2 × 10−16 | 0.91 | T | 464 | <2 × 10−16 | 0.98 |
| Bisabolol | 4.7 | 1.1 × 10−3 | 0.33 | T × C | 26.4 | 1 × 10−12 | 0.73 | C | 891 | <2 × 10−16 | 0.95 |
| TPC | 18.4 | 3.7 × 10−10 | 0.66 | T × C | 8.61 | 7.0 × 10−6 | 0.47 | T | 149 | 5.3 × 10−28 | 0.94 |
| AA (ABTS) | 2 | 0.09 | 0.17 | D × T | 4.5 | 0.002 | 0.32 | T | 61 | 1.3 × 10−19 | 0.86 |
| AA (FRAP) | 6 | 0.0002 | 0.39 | T × C | 20.9 | 4.9 × 10−11 | 0.69 | T | 56 | 6.9 × 10−19 | 0.85 |
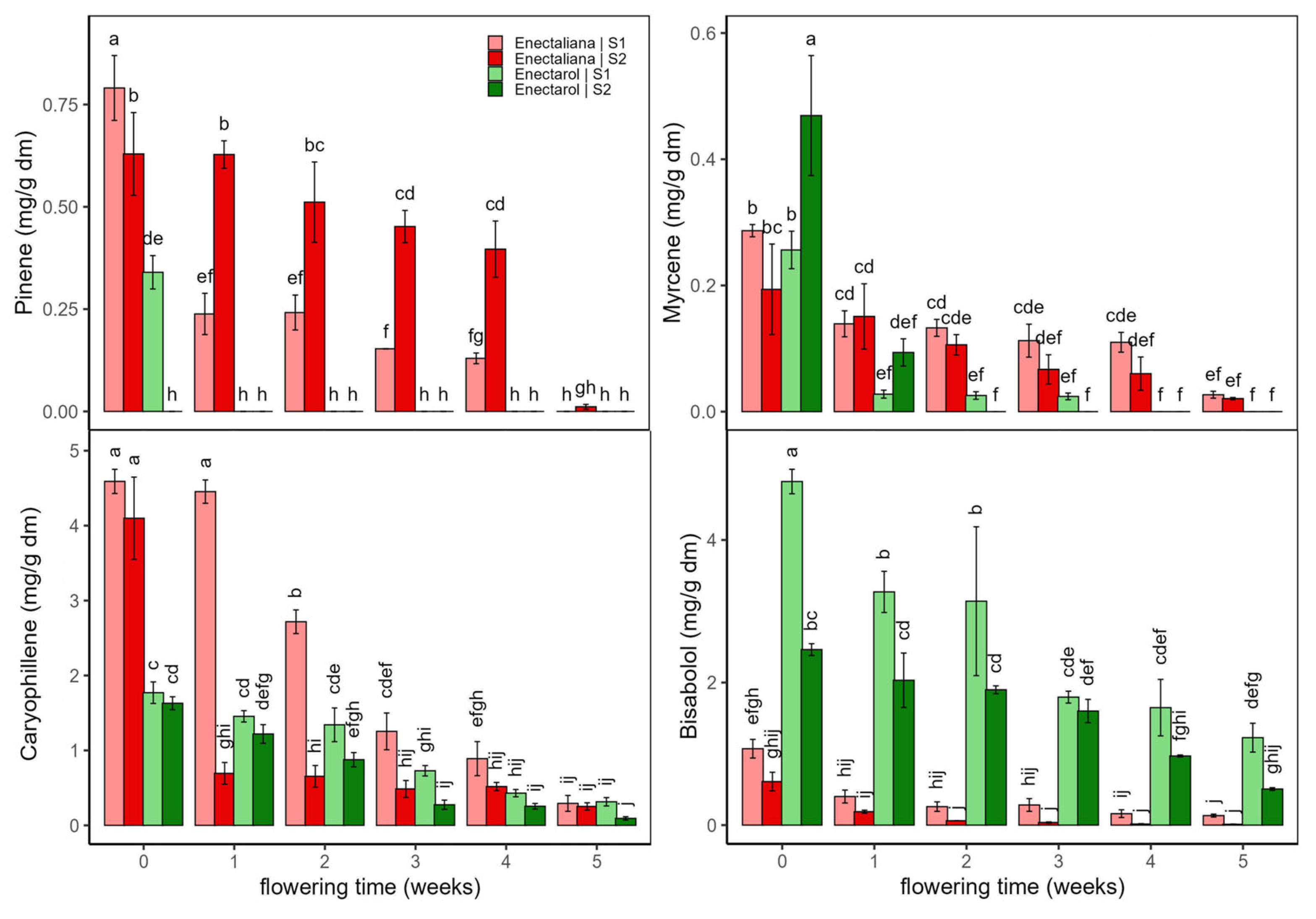
2.1.2. Terpenes
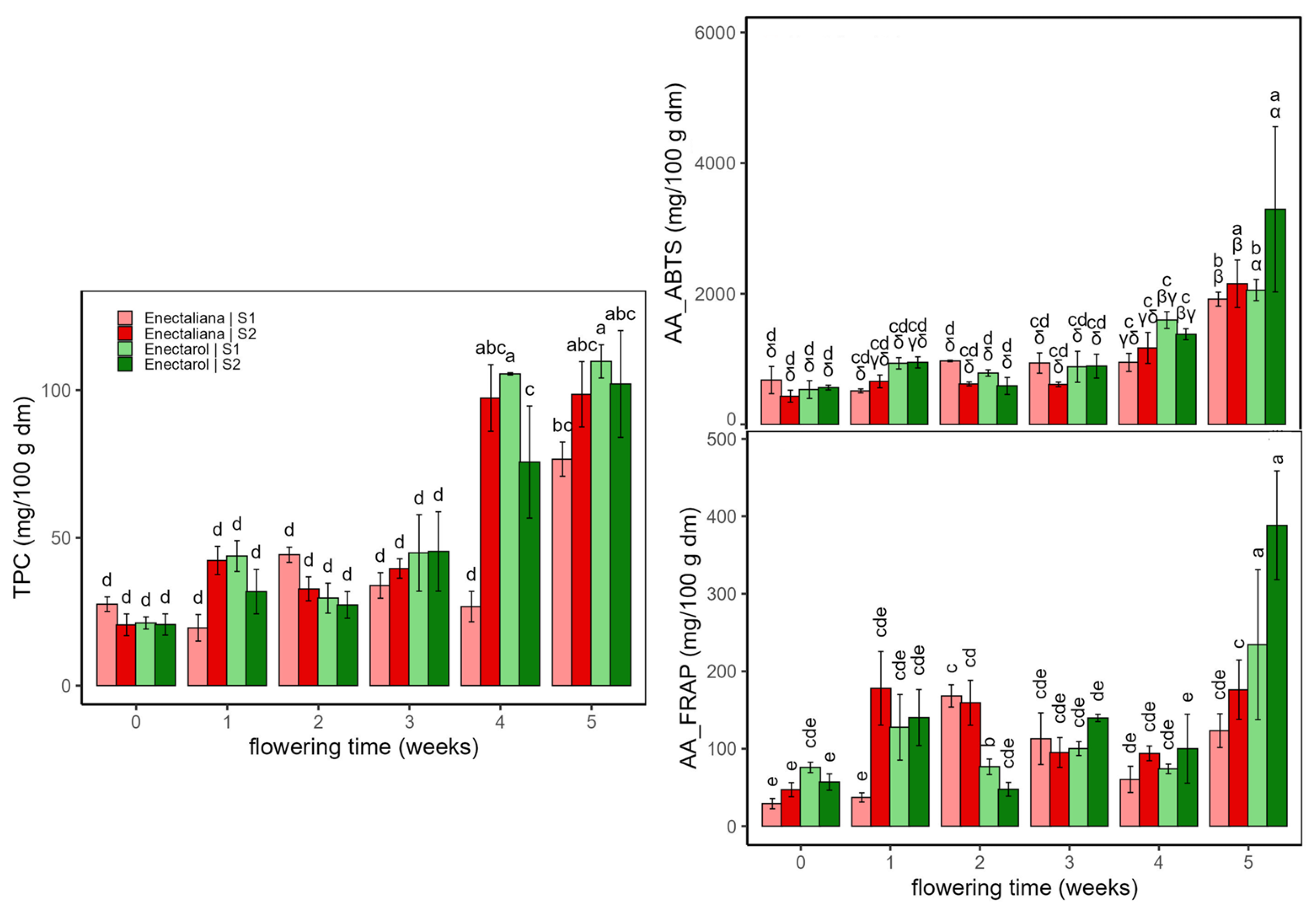
2.1.3. Antioxidant Compounds
2.2. Effect of Fertilizer Application, Planting Density, and Flowering Time in the Enectaliana Cultivar
2.2.1. Cannabinoids
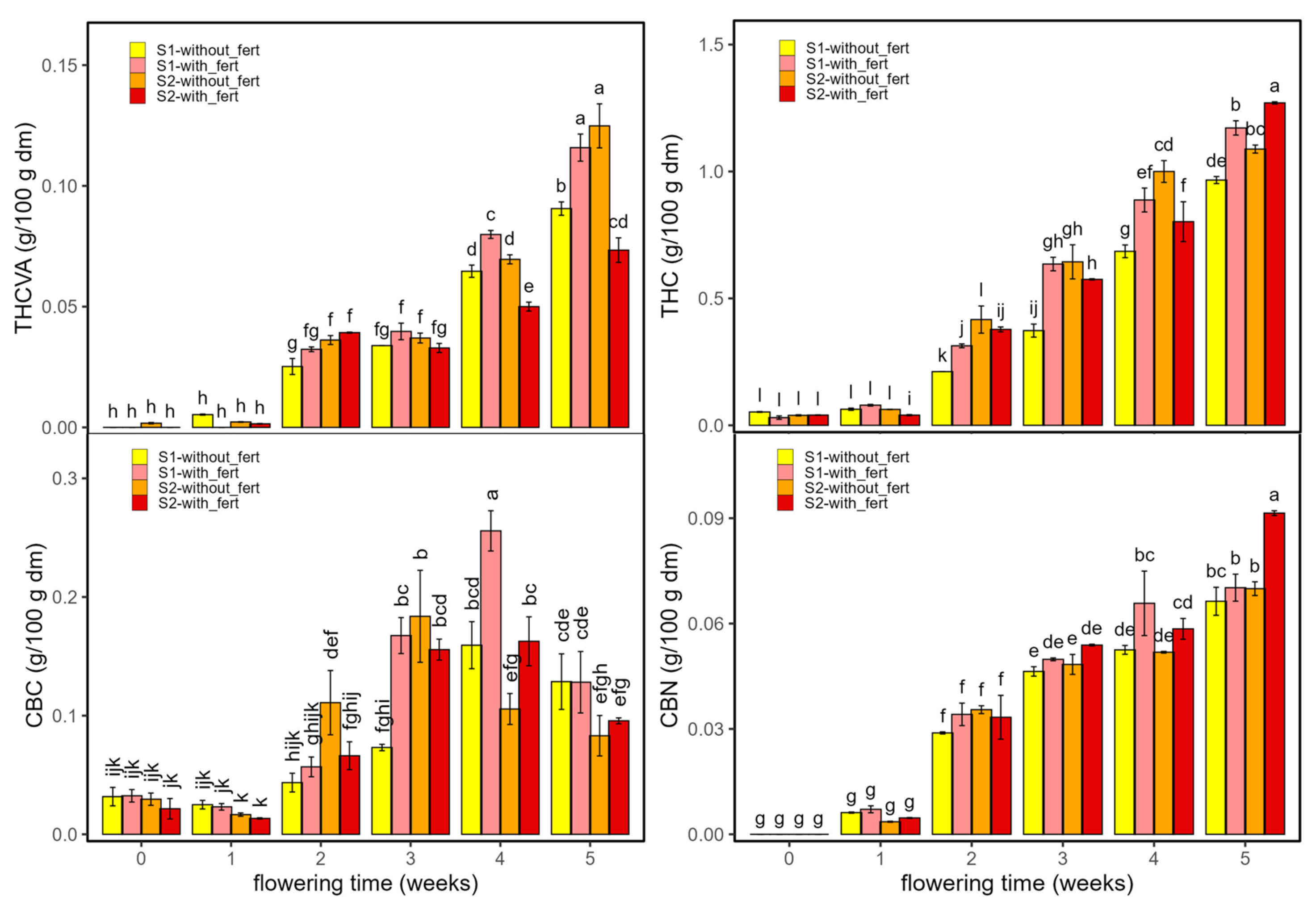
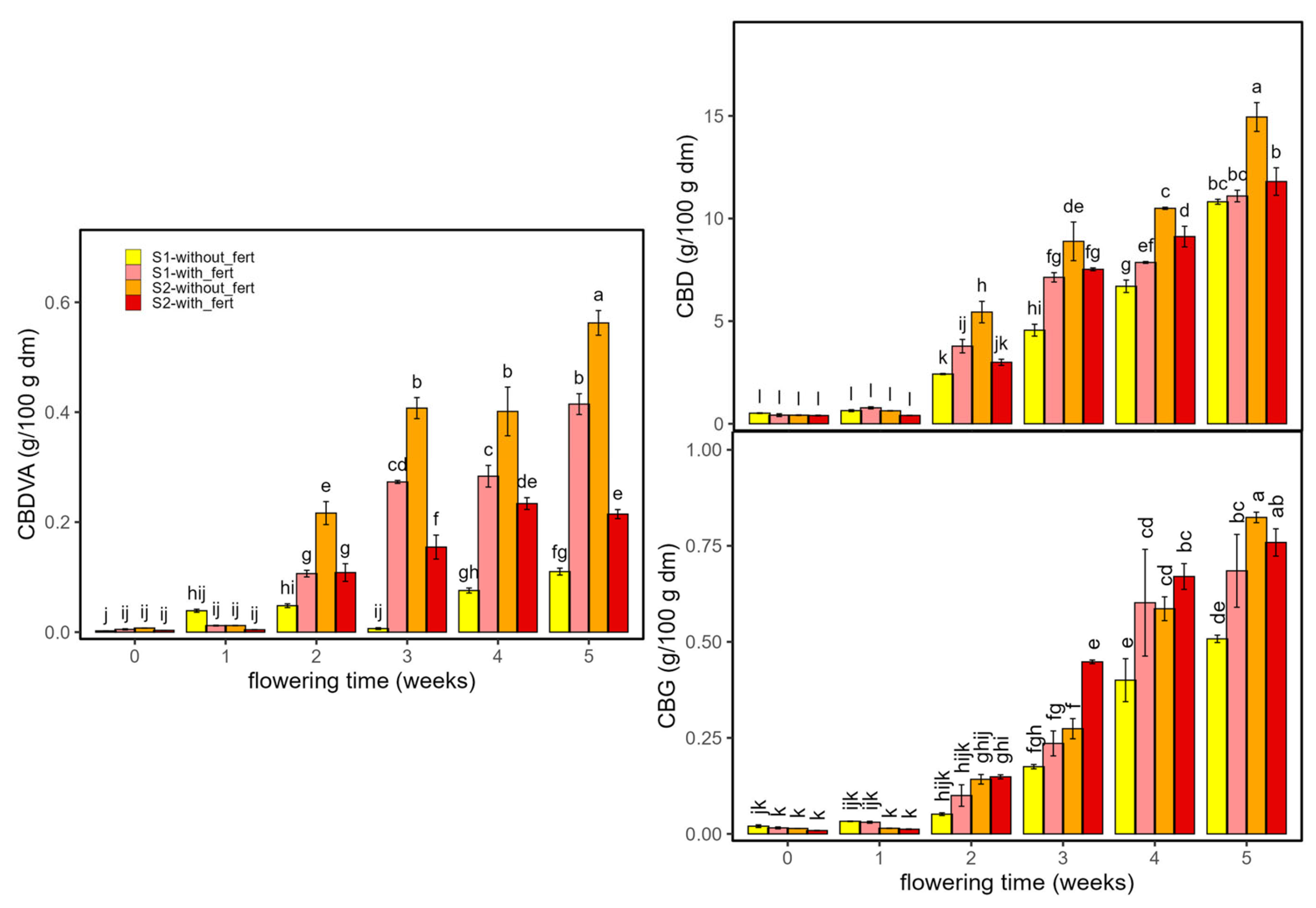
| F (D × T × f) | p (D × T × f) | η2p (D × T × f) | Two-Way Interaction | F (2-v) | p (2-v) | η2p (2-v) | Main Effect | F (Main) | p (Main) | η2p (Main) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| THCVA | 85 | <2 × 10−16 | 0.90 | D × f | 227 | <2 × 10−16 | 0.83 | T | 2160 | <2 × 10−16 | 1 |
| THC | 57 | <2 × 10−16 | 0.90 | T × f | 73 | <2 × 10−16 | 0.88 | T | 2485 | <2 × 10−16 | 1 |
| CBC | 10 | 1 × 10−6 | 0.59 | D × T | 24 | 6 × 10−12 | 0.71 | T | 776 | <2 × 10−16 | 0.99 |
| CBN | 8 | 2 × 10−5 | 0.49 | D × T | 13 | 6 × 10−8 | 0.57 | T | 1311 | <2 × 10−16 | 0.99 |
| CBDVA | 272 | <2 × 10−16 | 0.90 | D × f | 1715 | <2 × 10−16 | 0.97 | T | 954 | <2 × 10−16 | 0.99 |
| CBD | 20 | 1 × 10−10 | 0.70 | D × f | 208 | <2 × 10−16 | 0.81 | T | 2220 | <2 × 10−16 | 1 |
| CBG | 7 | 6 × 10−5 | 0.40 | D × T | 14 | 1 × 10−8 | 0.60 | T | 635 | <2 × 10−16 | 0.98 |
| α-Pinene | 5.8 | 3 × 10−4 | 0.4 | D × T | 41 | 3 × 10−16 | 0.8 | T | 142 | <2 × 10−16 | 0.94 |
| Myrcene | 10.3 | 10 × 10−7 | 0.5 | D × T | 21 | 4 × 10−11 | 0.7 | T | 65 | <2 × 10−16 | 0.87 |
