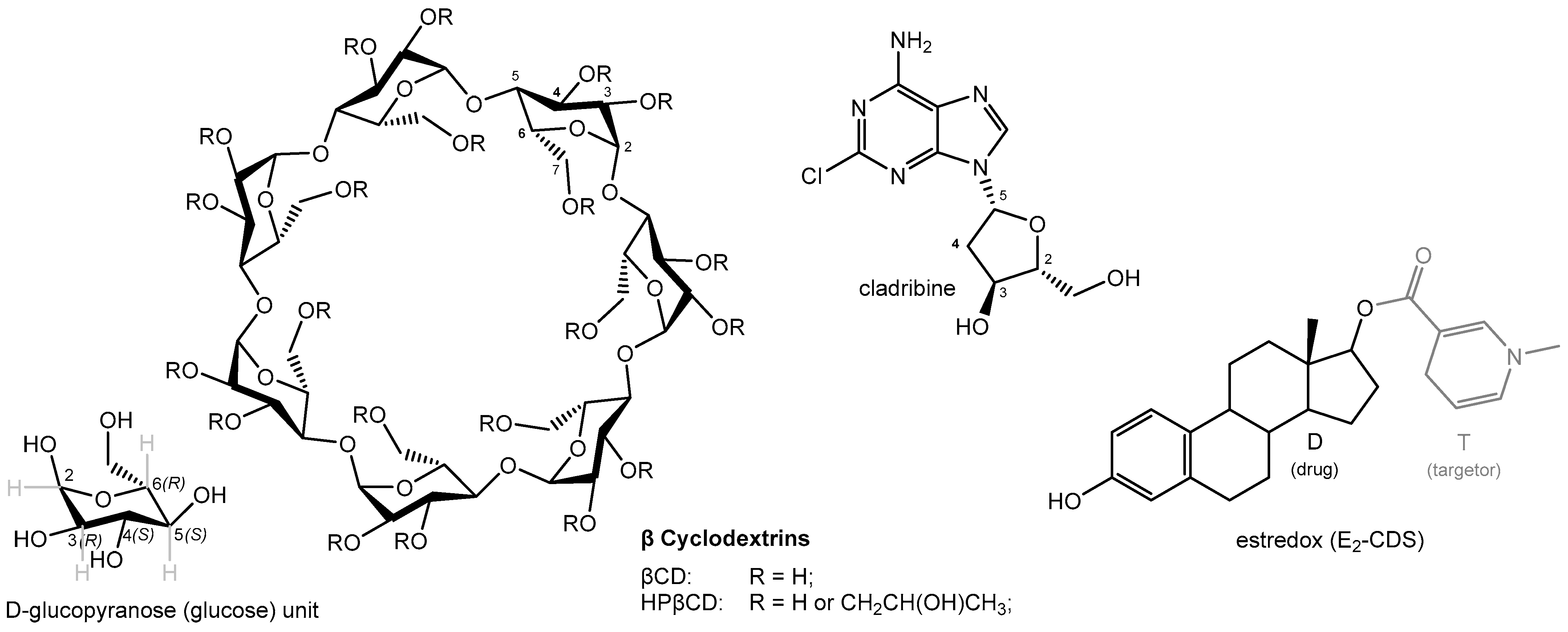
Cyclodextrin Complexes for Clinical Translatability: Applications for Cladribine and Retrometabolically Designed Estredox
Abstract
1. Introduction
2. CD Complex Stability
3. Improving Stability and Solubility: Estredox, a Brain-Targeting Retrometabolic Chemical Delivery System
3.1. Brain-Targeting Chemical Delivery Systems (CDSs)
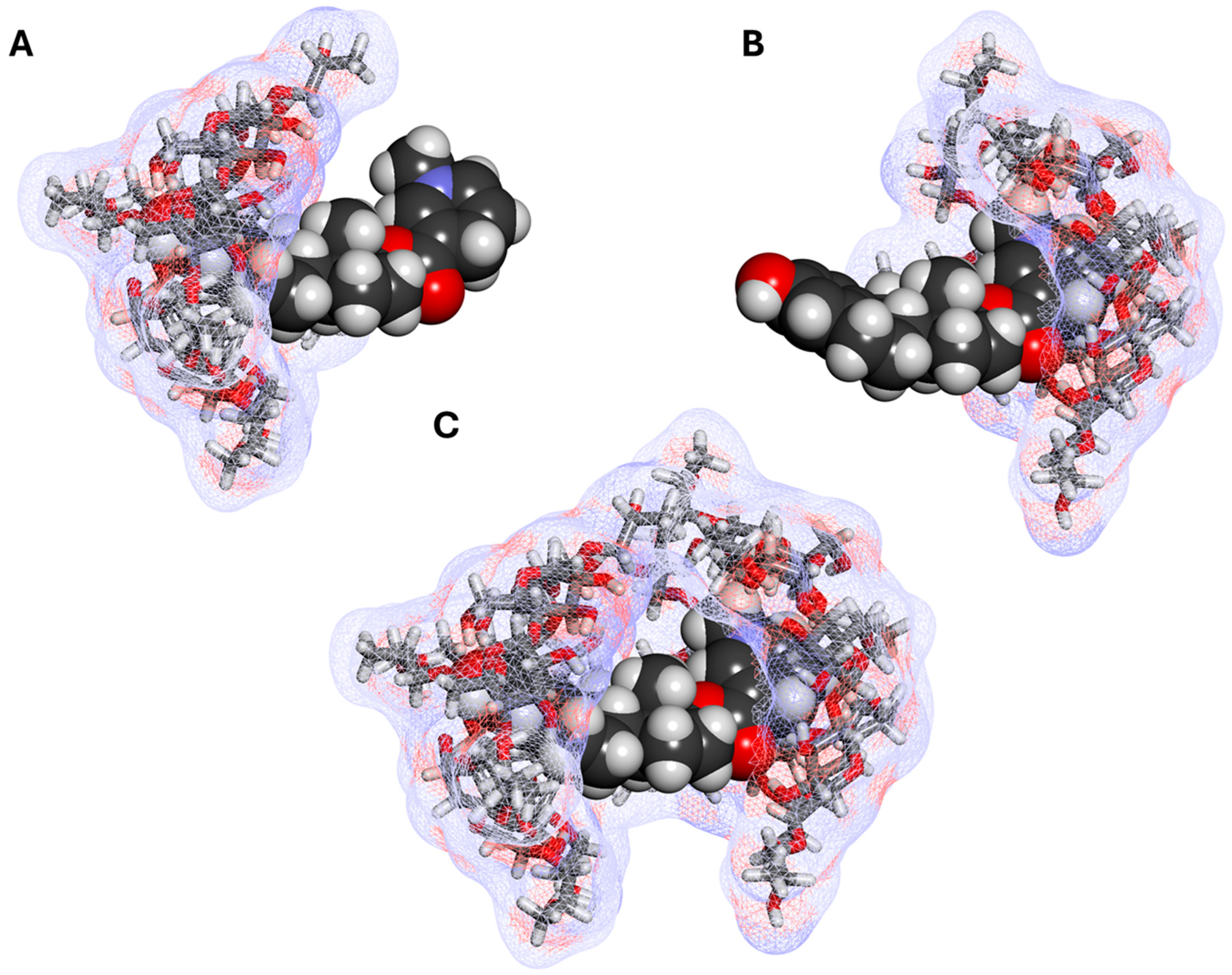
3.2. CDS—Cyclodextrin Complexes
3.3. Estredox—Clinical Applicability
4. Improving Oral Bioavailability: Cladribine
4.1. Mavenclad—Oral Cladribine, a Disease Modifying Therapy for Relapsing Multiple Sclerosis (MS)
4.2. Complex Dual Cladribine–Cyclodextrin Complex
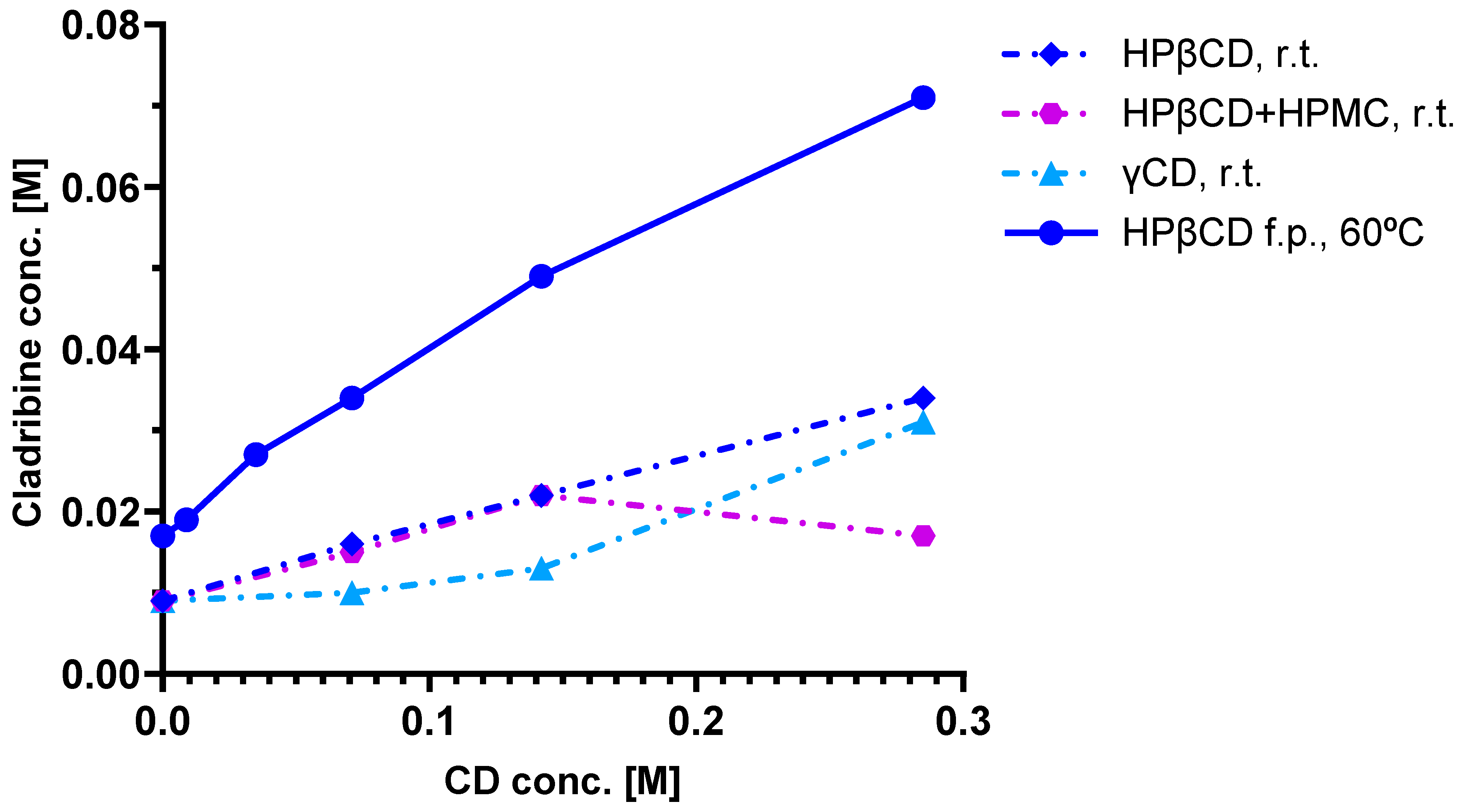
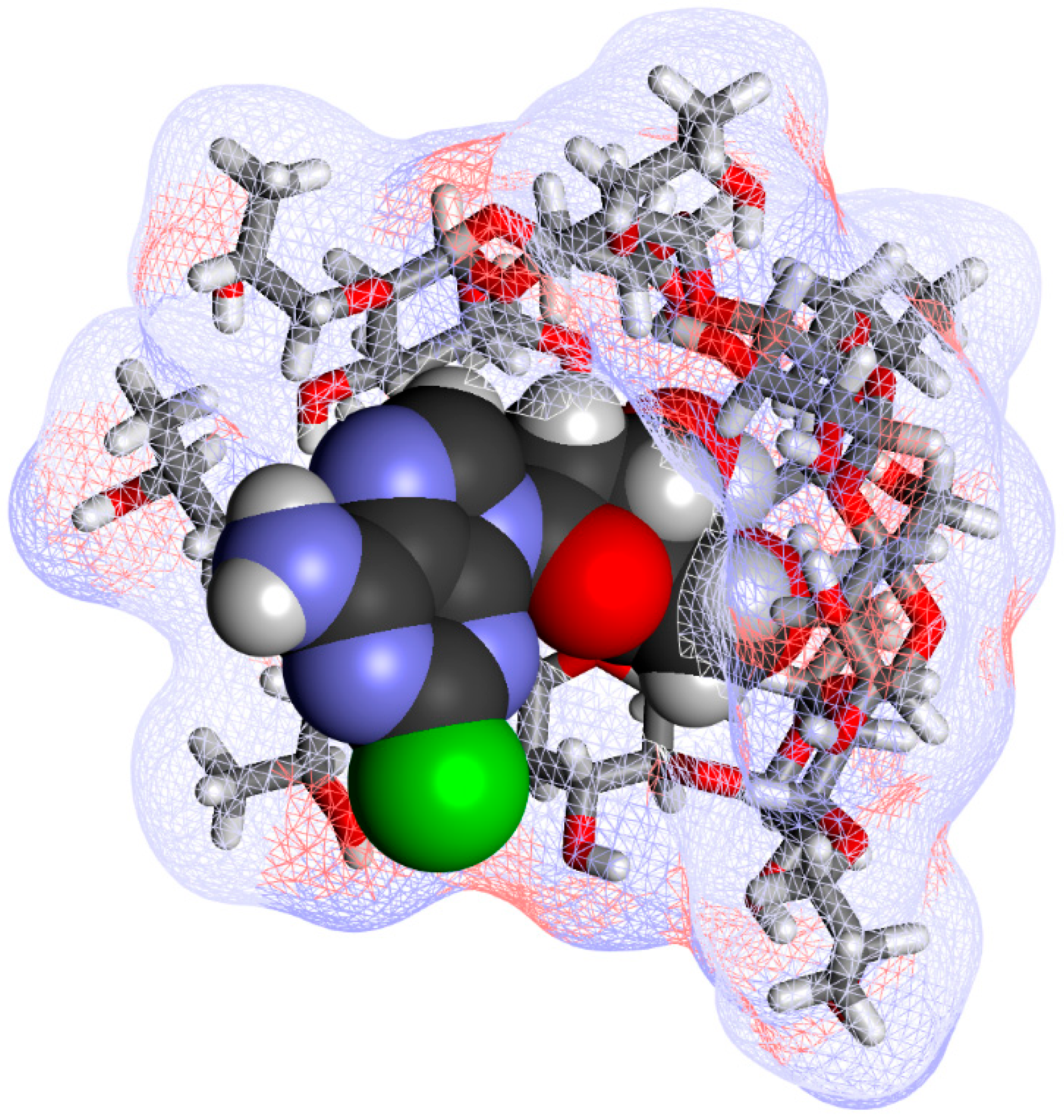
4.3. Phase Solubility Studies and Complex Structure
4.4. Preclinical Pharmacokinetics
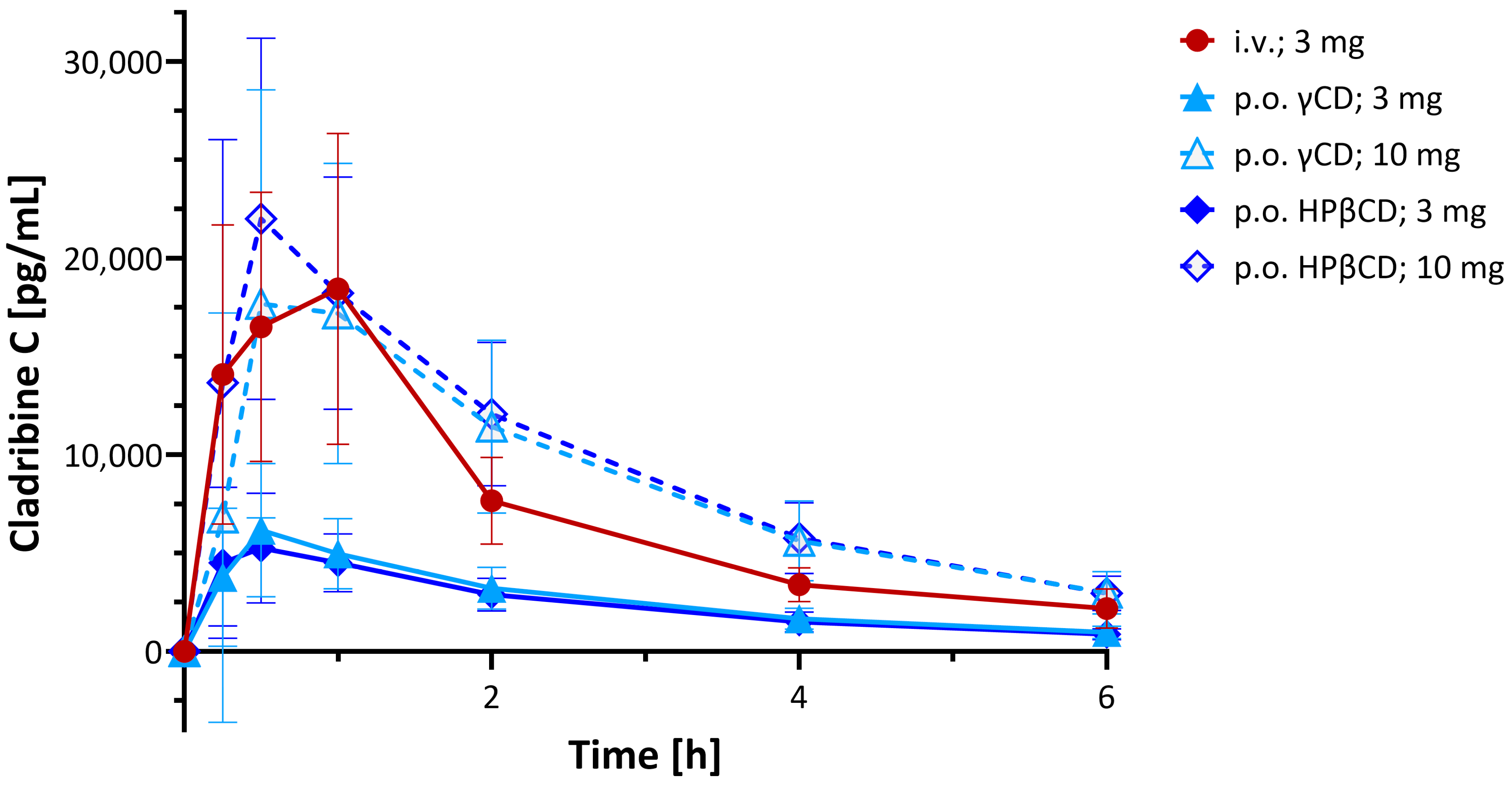
4.5. Clinical Pharmacokinetics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the concentration–time curve |
| BBB | Blood–brain barrier |
| CD | Cyclodextrin |
| CDS | Chemical delivery system |
| CNS | Central nervous system |
| CPK | Corey–Pauling–Koltun (model) |
| HPβCD | (2-Hydroxypropyl)-beta cyclodextrin; hydroxypropyl betadex |
| MS | Multiple sclerosis |
| PK | Pharmacokinetic |
References
- Saenger, W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Engl. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins and Their Inclusion Complexes; Akadémiai Kiadó: Budapest, Hungary, 1982; p. 296. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Musuc, A.M. Cyclodextrins: Advances in chemistry, toxicology, and multifaceted applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rau, G.; Pirvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocilteu, M.V. Cyclodextrins: Enhancing drug delivery, solubility and bioavailability for modern therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef]
