Special Issue “Fractalkine (CX3CL1) and Its Chemoattractant and Adhesion Molecule Properties in Health and Disease”
Conflicts of Interest
References
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef]
- Pan, Y.; Lloyd, C.; Zhou, H.; Dolich, S.; Deeds, J.; Gonzalo, J.A.; Vath, J.; Gosselin, M.; Ma, J.; Dussault, B.; et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997, 387, 611–617, Erratum in Nature 1997, 389, 100. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hoshino-Negishi, K.; Kuboi, Y.; Tago, F.; Yasuda, N.; Imai, T. Emerging Role of Fractalkine in the Treatment of Rheumatic Diseases. Immunotargets Ther. 2020, 9, 241–253. [Google Scholar] [CrossRef]
- Poniatowski, Ł.A.; Wojdasiewicz, P.; Krawczyk, M.; Szukiewicz, D.; Gasik, R.; Kubaszewski, Ł.; Kurkowska-Jastrzębska, I. Analysis of the Role of CX3CL1 (Fractalkine) and Its Receptor CX3CR1 in Traumatic Brain and Spinal Cord Injury: Insight into Recent Advances in Actions of Neurochemokine Agents. Mol. Neurobiol. 2017, 54, 2167–2188. [Google Scholar] [CrossRef]
- Foussat, A.; Coulomb-L’Hermine, A.; Gosling, J.; Krzysiek, R.; Durand-Gasselin, I.; Schall, T.; Balian, A.; Richard, Y.; Galanaud, P.; Emilie, D. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur. J. Immunol. 2000, 30, 87–97. [Google Scholar] [CrossRef]
- Szukiewicz, D. CX3CL1 (Fractalkine)-CX3CR1 Axis in Inflammation-Induced Angiogenesis and Tumorigenesis. Int. J. Mol. Sci. 2024, 25, 4679. [Google Scholar] [CrossRef]
- Frias-Anaya, E.; Gallego-Gutierrez, H.; Gongol, B.; Weinsheimer, S.; Lai, C.C.; Orecchioni, M.; Sriram, A.; Bui, C.M.; Nelsen, B.; Hale, P.; et al. Mild Hypoxia Accelerates Cerebral Cavernous Malformation Disease Through CX3CR1-CX3CL1 Signaling. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1246–1264. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, L.A.; Kotela, A.; Deszczyński, J.; Kotela, I.; Szukiewicz, D. The chemokine CX3CL1 (fractalkine) and its receptor CX3CR1: Occurrence and potential role in osteoarthritis. Arch. Immunol. Ther. Exp. 2014, 62, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.J.; Lysaght, J. CX3CL1 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. Structure and Function of Ligand CX3CL1 and its Receptor CX3CR1 in Cancer. Curr. Med. Chem. 2022, 29, 6228–6246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Zhuang, R.; Yang, K.; Chen, L.; Jin, B.; Ma, Y.; Zhang, Y.; Tang, K. Alterations in CX3CL1 Levels and Its Role in Viral Pathogenesis. Int. J. Mol. Sci. 2024, 25, 4451. [Google Scholar] [CrossRef]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Santos-Mendoza, T.; Sevilla-Reyes, E. The Role of the CX3CR1-CX3CL1 Axis in Respiratory Syncytial Virus Infection and the Triggered Immune Response. Int. J. Mol. Sci. 2024, 25, 9800. [Google Scholar] [CrossRef]
