Clofazimine Treatment Modulates Key Non-Coding RNAs Associated with Tumor Progression and Drug Resistance in Lethal Prostate Cancer
Abstract
1. Introduction
2. Results
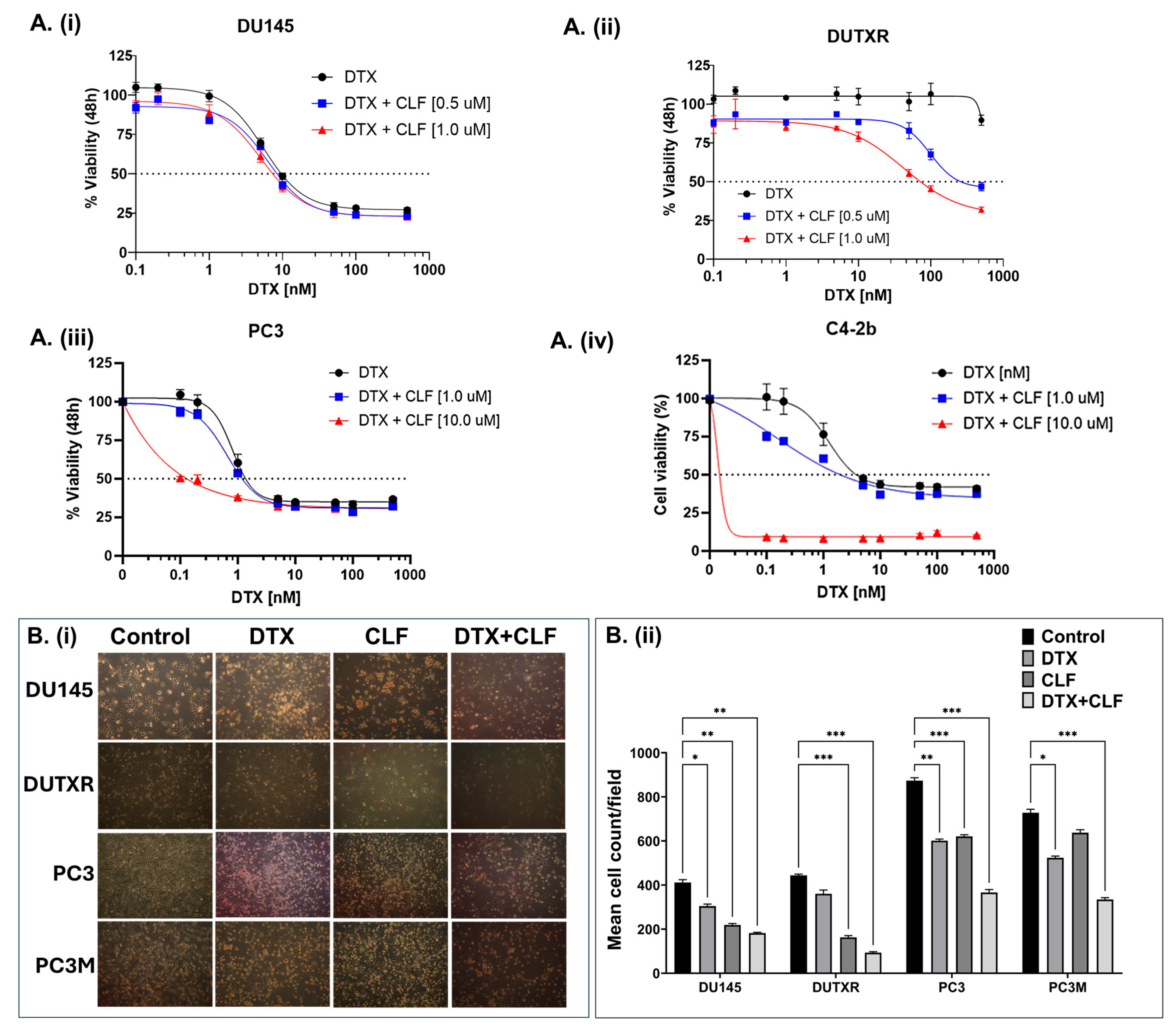
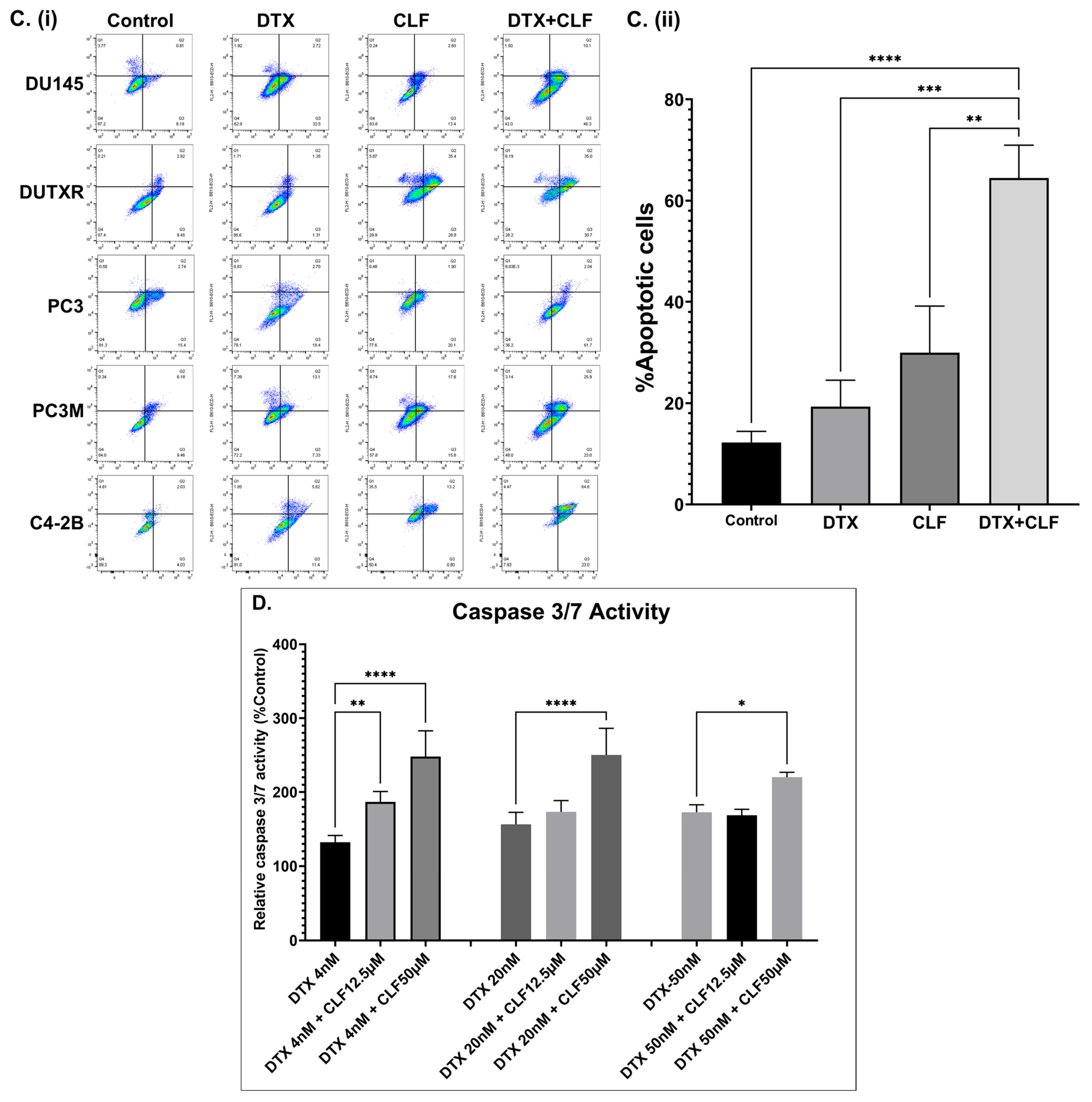
