Spectroscopic Probing of Solute–Solvent Interactions in Aqueous Methylsulphonylmethane (MSM) Solutions: An Integrated ATR-FTIR, Chemometric, and DFT Study
Abstract
1. Introduction
2. Results and Discussion
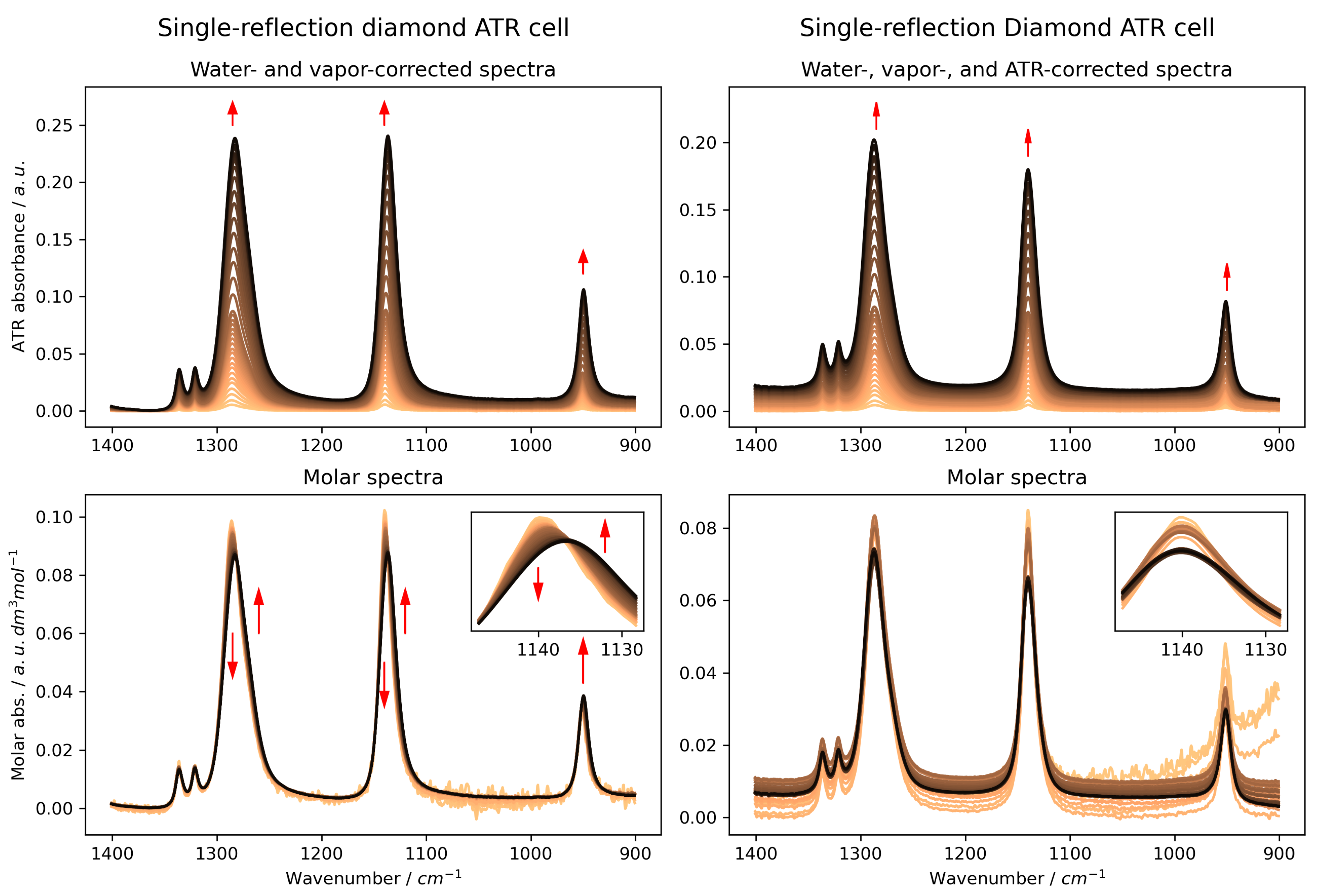
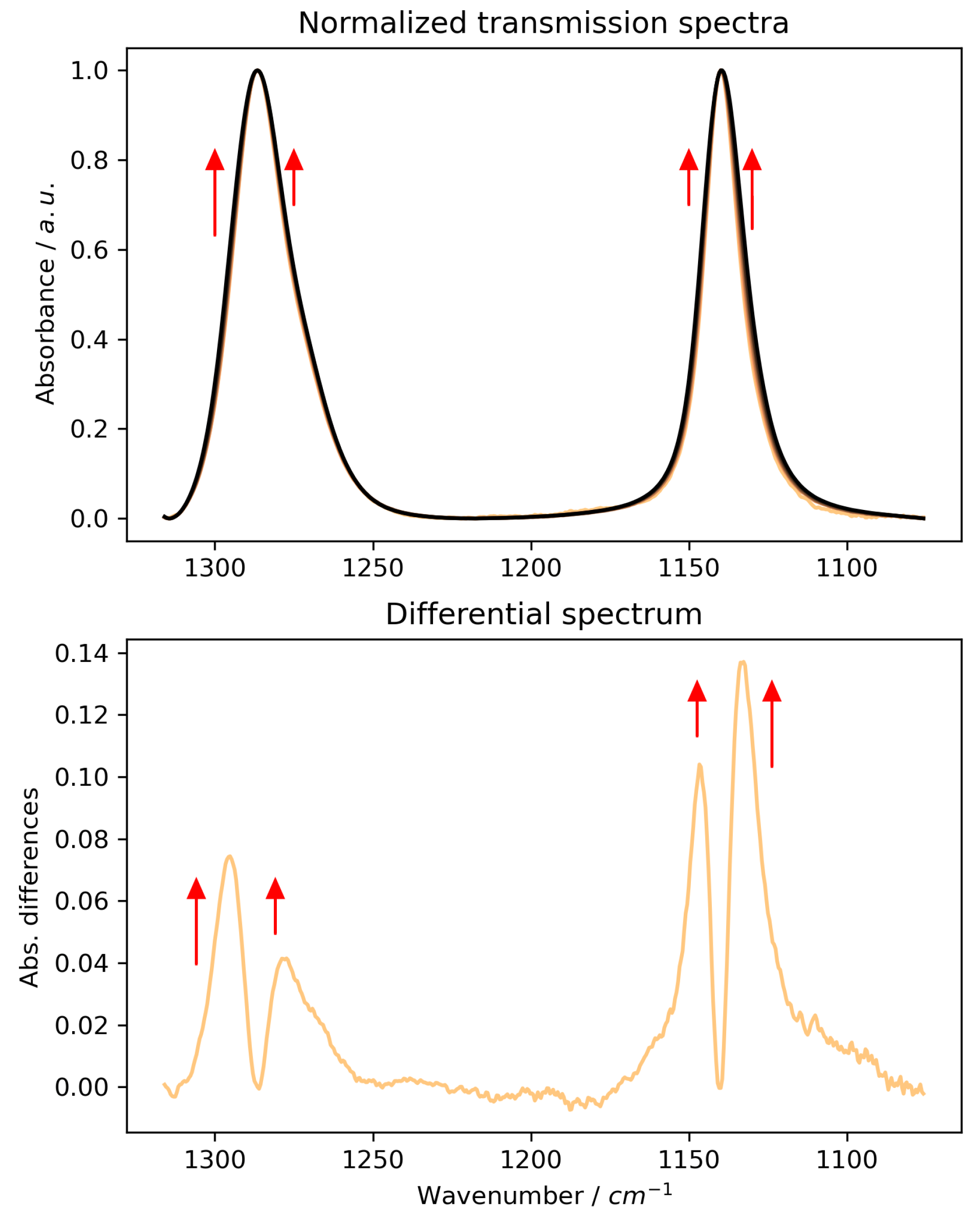
2.1. ATR-FTIR Spectra of MSM: Artefacts Versus Reality
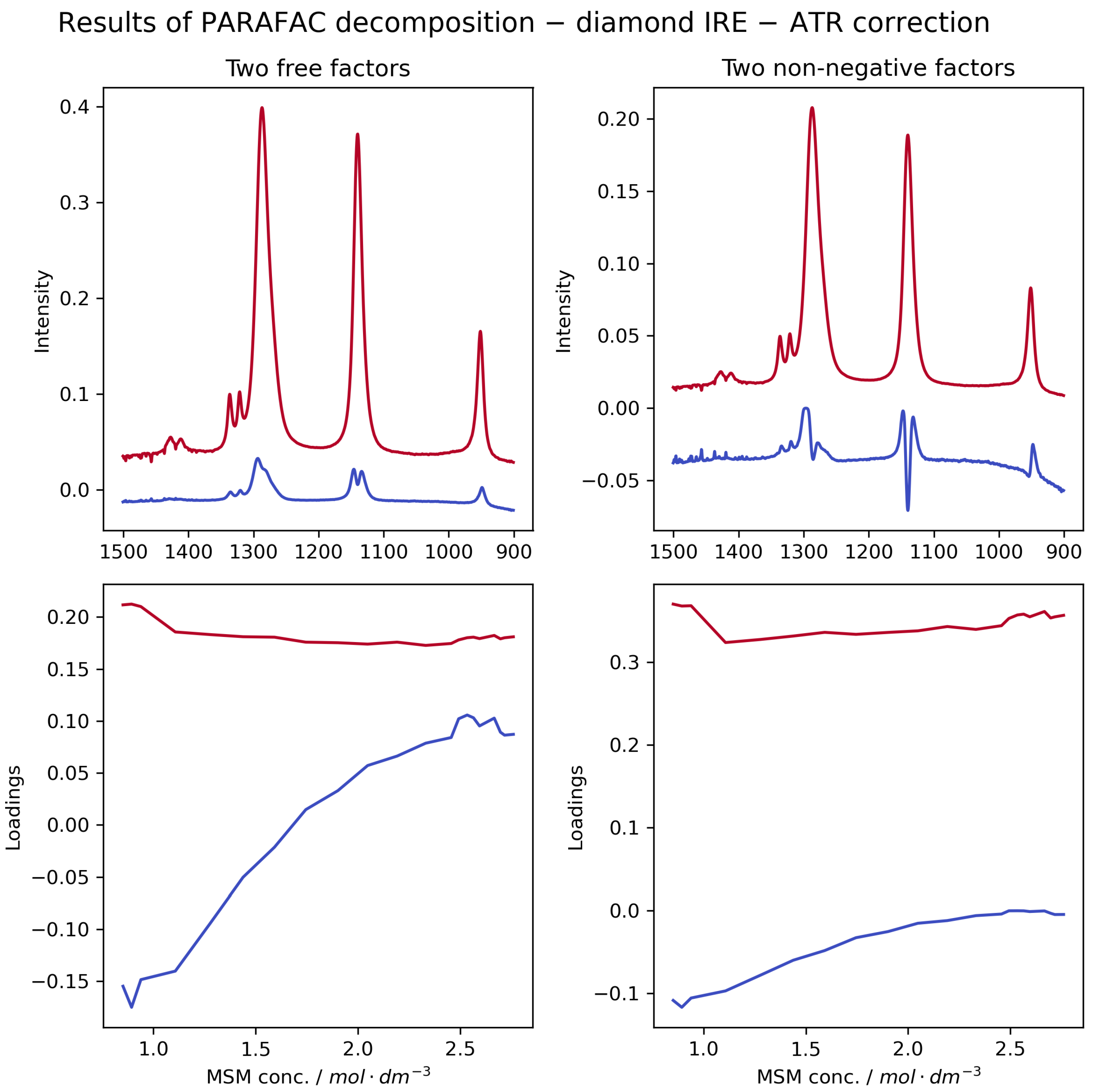
2.2. Chemometric and Theoretical Insights into MSM Solvation
3. Materials and Methods
3.1. Preparation of Solutions
3.2. Measurement Parameters
3.3. Software for Spectra Handling and Processing
3.4. DFT Calculations
4. Conclusions
- For concentration-dependent spectral series, uncorrected ATR-FTIR data are dominated by optical artefacts. In the case of aqueous MSM, these artefacts manifested as a misleading, systematic red-shift in all major bands, obscuring the true chemical information. This finding demonstrates that whilst these minor artefacts may be negligible for routine qualitative analysis, in studies focusing on subtle concentration-dependent effects to interpret molecular interactions, failing to perform a validated ATR correction leads to erroneous spectral interpretation.