- Buchwald, P. Complexation thermodynamics of cyclodextrins in the framework of a molecular size-based model for nonassociative organic liquids that includes a modified hydration-shell hydrogen-bond model for water. J. Phys. Chem. B 2002, 106, 6864–6870. [Google Scholar] [CrossRef]
- Bodor, N.; Buchwald, P. Theoretical insights into the formation, structure, and energetics of some cyclodextrin complexes. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 9–14. [Google Scholar] [CrossRef]
- Loftsson, T. Pharmaceutical applications of b-cyclodextrin. Pharm. Technol. 1999, 23, 40–50. [Google Scholar]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Loftsson, T. Cyclodextrins in parenteral formulations. J. Pharm. Sci. 2021, 110, 654–664. [Google Scholar] [CrossRef]
- Ferreira, L.; Campos, J.; Veiga, F.; Cardoso, C.; Paiva-Santos, A.C. Cyclodextrin-based delivery systems in parenteral formulations: A critical update review. Eur. J. Pharm. Biopharm. 2022, 178, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Sarabia-Vallejo, A.; Caja, M.D.M.; Olives, A.I.; Martin, M.A.; Menendez, J.C. Cyclodextrin inclusion complexes for improved drug bioavailability and activity: Synthetic and analytical aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Puskás, I.; Szente, L.; Szőcs, L.; Fenyvesi, É. Recent list of cyclodextrin-containing drug products. Per. Polytech. Chem. Eng. 2023, 67, 11–17. [Google Scholar] [CrossRef]
