The Role of IL-17 in Periodontitis and Its Systemic Connections
Abstract
1. Introduction
Search Strategy and Study Selection
2. IL-17 as Pro-Inflammatory Marker in Periodontitis
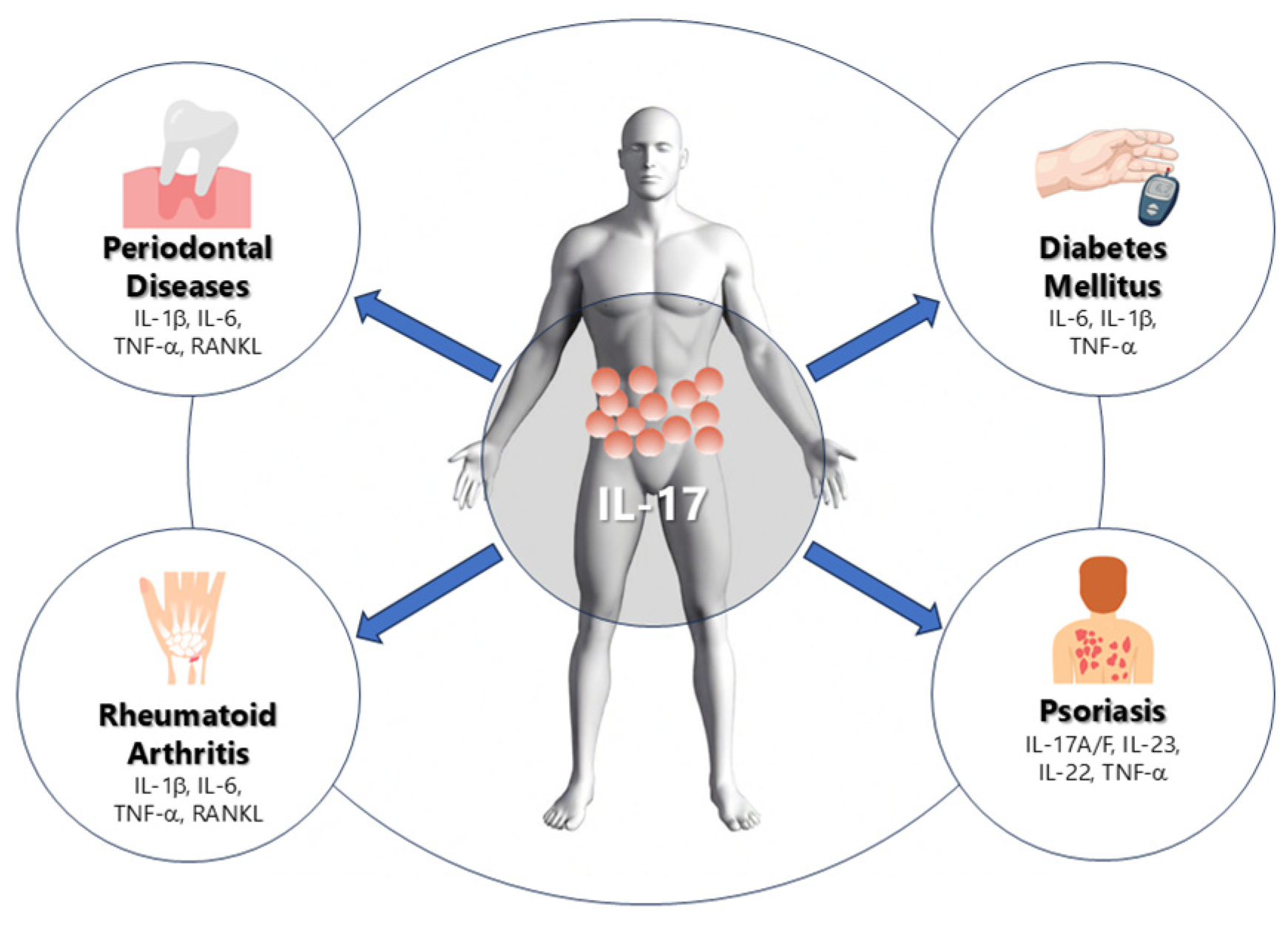
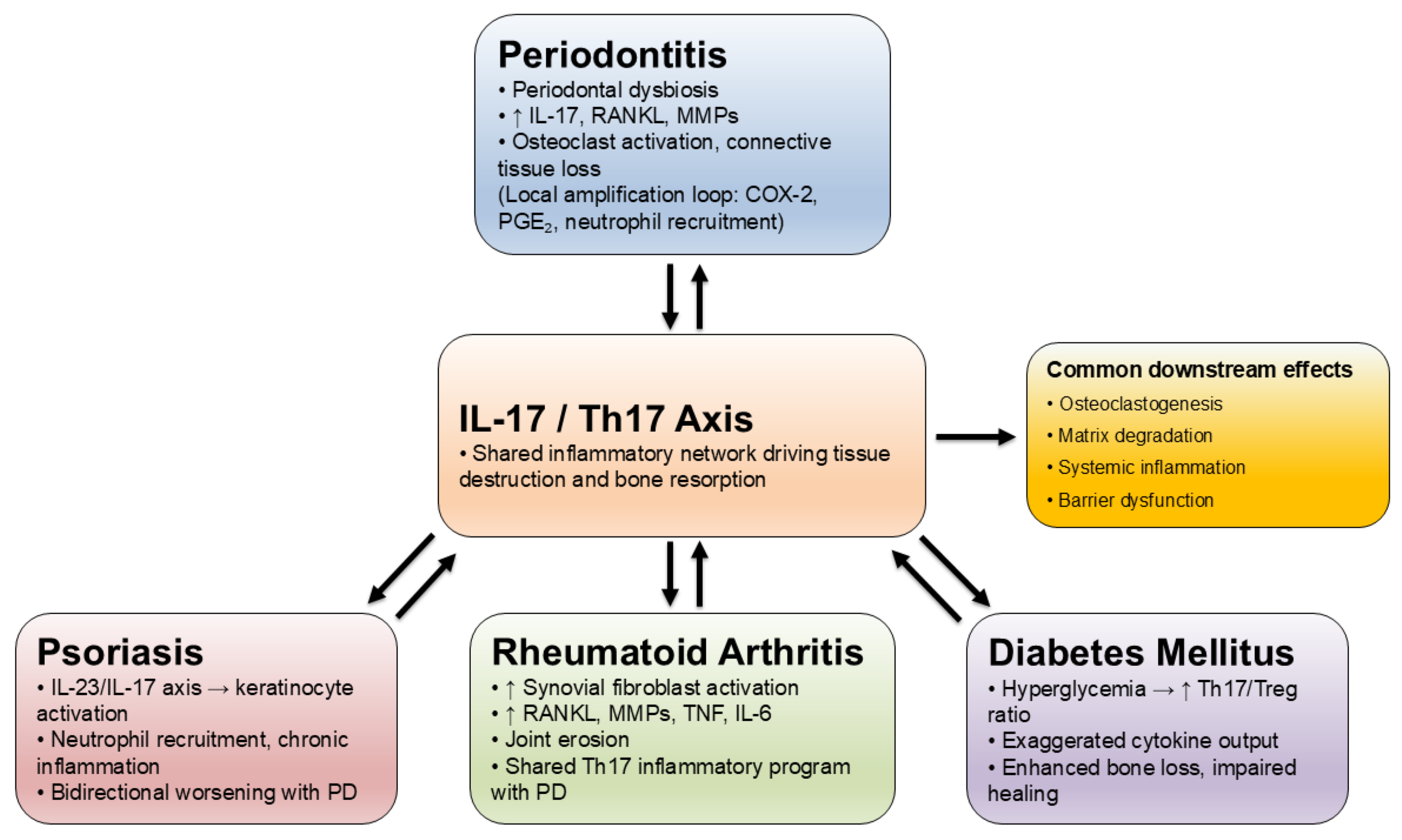
3. Relation Between IL-17 in Periodontitis and Other Systemic Diseases
3.1. IL-17 and Rheumatoid Arthritis
3.2. IL-17 and Diabetes
3.3. IL-17 and Psoriasis
4. IL-17 Polymorphisms in Periodontitis
4.1. IL-17A rs2275913
4.2. IL-17F rs763780
4.3. Clinical Implications
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgP | aggressive periodontitis |
| BI | bleeding index |
| CAL | clinical attachment loss |
| COX-2 | cyclooxygenase-2 |
| CP | chronic periodontitis |
| Del-1 | developmental endothelial locus-1 |
| F | female |
| FBG | fasting blood glucose |
| GAgP | generalized aggressive periodontitis |
| GCF | gingival crevicular fluid |
| GI | gingival index |
| HbA1c | glycated hemoglobin A1c |
| IL | interleukin |
| LAgP | localized aggressive periodontitis |
| M | male |
| NSPT | non-surgical periodontal treatment |
| OPG | osteoprotegerin |
| OPR | osteoporosis |
| PAL | periodontal attachment level |
| PASI | psoriasis area and severity index |
| PD | periodontitis |
| PGE2 | prostaglandin E2 |
| PPD | periodontal probing depth |
| RA | rheumatoid arthritis |
| RCT | randomized controlled trial |
| sRANKL | receptor activator of nuclear factor-kappa B ligand |
| SH | systemically healthy |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
References
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Kumar, S. Evidence-Based Update on Diagnosis and Management of Gingivitis and Periodontitis. Dent. Clin. N. Am. 2019, 63, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S44–S67. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Ozçaka, O.; Nalbantsoy, A.; Buduneli, N. Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. J. Periodontal Res. 2011, 46, 592–598. [Google Scholar] [CrossRef]
- Yu, J.J.; Ruddy, M.J.; Wong, G.C.; Sfintescu, C.; Baker, P.J.; Smith, J.B.; Evans, R.T.; Gaffen, S.L. An essential role for IL-17 in preventing pathogen-initiated bone destruction: Recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 2007, 109, 3794–3802. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Z.; Tu, S.-Q.; Wei, J.-M.; Hou, Y.-L.; Kuang, Z.-L.; Kang, X.-N.; Ai, H. Role of Interleukin-17A in the Pathomechanisms of Periodontitis and Related Systemic Chronic Inflammatory Diseases. Front. Immunol. 2022, 13, 862415. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, R.; Wang, R.; Wang, Y.; Guo, J. Global, regional, and national burden of periodontal diseases from 1990 to 2021 and predictions to 2040: An analysis of the global burden of disease study 2021. Front. Oral Health. 2025, 6, 1627746. [Google Scholar] [CrossRef]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Tang, Z.; Jin, L.; Yang, Y. The dual role of IL-17 in periodontitis regulating immunity and bone homeostasis. Front. Immunol. 2025, 16, 1578635. [Google Scholar] [CrossRef]
- Vernal, R.; Dutzan, N.; Chaparro, A.; Puente, J.; Valenzuela, M.A.; Gamonal, J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J. Clin. Periodontol. 2005, 32, 383–389. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Koertge, T.E.; Brooks, C.N.; Sabatini, R.; Purkall, D.E.; Tew, J.G. IL-17 in sera from patients with aggressive periodontitis. J. Dent. Res. 2010, 89, 943–947. [Google Scholar] [CrossRef]
- Gümüş, P.; Buduneli, E.; Bıyıkoğlu, B.; Aksu, K.; Saraç, F.; Nile, C.; Lappin, D.; Buduneli, N. Gingival crevicular fluid, serum levels of receptor activator of nuclear factor-κB ligand, osteoprotegerin, and interleukin-17 in patients with rheumatoid arthritis and osteoporosis and with periodontal disease. J. Periodontol. 2013, 84, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Mitani, A.; Niedbala, W.; Fujimura, T.; Mogi, M.; Miyamae, S.; Higuchi, N.; Abe, A.; Hishikawa, T.; Mizutani, M.; Ishihara, Y.; et al. Increased expression of interleukin (IL)-35 and IL-17, but not IL-27, in gingival tissues with chronic periodontitis. J. Periodontol. 2015, 86, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, H.L.; Warad, S.; Ashok, N.; Baroudi, K.; Tarakji, B. Association of Interleukin-17 polymorphism (-197G/A) in chronic and localized aggressive periodontitis. Braz. Oral Res. 2016, 30, S1806-83242016000100219. [Google Scholar] [CrossRef] [PubMed]
- Linhartova, P.B.; Kastovsky, J.; Lucanova, S.; Bartova, J.; Poskerova, H.; Vokurka, J.; Fassmann, A.; Kankova, K.; Holla, L.I. Interleukin-17A Gene Variability in Patients with Type 1 Diabetes Mellitus and Chronic Periodontitis: Its Correlation with IL-17 Levels and the Occurrence of Periodontopathic Bacteria. Mediat. Inflamm. 2016, 2016, 2979846. [Google Scholar] [CrossRef]
