Antioxidant Status of Cyanobacteria Strains During Long-Term Cultivation in Nitrogen-Free Media
Abstract
1. Introduction
2. Results
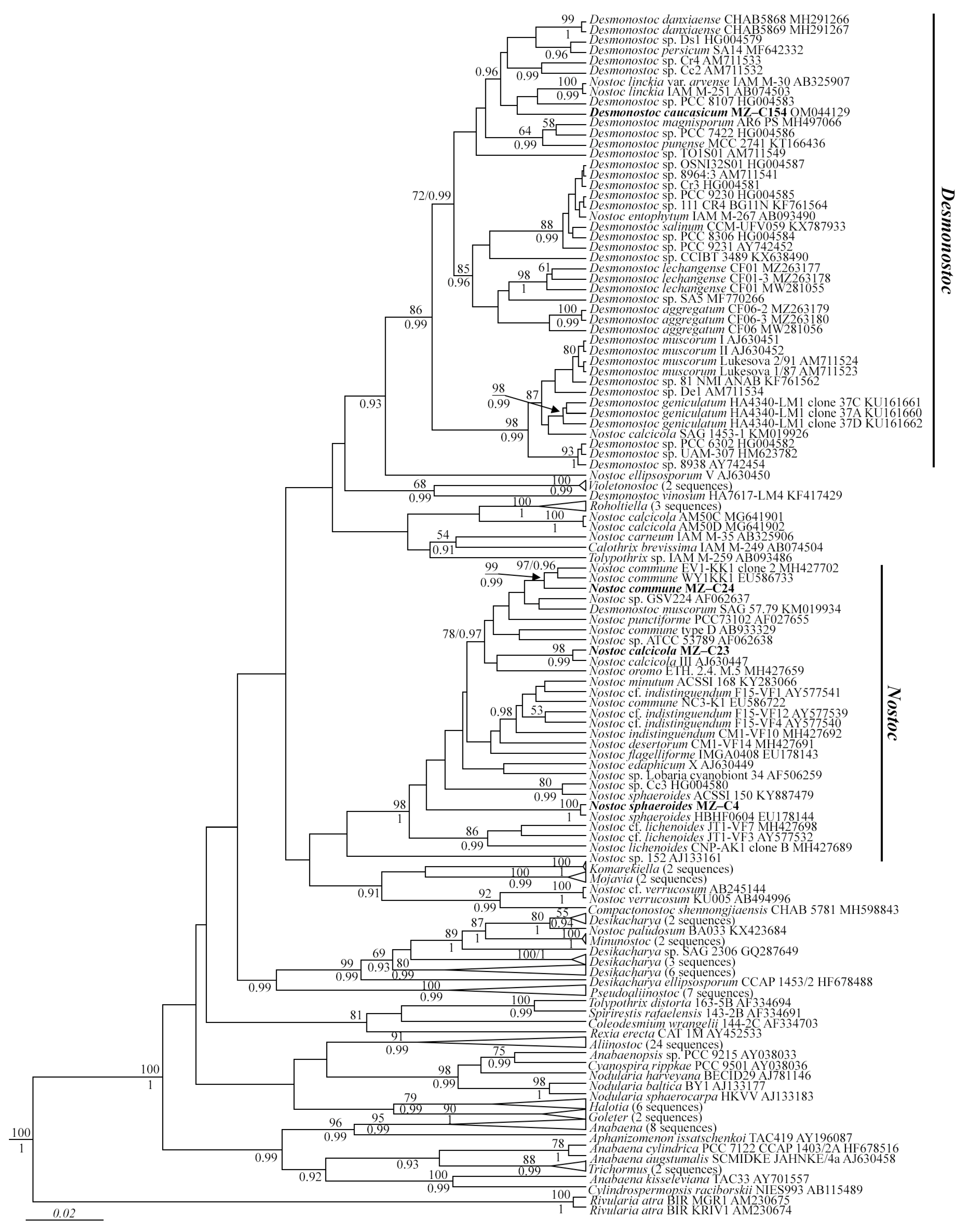
2.1. Molecular Analyses
2.2. Strain Growth
2.3. Fatty Acid Profiles
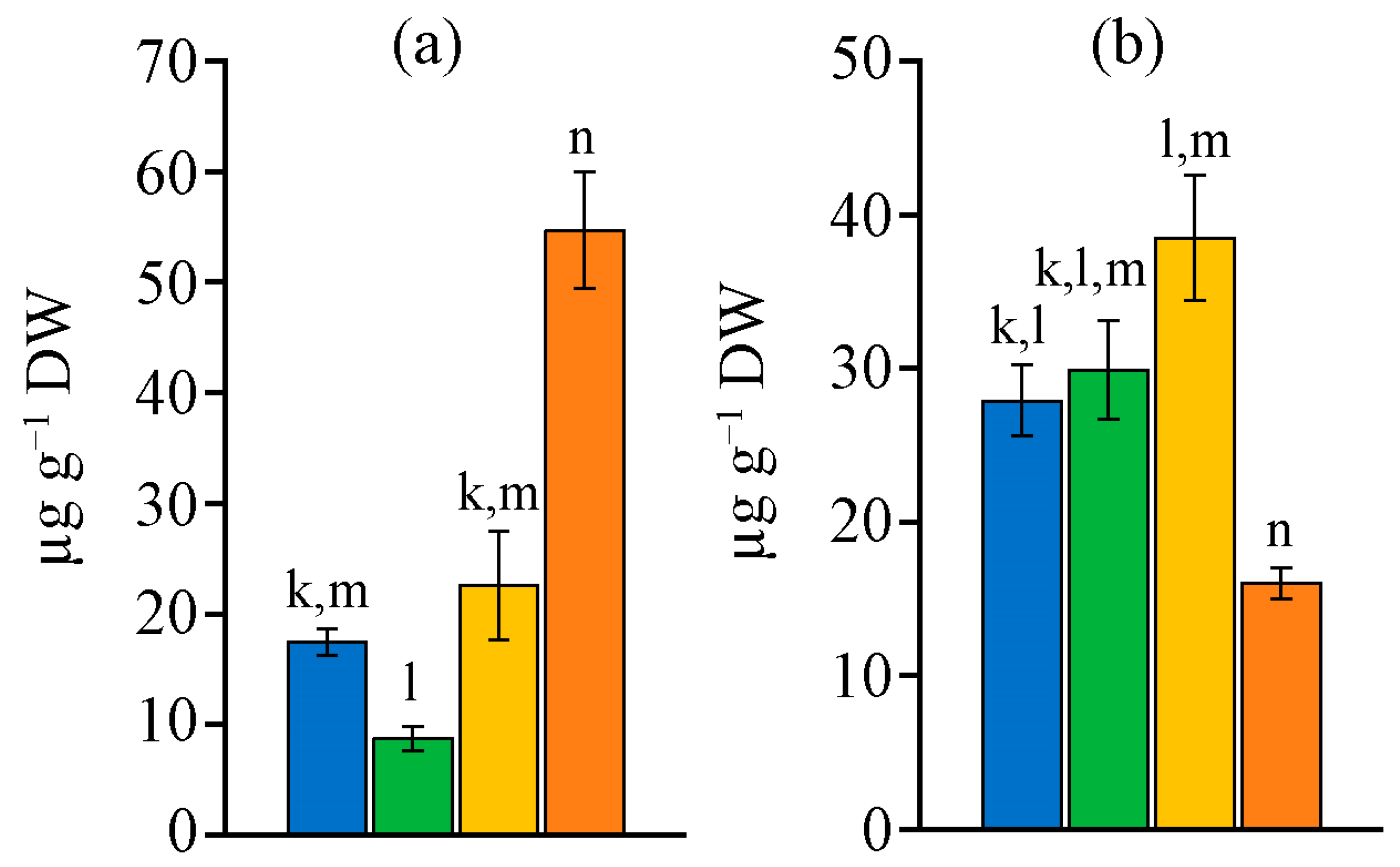
2.4. Retinol and α-Tocopherol Content
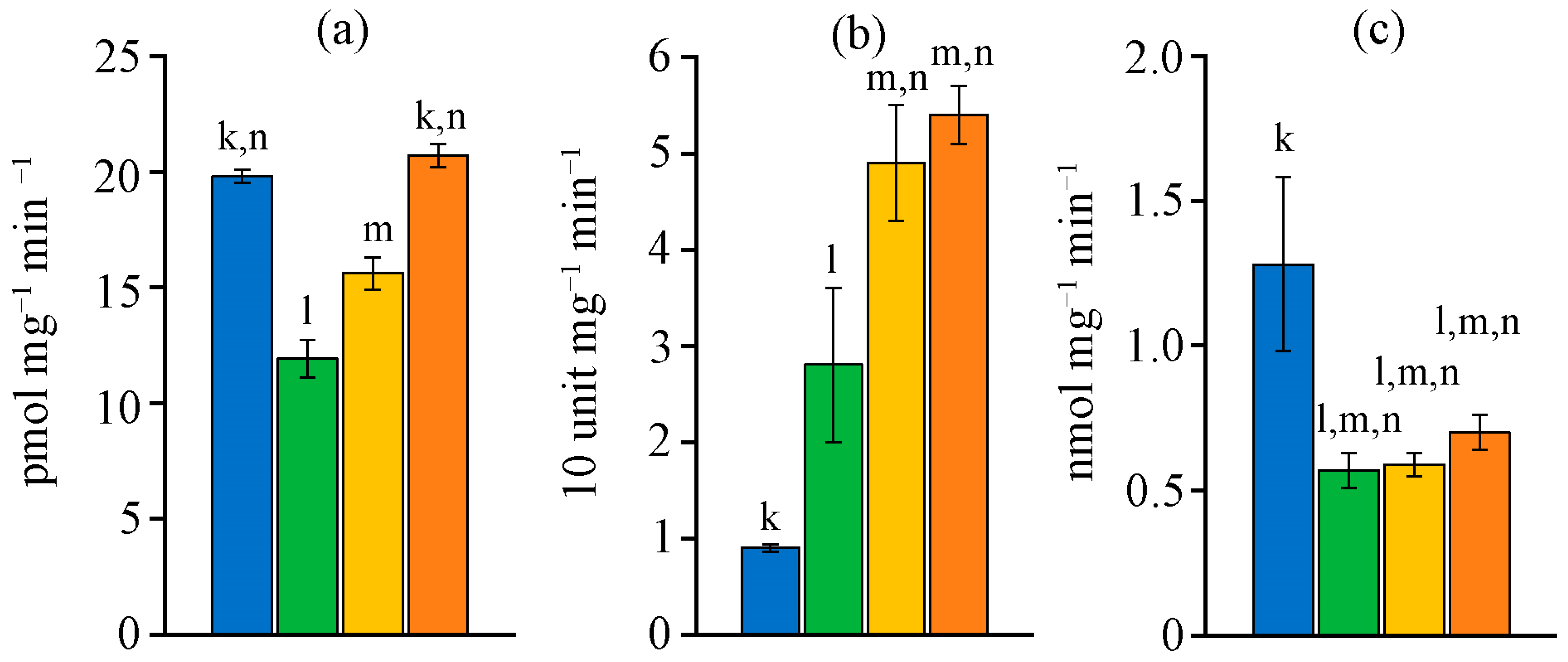
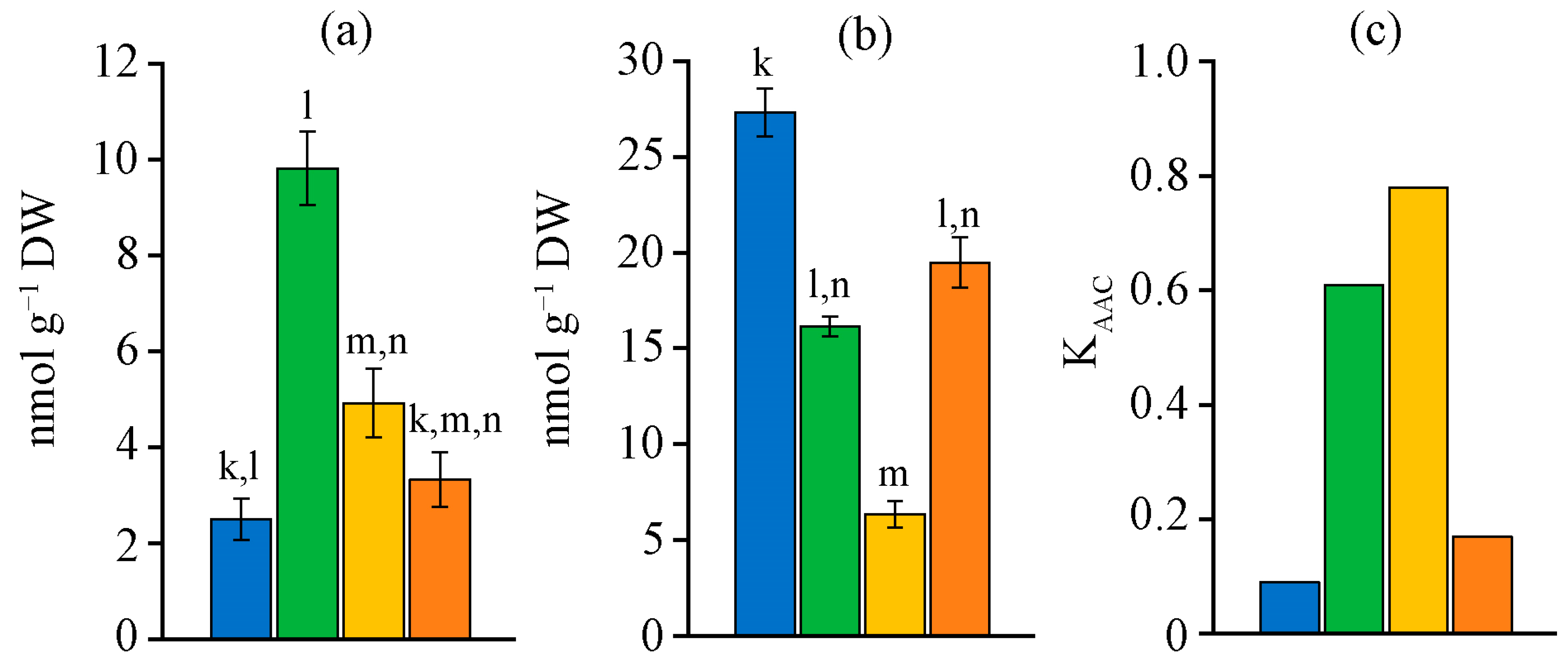
2.5. Antioxidant Defence System Characteristic
2.6. Succinate Dehydrogenase Activity
2.7. Evaluation of the Relationship of the Measured Parameters in the Studied Strains
3. Discussion
4. Material and Methods
4.1. Cyanobacterial Material
4.2. DNA Extraction, PCR Amplification and Sequencing
4.3. Growth Assessment
4.4. Biochemical Parameters
4.4.1. Vitamin A (Retinol) and E (α-Tocopherol) Content Measurement
4.4.2. Succinate Dehydrogenase and Antioxidant Enzyme Activity Measurement
4.4.3. Fatty Acid Analysis, Protein Content, and Thiobarbituric-Acid-Reactive Substance Content
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitton, B.A.; Potts, M. Introduction to the cyanobacteria. In Ecology of Cyanobacteria II; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. [Google Scholar]
- Maltsev, Y.; Maltseva, S.; Maltseva, I. Diversity of cyanobacteria and algae during primary succession in iron ore tailing dumps. Microb. Ecol. 2022, 83, 408–423. [Google Scholar] [CrossRef]
- Scherbina, V.V.; Maltseva, I.A.; Solonenko, A.N. Peculiarities of postpyrogene development of algae in steppe biocenoses at Askania Nova Biospheric National Park. Contemp. Probl. Ecol. 2014, 7, 187–191. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Y.; Fu, Y.; She, S.; Wang, X.; Hou, L.; Chen, C.; Chen, L. Differences in carbon and nitrogen cycling strategies and regional variability in biological soil crust types. Int. J. Mol. Sci. 2025, 26, 3989. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zanchett, G.; Oliveira-Filho, E.C. Cyanobacteria and cyanotoxins: From impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Martínez-Ruiz, F.E.; Andrade-Bustamante, G.; Holguín-Peña, R.J.; Renganathan, P.; Gaysina, L.A.; Sukhanova, N.V.; Puente, E.O.R. Microalgae as functional food ingredients: Nutritional benefits, challenges, and regulatory considerations for safe consumption. Biomass 2025, 5, 25. [Google Scholar] [CrossRef]
- Issa, A.A.; Abd-Alla, M.H.; Ohyama, T. Nitrogen fixing cyanobacteria: Future prospect. In Advances in Biology and Ecology of Nitrogen Fixation; Ohyama, T., Ed.; IntechOpen: London, UK, 2014; pp. 23–48. [Google Scholar]
