Synthesis and Evaluation of Powerful Antioxidant Dendrimers Derived from D-Mannitol and Syringaldehyde
Abstract
1. Introduction
2. Results
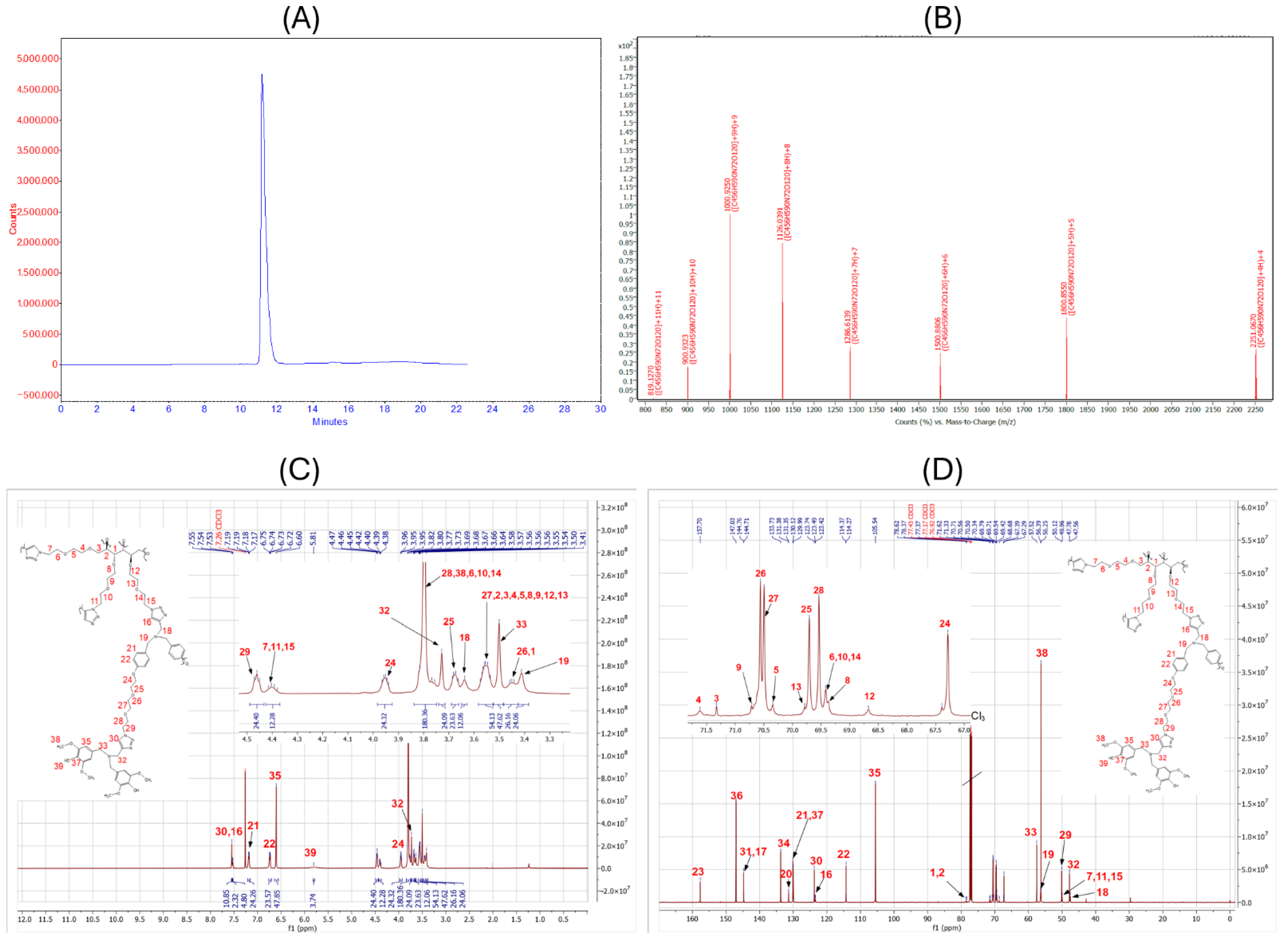
2.1. Chemistry
2.2. DPPH Assay Results
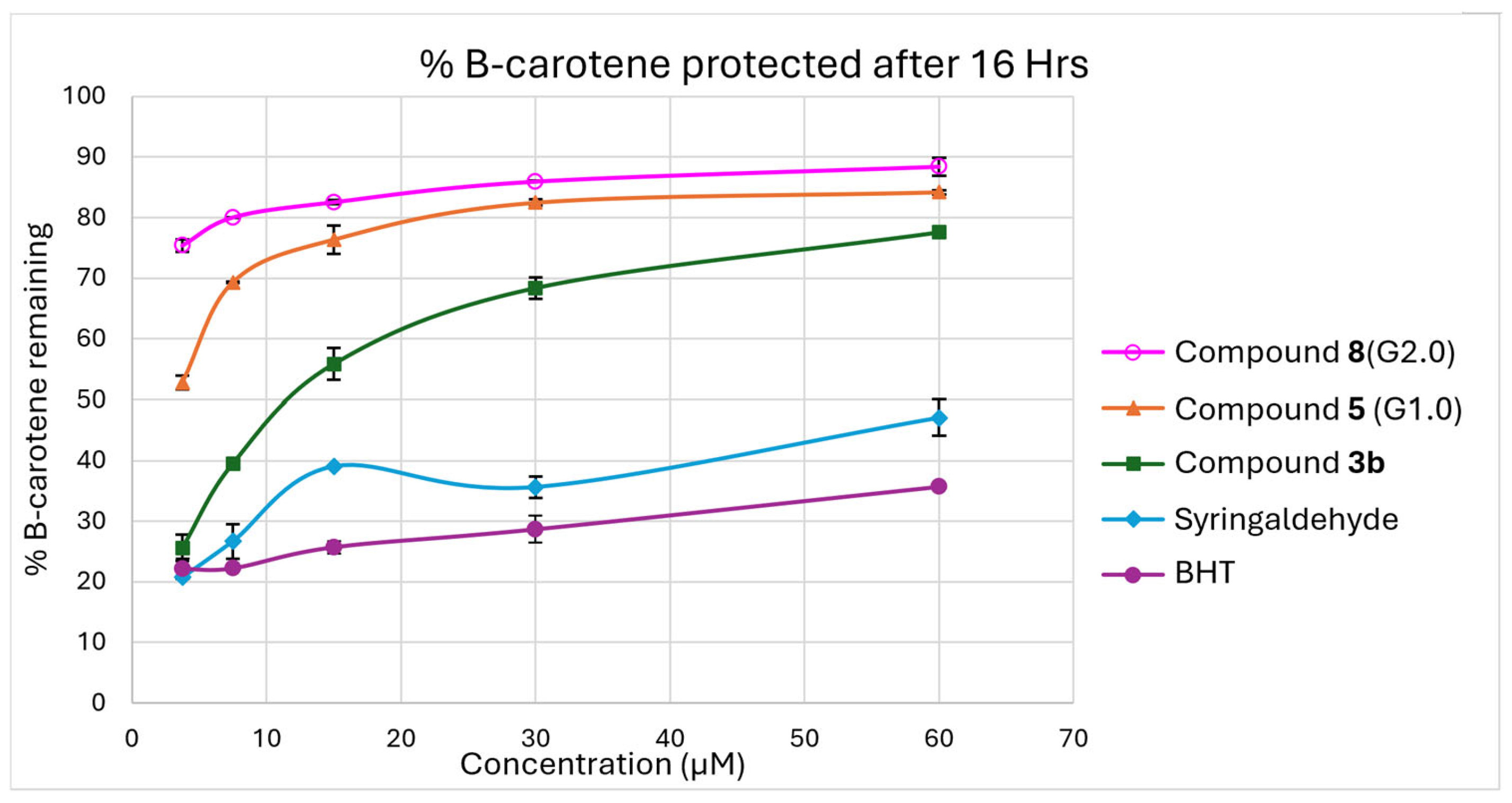
2.3. β-Carotene Bleaching Assay Results
2.4. Cell Viability Assay Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. General Information for Synthesis and Purification
4.3. Characterization
4.4. DPPH Assay
4.5. β-Carotene Bleaching Assay
4.6. Cell Viability Assay
4.7. Synthesis Methods and Analysis Results
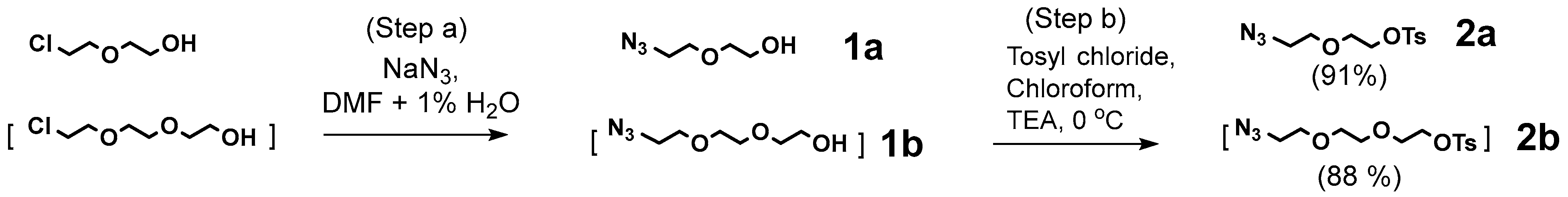
4.7.1. Compound 2a
4.7.2. Compound 2b
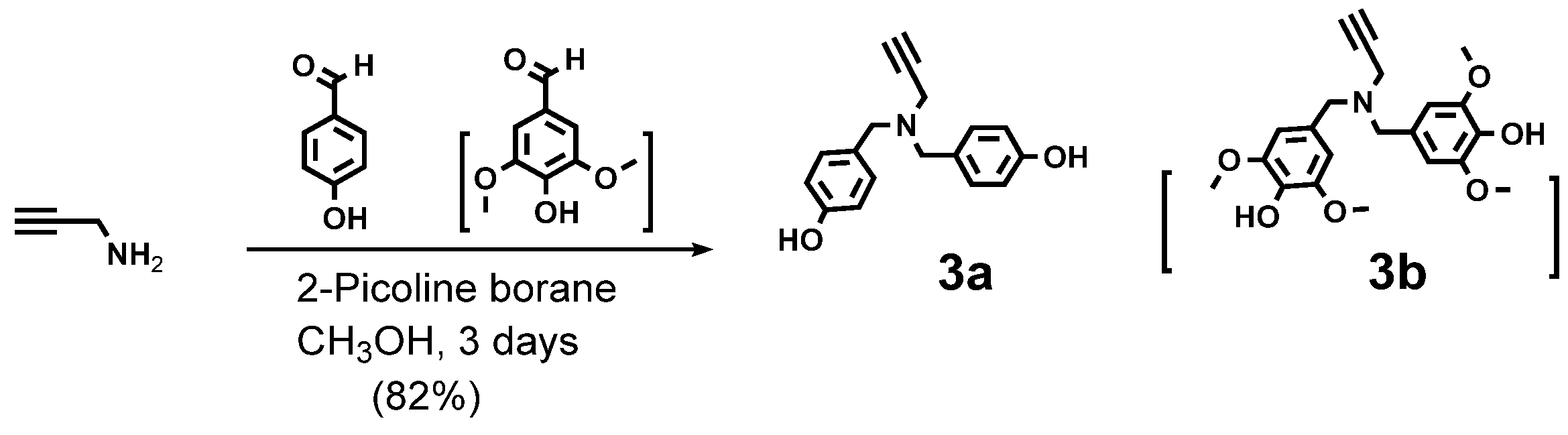
4.7.3. Compound 3a (Internal BB)
4.7.4. Compound 3b (Surface BB)
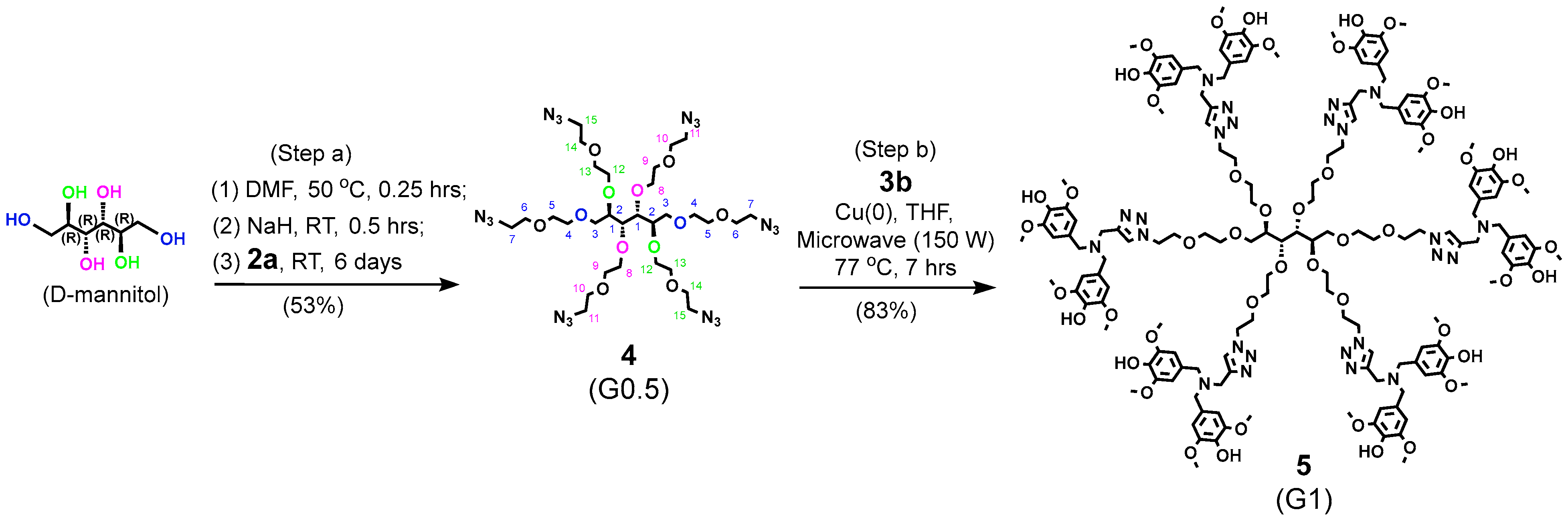
4.7.5. Compound 4 (G0.5)
4.7.6. Compound 5 (G1)
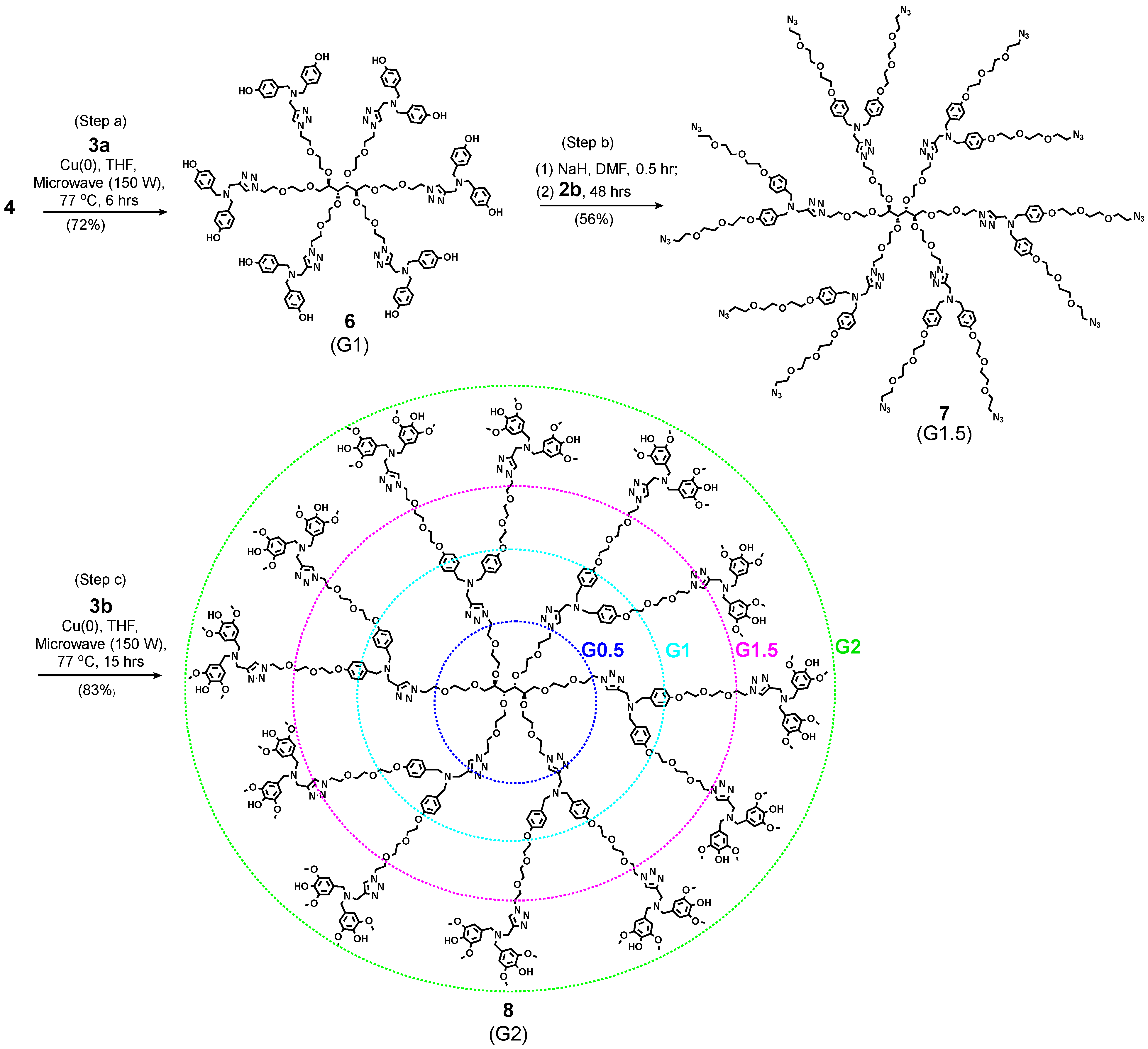
4.7.7. Compound 6 (G1)
4.7.8. Compound 7 (G1.5)
4.7.9. Compound 8 (G2)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Acknowledgments
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Robards, K.; Kerr, A.F.; Patsalides, E. Rancidity and its measurement in edible oils and snack foods. A review. Analyst 1988, 113, 213–224. [Google Scholar] [CrossRef] [PubMed]
- German, J.B. Food processing and lipid oxidation. Adv. Exp. Med. Biol. 1999, 459, 23–50. [Google Scholar] [PubMed]
- Andersen, F.A. Final report of the safety assessment of L-ascorbic acid, calcium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate as used in cosmetics. Int. J. Toxicol. 2005, 24, 51–111. [Google Scholar]
- Oh, S.; Stache, E.E. Recent advances in oxidative degradation of plastics. Chem. Soc. Rev. 2024, 53, 7309–7327. [Google Scholar] [CrossRef]
- Sharma, H.; Reshu Rajput, R. The science of food preservation: A comprehensive review of synthetic preservatives. J. Curr. Res. Food Sci. 2023, 4, 25–29. [Google Scholar]
- Petcu, C.D.; Tăpăloagă, D.; Mihai, O.D.; Gheorghe-Irimia, R.A.; Negoiță, C.; Georgescu, I.M.; Tăpăloagă, P.R.; Borda, C.; Ghimpețeanu, O.M. Harnessing natural antioxidants for enhancing food shelf life: Exploring sources and applications in the food industry. Foods 2023, 12, 3176. [Google Scholar] [CrossRef]