- Vahabi, S.; Nazemisalman, B.; Hosseinpour, S.; Salavitabar, S.; Aziz, A. Interleukin-2, -16, and -17 gene polymorphisms in Iranian patients with chronic periodontitis. J. Investig. Clin. Dent. 2018, 9, e12319. [Google Scholar] [CrossRef]
- Saxena, S.; Venugopal, R.; Chandrayan, R.R.; Yuwanati, M.B.; Awasthi, H.; Jain, M. Association of chronic periodontitis and type 2 diabetes mellitus with salivary Del-1 and IL-17 levels. J. Oral Biol. Craniofacial Res. 2020, 10, 529–534. [Google Scholar] [CrossRef]
- Jiménez, C.; Carvajal, D.; Hernández, M.; Valenzuela, F.; Astorga, J.; Fernández, A. Levels of the interleukins 17A, 22, and 23 and the S100 protein family in the gingival crevicular fluid of psoriatic patients with or without periodontitis. An. Bras. Dermatol. 2021, 25, 163–170. [Google Scholar] [CrossRef]
- Wu, K.J.; Tu, C.C.; Hu, J.X.; Chu, P.H.; Ma, K.S.K.; Chiu, H.Y.; Kuo, M.Y.P.; Tsai, T.F.; Chen, Y.W. Severity of periodontitis and salivary interleukin-1β are associated with psoriasis involvement. J. Formos. Med. Assoc. 2022, 121, 1908–1916. [Google Scholar] [CrossRef]
- Malvandi, M.; Jazi, M.S.; Fakhari, E. Association of interleukin-17A gene promoter polymorphism with the susceptibility to generalized chronic periodontitis in an Iranian population. Dent. Res. J. 2022, 19, 85. [Google Scholar] [CrossRef]
- Nair, V.; Grover, V.; Arora, S.; Das, G.; Ahmad, I.; Ohri, A.; Sainudeen, S.; Saluja, P.; Saha, A. Comparative Evaluation of Gingival Crevicular Fluid Interleukin-17, 18 and 21 in Different Stages of Periodontal Health and Disease. Medicina 2022, 58, 1042. [Google Scholar] [CrossRef]
- Wankhede, A.N.; Dhadse, P.V. Interleukin-17 levels in gingival crevicular fluid of aggressive periodontitis and chronic periodontitis patients. J. Indian Soc. Periodontol. 2022, 26, 552–556. [Google Scholar] [CrossRef]
- Mazurek-Mochol, M.; Serwin, K.; Bonsmann, T.; Kozak, M.; Piotrowska, K.; Czerewaty, M.; Safranow, K.; Pawlik, A. Expression of Interleukin 17A and 17B in Gingival Tissue in Patients with Periodontitis. J. Clin. Med. 2023, 12, 4614. [Google Scholar] [CrossRef]
- Xu, X.R.; Xu, J.L.; He, L.; Wang, X.E.; Lu, H.Y.; Meng, H.X. Comparison of the inflammatory states of serum and gingival crevicular fluid in periodontitis patients with or without type 2 diabetes mellitus. J. Dent. Sci. 2023, 18, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Altaca, M.; Cebesoy, E.I.; Kocak-Oztug, N.A.; Bingül, I.; Cifcibasi, E. Interleukin-6, -17, and -35 levels in association with clinical status in stage III and stage IV periodontitis: A cross-sectional study. BMC Oral Health 2024, 24, 1015. [Google Scholar] [CrossRef] [PubMed]
- Ezgi, S.T.; Gulay, T.; Aysegul, A.Y.; Melek, Y. Non-surgical periodontal treatment effects on IL-17 and IL-35 levels in smokers and non-smokers with periodontitis. Odontology 2025, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; van Asten, S.D.; Burns, L.A.; Evans, H.G.; Walter, G.J.; Hashim, A.; Hughes, F.J.; Taams, L.S. Periodontitis-associated pathogens P. gingivalis and A. actinomycetemcomitans activate human CD14(+) monocytes leading to enhanced Th17/IL-17 responses. Eur. J. Immunol. 2016, 46, 2211–2221. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Guerrero-Velázquez, C.; Becerra-Ruiz, J.S.; Rodríguez-Montaño, R.; Avetisyan, A.; Heboyan, A. IL-23/IL-17 axis levels in gingival crevicular fluid of subjects with periodontal disease: A systematic review. BMC Oral Health 2024, 24, 302. [Google Scholar] [CrossRef]
- Sidharthan, S.; Gopalakrishnan, D.; Kheur, S.; Mohapatra, S. Assessment of the role of Th17 cell and related biomarkers in periodontitis: A systematic review. Arch. Oral Biol. 2025, 175, 106272. [Google Scholar] [CrossRef]
- Baumeister, S.; Holtfreter, B.; Reckelkamm, S.L.; Hagenfeld, D.; Kocher, T.; Alayash, Z.; Ehmke, B.; Baurecht, H.; Nolde, M. Effect of interleukin-17 on periodontitis development: An instrumental variable analysis. J. Periodontol. 2023, 94, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The Oral Microbiota Is Modified by Systemic Diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Eskandari-Nasab, E.; Moghadampour, M.; Tahmasebi, A. Meta-Analysis of Risk Association Between Interleukin-17A and F Gene Polymorphisms and Inflammatory Diseases. J. Interferon Cytokine Res. 2017, 37, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Tandon, A.; AlQallaf, H.; John, V.; Sinha, M.; Gibson, M.P. Inflammatory Association between Periodontal Disease and Systemic Health. Inflammation 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gremese, E.; Tolusso, B.; Bruno, D.; Perniola, S.; Ferraccioli, G.; Alivernini, S. The forgotten key players in rheumatoid arthritis: IL-8 and IL-17—Unmet needs and therapeutic perspectives. Front. Med. 2023, 10, 956127. [Google Scholar] [CrossRef]