2.1. CLF Treatment Results in Loss of Viability and Increased Apoptosis in PCa Cell Lines
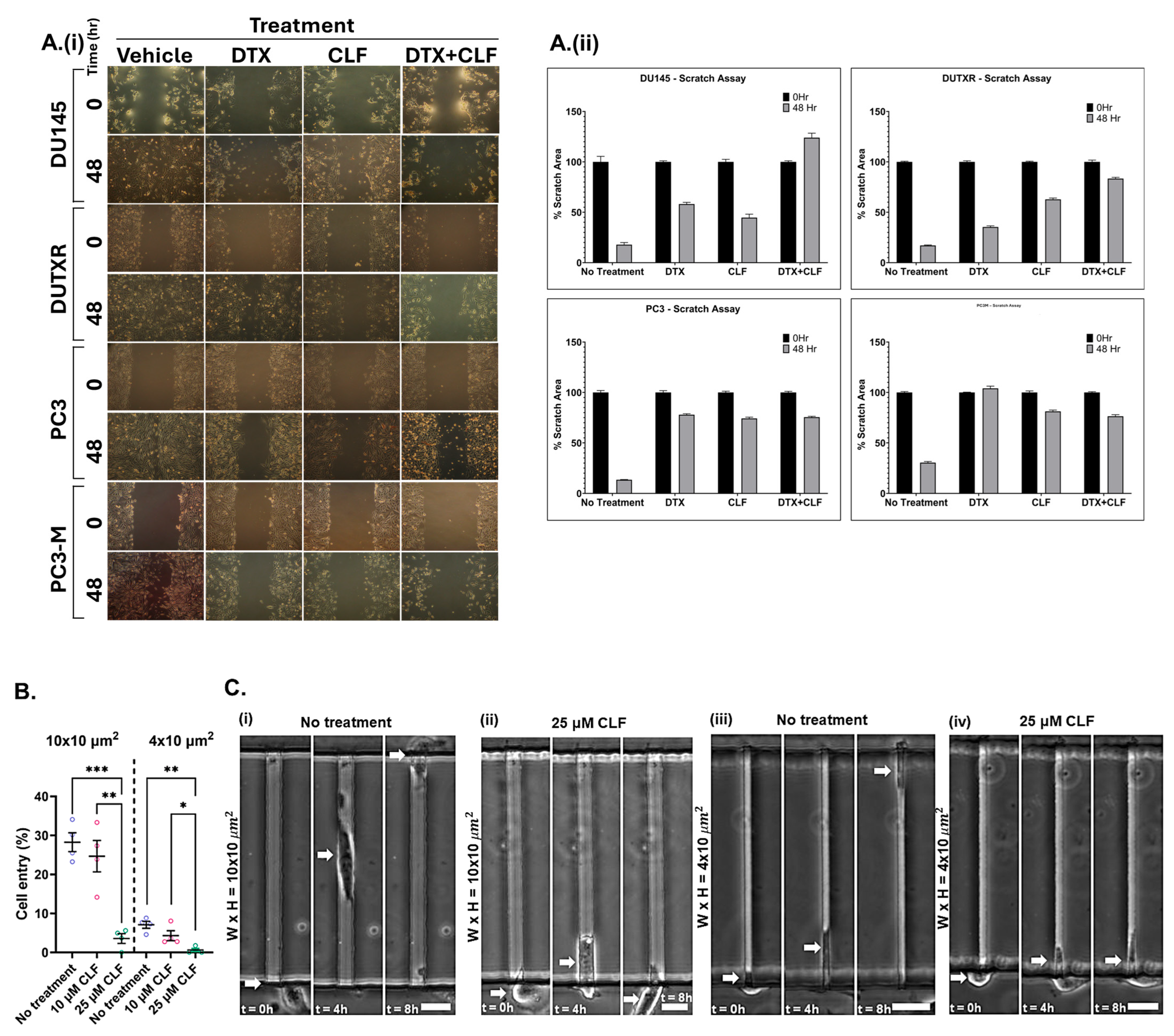
2.2. Cell Migration/Scratch Assay
