Cyanobacteria Under UV Radiation: General Insights into Stress Responses
Abstract
1. Introduction
2. UV Radiation and Its Influence on Cyanobacteria
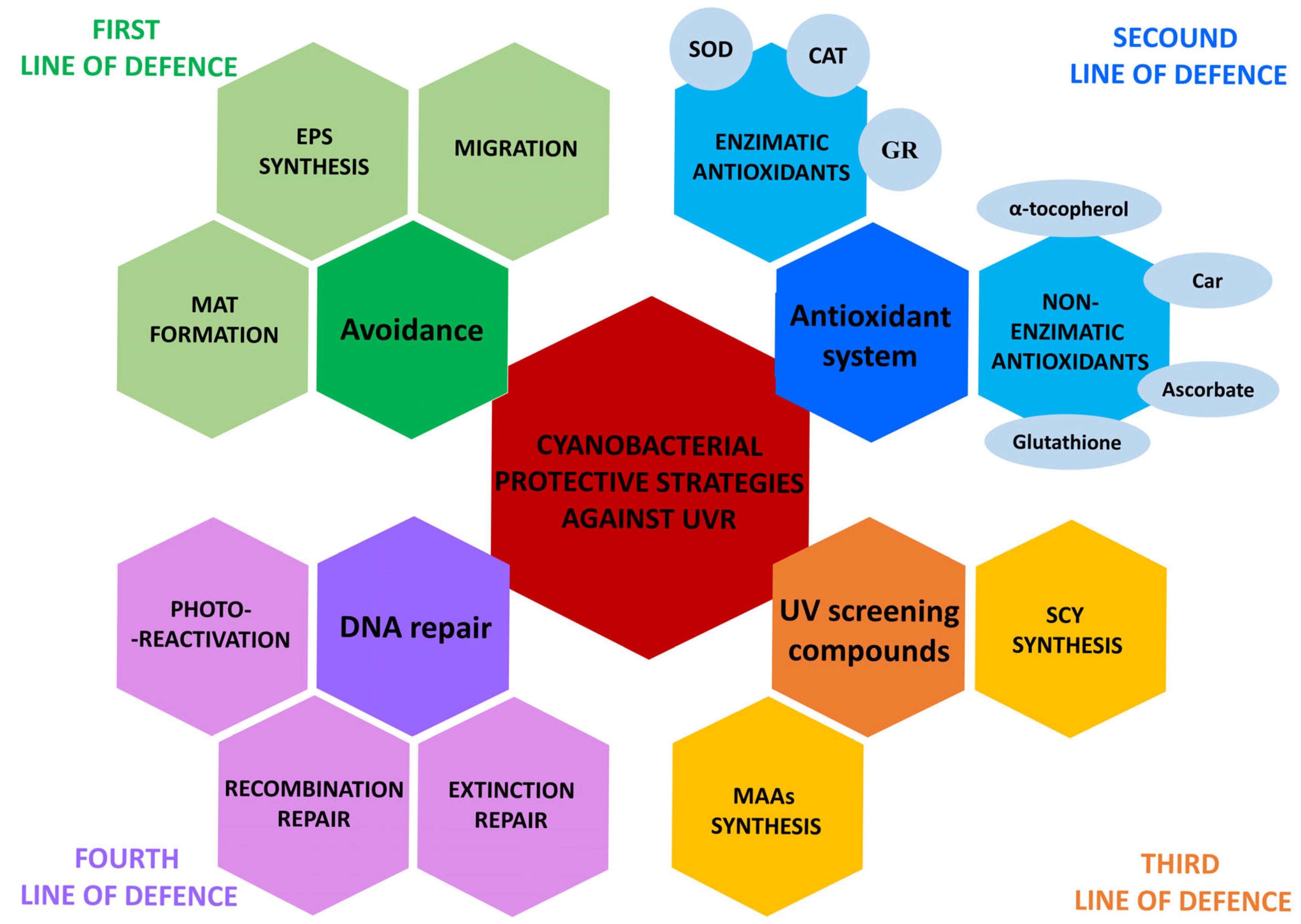
3. Cyanobacterial Adaptation Mechanisms Against UV Irradiation
3.1. Avoidance
3.2. Antioxidant System
3.3. UV-Absorbing Compounds
3.4. DNA Repair
4. The Ecological Context of UV Treatment on Cyanobacteria
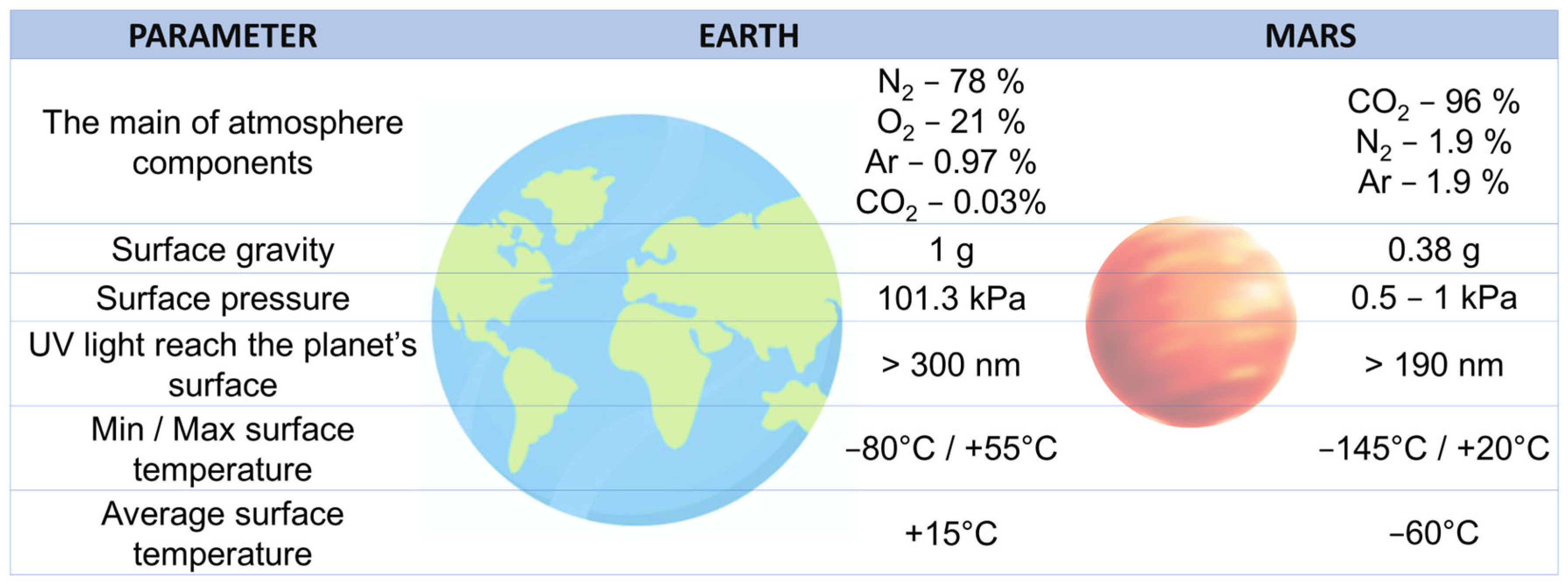
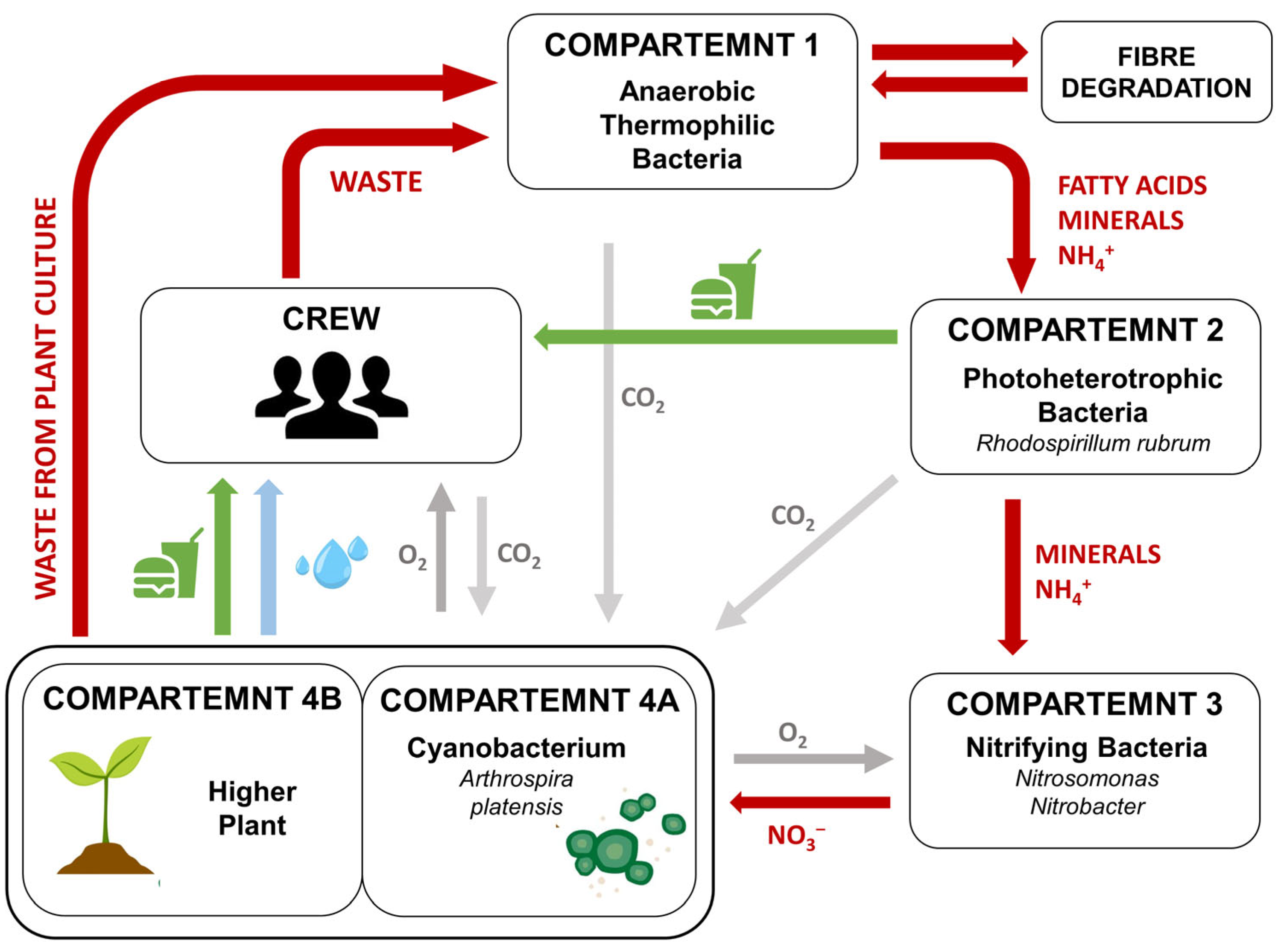
5. The Potential Use of Cyanobacteria in Biological Life-Support Systems (BLSSs) and as Extraterrestial Planet Terraforming Agents
6. Cyanobacteria in Space Mission Experiments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GOE | Great Oxidation Event |
| UVR | ultraviolet radiation |
| ROS | reactive oxygen species |
| MAAs | mycosporine-like amino acids |
| Scy | scytonemin |
| OP | oxygenic photosynthesis |
| MELiSSA project | Micro-Ecological Life-Support System Alternative project |
| BOSS experiment | Biofilm Organisms Surfing Space experiment |
| PQ | plastoquinone |
| Chl a | chlorophyll a |
| PSII | photosystem II |
| Fv/Fm | maximum photochemical efficiency of PSII |
| CPDs | cis-syn-cyclobutane pyrimidine dimers |
| (6-4)-PPs | pyrimidine-pyrimidone (6-4) photoproducts |
| NER | nucleotide excision repair |
| EPS | exopolysaccharides |
| PAR | photosynthetically active radiation (PAR) |
| PB | photosynthetically active radiation (PAR) in conjunction with UVB (PAR+UVB) |
| PA | photosynthetically active radiation (PAR) in conjunction with UVA (PAR+UVA) |
| PAB | photosynthetically active radiation (PAR) in conjunction with UVA and UVB (PAR+UVA+UVB) |
| Car | carotenoids |
| SOD | superoxide dismutase |
| CAT | catalase |
| GR | glutathione reductase |
| WspA | water-stress proteins |
| MC | microcystin |
| BLSSs | biological life-supported systems |
| ESA | European Space Agency |
| NASA | National Aeronautics and Space Administration |
| ISS | International Space Station |
| CBCR | cyanobacteriochrome |
| RubisCO | ribulose-1,5-bisphosphate carboxylase/oxygenase |
References
- Blaustein, R. The Great Oxidation Event. Bioscience 2016, 66, 189–195. [Google Scholar] [CrossRef]
- Sessions, A.L.; Doughty, D.M.; Welander, P.V.; Summons, R.E.; Newman, D.K. The Continuing Puzzle of the Great Oxidation Event. Curr. Biol. 2009, 19, R567–R574. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Cardona, T. On the Origin of Oxygenic Photosynthesis and Cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Lombard, J.; Soule, T.; Dunaj, S.; Wu, S.H.; Wojciechowski, M.F. Timing the Evolutionary Advent of Cyanobacteria and the Later Great Oxidation Event Using Gene Phylogenies of a Sunscreen. MBio 2019, 10, e00561-19. [Google Scholar] [CrossRef]
- Sajjad, W.; Ilahi, N.; Kang, S.; Bahadur, A.; Zada, S.; Iqbal, A. Endolithic Microbes of Rocks, Their Community, Function and Survival Strategies. Int. Biodeterior. Biodegrad. 2022, 169, 105387. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.P.; et al. Ultraviolet Radiation and Cyanobacteria. J. Photochem. Photobiol. B Biol. 2014, 141, 154–169. [Google Scholar] [CrossRef]
- Singh, H. Desiccation and Radiation Stress Tolerance in Cyanobacteria. J. Basic Microbiol. 2018, 58, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Cottin, H.; Rettberg, P. EXPOSE-R2 on the International Space Station (2014–2016): Results from the PSS and BOSS Astrobiology Experiments. Astrobiology 2019, 19, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Lasseur, C.; Lasseur, C.; Brunet, J.; De Weever, H.; Dixon, M.; Dussap, G.; Godia, F.; Leys, N.; Mergeay, M.; Van Der Straeten, D. Melissa: The European Project of a Closed Life Support System. Gravitational Space Biol. 2010, 23, 3. [Google Scholar]
- Mapstone, L.J.; Leite, M.N.; Purton, S.; Crawford, I.A.; Dartnell, L. Cyanobacteria and Microalgae in Supporting Human Habitation on Mars. Biotechnol. Adv. 2022, 59, 107946. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Albertini, R.; Colucci, M.E.; Coluccia, A.; Ibrahim, M.M.M.; Zoni, R.; Veronesi, L.; Affanni, P.; Pasquarella, C. An Overview on the Use of Ultraviolet Radiation to Disinfect Air and Surfaces. Acta Biomed. 2023, 94, e2023165. [Google Scholar] [CrossRef]
