The Plasminogen Activation System in the Central Nervous System: Implications for Epilepsy and Neuropsychiatric Disorders
Abstract
1. Introduction
2. The Plasminogen Activation System and Its Function in the Central Nervous System
2.1. Structure and Expression of PA System Components
2.1.1. Plasminogen/Plasmin Activation by tPA and uPA
2.1.2. The Inhibitors of Plasminogen Activators
2.2. Functional Roles and Molecular Pathways of the PA System in the CNS
2.2.1. The PA System and Extracellular Matrix, Blood–Brain Barrier, Neuroinflammation, and Neurodegeneration
2.2.2. PA Inhibitors in Regulating Neuroinflammation and Neurotoxicity
2.2.3. PA System in Neuronal Plasticity, Growth, and Repair
2.2.4. PA System and Cell Adhesion and Migration
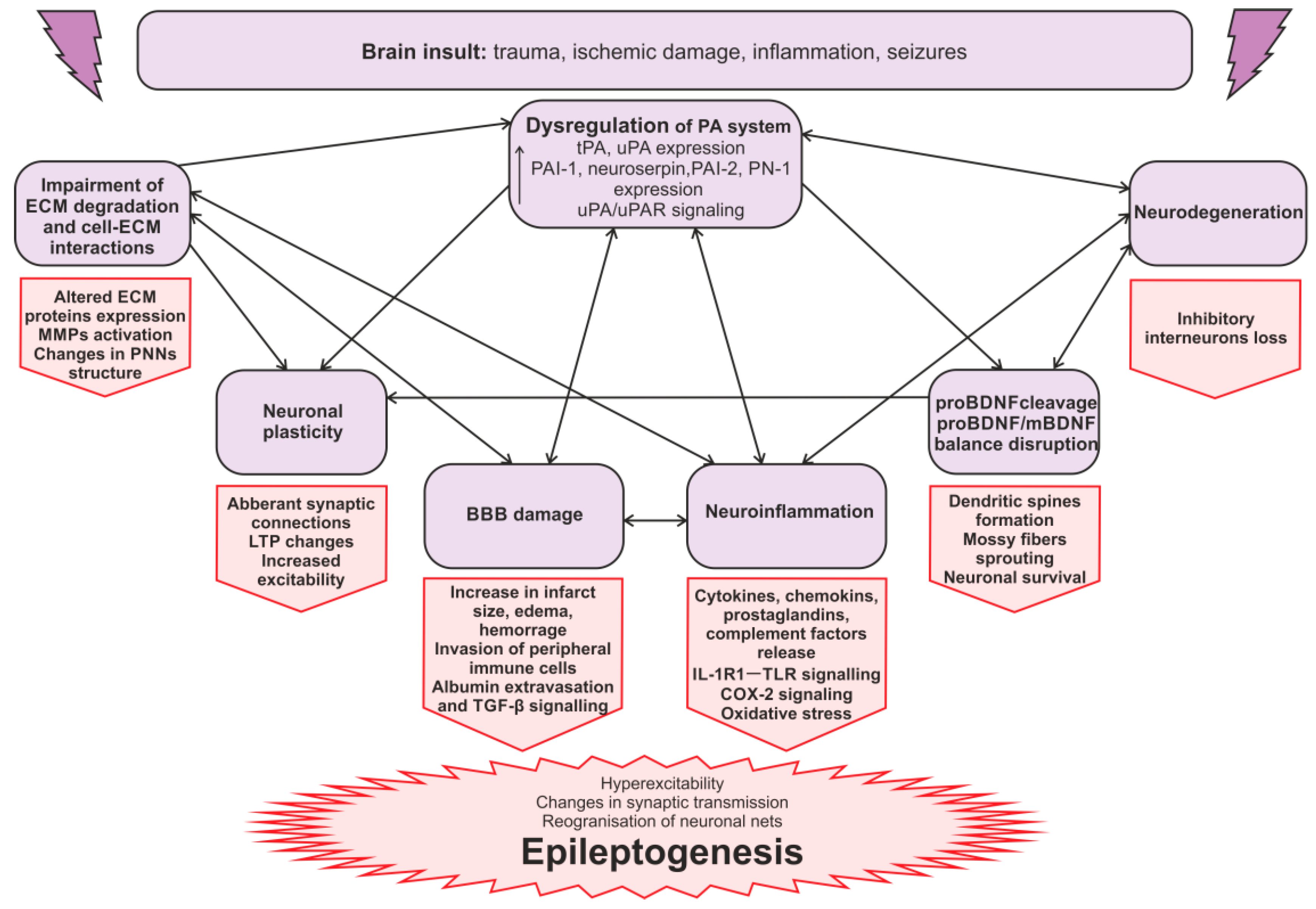
3. Plasminogen Activation System in Epilepsy
3.1. PA System in Epilepsy: Experimental Animal Models
3.2. PA System in Epilepsy: Clinical Studies
3.3. The Mechanisms of the PA System Involvement in Seizures and Epileptogenesis
3.3.1. The Regulation of ECM in Epilepsy
3.3.2. The Blood–Brain Barrier in Epilepsy
3.3.3. PA Activation System, Neuroinflammation, and Epilepsy
3.3.4. uPAR Signaling in Epileptogenesis
3.3.5. Synaptic Reorganization in Epileptogenesis
4. Plasminogen Activation System in Neuropsychiatric Disorders
4.1. Depression
4.2. Anxiety and Stress
4.3. PTSD
5. Prospective Role of the PA System in the Development of NeuropsyChiatric Comorbidities in Epilepsy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AED | Antiepileptic Drug |
| BBB | Blood–brain barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| COX-2 | Cyclooxygenase 2 |
| ECM | Extracellular Matrix |
| Erk | Extracellular signal-regulated kinase |
| FENIB | Familial Encephalopathy with Neuroserpin Inclusion Bodies |
| GFD | Growth Factor-like Domain |
| IL-1β | Interleukin 1 beta |
| iNOS | Inducible nitric oxide synthase |
| KA | Kainic Acid |
| LPS | Lipopolysaccharide |
| LTP | Long-Term Potentiation |
| mBDNF | Mature Brain-Derived Neurotrophic Factor |
| MDD | Major Depressive Disorder |
| mTBI | Mild Traumatic Brain Injury |
| NF-kB | Nuclear factor kB |
| NMDA | N-Methyl-D-Aspartate |
| PA | Plasminogen Activation/Plasminogen Activator |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PAI-2 | Plasminogen Activator Inhibitor-2 |
| PN-1 | Protease Nexin-1 |
| proBDNF | Precursor form of Brain-Derived Neurotrophic Factor |
| pro-uPA | single-chain zymogen form of uPA |
| PTSD | Post-Traumatic Stress Disorder |
| PTZ | Pentylenetetrazol |
| RCL | Reactive Center Loop |
| SE | Status Epilepticus |
| tPA | Tissue-type Plasminogen Activator |
| TBI | Traumatic Brain Injury |
| TGF-β | Transforming growth factor beta |
| TLE | Temporal Lobe Epilepsy |
| TNF-α | Tumor Necrosis Factor-alpha |
| TLR | Toll-like receptor |
| Trk | Tropomyosin receptor kinase |
| uPA | Urokinase-type Plasminogen Activator |
| uPAR | Urokinase-type Plasminogen Activator Receptor |
| WT | Wild Type |
References
- Shan, T.; Zhu, Y.; Fan, H.; Liu, Z.; Xie, J.; Li, M.; Jing, S. Global, Regional, and National Time Trends in the Burden of Epilepsy, 1990–2019: An Age-Period-Cohort Analysis for the Global Burden of Disease 2019 Study. Front. Neurol. 2024, 15, 1418926. [Google Scholar] [CrossRef] [PubMed]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of Epilepsy: Current Concepts and Future Perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef]
- Agrawal, N.; Bird, J.S.; von Oertzen, T.J.; Cock, H.; Mitchell, A.J.; Mula, M. Depression Correlates with Quality of Life in People with Epilepsy Independent of the Measures Used. Epilepsy Behav. 2016, 62, 246–250. [Google Scholar] [CrossRef]
- Josephson, C.B.; Jetté, N. Psychiatric Comorbidities in Epilepsy. Int. Rev. Psychiatry 2017, 29, 409–424. [Google Scholar] [CrossRef]
- Kanner, A.M. Depression in Epilepsy: A Complex Relation with Unexpected Consequences. Curr. Opin. Neurol. 2008, 21, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.; Francomme, L.; Vignal, J.P.; Jacquot, C.; Schwan, R.; Tyvaert, L.; Maillard, L.; Hingray, C. Interictal Psychiatric Comorbidities of Drug-Resistant Focal Epilepsy: Prevalence and Influence of the Localization of the Epilepsy. Epilepsy Behav. 2019, 94, 288–296. [Google Scholar] [CrossRef]
- Soncin, L.D.; Belquaid, S.; McGonigal, A.; Giusiano, B.; Bartolomei, F.; Faure, S. Post-Traumatic Stress Disorder (PTSD), Cognitive Control, and Perceived Seizure Control in Patients with Epilepsy: An Exploratory Study. Epilepsy Behav. 2023, 147, 109396. [Google Scholar] [CrossRef]
- Collen, D. The Plasminogen (Fibrinolytic) System. Thromb. Haemost. 1999, 82, 259–270. [Google Scholar] [CrossRef]
- Fay, W.P.; Garg, N.; Sunkar, M. Vascular Functions of the Plasminogen Activation System. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Tsirka, S.E.; Gualandris, A.; Amaral, D.G.; Strickland, S. Excitotoxin-Induced Neuronal Degeneration and Seizure Are Mediated by Tissue Plasminogen Activator. Nature 1995, 377, 340–344. [Google Scholar] [CrossRef]
- Masos, T.; Miskin, R. MRNAs Encoding Urokinase-Type Plasminogen Activator and Plasminogen Activator Inhibitor-1 Are Elevated in the Mouse Brain Following Kainate-Mediated Excitation. Brain Res. Mol. Brain Res. 1997, 47, 157–169. [Google Scholar] [CrossRef]
- Yepes, M.; Sandkvist, M.; Moore, E.G.; Bugge, T.H.; Strickland, D.K.; Lawrence, D.A. Tissue-Type Plasminogen Activator Induces Opening of the Blood-Brain Barrier via the LDL Receptor–Related Protein. J. Clin. Investig. 2003, 112, 1533–1540. [Google Scholar] [CrossRef]
- Pitkanen, A.; Lukasiuk, K. Molecular and Cellular Basis of Epileptogenesis in Symptomatic Epilepsy. Epilepsy Behav. 2009, 14 (Suppl. S1), 16–25. [Google Scholar] [CrossRef]
- Bouarab, C.; Roullot-Lacarrière, V.; Vallée, M.; Le Roux, A.; Guette, C.; Mennesson, M.; Marighetto, A.; Desmedt, A.; Piazza, P.V.; Revest, J.M. PAI-1 Protein Is a Key Molecular Effector in the Transition from Normal to PTSD-like Fear Memory. Mol. Psychiatry 2021, 26, 4968. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, S.; Li, C.; Lu, N.; Yue, Y.; Yin, Y.; Zhang, Y.; Zhi, X.; Zhang, D.; Yuan, Y. The Serum Protein Levels of the TPA–BDNF Pathway Are Implicated in Depression and Antidepressant Treatment. Transl. Psychiatry 2017, 7, e1079. [Google Scholar] [CrossRef]
- Douceau, S.; Lemarchand, E.; Hommet, Y.; Lebouvier, L.; Joséphine, C.; Bemelmans, A.P.; Maubert, E.; Agin, V.; Vivien, D. PKCδ-Positive GABAergic Neurons in the Central Amygdala Exhibit Tissue-Type Plasminogen Activator: Role in the Control of Anxiety. Mol. Psychiatry 2022, 27, 2197–2205. [Google Scholar] [CrossRef]
- Carmeliet, P.; Schoonjans, L.; Kieckens, L.; Ream, B.; Degen, J.; Bronson, R.; De Vos, R.; Van Den Oord, J.J.; Collen, D.; Mulligan, R.C. Physiological Consequences of Loss of Plasminogen Activator Gene Function in Mice. Nature 1994, 368, 419–424. [Google Scholar] [CrossRef]
- Melchor, J.P.; Strickland, S. Tissue Plasminogen Activator in Central Nervous System Physiology and Pathology. Thromb. Haemost. 2005, 93, 655. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yan, Y.; Cao, J.; Zhou, Y.; Zhang, H.; Xu, Q.; Zhou, J. A Family of Serine Protease Inhibitors (Serpins) and Its Expression Profiles in the Ovaries of Rhipicephalus Haemaphysaloides. Infect. Genet. Evol. 2020, 84, 104346. [Google Scholar] [CrossRef]
- Wright, H.T. The Structural Puzzle of How Serpin Serine Proteinase Inhibitors Work. Bioessays 1996, 18, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Ali, C.; Parcq, J.; Vivien, D.; Docagne, F. The Plasminogen Activation System in Neuroinflammation. Biochim. Et Biophys. Acta Mol. Basis Dis. 2016, 1862, 395–402. [Google Scholar] [CrossRef]
- Basham, M.E.; Seeds, N.W. Plasminogen Expression in the Neonatal and Adult Mouse Brain. J. Neurochem. 2001, 77, 318–325. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, A.; Parmer, R.J.; Miles, L.A. Plasminogen Gene Expression Is Regulated by Nerve Growth Factor. J. Thromb. Haemost. 2007, 5, 1715–1725. [Google Scholar] [CrossRef]
- Larsen, G.R.; Henson, K.; Blue, Y. Variants of Human Tissue-Type Plasminogen Activator. Fibrin Binding, Fibrinolytic, and Fibrinogenolytic Characterization of Genetic Variants Lacking the Fibronectin Finger-like and/or the Epidermal Growth Factor Domains. J. Biol. Chem. 1988, 263, 1023–1029. [Google Scholar] [CrossRef]
- van Zonneveld, A.-J.; Veerman, H.; MacDonald, M.E.; Pannekoek, H.; van Mourik, J.A. Structure and Function of Human Tissue-Type Plasminogen Activator (t-PA). J. Cell Biochem. 1986, 32, 169–178. [Google Scholar] [CrossRef]
- Carriero, M.V.; Franco, P.; Vocca, I.; Alfano, D.; Longanesi-Cattani, I.; Bifulco, K.; Mancini, A.; Caputi, M.; Stoppelli, M.P. Structure, Function and Antagonists of Urokinase-Type Plasminogen Activator. Front. Biosci. Landmark 2009, 14, 3782–3794. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V.; Wang, L.; Sun, N.; Haffke, S.; Verrall, S.; Seeds, N.W.; Fisher, M.J.; Schreiber, S.S. Expression of Tissue Plasminogen Activator in Cerebral Capillaries: Possible Fibrinolytic Function of the Blood-Brain Barrier. Neurosurgery 1995, 37, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Krystosek, A.; Seeds, N.W. Plasminogen Activator Secretion by Granule Neurons in Cultures of Developing Cerebellum. Proc. Natl. Acad. Sci. USA 1981, 78, 7810. [Google Scholar] [CrossRef]
- Sappino, A.P.; Madani, R.; Huarte, J.; Belin, D.; Kiss, J.Z.; Wohlwend, A.; Vassalli, J.D. Extracellular Proteolysis in the Adult Murine Brain. J. Clin. Investig. 1993, 92, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Sallés, F.J.; Strickland, S. Localization and Regulation of the Tissue Plasminogen Activator–Plasmin System in the Hippocampus. J. Neurosci. 2002, 22, 2125. [Google Scholar] [CrossRef]
- Rogove, A.D.; Siao, C.J.; Keyt, B.; Strickland, S.; Tsirka, S.E. Activation of Microglia Reveals a Non-Proteolytic Cytokine Function for Tissue Plasminogen Activator in the Central Nervous System. J. Cell Sci. 1999, 112 Pt 22, 4007–4016. [Google Scholar] [CrossRef]
- Siao, C.J.; Fernandez, S.R.; Tsirka, S.E. Cell Type-Specific Roles for Tissue Plasminogen Activator Released by Neurons or Microglia after Excitotoxic Injury. J. Neurosci. 2003, 23, 3234. [Google Scholar] [CrossRef] [PubMed]
