UVB-/Age-Dependent Upregulation of Inflammatory Factor Interleukin-6 Receptor (IL-6R) in Keratinocytes Stimulates Melanocyte Dendricity
Abstract
1. Introduction
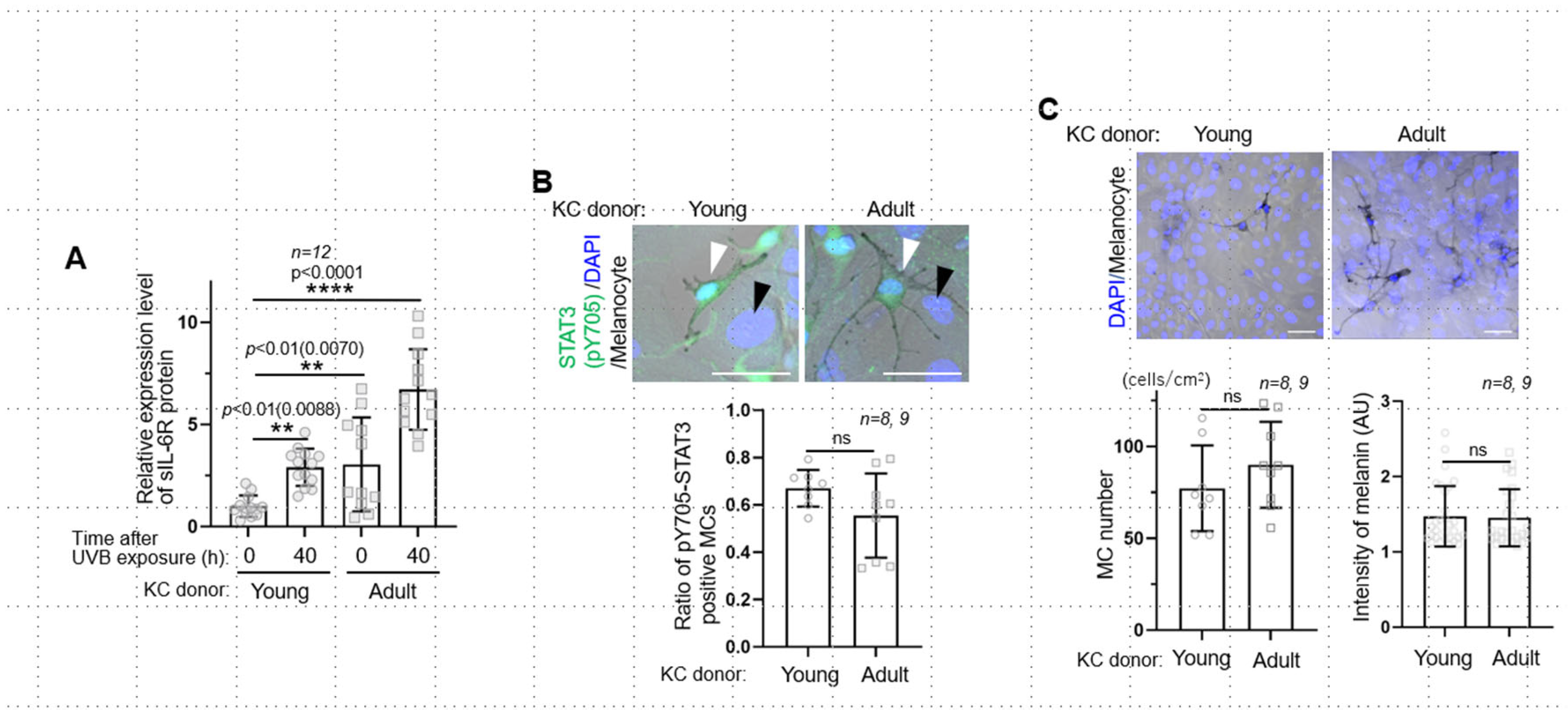
2. Results and Discussion
3. Materials and Methods
3.1. Assessment of Age Spots in Human Cutaneous Specimens
3.2. Cell Cultures
3.3. UVB Irradiation
3.4. Neutralization of the Function of Interleukin-6 Receptor (IL-6R) with Tocilizumab
3.5. ELISA Assay for Measurement of sIL-6R
3.6. Fluorescent Immunostaining of Cultured Cells and Skin Tissues
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flament, F.; Saint-Leger, D. Photoaging’s portrait: The road map towards its photoprotection. Int. J. Cosmet. Sci. 2023, 45, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Morita, A.; Seité, S.; Haarmann-Stemmann, T.; Grether-Beck, S.; Krutmann, J. Environment-induced lentigines: Formation of solar lentigines beyond ultraviolet radiation. Exp. Dermatol. 2015, 24, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Yamashita, T.; Shibata, T. Influence of environmental factors on facial pigmented spots: Epidemiological survey of women living in the northern and southern regions of Japan. Photodermatol. Photoimmunol. Photomed. 2023, 39, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Jo, S.Y.; Lee, M.H.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E. The Effect of MCP-1/CCR2 on the proliferation and senescence of epidermal constituent cells in solar lentigo. Int. J. Mol. Sci. 2016, 17, 948. [Google Scholar] [CrossRef]
- Meunier, M.; Bracq, M.; Chapuis, E.; Lapierre, L.; Humeau, A.; Bernard, S.; Lambert, C.; Paulus, C.; Auriol, P.; Lemagnen, P.; et al. Targeting SDF-1 as an efficient strategy to resolve skin hyperpigmentation issues with Himanthalia elongata extract. Cosmet. Dermatol. 2023, 22, 383–394. [Google Scholar] [CrossRef]
- Iriyama, S.; Ono, T.; Aoki, H.; Amano, S. Hyperpigmentation in human solar lentigo is promoted by heparanase-induced loss of heparan sulfate chains at the dermal-epidermal junction. J. Dermatol. Sci. 2018, 64, 223–228. [Google Scholar] [CrossRef]
- Inoue, D.; Nurani, A.M.; Maeno, K.; Kikuchi, K.; Aoki, H.; Onodera, T.; Shibata, T. Melanoaging: Uncovering and resolving an age-spot-specific metabolic change and cellular senescence caused by excessive melanin deposition. IFSCC Magazine 2025, 27, 4. [Google Scholar]
- Fu, C.; Chen, J.; Lu, J.; Yi, L.; Tong, X.; Kang, L.; Pei, S.; Ouyang, Y.; Jiang, L.; Ding, Y.; et al. Roles of inflammation factors in melanogenesis. Mol. Med. Rep. 2020, 21, 1421–1430. [Google Scholar] [CrossRef]
- Inoue, D.; Narita, T.; Ono, T.; Ishikawa, K.; Maeno, K.; Aoki, H.; Motoyama, A.; Shibata, T. A mechanism of melanogenesis mediated by E-cadherin downregulation and its involvement in solar lentigines. Int. J. Cosmet. Sci. 2023, 45, 775–790. [Google Scholar] [CrossRef]