- Vincent, W.F.; Neale, P.J. Mechanisms of UV Damage to Aquatic Organisms. In The Effects of UV Radiation in the Marine Environment; De Mora, S., Demers, S., Vernet, M., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 149–176. ISBN 9780511535444. [Google Scholar]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and Biochemical Responses to High Light and Temperature Stress in Plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Singh, P.R.; Gupta, A.; Singh, A.P.; Jaiswal, J.; Sinha, R.P. Effects of Ultraviolet Radiation on Cellular Functions of the Cyanobacterium Synechocystis sp. PCC 6803 and Its Recovery under Photosynthetically Active Radiation. J. Photochem. Photobiol. B Biol. 2024, 252, 112866. [Google Scholar] [CrossRef]
- Singh, V.K.; Jha, S.; Rana, P.; Mishra, S.; Kumari, N.; Singh, S.C.; Anand, S.; Upadhye, V.; Sinha, R.P. Resilience and Mitigation Strategies of Cyanobacteria under Ultraviolet Radiation Stress. Int. J. Mol. Sci. 2023, 24, 12381. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, J.; Wang, X.; Wang, X.; Cao, H.; Teng, F.; Yao, S.; Lin, Z.; Jiang, Y.; Tao, Y. Optimizing UVA and UVC Synergy for Effective Control of Harmful Cyanobacterial Blooms. Environ. Sci. Ecotechnology 2024, 22, 100455. [Google Scholar] [CrossRef]
- Tao, Y.; Mao, X.; Hu, J.; Mok, H.O.L.; Wang, L.; Au, D.W.T.; Zhu, J.; Zhang, X. Mechanisms of Photosynthetic Inactivation on Growth Suppression of Microcystis aeruginosa under UV-C Stress. Chemosphere 2013, 93, 637–644. [Google Scholar] [CrossRef]
- He, J.; Ou, H.; Chen, J.; Liu, J.; Lu, D. Intrinsic Mechanism of UV-C-Induced Inactivation of Microcystis aeruginosa: Impairment on Photosynthetic System. Water. Air. Soil Pollut. 2016, 227, 82. [Google Scholar] [CrossRef]
- Baqué, M.; Viaggiu, E.; Scalzi, G.; Billi, D. Endurance of the Endolithic Desert Cyanobacterium Chroococcidiopsis under UVC Radiation. Extremophiles 2013, 17, 161–169. [Google Scholar] [CrossRef]
- Ng, W.O.; Pakrasi, H.B. DNA Photolyase Homologs Are the Major UV Resistance Factors in the Cyanobacterium Synechocystis Sp. PCC 6803. Mol. Gen. Genet. 2001, 264, 924–930. [Google Scholar] [CrossRef]
- Domain, F.; Houot, L.; Chauvat, F.; Cassier-Chauvat, C. Function and Regulation of the Cyanobacterial Genes LexA, RecA and RuvB: LexA Is Critical to the Survival of Cells Facing Inorganic Carbon Starvation. Mol. Microbiol. 2004, 53, 65–80. [Google Scholar] [CrossRef]
- Irankhahi, P.; Riahi, H.; Hassani, S.B.; Eskafi, M.; Azimzadeh Irani, M.; Shariatmadari, Z. The Role of the Protective Shield against UV-C Radiation and Its Molecular Interactions in Nostoc Species (Cyanobacteria). Sci. Rep. 2024, 14, 19258. [Google Scholar] [CrossRef]
- Moon, Y.J.; Kim, S.I.; Chung, Y.H. Sensing and Responding to UV-A in Cyanobacteria. Int. J. Mol. Sci. 2012, 13, 16303–16332. [Google Scholar] [CrossRef]
- Vass, I.Z.; Kõs, P.B.; Sass, L.; Nagy, C.I.; Vass, I. The Ability of Cyanobacterial Cells to Restore UV-B Radiation Induced Damage to Photosystem II Is Influenced by Photolyase Dependent DNA Repair. Photochem. Photobiol. 2013, 89, 384–390. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Alwaleed, E.A.; Ibrahim, A.; Saber, H. Detrimental Effect of UV-B Radiation on Growth, Photosynthetic Pigments, Metabolites and Ultrastructure of Some Cyanobacteria and Freshwater Chlorophyta. Int. J. Radiat. Biol. 2021, 97, 265–275. [Google Scholar] [CrossRef]
- Noaman, N.H. Ultraviolet-B Irradiation Alters Amino Acids, Proteins, Fatty Acids Contents and Enzyme Activities of Synechococcus leopoliensis. Int. J. Bot. 2007, 3, 109–113. [Google Scholar] [CrossRef][Green Version]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively Generated Damage to Cellular DNA by UVB and UVA Radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Ultraviolet-B-Induced DNA Damage and Photorepair in the Cyanobacterium Anabaena variabilis PCC 7937. Environ. Exp. Bot. 2011, 74, 280–288. [Google Scholar] [CrossRef]
- Noyma, N.P.; Silva, T.P.; Chiarini-Garcia, H.; Amado, A.M.; Roland, F.; Melo, R.C.N. Potential Effects of UV Radiation on Photosynthetic Structures of the Bloom-Forming Cyanobacterium Cylindrospermopsis raciborskii CYRF-01. Front. Microbiol. 2015, 6, 1202. [Google Scholar] [CrossRef] [PubMed]