- Louessard, M.; Lacroix, A.; Martineau, M.; Mondielli, G.; Montagne, A.; Lesept, F.; Lambolez, B.; Cauli, B.; Mothet, J.P.; Vivien, D.; et al. Tissue Plasminogen Activator Expression Is Restricted to Subsets of Excitatory Pyramidal Glutamatergic Neurons. Mol. Neurobiol. 2016, 53, 5000–5012. [Google Scholar] [CrossRef]
- Delaunay-Piednoir, B.; Pouettre, E.; Etard, O.; Goux, D.; Baudron, E.; Docagne, F.; Maubert, E.; Vivien, D.; Bardou, I. Tissue-Type Plasminogen Activator Expression by Endothelial Cells and Oligodendrocytes Is Required for Proper CNS Myelination. Acta Neuropathol. Commun. 2025, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.K.; Lawrence, D.A. Characterization of Tissue Plasminogen Activator Expression and Trafficking in the Adult Murine Brain. eNeuro 2018, 5, ENEURO.0119-18.2018. [Google Scholar] [CrossRef]
- Wu, F.; Catano, M.; Echeverry, R.; Torre, E.; Haile, W.B.; An, J.; Chen, C.; Cheng, L.; Nicholson, A.; Tong, F.C.; et al. Urokinase-Type Plasminogen Activator Promotes Dendritic Spine Recovery and Improves Neurological Outcome Following Ischemic Stroke. J. Neurosci. 2014, 34, 14219. [Google Scholar] [CrossRef]
- Diaz, A.; Merino, P.; Manrique, L.G.; Cheng, L.; Yepes, M. Urokinase-Type Plasminogen Activator (UPA) Protects the Tripartite Synapse in the Ischemic Brain via Ezrin-Mediated Formation of Peripheral Astrocytic Processes. J. Cereb. Blood Flow Metab. 2018, 39, 2157. [Google Scholar] [CrossRef]
- Dent, M.A.R.; Sumi, Y.; Morris, R.J.; Seeley, P.J. Urokinase-type Plasminogen Activator Expression by Neurons and Oligodendrocytes During Process Outgrowth in Developing Rat Brain. Eur. J. Neurosci. 1993, 5, 633–647. [Google Scholar] [CrossRef]
- Petersen, L.C.; Lund, L.R.; Nielsen, L.S.; Danø, K.; Skriver, L. One-Chain Urokinase-Type Plasminogen Activator from Human Sarcoma Cells Is a Proenzyme with Little or No Intrinsic Activity. J. Biol. Chem. 1988, 263, 11189–11196. [Google Scholar] [CrossRef] [PubMed]
- Ellis, V.; Scully, M.F.; Kakkar, V. V Plasminogen Activation Initiated by Single-Chain Urokinase-Type Plasminogen Activator. Potentiation by U937 Monocytes. J. Biol. Chem. 1989, 264, 2185–2188. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Carmeliet, P. UPAR: A Versatile Signalling Orchestrator. Nat. Rev. Mol. Cell Biol. 2002, 3, 932–943. [Google Scholar] [CrossRef]
- Cunningham, O.; Campion, S.; Hugh Perry, V.; Murray, C.; Sidenius, N.; Docagne, F.; Cunningham, C. Microglia and the Urokinase Plasminogen Activator Receptor/UPA System in Innate Brain Inflammation. Glia 2009, 57, 1802–1814. [Google Scholar] [CrossRef]
- Yepes, M. The UPA/UPAR System in Astrocytic Wound Healing. Neural Regen. Res. 2022, 17, 2404. [Google Scholar] [CrossRef] [PubMed]
- Beschorner, R.; Schluesener, H.J.; Nguyen, T.D.; Magdolen, V.; Luther, T.; Pedal, I.; Mattern, R.; Meyermann, R.; Schwab, J.M. Lesion-Associated Accumulation of UPAR/CD87- Expressing Infiltrating Granulocytes, Activated Microglial Cells/Macrophages and Upregulation by Endothelial Cells Following TBI and FCI in Humans. Neuropathol. Appl. Neurobiol. 2000, 26, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Mahanivong, C.; Yu, J.; Huang, S. Elevated Urokinase-Specific Surface Receptor Expression Is Maintained through Its Interaction with Urokinase Plasminogen Activator. Mol. Carcinog. 2007, 46, 165–175. [Google Scholar] [CrossRef]
- Smith, H.W.; Marshall, C.J. Regulation of Cell Signalling by UPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Husain, S.S. Single-Chain Urokinase-Type Plasminogen Activator Does Not Possess Measurable Intrinsic Amidolytic or Plasminogen Activator Activities. Biochemistry 1991, 30, 5797–5805. [Google Scholar] [CrossRef] [PubMed]
- Anfray, A.; Brodin, C.; Drieu, A.; Potzeha, F.; Dalarun, B.; Agin, V.; Vivien, D.; Orset, C. Single- and Two- Chain Tissue Type Plasminogen Activator Treatments Differentially Influence Cerebral Recovery after Stroke. Exp. Neurol. 2021, 338, 113606. [Google Scholar] [CrossRef]
- Wittwer, A.J.; Howard, S.C. Glycosylation at Asn-184 Inhibits the Conversion of Single-Chain to Two-Chain Tissue-Type Plasminogen Activator by Plasmin. Biochemistry 1990, 29, 4175–4180. [Google Scholar] [CrossRef]
- Gonias, S.L. Plasminogen Activator Receptor Assemblies in Cell Signaling, Innate Immunity, and Inflammation. Am. J. Physiol. Cell Physiol. 2021, 321, C721. [Google Scholar] [CrossRef]
- Tjärnlund-Wolf, A.; Brogren, H.; Lo, E.H.; Wang, X. Plasminogen Activator Inhibitor-1 and Thrombotic Cerebrovascular Diseases. Stroke 2012, 43, 2833. [Google Scholar] [CrossRef]
- Gettins, P.G.W.; Olson, S.T. Inhibitory Serpins. New Insights into Their Folding, Polymerization, Regulation and Clearance. Biochem. J. 2016, 473, 2273. [Google Scholar] [CrossRef]
- Sharp, A.M.; Stein, P.E.; Pannu, N.S.; Carrell, R.W.; Berkenpas, M.B.; Ginsburg, D.; Lawrence, D.A.; Read, R.J. The Active Conformation of Plasminogen Activator Inhibitor 1, a Target for Drugs to Control Fibrinolysis and Cell Adhesion. Structure 1999, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hino, H.; Akiyama, H.; Iseki, E.; Kato, M.; Kondo, H.; Ikeda, K.; Kosaka, K. Immunohistochemical Localization of Plasminogen Activator Inhibitor-1 in Rat and Human Brain Tissues. Neurosci. Lett. 2001, 297, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Kwon, K.J.; Choi, C.S.; Jeon, S.J.; Cheong, K.C.; Park, J.H.; Ko, H.M.; Lee, S.H.; Cheong, J.H.; Ryu, J.H.; et al. Valproic Acid Induces Astrocyte-Dependent Neurite Outgrowth from Cultured Rat Primary Cortical Neuron via Modulation of TPA/PAI-1 Activity. Glia 2013, 61, 694–709. [Google Scholar] [CrossRef]
- Docagne, F.; Nicole, O.; Marti, H.H.; Mackenzie, E.T.; Buisson, A.; Vivien, D. Transforming Growth Factor-Βl as a Regulator of the Serpins/t-PA Axis in Cerebral Ischemia. FASEB J. 1999, 13, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, E.K.O. Regulation of Plasminogen Activator Inhibitor Type 1 Gene Expression by Inflammatory Mediators and Statins. Thromb. Haemost. 2008, 100, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Osterwalder, T.; Contartese, J.; Stoeckli, E.T.; Kuhn, T.B.; Sonderegger, P. Neuroserpin, an Axonally Secreted Serine Protease Inhibitor. EMBO J. 1996, 15, 2944–2953. [Google Scholar] [CrossRef]
- Hastings, G.A.; Coleman, T.A.; Haudenschild, C.C.; Stefansson, S.; Smith, E.P.; Barthlow, R.; Cherry, S.; Sandkvist, M.; Lawrence, D.A. Neuroserpin, a Brain-Associated Inhibitor of Tissue Plasminogen Activator Is Localized Primarily in Neurons. Implications for the Regulation of Motor Learning and Neuronal Survival. J. Biol. Chem. 1997, 272, 33062–33067. [Google Scholar] [CrossRef]
- D’Acunto, E.; Fra, A.; Visentin, C.; Manno, M.; Ricagno, S.; Galliciotti, G.; Miranda, E. Neuroserpin: Structure, Function, Physiology and Pathology. Cell Mol. Life Sci. 2021, 78, 6409. [Google Scholar] [CrossRef]
- Krueger, S.R.; Ghisu, G.P.; Cinelli, P.; Gschwend, T.P.; Osterwalder, T.; Wolfer, D.P.; Sonderegger, P. Expression of Neuroserpin, an Inhibitor of Tissue Plasminogen Activator, in the Developing and Adult Nervous System of the Mouse. J. Neurosci. 1997, 17, 8984–8996. [Google Scholar] [CrossRef]
- Yepes, M.; Sandkvist, M.; Wong, M.K.K.; Coleman, T.A.; Smith, E.; Cohan, S.L.; Lawrence, D.A. Neuroserpin Reduces Cerebral Infarct Volume and Protects Neurons from Ischemia-Induced Apoptosis. Blood 2000, 96, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Remold-O’Donnell, E. The Ovalbumin Family of Serpin Proteins. FEBS Lett. 1993, 315, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, E.K.O.; Baker, M.S.; Bunn, C.L. Biological and Clinical Aspects of Plasminogen Activator Inhibitor Type 2. Blood 1995, 86, 4007–4024. [Google Scholar] [CrossRef]
- Åstedt, B.; Bladh, B.; Christensen, U.; Lecander, I. Different Inhibition of One and Two Chain Tissue Plasminogen Activator by a Placental Inhibitor Studied with Two Tripeptide-p-Nitroanilide Substrates. Scand. J. Clin. Lab. Investig. 1985, 45, 429–435. [Google Scholar] [CrossRef]
- Akiyama, H.; Ikeda, K.; Kondo, H.; Kato, M.; McGeer, P.L. Microglia Express the Type 2 Plasminogen Activator Inhibitor in the Brain of Control Subjects and Patients with Alzheimer’s Disease. Neurosci. Lett. 1993, 164, 233–235. [Google Scholar] [CrossRef]
- Dietzmann, K.; Bossanyi, P.V.; Krause, D.; Wittig, H.; Mawrin, C.; Kirches, E. Expression of the Plasminogen Activator System and the Inhibitors PAI-1 and PAI-2 in Posttraumatic Lesions of the CNS and Brain Injuries Following Dramatic Circulatory Arrests: An Immunohistochemical Study. Pathol. Res. Pract. 2000, 196, 15–21. [Google Scholar] [CrossRef]
- Guenther, J.; Nick, H.; Monard, D. A Glia-Derived Neurite-Promoting Factor with Protease Inhibitory Activity. EMBO J. 1985, 4, 1963–1966. [Google Scholar] [CrossRef]
- Gioor, S.; Odink, K.; Guenther, J.; Nick, H.; Monard, D. A Glia-Derived Neurite Promoting Factor with Protease Inhibitory Activity Belongs to the Protease Nexins. Cell 1986, 47, 687–693. [Google Scholar] [CrossRef]
- Baker, J.B.; Low, D.A.; Simmer, R.L.; Cunningham, D.D. Protease-Nexin: A Cellular Component That Links Thrombin and Plasminogen Activator and Mediates Their Binding to Cells. Cell 1980, 21, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Vaughans, P.J.; Cunninghamq, D.D. Regulation of Protease Nexin-1 Synthesis and Secretion in Cultured Brain Cells by Injury-Related Factors. J. Biol. Chem. 1993, 268, 3720–3727. [Google Scholar] [CrossRef]
- Mirante, O.; Price, M.; Puentes, W.; Castillo, X.; Benakis, C.; Thevenet, J.; Monard, D.; Hirt, L. Endogenous Protease Nexin-1 Protects against Cerebral Ischemia. Int. J. Mol. Sci. 2013, 14, 16719. [Google Scholar] [CrossRef]
- Barros, C.S.; Franco, S.J.; Müller, U. Extracellular Matrix: Functions in the Nervous System. Cold Spring Harb. Perspect. Biol. 2011, 3, a005108. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The Extracellular Matrix: Structure, Composition, Age-Related Differences, Tools for Analysis and Applications for Tissue Engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Werb, Z. ECM and Cell Surface Proteolysis: Regulating Cellular Ecology. Cell 1997, 91, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.L.; Enghild, J.J.; Pizzo, S.V.; Stack, M.S. The Extracellular Matrix Proteins Laminin and Fibronectin Contain Binding Domains for Human Plasminogen and Tissue Plasminogen Activator. J. Biol. Chem. 1993, 268, 18917–18923. [Google Scholar] [CrossRef] [PubMed]
