Caraway Extract Increases Ucp-1 mRNA Expression in C3H10T1/2 Adipocytes Through Direct and Indirect Effects
Abstract
1. Introduction
2. Results
2.1. C. carvi Extract Enhances UCP-1 Expression by Increasing Adrenergic Sensitivity in C3H10T1/2 Adipocytes
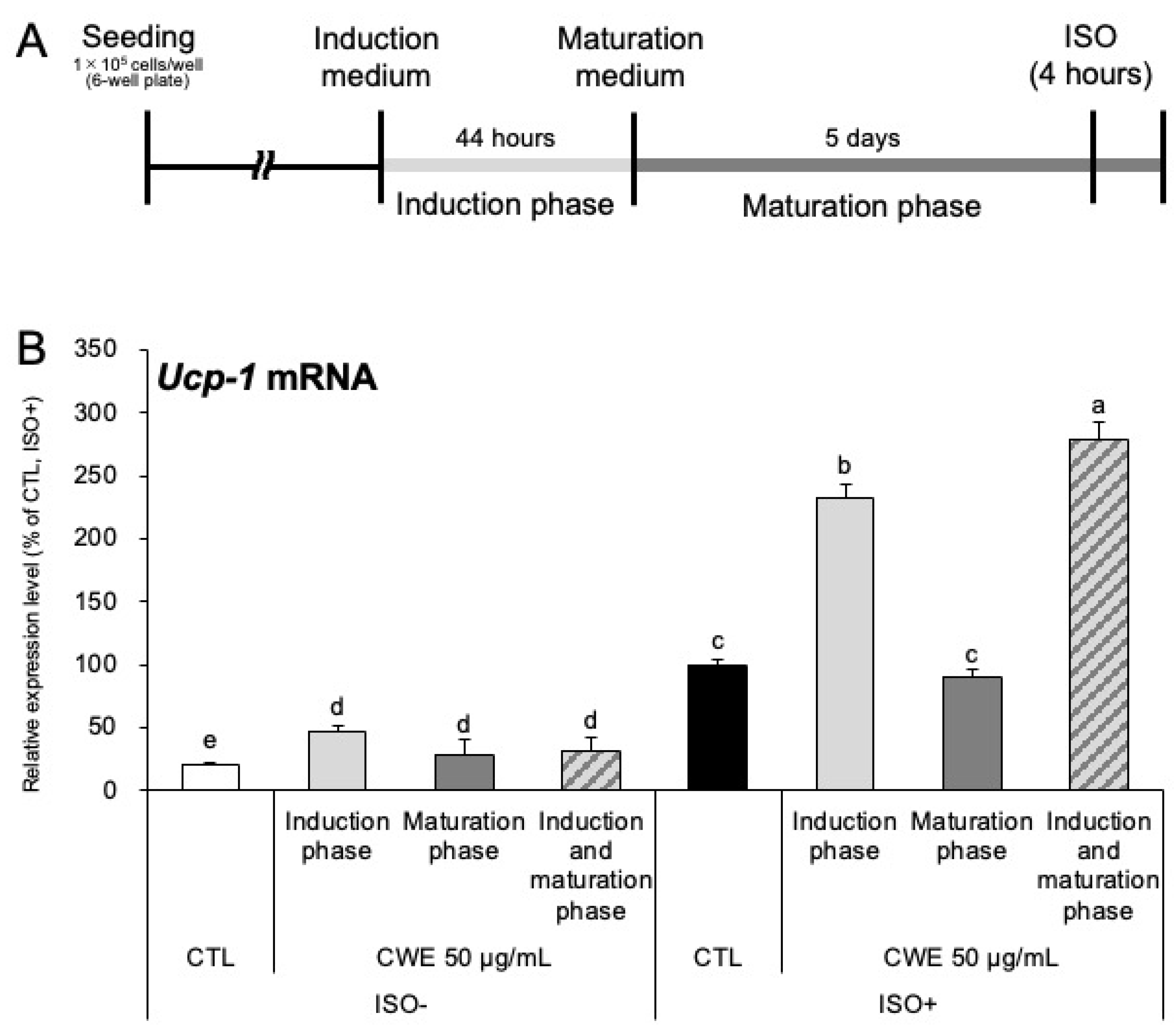
2.2. CWE Enhances Adrenergic Sensitivity During the Induction Phase of Adipocyte Differentiation
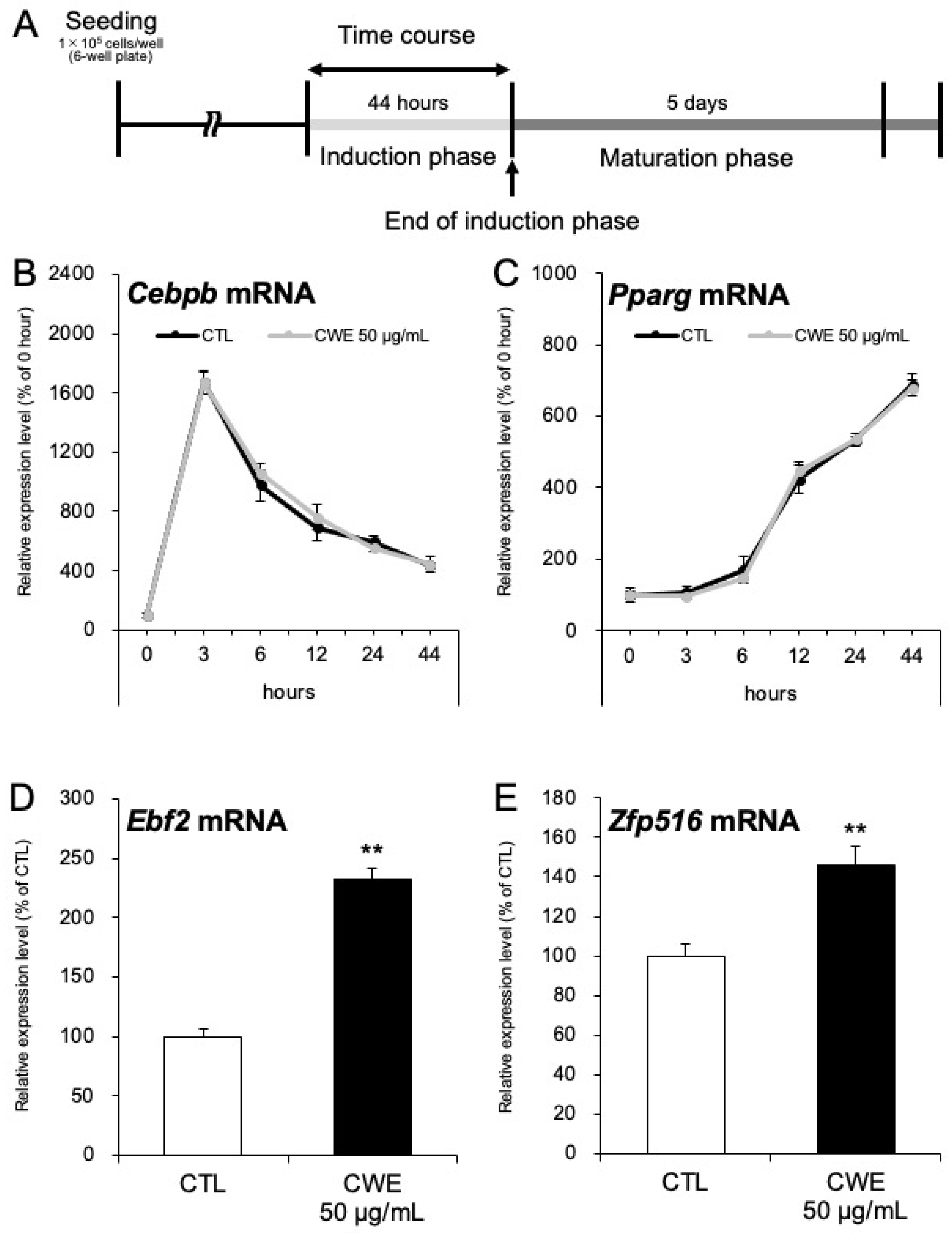
2.3. CWE Promotes Beige Adipocyte Differentiation During the Induction Phase
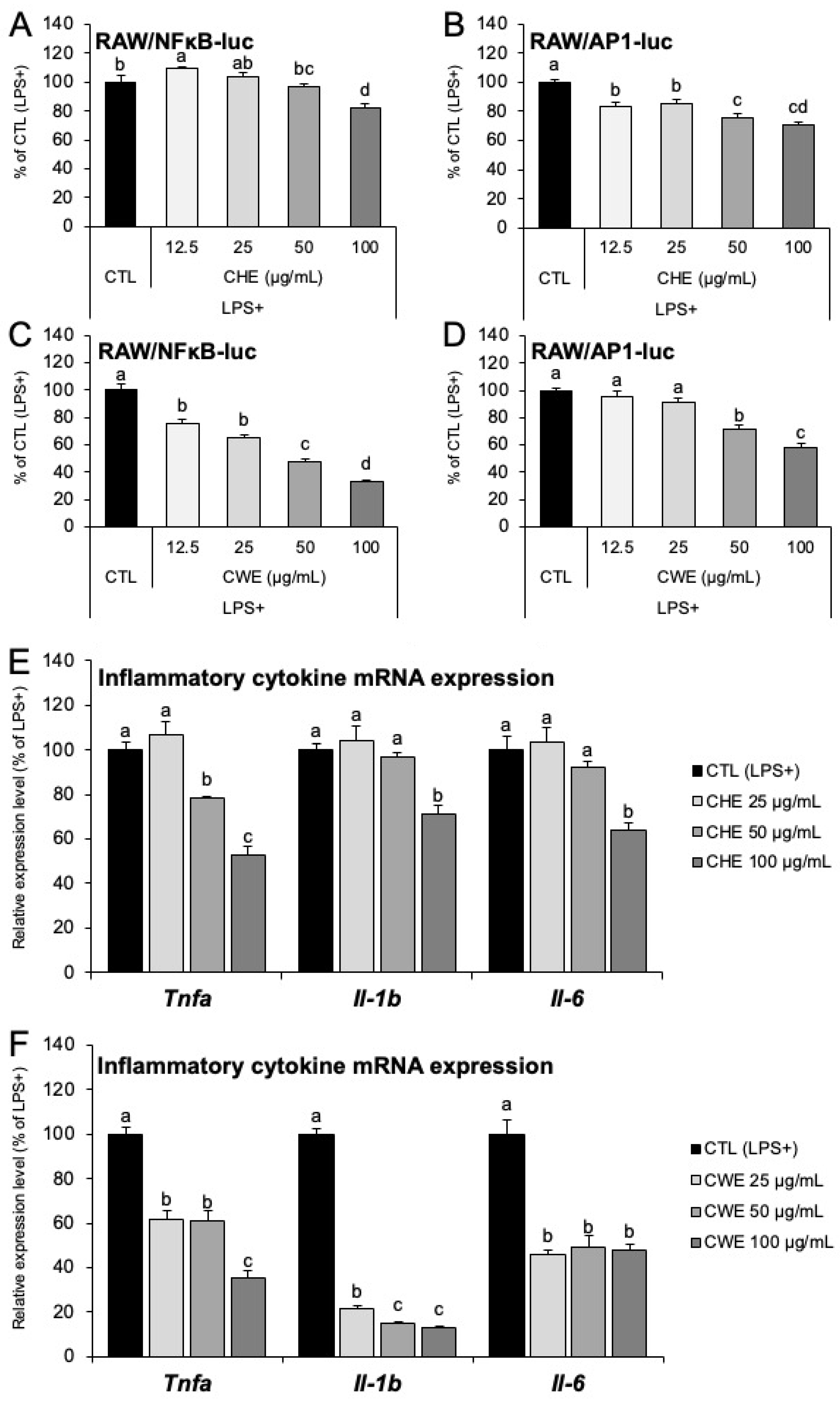
2.4. CHE and CWE Inhibit AP-1/NF-κB Activity and Suppress Inflammatory Gene Expression in RAW264.7 Macrophages
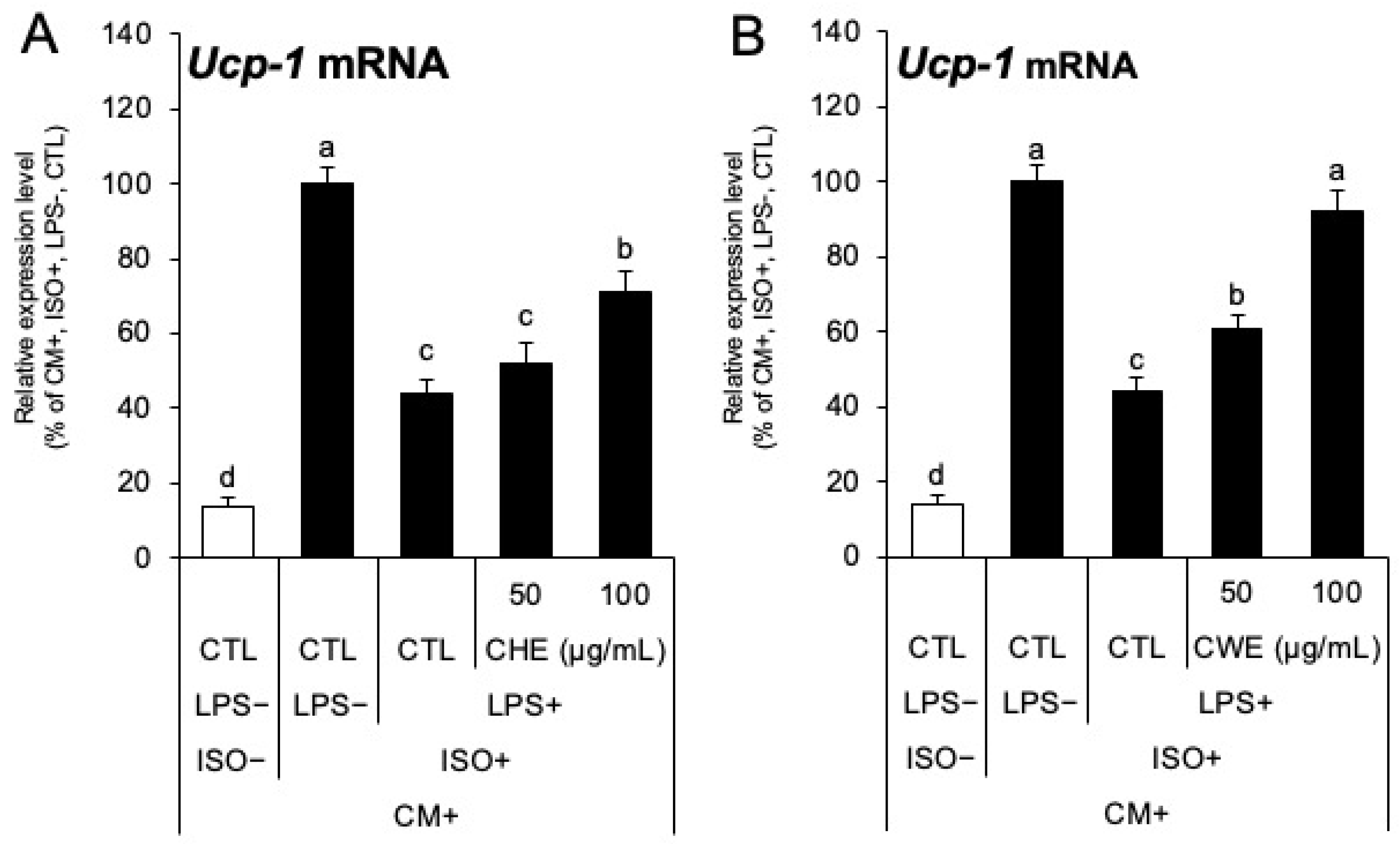
2.5. Anti-Inflammatory Effects of C. carvi Extracts Restore UCP-1 Expression Suppressed by Inflammation
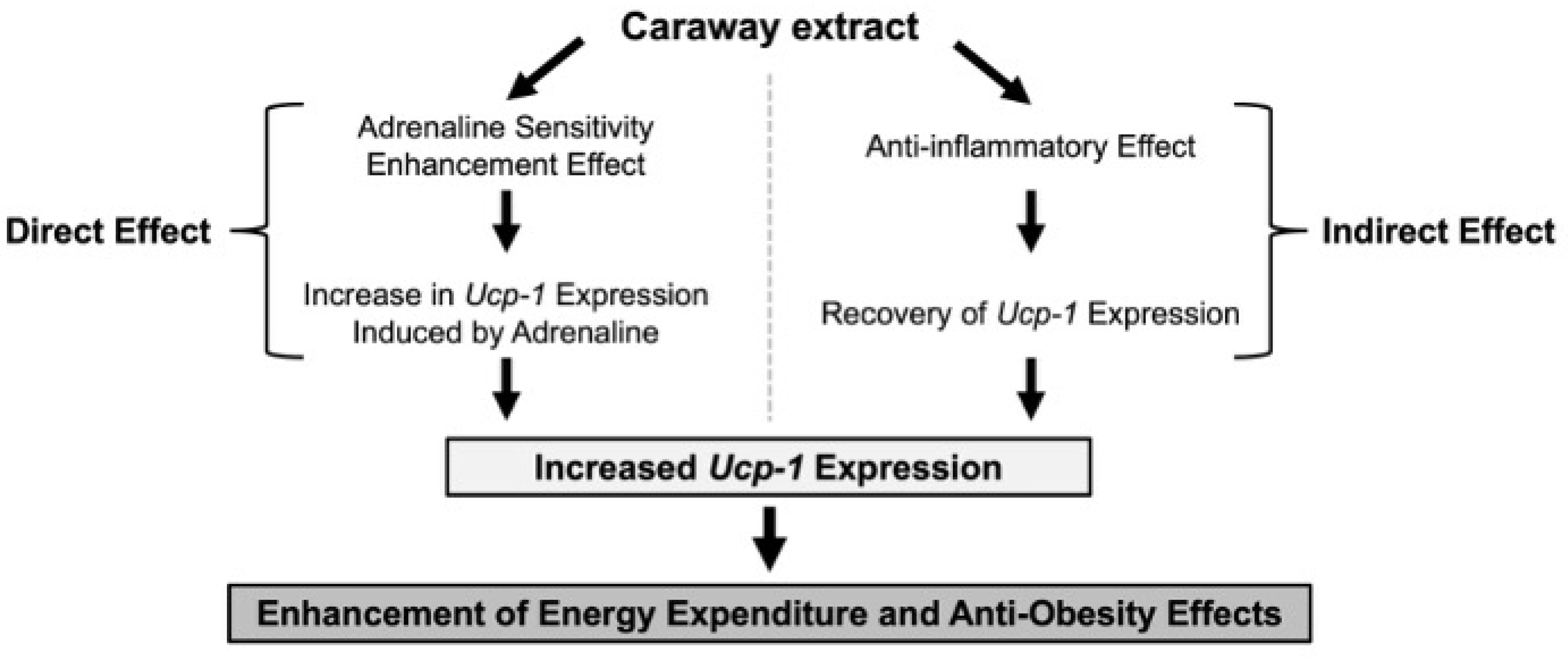
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Treatment of C3H10T1/2 Cells with Extracts
4.4. Treatment of RAW264.7 Macrophages with Extracts
4.5. Treatment of C3H10T1/2 Adipocytes with RAW264.7-Conditioned Medium
4.6. Preparation of Extracts
4.7. RNA Preparation and Quantitative RT-PCR Analysis
4.8. Luciferase Ligand Assay
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Kopecky, J.; Clarke, G.; Enerbäck, S.; Spiegelman, B.; Kozak, L.P. Expression of the Mitochondrial Uncoupling Protein Gene from the aP2 Gene Promoter Prevents Genetic Obesity. J. Clin. Investig. 