Clinical and Molecular Characteristics of KRAS Codon-Specific Mutations in Advanced Pancreatic Ductal Adenocarcinoma with Prognostic and Therapeutic Implications
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. KRAS Mutation Subtypes and Survival Outcomes
2.3. KRAS Allele Specific Mutations and Co-Mutations
2.4. KRAS Mutation Subtypes and Clinical Characteristics
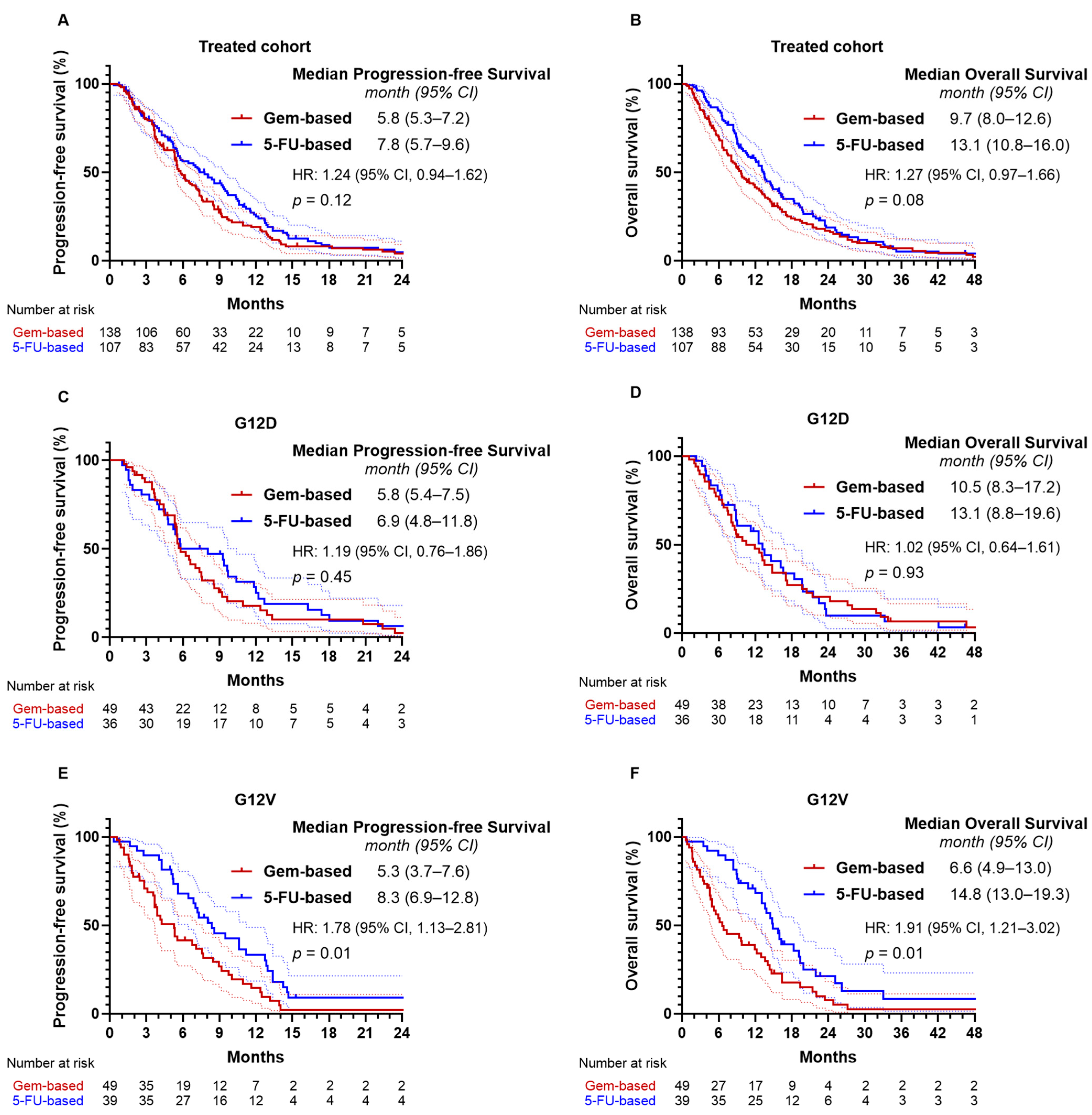
2.5. KRAS Mutation Subtypes and Treatment Outcomes
2.6. Effectiveness of Treatment Regimens
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Molecular Alteration Analyses
4.3. Mutational Status of HRD- and DDR-Related Genes
4.4. Assessments
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic Ductal Adenocarcinoma |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| TP53 | Tumor protein p53 |
| CDKN2A | Cyclin-Dependent Kinase Inhibitor 2A |
| SMAD4 | Mothers against decapentaplegic homolog 4 |
| NGS | Next-Generation Sequencing |
| SNV | Single Nucleotide Variant |
| CNV | Copy Number Variation |
| cfDNA | Cell-free DNA |
| HRD | Homologous Recombination Deficiency |
| DDR | DNA Damage Response |
| CA 19-9 | Carbohydrate Antigen 19-9 |
| CEA | Carcinoembryonic Antigen |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| 5-FU | 5-Fluorouracil |
| Nal-IRI/FL | Nanoliposomal Irinotecan + 5-Fluorouracil/Leucovorin |
| ORR | Objective Response Rate |
| DCR | Disease Control Rate |