- Zhang, F.; Jonsson, A.H.; Nathan, A.; Millard, N.; Curtis, M.; Xiao, Q.; Gutierrez-Arcelus, M.; Apruzzese, W.; Watts, G.F.M.; Weisenfeld, D.; et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 2023, 623, 616–624. [Google Scholar] [CrossRef]
- Vitales-Noyola, M.; Hernández-Castro, B.; Alvarado-Hernández, D.; Baranda, L.; Bernal-Silva, S.; Abud-Mendoza, C.; Niño-Moreno, P.; González-Amaro, R. Levels of Pathogenic Th17 and Th22 Cells in Patients with Rheumatoid Arthritis. J. Immunol. Res. 2022, 2022, 5398743. [Google Scholar] [CrossRef]
- Samaan, S.F.; Taha, S.I.; Mahmoud, F.A.; Elsaadawy, Y.; Khalil, S.A.; Gamal, D.M. Role of Interleukin-17 in Predicting Activity of Rheumatoid Arthritis and Systemic Lupus Erythematosus. Clin. Med. Insights. Arthritis Musculoskelet. Disord. 2024, 17, 11795441241276880. [Google Scholar] [CrossRef]
- Berthelot, J.M.; Le Goff, B. Rheumatoid arthritis and periodontal disease. Jt. Bone Spine 2010, 77, 537–541. [Google Scholar] [CrossRef]
- Xia, X.; He, C.; Xue, Z.; Wang, Y.; Qin, Y.; Ren, Z.; Huang, Y.; Luo, H.; Chen, H.-N.; Zhang, W.-H.; et al. Single cell immunoprofile of synovial fluid in rheumatoid arthritis with TNF/JAK inhibitor treatment. Nat. Commun. 2025, 16, 2152. [Google Scholar] [CrossRef]
- Huang, Z.; Pei, X.; Graves, D.T. The Interrelationship Between Diabetes, IL-17 and Bone Loss. Curr. Osteoporos. Rep. 2020, 18, 23–31. [Google Scholar] [CrossRef]
- Borgnakke, W.S. Current scientific evidence for why periodontitis should be included in diabetes management. Front. Clin. Diabetes Health 2024, 4, 1257087. [Google Scholar] [CrossRef] [PubMed]
- Brembilla, N.C.; Boehncke, W.H. Revisiting the interleukin 17 family of cytokines in psoriasis: Pathogenesis and potential targets for innovative therapies. Front. Immunol. 2023, 14, 1186455. [Google Scholar] [CrossRef] [PubMed]
- Marruganti, C.; Gaeta, C.; Falciani, C.; Cinotti, E.; Rubegni, P.; Alovisi, M.; Scotti, N.; Baldi, A.; Bellan, C.; Defraia, C.; et al. Are periodontitis and psoriasis associated? A pre-clinical murine model. J. Clin. Periodontol. 2024, 51, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Di Palo, M.P.; Rupe, A.; Piedepalumbo, F.; Sessa, A.; De Benedetto, G.; Barone, S.R.; Contaldo, M. Periodontitis in Psoriatic Patients: Epidemiological Insights and Putative Etiopathogenic Links. Epidemiologia 2024, 5, 479–498. [Google Scholar] [CrossRef]
- Majchrzycka, M.; Andrzejewska, M.; Surdacka, A.; Surdacki, M.; Adamski, Z. Evaluation of the relationship between psoriasis, periodontitis, and markers of inflammation. Postepy Dermatol. Alergol. 2022, 39, 1123–1127. [Google Scholar] [CrossRef]
- Rodríguez-Montaño, R.; Alarcón-Sánchez, M.A.; Lomelí-Martínez, S.M.; Martínez-Bugarin, C.H.; Heboyan, A. Genetic Variants of the IL-23/IL-17 Axis and Its Association With Periodontal Disease: A Systematic Review. Immun. Inflamm. Dis. 2025, 13, e70147. [Google Scholar] [CrossRef]
- Sasikumar, P.K.; Varghese, S.S.; Kumaran, T.; Devi, S.S. Meta-analysis of risk association between interleukin-17A rs2275913 and chronic periodontitis. Contemp. Clin. Dent. 2020, 11, 3–9. [Google Scholar] [CrossRef]
- Corrêa, J.D.; Madeira, M.F.M.; Resende, R.G.; Correia-Silva, J.d.F.; Gomez, R.S.; Souza, D.d.G.d.; Teixeira, M.M.; Queiroz-Junior, C.M.; da Silva, T.A. Association between polymorphisms in interleukin-17A and -17F genes and chronic periodontal disease. Mediat. Inflamm. 2012, 2012, 846052. [Google Scholar] [CrossRef]
- Farmohammadi, A.; Tavangar, A.; Ehteram, M.; Karimian, M. Association of A-197G polymorphism in interleukin-17 gene with chronic periodontitis: Evidence from six case-control studies with a computational biology approach. J. Investig. Clin. Dent. 2019, 10, e12424. [Google Scholar] [CrossRef]
- da Silva, F.R.P.; Pessoa, L.D.S.; Vasconcelos, A.C.C.G.; de Aquino, L.W.; Alves, E.H.P.; Vasconcelos, D.F.P. Polymorphisms in interleukins 17A and 17F genes and periodontitis: Results from a meta-analysis. Mol. Biol. Rep. 2017, 44, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Mazurek-Mochol, M.; Kozak, M.; Malinowski, D.; Safranow, K.; Pawlik, A. IL-17F Gene rs763780 and IL-17A rs2275913 Polymorphisms in Patients with Periodontitis. Int. J. Environ. Res. Public Health 2021, 18, 1081. [Google Scholar] [CrossRef] [PubMed]
- Alsherif, E.; Alhudiri, I.; ElJilani, M.; Ramadan, A.; Rutland, P.; Elzagheid, A.; Enattah, N. Screening of interleukin 17F gene polymorphisms and eight subgingival pathogens in chronic periodontitis in Libyan patients. Libyan J. Med. 2023, 18, 2225252. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.M.; e Silva, M.R.M.A.; Silva, J.d.F.C.; da Costa, J.E.; Gollob, K.J.; Dutra, W.O.; Moreira, P.R. Evaluation of IL17A expression and of IL17A, IL17F and IL23R gene polymorphisms in Brazilian individuals with periodontitis. Hum. Immunol. 2013, 74, 207–214. [Google Scholar] [CrossRef]