- Ramalingam, S.; Govindasamy, M.; Rajendran, S. The influence of natural and synthetic antioxidant on oxidation stability and emission of sapota oil methyl ester as fuel in CI engine. Therm. Sci. 2016, 20, S991–S997. [Google Scholar] [CrossRef]
- Pritchard, G. Plastics Additives: An A-Z Reference; Springer: Berlin/Heidelberg, Germany, 1998; pp. 64–68. [Google Scholar]
- He, J.B.; Shi, H.; Wang, Y.; Gao, X.L. Synthesis, characterization, and performance evaluation of sulfur-containing diphenylamines based on intramolecular synergism. Molecules 2018, 23, 401. [Google Scholar] [CrossRef]
- Anghinoni, J.M.; Birmann, P.T.; da Rocha, M.J.; Gomes, C.S.; Davies, M.J.; Brüning, C.A.; Savegnago, L.; Lenardão, E.J. Recent advances in the synthesis and antioxidant activity of low molecular mass organoselenium molecules. Molecules 2023, 28, 7349. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.N.E.; Krayem, N.; Derbala, H.A.; Kamal, S.; Bukhari, S.N.A.; El-Ashrey, M.K.; Almarhoon, Z.M.; Soliman Alterary, S.; Ben Bacha, A. New nitrogen-, oxygen-, and sulfur-containing heterocyclic compounds as anti-colon cancer agents: Synthesis, multitargeted evaluations, molecular docking simulations and ADMET predictions. Pharmaceuticals 2025, 18, 801. [Google Scholar] [CrossRef] [PubMed]
- Odrobińska, J.; Neugebauer, D. PEG grafted polymethacrylates bearing antioxidants as a new class of polymer conjugates for application in cosmetology. Materials 2020, 13, 3455. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Morelli, C.; Cirillo, G.; Curcio, M.; Parisi, O.I.; Maris, P.; Sisci, D.; Picci, N. Anticancer activity of a quercetin-based polymer towards HeLa cancer cells. Anticancer Res. 2012, 32, 2843–2847. [Google Scholar]
- Sunoqrot, S.; Al-Debsi, T.; Al-Shalabi, E.; Ibrahim, L.H.; Faruqu, F.N.; Walters, A.; Palgrave, R.G.; Al-Jamal, K.T. Bioinspired polymerization of quercetin to produce a curcumin-loaded nanomedicine with potent cytotoxicity and cancer-targeting potential in vivo. ACS Biomater. Sci. Eng. 2019, 5, 6036–6045. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, S.K.; Ahn, C.B.; Je, J.Y. Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr. Polym. 2011, 83, 1617–1622. [Google Scholar] [CrossRef]