| PARP | Poly(ADP-ribose) Polymerase |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Dougan, S.K.; Aguirre, A.J.; Maitra, A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat. Cancer 2022, 3, 272–286. [Google Scholar] [CrossRef]
- Cicenas, J.; Kvederaviciute, K.; Meskinyte, I.; Meskinyte-Kausiliene, E.; Skeberdyte, A.; Cicenas, J. KRAS, TP53, CDKN2A, SMAD4, BRCA1, and BRCA2 Mutations in Pancreatic Cancer. Cancers 2017, 9, 42. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; Yousef, M.; Chowdhury, S.; Abdilleh, K.; Knafl, M.; Edelkamp, P.; Alfaro-Munoz, K.; Chacko, R.; Peterson, J.; Smaglo, B.G.; et al. Impact of KRAS mutations and co-mutations on clinical outcomes in pancreatic ductal adenocarcinoma. NPJ Precis. Oncol. 2024, 8, 27. [Google Scholar]
- Ostrem, J.M.; Shokat, K.M. Direct small-molecule inhibitors of KRAS: From structural insights to mechanism-based design. Nat. Rev. Drug Discov. 2016, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.B.; Cheng, N.; Markosyan, N.; Sor, R.; Kim, I.K.; Hallin, J.; Shoush, J.; Quinones, L.; Brown, N.V.; Bassett, J.B.; et al. Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer. Cancer Discov. 2023, 13, 298–311. [Google Scholar] [CrossRef]
- Hallin, J.; Bowcut, V.; Calinisan, A.; Briere, D.M.; Hargis, L.; Engstrom, L.D.; Laguer, J.; Medwid, J.; Vanderpool, D.; Lifset, E.; et al. Anti-tumor efficacy of a potent and selective non-covalent KRAS(G12D) inhibitor. Nat. Med. 2022, 28, 2171–2182. [Google Scholar] [CrossRef]