- Liu, X.; Li, H. A Systematic Review and Meta-Analysis on Multiple Cytokine Gene Polymorphisms in the Pathogenesis of Periodontitis. Front. Immunol. 2022, 12, 713198. [Google Scholar] [CrossRef]
- Wilharm, A.; Binz, C.; Sandrock, I.; Rampoldi, F.; Lienenklaus, S.; Blank, E.; Winkel, A.; Demera, A.; Hovav, A.-H.; Stiesch, M.; et al. Interleukin-17 is disease promoting in early stages and protective in late stages of experimental periodontitis. PLoS ONE 2022, 17, e0265486. [Google Scholar] [CrossRef]
- Bai, Y.; Xie, P.; Jin, Z.; Qin, S.; Ma, G. Leveraging genetics to investigate causal effects of immune cell phenotypes in periodontitis: A mendelian randomization study. Front. Genet. 2024, 15, 1382270. [Google Scholar] [CrossRef]
- Raimondo, A.; Spirito, F.D.; Lembo, S. Oral Diseases During Systemic Psoriatic Drugs: A Review of the Literature and Case Series. Dermatol. Pr. Concept. 2024, 14, e2024107. [Google Scholar] [CrossRef]

| Authors, Year, Country | Participants | Periodontal Evaluation | Purpose | Study Limitations | |||
|---|---|---|---|---|---|---|---|
| Amount | Sex (M—Male, F—Female) | Age | |||||
| Healthy | Patients | ||||||
| Vernal et al., 2005, Chile [11] | 8 | 16 | Healthy: 5 M, 11 F Patients: 5 M, 9 F | Healthy: 36.4 ± 7.9 Patients: 38.4 ± 8.2 | A minimum of 5–6 teeth exhibited periodontal probing depths > 5 mm, attachment loss > 3 mm, and extensive bone loss evident on radiographs. | To evaluate IL-17 levels in gingival crevicular fluid and in the cultured supernatants of gingival cells derived from individuals diagnosed with chronic periodontitis. | Small cohort study (n = 24) and single-center recruitment limit statistical power. IL-17 quantification in GCF is prone to intra-individual variability and sampling bias. |
| Schenkein et al., 2010, USA [12] | 67 | 102 | N/A | Healthy: 22.9 ± 0.9 Patients: LAgP: 20.4 ± 1.0 GAgP: 31.2 ± 1.1 | A minimum of 8 teeth showed interproximal attachment loss of ≥5 mm with no fewer than 3 of these teeth being other than first molars or incisors. Individuals diagnosed with localized aggressive periodontitis had disease onset before the age of 30, exhibiting ≥ 5 mm interproximal attachment loss confined to the first molars and incisors, and affecting no more than 2 additional teeth. | Measurement of IL-17 serum concentrations in healthy and diseased patients for comparison. | Unequal group sizes and age distribution across LAgP, GAgP, and healthy groups may confound cytokine comparisons. Serum IL-17 reflects systemic rather than local inflammation, potentially masking periodontal specificity. |
| Gümüş et al., 2013, Turkey [13] | 17 | 49 | Healthy: 11 F Patients: 49 F | N/A | Patients with more than 10 teeth suffering from an inflammatory periodontal disease and osteoporosis or rheumatoid arthritis. | To assess the levels of soluble receptor activator of nuclear factor-kappa B ligand, interleukin (IL)-17A, IL-17E, IL-17F, IL-17A/F, and osteoprotegerin in gingival crevicular fluid and serum of women with rheumatoid arthritis, osteoporosis, and systemically healthy controls, all presenting with periodontal disease. | Female-only cohort; heterogeneity due to inclusion of RA and osteoporosis groups. Medication use (anti-inflammatory or anti-resorptive drugs) could suppress cytokine levels, introducing treatment-related bias. |
| Mitani et al., 2015 Japan [14] | 40 | 86 | N/A | N/A | A minimum of six teeth exhibited sites with PPD ≥ 5 mm, CAL ≥ 6 mm, as well as significant bone loss visible on radiographs. | Examination of the production and expression of IL-35, IL-17, and IL-27 in human gingival tissue and gingival crevicular fluid. | No demographic stratification (age, sex, systemic status) reported. Cross-sectional design without longitudinal follow-up. Cytokine detection limited to GCF and tissue—no serum comparison to verify systemic correspondence. |
| Chaudari et al., 2016, India [15] | 35 | 70 | LAgP patients: 19 M 16 F chronic P. patients: 18 M 17 F healthy subjects: 19 M 16 F | Patients: 37.20 ± 4.21 Healthy: 21.23 ± 4.56 | Localized aggressive periodontitis was defined as interproximal CAL impacting a minimum of two permanent teeth, including at least one first molar, and affecting no more than two additional teeth besides the first molars and incisors. CP patients had at least 20 natural teeth and a minimum of six periodontal pockets measuring ≥ 5 mm, or CAL of ≥3 mm, along with local factors associated with the destruction of periodontal tissues. | To investigate the association of IL-17 polymorphism (-197G/A) in CP and LAgP. | Limited genetic sample (n = 105) lacks power to detect small effects. No multivariate control for smoking or oral hygiene. Diagnostic criteria for CP and LAgP may overlap, risking misclassification. |