- Dewangan, H.K.; Sharma, R.; Shah, K.; Alam, P. Development of FA-conjugated PAMAM dendrimer as potential antioxidant therapy for cancer cells. J. Mater. Res. 2025, 40, 1405–1416. [Google Scholar] [CrossRef]
- Bacha, K.; Estager, J.; Brassart-Pasco, S.; Chemotti, C.; Fernandes, A.E.; Mbakidi, J.P.; Deleu, M.; Bouquillon, S. Synthesis and activity of ionic antioxidant-functionalized PAMAMs and PPIs dendrimers. Polymers 2022, 14, 3513. [Google Scholar] [CrossRef]
- Stadler, J.; Garmo, L.G.; Doyle, D.; Cheng, C.I.; Richardson, G.; Waheed, Z.; Tofan, T.; Srinageshwar, B.; Sharma, A.; Petersen, R.B.; et al. Curcumin encapsulated in PAMAM dendrimers for the therapeutic treatment of ischemic stroke in rats. Front. Cell Dev. Biol. 2025, 12, 1467417. [Google Scholar] [CrossRef]
- Bacha, K.; Chemotti, C.; Monboisse, J.C.; Robert, A.; Furlan, A.L.; Smeralda, W.; Damblon, C.; Estager, J.; Brassart-Pasco, S.; Mbakidi, J.P.; et al. Encapsulation of vitamin C by glycerol-derived dendrimers, their interaction with biomimetic models of Stratum corneum and their cytotoxicity. Molecules 2022, 27, 8022. [Google Scholar] [CrossRef]
- Boisselier, E.; Liang, L.; Dalko-Csiba, M.; Ruiz, J.; Astruc, D. Interactions and encapsulation of vitamins C, B3, and B6 with dendrimers in water. Chemistry 2010, 16, 6056–6068. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating antioxidant functionality into polymer materials: Fundamentals, strategies, and applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395. [Google Scholar] [CrossRef] [PubMed]
- Bacha, K.; Chemotti, C.; Mbakidi, J.P.; Deleu, M.; Bouquillon, S. Dendrimers: Synthesis, encapsulation applications and specific interaction with the Stratum Corneum—A review. Macromol 2023, 3, 343–370. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress”. Drug Deliv. Transl. Res. 2020, 10, 259–270. [Google Scholar] [CrossRef]