- Johnson, C.; Burkhart, D.L.; Haigis, K.M. Classification of KRAS-Activating Mutations and the Implications for Therapeutic Intervention. Cancer Discov. 2022, 12, 913–923. [Google Scholar] [CrossRef]
- Hobbs, G.A.; Der, C.J. RAS Mutations Are Not Created Equal. Cancer Discov. 2019, 9, 696–698. [Google Scholar] [CrossRef]
- Boilève, A.; Rousseau, A.; Hilmi, M.; Tarabay, A.; Mathieu, J.R.R.; Cartry, J.; Bedja, S.; Goudarzi, N.; Nicotra, C.; Ngo-Camus, M.; et al. Codon-specific KRAS mutations predict survival in advanced pancreatic cancer. ESMO Gastrointest. Oncol. 2024, 3, 100030. [Google Scholar] [CrossRef]
- Bournet, B.; Muscari, F.; Buscail, C.; Assenat, E.; Barthet, M.; Hammel, P.; Selves, J.; Guimbaud, R.; Cordelier, P.; Buscail, L. KRAS G12D Mutation Subtype Is A Prognostic Factor for Advanced Pancreatic Adenocarcinoma. Clin. Transl. Gastroenterol. 2016, 7, e157. [Google Scholar] [CrossRef]
- Ardalan, B.; Ciner, A.; Baca, Y.; Darabi, S.; Kasi, A.; Lou, E.; Azqueta, J.I.; Xiu, J.; Nabhan, C.; Shields, A.F.; et al. Prognostic indicators of KRAS G12X mutations in pancreatic cancer. J. Clin. Oncol. 2023, 41 (Suppl. S4), 735. [Google Scholar] [CrossRef]
- Kim, M.P.; Li, X.; Deng, J.; Zhang, Y.; Dai, B.; Allton, K.L.; Hughes, T.G.; Siangco, C.; Augustine, J.J.; Kang, Y.; et al. Oncogenic KRAS Recruits an Expansive Transcriptional Network through Mutant p53 to Drive Pancreatic Cancer Metastasis. Cancer Discov. 