Cellulose Plant-Derived Scaffolds as a Tool for Myometrium Modeling
Abstract
1. Introduction
2. Results
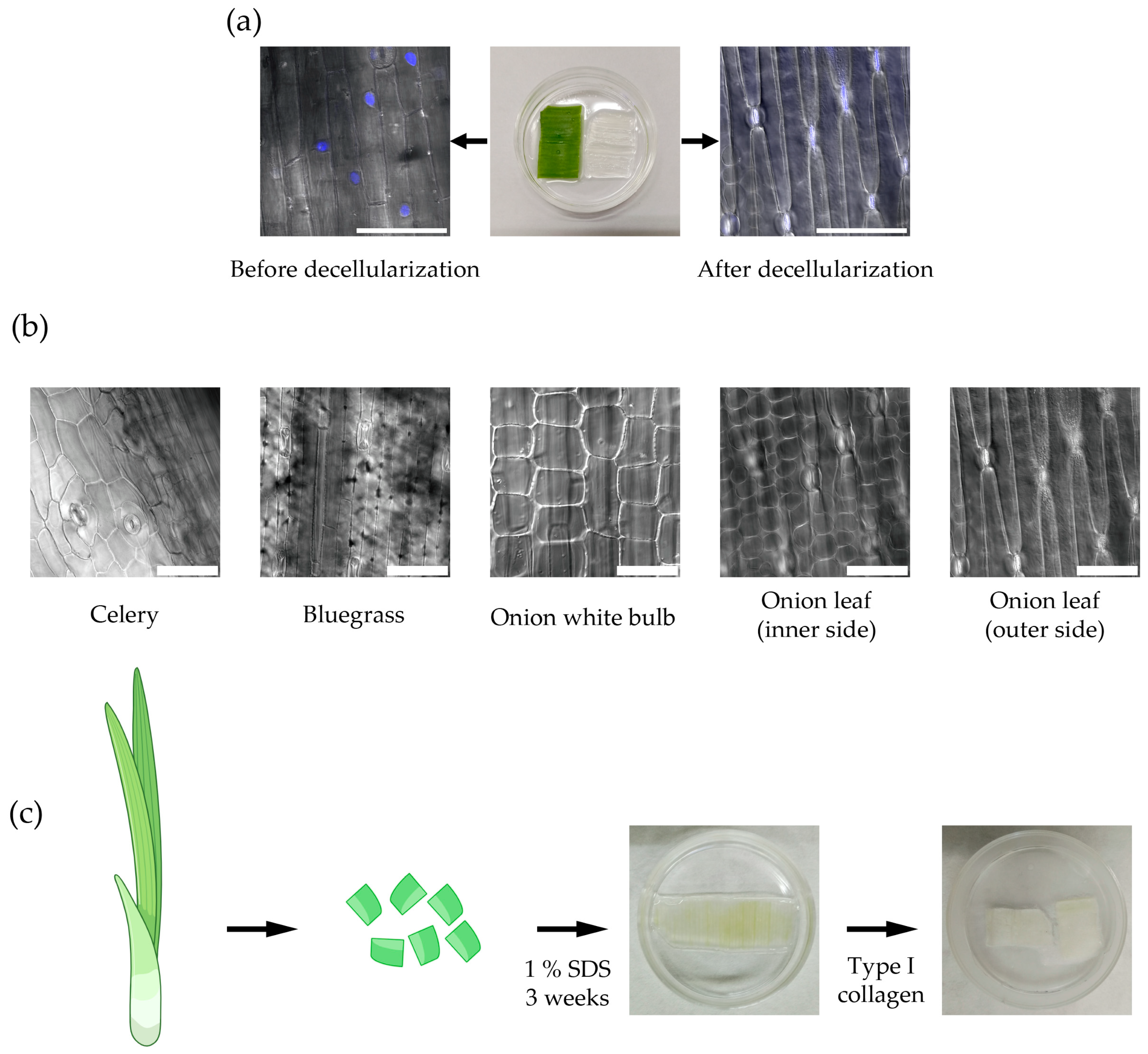
2.1. Plant-Derived Cellulose Scaffold for Myometrium Modeling In Vitro
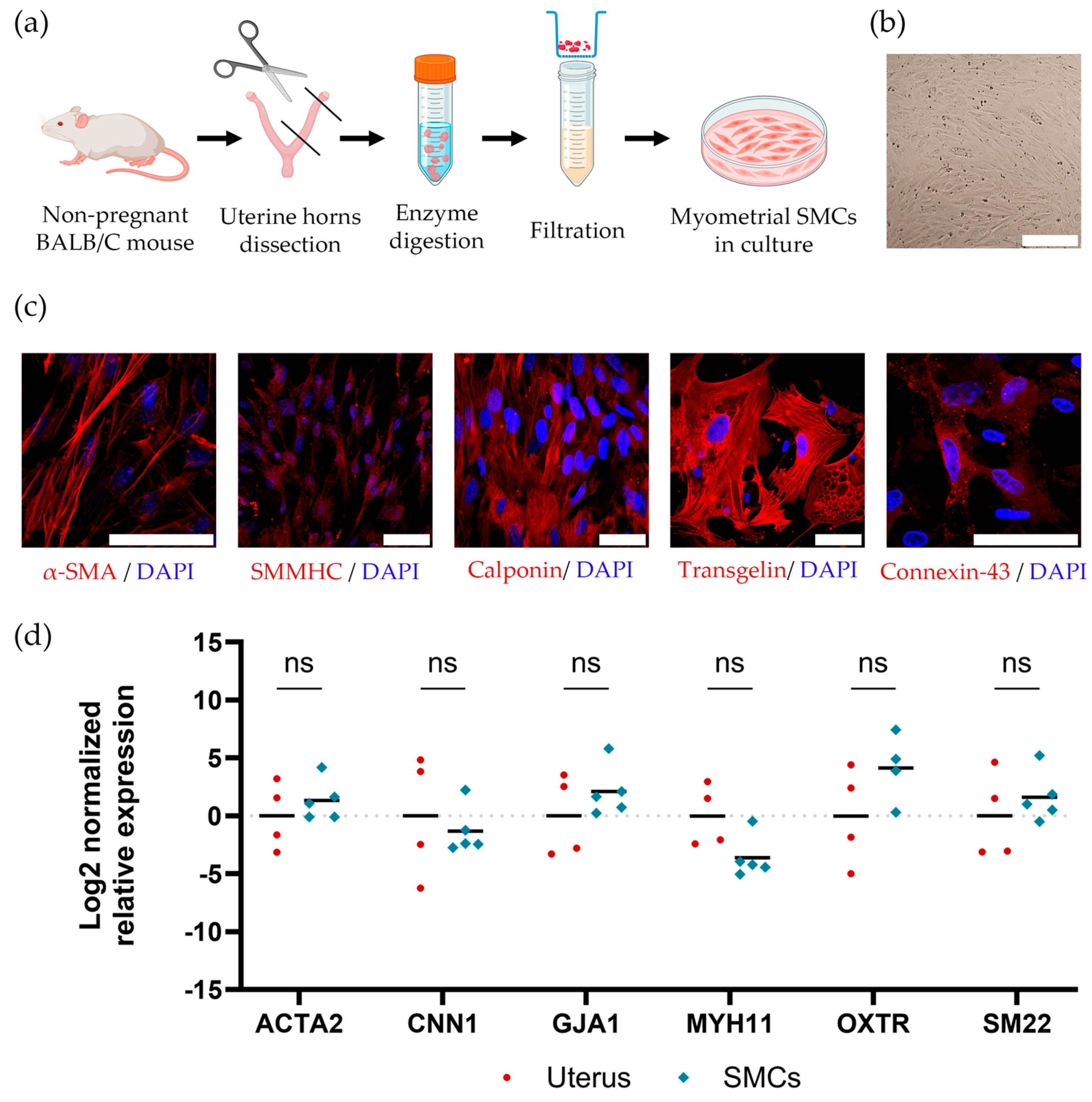
2.2. Isolating and Maintaining Primary Mouse Myometrial Smooth Muscle Cells
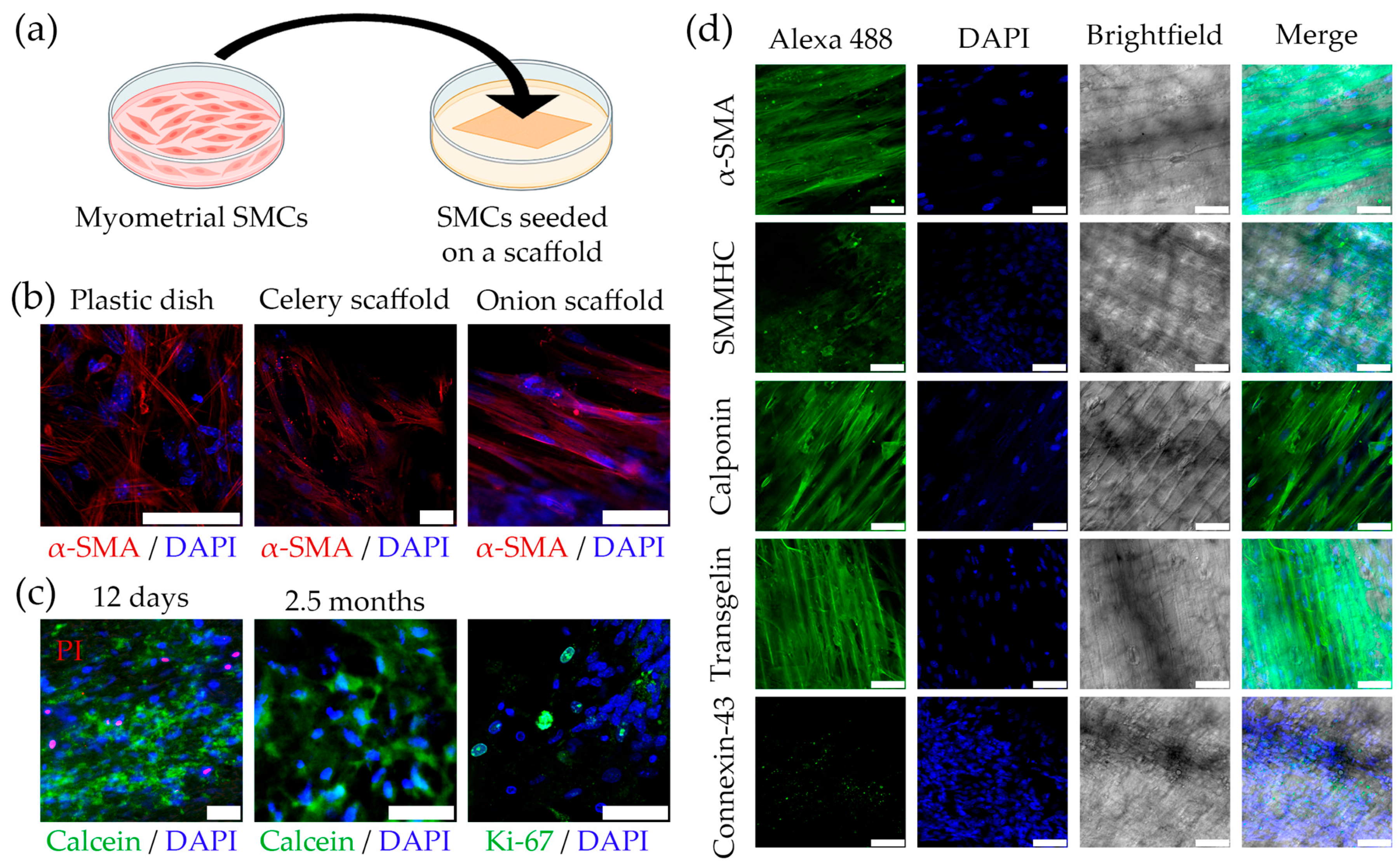
2.3. Cultivating Mouse SMCs on the Plant-Derived Cellulose Scaffolds
2.4. Developing a Multilayer Model of Myometrium Based on Plant-Derived Cellulose Scaffolds
3. Discussion
4. Materials and Methods
4.1. Preparation of Cellulose Scaffolds
4.2. Isolating and Cultivating of Mouse Myometrial SMCs
4.3. Seeding of Murine Myometrial SMCs onto Cellulose Scaffolds
4.4. Developing of a Multilayer Scaffold-Cell Construct
4.5. Immunocytochemical Staining
4.6. Western Blot Analysis
4.7. Gene Expression Analysis
4.7.1. RNA Isolation
4.7.2. Reverse Transcription and Real-Time Quantitative PCR (RT-qPCR)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| SMCs | smooth muscle cells |
| SDS | sodium dodecyl sulfate |
| α-SMA | α-smooth muscle actin |
| SMMHC | smooth muscle myosin heavy chains |
| PI | Propidium Iodide |
| ECM | extracellular matrix |
| PBS | phosphate-buffered saline |
| DAPI | 4′,6-diamidino-2-phenylindole |
| HBSS | Hanks balanced saline |
| FBS | fetal bovine serum |
References
- Dave, R.; Pandey, K.; Patel, R.; Gour, N.; Bhatia, D. Leveraging 3D cell culture and AI technologies for next-generation drug discovery. Cell Biomater. 2025, 1, 100050. [Google Scholar] [CrossRef]