- Grodzicka, M.; Michlewska, S.; Buczkowski, A.; Sekowski, S.; Pena-Gonzalez, C.E.; Ortega, P.; de la Mata, F.J.; Blasiak, J.; Bryszewska, M.; Ionov, M. A new class of polyphenolic carbosilane dendrimers binds human serum albumin in a structure-dependent fashion. Sci. Rep. 2024, 14, 5946. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Cheong, J.E.; Nelson, J.E. Synthesis and antioxidant properties of dendritic polyphenols. Bioorg. Med. Chem. Lett. 2009, 19, 6326–6330. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Uzarski, R.L.; Cheong, J.E.; Xu, H.; Held, R.A.; Upadhaya, S.K.; Nelson, J.L. Potent antioxidant dendrimers lacking pro-oxidant activity. Free Radic. Biol. Med. 2011, 50, 918–925. [Google Scholar] [CrossRef]
- Lee, C.Y.; Nanah, C.; Held, R.; Clark, A.; Huynh, U.; Pressnall, M.; Uzarski, R.L.; McGraken, J.; Sharma, A. Effect of electron donating groups on polyphenol-based antioxidant dendrimers. Biochimie 2015, 111, 125–134. [Google Scholar] [CrossRef]
- Cornea, A.C.; Marc, G.; Ionuț, I.; Moldovan, C.; Stana, A.; Oniga, S.D.; Pîrnău, A.; Vlase, L.; Oniga, I.; Oniga, O. Synthesis, characterization, and antioxidant activity evaluation of new N-methyl substituted thiazole-derived polyphenolic compounds. Molecules 2025, 30, 1345. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Semenya, J.; Anamoah, C.; Chapman, K.N.; Barone, V. Computational study of ortho-substituent effects on antioxidant activities of phenolic dendritic antioxidants. Antioxidants 2020, 9, 189. [Google Scholar] [CrossRef]
- Lee, C.Y.; Anamoah, C.; Semenya, J.; Chapman, K.N.; Knoll, A.N.; Brinkman, H.F.; Malone, J.I.; Sharma, A. Electronic (donating or withdrawing) effects of ortho-phenolic substituents in dendritic antioxidants. Tetrahedron Lett. 2020, 61, 151607. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Zhao, X.; Li, Y. Effects of nitro- and amino-group on the antioxidant activity of Genistein: A theoretical study. Food Chem. 2019, 275, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Held, R.; Sharma, A.; Baral, R.; Nanah, C.; Dumas, D.; Jenkins, S.; Upadhaya, S.; Du, W. Copper granule-catalyzed microwave-assisted click synthesis of polyphenol dendrimers. J. Org. Chem. 2013, 78, 11221–11228. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene assay revisited. Application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, L.; Meng, W.; Zhang, X.; Li, J.; Dong, P. Molecular weight distribution and structure analysis of phlorotannins in Sanhai kelp (Saccharina japonica) and evaluation of their antioxidant activities. Food Chem. 2025, 469, 142569. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. The dendritic effect. In Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012; pp. 276–292. [Google Scholar]
- Shehata, A.N.; Ashour, W.E. Competitive evaluation on antioxidant and antimicrobial properties for three different molecular weights of commercial chitosan biopolymer. Curr. Sci. Int. 2024, 13, 382–398. [Google Scholar] [CrossRef]
- Saallah, S.; Ishak, N.H.; Sarbon, N.M. Effect of different molecular weight on the antioxidant activity and physicochemical properties of golden apple snail (Ampullariidae) protein hydrolysates. Food Res. 2020, 4, 1363–1370. [Google Scholar] [CrossRef]
- Shahosseini, S.R.; Javadian, S.R.; Safari, R. Effects of molecular weights-assisted enzymatic hydrolysis on antioxidant and anticancer activities of Liza abu muscle protein hydrolysates. Int. J. Pept. Res. Ther. 2022, 28, 72. [Google Scholar] [CrossRef]
- Wang, J.M.; Sun, X.Y.; Ouyang, J.-M. Structural characterization, antioxidant activity, and biomedical application of Astragalus polysaccharide degradation products. Int. J. Polym. Sci. 2018, 5136185. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Wu, J.; Yang, X.; Yang, S.; Zhu, W.; Liu, Y.; Tang, W.; Nie, S.; Hassouna, A.; et al. Fucoidan extracted from sporophyll of Undaria Pinnatifida grown in Weihai, China—Chemical composition and comparison of antioxidant activity of different molecular weight fractions. Front. Nutr. 2021, 8, 636930. [Google Scholar] [CrossRef]
- Shojaee, M.S.; Moeenfard, M.; Farhoosh, R. Kinetics and stoichiometry of gallic acid and methyl gallate in scavenging DPPH radical as affected by the reaction solvent. Sci. Rep. 2022, 12, 8765. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural features of small molecule antioxidants and strategic modifications to improve potential bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef]