2021, 11, 2094–2111. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, R.; Gayet, O.; Duconseil, P.; Vanbrugghe, C.; Roques, J.; Bigonnet, M.; Blum, Y.; Elarouci, N.; Armenoult, L.; Ayadi, M.; et al. A transcriptomic signature to predict adjuvant gemcitabine sensitivity in pancreatic adenocarcinoma. Ann. Oncol. 2021, 32, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Satake, H.; George, T.J.; Yaeger, R.; Hollebecque, A.; Garrido-Laguna, I.; Schuler, M.; Burns, T.F.; Coveler, A.L.; Falchook, G.S.; et al. Sotorasib in KRAS p.G12C-Mutated Advanced Pancreatic Cancer. N. Engl. J. Med. 2023, 388, 33–43. [Google Scholar] [CrossRef]
- Linehan, A.; O’Reilly, M.; McDermott, R.; O’Kane, G.M. Targeting KRAS mutations in pancreatic cancer: Opportunities for future strategies. Front. Med. 2024, 11, 1369136. [Google Scholar] [CrossRef]
- Ben-Ammar, I.; Rousseau, A.; Nicolle, R.; Tarabay, A.; Boige, V.; Valery, M.; Pudlarz, T.; Malka, D.; Gelli, M.; Fernandez-De-Sevilla, E.; et al. Precision medicine for KRAS wild-type pancreatic adenocarcinomas. Eur. J. Cancer 2024, 197, 113497. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kyung, D.; Yuk, H.D.; Jeong, C.W.; Lee, W.; Yoon, J.K.; Kim, H.P.; Bang, D.; Kim, T.Y.; Lim, Y.; et al. Practical Utility of Liquid Biopsies for Evaluating Genomic Alterations in Castration-Resistant Prostate Cancer. Cancers 2023, 15, 2847. [Google Scholar] [CrossRef]