- Rauner, G.; Gupta, P.B.; Kuperwasser, C. From 2D to 3D and beyond: The evolution and impact of in vitro tumor models in cancer research. Nat. Methods. 2025, 22, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.Y.; Kim, G.; Son, J.S.; Han, J.; Kim, J.H. Recent Advances in Three-Dimensional In Vitro Models for Studies of Liver Fibrosis. Tissue Eng. Regen. Med. 2025, 22, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide—Identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Wang, L.; Hu, D.; Xu, J.; Hu, J.; Wang, Y. Complex in vitro Model: A Transformative Model in Drug Development and Precision Medicine. Clin. Transl. Sci. 2023, 17, e13695. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Górnicki, T.; Lambrinow, J.; Golkar-Narenji, A.; Data, K.; Domagała, D.; Niebora, J.; Farzaneh, M.; Mozdziak, P.; Zabel, M.; Antosik, P.; et al. Biomimetic Scaffolds-A Novel Approach to Three Dimensional Cell Culture Techniques for Potential Implementation in Tissue Engineering. Nanomaterials 2024, 14, 531. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple derived cellulose scaffolds for 3D mammalian cell culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of subcutaneously implanted plant-derived cellulose biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent advances in modified cellulose for tissue culture applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Q.; Wang, S.; Zhang, J.; Fan, S.; Lin, X. Current advances in the development of decellularized plant extracellular matrix. Front. Bioeng. Biotechnol. 