- The ATR correction is mandatory to reveal the true molecular interactions. Only after correction did our spectra become consistent with both transmission data and DFT predictions. The corrected data show that increasing MSM concentration primarily causes a broadening of the sulphone vibrational bands, not a shift in their position. This effect is attributed to an increased heterogeneity of the local hydration environments around MSM molecules, a conclusion strongly supported by transmission spectra, theoretical calculations, and previous studies [12] that revealed a complex, multi-population hydration shell structure.
- The choice of ATR internal reflection element (IRE) is critical for successful correction. Our results show that while Ge and diamond crystals yielded spectra that could be reliably corrected to reflect the true chemical phenomena, the correction for ZnSe was insufficient. This provides a practical guideline for future quantitative ATR-FTIR studies on aqueous systems.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients 2017, 9, 290. [Google Scholar] [CrossRef]
- Brien, S.; Prescott, P.; Bashir, N.; Lewith, H.; Lewith, G. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthr. Cartil. 2008, 16, 1277–1288. [Google Scholar] [CrossRef]
- Evans, M.S.; Reid, K.H.; Sharp, J.B. Dimethylsulfoxide (DMSO) blocks conduction in peripheral nerve C fibers: A possible mechanism of analgesia. Neurosci. Lett. 1993, 150, 145–148. [Google Scholar] [CrossRef]
- Paulus, H.E. FDA advisory committee meeting: Methotrexate; guidelines for the clinical evaluation of anti-inflammatory drugs; DMSO in scleroderma. Arthritis Rheum. 1986, 29, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Du, S.; Yu, C.; Tian, N.; Tang, W. The effect of solvents on solid-liquid phase equilibrium of Dimethyl sulfone. J. Mol. Liq. 2020, 302, 112448. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Kurczewska, D.; Domińska, K.; Urbanek, K.A.; Piastowska-Ciesielska, A.W. Methylsulfonylmethane sensitizes endometrial cancer cells to doxorubicin. Cell Biol. Toxicol. 2021, 37, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Feairheller, W.R.; Katon, J.E. The vibrational spectra of sulfones. Spectrochim. Acta 1964, 20, 1099–1108. [Google Scholar] [CrossRef]
- Beerwerth, J.; Storek, M.; Greim, D.; Lueg, J.; Siegel, R.; Cetinkaya, B.; Hiller, W.; Zimmermann, H.; Senker, J.; Böhmer, R. Two-site jumps in dimethyl sulfone studied by one- and two-dimensional 17O NMR spectroscopy. J. Magn. Reson. 2018, 288, 84–94. [Google Scholar] [CrossRef]
- Givan, A.; Grothe, H.; Loewenschuss, A.; Nielsen, C.J. Infrared spectra and ab initio calculations of matrix isolated dimethyl sulfone and its water complex. Phys. Chem. Chem. Phys. 2002, 4, 255–263. [Google Scholar] [CrossRef]
- Clark, T.; Murray, J.S.; Lane, P.; Politzer, P. Why are dimethyl sulfoxide and dimethyl sulfone such good solvents? J. Mol. Model. 2008, 14, 689–697. [Google Scholar] [CrossRef]
- Liana, S.; Gabrielyan, S.A.; Markarian, H.W. Dielectric spectroscopy of dimethylsulfone solutions in water and dimethylsulfoxide. J. Mol. Liq. 2014, 194, 37–40. [Google Scholar] [CrossRef]
- Panuszko, A.; Śmiechowski, M.; Pieloszczyk, M.; Malinowski, A.; Stangret, J. Weakly Hydrated Solute of Mixed Hydrophobic-Hydrophilic Nature. J. Phys. Chem. B 2024, 128, 6352–6361. [Google Scholar] [CrossRef] [PubMed]