- Marchina, E.; Barlati, S. Degradation of Human Plasma and Extracellular Matrix Fibronectin by Tissue Type Plasminogen Activator and Urokinase. Int. J. Biochem. Cell Biol. 1996, 28, 1141–1150. [Google Scholar] [CrossRef]
- Murphy, G.; Atkinson, S.; Ward, R.; Gavrilvoc, J.; Reynolds, J.J. The Role of Plasmhogen Activators in the Regulation of Connective Tissue Metalloproteinasesa. Ann. N. Y Acad. Sci. 1992, 667, 1–12. [Google Scholar] [CrossRef]
- Carmeliet, P.; Moons, L.; Lijnen, R.; Baes, M.; Lemaǐtre, V.; Tipping, P.; Drew, A.; Eeckhout, Y.; Shapiro, S.; Lupu, F.; et al. Urokinase-Generated Plasmin Activates Matrix Metalloproteinases during Aneurysm Formation. Nat. Genet. 1997, 17, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Wiera, G.; Nowak, D.; van Hove, I.; Dziegiel, P.; Moons, L.; Mozrzymas, J.W. Mechanisms of NMDA Receptor- and Voltage-Gated L-Type Calcium Channel-Dependent Hippocampal LTP Critically Rely on Proteolysis That Is Mediated by Distinct Metalloproteinases. J. Neurosci. 2017, 37, 1240. [Google Scholar] [CrossRef]
- Wiera, G.; Mozrzymas, J.W. Extracellular Metalloproteinases in the Plasticity of Excitatory and Inhibitory Synapses. Cells 2021, 10, 2055. [Google Scholar] [CrossRef]
- Vandooren, J.; Van Den Steen, P.E.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9): The next Decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Fredriksson, L.; Lawrence, D.A.; Medcalf, R.L. TPA Modulation of the Blood-Brain Barrier: A Unifying Explanation for the Pleiotropic Effects of TPA in the CNS? Semin. Thromb. Hemost. 2016, 43, 154. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The Blood–Brain Barrier in Health and Disease: Important Unanswered Questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef]
- Niego, B.; Medcalf, R.L. Plasmin-Dependent Modulation of the Blood–Brain Barrier: A Major Consideration during TPA-Induced Thrombolysis? J. Cereb. Blood Flow Metab. 2014, 34, 1283. [Google Scholar] [CrossRef] [PubMed]
- Sashindranath, M.; Sales, E.; Daglas, M.; Freeman, R.; Samson, A.L.; Cops, E.J.; Beckham, S.; Galle, A.; McLean, C.; Morganti-Kossmann, C.; et al. The Tissue-Type Plasminogen Activator–Plasminogen Activator Inhibitor 1 Complex Promotes Neurovascular Injury in Brain Trauma: Evidence from Mice and Humans. Brain 2012, 135, 3251. [Google Scholar] [CrossRef]
- Benchenane, K.; Berezowski, V.; Ali, C.; Fernández-Monreal, M.; López-Atalaya, J.P.; Brillault, J.; Chuquet, J.; Nouvelot, A.; MacKenzie, E.T.; Bu, G.; et al. Tissue-Type Plasminogen Activator Crosses the Intact Blood-Brain Barrier by Low-Density Lipoprotein Receptor-Related Protein-Mediated Transcytosis. Circulation 2005, 111, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Klecker, P.H.; Fritzen, L.; Mazura, A.D.; Weggen, S.; Pietrzik, C.U. Antibody-Mediated Inhibition of Tissue-Type Plasminogen Activator Binding to the Low-Density Lipoprotein Receptor-Related Protein 1 as a Potential Beneficial Modulator for Stroke Therapy. J. Cell Biochem. 2023, 124, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Parcq, J.; Bertrand, T.; Montagne, A.; Baron, A.F.; MacRez, R.; Billard, J.M.; Briens, A.; Hommet, Y.; Wu, J.; Yepes, M.; et al. Unveiling an Exceptional Zymogen: The Single-Chain Form of TPA Is a Selective Activator of NMDA Receptor-Dependent Signaling and Neurotoxicity. Cell Death Differ. 2012, 19, 1983. [Google Scholar] [CrossRef]
- Samson, A.L.; Nevin, S.T.; Croucher, D.; Niego, B.; Daniel, P.B.; Weiss, T.W.; Moreno, E.; Monard, D.; Lawrence, D.A.; Medcalf, R.L. Tissue-Type Plasminogen Activator Requires a Co-Receptor to Enhance N-Methyl-D-Aspartate Receptor Function. J. Neurochem. 2008, 107, 1091. [Google Scholar] [CrossRef]
- András, I.E.; Deli, M.A.; Veszelka, S.; Hayashi, K.; Hennig, B.; Toborek, M. The NMDA and AMPA/KA Receptors Are Involved in Glutamate-Induced Alterations of Occludin Expression and Phosphorylation in Brain Endothelial Cells. J. Cereb. Blood Flow Metab. 2007, 27, 1431–1443. [Google Scholar] [CrossRef]
- Miles, L.A.; Vago, J.P.; Sousa, L.P.; Parmer, R.J. Functions of the Plasminogen Receptor, Plg-RKT. J. Thromb. Haemost. 2020, 18, 2468. [Google Scholar] [CrossRef]
- Baker, S.K.; Chen, Z.L.; Norris, E.H.; Strickland, S. Plasminogen Mediates Communication between the Peripheral and Central Immune Systems during Systemic Immune Challenge with Lipopolysaccharide. J. Neuroinflammation 2019, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Jou, I.; Joe, E. Plasminogen-Induced IL-1β and TNF-α Production in Microglia Is Regulated by Reactive Oxygen Species. Biochem. Biophys. Res. Commun. 2003, 312, 969–974. [Google Scholar] [CrossRef]
- Zhang, C.; An, J.; Strickland, D.K.; Yepes, M. The Low-Density Lipoprotein Receptor-Related Protein 1 Mediates Tissue-Type Plasminogen Activator-Induced Microglial Activation in the Ischemic Brain. Am. J. Pathol. 2009, 174, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Pineda, D.; Ampurdanés, C.; Medina, M.G.; Serratosa, J.; Tusell, J.M.; Saura, J.; Planas, A.M.; Navarro, P. Tissue Plasminogen Activator Induces Microglial Inflammation via a Noncatalytic Molecular Mechanism Involving Activation of Mitogen-Activated Protein Kinases and Akt Signaling Pathways and AnnexinA2 and Galectin-1 Receptors. Glia 2012, 60, 526–540. [Google Scholar] [CrossRef]
- Starossom, S.C.; Mascanfroni, I.D.; Imitola, J.; Cao, L.; Raddassi, K.; Hernandez, S.F.; Bassil, R.; Croci, D.O.; Cerliani, J.P.; Delacour, D.; et al. Galectin-1 Deactivates Classically-Activated Microglia and Protects from Inflammation-Induced Neurodegeneration. Immunity 2012, 37, 249. [Google Scholar] [CrossRef] [PubMed]
- Siao, C.J.; Tsirka, S.E. Tissue Plasminogen Activator Mediates Microglial Activation via Its Finger Domain through Annexin II. J. Neurosci. 2002, 22, 3352. [Google Scholar] [CrossRef]
- Tian, X.; Yang, W.; Jiang, W.; Zhang, Z.; Liu, J.; Tu, H. Multi-Omics Profiling Identifies Microglial Annexin A2 as a Key Mediator of NF-ΚB Pro-Inflammatory Signaling in Ischemic Reperfusion Injury. Mol. Cell Proteom. 2024, 23, 100723. [Google Scholar] [CrossRef]
- Gur-Wahnon, D.; Mizrachi, T.; Maaravi-Pinto, F.Y.; Lourbopoulos, A.; Grigoriadis, N.; Higazi, A.A.R.; Brenner, T. The Plasminogen Activator System: Involvement in Central Nervous System Inflammation and a Potential Site for Therapeutic Intervention. J. Neuroinflammation 2013, 10, 124. [Google Scholar] [CrossRef]
- Wu, J.; Echeverry, R.; Guzman, J.; Yepes, M. Neuroserpin Protects Neurons from Ischemia-Induced Plasmin-Mediated Cell Death Independently of Tissue-Type Plasminogen Activator Inhibition. Am. J. Pathol. 2010, 177, 2576. [Google Scholar] [CrossRef]
- Nagai, N.; Suzuki, Y.; Van Hoef, B.; Lijnen, H.R.; Collen, D. Effects of Plasminogen Activator Inhibitor-1 on Ischemic Brain Injury in Permanent and Thrombotic Middle Cerebral Artery Occlusion Models in Mice. J. Thromb. Haemost. 2005, 3, 1379–1384. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, S.H.; Ko, H.M.; Kwon, K.J.; Cho, K.S.; Choi, C.S.; Park, J.H.; Kim, H.Y.; Lee, J.; Han, S.H.; et al. Biphasic Regulation of Tissue Plasminogen Activator Activity in Ischemic Rat Brain and in Cultured Neural Cells: Essential Role of Astrocyte-Derived Plasminogen Activator Inhibitor-1. Neurochem. Int. 2011, 58, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Praetner, M.; Zuchtriegel, G.; Holzer, M.; Uhl, B.; Schaubächer, J.; Mittmann, L.; Fabritius, M.; Fürst, R.; Zahler, S.; Funken, D.; et al. Plasminogen Activator Inhibitor-1 Promotes Neutrophil Infiltration and Tissue Injury on Ischemia-Reperfusion. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Griemert, E.V.; Recarte Pelz, K.; Engelhard, K.; Schäfer, M.K.; Thal, S.C. PAI-1 but Not PAI-2 Gene Deficiency Attenuates Ischemic Brain Injury After Experimental Stroke. Transl. Stroke Res. 2018, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Griemert, E.V.; Hedrich, J.; Hirnet, T.; Thal, S.C. Deficiency of Plasminogen Activator Inhibitor Type 2 Limits Brain Edema Formation after Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2272–2278. [Google Scholar] [CrossRef]
- Lee, J.A.; Yerbury, J.J.; Farrawell, N.; Shearer, R.F.; Constantinescu, P.; Hatters, D.M.; Schroder, W.A.; Suhrbier, A.; Wilson, M.R.; Saunders, D.N.; et al. SerpinB2 (PAI-2) Modulates Proteostasis via Binding Misfolded Proteins and Promotion of Cytoprotective Inclusion Formation. PLoS ONE 2015, 10, e0130136. [Google Scholar] [CrossRef]
- Ryu, J.; Pyo, H.; Jou, I.; Joe, E. Thrombin Induces NO Release from Cultured Rat Microglia via Protein Kinase C, Mitogen-Activated Protein Kinase, and NF-ΚB. J. Biol. Chem. 2000, 275, 29955–29959. [Google Scholar] [CrossRef]
- Ryu, J.; Min, K.; Rhim, T.Y.; Kim, T.H.; Pyo, H.; Jin, B.; Kim, S.-U.; Jou, I.; Kim, S.S.; Joe, E. Prothrombin Kringle-2 Activates Cultured Rat Brain Microglia. J. Immunol. 2002, 168, 5805–5810. [Google Scholar] [CrossRef]
- Qian, Z.; Gilbert, M.E.; Colicos, M.A.; Kandel, E.R.; Kuhl, D. Tissue-Plasminogen Activator Is Induced as an Immediate-Early Gene during Seizure, Kindling and Long-Term Potentiation. Nature 1993, 361, 453–457. [Google Scholar] [CrossRef]
- Seeds, N.W.; Basham, M.E.; Ferguson, J.E. Absence of Tissue Plasminogen Activator Gene or Activity Impairs Mouse Cerebellar Motor Learning. J. Neurosci. 2003, 23, 7368. [Google Scholar] [CrossRef]
- Zhuo, M.; Holtzman, D.M.; Li, Y.; Osaka, H.; DeMaro, J.; Jacquin, M.; Bu, G. Role of Tissue Plasminogen Activator Receptor LRP in Hippocampal Long-Term Potentiation. J. Neurosci. 2000, 20, 542. [Google Scholar] [CrossRef]
- Pang, P.T.; Teng, H.K.; Zaitsev, E.; Woo, N.T.; Sakata, K.; Zhen, S.; Teng, K.K.; Yung, W.H.; Hempstead, B.L.; Lu, B. Cleavage of ProBDNF by TPA/Plasmin Is Essential for Long-Term Hippocampal Plasticity. Science 2004, 306, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-Induced Local Protein Synthesis and Synaptic Plasticity. Neuropharmacology 2014, 76, 639–656. [Google Scholar] [CrossRef]
- Targett, I.L.; Crompton, L.A.; Conway, M.E.; Craig, T.J. Differentiation of SH-SY5Y Neuroblastoma Cells Using Retinoic Acid and BDNF: A Model for Neuronal and Synaptic Differentiation in Neurodegeneration. Vitr. Cell. Dev. Biol. Anim. 2024, 60, 1058–1067. [Google Scholar] [CrossRef]