- Nawaz, T.; Fahad, S.; Saud, S.; Zhou, R.; Abdelsalam, N.R.; Abdelhamid, M.M.A.; Jaremko, M. Sustainable nitrogen solutions: Cyanobacteria-powered plant biotechnology for conservation and metabolite production. Curr. Plant Biol. 2024, 40, 100399. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Řehaková, K.; Johansen, J.R.; Casamatta, D.A.; Xuesong, L.; Vincent, J. Morphological and molecular characterization of selected desert soil cyanobacteria: Three species new to science including Mojavia pulchra gen. et sp. nov. Phycologia 2007, 46, 481–502. [Google Scholar] [CrossRef]
- Hrouzek, P.; Lukešová, A.; Mareš, J.; Ventura, S. Description of the cyanobacterial genus Desmonostoc gen. nov. including D. muscorum comb. nov. as a distinct, phylogenetically coherent taxon related to the genus Nostoc. Fottea Olomouc 2013, 13, 201–213. [Google Scholar] [CrossRef]
- Sharma, M.; Sudheer, S.; Usmani, Z.; Rani, R.; Gupta, P. Deciphering the omics of plant-microbe interaction: Perspectives and new insights. Curr. Genom. 2020, 21, 343–362. [Google Scholar] [CrossRef]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of light intensity and quality on phycobiliprotein accumulation in the cyanobacterium Nostoc sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef]
- El Shafay, S.M.; Gaber, A.; Alsanie, W.F.; Elshobary, M.E. Influence of nutrient manipulation on growth and biochemical constituent in Anabaena variabilis and Nostoc muscorum to enhance biodiesel production. Sustainability 2021, 13, 9081. [Google Scholar] [CrossRef]
- Mouga, T.; Pereira, J.; Moreira, V.; Afonso, C. Unveiling the cultivation of Nostoc sp. under controlled laboratory conditions. Biology 2024, 13, 306. [Google Scholar] [CrossRef]
- Poveda, J. Cyanobacteria in plant health: Biological strategy against abiotic and biotic stresses. Crop Prot. 2021, 141, 105450. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Oxidative environment and redox homeostasis in plants: Dissecting out significant contribution of major cellular organelles. Front. Environ. Sci. 2015, 2, 70. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhargava, P.; Rai, L.C. Salinity and copper-induced oxidative damage and changes in the antioxidative defence systems of Anabaena doliolum. World J. Microbiol. Biotechnol. 2005, 21, 1291–1298. [Google Scholar] [CrossRef]
- López-Hernández, J.F.; García-Alamilla, P.; Palma-Ramírez, D.; Álvarez-González, C.A.; Paredes-Rojas, J.C.; Márquez-Rocha, F.J. Continuous microalgal cultivation for antioxidants production. Molecules 2020, 25, 4171. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.; Bhargava, P.; Thapar, R.; Srivastava, A.K.; Rai, L.C. A comparative study of antioxidative defense system in the copper and temperature acclimated strains of Anabaena doliolum. World J. Microbiol. Biotechnol. 2008, 24, 2997–3004. [Google Scholar] [CrossRef]