2.3. CLF Reduces the Migration of mCRPC Cells Through Microfluidic Channels
2.4. CLF Inhibited Aldehyde Dehydrogenase Activity in PCa Cells
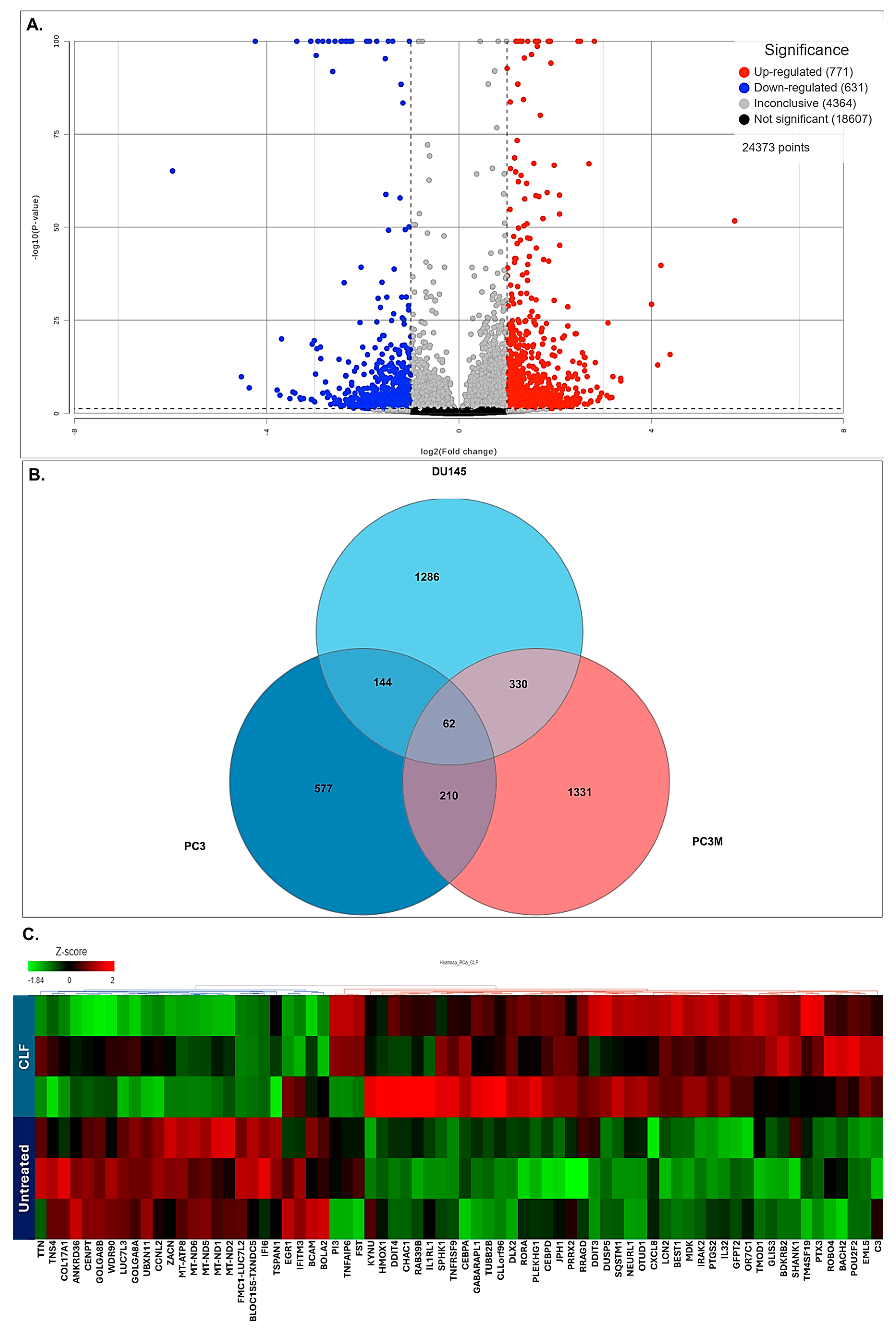
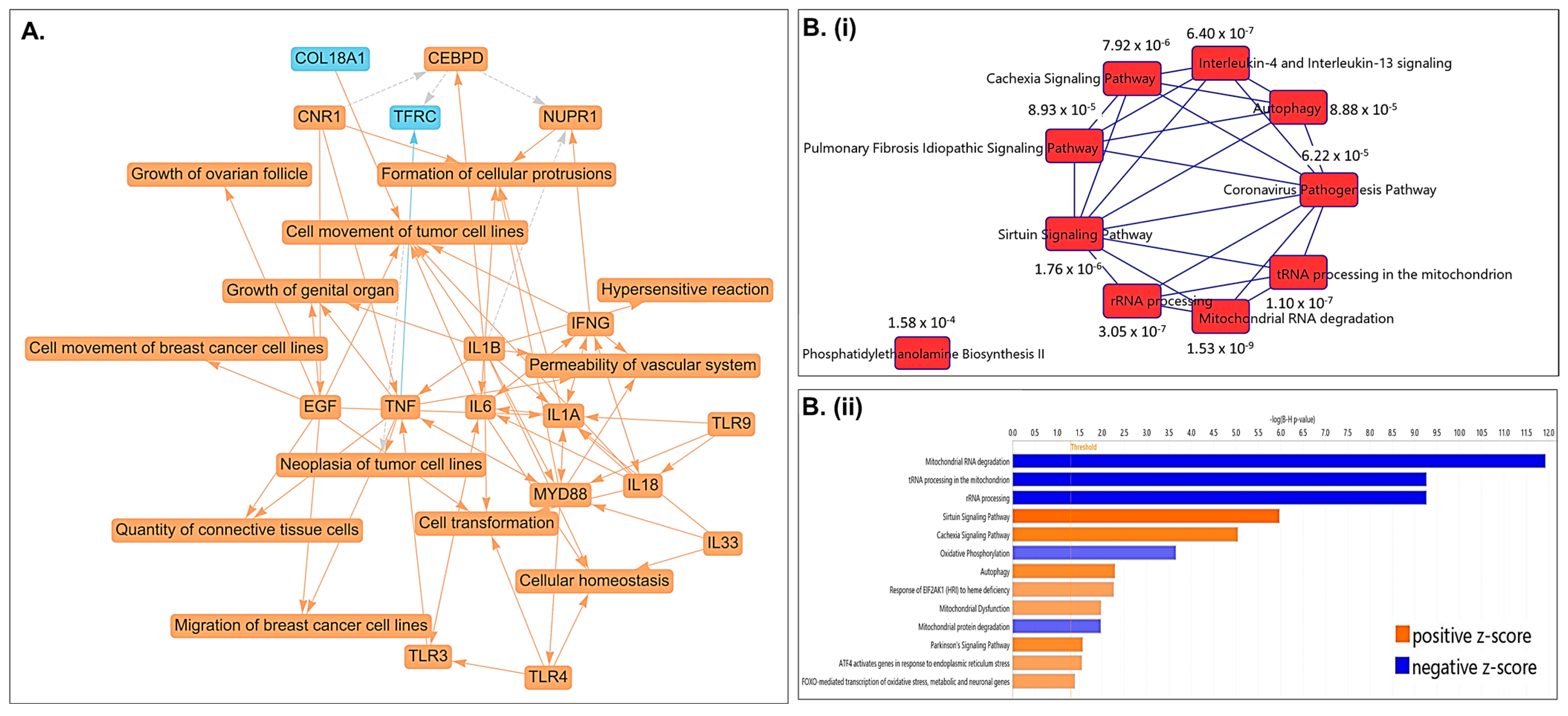
2.5. Pathway Analysis Revealed