1995, 96, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Katsumura, T.; Motoi, M.; Oota, H.; Watanuki, S. Experimental Evidence Reveals the UCP1 Genotype Changes the Oxygen Consumption Attributed to Non-Shivering Thermogenesis in Humans. Sci. Rep. 2017, 7, 5570. [Google Scholar] [CrossRef] [PubMed]
- Rousset, S.; Alves-Guerra, M.-C.; Mozo, J.; Miroux, B.; Cassard-Doulcier, A.-M.; Bouillaud, F.; Ricquier, D. The biology of mitochondrial uncoupling proteins. Diabetes 2004, 53 (Suppl. S1), S130–S135. [Google Scholar] [CrossRef]
- Sidossis, L.; Kajimura, S. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Investig. 2015, 125, 478–486. [Google Scholar] [CrossRef]
- Sakamoto, T.; Takahashi, N.; Sawaragi, Y.; Naknukool, S.; Yu, R.; Goto, T.; Kawada, T. Inflammation induced by RAW macrophages suppresses UCP1 mRNA induction via ERK activation in 10T1/2 adipocytes. Am. J. Physiol. Cell Physiol. 2013, 304, C729–C738. [Google Scholar] [CrossRef]
- Sakamoto, T.; Nitta, T.; Maruno, K.; Yeh, Y.-S.; Kuwata, H.; Tomita, K.; Goto, T.; Takahashi, N.; Kawada, T. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E676–E687. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Naknukool, S.; Yoshitake, R.; Hanafusa, Y.; Tokiwa, S.; Li, Y.; Sakamoto, T.; Nitta, T.; Kim, M.; Takahashi, N.; et al. Proinflammatory cytokine interleukin-1β suppresses cold-induced thermogenesis in adipocytes. Cytokine 2016, 77, 107–114. [Google Scholar] [CrossRef]