- Liu, H.; Guo, S.; Wei, S.; Liu, J.; Tian, B. Pharmacokinetics and pharmacodynamics of cyclodextrin-based oral drug delivery formulations for disease therapy. Carbohydr. Polym. 2024, 329, 121763. [Google Scholar] [CrossRef]
- Hoti, G.; Bajwa, N.; Caldera, F.; Singh, P.A.; Hussein, I.; Cecone, C.; Matencio, A.; Spagnolo, R.; Argenziano, M.; Cavalli, R.; et al. Cyclodextrin-based therapeutics delivery systems: A review of current clinical trials. Curr. Res. Pharmacol. Drug Discov. 2025, 9, 100232. [Google Scholar] [CrossRef] [PubMed]
- Munnangi, S.R.; Youssef, A.A.A.; Narala, N.; Lakkala, P.; Narala, S.; Vemula, S.K.; Repka, M. Drug complexes: Perspective from Academic Research and Pharmaceutical Market. Pharm. Res. 2023, 40, 1519–1540. [Google Scholar] [CrossRef]
- Buchwald, P.; Bodor, N. Simple model for nonassociative organic liquids and water. J. Am. Chem. Soc. 2000, 122, 10671–10679. [Google Scholar] [CrossRef]
- Buchwald, P. A general bilinear model to describe growth or decline time-profiles. Math. Biosci. 2007, 205, 108–136. [Google Scholar] [CrossRef]
- Angelova, S.; Pereva, S.; Dudev, T.; Spassov, T. Cyclodextrins’ internal cavity hydration: Insights from theory and experiment. Inorganics 2025, 13, 28. [Google Scholar] [CrossRef]
- Bodor, N.; Farag, H.H.; Brewster, M.E. Site-specific, sustained release of drugs to the brain. Science 1981, 214, 1370–1372. [Google Scholar] [CrossRef]
- Bodor, N.; Buchwald, P. Recent advances in the brain targeting of neuropharmaceuticals by chemical delivery systems. Adv. Drug Deliv. Rev. 1999, 36, 229–254. [Google Scholar] [CrossRef] [PubMed]
- Bodor, N.; Buchwald, P. Retrometabolic Drug Design and Targeting, 1st ed.; Wiley: Hoboken, NJ, USA, 2012; p. 418. [Google Scholar]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Rautio, J.; Meanwell, N.A.; Di, L.; Hageman, M.J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 2018, 17, 559–587. [Google Scholar] [CrossRef]