| Linhartova et al., 2016, Czech [16] | 154 | 407 | Healthy: 75 M 79 F Patients: 195 M 212 F | Healthy: 48.5 ± 10.7 Patients: CP: 52.5 ± 9.8 T1DM: 46.4 ± 13.8 T1DM + CP: 49.9 ± 10.6 | The determination of PD or non-PD was made using a thorough clinical examination, assessment of medical and dental history, evaluation of tooth mobility, and radiographic examination. | Investigation of IL-17A −197A/G and IL-17F +7488C/T polymorphisms in individuals with T1DM and CP; evaluation of their associations with IL-17 production and the presence of periodontal pathogens. | Potential population stratification not adjusted for. Glycemic control in T1DM participants not standardized. Cross-sectional framework cannot separate cause from effect regarding IL-17 and T1DM comorbidity. |
| Vahabi et al., 2018, Iran [17] | 75 | 99 | Healthy: 29 M 46 F Patients: 56 M 43 F | Patients: 40.28 (mean) Healthy: 31.67 (mean) | In the present study, CP cases with a CAL of ≥5 mm in more than 30% of their remaining teeth were classified as severe CP, those with 3–4 mm CAL as moderate CP, and those with 1–2 mm CAL as mild CP. | To examine the relationship between IL-2 (T-330G), IL-16 (T-295C), and IL-17 (A-7383G) gene variants and the risk of developing CP in an Iranian population. | Small, hospital-based sample. Possible genotyping errors unverified by replication. Environmental or lifestyle confounders (smoking, diet) not accounted for. Only mild/moderate CP cases analyzed, reducing phenotype spectrum. |
| Saxena et al., 2020, India [18] | 17 | 68 | N/A | N/A | Individuals were classified as having CP if they had sites with CAL of ≥4 mm PD of ≥5 mm at four or more teeth per jaw and alveolar bone loss of ≥50% in two or more quadrants. | Investigation of the relationship between salivary levels of developmental endothelial locus-1 and IL-17 in chronic periodontitis. | Small single-center sample; limited control of systemic comorbidities such as diabetes or smoking. Cross-sectional saliva-based design does not establish temporal relationships. |
| Jiménez et al., 2021, Chile [19] | 39 | 43 | Healthy: 21 M 18 F Patients: 18 M 25 F | Individuals with untreated PD: 42.95 (mean) Individuals with untreated psoriasis and untreated PD: 50.2 (mean) | Severe periodontal disease was classified as having at least two interproximal sites with CAL ≥ 6 mm on separate teeth along with at least one interproximal site with probing pocket depth ≥ 5 mm. Moderate PD was classified as having at least two interproximal sites with CAL ≥ 4 mm on different teeth and/or at least two interproximal sites with PPD ≥ 5 mm. Cases were considered no or mild PD if they did not fulfill the criteria for either severe or moderate PD. | To assess the concentrations of IL-17A, IL-22, IL-23, S100A7, S100A8, and S100A9 in gingival crevicular fluid of psoriatic and periodontally healthy individuals, both with and without periodontal disease, and to examine their association with psoriasis severity. | Moderate sample size, psoriasis severity and systemic therapy not controlled for possibly affecting cytokine output. Multiplex cytokine measurements increase multiple-testing risk without correction. |
| Wu et al., 2022, Taiwan [20] | 0 | 33 | Patients: 21 M 12 F | Patients: 48.58 ± 2.07 | The prevalence of periodontal disease was 100%, plaque score was 1.10 ± 0.11, gingival score was 1.37 ± 0.12, sites with probing depth around 4 mm were 9.88 ± 1.57%, sites with CAL around 4 mm were 52.15 ± 4.78%, and the mean number of remaining teeth was 25.91 ± 0.66. | To investigate the relationship between psoriasis severity and periodontal tissue destruction in patients with psoriasis. | No healthy or non-psoriatic control group, restricting generalizability. Periodontal diagnosis based on limited indices, lacking radiographic validation. Cannot determine if psoriasis precedes or follows PD changes. |
| Malvandi et al., 2022, Iran [21] | 118 | 54 | Healthy: 42 M 76 F Patients: 27 M 27 F | Patients: 39.89 ± 6.1 Healthy: 39.25 ± 10.7 | Presence of CAL measuring ≥ 5 mm and probing pocket depth of ≥4 mm affecting over 30% of the evaluated sites. | To examine the potential association of the IL-17 gene promoter polymorphic site 197 G > A (rs2275913) with generalized severe chronic periodontitis among individuals of Iranian origin. | Small cohort (n = 172) for genetic association. Lack of adjustment for smoking or microbial profile. Findings require replication in independent populations. |
| Nair et al., 2022, India [22] | 30 | 60 | M:F = 45:45 | 20–50 | GI, PPD, CAL. | Quantify GCF IL-17, IL-18, IL-21 across health, gingivitis, CP. | Sample size insufficient for multivariate analysis. Matching of controls not described. Cytokine assessment limited to GCF without confirmation in tissue or serum compartments. |