- Vass, I.; Turcsányi, E.; Touloupakis, E.; Ghanotakis, D.; Petrouleas, V. The Mechanism of UV-A Radiation-Induced Inhibition of Photosystem II Electron Transport Studied by EPR and Chlorophyll Fluorescence. Biochemistry 2002, 41, 10200–10208. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A.; Madamwar, D. Responses of a Rice-Field Cyanobacterium Anabaena siamensis TISTR-8012 upon Exposure to PAR and UV Radiation. J. Plant Physiol. 2014, 171, 1545–1553. [Google Scholar] [CrossRef]
- Kumar, D.; Kannaujiya, V.K.; Jaiswal, J.; Sinha, R.P. Effects of Ultraviolet and Photosynthetically Active Radiation on Phycocyanin of Habitat Specific Cyanobacteria. J. Sci. Res. 2020, 64, 74–79. [Google Scholar] [CrossRef]
- Jacinavicius, F.R.; Geraldes, V.; Crnkovic, C.M.; Delbaje, E.; Fiore, M.F.; Pinto, E. Effect of Ultraviolet Radiation on the Metabolomic Profiles of Potentially Toxic Cyanobacteria. FEMS Microbiol. Ecol. 2021, 97, fiaa243. [Google Scholar] [CrossRef]
- Cockell, C.S.; Raven, J.A. Ozone and Life on the Archaean Earth. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, V.R.; Bennett, P.C.; Engel, A.S.; Tyler, S.W.; Ferris, F.G. Chilean High-Altitude Hot-Spring Sinters: A Model System for UV Screening Mechanisms by Early Precambrian Cyanobacteria. Geobiology 2006, 4, 15–28. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Multiple Defense Systems in Cyanobacteria in Response to Solar UV Radiation. In Cyanobacteria: Ecological Importance, Biotechnological Uses and Risk Management; Davison, D., Ed.; Nova Science Publisher: New York, NY, USA, 2015; pp. 125–158. ISBN 9781634631426. [Google Scholar]
- Biddanda, B.A.; McMillan, A.C.; Long, S.A.; Snider, M.J.; Weinke, A.D. Seeking Sunlight: Rapid Phototactic Motility of Filamentous Mat-Forming Cyanobacteria Optimize Photosynthesis and Enhance Carbon Burial in Lake Huron’s Submerged Sinkholes. Front. Microbiol. 2015, 6, 930. [Google Scholar] [CrossRef]
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial Mat Ecosystems: Structure Types, Functional Diversity, and Biotechnological Application. Electron. J. Biotechnol. 2018, 31, 48–56. [Google Scholar] [CrossRef]
- Quesada, A.; Vincent, W.F. Adaptation of Cyanobacteria to the Light Regime within Antarctic Microbial Mats. SIL Proc. 1922–2010 1993, 25, 960–965. [Google Scholar] [CrossRef]
- Vincent, W.F.; Quesada, A. Ultraviolet Radiation Effects on Cyanobacteria: Implications for Antarctic Microbial Ecosystems. In Ultraviolet Radiation in Antarctica: Measurements and Biological Effects Antarctic Research Series; Weiler, C.S., Penhale, P.A., Eds.; American Geophysical Union: Washington, DC, USA, 1994; Volume 62, pp. 111–124. [Google Scholar]
- Karsten, U.; Maier, J.; Garcia-Pichel, F. Seasonality in UV-Absorbing Compounds of Cyanobacterial Mat Communities from an Intertidal Mangrove Flat. Aquat. Microb. Ecol. 1998, 16, 37–44. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Castenholz, R.W. Characterization and Biological Implications of Scytonemin, a Cyanobacterial Sheath Pigment. J. Phycol. 1991, 27, 395–409. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Garcia-Pichel, F.; Mechling, M.; Mechling, M.; Castenholtz, R.W.; Castenholtz, R.W. Diel Migrations of Microorganisms in a Hypersaline Microbial Mat. Appl. Environ. Microbiol. 1994, 60, 1500–1511. [Google Scholar] [CrossRef]
- Choi, J.S.; Chung, Y.H.; Moon, Y.J.; Kim, C.; Watanabe, M.; Song, P.S.; Joe, C.O.; Bogorad, L.; Park, Y.M. Photomovement of the Gliding Cyanobacterium Synechocystis Sp. PCC 6803. Photochem. Photobiol. 1999, 70, 95–102. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, K. Photosynthetically Active and UV Radiation Act in an Antagonistic Way in Regulating Buoyancy of Arthrospira (Spirulina) platensis (Cyanobacterium). Environ. Exp. Bot. 2009, 66, 265–269. [Google Scholar] [CrossRef]
- Moon, Y.J.; Kim, S.Y.; Jung, K.H.; Choi, J.S.; Park, Y.M.; Chung, Y.H. Cyanobacterial Phytochrome Cph2 Is a Negative Regulator in Phototaxis toward UV-A. FEBS Lett. 2011, 585, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Cho, H.S.; Cho, J.I.; Jeon, J.S.; Lagarias, J.C.; Park, Y.I. Near-UV Cyanobacteriochrome Signaling System Elicits Negative Phototaxis in the Cyanobacterium Synechocystis Sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2011, 108, 10780–10785. [Google Scholar] [CrossRef]