- de Boer, A.G.; Gaillard, P.J. Drug targeting to the brain. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. A historical review of brain drug delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef]
- Rydström, J.; Hoek, J.B.; Ernster, L. The nicotinamide nucleotide transhydrogenases. In The Enzymes; Boyer, P.D., Ed.; Academic Press: New York, NY, USA, 1976; Volume 13, pp. 51–88. [Google Scholar]
- Hoek, J.B.; Rydström, J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem. J. 1988, 254, 1–10. [Google Scholar] [CrossRef]
- Anderson, W.R.; Simpkins, J.W.; Brewster, M.E.; Bodor, N. Brain-enhanced delivery of testosterone using a chemical delivery system complexed with 2-hydroxypropyl-ß-cyclodextrin. Drug Des. Del. 1988, 2, 287–298. [Google Scholar]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-beta-cyclodextrin (HP-b-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Pitha, J.; Pitha, J. Amorphous water-soluble derivatives of cyclodextrins: Nontoxic dissolution enhancing excipients. J. Pharm. Sci. 1985, 74, 987–990. [Google Scholar] [CrossRef]
- Pitha, J.; Milecki, J.; Fales, H.; Pannell, L.; Uekama, K. Hydroxypropyl-ß-cyclodextrin: Preparation and characterization, effects on solubility of drugs. Int. J. Pharm. 1986, 29, 73–82. [Google Scholar] [CrossRef]
- Yoshida, A.; Arima, H.; Uekama, K.; Pitha, J. Pharmaceutical evaluation of hydroxyalkyl ethers of ß-cyclodextrins. Int. J. Pharm. 1988, 46, 217–222. [Google Scholar] [CrossRef]
- Brewster, M.E.; Estes, K.S.; Bodor, N. An intravenous toxicity study of 2-hydroxypropyl-ß-cyclodextrin, a useful drug solubilizer, in rats and monkeys. Int. J. Pharm. 1990, 59, 231–243. [Google Scholar] [CrossRef]