- Park, W.; Chen, J.; Chou, J.F.; Varghese, A.M.; Yu, K.H.; Wong, W.; Capanu, M.; Balachandran, V.; McIntyre, C.A.; El Dika, I.; et al. Genomic Methods Identify Homologous Recombination Deficiency in Pancreas Adenocarcinoma and Optimize Treatment Selection. Clin. Cancer Res. 2020, 26, 3239–3247. [Google Scholar] [CrossRef]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef]
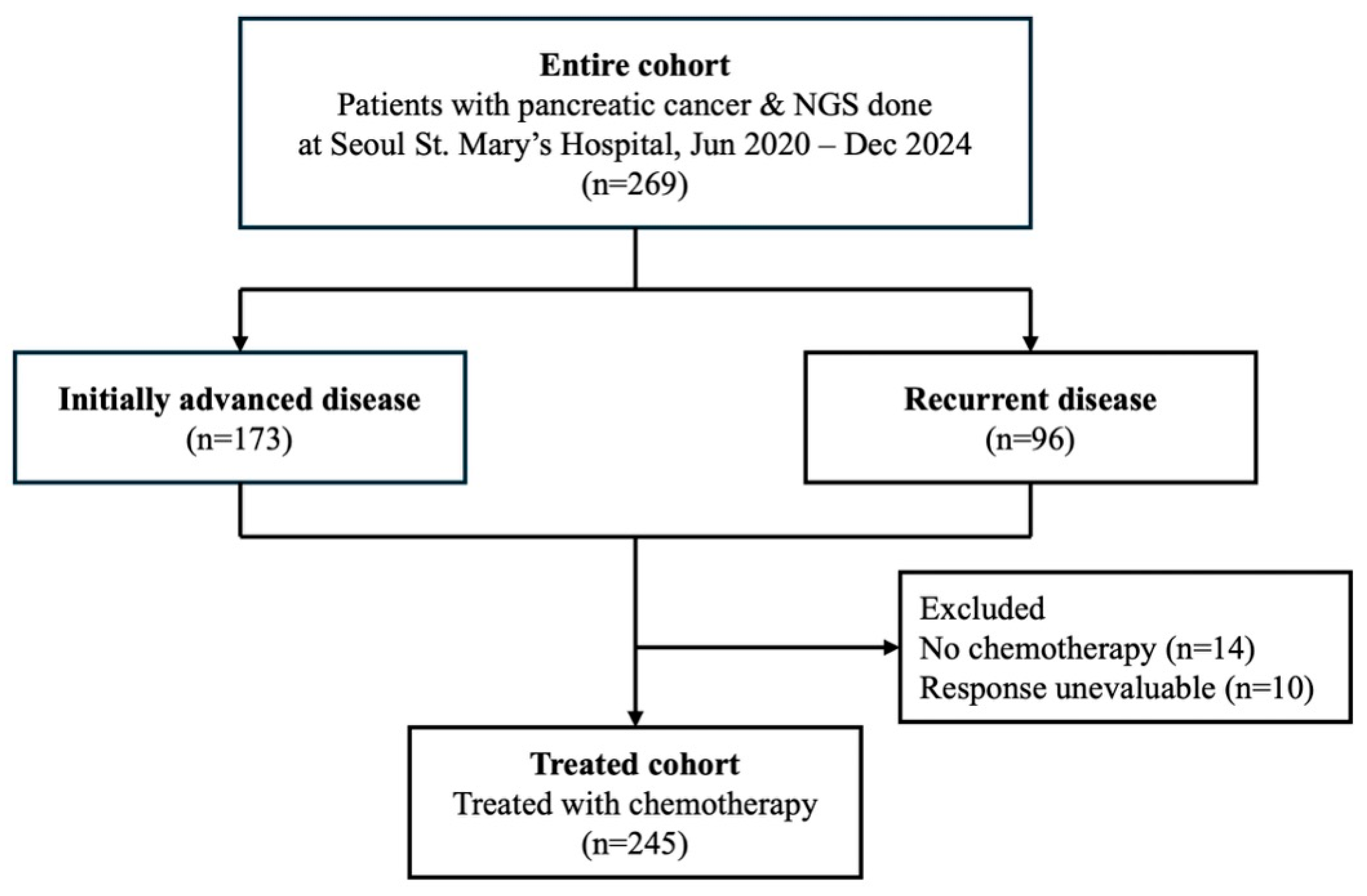
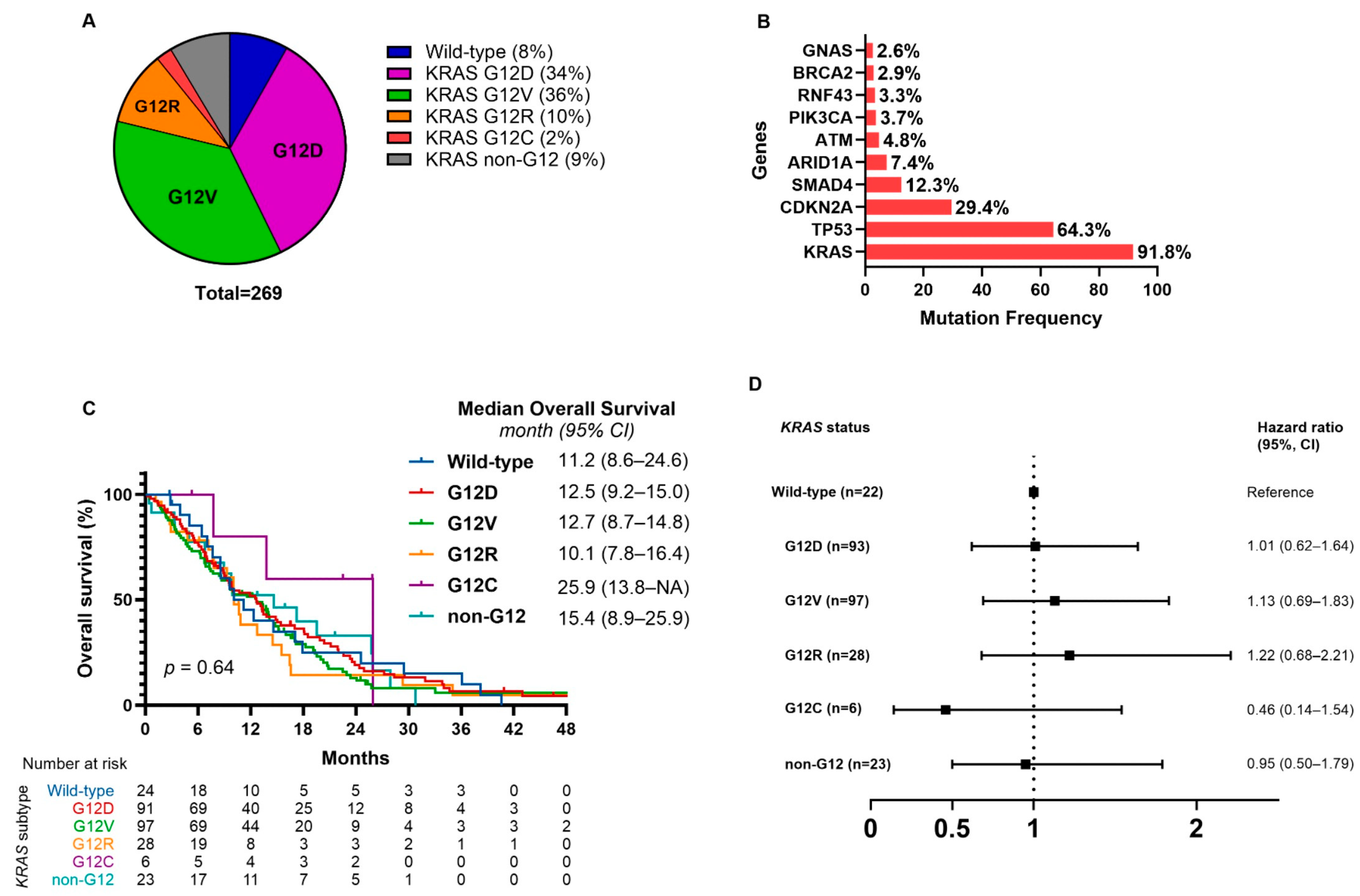
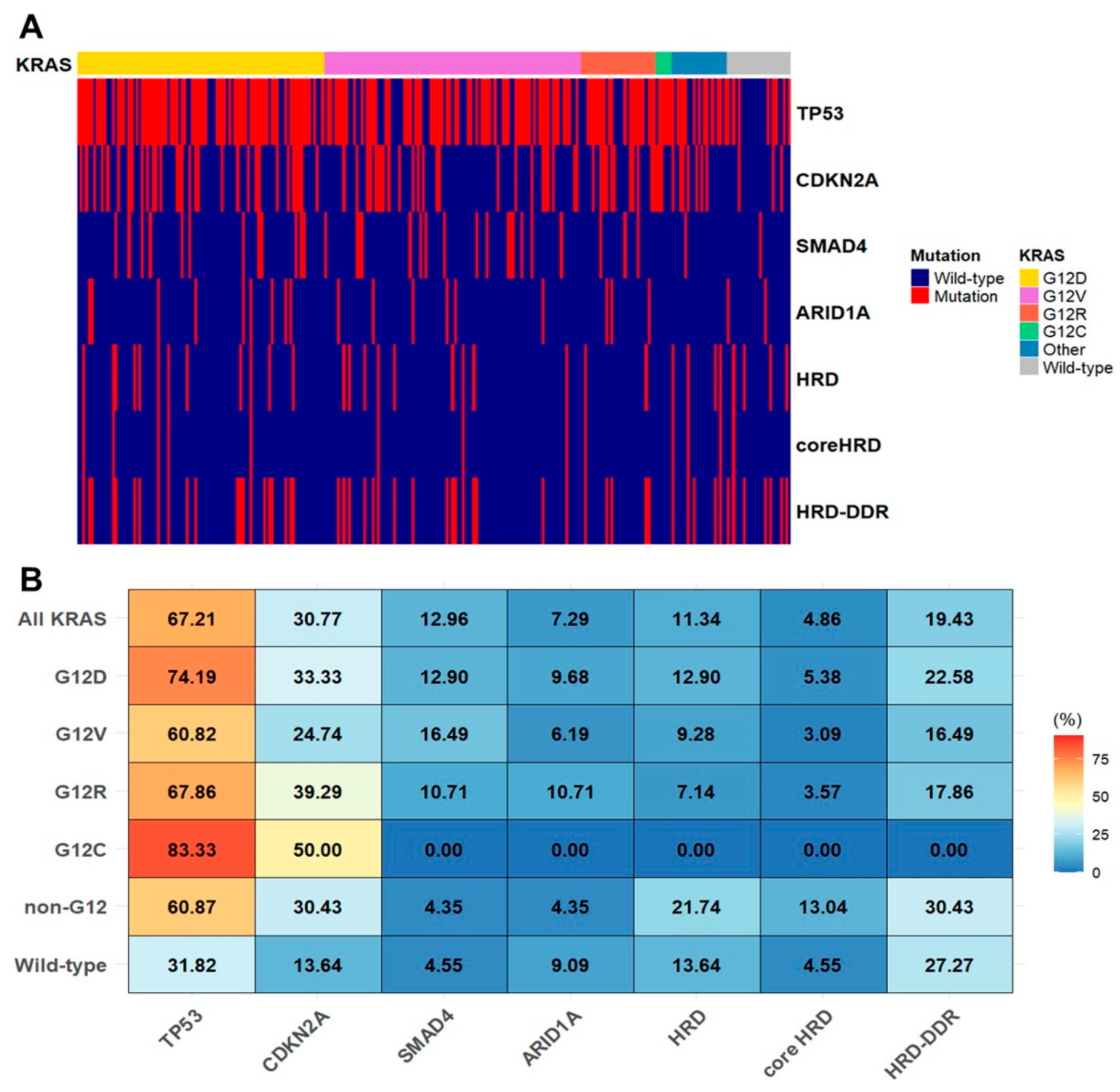
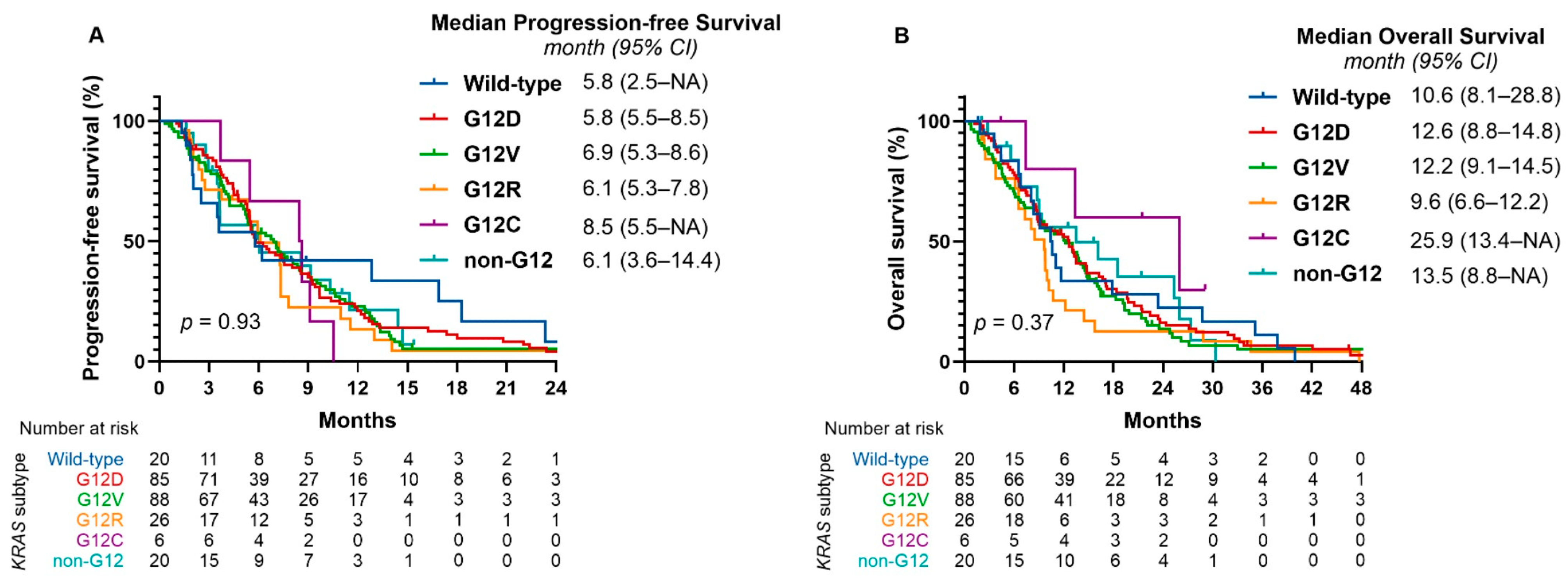
| Variable | Entire Cohort (n = 269) |
|---|---|
| Age, years | 66 (61–73) |
| Gender | |
| Male | 141 (52.4) |
| Female | 128 (47.6) |
| Stage at diagnosis | |
| Resectable | 96 (35.7) |
| Advanced | 173 (64.3) |
| Disease stage | |
| Locally advanced | 48 (17.8) |
| Metastatic | 221 (82.2) |
| Tumor location | |
| Head | 135 (50.2) |
| Body/Tail | 134 (49.8) |
| Histology | |
| Adenocarcinoma | 240 (89.2) |
| Others * | 29 (10.8) |
| Grade of differentiation | |
| Well to moderately | 153 (56.9) |
| Poorly | 53 (19.7) |
| Not specified | 63 (23.4) |
| Specimen type for NGS | |
| Primary tumor | 176 (65.4) |
| Metastatic sites | 76 (28.3) |
| Liquid biopsy | 17 (6.3) |
| Method of specimen acquisition | |
| Surgical resection | 115 (42.8) |
| Needle biopsy | 137 (50.9) |
| Peripheral blood | 17 (6.3) |
| KRAS status | |
| Wild type | 22 (8.2) |
| Mutant | 247 (91.8) |
| TP53 status | |
| Wild type | 96 (35.7) |
| Mutant | 173 (64.3) |