- Maltsev, Y.I.; Pakhomov, A.Y.; Maltseva, I.A. Specific features of algal communities in forest litter of forest biogeocenoses of the Steppe zone. Contemp. Probl. Ecol. 2017, 10, 71–76. [Google Scholar] [CrossRef]
- Maltsev, Y.I.; Didovich, S.V.; Maltseva, I.A. Seasonal changes in the communities of microorganisms and algae in the litters of tree plantations in the Steppe zone. Eurasian Soil Sci. 2017, 50, 935–942. [Google Scholar] [CrossRef]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef]
- Temina, M.; Rezankova, H.; Rezanka, T.; Dembitsky, V.M. Diversity of the fatty acids of the Nostoc species and their statistical analysis. Microbiol. Res. 2007, 162, 308–321. [Google Scholar] [CrossRef]
- Liu, X.J.; Chen, F.; Jiang, Y. Differentiation of Nostoc flagelliforme and its neighboring species using fatty-acid profiling as a chemotaxonomic tool. Curr. Microbiol. 2003, 47, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Deschoenmaeker, F.; Bayon-Vicente, G.; Sachdeva, N.; Depraetere, O.; Cabrera Pino, J.C.; Leroy, B.; Muylaert, K.; Wattiez, R. Impact of different nitrogen sources on the growth of Arthrospira sp. PCC 8005 under batch and continuous cultivation—A biochemical, transcriptomic and proteomic profile. Bioresour. Technol. 2017, 237, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Christman, H.D.; Campbell, E.L.; Meeks, J.C. Global transcription profiles of the nitrogen stress response resulting in heterocyst or hormogonium development in Nostoc punctiforme. J. Bacteriol. 2011, 193, 6874–6886. [Google Scholar] [CrossRef]
- Mishra, Y.; Bhargava, P.; Rai, L.C. Differential induction of enzymes and antioxidants of the antioxidative defense system in Anabaena doliolum exposed to heat stress. J. Therm. Biol. 2005, 30, 524–531. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Oliveira, O.; Oliveira, S.; Mendes, A.M.; Caetano, N.S. Lipid content and productivity of Arthrospira platensis and Chlorella vulgaris under mixotrophic conditions and salinity stress. Chem. Eng. Trans. 2016, 49, 187–192. [Google Scholar] [CrossRef]
- Sopandi, T.; Rohmah, S.; Agustina, S.A.T. Biomass and nutrient composition of Spirulina platensis grown in goat manure media. Asian J. Agric. Biol. 2020, 8, 158–167. [Google Scholar] [CrossRef]