| β-Caryophyllene | 11.5 | 2 × 10−7 | 0.5 | D × T | 113 | <2 × 10−16 | 0.9 | T | 482 | <2 × 10−16 | 0.98 |
| Bisabolol | 9.8 | 2 × 10−6 | 0.5 | D × T | 32 | 3 × 10−14 | 0.8 | T | 233 | <2 × 10−16 | 0.96 |
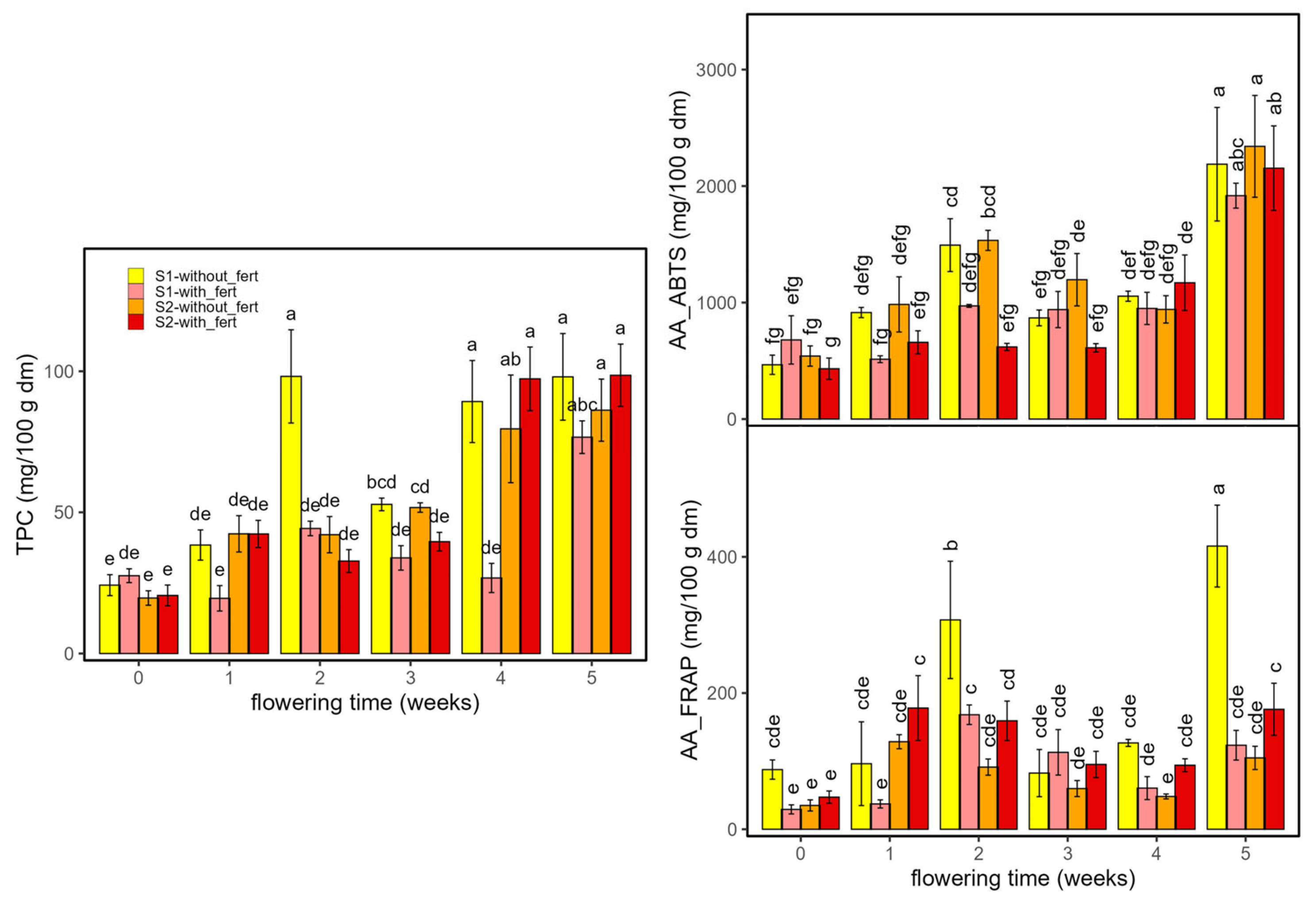
| TPC | 38.7 | 1 × 10−15 | 0.8 | D × T | 129 | <2 × 10−16 | 0.93 | T | 745 | <2 × 10−16 | 0.99 |
| AA (ABTS) | 12.7 | 7.3 × 10−8 | 0.57 | T × f | 69 | <2 × 10−16 | 0.88 | T | 869 | <2 × 10−16 | 0.99 |
| AA (FRAP) | 78.8 | <2 × 10−16 | 0.89 | D × f | 616 | <2 × 10−16 | 0.93 | T | 256 | <2 × 10−16 | 0.96 |
2.2.2. Terpenes
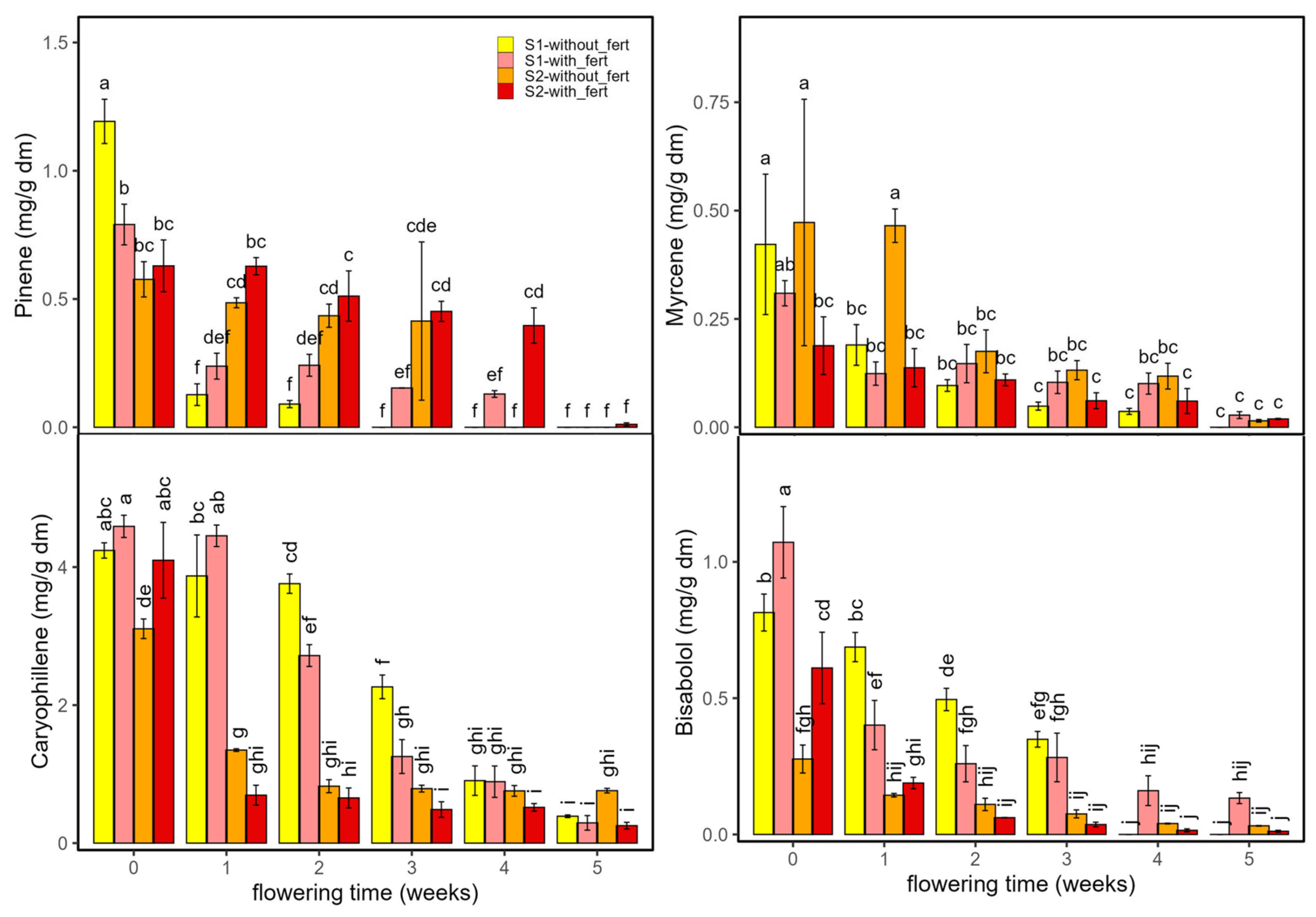
2.2.3. Antioxidant Compounds
3. Materials and Methods
3.1. Experimental Site
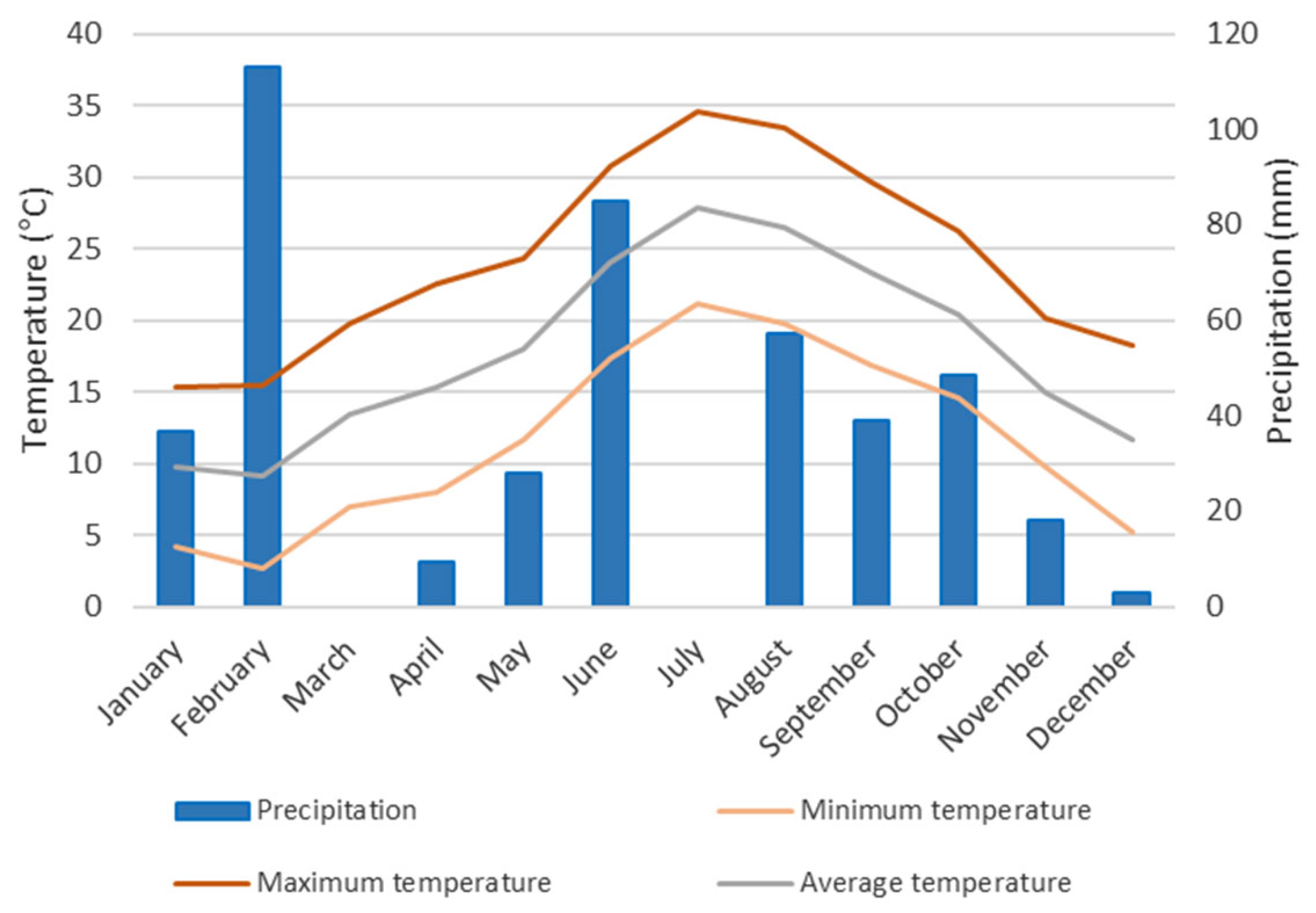
3.2. Samples
3.3. Analytical Methodology
3.3.1. Moisture Content
3.3.2. Extraction of Bioactive Compounds
3.3.3. Cannabinoid Analysis by GC-FID
3.3.4. Terpene Analysis by GC-MS
3.3.5. Antioxidant Compounds
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Antioxidant activity (mg trolox/100 d dry matter) |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| CBC | Cannabichromene |
| CBD | Cannabigerol |
| CBDV | Cannabidivarin |
| CBG | Cannabigerol |
| CBN | Cannabinol |
| FRAP | Ferric Reducing Antioxidant Power |
| GC-FID | Gas Chromatography with Flame ionization detector |
| CG-MS | Gas Chromatography with Mass Spectrometry |
| THC | Tetrahydrocannabinol |
| THCV | Tetrahydrocannabivarin |
| TPC | Total polyphenol content (mg gallic acid/100 d dry matter) |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
References
- André, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the Polyphenol and Terpene Content, Antioxidant Activity and Plant Morphology of Eight Different Fiber-Type Cultivars of Cannabis sativa L. Cultivated at Three Sowing Densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef]