- Bertie, J.E.; Eysel, H.H. Infrared Intensities of Liquids I: Determination of Infrared Optical and Dielectric Constants by FT-IR Using the CIRCLE ATR Cell. Appl. Spectrosc. 1985, 39, 392–401. [Google Scholar] [CrossRef]
- Chalmers, J.M.; Griffiths, P.R. (Eds.) Handbook of Vibrational Spectroscopy, Volume III; John Wiley & Sons, Ltd.: Chichester, UK, 2002; Chapter Mid-Infrared Spectroscopy: Anomalies, Artifacts and Common Errors; pp. 2327–2347. [Google Scholar] [CrossRef]
- Mayerhöfer, T.G.; Costa, W.D.P.; Popp, J. Sophisticated Attenuated Total Reflection Correction Within Seconds for Unpolarized Incident Light at 45°. Appl. Spectrosc. 2024, 78, 321–328. [Google Scholar] [CrossRef]
- Bruździak, P. Vapor correction of FTIR spectra—A simple automatic least squares approach. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2019, 223, 117373. [Google Scholar] [CrossRef]
- Pastwa, P.; Bruździak, P. VaporFit: An open-source software for accurate atmospheric correction of FTIR spectra. Phys. Chem. Chem. Phys. 2025, 27, 12689–12698. [Google Scholar] [CrossRef]
- Kossaifi, J.; Panagakis, Y.; Anandkumar, A.; Pantic, M. TensorLy: Tensor Learning in Python. J. Mach. Learn. Res. 2019, 20, 1–6. [Google Scholar]
- Bro, R. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Malinowski, E.R. Factor Analysis in Chemistry, 3rd ed.; Wiley-Interscience: New York, NY, USA, 2002. [Google Scholar]
- Davidson, E.R. Comment on Dunning’s correlation-consistent basis sets. Chem. Phys. Lett. 1996, 260, 514–518. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Accounts 2008, 120, 215–241, Erratum in Theor. Chem. Accounts 2008, 119, 525. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.02; Gaussian Inc.: Wallingford, UK, 2016. [Google Scholar]
- Barroso, J.; Šakić, D. Error for Gaussian16 .log files and GaussView5. 2018. Available online: https://joaquinbarroso.com/2018/01/29/chk-files-gaussview (accessed on 30 October 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panuszko, A.; Pastwa, P.; Giemza, P.; Bruździak, P. Spectroscopic Probing of Solute–Solvent Interactions in Aqueous Methylsulphonylmethane (MSM) Solutions: An Integrated ATR-FTIR, Chemometric, and DFT Study. Int. J. Mol. Sci. 2025, 26, 10953. https://doi.org/10.3390/ijms262210953
Panuszko A, Pastwa P, Giemza P, Bruździak P. Spectroscopic Probing of Solute–Solvent Interactions in Aqueous Methylsulphonylmethane (MSM) Solutions: An Integrated ATR-FTIR, Chemometric, and DFT Study. International Journal of Molecular Sciences. 2025; 26(22):10953. https://doi.org/10.3390/ijms262210953
Chicago/Turabian StylePanuszko, Aneta, Przemysław Pastwa, Paulina Giemza, and Piotr Bruździak. 2025. "Spectroscopic Probing of Solute–Solvent Interactions in Aqueous Methylsulphonylmethane (MSM) Solutions: An Integrated ATR-FTIR, Chemometric, and DFT Study" International Journal of Molecular Sciences 26, no. 22: 10953. https://doi.org/10.3390/ijms262210953
APA StylePanuszko, A., Pastwa, P., Giemza, P., & Bruździak, P. (2025). Spectroscopic Probing of Solute–Solvent Interactions in Aqueous Methylsulphonylmethane (MSM) Solutions: An Integrated ATR-FTIR, Chemometric, and DFT Study. International Journal of Molecular Sciences, 26(22), 10953. https://doi.org/10.3390/ijms262210953