- Matsuda, T. The physiological and pathophysiological role of IL-6/STAT3-mediated signal transduction and STAT3 binding partners in therapeutic applications. Biol. Pharm. Bull. 2023, 46, 364–378. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.; Niu, M.; Song, S.; Wei, L.; Chen, G.; Ding, Y.; Wang, Y.; Su, Z.; Wang, H. Soluble IL-6R-mediated IL-6 trans-signaling activation contributes to the pathological development of psoriasis. J. Mol. Med. 2021, 99, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, N.; Fukuda, M. Recent advances in understanding the molecular basis of melanogenesis in melanocytes. F1000Research 2020, 9, Rev-608. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Kasutani, K.; Okazaki, M.; Nakamura, A.; Kawai, S.; Sugimoto, M.; Matsumoto, Y.; Ohsugi, Y. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int. Immunopharmacol. 2005, 5, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Schmertl, T.; Lokau, J.; Garbers, C. IL-6 Signaling in Immunopathology: From Basic Biology to Selective Therapeutic Intervention. ImmunoTargets Ther. 2025, 14, 681–695. [Google Scholar] [CrossRef]
- Cao, C.; Xu, W.; Lei, J.; Zheng, Y.; Zhang, A.; Xu, A.; Lin, F.; Zhou, M. The IL-6 autocrine loop promoting IFN-γ-induced fibroblast senescence is involved in psychological stress-mediated exacerbation of vitiligo. Inflamm. Res. 2025, 74, 72. [Google Scholar] [CrossRef]
- Swope, V.B.; Abdel-Malek, Z.; Kassem, L.M.; Nordlund, J.J. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J. Investig. Dermatol. 1991, 96, 180–185. [Google Scholar] [CrossRef]
- Ihara, S.; Nakajima, K.; Fukada, T.; Hibi, M.; Nagata, S.; Hirano, T.; Fukui, Y. Dual control of neurite outgrowth by STAT3 and MAP kinase in PC12 cells stimulated with interleukin-6. EMBO J. 1997, 16, 5345–5352. [Google Scholar] [CrossRef]
- Hakozaki, T.; Wang, J.; Laughlin, T.; Jarrold, B.; Zhao, W.; Furue, M. Role of interleukin-6 and endothelin-1 receptors in enhanced melanocyte dendricity of facial spots and suppression of their ligands by niacinamide and tranexamic acid. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 3–10. [Google Scholar] [CrossRef]
- Balato, N.; Di Costanzo, L.; Balato, A.; Patruno, C.; Scalvenzi, M.; Ayala, F. Psoriasis and melanocytic naevi: Does the first confer a protective role against melanocyte progression to naevi? Br. J. Dermatol. 2011, 164, 1262–1270. [Google Scholar] [CrossRef]
- Furukawa, J.Y.; Martinez, R.M.; Morocho-Jácome, A.L.; Castillo-Gómez, T.S.; Pereda-Contreras, V.J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Skin impacts from exposure to ultraviolet, visible, infrared, and artificial lights—A review. J. Cosmet. Laser Ther. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, D.; Ohba, K.; Shibata, T. UVB-/Age-Dependent Upregulation of Inflammatory Factor Interleukin-6 Receptor (IL-6R) in Keratinocytes Stimulates Melanocyte Dendricity. Int. J. Mol. Sci. 2025, 26, 10971. https://doi.org/10.3390/ijms262210971
Inoue D, Ohba K, Shibata T. UVB-/Age-Dependent Upregulation of Inflammatory Factor Interleukin-6 Receptor (IL-6R) in Keratinocytes Stimulates Melanocyte Dendricity. International Journal of Molecular Sciences. 2025; 26(22):10971. https://doi.org/10.3390/ijms262210971
Chicago/Turabian StyleInoue, Daigo, Koji Ohba, and Takako Shibata. 2025. "UVB-/Age-Dependent Upregulation of Inflammatory Factor Interleukin-6 Receptor (IL-6R) in Keratinocytes Stimulates Melanocyte Dendricity" International Journal of Molecular Sciences 26, no. 22: 10971. https://doi.org/10.3390/ijms262210971
APA StyleInoue, D., Ohba, K., & Shibata, T. (2025). UVB-/Age-Dependent Upregulation of Inflammatory Factor Interleukin-6 Receptor (IL-6R) in Keratinocytes Stimulates Melanocyte Dendricity. International Journal of Molecular Sciences, 26(22), 10971. https://doi.org/10.3390/ijms262210971