- Stoia, M.; Oancea, S. Low-molecular-weight synthetic antioxidants: Classification, pharmacological profile, effectiveness and trends. Antioxidants 2022, 11, 638. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Oliver, S.; Vittorio, O.; Cirilloe, G.; Boyer, C. Enhancing the therapeutic effects of polyphenols with macromolecules. Polym. Chem. 2016, 7, 1529–1544. [Google Scholar] [CrossRef]
- Morici, E.; Arrigo, R.; Dintcheva, N.T. Quercetin as natural stabilizing agent for bio-polymer. AIP Conf. Proc. 2014, 1599, 314–317. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Fernández-Bravo, S.; Bravo-Díaz, C. Innovative delivery and release systems for antioxidants and other active substances in the treatment of cancer. Pharmaceuticals 2023, 16, 1038. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440–112458. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Chan, Y.S.; Nandong, J. Insights into the release mechanisms of antioxidants from nanoemulsion droplets. J. Food Sci. Technol. 2022, 59, 1677–1691. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Sam, J.H.; Jeevanandam, J.; Chan, Y.S.; Nandong, J. Thermal degradation of antioxidant compounds: Effects of parameters, thermal degradation kinetics, and formulation strategies. Food Bioprocess Technol. 2022, 15, 1919–1935. [Google Scholar] [CrossRef]
- He, L.; Lou, J.; Nie, X.; He, M.; Fan, Q.; Yang, J.; Liu, Y.; Qin, S.; Yu, J. Highly enhanced toughness and thermal oxygen aging resistance of PA 6 via novel designed macromolecular antioxidants. Polymer 2024, 312, 127614. [Google Scholar] [CrossRef]
- Kurtoglu, Y.E.; Navath, R.S.; Wang, B.; Kannan, S.; Romero, R.; Kannan, R.M. Poly(amidoamine) dendrimer–drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials 2009, 30, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, A.; Roy, S.S.; Satsangi, R.K.; Vadlamudi, R.K.; Ong, J.L. Design of a paclitaxel prodrug conjugate for active targeting of an enzyme upregulated in breast cancer cells. Mol. Pharm. 