- Quesseveur, G.; David, D.J.; Gaillard, M.C.; Pla, P.; Wu, M.V.; Nguyen, H.T.; Nicolas, V.; Auregan, G.; David, I.; Dranovsky, A.; et al. BDNF Overexpression in Mouse Hippocampal Astrocytes Promotes Local Neurogenesis and Elicits Anxiolytic-like Activities. Transl. Psychiatry 2013, 3, e253. [Google Scholar] [CrossRef]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of Cell Survival by Secreted Proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef]
- Guo, J.; Ji, Y.; Ding, Y.; Jiang, W.; Sun, Y.; Lu, B.; Nagappan, G. BDNF Pro-Peptide Regulates Dendritic Spines via Caspase-3. Cell Death Dis. 2016, 7, e2264. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Selakovic, D.; Jovicic, N.; Ljujic, B.; Rosic, G. BDNF/ProBDNF Interplay in the Mediation of Neuronal Apoptotic Mechanisms in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 4926. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Mantuano, E.; Inoue, G.; Campana, W.M.; Gonias, S.L. Ligand Binding to LRP1 Transactivates Trk Receptors by a Src Family Kinase–Dependent Pathway. Sci. Signal 2009, 2, ra18. [Google Scholar] [CrossRef]
- Qiu, Z.; Hyman, B.T.; Rebeck, G.W. Apolipoprotein E Receptors Mediate Neurite Outgrowth through Activation of P44/42 Mitogen-Activated Protein Kinase in Primary Neurons. J. Biol. Chem. 2004, 279, 34948–34956. [Google Scholar] [CrossRef]
- Mantuano, E.; Lam, M.S.; Gonias, S.L. LRP1 Assembles Unique Co-Receptor Systems to Initiate Cell Signaling in Response to Tissue-Type Plasminogen Activator and Myelin-Associated Glycoprotein. J. Biol. Chem. 2013, 288, 34009. [Google Scholar] [CrossRef] [PubMed]
- Merino, P.; Diaz, A.; Jeanneret, V.; Wu, F.; Torre, E.; Cheng, L.; Yepes, M. Urokinase-Type Plasminogen Activator (UPA) Binding to the UPA Receptor (UPAR) Promotes Axonal Regeneration in the Central Nervous System. J. Biol. Chem. 2016, 292, 2741. [Google Scholar] [CrossRef]
- Ismail, A.A.; Shaker, B.T.; Bajou, K. The Plasminogen–Activator Plasmin System in Physiological and Pathophysiological Angiogenesis. Int. J. Mol. Sci. 2021, 23, 337. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ikeda, N.; Nakashima, M.; Ikeshima-Kataoka, H.; Miyamoto, Y. Vitronectin Regulates the Fibrinolytic System during the Repair of Cerebral Cortex in Stab-Wounded Mice. J. Neurotrauma 2017, 34, 3183–3191. [Google Scholar] [CrossRef]
- Van Aken, B.E.; Seiffert, D.; Thinnes, T.; Loskutoff, D.J. Localization of Vitronectin in the Normal and Atherosclerotic Human Vessel Wall. Histochem. Cell Biol. 1997, 107, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.D.; Ferraris, G.M.S.; Andolfo, A.; Cunningham, O.; Sidenius, N. UPAR-Induced Cell Adhesion and Migration: Vitronectin Provides the Key. J. Cell Biol. 2007, 177, 927–939. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, D.; Wang, B.; Knabe, W.E.; Meroueh, S.O. A New Class of Orthosteric UPAR·uPA Small-Molecule Antagonists Are Allosteric Inhibitors of the UPAR·vitronectin Interaction. ACS Chem. Biol. 2015, 10, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9, Erratum in Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Yebra, M.; Parry, G.C.N.; Strömblad, S.; Mackman, N.; Rosenberg, S.; Mueller, B.M.; Cheresh, D.A. Requirement of Receptor-Bound Urokinase-Type Plasminogen Activator for Integrin Avβ5-Directed Cell Migration. J. Biol. Chem. 1996, 271, 29393–29399. [Google Scholar] [CrossRef]
- Hamada, M.; Varkoly, K.S.; Riyadh, O.; Beladi, R.; Munuswamy-Ramanujam, G.; Rawls, A.; Wilson-Rawls, J.; Chen, H.; McFadden, G.; Lucas, A.R. Urokinase-Type Plasminogen Activator Receptor (UPAR) in Inflammation and Disease: A Unique Inflammatory Pathway Activator. Biomedicines 2024, 12, 1167. [Google Scholar] [CrossRef]
- Roy, A.; Coum, A.; Marinescu, V.D.; Põlajeva, J.; Smits, A.; Nelander, S.; Uhrbom, L.; Westermark, B.; Forsberg-Nilsson, K.; Pontén, F.; et al. Glioma-Derived Plasminogen Activator Inhibitor-1 (PAI-1) Regulates the Recruitment of LRP1 Positive Mast Cells. Oncotarget 2015, 6, 23647. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; De Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Primers 2018, 4, 18024. [Google Scholar] [CrossRef]
- Leite, J.P.; Garcia-Cairasco, N.; Cavalheiro, E.A. New Insights from the Use of Pilocarpine and Kainate Models. Epilepsy Res. 2002, 50, 93–103. [Google Scholar] [CrossRef]
- Löscher, W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem. Res. 2017, 42, 1873–1888. [Google Scholar] [CrossRef]
- Pitkänen, A.; Lukasiuk, K. Mechanisms of Epileptogenesis and Potential Treatment Targets. Lancet Neurol. 2011, 10, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.J.; Lazarowski, A.; Vega-García, A.; Buriticá-Ramírez, E.; Auzmendi, J.; Becerra-Hernández, L.V.; Nuñez-Lumbreras, M.; de Los, A.; Orozco-Suárez, S.A.; Alves, S.S.; et al. Modulation of Neuroinflammation as a Therapeutic Strategy for the Control of Epilepsy. Seizure Eur. J. Epilepsy 2025, 131, 458–470. [Google Scholar] [CrossRef]
- Nagai, N.; Urano, T.; Endo, A.; Takahashi, H.; Takada, Y.; Takada, A. Neuronal Degeneration and a Decrease in Laminin-like Immunoreactivity Is Associated with Elevated Tissue-Type Plasminogen Activator in the Rat Hippocampus after Kainic Acid Injection. Neurosci. Res. 1999, 33, 147–154. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Rubina, K.A.; Rysenkova, K.D.; Gruzdeva, A.M.; Ivashkina, O.I.; Anokhin, K.V.; Tkachuk, V.A.; Semina, E.V. Urokinase Receptor and Tissue Plasminogen Activator as Immediate-Early Genes in Pentylenetetrazole-Induced Seizures in the Mouse Brain. Eur. J. Neurosci. 2020, 51, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Fischer, B.; Platt, D.; Schmoll, H.; Kessler, C. Delayed and Blunted Induction of MRNA for Tissue Plasminogen Activator in the Brain of Old Rats Following Pentylenetetrazole-Induced Seizure Activity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, B242–B248. [Google Scholar] [CrossRef][Green Version]
- Tan, M.L.; Ng, A.; Pandher, P.S.; Sashindranath, M.; Hamilton, J.A.; Davis, S.M.; O’Brien, T.J.; Medcalf, R.L.; Yan, B.; Jones, N.C. Tissue Plasminogen Activator Does Not Alter Development of Acquired Epilepsy. Epilepsia 2012, 53, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Yepes, M.; Sandkvist, M.; Coleman, T.A.; Moore, E.; Wu, J.-Y.; Mitola, D.; Bugge, T.H.; Lawrence, D.A. Regulation of Seizure Spreading by Neuroserpin and Tissue-Type Plasminogen Activator Is Plasminogen-Independent. J. Clin. Investig. 2002, 109, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Animal Models of Epilepsy for the Development of Antiepileptogenic and Disease-Modifying Drugs. A Comparison of the Pharmacology of Kindling and Post-Status Epilepticus Models of Temporal Lobe Epilepsy. Epilepsy Res. 2002, 50, 105–123. [Google Scholar] [CrossRef]
- Wu, Y.P.; Siao, C.J.; Lu, W.; Sung, T.C.; Frohman, M.A.; Milev, P.; Bugge, T.H.; Degen, J.L.; Levine, J.M.; Margolis, R.U.; et al. The Tissue Plasminogen Activator (Tpa/Plasmin) Extracellular Proteolytic System Regulates Seizure-Induced Hippocampal Mossy Fiber Outgrowth through a Proteoglycan Substrate. J. Cell Biol. 2000, 148, 1295. [Google Scholar] [CrossRef]
- Wan, D.; Yang, L.; Ren, J.; Huang, H.; Zhang, C.; Chen, L.; Su, X.; Huang, Q.; Niu, J.; Sun, T.; et al. Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in the Hippocampus of Lithium-Pilocarpine-Induced Acute Epileptic Rats. Mol. Biol. Rep. 2022, 49, 5805–5810. [Google Scholar] [CrossRef]
- Gorter, J.A.; Van Vliet, E.A.; Aronica, E.; Breit, T.; Rauwerda, H.; Lopes Da Silva, F.H.; Wadman, W.J. Potential New Antiepileptogenic Targets Indicated by Microarray Analysis in a Rat Model for Temporal Lobe Epilepsy. J. Neurosci. 2006, 26, 11083–11110. [Google Scholar] [CrossRef]
- Gorter, J.A.; Van Vliet, E.A.; Rauwerda, H.; Breit, T.; Stad, R.; Van Schaik, L.; Vreugdenhil, E.; Redeker, S.; Hendriksen, E.; Aronica, E.; et al. Dynamic Changes of Proteases and Protease Inhibitors Revealed by Microarray Analysis in CA3 and Entorhinal Cortex during Epileptogenesis in the Rat. Epilepsia 2007, 48 (Suppl. S5), 53–64. [Google Scholar] [CrossRef]
- Blackmon, T.J.; MacMahon, J.A.; Bernardino, P.N.; Hogans, R.E.; Cheng, M.-Y.; Vu, J.; Lee, R.D.; Saito, N.H.; Grodzki, A.C.; Bruun, D.A.; et al. Spatiotemporal perturbations of the plasminogen activation system in a rat model of acute organophosphate intoxication. Acta Neuropathol. Commun. 2025, 13, 1–16. [Google Scholar] [CrossRef]
- Karan, A.; Selivanova, E.; Spivak, Y.; Suleymanova, E. Region-Specific Long-Term Transcriptional Changes in the Plasminogen Activation System and Neuroinflammation in the Rat Brain After Status Epilepticus: Association with Depressive-like Behavior. Brain Sci. 2025, 15, 1083. [Google Scholar] [CrossRef]
- Fredriksson, L.; Stevenson, T.K.; Su, E.J.; Ragsdale, M.; Moore, S.; Craciun, S.; Schielke, G.P.; Murphy, G.G.; Lawrence, D.A. Identification of a Neurovascular Signaling Pathway Regulating Seizures in Mice. Ann. Clin. Transl. Neurol. 2015, 2, 722. [Google Scholar] [CrossRef]
- Thomas, A.X.; Cruz Del Angel, Y.; Gonzalez, M.I.; Carrel, A.J.; Carlsen, J.; Lam, P.M.; Hempstead, B.L.; Russek, S.J.; Brooks-Kayal, A.R. Rapid Increases in ProBDNF after Pilocarpine-Induced Status Epilepticus in Mice Are Associated with Reduced ProBDNF Cleavage Machinery. eNeuro 2016, 3, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Lukasiuk, K.; Kontula, L.; Pitkänen, A. CDNA Profiling of Epileptogenesis in the Rat Brain. Eur. J. Neurosci. 2003, 17, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, L.; Lukasiuk, K.; Pitkänen, A. Increased Expression and Activity of Urokinase-Type Plasminogen Activator during Epileptogenesis. Eur. J. Neurosci. 2006, 24, 1935–1945. [Google Scholar] [CrossRef]
- Powell, E.M.; Campbell, D.B.; Stanwood, G.D.; Davis, C.; Noebels, J.L.; Levitt, P. Genetic Disruption of Cortical Interneuron Development Causes Region- and GABA Cell Type-Specific Deficits, Epilepsy, and Behavioral Dysfunction. J. Neurosci. 2003, 23, 622–631. [Google Scholar] [CrossRef]
- Ndode-Ekane, X.E.; Pitkänen, A. Urokinase-Type Plasminogen Activator Receptor Modulates Epileptogenesis in Mouse Model of Temporal Lobe Epilepsy. Mol. Neurobiol. 2013, 47, 914–937. [Google Scholar] [CrossRef]
- Bolkvadze, T.; Puhakka, N.; Pitkänen, A. Epileptogenesis after Traumatic Brain Injury in Plaur-Deficient Mice. Epilepsy Behav. 2016, 60, 187–196. [Google Scholar] [CrossRef]
- Kyyriäinen, J.; Bolkvadze, T.; Koivisto, H.; Lipponen, A.; Pérez, L.O.; Ekolle Ndode-Ekane, X.; Tanila, H.; Pitkänen, A. Deficiency of Urokinase-Type Plasminogen Activator and Its Receptor Affects Social Behavior and Increases Seizure Susceptibility. Epilepsy Res. 2019, 151, 67–74. [Google Scholar] [CrossRef]