- El Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Food ingredients involved in white-to-brown adipose tissue conversion and in calorie burning. Front. Physiol. 2018, 9, 1954. [Google Scholar] [CrossRef]
- Kim, N.; Nam, M.; Kang, M.S.; Lee, J.O.; Lee, Y.W.; Hwang, G.-S.; Kim, H.S. Piperine regulates UCP1 through the AMPK pathway by generating intracellular lactate production in muscle cells. Sci. Rep. 2017, 7, 41066. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Horiguchi-Babamoto, E.; Suzuki, M.; Makihara, H.; Tomozawa, H.; Tsubata, M.; Shimada, T.; Sugiyama, K.; Aburada, M. Effects of ethyl acetate extract of Kaempferia parviflora on brown adipose tissue. J. Nat. Med. 2016, 70, 54–61. [Google Scholar] [CrossRef]
- Kim, M.; Goto, T.; Yu, R.; Uchida, K.; Tominaga, M.; Kano, Y.; Takahashi, N.; Kawada, T. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci. Rep. 2015, 5, 18013. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef]
- Lee, M.-S.; Shin, Y.; Jung, S.; Kim, Y. Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice. Food Nutr. Res. 2017, 61, 1325307. [Google Scholar] [CrossRef]
- Hara, H.; Takahashi, H.; Mohri, S.; Murakami, H.; Kawarasaki, S.; Iwase, M.; Takahashi, N.; Sugiura, M.; Goto, T.; Kawada, T. β-Cryptoxanthin Induces UCP-1 Expression via a RAR Pathway in Adipose Tissue. J. Agric. Food Chem. 2019, 67, 10595–10603. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Lee, N.H.; Hyun, C.-G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef]