- Chau, R.M.W.; Bhaya, D.; Huanga, K.C. Emergent Phototactic Responses of Cyanobacteria under Complex Light Regimes. MBio 2017, 8, e02330-16. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.N.; Varuni, P.; Bunbury, F.; Bhaya, D.; Menon, G.I. Phototaxis in Cyanobacteria: From Mutants to Models of Collective Behavior. MBio 2021, 12, e0239821. [Google Scholar] [CrossRef]
- Han, Y.; Hammerl, J.; Flemming, F.E.; Schuergers, N.; Wilde, A. A Cyanobacterial Chemotaxis-like System Controls Phototactic Orientation via Phosphorylation of Two Antagonistic Response Regulators. MicroLife 2024, 5, uqae012. [Google Scholar] [CrossRef]
- Rockwell, N.C.; Lagarias, J.C. Cyanobacteriochromes: A Rainbow of Photoreceptors. Annu. Rev. Microbiol. 2024, 78, 61–81. [Google Scholar] [CrossRef]
- Bunbury, F.; Rivas, C.; Calatrava, V.; Shelton, A.N.; Grossman, A.; Bhaya, D. Differential Phototactic Behavior of Closely Related Cyanobacterial Isolates from Yellowstone Hot Spring Biofilms. Appl. Environ. Microbiol. 2022, 88, e0019622. [Google Scholar] [CrossRef]
- Yang, Y.; Lam, V.; Adomako, M.; Simkovsky, R.; Jakob, A.; Rockwell, N.C.; Cohen, S.E.; Taton, A.; Wang, J.; Clark Lagarias, J.; et al. Phototaxis in a Wild Isolate of the Cyanobacterium Synechococcus elongatus. Proc. Natl. Acad. Sci. USA 2018, 115, E12378–E12387. [Google Scholar] [CrossRef]
- Li, S.Y.; He, C.; Valades-Cruz, C.A.; Zhang, C.C.; Yang, Y. Phototactic Signaling Network in Rod-Shaped Cyanobacteria: A Study on Synechococcus elongatus UTEX 3055. Microbiol. Res. 2025, 292, 127967. [Google Scholar] [CrossRef] [PubMed]
- Lamparter, T. Photosystems and Photoreceptors in Cyanobacterial Phototaxis and Photophobotaxis. FEBS Lett. 2024, 598, 1899–1908. [Google Scholar] [CrossRef]
- Schwabenland, E.; Jelen, C.J.; Weber, N.; Lamparter, T. Photophobotaxis in the Filamentous Cyanobacterium Phormidium lacuna: Mechanisms and Implications for Photosynthesis-Based Light Direction Sensing. Photochem. Photobiol. 2024, 100, 1290–1309. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-Induced Synthesis of Photoprotective Pigments and Extracellular Polysaccharides in the Terrestrial Cyanobacterium Nostoc commune. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qiu, B. Photosynthetic Adaptation of a Bloom-Forming Cyanobacterium Microcystis aeruginosa (Cyanophyceae) to Prolonged UV-B Exposure. J. Phycol. 2005, 41, 983–992. [Google Scholar] [CrossRef]
- Berera, R.; Van Stokkum, I.H.M.; D’Haene, S.; Kennis, J.T.M.; Van Grondelle, R.; Dekker, J.P. A Mechanism of Energy Dissipation in Cyanobacteria. Biophys. J. 2009, 96, 2261–2267. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Sinha, R.P.; Singh, S.P.; Häder, D.P. Photoprotective Compounds from Marine Organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558, Erratum in J. Ind. Microbiol. Biotechnol. 2010, 37, 991.. [Google Scholar] [CrossRef]
- Wright, D.J.; Smith, S.C.; Joardar, V.; Scherer, S.; Jervis, J.; Warren, A.; Helm, R.F.; Potts, M. UV Irradiation and Desiccation Modulate the Three-Dimensional Extracellular Matrix of Nostoc commune (Cyanobacteria). J. Biol. Chem. 2005, 280, 40271–40281. [Google Scholar] [CrossRef]
- Prasad, S.M.; Zeeshan, M. UV-B Radiation and Cadmium Induced Changes in Growth, Photosynthesis, and Antioxidant Enzymes of Cyanobacterium Plectonema boryanum. Biol. Plant. 2005, 49, 229–236. [Google Scholar] [CrossRef]
- Ahmed, H.; Pathak, J.; Rajneesh; Sonkar, P.K.; Ganesan, V.; Häder, D.P.; Sinha, R.P. Responses of a Hot Spring Cyanobacterium under Ultraviolet and Photosynthetically Active Radiation: Photosynthetic Performance, Antioxidative Enzymes, Mycosporine-like Amino Acid Profiling and Its Antioxidative Potentials. 3 Biotech 2021, 11, 10. [Google Scholar] [CrossRef]
- Jaiswal, J.; Kumari, N.; Gupta, A.; Singh, A.P.; Sinha, R.P. Impacts of Ultraviolet and Photosynthetically Active Radiations on Photosynthetic Efficiency and Antioxidant Systems of the Cyanobacterium Spirulina subsalsa HKAR-19. Folia Microbiol. 2024, 69, 747–765. [Google Scholar] [CrossRef]