- Brewster, M.E.; Estes, K.E.; Loftsson, T.; Perchalski, R.; Derendorf, H.; Mullersman, G.; Bodor, N. Improved delivery through biological membranes. XXXI. Solubilization and stabilization of an estradiol chemical delivery system by modified ß-cyclodextrins. J. Pharm. Sci. 1988, 77, 981–985. [Google Scholar] [CrossRef]
- Pop, E.; Loftsson, T.; Bodor, N. Solubilization and stabilization of a benzylpenicillin chemical delivery system by 2-hydroxypropyl-ß-cyclodextrin. Pharm. Res. 1991, 8, 1044–1049. [Google Scholar] [CrossRef]
- Buchwald, P.; Bodor, N. Brain-targeting chemical delivery systems and their cyclodextrin-based formulations in light of the contributions of Marcus E. Brewster. J. Pharm. Sci. 2016, 105, 2589–2600. [Google Scholar] [CrossRef]
- Greendale, G.A.; Lee, N.P.; Arriola, E.R. The menopause. Lancet 1999, 353, 571–580. [Google Scholar] [CrossRef]
- Crandall, C.J.; Mehta, J.M.; Manson, J.E. Management of menopausal symptoms: A review. J. Am. Med. Assoc. (JAMA) 2023, 329, 405–420. [Google Scholar] [CrossRef]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440. [Google Scholar] [CrossRef]
- Shepherd, J.E. Therapeutic options in female sexual dysfunction. J. Am. Pharm. Assoc. 2002, 42, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.M.; Houman, J.; Caron, A.T.; Anger, J. Female sexual dysfunction: A systematic review of outcomes across various treatment modalities. Sex. Med. Rev. 2019, 7, 223–250. [Google Scholar] [CrossRef]
- Garcia-Segura, L.M.; Azcoitia, I.; DonCarlos, L.L. Neuroprotection by estradiol. Prog. Neurobiol. 2001, 63, 29–60. [Google Scholar] [CrossRef]