| CDKN2A status | |
| Wild type | 190 (70.6) |
| Mutant | 79 (29.4) |
| SMAD4 status | |
| Wild type | 236 (87.7) |
| Mutant | 33 (12.3) |
| Variable | Entire Cohort (n = 245) |
|---|---|
| Age, years | 66 (61–73) |
| <65 | 99 (40.4) |
| ≥65 | 146 (59.6) |
| Gender | |
| Male | 126 (51.4) |
| Female | 119 (48.6) |
| ECOG performance status | |
| 0–1 | 214 (87.4) |
| 2 | 31 (12.6) |
| Disease stage | |
| Locally advanced | 43 (17.5) |
| Metastatic | 202 (82.5) |
| Previous tumor resection | |
| No (Initially advanced) | 158 (64.5) |
| Yes (Recurrent disease) | 87 (35.5) |
| Grade of differentiation | |
| Well to moderately | 140 (57.1) |
| Poorly | 48 (19.6) |
| Not specified | 57 (23.3) |
| Number of metastatic organ sites | |
| 0–1 | 170 (69.4) |
| ≥2 | 75 (30.6) |
| Site of metastatic disease | |
| Liver | 131 (53.4) |
| Lung | 40 (16.3) |
| Peritoneum | 61 (24.9) |
| Baseline CA 19-9 level * | |
| <59 × ULN (U/mL) | 63 (25.7) |
| ≥59 × ULN (U/mL) | 176 (71.8) |
| Unknown | 6 (2.5) |
| First-line palliative chemotherapy | |
| Gemcitabine-based | 138 (56.3) |
| Gemcitabine/nab-paclitaxel | 132 (53.8) |
| Gemcitabine | 6 (2.5) |
| Fluorouracil-based | 107 (43.7) |
| FOLFIRINOX | 95 (38.7) |
| Nal-IRI/FL | 12 (5.0) |
| KRAS mutation status | |
| Wild type | 20 (8.2) |
| G12D | 85 (34.7) |
| G12V | 88 (35.9) |
| G12R | 26 (10.5) |
| G12C | 6 (2.5) |
| Non-G12 † | 20 (8.2) |