- Seddighfar, M.; Mirghazanfari, S.M.; Dadpay, M. Analgesic and anti-inflammatory properties of hydroalcoholic extracts of Malva sylvestris, Carum carvi or Medicago sativa, and their combination in a rat model. J. Integr. Med. 2020, 18, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Neves, B.M.; Leitão, A.J.; Mendes, A.F. Molecular mechanisms underlying the anti-inflammatory properties of (R)-(-)-carvone: Potential roles of JNK1, Nrf2 and NF-κB. Pharmaceutics 2023, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, A.; Minaiyan, M.; Ghannadi, A.; Mahzouni, P. Effects of Carum carvi L. (Caraway) Extract and Essential Oil on TNBS-Induced Colitis in Rats. Res. Pharm. Sci. 2013, 8, 1–8. [Google Scholar]
- Takahashi, A.; Adachi, S.; Morita, M.; Tokumasu, M.; Natsume, T.; Suzuki, T.; Yamamoto, T. Post-Transcriptional Stabilization of Ucp1 mRNA Protects Mice from Diet-Induced Obesity. Cell Rep. 2015, 13, 2756–2767. [Google Scholar] [CrossRef]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Iida, S.; Chen, W.; Nakadai, T.; Ohkuma, Y.; Roeder, R.G. PRDM16 enhances nuclear receptor-dependent transcription of the brown fat-specific Ucp1 gene through interactions with Mediator subunit MED1. Genes Dev. 2015, 29, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Roemmich, J.N.; Claycombe, K.J. Evaluation of markers of beige adipocytes in white adipose tissue of the mouse. Nutr. Metab. 2016, 13, 24. [Google Scholar] [CrossRef]