- Chakravarty, D.; Banerjee, M.; Waghmare, N.; Ballal, A. Cyanobacterial Mn-Catalase ‘KatB’: Molecular Link between Salinity and Oxidative Stress Resistance. Commun. Integr. Biol. 2016, 9, e1216738. [Google Scholar] [CrossRef]
- Ślesak, I.; Kula, M.; Ślesak, H.; Miszalski, Z.; Strzałka, K. How to Define Obligatory Anaerobiosis? An Evolutionary View on the Antioxidant Response System and the Early Stages of the Evolution of Life on Earth. Free Radic. Biol. Med. 2019, 140, 61–73. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Y.; Liu, Y.; Liu, Y. Ultraviolet-B Exposure Induces Photo-Oxidative Damage and Subsequent Repair Strategies in a Desert Cyanobacterium Microcoleus vaginatus Gom. Eur. J. Soil Biol. 2009, 45, 377–382. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sinha, R.P. Impacts of Diurnal Variation of Ultraviolet-B and Photosynthetically Active Radiation on Phycobiliproteins of the Hot-Spring Cyanobacterium Nostoc Sp. Strain HKAR-2. Protoplasma 2017, 254, 423–433. [Google Scholar] [CrossRef]
- Yan, F.; Li, M.; Zang, S.; Xu, Z.; Bao, M.; Wu, H. UV Radiation and Temperature Increase Alter the PSII Function and Defense Mechanisms in a Bloom-Forming Cyanobacterium Microcystis aeruginosa. Front. Microbiol. 2024, 15, 1351796. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Sharma, P.K. High-light-induced Changes on Photosynthesis, Pigments, Sugars, Lipids and Antioxidant Enzymes in Freshwater (Nostoc spongiaeforme) and Marine (Phormidium corium) Cyanobacteria. Photochem. Photobiol. 2006, 82, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Ahmed, H.; Singh, P.R.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Mechanisms of Photoprotection in Cyanobacteria; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128146682. [Google Scholar]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative Stress in Cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Bernroitner, M.; Zamocky, M.; Furtmüller, P.G.; Peschek, G.A.; Obinger, C. Occurrence, Phylogeny, Structure, and Function of Catalases and Peroxidases in Cyanobacteria. J. Exp. Bot. 2009, 60, 423–440. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Gao, X. Scytonemin Plays a Potential Role in Stabilizing the Exopolysaccharidic Matrix in Terrestrial Cyanobacteria. Microb. Ecol. 2017, 73, 255–258. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-Protectants in Cyanobacteria. Plant Sci. 2008, 174, 278–289. [Google Scholar] [CrossRef]
- Raj, S.; Kuniyil, A.M.; Sreenikethanam, A.; Gugulothu, P. Microalgae as a Source of Mycosporine-like Amino Acids (MAAs); Advances and Future Prospects. Int. J. Environ. Res. Public Health 2021, 18, 12402. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, V.; de Medeiros, L.S.; Jacinavicius, F.R.; Long, P.F.; Pinto, E. Development and Validation of a Rapid LC-MS/MS Method for the Quantification of Mycosporines and Mycosporine-like Amino Acids (MAAs) from Cyanobacteria. Algal Res. 2020, 46, 101796. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Shree, A.; Patel, H.M.; Chaudhry, S.; Madamwar, D. Characterization, UV-Induction, Antioxidant Function and Role in Photo-Protection of Mycosporine-like Amino Acids (MAAs) in a Unicellular Cyanobacterium, Euhalothece sp.WR7. Algal Res. 2023, 70, 103030. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Wingard, C.E.; Castenholz, R.W. Evidence Regarding the UV Sunscreen Role of a Mycosporine-like Compound in the Cyanobacterium Gloeocapsa Sp. Appl. Environ. Microbiol. 1993, 59, 170–176. [Google Scholar] [CrossRef]
- Sinha, R.P.; Ambasht, N.K.; Sinha, J.P.; Klisch, M.; Häder, D.P. UV-B-Induced Synthesis of Mycosporine-like Amino Acids in Three Strains of Nodularia (Cyanobacteria). J. Photochem. Photobiol. B Biol. 2003, 71, 51–58. [Google Scholar] [CrossRef]
- Portwich, A.; Garcia-Pichel, F. A Novel Prokaryotic UVB Photoreceptor in the Cyanobacterium Chlorogloeopsis PCC 6912. Photochem. Photobiol. 2000, 71, 493–498. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Sherry, N.D.; Castenholz, R.W. Evidence for an Ultraviolet Sunscreen Role of the Extracellular Pigment Scytonemin in the Terrestrial Cyanobacterium Chlorogloeopsis Sp. Photochem. Photobiol. 1992, 56, 17–23. [Google Scholar] [CrossRef]