| Wankhede et al., 2022, India [23] | 30 | 60 | N/A | Average of all participants = 37.5 years. Participants between 20 and 65 years | Group 1 consisted of participants under 35 years of age who had at least six permanent teeth, excluding the incisors and first molars, with probing pocket depths and periodontal attachment levels of ≥5 mm. Group 2 (CP) included participants exhibiting clinical signs of gingival inflammation, with at least six teeth in each jaw, PPD ≥ 4 mm, and PAL ≥ 4 mm. | To show the relation between IL-17 and severity of PD (AgP, CP). | Small, convenience sample with wide age range. Clinical measurements may vary by examiner. No information on systemic health or medications; potential inter-group differences in oral hygiene. |
| Mazurek-Mochol et al., 2023, Poland [24] | 14 | 14 | Healthy: 5 M, 9 F Patients: 5 M, 9 F | Healthy: 54.2 ± 12.5 Patients: 52.8 ± 11.3 | Interdental CAL of ≥2 mm was present at two or more non-adjacent teeth, or buccal/lingual CAL ≥ 3 mm; periodontal pockets exceeding 3 mm were found at two or more teeth, with the attachment loss not explained by non-periodontal factors; bleeding on probing was noted at sites with deep probing depths ≥ 5 mm; and radiographs showed a minimum of 15% bone loss. | Investigation of the expression patterns and spatial distribution of IL-17A and IL-17B within gingival tissues of individuals affected by periodontal disease. | Very small (n = 28) exploratory sample. Immunohistochemical quantification semi-quantitative and subjective. |
| Xu et al., 2023, China [25] | 20 | 80 | Healthy: 8 M, 12 F Patients: 40 M, 40 F | Healthy: 33.55 ± 6.98 Patients: 52.23 ± 9.65 (CP) 57.23 ± 11.63 (CP+T2DM) | PPD, bleeding index (BI), HbA1c, CAL, FBG. | Compare serum and GCF inflammatory states in CP with/without T2DM vs. healthy. | Cross-sectional design; glycemic control and antidiabetic therapy non-standardized. Potential ethnic limitation to East Asian population. Serum vs. GCF cytokine comparison may be influenced by sampling time. |
| Altaca et al., 2024, Turkey [26] | 30 | 30 | Healthy: 10 M, 20 F Patients: 18 M, 12 F | Healthy: 28.83 ± 9.95 Patients: 40.93 ± 10.14 | Full-mouth, GI, PPD, CAL. | Compare GCF IL-6, IL-17, IL-35 in healthy vs. stage III, IV; correlate with indices. | Modest sample size (n = 60) and stage grouping only (III–IV) without full staging–grading framework. Lack of longitudinal assessment to determine IL-17 modulation post-therapy. |
| Ezgi et al., 2025, Turkey [27] | 18 | 37 | N/A | N/A | Baseline and 4 weeks post-non-surgical periodontal treatment (NSPT). | Evaluate the impact of non-surgical periodontal therapy on salivary and gingival crevicular fluid levels of IL-17 and IL-35 in smokers compared to non-smokers. | Small sample size (n = 55) with short follow-up (4 weeks). Possible assay variability between saliva and GCF samples. No blinding or placebo control; smoking status confounds cytokine changes after NSPT. |
| Authors, Year, Country | Method of Analysis | Collected Sample | Main Results | Conclusion |
|---|---|---|---|---|
| Vernal et al., 2005, Chile [11] | ELISA | GCF | 1. The concentration of interleukin-17 (IL-17) in the gingival crevicular fluid of patients with periodontitis is markedly elevated compared to that observed in healthy individuals. 2. T lymphocytes contribute to the synthesis of IL-17 within gingival tissues. 3. IL-17 has been shown to induce macrophages to release several pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β). | An elevation in IL-17 levels within the gingival crevicular fluid of patients with periodontitis suggests that this cytokine may play a contributory role in the pathogenesis of chronic periodontitis (CP). |
| Schenkein et al., 2010, USA [12] | ELISA | Serum | 1. Serum IL-17 levels were scarcely detectable in periodontally healthy individuals, whereas markedly elevated concentrations were observed in patients with localized and generalized aggressive periodontitis. 2. Multivariate analysis revealed a significant association between IL-17 concentrations and periodontal attachment loss, while no correlation was found with smoking status. 3. Th17-mediated immune responses appear to be a distinctive feature of aggressive periodontitis, suggesting a potential pathogenic role of IL-17 in this condition. | It is plausible that elevated serum IL-17 levels, originating primarily from gingival tissues, may contribute to the propagation of inflammatory responses in sites distant from the oral cavity. |
| Gümüş et al., 2013, Turkey [13] | ELISA | GCF + Serum | Although the total gingival crevicular fluid levels of albumin, osteoprotegerin (OPG), IL-17A, and IL-17A/F did not differ significantly among the study groups, notable differences were observed in the GCF concentrations of soluble RANKL (sRANKL), OPG, IL-17A, IL-17E, IL-17F, and IL-17A/F. The sRANKL/OPG ratio was markedly higher in the rheumatoid arthritis group compared with the osteoporotic and systemically healthy (SH) groups. | The elevated levels of inflammatory mediators observed in patients with rheumatoid arthritis, despite the prolonged administration of anti-inflammatory therapy, suggest an inherent tendency of these individuals to overproduce such mediators. |