2021, 9, 712262. [Google Scholar] [CrossRef] [PubMed]
- Contessi, N.N.; Toffoletto, N.; Farè, S.; Altomare, L. Plant tissues as 3D natural scaffolds for adipose, bone and tendon tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.K.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W. Engineering Aligned Skeletal Muscle Tissue Using Decellularized Plant-Derived Scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Mastrodimos, M.; Jain, S.; Badv, M.; Shen, J.; Montazerian, H.; Meyer, C.E.; Annabi, N.; Weiss, P.S. Human Skeletal Muscle Myoblast Culture in Aligned Bacterial Nanocellulose and Commercial Matrices. ACS Appl. Mater. Interfaces 2024, 16, 47150–47162. [Google Scholar] [CrossRef]
- Fong, E.L.S.; Toh, T.B.; Yu, H.; Chow, E.K. 3D Culture as a Clinically Relevant Model for Personalized Medicine. SLAS Technol. 2017, 22, 245–253. [Google Scholar] [CrossRef]
- Cacciamali, A.; Villa, R.; Dotti, S. 3D Cell Cultures: Evolution of an Ancient Tool for New Applications. Front. Physiol. 2022, 13, 836480. [Google Scholar] [CrossRef]
- Ferronato, G.A.; Vit, F.F.; da Silveira, J.C. 3D culture applied to reproduction in females: Possibilities and perspectives. Anim. Reprod. 2024, 21, e20230039. [Google Scholar] [CrossRef]