2014, 11, 1906–1918. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied. Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today. 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Thomas, T.P.; Lee, K.H.; Li, M.; Zong, H.; Desai, A.M.; Kotlyar, A.; Huang, B.; Banaszak Holl, M.M.; Baker, J.R., Jr. Polyvalent saccharide-functionalized generation 3 poly(amidoamine) dendrimer-methotrexate conjugate as a potential anticancer agent. Bioorg. Med. Chem. 2011, 19, 2557–2564. [Google Scholar] [CrossRef]
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The size-dependent cytotoxicity of amorphous silica nanoparticles: A systematic review of in vitro studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Yan, Z.Y.; Yan, X.P. Size- and shape-dependent cytotoxicity of nano-sized Zr-based porphyrinic metal-organic frameworks to macrophages. Sci. Total Environ. 2022, 833, 155309. [Google Scholar] [CrossRef] [PubMed]
- Awashra, M.; Młynarz, P. The toxicity of nanoparticles and their interaction with cells: An in vitro metabolomic perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D.G. The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res. Lett. 2009, 4, 1409–1420. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, L.; Thompson, D.M.; Montoya, L.D. Effect of particle size on in vitro cytotoxicity of titania and alumina nanoparticles. J. Exp. Nanosci. 2014, 9, 625–638. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yan, Y.; Zhang, J.; Li, Z. Understanding the role of hydrophobicity arrangement in cellular uptake of synthetic virus-like nanoparticles. Chem. Phys. Lett. 2021, 766, 138336. [Google Scholar] [CrossRef]
- Muthukumarasamyvel, T.; Rajendran, G.; Santhana Panneer, D.; Kasthuri, J.; Kathiravan, K.; Rajendiran, N. Role of surface hydrophobicity of dicationic amphiphile stabilized gold nanoparticles on A549 lung cancer cells. ACS Omega 2017, 2, 3527–3538. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Y.; Bai, X.; Zhou, X.; Zhou, H.; Liu, S.; Yan, B. Induction of oxidative stress and sensitization of cancer cells to paclitaxel by gold nanoparticles with different charge densities and hydrophobicities. J. Mater. Chem. B 2018, 6, 1633–1639. [Google Scholar] [CrossRef]