- Baracho, D.H.; Lombardi, A.T. Study of the growth and biochemical composition of 20 species of cyanobacteria cultured in cylindrical photobioreactors. Microb. Cell Fact. 2023, 22, 36. [Google Scholar] [CrossRef]
- Sero, E.T.; Siziba, N.; Bunhu, T.; Shoko, R. Isolation and screening of microalgal species, native to Zimbabwe, with potential use in biodiesel production. All Life 2021, 14, 256–264. [Google Scholar] [CrossRef]
- Toumi, A.; Politaeva, N.A. Impact of the nitrate concentration on the biomass growth and the fatty acid profiles of microalgae Chlorella sorokiniana. IOP Conf. Ser. Earth Environ. Sci. 2021, 689, 012026. [Google Scholar] [CrossRef]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 2012, 24, 989–1001. [Google Scholar] [CrossRef]
- Gugger, M.; Lyra, C.; Suominen, I.; Tsitko, I.; Humbert, J.F.; Salkinoja-Salonen, M.S.; Sivonen, K. Cellular fatty acids as chemotaxonomic markers of the genera Anabaena, Aphanizomenon, Microcystis, Nostoc and Planktothrix (cyanobacteria). Int. J. Syst. Evol. Microbiol. 2002, 52, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Miao, X. Short chain fatty acid biosynthesis in microalgae Synechococcus sp. PCC 7942. Mar. Drugs 2019, 17, 255. [Google Scholar] [CrossRef]
- Hu, H.; Gao, K. Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol. Lett. 2006, 28, 987–992. [Google Scholar] [CrossRef]
- Kudahettige, N.P.; Pickova, J.; Gentili, F.G. Stressing algae for biofuel production: Biomass and biochemical composition of Scenedesmus dimorphus and Selenastrum minutum grown in municipal untreated wastewater. Front. Energy Res. 2018, 6, 132. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Stupák, M.; Vidová Ugurbaş, M.; Židzik, J.; Mičková, H. Therapeutic uses of retinol and retinoid-related antioxidants. Molecules 2025, 30, 2191. [Google Scholar] [CrossRef]
- Mudimu, O.; Koopmann, I.K.; Rybalka, N.; Friedl, T.; Schulz, R.; Bilger, W. Screening of microalgae and cyanobacteria strains for α-tocopherol content at different growth phases and the influence of nitrate reduction on α-tocopherol production. J. Appl. Phycol. 2017, 29, 2867–2875. [Google Scholar] [CrossRef]
- Aaronson, S.; Dhawale, S.W.; Patni, N.J.; Deangelis, B.; Frank, O.; Baker, H. The cell content and secretion of water-soluble vitamins by several freshwater algae. Arch. Microbiol. 1977, 112, 57–59. [Google Scholar] [CrossRef]
- Sakamoto, T.; Wei, Y.; Yuasa, K.; Nishiyama, Y. Recovery of photosynthesis after long-term storage in the terrestrial cyanobacterium Nostoc commune. J. Gen. Appl. Microbiol. 2022, 68, 169–174. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Fact. 2020, 19, 201. [Google Scholar] [CrossRef]