- Callado, C.S.-C.; Núñez-Sánchez, N.; Casano, S.; Ferreiro-Vera, C. The Potential of near Infrared Spectroscopy to Estimate the Content of Cannabinoids in Cannabis sativa L.: A Comparative Study. Talanta 2018, 190, 147–157. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Wang, M.; Radwan, M.M.; Wanas, A.S.; Majumdar, C.G.; Avula, B.; Wang, Y.H.; Khan, I.A.; Chandra, S.; Lata, H.; et al. Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application. Planta Medica 2019, 85, 431–438. [Google Scholar] [CrossRef]
- Brkljača, N.; Đurović, S.; Milošević, S.; Gašić, U.; Panković, D.; Zeković, Z.; Pavlić, B. Sequential Extraction Approach for Sustainable Recovery of Various Hemp (Cannabis sativa L.) Bioactive Compounds. Sustain. Chem. Pharm. 2023, 35, 101213. [Google Scholar] [CrossRef]
- Trovato, E.; Arena, K.; Tella, R.L.; Rigano, F.; Vinci, R.L.; Dugo, P.; Mondello, L.; Guarnaccia, P. Hemp Seed-Based Food Products as Functional Foods: A Comprehensive Characterization of Secondary Metabolites Using Liquid and Gas Chromatography Methods. J. Food Compos. Anal. 2023, 117, 105151. [Google Scholar] [CrossRef]
- Parlamento Europeo y Consejo de la Unión Europea. Reglamento (UE) 2021/2115 del Parlamento Europeo y del Consejo, de 2 de diciembre de 2021, por el que se establecen normas relativas a los planes estratégicos que deben elaborar los Estados miembros en el marco de la política agrícola común (PAC). D. Of. Unión Eur. 2021, L 435, 1–186. [Google Scholar]
- Ostadi, A.; Javanmard, A.; Machiani, M.A.; Morshedloo, M.R.; Nouraein, M.; Rasouli, F.; Maggi, F. Effect of Different Fertilizer Sources and Harvesting Time on the Growth Characteristics, Nutrient Uptakes, Essential Oil Productivity and Composition of Mentha x piperita L. Ind. Crops Prod. 2020, 148, 112290. [Google Scholar] [CrossRef]
