Selected Post-Translational Modifications—Phosphorylation and Glutathionylation—As Factors Involved in the Regulation During the Pregnancy Course and Foetal Membrane Release in Cows
Abstract
1. Introduction
2. Results
2.1. Phosphoprotein Quantification
2.2. Post-Translational Modifications
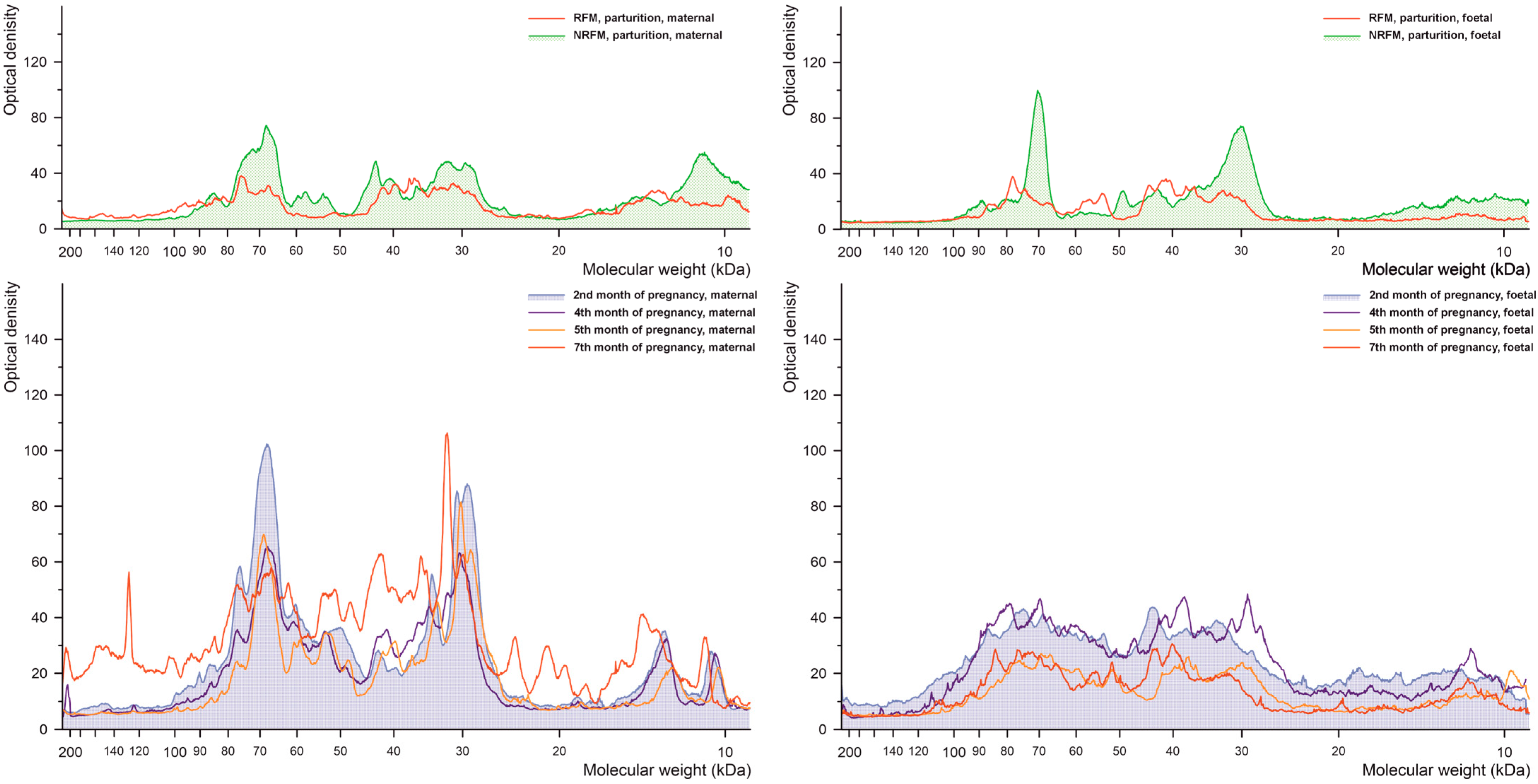
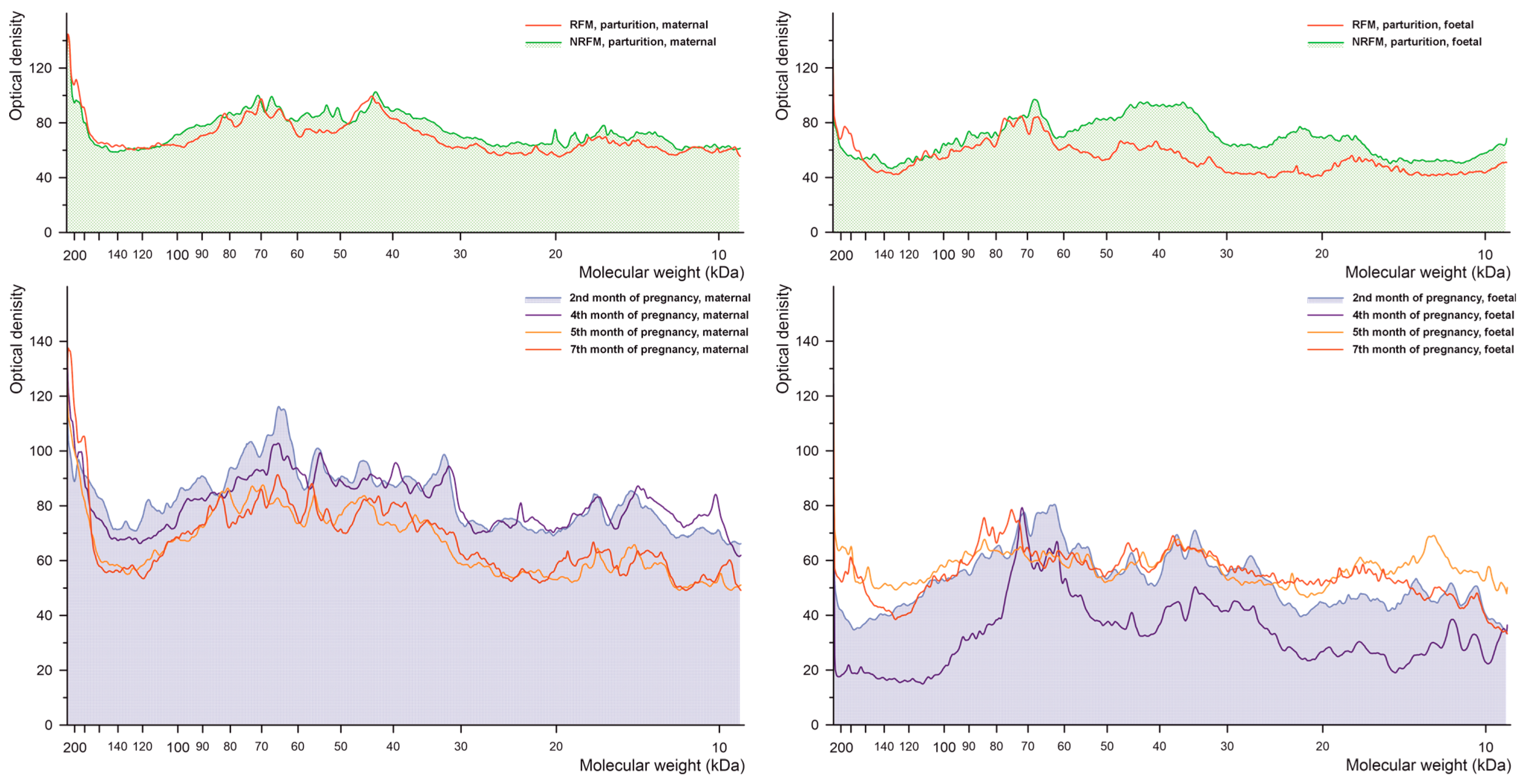
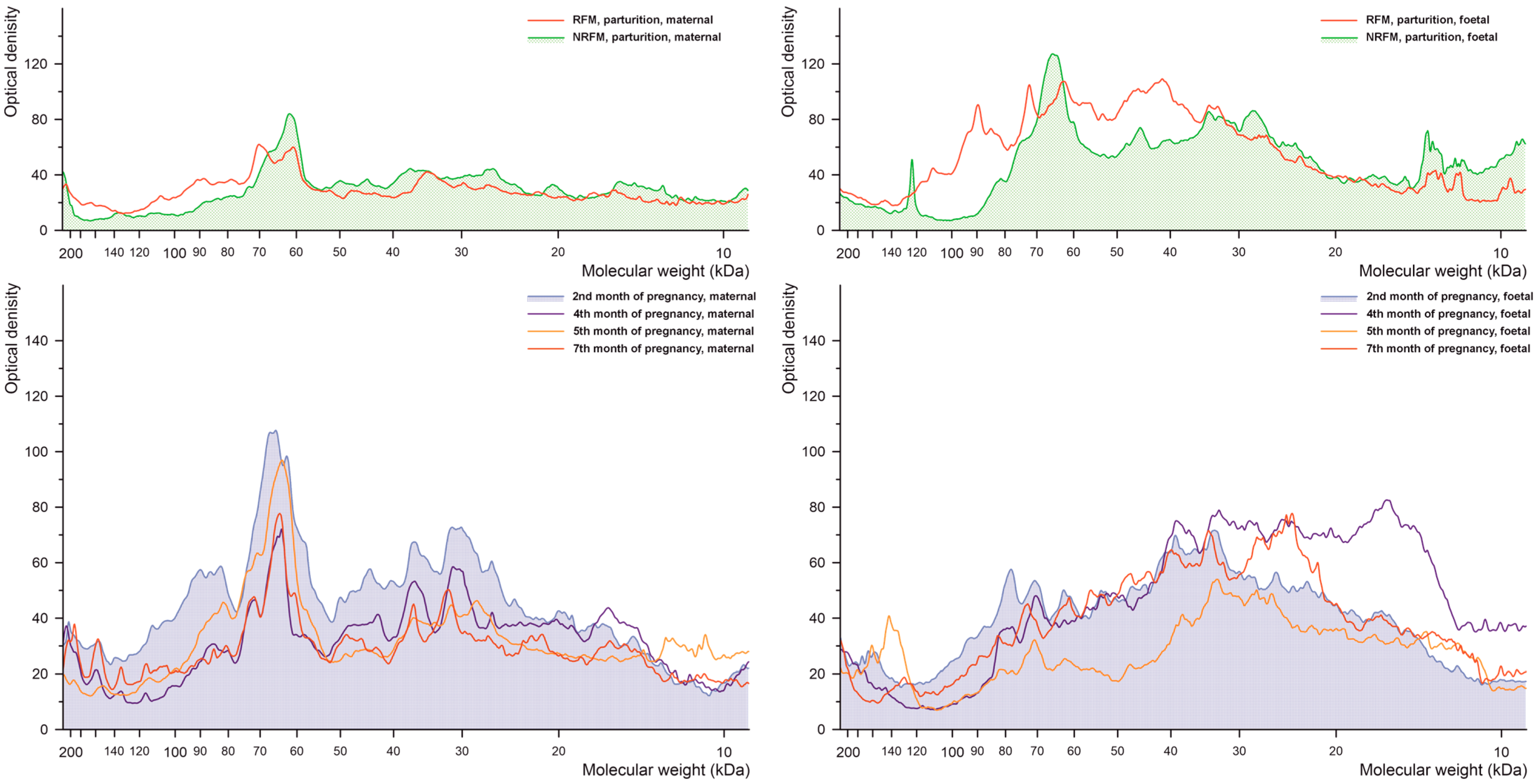
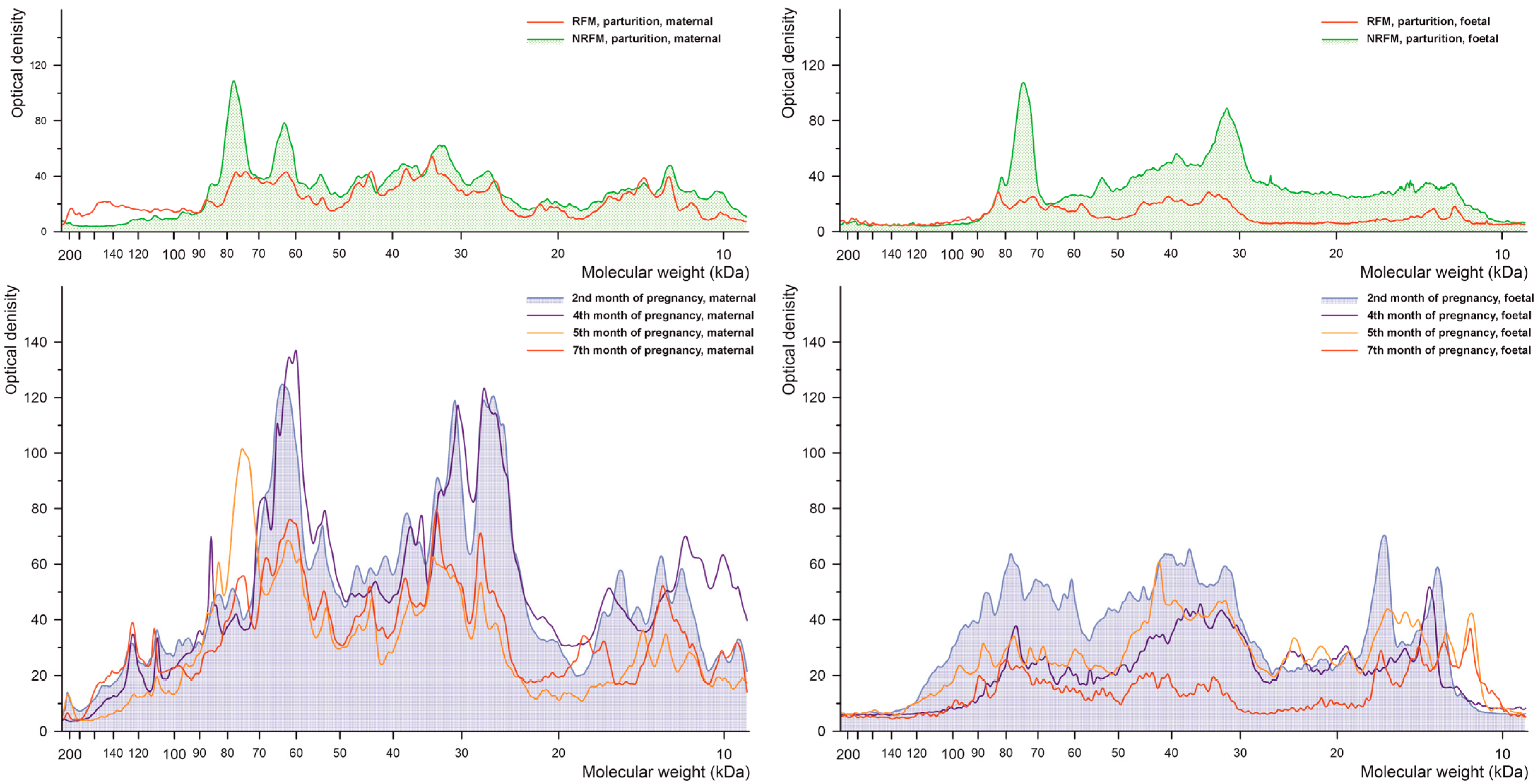
2.2.1. Phosphorylated Tyrosine
2.2.2. Phosphorylated Serine
2.2.3. Phosphorylated Threonine
2.2.4. Glutathionylated Proteins
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Preparation of Homogenates
4.3. Phosphoprotein Quantification
4.4. Preparation of Homogenates Protein Preparation and Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTM | post-translational modification |
| GS-ylation | protein glutathionylation |
| ECM | extracellular matrix |
| RFM | retention of foetal membranes/retained foetal membranes |
| NRFM | not-retained foetal membranes |
| CI | confidence interval |
References
- Dutta, H.; Jain, N. Post-Translational Modifications and Their Implications in Cancer. Front. Oncol. 2023, 13, 1240115. [Google Scholar] [CrossRef]
- Zhong, Q.; Xiao, X.; Qiu, Y.; Xu, Z.; Chen, C.; Chong, B.; Zhao, X.; Hai, S.; Li, S.; An, Z.; et al. Protein Posttranslational Modifications in Health and Diseases: Functions, Regulatory Mechanisms, and Therapeutic Implications. MedComm 2023, 4, e261. [Google Scholar] [CrossRef]