- Ilieva, Y.; Zaharieva, M.M.; Najdenski, H.; Kroumov, A.D. Antimicrobial activity of Arthrospira (former Spirulina) and Dunaliella related to recognized antimicrobial bioactive compounds. Int. J. Mol. Sci. 2024, 25, 5548. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Chen, H.; He, C.L.; Wang, Q. Nitrogen starvation induced oxidative stress in an oil-producing green alga Chlorella sorokiniana C3. PLoS ONE 2013, 8, e69225. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol. Biofuels 2017, 10, 60. [Google Scholar] [CrossRef]
- Bela, K.; Riyazuddin, R.; Csiszár, J. Plant glutathione peroxidases: Non-heme peroxidases with large functional flexibility as a core component of ROS-processing mechanisms and signalling. Antioxidants 2022, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Bernroitner, M.; Peschek, G.A.; Obinger, C. Hydrogen peroxide degradation in cyanobacteria. In Bioenergetic Processes of Cyanobacteria: From Evolutionary Singularity to Ecological Diversity; Springer: Dordrecht, The Netherlands, 2011; pp. 159–185. [Google Scholar] [CrossRef]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. The ‘stress’ concept in microalgal biology—Homeostasis, acclimation and adaptation. J. Appl. Phycol. 2018, 30, 2815–2825. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae–mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Maltseva, S.; Bachura, Y.; Erst, T.; Kulikovskiy, M.; Maltsev, Y. Description of Desmonostoc caucasicum sp. nov. (Cyanobacteria) using an integrative taxonomic approach. Phycologia 2022, 61, 514–527. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Wiley: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Lepère, C.; Wilmotte, A.; Meyer, B. Molecular diversity of Microcystis strains (Cyanophyceae, Chroococcales) based on 16S rDNA sequences. Syst. Geogr. Plants 2000, 70, 275–283. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef]
- Flechtner, V.R.; Boyer, S.L.; Johansen, J.R.; DeNoble, M.L. Spirirestis rafaelensis gen. et sp. nov. (Cyanophyceae), a new cyanobacterial genus from arid soils. Nova Hedwig. 2002, 74, 1–24. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nature Meth. 2012, 9, 772. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.M.; Mehta, S.K. Antimicrobial activities of freshwater cyanobacterium, Nostoc sp. from Tamdil Wetland of Mizoram, India: An identification of bioactive compounds by GC-MS. Int. J. Pharm. Sci. Res. 2016, 7, 1179–1191. [Google Scholar] [CrossRef]