- Gao, X.; Jing, X.; Liu, X.; Lindblad, P. Biotechnological Production of the Sunscreen Pigment Scytonemin in Cyanobacteria: Progress and Strategy. Mar. Drugs 2021, 19, 129. [Google Scholar] [CrossRef]
- Orellana, G.; Gómez-Silva, B.; Urrutia, M.; Galetović, A. UV-A Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert. Microorganisms 2020, 8, 1690. [Google Scholar] [CrossRef]
- Dillon, J.G.; Tatsumi, C.M.; Tandingan, P.G.; Castenholz, R.W. Effect of Environmental Factors on the Synthesis of Scytonemin, a UV-Screening Pigment, in a Cyanobacterium (Chroococcidiopsis sp.). Arch. Microbiol. 2002, 177, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Rath, J.; Mandal, S.; Adhikary, S.P. Salinity Induced Synthesis of UV-Screening Compound Scytonemin in the Cyanobacterium Lyngbya aestuarii. J. Photochem. Photobiol. B Biol. 2012, 115, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Madrahi, G.S.; Naeimpoor, F. UV Induced Biosynthesis of Cyano-Sunscreen “Scytonemin” by Leptolyngbya mycodia and Its Effectual Antioxidant Activity. Iran. J. Pharm. Sci. 2022, 18, 19–33. [Google Scholar]
- Ng, W.O.; Zentella, R.; Wang, Y.; Taylor, J.S.A.; Pakrasi, H.B. PhrA, the Major Photoreactivating Factor in the Cyanobacterium Synechocystis Sp. Strain PCC 6803 Codes for a Cyclobutane-Pyrimidine-Dimer- Specific DNA Photolyase. Arch. Microbiol. 2000, 173, 412–417. [Google Scholar] [CrossRef]
- Yang, W. Surviving the Sun: Repair and Bypass of DNA UV Lesions. Protein Sci. 2011, 20, 1781–1789. [Google Scholar] [CrossRef]
- Truglio, J.J.; Croteau, D.L.; van Houten, B.; Kisker, C. Prokaryotic Nucleotide Excision Repair: The UvrABC System. Chem. Rev. 2006, 106, 233–252. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Veaudor, T.; Chauvat, F. Comparative Genomics of DNA Recombination and Repair in Cyanobacteria: Biotechnological Implications. Front. Microbiol. 2016, 7, 1809. [Google Scholar] [CrossRef]
- Singh, P.R.; Gupta, A.; Rajneesh; Pathak, J.; Sinha, R.P. Phylogenetic Distribution, Structural Analysis and Interaction of Nucleotide Excision Repair Proteins in Cyanobacteria. DNA Repair 2023, 126, 103487. [Google Scholar] [CrossRef]
- Badri, H.; Monsieurs, P.; Coninx, I.; Wattiez, R.; Leys, N. Molecular Investigation of the Radiation Resistance of Edible Cyanobacterium Arthrospira Sp. PCC 8005. Microbiologyopen 2015, 4, 187–207. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.; Shi, X.; Yu, Y.; Zhang, M. Effects of UV-B Radiation on Microcystin Production of a Toxic Strain of Microcystis aeruginosa and Its Competitiveness against a Non-Toxic Strain. J. Hazard. Mater. 2015, 283, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Meissner, S.; Steinhauser, D.; Dittmann, E. Metabolomic Analysis Indicates a Pivotal Role of the Hepatotoxin Microcystin in High Light Adaptation of Microcystis. Environ. Microbiol. 2015, 17, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tao, Y.; Zhan, X.M.; Dao, G.H.; Hu, H.Y. UV-C Irradiation for Harmful Algal Blooms Control: A Literature Review on Effectiveness, Mechanisms, Influencing Factors and Facilities. Sci. Total Environ. 2020, 723, 137986. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro-Araújo, M.K.; Lorenzi, A.S.; Chia, M.A.; Mota, E.C.; do Carmo Bittencourt-Oliveira, M. Insights into the Impact of Increasing Temperature, Light Intensity, and UV-B Exposure on the Circadian Rhythm of Microcystin Production and Release, and the Expression of Mcy Genes in the Cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 2022, 34, 231–242. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, J.; Zhou, J.; Han, Z. Algae Removal and Degradation of Microcystins by UV-C System: A Review. Water Environ. Res. 2025, 97, e70049. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Börner, T.; Dittmann, E. The Cyanobacterial Hepatotoxin Microcystin Binds to Proteins and Increases the Fitness of Microcystis under Oxidative Stress Conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef]
- Jacinavicius, F.R.; Pacheco, A.B.F.; Chow, F.; Verissimo da Costa, G.C.; Kalume, D.E.; Rigonato, J.; Schmidt, E.C.; Sant’Anna, C.L. Different Ecophysiological and Structural Strategies of Toxic and Non-Toxic Microcystis aeruginosa (Cyanobacteria) Strains Assessed under Culture Conditions. Algal Res. 2019, 41, 101548. [Google Scholar] [CrossRef]