- Rantala, J.; Kemppainen, S.; Ndode-Ekane, X.E.; Lahtinen, L.; Bolkvadze, T.; Gurevicius, K.; Tanila, H.; Pitkänen, A. Urokinase-Type Plasminogen Activator Deficiency Has Little Effect on Seizure Susceptibility and Acquired Epilepsy Phenotype but Reduces Spontaneous Exploration in Mice. Epilepsy Behav. 2015, 42, 117–128. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Bates, E.J.; Ferrante, A.; Antalis, T.M. Plasminogen Activator Inhibitor Type 2 Inhibits Tumor Necrosis Factor α-Induced Apoptosis. J. Biol. Chem. 1995, 270, 27894–27904. [Google Scholar] [CrossRef] [PubMed]
- Buchthal, B.; Weiss, U.; Bading, H. Post-Injury Nose-to-Brain Delivery of Activin A and SerpinB2 Reduces Brain Damage in a Mouse Stroke Model. Mol. Ther. 2018, 26, 2357–2365. [Google Scholar] [CrossRef]
- Sharon, R.; Abramovitz, R.; Miskin, R. Plasminogen MRNA Induction in the Mouse Brain after Kainate Excitation: Codistribution with Plasminogen Activator Inhibitor-2 (PAI-2) MRNA. Brain Res. Mol. Brain Res. 2002, 104, 170–175. [Google Scholar] [CrossRef]
- Itsekson-Hayosh, Z.; Shavit-Stein, E.; Katzav, A.; Rubovitch, V.; Maggio, N.; Chapman, J.; Harnof, S.; Pick, C.G. Minimal Traumatic Brain Injury in Mice: Protease-Activated Receptor 1 and Thrombin-Related Changes. J. Neurotrauma 2016, 33, 1848–1854. [Google Scholar] [CrossRef]
- De Castro Ribeiro, M.; Badaut, J.; Price, M.; Meins, M.; Bogousslavsky, J.; Monard, D.; Hirt, L. Thrombin in Ischemic Neuronal Death. Exp. Neurol. 2006, 198, 199–203. [Google Scholar] [CrossRef]
- Lee, K.R.; Drury, I.; Vitarbo, E.; Hoff, J.T. Seizures Induced by Intracerebral Injection of Thrombin: A Model of Intracerebral Hemorrhage. J. Neurosurg. 1997, 87, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ben Shimon, M.; Shavit-Stein, E.; Altman, K.; Pick, C.G.; Maggio, N. Thrombin as Key Mediator of Seizure Development Following Traumatic Brain Injury. Front. Pharmacol. 2020, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Savotchenko, A.; Klymenko, M.; Shypshyna, M.; Isaev, D. The Role of Thrombin in Early-Onset Seizures. Front. Cell Neurosci. 2023, 17, 1101006. [Google Scholar] [CrossRef]
- Winokur, P.N.; Subramanian, P.; Bullock, J.L.; Arocas, V.; Becerra, S.P. Comparison of Two Neurotrophic Serpins Reveals a Small Fragment with Cell Survival Activity. Mol. Vis. 2017, 23, 372. [Google Scholar]
- Lahtinen, L.; Huusko, N.; Myöhänen, H.; Lehtivarjo, A.K.; Pellinen, R.; Turunen, M.P.; Ylä-Herttuala, S.; Pirinen, E.; Pitkänen, A. Expression of Urokinase-Type Plasminogen Activator Receptor Is Increased during Epileptogenesis in the Rat Hippocampus. Neuroscience 2009, 163, 316–328. [Google Scholar] [CrossRef]
- Leo, A.; Nesci, V.; Tallarico, M.; Amodio, N.; Gallo Cantafio, E.M.; De Sarro, G.; Constanti, A.; Russo, E.; Citraro, R. IL-6 Receptor Blockade by Tocilizumab Has Anti-Absence and Anti-Epileptogenic Effects in the WAG/Rij Rat Model of Absence Epilepsy. Neurotherapeutics 2020, 17, 2004–2014. [Google Scholar] [CrossRef]
- Kovács, Z.; Dobolyi, Á.; Juhász, G.; Kékesi, K.A. Lipopolysaccharide Induced Increase in Seizure Activity in Two Animal Models of Absence Epilepsy WAG/Rij and GAERS Rats and Long Evans Rats. Brain Res. Bull. 2014, 104, 7–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sutton, R.; Keohane, M.E.; VandenBerg, S.R.; Gonias, S.L. Plasminogen Activator Inhibitor-1 in the Cerebrospinal Fluid as an Index of Neurological Disease. Blood Coagul. Fibrinolysis 1994, 5, 167–171. [Google Scholar] [CrossRef]
- Akenami, F.O.T.; Koskiniemi, M.; Färkkilä, M.; Vaheri, A. Cerebrospinal Fluid Plasminogen Activator Inhibitor-1 in Patients with Neurological Disease. J. Clin. Pathol. 1997, 50, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Eruysal, E.; Ravdin, L.; Zhang, C.; Kamel, H.; Iadecola, C.; Ishii, M. Sexually Dimorphic Association of Circulating Plasminogen Activator Inhibitor-1 Levels and Body Mass Index with Cerebrospinal Fluid Biomarkers of Alzheimer’s Pathology in Preclinical Alzheimer’s Disease. J. Alzheimers Dis. 2023, 91, 1073. [Google Scholar] [CrossRef]
- De Paula Sabino, A.; Ribeiro, D.D.; Domingueti, C.P.; Dos Santos, M.S.; Gadelha, T.; SantAna Dusse, L.M.; Das Graças Carvalho, M.; Fernandes, A.P. Plasminogen Activator Inhibitor-1 4G/5G Promoter Polymorphism and PAI-1 Plasma Levels in Young Patients with Ischemic Stroke. Mol. Biol. Rep. 2011, 38, 5355–5360. [Google Scholar] [CrossRef]
- Nakae, R.; Murai, Y.; Wada, T.; Fujiki, Y.; Kanaya, T.; Takayama, Y.; Suzuki, G.; Naoe, Y.; Yokota, H.; Yokobori, S. Hyperfibrinolysis and Fibrinolysis Shutdown in Patients with Traumatic Brain Injury. Sci. Rep. 2022, 12, 19107. [Google Scholar] [CrossRef]
- Du, Y.; Xiao, X.; You, H.Z.; Hou, Z.Y.; Yang, X.D.; Wang, J.; Tang, J.; Wang, Y. Association of High Plasma Levels of Serpin E1, IGFBP2, and CCL5 With Refractory Epilepsy in Children by Cytokine Profiling. Clin. Pediatr. 2024, 63, 953–962. [Google Scholar] [CrossRef]
- Iyer, A.M.; Zurolo, E.; Boer, K.; Baayen, J.C.; Giangaspero, F.; Arcella, A.; Di Gennaro, G.C.; Esposito, V.; Spliet, W.G.M.; van Rijen, P.C.; et al. Tissue Plasminogen Activator and Urokinase Plasminogen Activator in Human Epileptogenic Pathologies. Neuroscience 2010, 167, 929–945. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Wang, T.; Liang, Q.C.; Jing, X.R.; Zheng, J.; Wang, C.; Meng, Q.; Wang, L.; Wang, W.; et al. Increased Expression of Urokinase-Type Plasminogen Activator Receptor in the Frontal Cortex of Patients with Intractable Frontal Lobe Epilepsy. J. Neurosci. Res. 2010, 88, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Quirico-Santos, T.; Nascimento Mello, A.; Casimiro Gomes, A.; De Carvalho, L.P.; De Souza, J.M.; Alves-Leon, S. Increased Metalloprotease Activity in the Epileptogenic Lesion—Lobectomy Reduces Metalloprotease Activity and Urokinase-Type UPAR Circulating Levels. Brain Res. 2013, 1538, 172–181. [Google Scholar] [CrossRef]
- Han, W.; Jiang, P.; Guo, Y.; Xu, P.; Dang, R.; Li, G.; He, X.; Liao, D.; Yan, G. Role of T-PA and PAI-1 Variants in Temporal Lobe Epilepsy in Chinese Han Population. BMC Neurol. 2019, 19, 13. [Google Scholar] [CrossRef]
- Hassanien, S.M.; Awadalla, M.M.; Saad And, A.A.; Abdel Aziz, N.A. Tissue Plasminogen Activator in Children with Idiopathic and Intractable Epilepsies. J. Pediatr. Neurol. 2010, 8, 193–197. [Google Scholar] [CrossRef]
- Keller, L.; Hobohm, C.; Zeynalova, S.; Classen, J.; Baum, P. Does Treatment with T-PA Increase the Risk of Developing Epilepsy after Stroke? J. Neurol. 2015, 262, 2364–2372. [Google Scholar] [CrossRef]
- Torrente, D.; Su, E.J.; Citalán-Madrid, A.F.; Schielke, G.P.; Magaoay, D.; Warnock, M.; Stevenson, T.; Mann, K.; Lesept, F.; Delétage, N.; et al. The Interaction of TPA with NMDAR1 Drives Neuroinflammation and Neurodegeneration in α-Synuclein-Mediated Neurotoxicity. J. Neuroinflammation 2025, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Tsirka, S.E.; Strickland, S.; Stieg, P.E.; Soriano, S.G.; Lipton, S.A. Tissue Plasminogen Activator (TPA) Increases Neuronal Damage after Focal Cerebral Ischemia in Wild-Type and TPA-Deficient Mice. Nat. Med. 1998, 4, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Villarán, R.F.; de Pablos, R.M.; Argüelles, S.; Espinosa-Oliva, A.M.; Tomás-Camardiel, M.; Herrera, A.J.; Cano, J.; Machado, A. The Intranigral Injection of Tissue Plasminogen Activator Induced Blood-Brain Barrier Disruption, Inflammatory Process and Degeneration of the Dopaminergic System of the Rat. Neurotoxicology 2009, 30, 403–413. [Google Scholar] [CrossRef]
- Ishihara, H.; Kohyama, S.; Nishida, S.; Kumagai, K.; Hayashi, S.; Kato, H. Effect of Intravenous Thrombolysis and Mechanical Thrombectomy on the Incidence of Acute Symptomatic Seizure and Post-Stroke Epilepsy in Patients with Acute Large-Vessel Occlusion. JNET J. Neuroendovascular Ther. 2024, 18, 207. [Google Scholar] [CrossRef]
- Eriksson, H.; Nordanstig, A.; Rentzos, A.; Zelano, J.; Redfors, P. Risk of Poststroke Epilepsy after Reperfusion Therapies: A National Cohort Study. Eur. J. Neurol. 2023, 30, 1303–1311. [Google Scholar] [CrossRef]
- Nesselroth, D.; Gilad, R.; Namneh, M.; Avishay, S.; Eilam, A. Estimation of Seizures Prevalence in Ischemic Strokes after Thrombolytic Therapy. Seizure 2018, 62, 91–94. [Google Scholar] [CrossRef]
- Brondani, R.; de Almeida, A.G.; Cherubini, P.A.; Secchi, T.L.; de Oliveira, M.A.; Martins, S.C.O.; Bianchin, M.M. Risk Factors for Epilepsy After Thrombolysis for Ischemic Stroke: A Cohort Study. Front. Neurol. 2020, 10, 1256. [Google Scholar] [CrossRef]
- Naylor, J.; Thevathasan, A.; Churilov, L.; Guo, R.; Xiong, Y.; Koome, M.; Chen, Z.; Chen, Z.; Liu, X.; Kwan, P.; et al. Association between Different Acute Stroke Therapies and Development of Post Stroke Seizures. BMC Neurol. 2018, 18, 61. [Google Scholar] [CrossRef]
- Roussel, B.D.; Lomas, D.A.; Crowther, D.C. Progressive Myoclonus Epilepsy Associated with Neuroserpin Inclusion Bodies (Neuroserpinosis). Epileptic Disord. 2016, 18, S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.; Shrimpton, A.E.; Carrell, R.W.; Lomas, D.A.; Gerhard, L.; Baumann, B.; Lawrence, D.A.; Yepes, M.; Kim, T.S.; Ghetti, B.; et al. Association between Conformational Mutations in Neuroserpin and Onset and Severity of Dementia. Lancet 2002, 359, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Belorgey, D.; Crowther, D.C.; Mahadeva, R.; Lomas, D.A. Mutant Neuroserpin (S49P) That Causes Familial Encephalopathy with Neuroserpin Inclusion Bodies Is a Poor Proteinase Inhibitor and Readily Forms Polymers in Vitro. J. Biol. Chem. 2002, 277, 17367–17373. [Google Scholar] [CrossRef]
- Mcrae, P.A.; Baranov, E.; Rogers, S.L.; Porter, B.E. Persistent Decrease in Multiple Components of the Perineuronal Net Following Status Epilepticus. Eur. J. Neurosci. 2012, 36, 3471. [Google Scholar] [CrossRef] [PubMed]
- Naffah-Mazzacoratti, M.G.; Porcionatto, G.A.A.; Scorza, F.A.; Amado, D.; Silva, R.; Bellissimo, M.I.; Nader, H.B.; Cavalheiro, E.A. Selective Alterations of Glycosaminoglycans Synthesis and Proteoglycan Expression in Rat Cortex and Hippocampus in Pilocarpine-Induced Epilepsy. Brain Res. Bull. 1999, 50, 229–239. [Google Scholar] [CrossRef]