- Karve, T.M.; Cheema, A.K. Small Changes Huge Impact: The Role of Protein Posttranslational Modifications in Cellular Homeostasis and Disease. J. Amino Acids 2011, 2011, 207691. [Google Scholar] [CrossRef] [PubMed]
- Conibear, A.C. Deciphering Protein Post-Translational Modifications Using Chemical Biology Tools. Nat. Rev. Chem. 2020, 4, 674–695. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Zahiri, J. Post-Translational Modifications in Proteins: Resources, Tools and Prediction Methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L. Lo The Crucial Role of Protein Phosphorylation in Cell Signaling and Its Use as Targeted Therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Poerschke, R.L.; Fritz, K.S.; Franklin, C.C. Methods to Detect Protein Glutathionylation. Curr. Protoc. Toxicol. 2013, 57, 6.17.1–6.17.18. [Google Scholar] [CrossRef]
- Ghezzi, P. Protein Glutathionylation in Health and Disease. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3165–3172. [Google Scholar] [CrossRef]
- Pastore, A.; Piemonte, F. S-Glutathionylation Signaling in Cell Biology: Progress and Prospects. Eur. J. Pharm. Sci. 2012, 46, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Duan, X.; Lv, H.; Yang, Y.; Liu, Y.; Gao, X.; Hou, X. The Effects of L-Type Amino Acid Transporter 1 on Milk Protein Synthesis in Mammary Glands of Dairy Cows. J. Dairy Sci. 2018, 101, 1687–1696. [Google Scholar] [CrossRef]
- Toerien, C.A.; Cant, J.P. Abundance and Phosphorylation State of Translation Initiation Factors in Mammary Glands of Lactating and Nonlactating Dairy Cows. J. Dairy Sci. 2007, 90, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.M.M.C.; Campos, D.B.; Buratini, J., Jr.; Binelli, M.; Papa, P.C. Growth Factors and Steroidogenesis in the Bovine Placenta. Anim. Reprod. 2008, 5, 3–15. [Google Scholar]
- Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Spencer, T.E. Osteopontin: Roles in Implantation and Placentation. Biol. Reprod. 2003, 69, 1458–1471. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining Postpartum Uterine Disease in Cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Yin, Y.; Liao, L.; Xu, Q.; Xie, S.; Yuan, L.; Zhou, R. Insight into the Post-Translational Modifications in Pregnancy and Related Complications. Biol. Reprod. 2025, 112, 204–224. [Google Scholar] [CrossRef]
- Monfardini, E.; Paape, M.J.; Wang, Y.; Capuco, A.V.; Husheem, M.; Wood, L.; Burvenich, C. Evaluation of L-Selectin Expression and Assessment of Protein Tyrosine Phosphorylation in Bovine Polymorphonuclear Neutrophil Leukocytes around Parturition. Vet. Res. 2002, 33, 271–281. [Google Scholar] [CrossRef][Green Version]
- Galski, H.; De Groot, N.; Ilan, J.; Hochberg, A.A. Phosphorylation of Tyrosine in Cultured Human Placenta. BBA—Mol. Cell Res. 1984, 804, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Shi, Z.; Liang, G. Comparative N-Glycoproteomic and Phosphoproteomic Profiling of Human Placental Plasma Membrane between Normal and Preeclampsia Pregnancies with High-Resolution Mass Spectrometry. PLoS ONE 2013, 8, e80480. [Google Scholar] [CrossRef]