| Mitani et al., 2015, Japan [14] | ELISA | GCF + gingival tissue | 1. Concentrations of IL-35 and IL-17 in gingival crevicular fluid were significantly elevated in patients with periodontal disease compared with periodontally healthy participants. 2. A positive correlation was identified between IL-17 levels and CAL. 3. Expression of IL-17A mRNA was markedly upregulated in inflamed gingival tissues relative to healthy control samples. | Interleukin-35 (IL-35) and interleukin-17 (IL-17), but not interleukin-27 (IL-27), appear to be involved in the pathogenic mechanisms underlying periodontal disease. |
| Chaudari et al., 2016, India [15] | DNA genotyping | Serum | A statistically significant difference in genotype distribution was observed among patients with chronic periodontitis, localized aggressive periodontitis, and healthy subjects. Additionally, a significant variation in allele frequencies was detected within the chronic periodontitis (CP) group. | Polymorphic variation in the IL-17A gene at the −197A/G locus has been implicated in the development of chronic periodontitis and localized aggressive periodontitis within the Indian population. Possession of the A allele at this site appears to confer an increased genetic predisposition to both forms of periodontal disease. |
| Linhartova et al., 2016, Czech [16] | Immunohistochemical analysis | Subgingival and blood samples | No significant differences were observed in allele or genotype frequencies between patients with chronic periodontitis and those with both type 1 diabetes mellitus and chronic periodontitis. However, the presence of the A allele at the IL-17A−197 locus was associated with an increased risk of developing type 1 diabetes mellitus. Furthermore, carriers of the A allele among patients with type 1 diabetes mellitus exhibited significantly higher HbA1c levels. | Variations within the IL-17A gene may partially affect the metabolic control of type 1 diabetes mellitus and the prevalence of “red complex” bacteria in patients with chronic periodontitis, including those with diabetes. Moreover, the findings support the functional significance of the IL-17A polymorphism, as individuals carrying the A allele exhibited increased IL-17 secretion. |
| Vahabi et al., 2018, Iran [17] | DNA genotyping | Serum | No polymorphic variation in IL-2 (T-330G) was identified among the examined patients, as the TT genotype was detected in both study groups. Furthermore, none of the evaluated genotypes or alleles of IL-16 (T-295C) and IL-17 (A-7383G) demonstrated a significant association with chronic periodontitis. | Analysis of the Iranian population revealed no evidence of a relationship between the IL-2 (T-330G), IL-16 (T-295C), or IL-17 (A-7383G) genotypes and the occurrence of chronic periodontitis. |
| Saxena et al., 2020, India [18] | ELISA | Saliva | 1. Levels of developmental endothelial locus-1 (Del-1) and interleukin-17 were correlated with the degree of inflammation in chronic periodontitis, and the coexistence of type 2 diabetes mellitus further intensified disease severity. An inverse relationship between salivary Del-1 and IL-17 concentrations was also observed. 2. The coexistence of type 2 diabetes mellitus and chronic periodontitis was associated with elevated salivary IL-17 levels and a concomitant reduction in Del-1 levels. | An upregulation of salivary IL-17 and a concomitant downregulation of salivary Del-1 were observed with increasing severity of periodontal disease and type 2 diabetes mellitus. Moreover, the coexistence of type 2 diabetes mellitus in patients with chronic periodontitis may exacerbate inflammation-driven periodontal tissue destruction. |
| Jiménez et al., 2021, Chile [19] | ELISA | GCF | No significant differences were observed among the groups in the concentrations of IL-17A, IL-22, IL-23, or S100A7. | Findings from the analysis of immune components in gingival crevicular fluid suggest no evident immunological association between periodontal disease and psoriasis. |
| Wu et al., 2022, Taiwan [20] | Immunohistochemical analysis | Saliva | Baseline psoriasis severity showed no significant relationship with salivary concentrations of IL-1β, IL-12, IL-17A, interferon-γ, or tumor necrosis factor-α. | Salivary IL-1β, plaque accumulation, and CAL demonstrated associations with the Psoriasis Area and Severity Index. Greater periodontal impairment corresponded to more extensive psoriatic manifestations. The inflammatory burden within periodontal tissues in psoriasis appears to be modulated by anti-inflammatory medication and smoking habits. IL-17, however, did not exhibit any measurable impact on psoriasis severity. |