- Guljamow, A.; Kreische, M.; Ishida, K.; Liaimer, A.; Altermark, B.; Bähr, L.; Hertweck, C.; Ehwald, R.; Dittmann, E. High-density cultivation of terrestrial nostoc strains leads to reprogramming of secondary metabolome. Appl. Environ. Microbiol. 2017, 83, e01510-17. [Google Scholar] [CrossRef]
- Kotai, J. Instructions for preparation of modified nutrient solution Z8 for algae. Nor. Inst. Water Res. 1972, 11, 5. [Google Scholar]
- Komárek, J. Cyanoprokaryota: 3rd Part: Heterocystous Genera. In Süßwasserflora von Mitteleuropa; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Springer Spektrum: Berlin/Heidelberg, Germany, 2013; Volume 19, pp. 1–1130. [Google Scholar]
- Maltseva, S.Y.; Kulikovskiy, M.S.; Maltsev, Y.I. Functional state of Coelastrella multistriata (Sphaeropleales, Chlorophyta) in an enrichment culture. Microbiology 2022, 91, 523–532. [Google Scholar] [CrossRef]
- Yakoviichuk, A.; Krivova, Z.; Maltseva, S.; Kochubey, A.; Kulikovskiy, M.; Maltsev, Y. Antioxidant status and biotechnological potential of new Vischeria vischeri (Eustigmatophyceae) soil strains in enrichment cultures. Antioxidants 2023, 12, 654. [Google Scholar] [CrossRef]
- Maltseva, I.; Yakoviichuk, A.; Maltseva, S.; Cherkashina, S.; Kulikovskiy, M.; Maltsev, Y. Biochemical and antioxidant characteristics of Chlorococcum oleofaciens (Chlorophyceae, Chlorophyta) under various cultivation conditions. Plants 2024, 13, 2413. [Google Scholar] [CrossRef]
- Dewi, R.N.; Mahreni; Azimatun Nur, M.M.; Siahaan, A.A.; Ardhi, A.C. Enhancing the biomass production of microalgae by mixotrophic cultivation using virgin coconut oil mill effluent. Environ. Eng. Res. 2023, 28, 220059. [Google Scholar] [CrossRef]
- Delgado-Zamarreño, M.M.; Bustamante-Rangel, M.; García-Jiménez, M.; Sánchez-Pérez, A.; Carabias-Martínez, R. Off-line coupling of pressurized liquid extraction and LC/ED for the determination of retinyl acetate and tocopherols in infant formulas. Talanta 2006, 70, 1094–1099. [Google Scholar] [CrossRef]
- Hossu, A.-M.; Radulescu, C.; Ilie, M.; Balalau, D.; Magearu, V. Qualitative and semiquantitative TLC analysis of vitamins A, D and E. Rev. Chim. 2006, 57, 1188–1189. [Google Scholar]
- Munujos, P.; Coll-Cantí, J.; González-Sastre, F.; Gella, F.J. Assay of succinate dehydrogenase activity by a colorimetric-continuous method using iodonitrotetrazolium chloride as electron acceptor. Anal. Biochem. 1993, 212, 506–509. [Google Scholar] [CrossRef]
- Hamza, T.A.; Hadwan, M.H. New spectrophotometric method for the assessment of catalase enzyme activity in biological tissues. CAC 2020, 16, 1054–1062. [Google Scholar] [CrossRef]