- Ueno, H.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Takahashi, Y.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Alteration of Extracellular Matrix Molecules and Perineuronal Nets in the Hippocampus of Pentylenetetrazol-Kindled Mice. Neural Plast. 2019, 2019, 8924634. [Google Scholar] [CrossRef]
- Okamoto, M.; Sakiyama, J.; Mori, S.; Kurazono, S.; Usui, S.; Hasegawa, M.; Oohira, A. Kainic Acid-Induced Convulsions Cause Prolonged Changes in the Chondroitin Sulfate Proteoglycans Neurocan and Phosphacan in the Limbic Structures. Exp. Neurol. 2003, 184, 179–195. [Google Scholar] [CrossRef]
- Perosa, S.R.; Porcionatto, M.A.; Cukiert, A.; Martins, J.R.M.; Amado, D.; Nader, H.B.; Cavalheiro, E.A.; Leite, J.P.; Naffah-Mazzacoratti, M.G. Extracellular Matrix Components Are Altered in the Hippocampus, Cortex, and Cerebrospinal Fluid of Patients with Mesial Temporal Lobe Epilepsy. Epilepsia 2002, 43 (Suppl. S5), 159–161. [Google Scholar] [CrossRef]
- Peixoto-Santos, J.E.; Velasco, T.R.; Galvis-Alonso, O.Y.; Araujo, D.; Kandratavicius, L.; Assirati, J.A.; Carlotti, C.G.; Scandiuzzi, R.C.; Dos Santos, A.C.; Leite, J.P. Temporal Lobe Epilepsy Patients with Severe Hippocampal Neuron Loss but Normal Hippocampal Volume: Extracellular Matrix Molecules Are Important for the Maintenance of Hippocampal Volume. Epilepsia 2015, 56, 1562–1570. [Google Scholar] [CrossRef]
- Celio, M.R.; Spreafico, R.; De Biasi, S.; Vitellaro-Zuccarello, L. Perineuronal Nets: Past and Present. Trends Neurosci. 1998, 21, 510–515. [Google Scholar] [CrossRef]
- Chaunsali, L.; Tewari, B.P.; Sontheimer, H. Perineuronal Net Dynamics in the Pathophysiology of Epilepsy. Epilepsy Curr. 2021, 21, 273. [Google Scholar] [CrossRef] [PubMed]
- Balmer, T.S. Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro 2016, 3, ENEURO.0112-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Bozzelli, P.L.; Caccavano, A.; Allen, M.; Balmuth, J.; Vicini, S.; Wu, J.Y.; Conant, K. Disruption of Perineuronal Nets Increases the Frequency of Sharp Wave Ripple Events. Hippocampus 2018, 28, 42–52. [Google Scholar] [CrossRef]
- Shi, W.; Wei, X.; Wang, X.; Du, S.; Liu, W.; Song, J.; Wang, Y. Perineuronal Nets Protect Long-Term Memory by Limiting Activity-Dependent Inhibition from Parvalbumin Interneurons. Proc. Natl. Acad. Sci. USA 2019, 116, 27063–27073. [Google Scholar] [CrossRef]
- Woo, A.L.M.; Fleischel, E.J.; Patel, D.C.; Sontheimer, H. Contribution of Perineuronal Nets to Hyperexcitability in Pilocarpine-Induced Status Epilepticus. Epilepsia 2025, 66, 3528–3543. [Google Scholar] [CrossRef]
- Lépine, M.; Douceau, S.; Devienne, G.; Prunotto, P.; Lenoir, S.; Regnauld, C.; Pouettre, E.; Piquet, J.; Lebouvier, L.; Hommet, Y.; et al. Parvalbumin Interneuron-Derived Tissue-Type Plasminogen Activator Shapes Perineuronal Net Structure. BMC Biol. 2022, 20, 218. [Google Scholar] [CrossRef]
- Jourquin, J.; Tremblay, E.; Décanis, N.; Charton, G.; Hanessian, S.; Chollet, A.M.; Le Diguardher, T.; Khrestchatisky, M.; Rivera, S. Neuronal Activity-Dependent Increase of Net Matrix Metalloproteinase Activity Is Associated with MMP-9 Neurotoxicity after Kainate. Eur. J. Neurosci. 2003, 18, 1507–1517. [Google Scholar] [CrossRef]
- Konopka, A.; Grajkowska, W.; Ziemiańska, K.; Roszkowski, M.; Daszkiewicz, P.; Rysz, A.; Marchel, A.; Koperski, Ł.; Wilczyński, G.M.; Dzwonek, J. Matrix Metalloproteinase-9 (MMP-9) in Human Intractable Epilepsy Caused by Focal Cortical Dysplasia. Epilepsy Res. 2013, 104, 45–58. [Google Scholar] [CrossRef]
- Pitkänen, A.; Ndode-Ekane, X.E.; Łukasiuk, K.; Wilczynski, G.M.; Dityatev, A.; Walker, M.C.; Chabrol, E.; Dedeurwaerdere, S.; Vazquez, N.; Powell, E.M. Neural ECM and Epilepsy. Prog. Brain Res. 2014, 214, 229–262. [Google Scholar] [CrossRef]
- Mendes, N.F.; Pansani, A.P.; Carmanhães, E.R.F.; Tange, P.; Meireles, J.V.; Ochikubo, M.; Chagas, J.R.; da Silva, A.V.; Monteiro de Castro, G.; Le Sueur-Maluf, L. The Blood-Brain Barrier Breakdown During Acute Phase of the Pilocarpine Model of Epilepsy Is Dynamic and Time-Dependent. Front. Neurol. 2019, 10, 382, Erratum in Front. Neurol. 2019, 10, 603. [Google Scholar] [CrossRef]
- Horowitz, S.W.; Merchut, M.; Fine, M.; Azar-Kia, B. Complex Partial Seizure-Induced Transient MR Enhancement. J. Comput. Assist. Tomogr. 1992, 16, 814–816. [Google Scholar] [CrossRef]
- Alvarez, V.; Maeder, P.; Rossetti, A.O. Postictal Blood-Brain Barrier Breakdown on Contrast-Enhanced MRI. Epilepsy Behav. 2010, 17, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Chung, J.I.; Yoon, P.H.; Kim, D.I.; Chung, T.S.; Kim, E.J.; Jeong, E.K. Transient MR Signal Changes in Patients with Generalized Tonicoclonic Seizure or Status Epilepticus: Periictal Diffusion-Weighted Imaging. AJNR Am. J. Neuroradiol. 2001, 22, 1149. [Google Scholar]
- Rüber, T.; David, B.; Lüchters, G.; Nass, R.D.; Friedman, A.; Surges, R.; Stöcker, T.; Weber, B.; Deichmann, R.; Schlaug, G.; et al. Evidence for Peri-Ictal Blood-Brain Barrier Dysfunction in Patients with Epilepsy. Brain 2018, 141, 2952–2965. [Google Scholar] [CrossRef]
- Reiter, J.T.; Schulte, F.; Bauer, T.; David, B.; Endler, C.; Isaak, A.; Schuch, F.; Bitzer, F.; Witt, J.A.; Hattingen, E.; et al. Evidence for Interictal Blood–Brain Barrier Dysfunction in People with Epilepsy. Epilepsia 2024, 65, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Bronisz, E.; Cudna, A.; Wierzbicka, A.; Kurkowska-Jastrzębska, I. Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients. Cells 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-Promoting Effect of Blood–Brain Barrier Disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef]
- Van Vliet, E.A.; Araújo, S.D.C.; Redeker, S.; Van Schaik, R.; Aronica, E.; Gorter, J.A. Blood-Brain Barrier Leakage May Lead to Progression of Temporal Lobe Epilepsy. Brain 2007, 130, 521–534. [Google Scholar] [CrossRef]
- Bar-Klein, G.; Lublinsky, S.; Kamintsky, L.; Noyman, I.; Veksler, R.; Dalipaj, H.; Senatorov, V.V.; Swissa, E.; Rosenbach, D.; Elazary, N.; et al. Imaging Blood-Brain Barrier Dysfunction as a Biomarker for Epileptogenesis. Brain 2017, 140, 1692–1705. [Google Scholar] [CrossRef]
- Han, H.; Mann, A.; Ekstein, D.; Eyal, S. Breaking Bad: The Structure and Function of the Blood-Brain Barrier in Epilepsy. AAPS J. 2017, 19, 973–988. [Google Scholar] [CrossRef]
- Meijer, W.C.; Gorter, J.A. Role of Blood–Brain Barrier Dysfunction in the Development of Poststroke Epilepsy. Epilepsia 2024, 65, 2519–2536. [Google Scholar] [CrossRef]
- Weissberg, I.; Wood, L.; Kamintsky, L.; Vazquez, O.; Milikovsky, D.Z.; Alexander, A.; Oppenheim, H.; Ardizzone, C.; Becker, A.; Frigerio, F.; et al. Albumin Induces Excitatory Synaptogenesis through Astrocytic TGF-β/ALK5 Signaling in a Model of Acquired Epilepsy Following Blood-Brain Barrier Dysfunction. Neurobiol. Dis. 2015, 78, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Friedman, A. Structural, Molecular, and Functional Alterations of the Blood-Brain Barrier during Epileptogenesis and Epilepsy: A Cause, Consequence, or Both? Int. J. Mol. Sci. 2020, 21, 591. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory Pathways as Treatment Targets and Biomarkers in Epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef]
- Vezzani, A.; Viviani, B. Neuromodulatory Properties of Inflammatory Cytokines and Their Impact on Neuronal Excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Aronica, E.; Vezzani, A.; Ravizza, T. Review: Neuroinflammatory Pathways as Treatment Targets and Biomarker Candidates in Epilepsy: Emerging Evidence from Preclinical and Clinical Studies. Neuropathol. Appl. Neurobiol. 2018, 44, 91–111. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The Role of Inflammation in Epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Vezzani, A.; Lang, B.; Aronica, E. Immunity and Inflammation in Epilepsy. Cold Spring Harb. Perspect. Med. 2016, 6, a022699. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Woodhurst, W.B.; MacDonald, D.B.; Jones, M.W. Reactive Microglia in Hippocampal Sclerosis Associated with Human Temporal Lobe Epilepsy. Neurosci. Lett. 1995, 191, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.G.; Boop, F.A.; Mrak, R.E.; Griffin, W.S.T. Increased Neuronal Beta-Amyloid Precursor Protein Expression in Human Temporal Lobe Epilepsy: Association with Interleukin-1 Alpha Immunoreactivity. J. Neurochem. 1994, 63, 1872–1879. [Google Scholar] [CrossRef]
- Leal, B.; Chaves, J.; Carvalho, C.; Rangel, R.; Santos, A.; Bettencourt, A.; Lopes, J.; Ramalheira, J.; Silva, B.M.; da Silva, A.M.; et al. Brain Expression of Inflammatory Mediators in Mesial Temporal Lobe Epilepsy Patients. J. Neuroimmunol. 2017, 313, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, T.; Scheper, M.; Di Sapia, R.; Gorter, J.; Aronica, E.; Vezzani, A. MTOR and Neuroinflammation in Epilepsy: Implications for Disease Progression and Treatment. Nat. Rev. Neurosci. 2024, 25, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Alfano, D.; Franco, P.; Stoppelli, M.P. Modulation of Cellular Function by the Urokinase Receptor Signalling: A Mechanistic View. Front. Cell Dev. Biol. 2022, 10, 818616. [Google Scholar] [CrossRef]
- Spalice, A.; Parisi, P.; Nicita, F.; Pizzardi, G.; Del Balzo, F.; Iannetti, P. Neuronal Migration Disorders: Clinical, Neuroradiologic and Genetics Aspects. Acta Paediatr. 2009, 98, 421–433. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Dudek, F.E. Primary and Secondary Mechanisms of Epileptogenesis in the Temporal Lobe: There Is a before and an After. Epilepsy Curr. 2010, 10, 118–125. [Google Scholar] [CrossRef]
- Cavazos, J.E.; Zhang, P.; Qazi, R.; Sutula, T.P. Ultrastructural Features of Sprouted Mossy Fiber Synapses in Kindled and Kainic Acid-Treated Rats. J. Comp. Neurol. 2003, 458, 272–292. [Google Scholar] [CrossRef]
- Mello, L.E.; Cavalheiro, E.A.; Tan, A.M.; Kupfer, W.R.; Pretorius, J.K.; Babb, T.L.; Finch, D.M. Circuit Mechanisms of Seizures in the Pilocarpine Model of Chronic Epilepsy: Cell Loss and Mossy Fiber Sprouting. Epilepsia 1993, 34, 985–995. [Google Scholar] [CrossRef]
- Cronin, J.; Dudek, F.E. Chronic Seizures and Collateral Sprouting of Dentate Mossy Fibers after Kainic Acid Treatment in Rats. Brain Res. 1988, 474, 181–184. [Google Scholar] [CrossRef]
- Buckmaster, P.S.; Zhang, G.F.; Yamawaki, R. Axon Sprouting in a Model of Temporal Lobe Epilepsy Creates a Predominantly Excitatory Feedback Circuit. J. Neurosci. 2002, 22, 6650–6658. [Google Scholar] [CrossRef]