- Albert, K.M.; Newhouse, P.A. Estrogen, stress, and depression: Cognitive and biological Interactions. Annu. Rev. Clin. Psychol. 2019, 15, 399–423. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Song, X.; Lai, S.; Zhong, S.; Jia, Y. The effect of exogenous estrogen on depressive mood in women: A systematic review and meta-analysis of randomized controlled trials. J. Psychiatr. Res. 2023, 162, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogen and cognitive functioning in women. Endocr. Rev. 2003, 24, 133–151. [Google Scholar] [CrossRef]
- Cipriano, G.L.; Mazzon, E.; Anchesi, I. Estrogen receptors: A new frontier in Alzheimer’s disease therapy. Int. J. Mol. Sci. 2024, 25, 9077. [Google Scholar] [CrossRef]
- Bodor, N.; McCornack, J.; Brewster, M.E. Improved delivery through biological membranes. XXII. Synthesis and distribution of brain-selective estrogen delivery systems. Int. J. Pharm. 1987, 35, 47–59. [Google Scholar] [CrossRef]
- Bodor, N.S. Cladribine Formulations for Improved Oral and Transmucosal Delivery. U.S. Patent 8,623,408 B2, 7 January 2014. [Google Scholar]
- Bodor, N.S.; Dandiker, Y. Oral Formulations of Cladribine. U.S. Patent 8,785,415 B2, 22 July 2014. [Google Scholar]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Soelberg Sorensen, P.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef]
- Leist, T.P.; Weissert, R. Cladribine: Mode of action and implications for treatment of multiple sclerosis. Clin. Neuropharmacol. 2011, 34, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Leist, T.P.; Comi, G.; Cree, B.A.; Coyle, P.K.; Freedman, M.S.; Hartung, H.P.; Vermersch, P.; Casset-Semanaz, F.; Scaramozza, M.; Oral, cladribine for early MS Study Group. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): A phase 3 randomised trial. Lancet Neurol. 