- Katoh, N. Signal Transduction Systems Mediated by Protein Kinase-C and Estradiol Receptors in Cow Placenta and Caruncle. J. Vet. Med. Sci. 1992, 54, 81–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kankofer, M. Protein Peroxidation Processes in Bovine Retained and Not-Retained Placenta. J. Vet. Med. Ser. A 2001, 48, 207–212. [Google Scholar] [CrossRef]
- Kankofer, M. Non-Enzymatic Antioxidative Defence Mechanisms against Reactive Oxygen Species in Bovine-Retained and Not-Retained Placenta: Vitamin C and Glutathione. Reprod. Domest. Anim. 2001, 36, 203–206. [Google Scholar] [CrossRef]
- Li, W.; Moretti, L.; Su, X.; Yeh, C.R.; Torres, M.P.; Barker, T.H. Strain-Dependent Glutathionylation of Fibronectin Fibers Impacts Mechano-Chemical Behavior and Primes an Integrin Switch. Nat. Commun. 2024, 15, 8751. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Zhang, Y.; Kim, Y.J.; Hamilton, E.J.; Xu, B.; Limas, J.; McCracken, S.A.; Morris, J.M.; Makris, A.; Hennessy, A.; et al. B3-Adrenergic Agonist Counters Oxidative Stress and Na1 -K1 Pump Inhibitory S-Glutathionylation of Placental Cells: Implications for Preeclampsia. Am. J. Physiol.—Cell Physiol. 2025, 328, C27–C39. [Google Scholar] [CrossRef] [PubMed]
- Wawrzykowski, J.; Franczyk, M.; Kankofer, M. Patterns of Protein Glycosylation in Bovine Placentomes as a Function of Gestational Age and in Retained versus Non-Retained Placenta. Reprod. Domest. Anim. 2019, 54, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Jamioł, M.; Wawrzykowski, J.; Mojsym, W.; Hoedemaker, M.; Kankofer, M. Activity of Selected Glycosidases and Availability of Their Substrates in Bovine Placenta during Pregnancy and Parturition with and without Retained Foetal Membranes. Reprod. Domest. Anim. 2020, 55, 1093–1102. [Google Scholar] [CrossRef]