Top Differentially Regulated Molecular Pathways Associated with PCa Cells Treated with CLF
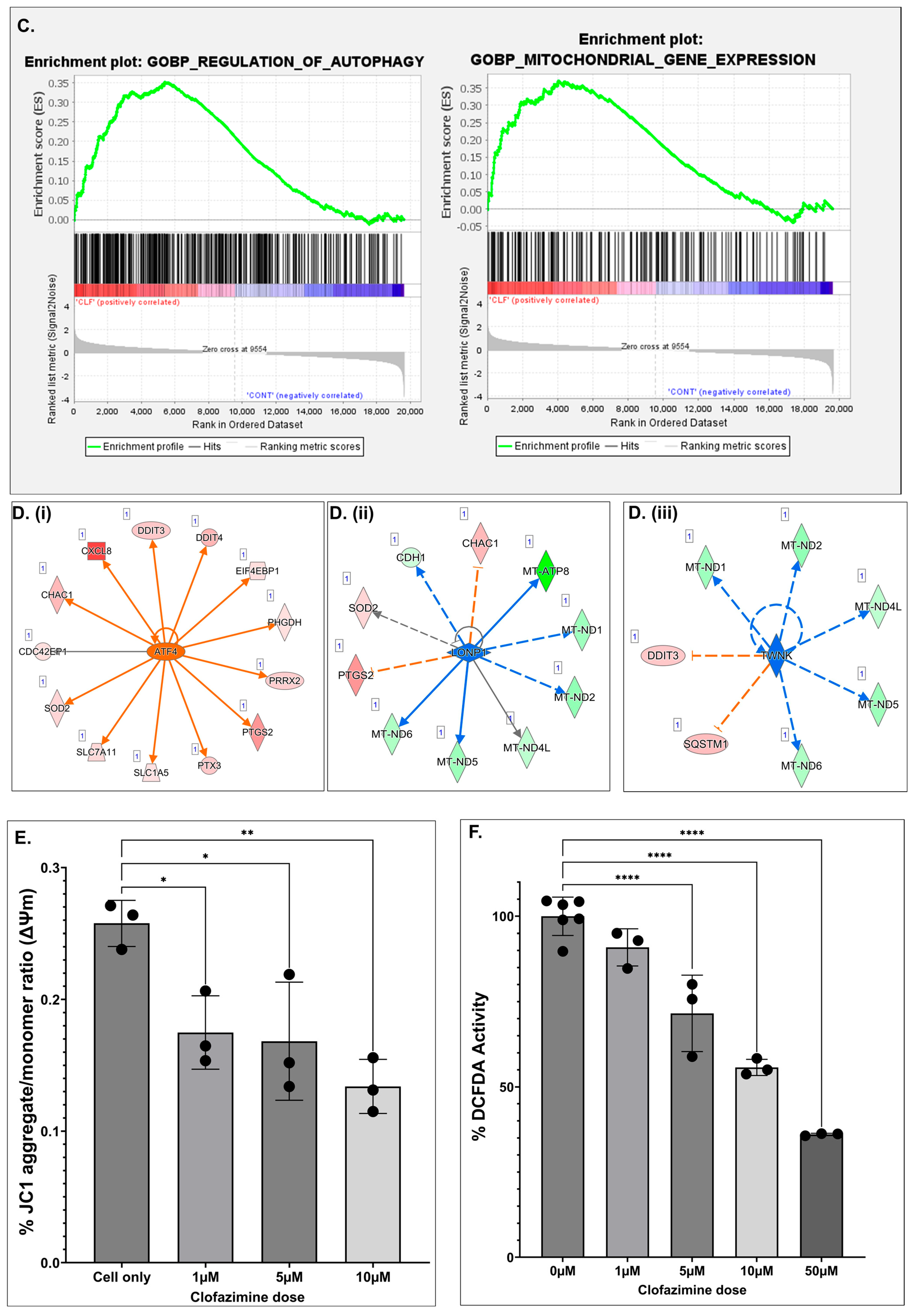