- Gutiérrez, I.L.; Martín-Hernández, D.; MacDowell, K.S.; García-Bueno, B.; Caso, J.R.; Leza, J.C.; Madrigal, J.L.M. CX3CL1 Regulation of Gliosis in Neuroinflammatory and Neuroprotective Processes. Int. J. Mol. Sci. 2025, 26, 959. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.J.; Muñetón-Gómez, V.; Alvárez, M.I.; Toledano-Díaz, A. Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer’s Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD? Curr. Alzheimer Res. 2016, 13, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhang, C.; Zhao, J.; Xu, J.; Zhang, P.; Ding, Q.; Zhang, J. Microvascular destabilization and intricated network of the cytokines in diabetic retinopathy: From the perspective of cellular and molecular components. Cell Biosci. 2024, 14, 85. [Google Scholar] [CrossRef]
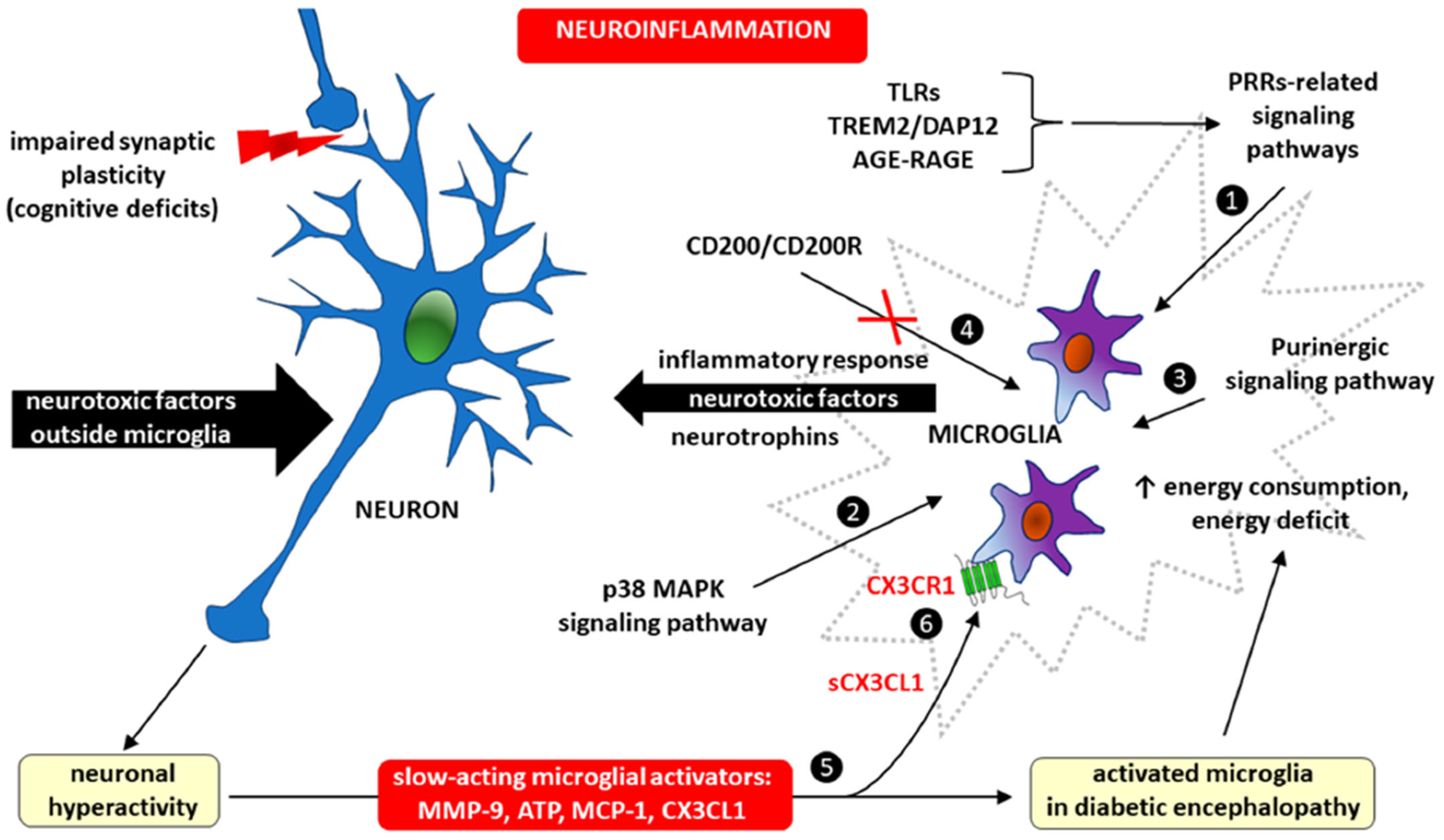
- Wątroba, M.; Grabowska, A.D.; Szukiewicz, D. Chemokine CX3CL1 (Fractalkine) Signaling and Diabetic Encephalopathy. Int. J. Mol. Sci. 2024, 25, 7527. [Google Scholar] [CrossRef]
- Stangret, A.; Sadowski, K.A.; Jabłoński, K.; Kochman, J.; Opolski, G.; Grabowski, M.; Tomaniak, M. Chemokine Fractalkine and Non-Obstructive Coronary Artery Disease-Is There a Link? Int. J. Mol. Sci. 2024, 25, 3885. [Google Scholar] [CrossRef]
- Sénécal, V.; Barat, C.; Gagnon, M.T.; Vanasse, F.; Leboeuf, M.; Gosselin, D.; Tremblay, M.J. Altered expression of fractalkine in HIV-1-infected astrocytes and consequences for the virus-related neurotoxicity. J. Neurovirol. 2021, 27, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.S.; Chirkova, T.; Slaunwhite, C.G.; Qiu, X.; Walsh, E.E.; Anderson, L.J.; Mariani, T.J. CX3CR1 Engagement by Respiratory Syncytial Virus Leads to Induction of Nucleolin and Dysregulation of Cilia-related Genes. J. Virol. 2021, 95, e00095-21. [Google Scholar] [CrossRef]
- Meineke, R.; Agac, A.; Knittler, M.C.; Ludlow, M.; Osterhaus, A.D.M.E.; Rimmelzwaan, G.F. Respiratory syncytial virus glycoprotein G impedes CX3CR1-activation by CX3CL1 and monocyte function. Npj Viruses 2024, 2, 63. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal. Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, W.; Guo, Y.; Xu, J.; Yin, M. CX3CL1/CX3CR1 in Alzheimer’s Disease: A Target for Neuroprotection. BioMed Res. Int. 2016, 2016, 8090918. [Google Scholar] [CrossRef]