- Hoang, A.C.; Yu, H.; Röszer, T. Transcriptional landscaping identifies a beige adipocyte depot in the newborn mouse. Cells 2021, 10, 2368. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; Da Silva, D.S.; Farias, G.R.; De Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Tabuchi, C.; Sul, H.S. Signaling Pathways Regulating Thermogenesis. Front. Endocrinol. 2021, 12, 595020. [Google Scholar] [CrossRef]
- Stine, R.R.; Shapira, S.N.; Lim, H.-W.; Ishibashi, J.; Harms, M.; Won, K.-J.; Seale, P. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol. Metab. 2016, 5, 57–65. [Google Scholar] [CrossRef]
- Angueira, A.R.; Shapira, S.N.; Ishibashi, J.; Sampat, S.; Sostre-Colón, J.; Emmett, M.J.; Titchenell, P.M.; Lazar, M.A.; Lim, H.W.; Seale, P. Early B cell factor activity controls developmental and adaptive thermogenic gene programming in adipocytes. Cell Rep. 2020, 30, 2869–2878.e4. [Google Scholar] [CrossRef]
- Dempersmier, J.; Sambeat, A.; Gulyaeva, O.; Paul, S.M.; Hudak, C.S.S.; Raposo, H.F.; Kwan, H.Y.; Kang, C.; Wong, R.H.F.; Sul, H.S. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol. Cell 2015, 57, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Morimoto, H.; Tanaka, M.; Inoue, H.; Goto, T.; Kawada, T.; Uehara, M.; Takahashi, N. Myricetin and myricitrin indirectly and directly increases uncoupling protein-1 mRNA expression in C3H10T1/2 beige adipocytes. Biochem. Biophys. Res. Commun. 