| Variable | Total (n = 245) | Wild Type (n = 20) | G12D (n = 85) | G12V (n = 88) | G12R (n = 26) | Non-G12 (n = 20) |
|---|---|---|---|---|---|---|
| Best overall response, n (%) | ||||||
| Partial response | 87 (35.5) | 4 (20.0) | 33 (38.8) | 30 (34.1) | 7 (26.9) | 11 (55.0) |
| Stable disease | 122 (49.8) | 11 (55.0) | 42 (49.4) | 45 (51.1) | 12 (46.2) | 8 (40.0) |
| Progressive disease | 36 (14.7) | 5 (25.0) | 10 (11.8) | 13 (14.8) | 7 (26.9) | 1 (5.0) |
| Objective response rate, n (%) | 87 (35.5) | 4 (20.0) | 33 (38.8) | 30 (34.1) | 7 (26.9) | 11 (55.0) |
| p value vs. G12D | 0.128 | NA | 0.532 | 0.352 | 0.214 | |
| Disease control rate, n (%) | 209 (85.3) | 15 (75.0) | 75 (88.2) | 75 (85.2) | 19 (73.1) | 19 (95.0) |
| p value vs. G12D | 0.156 | NA | 0.656 | 0.115 | 0.686 | |
| Median PFS, months [95% CI] | 6.3 [5.5–7.3] | 5.8 [2.5–NA] | 5.8 [5.5–8.5] | 6.9 [5.3–8.6] | 6.1 [5.3–7.8] | 6.1 [3.6–14.4] |
| p value vs. G12D | 0.718 | NA | 0.838 | 0.400 | 0.836 | |
| 6-month PFS, % [95% CI] | 51.8 [45.7–58.6] | 47.9 [29.1–78.6] | 49.1 [39.4–61.3] | 53.5 [43.7–65.4] | 53.7 [36.9–78.3] | 51.1 [32.5–80.2] |
| Median OS, months [95% CI] | 11.2 [9.6–13.1] | 10.6 [8.1–28.8] | 12.6 [8.8–14.8] | 12.2 [9.1–14.5] | 9.6 [6.6–12.2] | 13.5 [8.8–NA] |
| p value vs. G12D | 0.957 | NA | 0.496 | 0.146 | 0.714 | |