- Subbarayan, M.S.; Joly-Amado, A.; Bickford, P.C.; Nash, K.R. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol. Ther. 2022, 231, 107989. [Google Scholar] [CrossRef]
- Kawamura, N.; Katsuura, G.; Yamada-Goto, N.; Nakama, R.; Kambe, Y.; Miyata, A.; Furuyashiki, T.; Narumiya, S.; Ogawa, Y.; Inui, A. Brain fractalkine-CX3CR1 signalling is anti-obesity system as anorexigenic and anti-inflammatory actions in diet-induced obese mice. Sci. Rep. 2022, 12, 12604. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.Z. Diabetes and its drivers: The largest epidemic in human history? Clin. Diabetes Endocrinol. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Szukiewicz, D. Molecular Mechanisms for the Vicious Cycle between Insulin Resistance and the Inflammatory Response in Obesity. Int. J. Mol. Sci. 2023, 24, 9818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Liu, S.; Yang, M. Macrophage and inflammation in diabetes and metabolic dysfunction-associated steatotic liver disease: From mechanisms to therapeutic strategies. World J. Diabetes 2025, 16, 110515. [Google Scholar] [CrossRef]
- Sindhu, S.; Akhter, N.; Arefanian, H.; Al-Roub, A.A.; Ali, S.; Wilson, A.; Al-Hubail, A.; Al-Beloushi, S.; Al-Zanki, S.; Ahmad, R. Increased circulatory levels of fractalkine (CX3CL1) are associated with inflammatory chemokines and cytokines in individuals with type-2 diabetes. J. Diabetes Metab. Disord. 2017, 16, 15. [Google Scholar] [CrossRef]
- Schinzari, F.; Tesauro, M.; Campia, U.; Cardillo, C. Increased fractalkine and vascular dysfunction in obesity and in type 2 diabetes. Effects of oral antidiabetic treatment. Vascul. Pharmacol. 2020, 128–129, 106676. [Google Scholar] [CrossRef]
- Cardona, S.M.; Mendiola, A.S.; Yang, Y.C.; Adkins, S.L.; Torres, V.; Cardona, A.E. Disruption of Fractalkine Signaling Leads to Microglial Activation and Neuronal Damage in the Diabetic Retina. ASN Neuro 2015, 7, 1759091415608204. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.A.; Jobling, A.I.; Dixon, M.A.; Bui, B.V.; Vessey, K.A.; Phipps, J.A.; Greferath, U.; Venables, G.; Wong, V.H.Y.; Wong, C.H.Y.; et al. Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic retinopathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2112561118. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Schmidt, M.H.H.; Heinig, N. Microglia in retinal angiogenesis and diabetic retinopathy. Angiogenesis 2024, 27, 311–331. [Google Scholar] [CrossRef]