- Bloise, N.; Giannaccari, M.; Guagliano, G.; Peluso, E.; Restivo, E.; Strada, S.; Volpini, C.; Petrini, P.; Visai, L. Growing Role of 3D In Vitro Cell Cultures in the Study of Cellular and Molecular Mechanisms: Short Focus on Breast Cancer, Endometriosis, Liver and Infectious Diseases. Cells 2024, 13, 1054. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Mori, T. Pathogenesis of Human Adenomyosis: Current Understanding and Its Association with Infertility. J. Clin. Med. 2022, 11, 4057. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Santulli, P.; Oliveira, J.; Marcellin, L.; Maignien, C.; Melka, L.; Bordonne, C.; Millisher, A.E.; Plu-Bureau, G.; Cormier, J.; et al. Focal adenomyosis is associated with primary infertility. Fertil. Steril. 2020, 114, 1271–1277. [Google Scholar] [CrossRef]
- Donnez, J.; Taylor, H.S.; Marcellin, L.; Dolmans, M.M. Uterine fibroid-related infertility: Mechanisms and management. Fertil. Steril. 2024, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Roh, M.; England, S.K. Uterine contractions in rodent models and humans. Acta Physiol. 2021, 231, e13607. [Google Scholar] [CrossRef] [PubMed]
- Menon, R. Preterm birth: A global burden on maternal and child health. Pathog. Glob. Health 2012, 106, 139–140. [Google Scholar] [CrossRef]
- WHO Caesarean Section Rates Continue to Rise, Amid Growing Inequalities in Access. 2021. Available online: https://www.who.int/news/item/16-06-2021-caesarean-section-rates-continue-to-rise-amid-growing-inequalities-in-access (accessed on 5 November 2025).