| Malvandi et al., 2022, Iran [21] | DNA genotyping | Serum | Although none of the inheritance models revealed a significant association between periodontal disease risk and genotype distribution, certain patterns were noted. The GG genotype occurred more frequently among periodontally healthy controls, whereas the AG genotype was predominant in patients under the codominant model. Under the overdominant model, the GGAA and AG genotypes were more common in healthy individuals. | The A allele and AG genotype of IL-17 may represent potential contributors to an increased susceptibility to generalized chronic periodontitis. Although noticeable differences in allele and genotype distributions were observed between the groups, these differences did not reach statistical significance. |
| Nair et al., 2022, India [22] | ELISA | GCF | IL-17, IL-18, IL-21 lowest in health, highest in CP. IL-17 and IL-21 correlated with CAL. | IL-17 in GCF tracks periodontal inflammation and severity |
| Wankhede et al. 2022, India [23] | Immunohistochemical analysis | GCF | 1. A significant positive correlation was observed between IL-17 concentrations and both probing attachment loss and probing pocket depth in aggressive periodontitis. 2. In chronic periodontitis, IL-17 levels correlated positively with probing attachment loss, whereas no significant association was found with probing pocket depth. | IL-17 concentrations in gingival crevicular fluid were elevated in both aggressive and chronic periodontitis compared with periodontally healthy sites with higher levels detected in chronic periodontitis than in aggressive periodontitis. |
| Mazurek-Mochol et al., 2023, Poland [24] | Immunohistochemical analysis | Gingival tissue | 1. Enhanced IL-17 protein expression within gingival tissues of individuals affected by periodontal disease appears to result from post-translational regulatory mechanisms. 2. A direct association was identified between IL-17A expression and the local plaque index. 3. Transcriptional activity of IL-17B was markedly reduced in gingival tissues from patients with periodontal disease compared with those from periodontally healthy controls. 4. Expression of IL-17B at the mRNA level demonstrated a positive correlation with CAL. | Upregulation of IL-17 protein expression in gingival tissues of patients with periodontal disease appears to arise from post-translational modifications. |
| Xu et al., 2023, China [25] | ELISA | GCF + serum | Gingival crevicular fluid volume, total IL-17 concentration, and the RANKL/OPG ratio were elevated in patients with chronic periodontitis and in those with both chronic periodontitis and type 2 diabetes mellitus compared with periodontally healthy controls. These parameters were also higher in patients with chronic periodontitis and type 2 diabetes mellitus than in those with chronic periodontitis alone and showed a positive correlation with fasting blood glucose levels. | Diabetes amplifies local and systemic inflammation, including GCF IL-17, in PD. |
| Altaca et al., 2024, Turkey [26] | ELISA | GCF | PD—higher IL-6, IL-17, IL-35 vs. healthy. IL-17 correlated with PD and with GI, PPD, CAL in stage IV. | GCF IL-17 is elevated in severe PD and is associated with disease severity. |
| Ezgi et al., 2025, Turkey [27] | ELISA | Saliva + GCF | Following non-surgical periodontal therapy, clinical parameters showed improvement. In gingival crevicular fluid, concentrations of IL-17 and IL-35 increased in both groups; however, the total IL-17 content in gingival crevicular fluid decreased exclusively among non-smokers. | NSPT modulates IL-17/IL-35; smoking status influences biomarker dynamics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonsmann, T.; Mochol, M.; Bonsmann, E.; Jablonowski, L.; Pawlik, A.; Rasławska-Socha, J.; Lipski, M.; Mazurek-Mochol, M. The Role of IL-17 in Periodontitis and Its Systemic Connections. Int. J. Mol. Sci. 2025, 26, 10902. https://doi.org/10.3390/ijms262210902
Bonsmann T, Mochol M, Bonsmann E, Jablonowski L, Pawlik A, Rasławska-Socha J, Lipski M, Mazurek-Mochol M. The Role of IL-17 in Periodontitis and Its Systemic Connections. International Journal of Molecular Sciences. 2025; 26(22):10902. https://doi.org/10.3390/ijms262210902
Chicago/Turabian StyleBonsmann, Tobias, Martyna Mochol, Ewa Bonsmann, Lukasz Jablonowski, Andrzej Pawlik, Joanna Rasławska-Socha, Mariusz Lipski, and Małgorzata Mazurek-Mochol. 2025. "The Role of IL-17 in Periodontitis and Its Systemic Connections" International Journal of Molecular Sciences 26, no. 22: 10902. https://doi.org/10.3390/ijms262210902
APA StyleBonsmann, T., Mochol, M., Bonsmann, E., Jablonowski, L., Pawlik, A., Rasławska-Socha, J., Lipski, M., & Mazurek-Mochol, M. (2025). The Role of IL-17 in Periodontitis and Its Systemic Connections. International Journal of Molecular Sciences, 26(22), 10902. https://doi.org/10.3390/ijms262210902