- Jia, P.; Zhou, Y.; Zhang, X.; Zhang, Y.; Dai, R. Cyanobacterium Removal and Control of Algal Organic Matter (AOM) Release by UV/H2O2 Pre-Oxidation Enhanced Fe(II) Coagulation. Water Res. 2018, 131, 122–130. [Google Scholar] [CrossRef]
- Urtubia, H.O.; Betanzo, L.B.; Vásquez, M. Microalgae and Cyanobacteria as Green Molecular Factories: Tools and Perspectives. In Algae—Organisms for Imminent Biotechnology; IntechOpen: Rijeka, Croatia, 2016; pp. 1–28. [Google Scholar]
- Verseux, C.; Baqué, M.; Lehto, K.; De Vera, J.P.P.; Rothschild, L.J.; Billi, D. Sustainable Life Support on Mars—The Potential Roles of Cyanobacteria. Int. J. Astrobiol. 2016, 15, 65–92. [Google Scholar] [CrossRef]
- Singh, S. A Review on Possible Elicitor Molecules of Cyanobacteria: Their Role in Improving Plant Growth and Providing Tolerance against Biotic or Abiotic Stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef]
- Arai, M.; Tomita-Yokotani, K.; Sato, S.; Hashimoto, H.; Ohmori, M.; Yamashita, M. Growth of Terrestrial Cyanobacterium, Nostoc Sp., on Martian Regolith Simulant and Its Vacuum Tolerance. Biol. Sci. Space 2008, 22, 8–17. [Google Scholar] [CrossRef][Green Version]
- Baqué, M.; Verseux, C.; Böttger, U.; Rabbow, E.; de Vera, J.P.P.; Billi, D. Preservation of Biomarkers from Cyanobacteria Mixed with Mars like Regolith under Simulated Martian Atmosphere and UV Flux. Orig. Life Evol. Biosph. 2016, 46, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Billi, D.; Gallego Fernandez, B.; Fagliarone, C.; Chiavarini, S.; Rothschild, L.J. Exploiting a Perchlorate-Tolerant Desert Cyanobacterium to Support Bacterial Growth for in Situ Resource Utilization on Mars. Int. J. Astrobiol. 2021, 20, 29–35. [Google Scholar] [CrossRef]
- Fagliarone, C.; Napoli, A.; Chiavarini, S.; Baqué, M.; de Vera, J.P.; Billi, D. Biomarker Preservation and Survivability under Extreme Dryness and Mars-like UV Flux of a Desert Cyanobacterium Capable of Trehalose and Sucrose Accumulation. Front. Astron. Space Sci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Napoli, A.; Micheletti, D.; Pindo, M.; Larger, S.; Cestaro, A.; de Vera, J.P.; Billi, D. Absence of Increased Genomic Variants in the Cyanobacterium Chroococcidiopsis Exposed to Mars-like Conditions Outside the Space Station. Sci. Rep. 2022, 12, 8437. [Google Scholar] [CrossRef]
- Verseux, C.; Baqué, M.; Cifariello, R.; Fagliarone, C.; Raguse, M.; Moeller, R.; Billi, D. Evaluation of the Resistance of Chroococcidiopsis Spp. to Sparsely and Densely Ionizing Irradiation. Astrobiology 2017, 17, 118–125. [Google Scholar] [CrossRef]
- Verseux, C.; Heinicke, C.; Ramalho, T.P.; Determann, J.; Duckhorn, M.; Smagin, M.; Avila, M. A Low-Pressure, N2/CO2 Atmosphere Is Suitable for Cyanobacterium-Based Life-Support Systems on Mars. Front. Microbiol. 2021, 12, 611798. [Google Scholar] [CrossRef]
- Ramalho, T.P.; Chopin, G.; Pérez-Carrascal, O.M.; Tromas, N.; Verseux, C. Selection of Anabaena Sp. PCC 7938 as a Cyanobacterium Model for Biological ISRU on Mars. Appl. Environ. Microbiol. 2022, 88, e0059422. [Google Scholar] [CrossRef]
- Ramalho, T.P.; Chopin, G.; Salman, L.; Baumgartner, V.; Heinicke, C.; Verseux, C. On the Growth Dynamics of the Cyanobacterium Anabaena Sp. PCC 7938 in Martian Regolith. Npj Microgravity 2022, 8, 43. [Google Scholar] [CrossRef]
- Verseux, C.; Ramalho, T.P.; Bohuon, E.; Kunst, N.; Lang, V.; Heinicke, C. Dependence of Cyanobacterium Growth and Mars-Specific Photobioreactor Mass on Total Pressure, PN2 and PCO2. Npj Microgravity 2024, 10, 101. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Veloso, T.; Frankenbach, S.; Serôdio, J.; Passos, H.; Sousa, C.; Gonçalves, F.J.M.; Ventura, S.P.M.; Pereira, J.L. Cyanobacteria as Candidates to Support Mars Colonization: Growth and Biofertilization Potential Using Mars Regolith as a Resource. Front. Microbiol. 2022, 13, 840098. [Google Scholar] [CrossRef] [PubMed]