- Hooshmandabbasi, R.; Zerbe, H.; Bauersachs, S.; de Sousa, N.M.; Boos, A.; Klisch, K. Pregnancy-Associated Glycoproteins in Cows with Retained Fetal Membranes. Theriogenology 2018, 105, 158–163. [Google Scholar] [CrossRef]
- Krog, C.H.; Agerholm, J.S.; Nielsen, S.S. Fetal Age Assessment for Holstein Cattle. PLoS ONE 2018, 13, e0207682. [Google Scholar] [CrossRef]
- Shapiro, A.S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Stanford University Press: Redwood City, CA, USA, 1960. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Rothman, K.J. No Adjustments Are Needed for Multiple Comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Zhouheng, Y.; Bradley, P.A.; Frank, R.S.; Xinhua, Z. Cleaved β-Actin May Contribute to DNA Fragmentation Following Very Brief Focal Cerebral Ischemia. J. Neuropathol. Exp. Neurol. 2018, 77, 260–265. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Company, Catalog Number | Host; Reactivity | Dilution |
|---|---|---|---|
| Phosphoserine Monoclonal Antibody (3C171) | ThermoFisher Scientific, MA1-91608 | Mouse monoclonal/IgG1; chemical | 1:2000 |
| Phosphothreonine Monoclonal Antibody (PT-5H5) | ThermoFisher Scientific, 13-9200 | Mouse monoclonal/IgG2a, kappa; chemical | 1:1000 |
| Phospho-Tyrosine Monoclonal Antibody (pY20) | ThermoFisher Scientific, 14-5001-82 | Mouse monoclonal/IgG2b, kappa; chemical | 1:2000 |
| Glutathione Monoclonal Antibody (D8) | ThermoFisher Scientific, MA1-7620 | Mouse monoclonal/IgG2a; chemical | 1:2000 |