- Mazumdar, D.; Singh, S. Understanding diabetic encephalopathy: A model-based approach to explore cognitive and motor impairments through glutamatergic dysregulation. Mol. Biol. Rep. 2025, 52, 945. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Zhang, Z.; Ye, Y.; Zhou, J. Role of microglia-neuron interactions in diabetic encephalopathy. Ageing Res. Rev. 2018, 42, 28–39. [Google Scholar] [CrossRef]
- Rütsche, D.; Michalak-Micka, K.; Zielinska, D.; Moll, H.; Moehrlen, U.; Biedermann, T.; Klar, A.S. The Role of CD200-CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation. Cells 2022, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Cheng, K.; Ming, Y. CX3CL1/CX3CR1 Axis, as the Therapeutic Potential in Renal Diseases: Friend or Foe? Curr. Gene Ther. 2017, 17, 442–452. [Google Scholar] [CrossRef]
- Stangret, A.; Dykacz, W.; Jabłoński, K.; Wesołowska, A.; Klimczak-Tomaniak, D.; Kochman, J.; Tomaniak, M. The cytokine trio—Visfatin, placental growth factor and fractalkine—And their role in myocardial infarction with non-obstructive coronary arteries (MINOCA). Cytokine Growth Factor Rev. 2023, 74, 76–85. [Google Scholar] [CrossRef]
- Ekinci, Y.; Richardson, G.; Spyridopoulos, I. A Phase IIa Clinical Trial of KAND567, Fractalkine Receptor Inhibitor, in Patients with ST-Elevation Acute Myocardial Infarction after Percutaneous Coronary Intervention. J. Pharmacol. Exp. Ther. 2024, 389 (Suppl. 3), 472–473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szukiewicz, D. Special Issue “Fractalkine (CX3CL1) and Its Chemoattractant and Adhesion Molecule Properties in Health and Disease”. Int. J. Mol. Sci. 2025, 26, 10899. https://doi.org/10.3390/ijms262210899
Szukiewicz D. Special Issue “Fractalkine (CX3CL1) and Its Chemoattractant and Adhesion Molecule Properties in Health and Disease”. International Journal of Molecular Sciences. 2025; 26(22):10899. https://doi.org/10.3390/ijms262210899
Chicago/Turabian StyleSzukiewicz, Dariusz. 2025. "Special Issue “Fractalkine (CX3CL1) and Its Chemoattractant and Adhesion Molecule Properties in Health and Disease”" International Journal of Molecular Sciences 26, no. 22: 10899. https://doi.org/10.3390/ijms262210899
APA StyleSzukiewicz, D. (2025). Special Issue “Fractalkine (CX3CL1) and Its Chemoattractant and Adhesion Molecule Properties in Health and Disease”. International Journal of Molecular Sciences, 26(22), 10899. https://doi.org/10.3390/ijms262210899