- Arribas Tiemblo, M.; Macário, I.P.E.; Tornero, A.; Yáñez, A.; Andrejkovičová, S.; Gómez, F. Survival of Filamentous Cyanobacteria through Martian ISRU: Combined Effects of Desiccation and UV-B Radiation. Microorganisms 2025, 13, 1083. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Wang, B.; Li, C.; Bian, P.; Chen, L.; Wang, G. Exposure of Cyanobacterium Nostoc Sp. to the Mars-like Stratosphere Environment. J. Photochem. Photobiol. B Biol. 2021, 224, 112307. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Monsieurs, P.; Misztak, A.; Waleron, K.; Leys, N.; Cuypers, A.; Janssen, P.J. Helical and Linear Morphotypes of Arthrospira Sp. PCC 8005 Display Genomic Differences and Respond Differently to 60Co Gamma Irradiation. Eur. J. Phycol. 2020, 55, 129–146. [Google Scholar] [CrossRef]
- Verbeelen, T.; Leys, N.; Ganigué, R.; Mastroleo, F. Development of Nitrogen Recycling Strategies for Bioregenerative Life Support Systems in Space. Front. Microbiol. 2021, 12, 700810. [Google Scholar] [CrossRef]
- Harper, L.D.; Neal, C.R.; Poynter, J.; Schalkwyk, J.D.; Wingo, D.R. Life Support for a Low-Cost Lunar Settlement: No Showstoppers. New Space 2016, 4, 40–49. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef]
- Hendrickx, L.; De Wever, H.; Hermans, V.; Mastroleo, F.; Morin, N.; Wilmotte, A.; Janssen, P.; Mergeay, M. Microbial Ecology of the Closed Artificial Ecosystem MELiSSA (Micro-Ecological Life Support System Alternative): Reinventing and Compartmentalizing the Earth’s Food and Oxygen Regeneration System for Long-Haul Space Exploration Missions. Res. Microbiol. 2006, 157, 77–86. [Google Scholar] [CrossRef]
- Billi, D.; Staibano, C.; Verseux, C.; Fagliarone, C.; Mosca, C.; Baqué, M.; Rabbow, E.; Rettberg, P. Dried Biofilms of Desert Strains of Chroococcidiopsis Survived Prolonged Exposure to Space and Mars-like Conditions in Low Earth Orbit. Astrobiology 2019, 19, 1008–1017. [Google Scholar] [CrossRef]
- Wadsworth, J.; Rettberg, P.; Cockell, C.S. Aggregated Cell Masses Provide Protection against Space Extremes and a Microhabitat for Hitchhiking Co-Inhabitants. Astrobiology 2019, 19, 995–1007. [Google Scholar] [CrossRef]
| Species/Strain | Light | Phototactic Direction | Photoreceptors | Ecological Context | References |
|---|---|---|---|---|---|
| Synechocystis sp. PCC 6803 | red, green | positive | TaxD1 (PixJ1), PixD | freshwater, biofilms, fluctuating light | [45,48,49,50,51,52] |
| blue, UV | negative or inhibited | UirS, UirR (cyanobacteriochromes, CBCR), Cph2 (cyanobacterial phytochrome) | |||
| Synechococcus OS-B′ | UVA, blue, red, green | positive | PixJ, UirS | hot springs mats (50–55 °C) | [53] |
| Synechococcus elongatus UTEX 3055 | blue, green | both (bidirectional) | PixJSe (CBCR) | soil, biofilms | [54,55] |
| Phormidium lacuna | broad spectrum (PAR) | positive (weak light), negative (strong light) | CphA (cyanobacterial phytochrome), PixJ (CBCR), PSII | filamentous, biofilms | [56,57] |
| Species/Strains | Key Features | References |
|---|---|---|
| Chroococcidiopsis sp. CCMEE 029 Chroococcidiopsis sp. CCMEE 029 P-MRS | resistance to desiccation, temperature fluctuations, UVR, and perchlorate, nutritional value for heterotrophic bacteria | [108,109,110,111,112] |
| Anabaena sp. PCC 7938 | diazotrophy and rock-leaching abilities, as well as tolerance to perchlorates, feedstock for other organisms | [113,114,115,116] |
| Desmonostoc muscorum (Nostoc muscorum) UTAD N213 Desmonostoc sp. Nostoc sp. FACHB 892 | resistance to desiccation and Mars-like conditions, food source | [117,118,119] |
| Arthrospira sp. PCC 8005 Arthrospira platensis | resistance to ionising radiation, food source | [117,120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, Z.; Ślesak, I. Cyanobacteria Under UV Radiation: General Insights into Stress Responses. Int. J. Mol. Sci. 2025, 26, 10926. https://doi.org/10.3390/ijms262210926
Mazur Z, Ślesak I. Cyanobacteria Under UV Radiation: General Insights into Stress Responses. International Journal of Molecular Sciences. 2025; 26(22):10926. https://doi.org/10.3390/ijms262210926
Chicago/Turabian StyleMazur, Zofia, and Ireneusz Ślesak. 2025. "Cyanobacteria Under UV Radiation: General Insights into Stress Responses" International Journal of Molecular Sciences 26, no. 22: 10926. https://doi.org/10.3390/ijms262210926
APA StyleMazur, Z., & Ślesak, I. (2025). Cyanobacteria Under UV Radiation: General Insights into Stress Responses. International Journal of Molecular Sciences, 26(22), 10926. https://doi.org/10.3390/ijms262210926