2024, 734, 150771. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Ishikawa, T.; Kitajima, J. Water-soluble constituents of caraway: Aromatic compound, aromatic compound glucoside and glucides. Phytochemistry 2002, 61, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Choi, H.-J.; Chung, M.-S.; Ko, M.-J. Selective extraction of oxygenated terpene in caraway (Carum carvi L.) using subcritical water extraction (SWE) technique. Food Chem. 2022, 381, 132192. [Google Scholar] [CrossRef]
- Nøhr, M.K.; Bobba, N.; Richelsen, B.; Lund, S.; Pedersen, S.B. Inflammation downregulates UCP1 expression in brown adipocytes potentially via SIRT1 and DBC1 interaction. Int. J. Mol. Sci. 2017, 18, 1006. [Google Scholar] [CrossRef]
- Alomar, H.A.; Fathallah, N.; Abdel-Aziz, M.M.; Ibrahim, T.A.; Elkady, W.M. GC-MS profiling, anti-Helicobacter pylori, and anti-inflammatory activities of three apiaceous fruits’ essential oils. Plants 2022, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Morikawa, M.; Ozaki, E.; Numasaki, M.; Morimoto, H.; Tanaka, M.; Inoue, H.; Goto, T.; Kawada, T.; Eguchi, F.; et al. A modified system using macrophage-conditioned medium revealed that the indirect effects of anti-inflammatory food-derived compounds improve inflammation-induced suppression of UCP-1 mRNA expression in 10T1/2 adipocytes. Biosci. Biotechnol. Biochem. 2024, 88, 679–688. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, H.; Tomishima, N.; Suzuki, T.; Morimoto, H.; Inoue, H.; Kaneko, K.; Goto, T.; Kawada, T.; Uehara, M.; Takahashi, N. Caraway Extract Increases Ucp-1 mRNA Expression in C3H10T1/2 Adipocytes Through Direct and Indirect Effects. Int. J. Mol. Sci. 2025, 26, 10970. https://doi.org/10.3390/ijms262210970
Takahashi H, Tomishima N, Suzuki T, Morimoto H, Inoue H, Kaneko K, Goto T, Kawada T, Uehara M, Takahashi N. Caraway Extract Increases Ucp-1 mRNA Expression in C3H10T1/2 Adipocytes Through Direct and Indirect Effects. International Journal of Molecular Sciences. 2025; 26(22):10970. https://doi.org/10.3390/ijms262210970
Chicago/Turabian StyleTakahashi, Hisako, Nanami Tomishima, Toshihiro Suzuki, Hiromu Morimoto, Hirofumi Inoue, Kentaro Kaneko, Tsuyoshi Goto, Teruo Kawada, Mariko Uehara, and Nobuyuki Takahashi. 2025. "Caraway Extract Increases Ucp-1 mRNA Expression in C3H10T1/2 Adipocytes Through Direct and Indirect Effects" International Journal of Molecular Sciences 26, no. 22: 10970. https://doi.org/10.3390/ijms262210970
APA StyleTakahashi, H., Tomishima, N., Suzuki, T., Morimoto, H., Inoue, H., Kaneko, K., Goto, T., Kawada, T., Uehara, M., & Takahashi, N. (2025). Caraway Extract Increases Ucp-1 mRNA Expression in C3H10T1/2 Adipocytes Through Direct and Indirect Effects. International Journal of Molecular Sciences, 26(22), 10970. https://doi.org/10.3390/ijms262210970