2014, 13, 257–267. [Google Scholar] [CrossRef]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Boyko, A.; Correale, J.; Edan, G.; Freedman, M.S.; Montalban, X.; Rammohan, K.; Stefoski, D.; Yamout, B.; Leist, T.; et al. Long-term follow-up of patients with relapsing multiple sclerosis from the CLARITY/CLARITY Extension cohort of CLASSIC-MS: An ambispective study. Mult. Scler. 2023, 29, 719–730. [Google Scholar] [CrossRef]
- Merck, KGaA. Merck Unveils New MAVENCLAD® Four-Year Data Highlighting Benefits of Early Treatment and Sustained Efficacy Across Multiple Measures of Disease Activity; Press Release 12 September 2024; Merck KGaA: Darmstadt, Germany, 2024. [Google Scholar]
- Merck, KGaA. Fiscal 2023: Proven Resilience During Transitional Year; Press Release 7 March 2024; Merck KGaA: Darmstadt, Germany, 2024. [Google Scholar]
- Barclay, N.; Tarallo, M.; Hendrikx, T.; Marett, S. Patient preference for oral versus injectable and intravenous methods of treatment for rheumatoid arthritis. Value Health 2013, 16, A568. [Google Scholar] [CrossRef]
- Hincapie, A.L.; Penm, J.; Burns, C.F. Factors associated with patient preferences for disease-modifying therapies in multiple sclerosis. J. Manag. Care Spec. Pharm. 2017, 23, 822–830. [Google Scholar] [CrossRef]
- Baryakova, T.H.; Pogostin, B.H.; Langer, R.; McHugh, K.J. Overcoming barriers to patient adherence: The case for developing innovative drug delivery systems. Nat. Rev. Drug Discov. 2023, 22, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Liliemark, J. The clinical pharmacokinetics of cladribine. Clin. Pharmacokinet. 1997, 32, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.W.; Naeff, R. Cyclodextrin Cladribine Formulations. U.S. Patent 6,194,395 B1, 27 February 2001. [Google Scholar]