| beta Actin Monoclonal Antibody (BA3R) | ThermoFisher Scientific, MA5-15739-HRP | Mouse monoclonal/IgG2b; dog, chicken, human, mouse, non-human primate, rabbit, rat | 1:5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzykowski, J.; Jamioł, M.A.; Kosztowny, E.; Kankofer, M. Selected Post-Translational Modifications—Phosphorylation and Glutathionylation—As Factors Involved in the Regulation During the Pregnancy Course and Foetal Membrane Release in Cows. Int. J. Mol. Sci. 2025, 26, 10984. https://doi.org/10.3390/ijms262210984
Wawrzykowski J, Jamioł MA, Kosztowny E, Kankofer M. Selected Post-Translational Modifications—Phosphorylation and Glutathionylation—As Factors Involved in the Regulation During the Pregnancy Course and Foetal Membrane Release in Cows. International Journal of Molecular Sciences. 2025; 26(22):10984. https://doi.org/10.3390/ijms262210984
Chicago/Turabian StyleWawrzykowski, Jacek, Monika A. Jamioł, Ewelina Kosztowny, and Marta Kankofer. 2025. "Selected Post-Translational Modifications—Phosphorylation and Glutathionylation—As Factors Involved in the Regulation During the Pregnancy Course and Foetal Membrane Release in Cows" International Journal of Molecular Sciences 26, no. 22: 10984. https://doi.org/10.3390/ijms262210984
APA StyleWawrzykowski, J., Jamioł, M. A., Kosztowny, E., & Kankofer, M. (2025). Selected Post-Translational Modifications—Phosphorylation and Glutathionylation—As Factors Involved in the Regulation During the Pregnancy Course and Foetal Membrane Release in Cows. International Journal of Molecular Sciences, 26(22), 10984. https://doi.org/10.3390/ijms262210984