- Gnecco, J.S.; Brown, A.T.; Kan, E.L.; Baugh, L.; Ives, C.; Loring, M.; Griffith, L.G. Physiomimetic Models of Adenomyosis. Semin. Reprod. Med. 2020, 38, 179–196. [Google Scholar] [CrossRef]
- Antero, M.F.; Ayhan, A.; Segars, J.; Shih, I.M. Pathology and Pathogenesis of Adenomyosis. Semin. Reprod. Med. 2020, 38, 108–118. [Google Scholar] [CrossRef]
- Sabry, M.; Al-Hendy, A. Medical treatment of uterine leiomyoma. Reprod. Sci. 2012, 19, 339–353. [Google Scholar] [CrossRef]
- Banerjee, S.; Xu, W.; Chowdhury, I.; Driss, A.; Ali, M.; Yang, Q.; Al-Hendy, A.; Thompson, W.E. Human Myometrial and Uterine Fibroid Stem Cell-Derived Organoids for Intervening the Pathophysiology of Uterine Fibroid. Reprod. Sci. 2022, 29, 2607–2619. [Google Scholar] [CrossRef]
- Bulun, S.E. Tissue stem cells and uterine physiology and pathology. Semin. Reprod. Med. 2015, 33, 313–314. [Google Scholar] [CrossRef]
- Barnett, S.D.; Smith, C.R.; Ulrich, C.C.; Baker, J.E.; Buxton, I.L.O. S-Nitrosoglutathione Reductase Underlies the Dysfunctional Relaxation to Nitric Oxide in Preterm Labor. Sci. Rep. 2018, 8, 5614. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.A.; Nicholson, S.M.; Crankshaw, D.J.; Morrison, J.J. Maternal parity and functional contractility of human myometrium in vitro in the third trimester of pregnancy. J. Perinatol. 2019, 39, 439–444. [Google Scholar] [CrossRef]
- Malik, M.; Catherino, W.H. Development and validation of a three-dimensional in vitro model for uterine leiomyoma and patient-matched myometrium. Fertil. Steril. 2012, 97, 1287–1293. [Google Scholar] [CrossRef]
- Weigelt, B.; Bissell, M.J. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin. Cancer Biol. 2008, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Adissu, H.A.; Asem, E.K.; Lelièvre, S.A. Three-dimensional cell culture to model epithelia in the female reproductive system. Reprod. Sci. 2007, 14, 11–19. [Google Scholar] [CrossRef]
- Souza, G.R.; Tseng, H.; Gage, J.A.; Mani, A.; Desai, P.; Leonard, F.; Liao, A.; Longo, M.; Refuerzo, J.S.; Godin, B. Magnetically Bioprinted Human Myometrial 3D Cell Rings as A Model for Uterine Contractility. Int. J. Mol. Sci. 2017, 18, 683. [Google Scholar] [CrossRef]
- Kuneev, I.K.; Ivanova, Y.S.; Nashchekina, Y.A.; Patronova, E.K.; Sokolova, A.V.; Domnina, A.P. Development of a Method for Three-Dimensional Culturing of Human Mesenchymal Stem (Stromal) Cells Using a Cellulose Matrix. Cell Tissue Biol. 2023, 17, 388–397. [Google Scholar] [CrossRef]
- Durgin, B.G.; Straub, A.C. Redox control of vascular smooth muscle cell function and plasticity. Lab. Investig. 2018, 98, 1254–1262. [Google Scholar] [CrossRef]
- Liu, M.; Gomez, D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef]
- Huber, A.; Badylak, S.F. Phenotypic changes in cultured smooth muscle cells: Limitation or opportunity for tissue engineering of hollow organs? J. Tissue Eng. Regen. Med. 2012, 6, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Heidari Kani, M.; Chan, E.C.; Young, R.C.; Butler, T.; Smith, R.; Paul, J.W. 3D Cell Culturing and Possibilities for Myometrial Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Khoshnazar, S.M.; Asadi, A.; Roshancheshm, S.; Karimian, A.; Abdolmaleki, A. Application of 3D Scaffolds in Tissue Engineering. Cell Tissue Biol. 2023, 17, 454–464. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Young, R.C.; Schumann, R.; Zhang, P. Three-dimensional culture of human uterine smooth muscle myocytes on a resorbable scaffolding. Tissue Eng. 2003, 9, 451–459. [Google Scholar] [CrossRef]