- Menot, B.; Stopinski, J.; Martinez, A.; Oudart, J.B.; Maquart, F.X.; Bouquillon, S. Synthesis of surface-modified PAMAMs and PPIs for encapsulation purposes: Influence of the decoration on their sizes and toxicity. Tetrahedron 2015, 71, 3439–3446. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
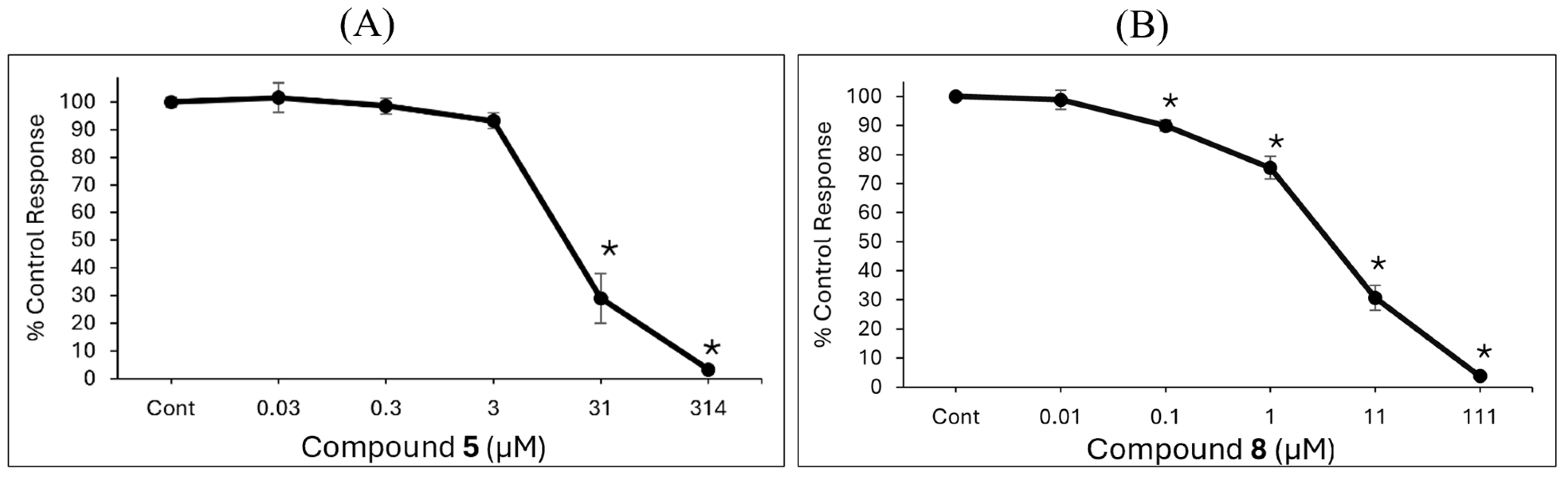
| Antioxidant | IC50 (µM) |
|---|---|
| Compound 8 (G2) | 0.7 |
| Compound 5 (G1) | 1.36 |
| Syringaldehyde | 260 |
| BHT | 880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbemade, B.; Clark, A.R.; Nanah, C.N.; Haruna, F.; Stengard, A.E.; Medes, S.A.; Lapratt, A.M.; Morehouse, S.L.; Uzarski, R.L.; Lee, C.Y. Synthesis and Evaluation of Powerful Antioxidant Dendrimers Derived from D-Mannitol and Syringaldehyde. Int. J. Mol. Sci. 2025, 26, 10966. https://doi.org/10.3390/ijms262210966
Agbemade B, Clark AR, Nanah CN, Haruna F, Stengard AE, Medes SA, Lapratt AM, Morehouse SL, Uzarski RL, Lee CY. Synthesis and Evaluation of Powerful Antioxidant Dendrimers Derived from D-Mannitol and Syringaldehyde. International Journal of Molecular Sciences. 2025; 26(22):10966. https://doi.org/10.3390/ijms262210966
Chicago/Turabian StyleAgbemade, Blessed, Amanda R. Clark, Cyprien N. Nanah, Fati Haruna, Aundrea E. Stengard, Skylar A. Medes, Ashlyn M. Lapratt, Samara L. Morehouse, Rebecca L. Uzarski, and Choon Young Lee. 2025. "Synthesis and Evaluation of Powerful Antioxidant Dendrimers Derived from D-Mannitol and Syringaldehyde" International Journal of Molecular Sciences 26, no. 22: 10966. https://doi.org/10.3390/ijms262210966
APA StyleAgbemade, B., Clark, A. R., Nanah, C. N., Haruna, F., Stengard, A. E., Medes, S. A., Lapratt, A. M., Morehouse, S. L., Uzarski, R. L., & Lee, C. Y. (2025). Synthesis and Evaluation of Powerful Antioxidant Dendrimers Derived from D-Mannitol and Syringaldehyde. International Journal of Molecular Sciences, 26(22), 10966. https://doi.org/10.3390/ijms262210966