- Sattar, A.A.; Matin, A.A.; Hadwan, M.H.; Hadwan, A.M.; Mohammed, R.M. Rapid and effective protocol to measure glutathione peroxidase activity. Bull. Natl. Res. Cent. 2024, 48, 100. [Google Scholar] [CrossRef]
- Sirota, T.V. Use of nitro blue tetrazolium in the reaction of adrenaline autooxidation for the determination of superoxide dismutase activity. Biochem. Moscow Suppl. Ser. B 2012, 6, 254–260. [Google Scholar] [CrossRef]
- Maltseva, S.; Kezlya, E.; Krivova, Z.; Gusev, E.; Kulikovskiy, M.; Maltsev, Y. Phylogeny and fatty acid profiles of Aliinostoc vietnamicum sp. nov. (Cyanobacteria) from the soils of Vietnam. J. Phycol. 2022, 58, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Marques de Sá, J.P. Applied Statistics: Using SPSS, Statistica, MATLAB, and R, 2nd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]

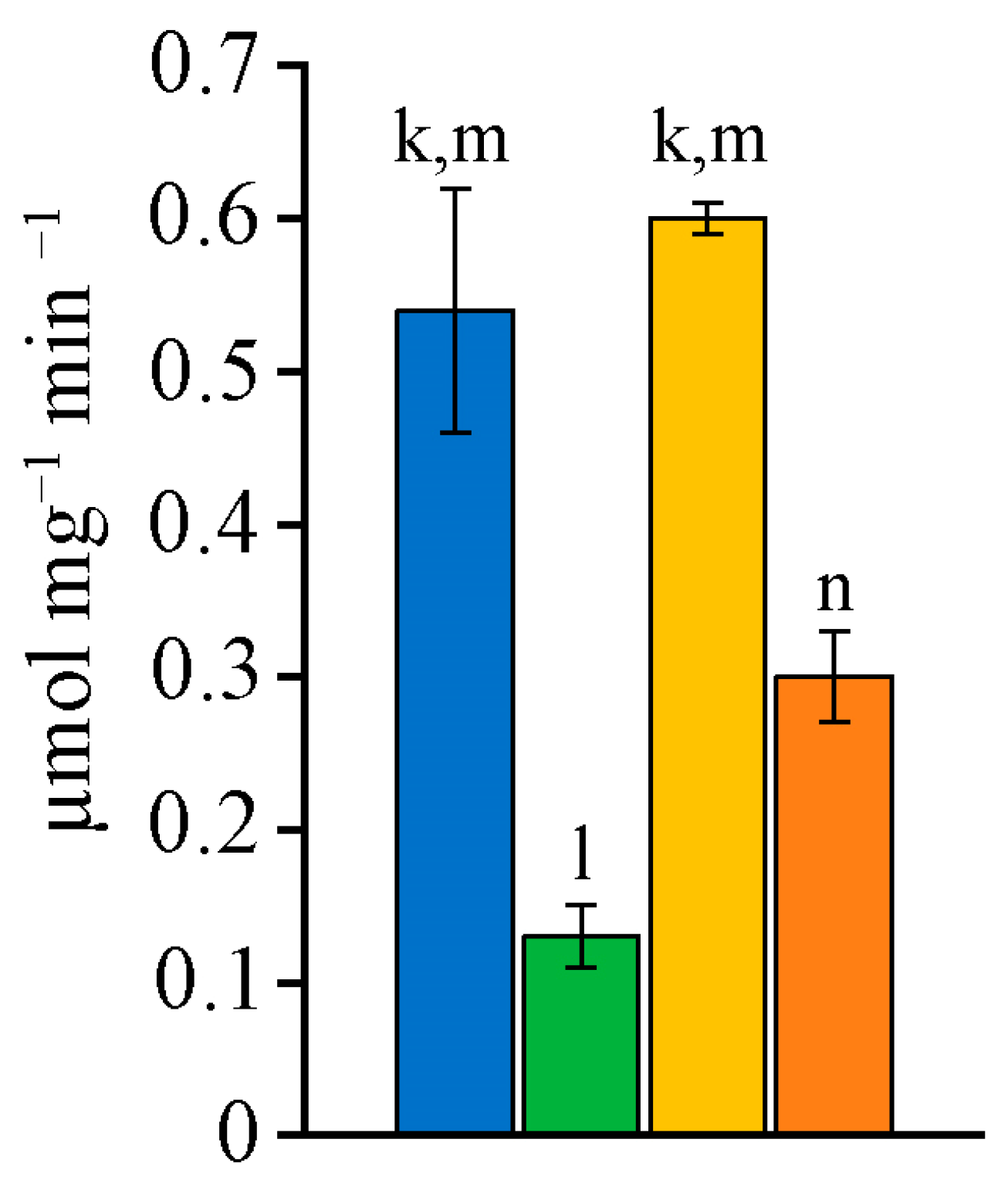
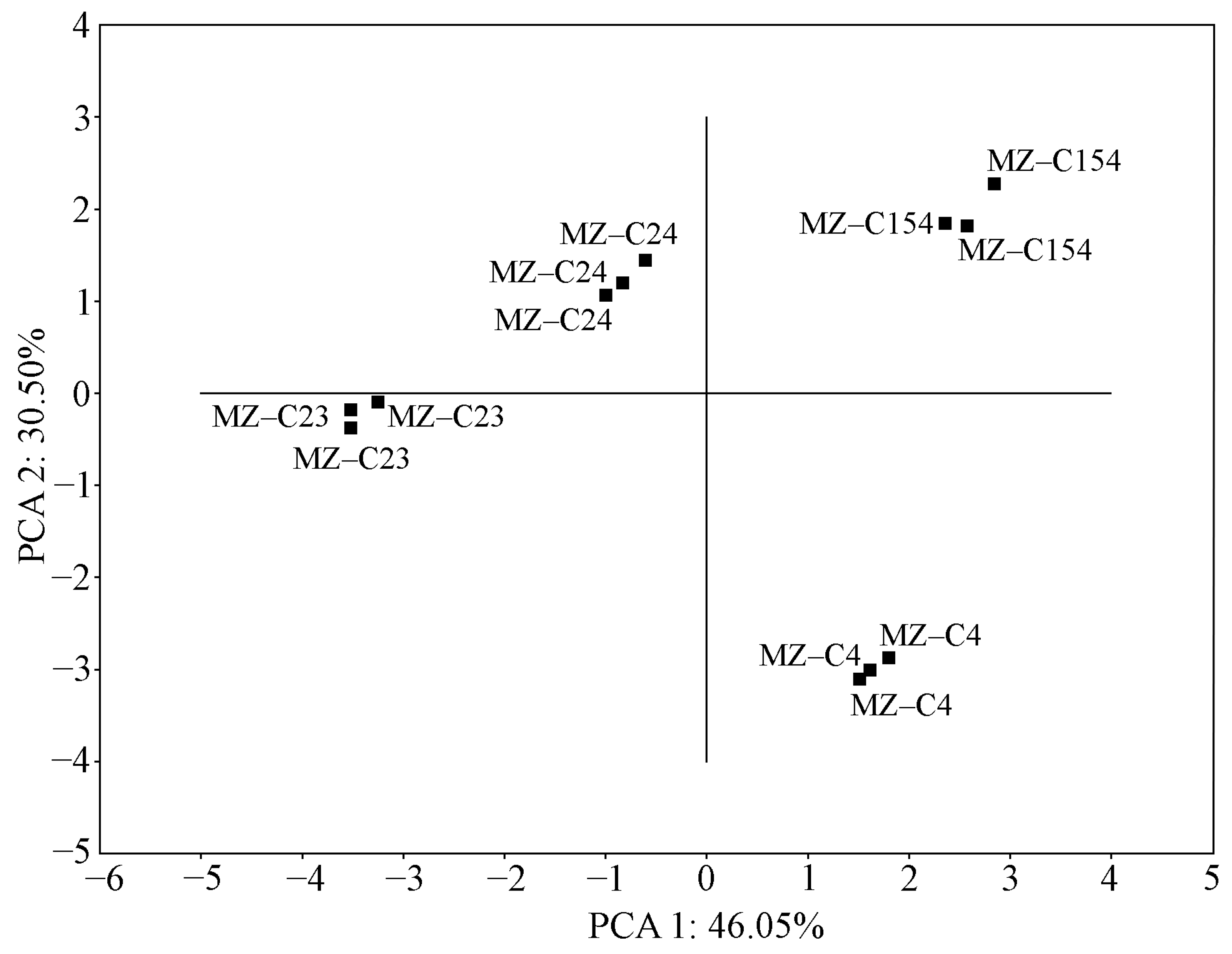

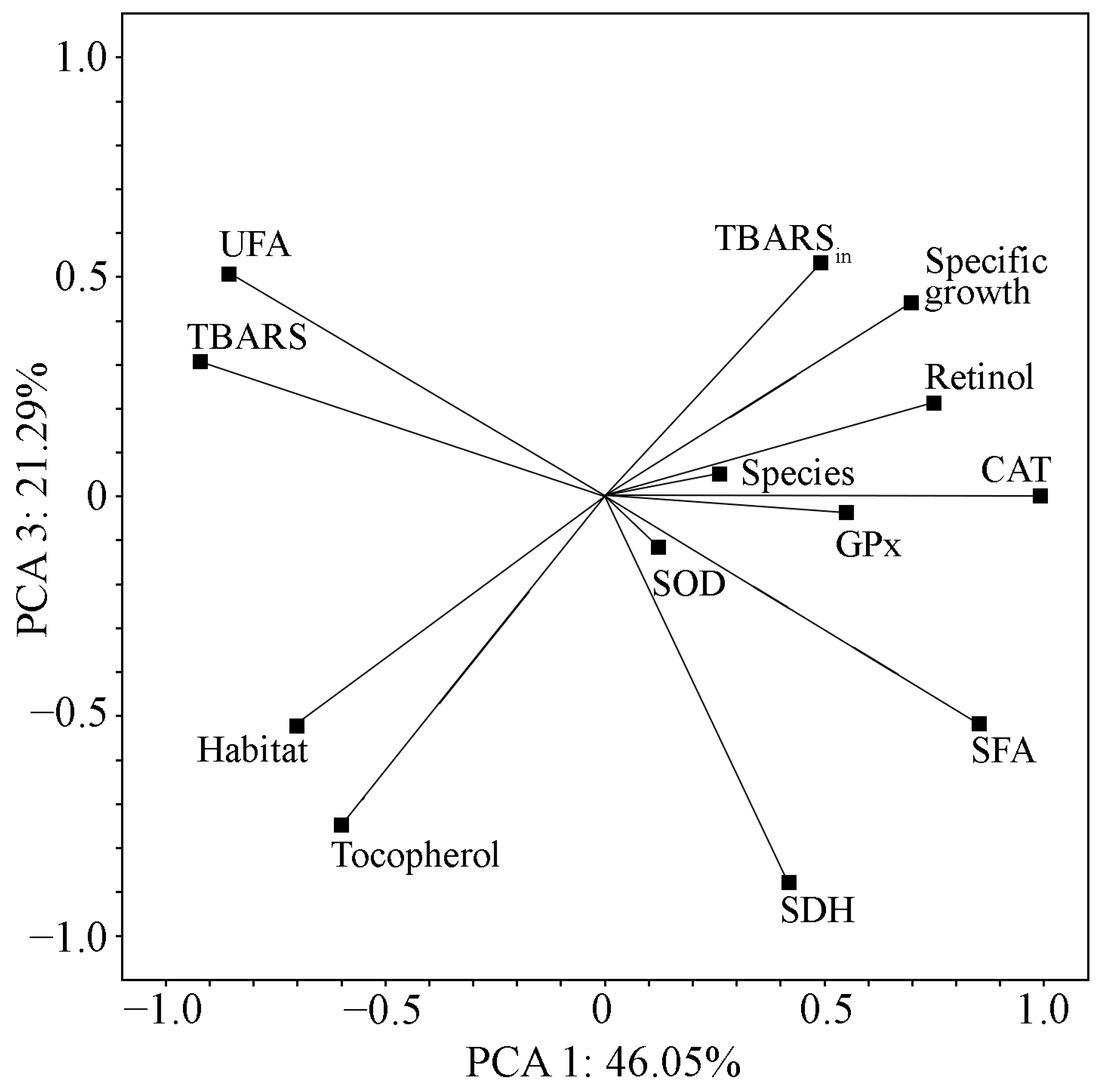
| Fatty Acid | Strain | N. sphaeroides MZ–C4 a | N. calcicola MZ–C23 a | N. commune MZ–C24 a | D. caucasicum MZ–C154 a | N. calcicola SAG 1453–1 | N. commune SAG 1453–5 | N. commune | D. muscorum UTEX 389 | D. muscorum SAG 57.79 |
|---|---|---|---|---|---|---|---|---|---|---|
| growth phase | stationary | stationary | stationary | stationary | stationary | stationary | stationary | stationary | stationary | |
| medium | Z8-N | Z8-N | Z8-N | Z8-N | ES Ag | ES Ag | BG-11 | BG-11 | ES Ag | |
| references | Present study | Present study | Present study | Present study | [26] | [26] | [27] | [28] | [26] | |
| 12:0 | 0.49 ± 0.03 | |||||||||
| 14:0 | 3.37 ± 0.16 | 3.58 ± 0.05 | 3.13 ± 0.04 | 0.92 | ||||||
| 14:1 | 0.3 | |||||||||
| 15:0 | 0.6 ± 0.01 | |||||||||
| 16:0 | 23.7 ± 0.84 | 27.28 ± 0.75 | 23.46 ± 0.73 | 24.3 ± 0.66 | 25.3 | 24.97 | 15.69 | 19.74 | ||
| 18:0 | 69.83 ± 1.16 | 67.58 ± 1.34 | 71.0 ± 1.43 | 8.34 | 2.49 | |||||
| 16:1 b | 27.87 | |||||||||
| 16:1n-7 | 1.4 ± 0.04 | 8.57 ± 0.11 | 0.85 ± 0.01 | 7.6 | 23.84 | 5.1 | 19.41 | |||
| 16:1n-11 | 2.9 | |||||||||
| 16:1n-9 | 3.6 | |||||||||
| 16:1n-5 | 0.6 | |||||||||
| 17:1 b | 0.4 | |||||||||
| 18:1 b | 21.80 | |||||||||
| 18:1n-9 | 7.01 ± 0.08 | 0.66 ± 0.03 | 6.0 | 11.2 | 5.75 | |||||
| 18:1n-5 | 0.89 ± 0.02 | |||||||||
| 18:1n-13 | 1.3 | |||||||||
| 18:1n-11 | 2.6 | |||||||||
| 16:2n-4 | 3.47 | |||||||||
| 16:2n-6 | 2.1 | |||||||||
| 17:2n-5 | 3.58 | |||||||||
| 18:2n-6 | 1.7 ± 0.02 | 17.82 ± 0.45 | 2.09 ± 0.04 | 0.72 ± 0.02 | 16.8 | 12.37 | 14.5 | 14.09 | 7.38 | |
| 18:2n-5 | 2.1 | |||||||||
| 18:2n-3 | 1.1 | |||||||||
| 16:3n-4 | 2.17 | |||||||||