- Malik, M.; Britten, J.; Catherino, W.H. A 3D Culture System of Human Immortalized Myometrial Cells. Bio-Protocol 2016, 6, e1970. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Zenhausern, F. The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Natural cellulose-based scaffold for improvement of stem cell osteogenic differentiation. J. Drug Deliv. Sci. Technol. 2021, 63, 102453. [Google Scholar] [CrossRef]
- Salehi, A.; Ernez, M.; Salido, G.L.; Cattaneo, G. Toward “Green” Vessels: Characterization of Microstructure, Mechanics, and Endothelial Cell Interaction on Three Macro-Tubular Plants for Vascular Tissue Engineering Applications. Adv. Mater. Technol. 2025, 10, 2401129. [Google Scholar] [CrossRef]
- Hasan, M.M.; Swapon, A.R.; Dipti, T.I.; Choi, Y.J.; Yi, H.G. Plant-Based Decellularization: A Novel Approach for Perfusion-Compatible Tissue Engineering Structures. J. Microbiol. Biotechnol. 2024, 34, 1003–1016. [Google Scholar] [CrossRef]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-Based Scaffolds in Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Vargas-Ovalle, M.I.; Demitri, C.; Madaghiele, M. Plant-Based Scaffolds for Tissue Engineering: A Review. Polymers 2025, 17, 2705. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Cao, R.; Yang, Z.S.; Hu, S.L.; Liang, S.J.; Zhang, S.M.; Zhu, S.Q.; Lu, L.; Long, C.H.; Yao, S.T.; Ma, Y.J.; et al. Molecular Mechanism of Mouse Uterine Smooth Muscle Regulation on Embryo Implantation. Int. J. Mol. Sci. 2022, 23, 12494. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar]

| Target Name | Gene | Direction | Primer Sequence (5′-3′) |
|---|---|---|---|
| Mouse 18S | 18S | Forward | GCAATTATTCCCCATGAACG |
| Reverse | GGCCTCACTAAACCATCCAA | ||
| Mouse Connexin 43 (Gap junction alpha-1 protein (GJA1)) | GJA1 | Forward | GTGCCGGCTTCACTTTCA |
| Reverse | GGAGTAGGCTTGGACCTTGTC | ||
| Mouse Oxytocin Receptor | OXTR | Forward | GTGCAGATGTGGAGCGTCT |
| Reverse | GTTGAGGCTGGCCAAGAG | ||
| Mouse Alpha SMA-2 | ACTA2 | Forward | GTCCCAGACATCAGGGAGTAA |
| Reverse | TCGGATACTTCAGCGTCAGGA | ||
| Mouse Calponin | CNN1 | Forward | GGTGAAACCCCACGACATCTT |
| Reverse | TTTGTCTTGGCCATGCTGG | ||
| Mouse Myosin heavy chain 11 | MYH11 | Forward | CATCCTGACCCCACGTATCAA |
| Reverse | ATCGGAAAAGGCGCTCATAGG | ||
| Mouse Transgelin (TAGLN) | SM22 alpha | Forward | CGATGGAAACTACCGTGGAGA |
| Reverse | TGAAGGCCAATGACGTGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolova, A.V.; Kuneev, I.K.; Nashchekina, Y.A.; Domnina, A.P. Cellulose Plant-Derived Scaffolds as a Tool for Myometrium Modeling. Int. J. Mol. Sci. 2025, 26, 10995. https://doi.org/10.3390/ijms262210995
Sokolova AV, Kuneev IK, Nashchekina YA, Domnina AP. Cellulose Plant-Derived Scaffolds as a Tool for Myometrium Modeling. International Journal of Molecular Sciences. 2025; 26(22):10995. https://doi.org/10.3390/ijms262210995
Chicago/Turabian StyleSokolova, Anastasiia V., Ivan K. Kuneev, Yuliya A. Nashchekina, and Alisa P. Domnina. 2025. "Cellulose Plant-Derived Scaffolds as a Tool for Myometrium Modeling" International Journal of Molecular Sciences 26, no. 22: 10995. https://doi.org/10.3390/ijms262210995
APA StyleSokolova, A. V., Kuneev, I. K., Nashchekina, Y. A., & Domnina, A. P. (2025). Cellulose Plant-Derived Scaffolds as a Tool for Myometrium Modeling. International Journal of Molecular Sciences, 26(22), 10995. https://doi.org/10.3390/ijms262210995