- Yazici, L. Optimizing Plant Density for Fiber and Seed Production in Industrial Hemp (Cannabis sativa L.). J. King Saud. Univ. -Sci. 2023, 35, 102419. [Google Scholar] [CrossRef]
- Dowling, C.A.; Melzer, R.; Schilling, S. Timing Is Everything: The Genetics of Flowering Time in Cannabis sativa. Biochemist 2021, 43, 34–38. [Google Scholar] [CrossRef]
- European Union. No 1177/2000, R. (CE) Regulation (CE) No 1177/2000 of the Commission, of 31 May 2000. Off. J. Eur. Communities 2000, L131, 38–40. [Google Scholar]
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Benković, E.T. Cannabinoid Content in Industrial Hemp (Cannabis sativa L.) Varieties Grown in Slovenia. Ind. Crops Prod. 2020, 145, 112082. [Google Scholar] [CrossRef]
- Tahir, M.N.; Raz, F.S.; Rondeau-Gagné, S.; Trant, J.F. The Biosynthesis of the Cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Jaidee, W.; Siridechakorn, I.; Nessopa, S.; Wisuitiprot, V.; Chaiwangrach, N.; Ingkaninan, K.; Waranuch, N. Kinetics of CBD, Δ9-THC Degradation and Cannabinol Formation in Cannabis Resin at Various Temperature and pH Conditions. Cannabis Cannabinoid Res. 2022, 7, 537–547. [Google Scholar] [CrossRef]
- Yang, R.; Yang, R.; Berthold, E.C.; McCurdy, C.R.; McCurdy, C.R.; Benevenute, S.D.S.; Brym, Z.T.; Freeman, J.H. Development of Cannabinoids in Flowers of Industrial Hemp (Cannabis sativa L.)-a Pilot Study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef]
- Amaducci, S.; Errani, M.; Venturi, G. Response of Hemp to Plant Population and Nitrogen Fertilisation. Ital. J. Agron. 2002, 6, 103–111. [Google Scholar]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant Density and Nitrogen Fertilization Affect Agronomic Performance of Industrial Hemp (Cannabis sativa L.) in Mediterranean Environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- Khatri, D.; Sandhu, S.S.; Johns, F.; Sandhu, H.; Chiluwal, A. Plant Spacing and Cultivar Effect on Industrial Hemp Growth, Biomass, and Cannabinoids Yield. Agrosyst. Geosci. Environ. 