- Sutula, T.; Cascino, G.; Cavazos, J.; Parada, I.; Ramirez, L. Mossy Fiber Synaptic Reorganization in the Epileptic Human Temporal Lobe. Ann. Neurol. 1989, 26, 321–330. [Google Scholar] [CrossRef]
- Cavazos, J.E.; Golarai, G.; Sutula, T.P. Septotemporal Variation of the Supragranular Projection of the Mossy Fiber Pathway in the Dentate Gyrus of Normal and Kindled Rats. Hippocampus 1992, 2, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Maglóczky, Z. Sprouting in Human Temporal Lobe Epilepsy: Excitatory Pathways and Axons of Interneurons. Epilepsy Res. 2010, 89, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, H.; Molnár, J.E.; Sóki, N.; Gyimesi, C.; Horváth, Z.; Janszky, J.; Dóczi, T.; Seress, L. Etiology-Related Degree of Sprouting of Parvalbumin-Immunoreactive Axons in the Human Dentate Gyrus in Temporal Lobe Epilepsy. Neuroscience 2020, 448, 55–70. [Google Scholar] [CrossRef]
- Harward, S.C.; Huang, Y.Z.; McNamara, J.O. BDNF/TrkB Signaling and Epileptogenesis. In Book Jasper’s Basic Mechanisms of the Epilepsies, 5th ed.; Noebels, J.L., Avoli, M., Eds.; Oxford University Press: New York, NY, USA, 2024; Chapter 32; pp. 665–680. [Google Scholar] [CrossRef]
- Danzer, S.C.; Crooks, K.R.C.; Lo, D.C.; McNamara, J.O. Increased Expression of Brain-Derived Neurotrophic Factor Induces Formation of Basal Dendrites and Axonal Branching in Dentate Granule Cells in Hippocampal Explant Cultures. J. Neurosci. 2002, 22, 9754. [Google Scholar] [CrossRef]
- Orefice, L.L.; Shih, C.C.; Xu, H.; Waterhouse, E.G.; Xu, B. Control of Spine Maturation and Pruning through ProBDNF Synthesized and Released in Dendrites. Mol. Cell. Neurosci. 2016, 71, 66–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kellner, Y.; Gödecke, N.; Dierkes, T.; Thieme, N.; Zagrebelsky, M.; Korte, M. The BDNF Effects on Dendritic Spines of Mature Hippocampal Neurons Depend on Neuronal Activity. Front. Synaptic Neurosci. 2014, 6, 5. [Google Scholar] [CrossRef]
- Lu, Y.; Christian, K.; Lu, B. BDNF: A Key Regulator for Protein-Synthesis Dependent LTP and Long-Term Memory? Neurobiol. Learn. Mem. 2007, 89, 312. [Google Scholar] [CrossRef]
- Mula, M.; Kanner, A.M.; Jetté, N.; Sander, J.W. Psychiatric Comorbidities in People With Epilepsy. Neurol. Clin. Pract. 2021, 11, e112–e120. [Google Scholar] [CrossRef]
- Pham, T.; Sauro, K.M.; Patten, S.B.; Wiebe, S.; Fiest, K.M.; Bulloch, A.G.M.; Jetté, N. The Prevalence of Anxiety and Associated Factors in Persons with Epilepsy. Epilepsia 2017, 58, e107–e110. [Google Scholar] [CrossRef]
- Kanner, A.M. Depression in Epilepsy: Prevalence, Clinical Semiology, Pathogenic Mechanisms, and Treatment. Biol. Psychiatry 2003, 54, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Pietraszek, M.H.; Takada, Y.; Nishimoto, M.; Ohara, K.; Ohara, K.; Takada, A. Fibrinolytic Activity in Depression and Neurosis. Thromb. Res. 1991, 63, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Brostedt, E.M.; de Faire, U.; Westerholm, P.; Knutsson, A.; Alfredsson, L. Job Strain and Plasminogen Activator Inhibitor-1: Results from the Swedish WOLF Study. Int. Arch. Occup. Environ. Health 2004, 77, 341–344. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Liu, Y.; Hou, Z.; Yue, Y.; Zhang, Y.; Zhao, F.; Xu, Z.; Li, Y.; Mou, X.; et al. Combined Serum Levels of Multiple Proteins in TPA-BDNF Pathway May Aid the Diagnosis of Five Mental Disorders. Sci. Rep. 2017, 7, 6871. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, C.; Li, Z.; Jiang, T.; Liang, Y.; Jiang, T.; Yu, C.; Yan, S.; Li, P.; Zhou, L. The Changes of TPA/PAI-1 System Are Associated with the Ratio of BDNF/ProBDNF in Major Depressive Disorder and SSRIs Antidepressant Treatment. Neuroscience 2024, 559, 220–228. [Google Scholar] [CrossRef]
- Girard, R.A.; Chauhan, P.S.; Tucker, T.A.; Allen, T.; Kaur, J.; Jeffers, A.; Koenig, K.; Florova, G.; Komissarov, A.A.; Gaidenko, T.A.; et al. Increased Expression of Plasminogen Activator Inhibitor-1 (PAI-1) Is Associated with Depression and Depressive Phenotype in C57Bl/6J Mice. Exp. Brain Res. 2019, 237, 3419–3430. [Google Scholar] [CrossRef]
- Lahlou-Laforet, K.; Alhenc-Gelas, M.; Pornin, M.; Bydlowski, S.; Seigneur, E.; Benetos, A.; Kierzin, J.M.; Scarabin, P.Y.; Ducimetiere, P.; Aiach, M.; et al. Relation of Depressive Mood to Plasminogen Activator Inhibitor, Tissue Plasminogen Activator, and Fibrinogen Levels in Patients With Versus Without Coronary Heart Disease. Am. J. Cardiol. 2006, 97, 1287–1291. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, C.; Ko, Y.-H.; Lee, M.-S.; Park, M.H.; Pae, C.-U.; Yoon, H.-K.; Han, C. Plasminogen Activator Inhibitor-1: Potential Inflammatory Marker in Late-Life Depression. Clin. Psychopharmacol. Neurosci. 2023, 21, 147–161. [Google Scholar] [CrossRef]
- Tsai, S.J.; Hong, C.J.; Liou, Y.J.; Yu, Y.W.Y.; Chen, T.J. Plasminogen Activator Inhibitor-1 Gene Is Associated with Major Depression and Antidepressant Treatment Response. Pharmacogenet Genom. 2008, 18, 869–875. [Google Scholar] [CrossRef]
- Party, H.; Dujarrier, C.; Hébert, M.; Lenoir, S.; Martinez De Lizarrondo, S.; Delépée, R.; Fauchon, C.; Bouton, M.C.; Obiang, P.; Godefroy, O.; et al. Plasminogen Activator Inhibitor-1 (PAI-1) Deficiency Predisposes to Depression and Resistance to Treatments. Acta Neuropathol. Commun. 2019, 7, 153. [Google Scholar] [CrossRef]
- Han, W.; Dang, R.; Xu, P.; Li, G.; Zhou, X.; Chen, L.; Guo, Y.; Yang, M.; Chen, D.; Jiang, P. Altered Fibrinolytic System in Rat Models of Depression and Patients with First-Episode Depression. Neurobiol. Stress. 2019, 11, 100188. [Google Scholar] [CrossRef]
- Silva, Z.M.; Toledo, D.N.M.; Pio, S.; Machado, B.A.A.; Santos, P.V.D.; Hó, F.G.; Medina, Y.N.; Cordeiro, P.H.D.M.; Perucci, L.O.; Pinto, K.M.D.C.; et al. Neuroserpin, IL-33 and IL-17A as Potential Markers of Mild Symptoms of Depressive Syndrome in Toxoplasma Gondii-Infected Pregnant Women. Front. Immunol. 2024, 15, 1394456. [Google Scholar] [CrossRef]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Li, P.; Du, X.; Zhao, M.; Wang, H.; Yang, D.; Wu, M.; Jing, W. Risk Factors for Anxiety in Patients with Epilepsy: A Meta-Analysis. Epilepsy Behav. 2024, 153, 109665. [Google Scholar] [CrossRef]
- Frasure-Smith, N. In-Hospital Symptoms of Psychological Stress as Predictors of Long-Term Outcome after Acute Myocardial Infarction in Men. Am. J. Cardiol. 1991, 67, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.L.; Abbey, S.E.; Irvine, J.; Shnek, Z.M.; Stewart, D.E. Prospective Examination of Anxiety Persistence and Its Relationship to Cardiac Symptoms and Recurrent Cardiac Events. Psychother. Psychosom. 2004, 73, 344–352. [Google Scholar] [CrossRef]
- Geiser, F.; Meier, C.; Wegener, I.; Imbierowicz, K.; Conrad, R.; Liedtke, R.; Oldenburg, J.; Harbrecht, U. Association between Anxiety and Factors of Coagulation and Fibrinolysis. Psychother. Psychosom. 2008, 77, 377–383. [Google Scholar] [CrossRef]
- Konkle, B.; Schuster, S.; Kelly, M.; Harjes, K.; Hassett, D.; Bohrer, M.; Tavassoli, M. Plasminogen Activator Inhibitor-1 Messenger RNA Expression Is Induced in Rat Hepatocytes In Vivo by Dexamethasone. Blood 1992, 79, 2636–2642. [Google Scholar] [CrossRef]
- Heaton, J.H.; Nebes, V.L.; O’Dell, L.G.; Morris, S.M.; Gelehrter, T.D. Glucocorticoid and Cyclic Nucleotide Regulation of Plasminogen Activator and Plasminogen Activator-Inhibitor Gene Expression in Primary Cultures of Rat Hepatocytes. Mol. Endocrinol. 1989, 3, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takeshita, K.; Shimokawa, T.; Yi, H.; Isobe, K.I.; Loskutoff, D.J.; Saito, H. Plasminogen Activator Inhibitor-1 Is a Major Stress-Regulated Gene: Implications for Stress-Induced Thrombosis in Aged Individuals. Proc. Natl. Acad. Sci. USA 2002, 99, 890. [Google Scholar] [CrossRef]
- Mennesson, M.; Revest, J.M. Glucocorticoid-Responsive Tissue Plasminogen Activator (TPA) and Its Inhibitor Plasminogen Activator Inhibitor-1 (PAI-1): Relevance in Stress-Related Psychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 4496. [Google Scholar] [CrossRef]
- Pawlak, R.; Magarinos, A.M.; Melchor, J.; McEwen, B.; Strickland, S. Tissue Plasminogen Activator in the Amygdala Is Critical for Stress-Induced Anxiety-like Behavior. Nat. Neurosci. 2003, 6, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Madani, R.; Kozlov, S.; Akhmedov, A.; Cinelli, P.; Kinter, J.; Lipp, H.P.; Sonderegger, P.; Wolfer, D.P. Impaired Explorative Behavior and Neophobia in Genetically Modified Mice Lacking or Overexpressing the Extracellular Serine Protease Inhibitor Neuroserpin. Mol. Cell. Neurosci. 2003, 23, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Reumann, R.; Vierk, R.; Zhou, L.; Gries, F.; Kraus, V.; Mienert, J.; Romswinkel, E.; Morellini, F.; Ferrer, I.; Nicolini, C.; et al. The Serine Protease Inhibitor Neuroserpin Is Required for Normal Synaptic Plasticity and Regulates Learning and Social Behavior. Learn. Mem. 2017, 24, 650–659. [Google Scholar] [CrossRef]
- Lu, E.; Pyatka, N.; Burant, C.J.; Sajatovic, M. Systematic Literature Review of Psychiatric Comorbidities in Adults with Epilepsy. J. Clin. Neurol. 2021, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Ertan, D.; Tarrada, A.; El-Hage, W.; Sanchez, S.; Four, E.; Mezouar, N.; Maillard, L.; Chrusciel, J.; Hingray, C. Prevalence of Posttraumatic Stress Disorder in Adults with Epilepsy: A Meta-Analysis. Seizure 2025, 126, 32–42. [Google Scholar] [CrossRef]
- Pepi, C.; Mercier, M.; Salimbene, L.; Galati, C.; Specchio, N.; de Palma, L. Post-Traumatic Stress-Disorder in Epilepsy: Meta-Analysis of Current Evidence. Epilepsy Behav. 2024, 157, 109833. [Google Scholar] [CrossRef]
- Von Känel, R.; Hepp, U.; Buddeberg, C.; Keel, M.; Mica, L.; Aschbacher, K.; Schnyder, U. Altered Blood Coagulation in Patients with Posttraumatic Stress Disorder. Psychosom. Med. 2006, 68, 598–604. [Google Scholar] [CrossRef]
- Règue, M.; Poilbout, C.; Martin, V.; Franc, B.; Lanfumey, L.; Mongeau, R. Increased 5-HT2C Receptor Editing Predisposes to PTSD-like Behaviors and Alters BDNF and Cytokines Signaling. Transl. Psychiatry 2019, 9, 100. [Google Scholar] [CrossRef]
- Aksu, S.; Unlu, G.; Kardesler, A.C.; Cakaloz, B.; Aybek, H. Altered Levels of Brain-Derived Neurotrophic Factor, ProBDNF and Tissue Plasminogen Activator in Children with Posttraumatic Stress Disorder. Psychiatry Res. 2018, 268, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Maguire, D.; Watt, J.; Armour, C.; Milanak, M.; Lagdon, S.; Lamont, J.V.; Kurth, M.J.; Fitzgerald, P.; Moore, T.; Ruddock, M.W. Post-Traumatic Stress Disorder: A Biopsychosocial Case-Control Study Investigating Peripheral Blood Protein Biomarkers. Biomark. Neuropsychiatry 2021, 5, 100042. [Google Scholar] [CrossRef]