- Van Axel Castelli, V.; Trivieri, G.; Zucchelli, I.; Brambilla, L.; Barbuzzi, T.; Castiglioni, C.; Paci, M.; Zerbi, G.; Zanol, M. Characterisation of an inclusion complex between cladribine and 2-hydroxypropyl-beta-cyclodextrin. J. Pharm. Sci. 2008, 97, 3897–3906. [Google Scholar] [CrossRef]
- Hermann, R.; Karlsson, M.O.; Novakovic, A.M.; Terranova, N.; Fluck, M.; Munafo, A. The clinical pharmacology of cladribine tablets for the treatment of relapsing multiple sclerosis. Clin. Pharmacokinet. 2019, 58, 283–297, Erratum in Clin. Pharmacokinet. 2019, 58, 401. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodor, N.; Buchwald, P. Cyclodextrin Complexes for Clinical Translatability: Applications for Cladribine and Retrometabolically Designed Estredox. Int. J. Mol. Sci. 2025, 26, 10976. https://doi.org/10.3390/ijms262210976
Bodor N, Buchwald P. Cyclodextrin Complexes for Clinical Translatability: Applications for Cladribine and Retrometabolically Designed Estredox. International Journal of Molecular Sciences. 2025; 26(22):10976. https://doi.org/10.3390/ijms262210976
Chicago/Turabian StyleBodor, Nicholas, and Peter Buchwald. 2025. "Cyclodextrin Complexes for Clinical Translatability: Applications for Cladribine and Retrometabolically Designed Estredox" International Journal of Molecular Sciences 26, no. 22: 10976. https://doi.org/10.3390/ijms262210976
APA StyleBodor, N., & Buchwald, P. (2025). Cyclodextrin Complexes for Clinical Translatability: Applications for Cladribine and Retrometabolically Designed Estredox. International Journal of Molecular Sciences, 26(22), 10976. https://doi.org/10.3390/ijms262210976