2025, 8, e70128. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Too Dense or Not Too Dense: Higher Planting Density Reduces Cannabinoid Uniformity but Increases Yield/Area in Drug-Type Medical Cannabis. Front. Plant Sci. 2022, 13, 713481. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and Storage Stability of Polyphenols from Vitis vinifera Grape Wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, A.; Lebaschi, M.H.; Ardakani, M.R.; Sharifabad, H.H.; Mirza, M. Plant Density and Manure Application Affected Yield and Essential Oil Composition of Bakhtiari Savory (Satureja bachtiarica Bunge.). Ind. Crops Prod. 2022, 177, 114516. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Cikoš, A.-M.; Jakovljević, M.; Šafranko, S.; Jerković, I. Separation of Selected Bioactive Compounds from Orange Peel Using the Sequence of Supercritical CO2 Extraction and Ultrasound Solvent Extraction: Optimization of Limonene and Hesperidin Content. Sep. Sci. Technol. 2019, 55, 2799–2811. [Google Scholar] [CrossRef]
- Mudge, E.M.; Murch, S.J.; Brown, P.N. Chemometric Analysis of Cannabinoids: Chemotaxonomy and Domestication Syndrome. Sci. Rep. 2018, 8, 13090. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Pirzad, A.; Shakiba, M.R.; Zehtab-Salmasi, S.; Mohammadi, S.A.; Sharifi, R.S.; Hassani, A. Effects of Irrigation Regime and Plant Density on Essential Oil Composition of German Chamomile (Matricaria chamomilla). J. Herbs Spices Med. Plants 2011, 17, 107–118. [Google Scholar] [CrossRef]