| 12-month OS, % [95%] | 48.0 [41.9–54.8] | 33.4 [17.4–64.2] | 51.8 [42.1–63.8] | 50.0 [40.4–61.8] | 25.4 [12.8–50.6] | 55.9 [37.1–84.2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, D.; Shin, K.; Hong, T.H.; Lee, S.H.; Kim, Y.; Kim, I.-H.; Hong, S.-H.; Lee, M.; Park, S.J. Clinical and Molecular Characteristics of KRAS Codon-Specific Mutations in Advanced Pancreatic Ductal Adenocarcinoma with Prognostic and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 10908. https://doi.org/10.3390/ijms262210908
Cho D, Shin K, Hong TH, Lee SH, Kim Y, Kim I-H, Hong S-H, Lee M, Park SJ. Clinical and Molecular Characteristics of KRAS Codon-Specific Mutations in Advanced Pancreatic Ductal Adenocarcinoma with Prognostic and Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(22):10908. https://doi.org/10.3390/ijms262210908
Chicago/Turabian StyleCho, Dongwoo, Kabsoo Shin, Tae Ho Hong, Sung Hak Lee, Younghoon Kim, In-Ho Kim, Sook-Hee Hong, MyungAh Lee, and Se Jun Park. 2025. "Clinical and Molecular Characteristics of KRAS Codon-Specific Mutations in Advanced Pancreatic Ductal Adenocarcinoma with Prognostic and Therapeutic Implications" International Journal of Molecular Sciences 26, no. 22: 10908. https://doi.org/10.3390/ijms262210908
APA StyleCho, D., Shin, K., Hong, T. H., Lee, S. H., Kim, Y., Kim, I.-H., Hong, S.-H., Lee, M., & Park, S. J. (2025). Clinical and Molecular Characteristics of KRAS Codon-Specific Mutations in Advanced Pancreatic Ductal Adenocarcinoma with Prognostic and Therapeutic Implications. International Journal of Molecular Sciences, 26(22), 10908. https://doi.org/10.3390/ijms262210908