| 18:3n-3 | 39.32 ± 0.88 | 37.1 | 37.68 | 13.5 | 11.29 | 26.9 | ||||
| 18:3n-6 | 3.2 | |||||||||
| 16:4n-3 | 6.94 | |||||||||
| 20:0 | 0.65 ± 0.03 | |||||||||
| 9-octadecanamid | 7.2 | |||||||||
| dioic fatty acids | 1.4 | |||||||||
| branched SFA | 4.1 | |||||||||
| total SFA | 96.9 ± 0.95 | 27.28 ± 0.75 | 96.36 ± 0.86 | 98.43 ± 1.15 | 25.3 | 24.97 | 30.0 | 24.95 | 22.23 | |
| total MUFA | 1.4 ± 0.04 | 15.58 ± 0.09 | 1.55 ± 0.02 | 0.85 ± 0.01 | 13.6 | 23.84 | 28.0 | 49.67 | 25.16 | |
| total PUFA | 1.7 ± 0.02 | 57.14 ± 0.68 | 2.09 ± 0.02 | 0.72 ± 0.02 | 53.9 | 50.05 | 36.5 | 25.38 | 50.44 | |
| total omega-3 | 39.32 ± 0.72 | 37.1 | 37.68 | 13.5 | 11.29 | 29.07 | ||||
| total omega-6 | 1.7 ± 0.02 | 17.82 ± 0.11 | 2.09 ± 0.02 | 0.72 ± 0.02 | 16.8 | 12.37 | 14.5 | 14.9 | 7.38 | |
| omega-3/omega-6 | 2.21 | 2.21 | 3.05 | 0.93 | 0.76 | 3.94 |
| Variable | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| Specific growth | 0.697 | 0.499 | 0.441 |
| Retinol | 0.749 | 0.619 | 0.214 |
| Tocopherol | −0.599 | −0.175 | −0.748 |
| SDH | 0.417 | −0.214 | −0.879 |
| GPx | 0.549 | −0.819 | −0.036 |
| CAT | 0.989 | −0.063 | 0.002 |
| SOD | 0.120 | 0.968 | −0.115 |
| TBARS | −0.921 | 0.142 | 0.307 |
| TBARSin | 0.491 | −0.679 | 0.5320 |
| SFA | 0.851 | 0.072 | −0.517 |
| UFA | −0.857 | −0.068 | 0.508 |
| Species | 0.260 | 0.959 | 0.052 |
| Habitat | −0.703 | 0.479 | −0.524 |
| Total variance % | 46.05 | 30.5 | 21.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltseva, I.; Yakoviichuk, A.; Maltseva, S.; Kulikovskiy, M.; Maltsev, Y. Antioxidant Status of Cyanobacteria Strains During Long-Term Cultivation in Nitrogen-Free Media. Int. J. Mol. Sci. 2025, 26, 10891. https://doi.org/10.3390/ijms262210891
Maltseva I, Yakoviichuk A, Maltseva S, Kulikovskiy M, Maltsev Y. Antioxidant Status of Cyanobacteria Strains During Long-Term Cultivation in Nitrogen-Free Media. International Journal of Molecular Sciences. 2025; 26(22):10891. https://doi.org/10.3390/ijms262210891
Chicago/Turabian StyleMaltseva, Irina, Aleksandr Yakoviichuk, Svetlana Maltseva, Maxim Kulikovskiy, and Yevhen Maltsev. 2025. "Antioxidant Status of Cyanobacteria Strains During Long-Term Cultivation in Nitrogen-Free Media" International Journal of Molecular Sciences 26, no. 22: 10891. https://doi.org/10.3390/ijms262210891
APA StyleMaltseva, I., Yakoviichuk, A., Maltseva, S., Kulikovskiy, M., & Maltsev, Y. (2025). Antioxidant Status of Cyanobacteria Strains During Long-Term Cultivation in Nitrogen-Free Media. International Journal of Molecular Sciences, 26(22), 10891. https://doi.org/10.3390/ijms262210891