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.B.; Amaducci, S. High Added-Value Compounds from Cannabis Threshing Residues. Ind. Crops Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary Metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Grevsen, K.; Fretté, X.; Christensen, L.P. Content and Composition of Volatile Terpenes, Flavonoids and Phenolic Acids in Greek Oregano (Origanum vulgare L. ssp. hirtum) at Different Development Stages during Cultivation in Cool Temperate Climate. Eur. J. Hortic. Sci. 2009, 74, 193–203. [Google Scholar]
- Assaggaf, H.M.; Naceiri Mrabti, H.; Rajab, B.S.; Attar, A.A.; Alyamani, R.A.; Hamed, M.; El Omari, N.; El Menyiy, N.; Hazzoumi, Z.; Benali, T.; et al. Chemical Analysis and Investigation of Biological Effects of Salvia Officinalis Essential Oils at Three Phenological Stages. Molecules 2022, 27, 5157. [Google Scholar] [CrossRef]
- Linder, E.R.; Young, S.; Li, X.; Henriquez Inoa, S.; Suchoff, D.H. The Effect of Harvest Date on Temporal Cannabinoid and Biomass Production in the Floral Hemp (Cannabis sativa L.) Cultivars BaOx and Cherry Wine. Horticulturae 2022, 8, 959. [Google Scholar] [CrossRef]
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of Nitrogen, Phosphorus, and Potassium for Soilless Production of Cannabis sativa in the Flowering Stage Using Response Surface Analysis. Front. Plant Sci. 2021, 12, 764103. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen Source Matters: High NH4/NO3 Ratio Reduces Cannabinoids, Terpenoids, and Yield in Medical Cannabis. Front. Plant Sci. 2022, 13, 830224. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, D.; Mercogliano, F.; Rebucci, R.; Bani, C.; Pinotti, L.; Di Lorenzo, C.; Savoini, G.; Baldi, A.; Giromini, C. Phenolic Profile and Antioxidant Activity of Hemp Co-Products Following Green Chemical Extraction and Ex Vivo Digestion. Ital. J. Anim. Sci. 2024, 23, 651–663. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Beleggia, R.; Menga, V.; Fulvio, F.; Fares, C.; Trono, D. Effect of Genotype, Year, and Their Interaction on the Accumulation of Bioactive Compounds and the Antioxidant Activity in Industrial Hemp (Cannabis sativa L.) Inflorescences. Int. J. Mol. Sci. 2023, 24, 8969. [Google Scholar] [CrossRef]
- Farouk, S.; Omar, M.M. Sweet Basil Growth, Physiological and Ultrastructural Modification, and Oxidative Defense System Under Water Deficit and Silicon Forms Treatment. J. Plant Growth Regul. 2020, 39, 1307–1331. [Google Scholar] [CrossRef]
- Soundararajan, P.; Sivanesan, I.; Jana, S.; Jeong, B.R. Influence of Silicon Supplementation on the Growth and Tolerance to High Temperature in Salvia splendens. Hortic. Environ. Biotechnol. 2014, 55, 271–279. [Google Scholar] [CrossRef]
- Giannoulis, K.D.; Kamvoukou, C.-A.; Gougoulias, N.; Wogiatzi, E. Irrigation and Nitrogen Application Affect Greek Oregano (Origanum vulgare ssp. hirtum) Dry Biomass, Essential Oil Yield and Composition. Ind. Crops Prod. 2020, 150, 112392. [Google Scholar] [CrossRef]
- Sytar, O.; Hajihashemi, S. Specific Secondary Metabolites of Medicinal Plants and Their Role in Stress Adaptation. In Plant Secondary Metabolites and Abiotic Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 425–479. ISBN 978-1-394-18645-7. [Google Scholar]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Prato, L.D.; Ansari, O.; Hardy, G.E.S.J.; Howieson, J.; O’Hara, G.; Ruthrof, K.X. The Cannabinoid Profile and Growth of Hemp (Cannabis sativa L.) Is Influenced by Tropical Daylengths and Temperatures, Genotype and Nitrogen Nutrition. Ind. Crops Prod. 2022, 178, 114605. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Gutiérrez-González, J.A. A Comprehensive and Critical Review of the Unstandardized Folin-Ciocalteu Assay to Determine the Total Content of Polyphenols: The Conundrum of the Experimental Factors and Method Validation. Talanta 2024, 272, 125771. [Google Scholar] [CrossRef] [PubMed]