- Price, R.B.; Duman, R. Neuroplasticity in Cognitive and Psychological Mechanisms of Depression: An Integrative Model. Mol. Psychiatry 2019, 25, 530. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Tu, J.L.; Li, X.H.; Hua, Q.; Liu, W.Z.; Liu, Y.; Pan, B.X.; Hu, P.; Zhang, W.H. Neuroinflammation Induces Anxiety- and Depressive-like Behavior by Modulating Neuronal Plasticity in the Basolateral Amygdala. Brain Behav. Immun. 2020, 91, 505–518. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. Is Depression an Inflammatory Disorder? Curr. Psychiatry Rep. 2011, 13, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Manji, H.; Lu, B. New Insights into BDNF Function in Depression and Anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Corsi-Travali, S.; Neumeister, A. The Role of BDNF-TrkB Signaling in the Pathogenesis of PTSD. J. Depress. Anxiety 2013, 2013, 6. [Google Scholar] [CrossRef]


| Component of the PA System | Expression Site | Main Function in CNS | Effect in the CNS |
|---|---|---|---|
| tPA | Endothelial cells Neurons Activated microglia Oligodendrocytes Mastocytes Ependymocytes. The most prominent expression in the limbic structures | Conversion of plasminogen to its active form, plasmin Cell signaling | Activation of microglia Synaptic remodeling Long-term potentiation formation Regulation of vascular permeability and BBB integrity Neuronal migration in developing brain Regulation of neurodegeneration and neuronal survival in various pathologic conditions |
| uPA/uPAR | uPA: neurons uPAR: microglia, endothelial cells, dendritic and axonal growth cones, astrocytes | Conversion of plasminogen to its active form, plasmin Cell signaling | Neuroplasticity Cell migration Cell proliferation and survival Neuroinflammation |
| PAI-1 | Astrocytes Neurons | tPA and uPA inhibition Cell cignaling | Neuroinflammation and neurodegeneration control Cell migration |
| PAI-2 | Microglia Astrocytes Endothelial cells | Inhibition of uPA and two-chain form of tPA | Neuroprotection |
| Neuroserpin | Neurons Predominantly in the neocortex, hippocampus, olfactory bulb, the amygdala | tPA and uPA inhibition. Regulation of basal levels of tPA in the neural tissues | Neuroplasticity Neuronal survival |
| PN-1 | Neurons Astrocytes | Inhibition of uPA and thrombin | Neuroprotection |
| Plasminogen/Plasmin | Neurons Low levels in the brain tissues including the hippocampus, cortex, cerebellum and neuroendocrine tissues | Extracellular proteolysis | Recruitment of peripheral immune cells Microglia activation Neuroinflammation Hemostasis and vascular function Degradation of ECM components Long-term plasticity |
| PA System Component | Model/Object | PA System Role | References |
|---|---|---|---|
| TPA | PTZ-induced acute seizures/rat | Increase in mRNA expression in the hippocampus and cortex | [111,141] |
| Electric kindling/rat | Increase in mRNA expression in the hippocampus | [111] | |
| KA-induced seizures/WT mice | Increase in tPA protein and enzymatic activity in the hippocampus and amygdala | [139,143] | |
| KA-induced seizures/tPA−/− mice | Increased resistance to seizures and reduced neuronal damage in tPA deficient mice Decreased mossy fiber outgrowth and sprouting in the dentate gyrus of tPA deficient mice | [10,143,145,151] | |
| Lithium-pilocarpine model of SE/rat | Increase in mRNA expression in the hippocampus on day 1 and day 3 after SE | [146] | |
| Electrically induced SE/rat | Increase in mRNA expression in the hippocampus during acute and latent phase after SE | [147,148] | |
| Amygdala kindling/transgenic heterozygous T4 mice (tPA overexpression) | Overexpression of tPA lowers the threshold for electrically induced seizures but does not affect epileptogenesis | [142] | |
| PTZ-induced seizures/WT mice | Increase in mRNA expression in the hippocampus, cortex, and amygdala 3 h after seizures | [140] | |
| Acute organophosphate intoxication-induced seizures/rat | Increase in tPA protein level in the hippocampus and cortex on days 1–7 after intoxication and up to day 28 in the hippocampus | [149] | |
| PAI-1 | KA-induced seizures/WT mice | Increase in mRNA expression in the limbic system and cortex | [11] |
| Pilocarpine SE model/WT mice | Increase in PAI-1 protein expression up to 3 days after SE | [152] | |
| PTZ-induced seizures/WT mice | Increase in mRNA expression in the anterior cortex 72 h after seizures | [140] | |
| NEUROSERPIN | KA-induced seizures/WT mice | Increase in protein level in response to KA-induced seizures Administration of exogenous neuroserpin delays the progression of seizure activity | [143] |
| KA-induced seizures/Nsp−/− mice | Increase in seizure severity and decrease in the latency to onset of seizures in neuroserpin-deficient mice | [151] | |
| UPA/UPAR | KA-induced seizures/WT mice | Increase in uPA mRNA expression in the limbic system and cortex | [11] |
| Electrically induced SE/rat | Increase in uPA protein level and enzymatic activity Increase in uPAR expression in hippocampal interneurons | [153,154,169] | |
| PTZ-induced seizures/WT mice | Increased uPA and uPAR mRNA expression in the hippocampus and cortex | [140] | |
| PTZ-induced seizures/uPAR−/− mice | Spontaneous seizure activity in uPAR−/− mice Increased sensitivity to PTZ-induced seizures in uPAR−/− mice | [155] | |
| KA-induced epileptogenesis/uPAR−/− mice | No changes in sensitivity to KA in uPAR−/− mice but higher severity of spontaneous seizures and neurodegeneration | [156] | |
| Lithium-pilocarpine model of SE/rat | Increase in uPAR mRNA expression up to 5 months after SE | [150] | |
| PAI-2 | KA-induced seizures/WT mice | Increase in PAI-2 mRNA expression in the cortex, hippocampus, and amygdala | [162] |
| NEXIN-1 | mTBI/WT mice | Increase in PN-1 expression in the later post mTBI stages Increase in sensitivity to seizure-like activity after thrombin injection | [163] |
| Disorder | Reported Changes in the PA System | References |
|---|---|---|
| Drug-resistant epilepsy in children | Increase in PAI-1 plasma level | [177] |
| Focal chronic refractory epilepsy | Increase in tPA, uPA, uPAR, and PAI-1 mRNA and protein expression in surgical specimens from patients with focal epilepsy | [178] |
| Intractable frontal lobe epilepsy | Increase in uPAR expression in frontal lobe | [179] |
| TLE | Increased plasma uPAR level, reversed after the removal of epileptogenic lesion Significant differences in genotypic and allelic frequencies of polymorphic sites of tPA gene PLAT between TLE patients and controls | [180,181] |
| Idiopathic epilepsy in children | Increase in serum tPA levels | [182] |
| FENIB | Polymerization of mutated neuroserpin leading to neurodegeneration and progressive myoclonus epilepsy | [192,193] |
| Disorder | Model(Condition)/Object | PA System Changes | References |
|---|---|---|---|
| Depression | Depression and depressive neurosis/patients | Decrease in tPA plasma levels, Decrease in plasma levels of total PAI-1 and tPA-PAI-1 complex | [253] |
| Decrease in serum tPA and BDNF, decrease in proBDNF, reverse after antidepressant treatment | [15,255] | ||
| MDD/patients | Increase in PAI-1 plasma level and mRNA expression | [256,257] | |
| MDD/geriatric patients | Decrease in PAI-1 plasma levels | [259] | |
| MDD/patients | rs2227684-G and rs7242-T alleles of the SERPINE1 gene encoding PAI-1 associated with MDD | [260] | |
| MDD/human brain tissue | Increased PAI-1 expression in the hippocampal astrocytes of MDD patients | [257] | |
| LPS-induced depression model/WT mice | Increased PAI-1 plasma levels and expression in hippocampus | [257] | |
| PAI-1−/− mice | PAI-1 deficiency led to the development of depressive-like phenotype with resistance to antidepressants | [261] | |
| First-episode depression/patients | Neuroserpin downregulation was found in the peripheral blood mononuclear cells | [262] | |
| Depressive syndrome during pregnancy/patients | Increase in neuroserpin plasma levels | [263] | |
| LPS-induced depression/rat | Decrease in mRNA expression of neuroserpin in the hippocampus and prefrontal cortex | [262] | |
| Anxiety and stress | Anxiety disorders (panic disorder with agoraphobia or social phobia)/patients | Increase in plasma PAI-1 levels | [268] |
| Panic disorder/patients | No changes in PAI-1 and tPA serum levels | [255] | |
| Restraint stress/WT mice | Increase in tPA expression and activity in the amygdala Increase in PAI-1 expression | [273] | |
| Restraint stress/tPA−/− mice | Anxiety-like behavior in response to stress is reduced compared to WT mice | [273] | |
| tPAnull mice | Increased locomotor hyperactivity and reduced anxiety | [16] | |
| PTSD | PTSD-like fear memory/ WT mice | Increased PAI-1 expression in response to corticosterone + fear conditioning | [14] |
| VGV transgenic mice (5-HT2CR overexpression) | Decreased tPA and BDNF mRNA expression in the hippocampus, increased in the amygdala | [280] | |
| PTSD/pediatric patients | Decreased serum levels of BDNF and proBDNF, increased serum tPA levels | [281] | |
| PTSD/patients | Increased serum levels of tPA | [282] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleymanova, E.; Karan, A. The Plasminogen Activation System in the Central Nervous System: Implications for Epilepsy and Neuropsychiatric Disorders. Int. J. Mol. Sci. 2025, 26, 10893. https://doi.org/10.3390/ijms262210893
Suleymanova E, Karan A. The Plasminogen Activation System in the Central Nervous System: Implications for Epilepsy and Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2025; 26(22):10893. https://doi.org/10.3390/ijms262210893
Chicago/Turabian StyleSuleymanova, Elena, and Anna Karan. 2025. "The Plasminogen Activation System in the Central Nervous System: Implications for Epilepsy and Neuropsychiatric Disorders" International Journal of Molecular Sciences 26, no. 22: 10893. https://doi.org/10.3390/ijms262210893
APA StyleSuleymanova, E., & Karan, A. (2025). The Plasminogen Activation System in the Central Nervous System: Implications for Epilepsy and Neuropsychiatric Disorders. International Journal of Molecular Sciences, 26(22), 10893. https://doi.org/10.3390/ijms262210893