- Eim, V.S.; Urrea, D.; Rosselló, C.; García-Pérez, J.V.; Femenia, A.; Simal, S. Optimization of the Drying Process of Carrot (Daucus carota v. Nantes) on the Basis of Quality Criteria. Dry. Technol. 2013, 31, 951–962. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different in Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay-Electron-Transfer Reactions with Organic Compounds in Solutions Containing Nitrite or Nitrate. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.-L. Proanthocyanidin Composition and Antioxidant Potential of the Stem Winemaking Byproducts from 10 Different Grape Varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalmau, E.; Umaña, M.; Eim, V.; Bon, J.; Simal, S. Influence of Agronomic Practices on the Bioactive Compound Production in Cannabis sativa L. Int. J. Mol. Sci. 2025, 26, 10999. https://doi.org/10.3390/ijms262210999
Dalmau E, Umaña M, Eim V, Bon J, Simal S. Influence of Agronomic Practices on the Bioactive Compound Production in Cannabis sativa L. International Journal of Molecular Sciences. 2025; 26(22):10999. https://doi.org/10.3390/ijms262210999
Chicago/Turabian StyleDalmau, Esperanza, Mónica Umaña, Valeria Eim, José Bon, and Susana Simal. 2025. "Influence of Agronomic Practices on the Bioactive Compound Production in Cannabis sativa L." International Journal of Molecular Sciences 26, no. 22: 10999. https://doi.org/10.3390/ijms262210999
APA StyleDalmau, E., Umaña, M., Eim, V., Bon, J., & Simal, S. (2025). Influence of Agronomic Practices on the Bioactive Compound Production in Cannabis sativa L. International Journal of Molecular Sciences, 26(22), 10999. https://doi.org/10.3390/ijms262210999