2.6. CLF Treatment Results in Altered Mitochondrial Metabolism
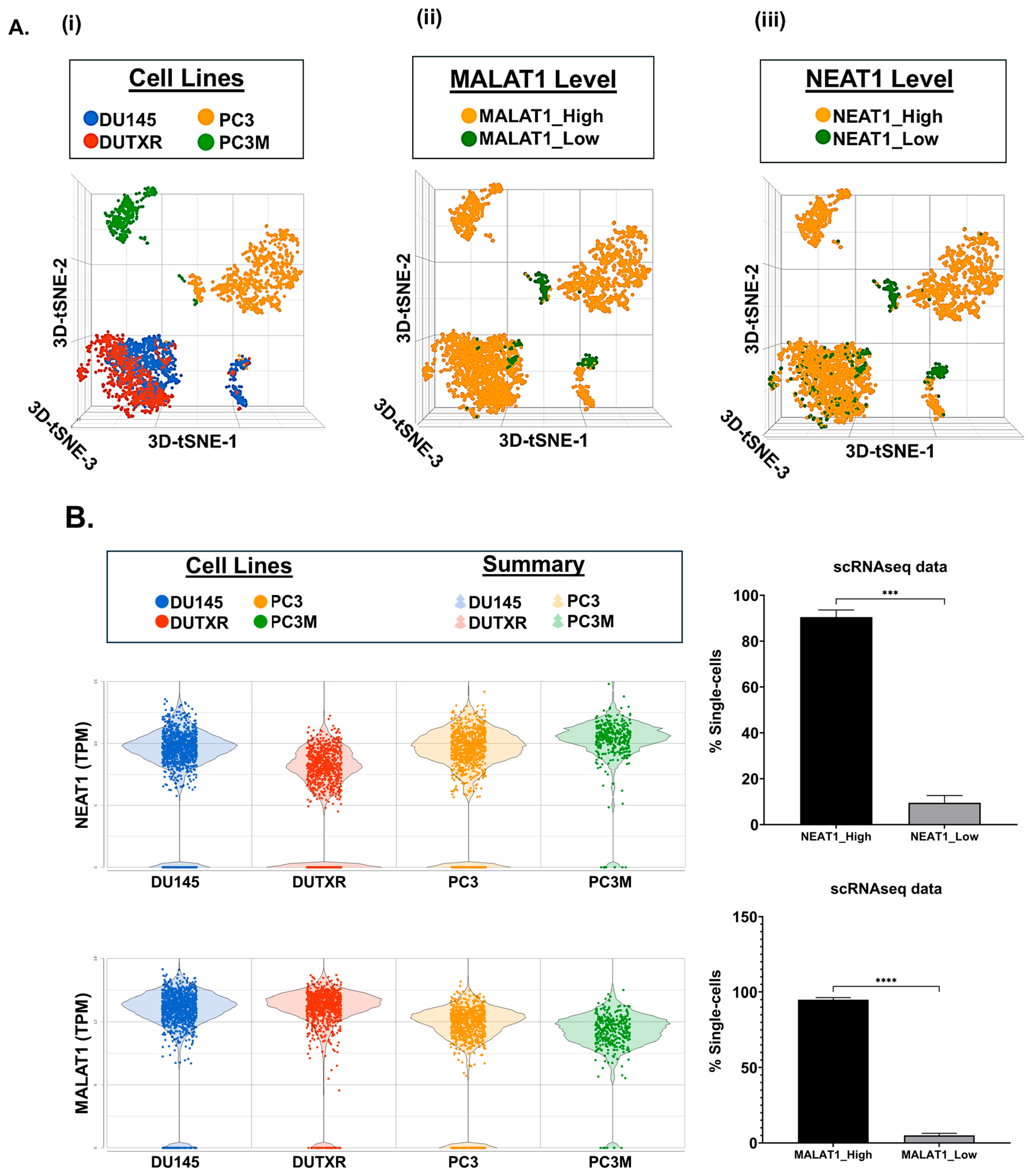
2.7. Single-Cell Transcriptomics (scRNAseq) Showed High Expression of Top Non-Coding RNAs MALAT1 and NEAT1 in mCRPC Cells
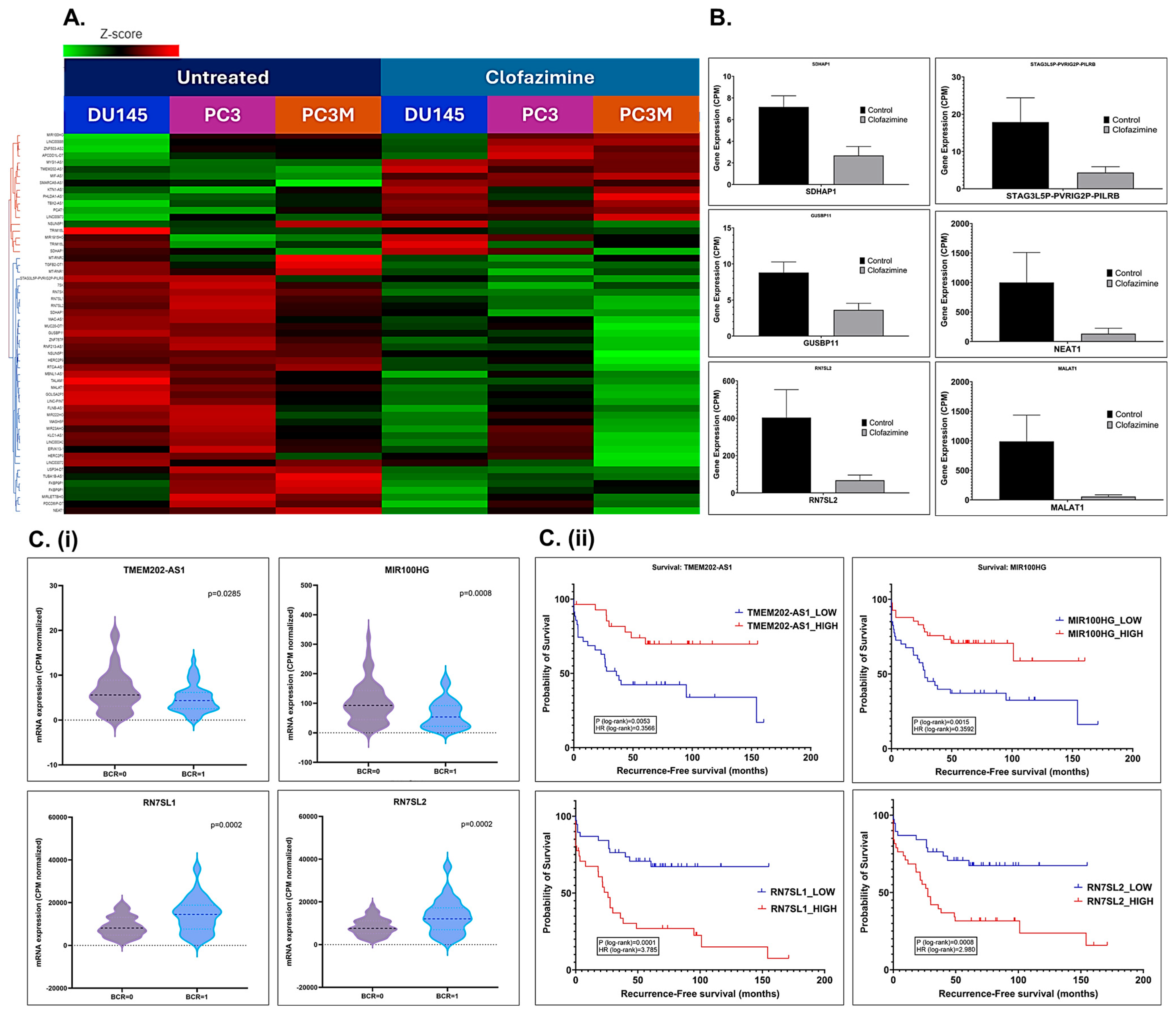
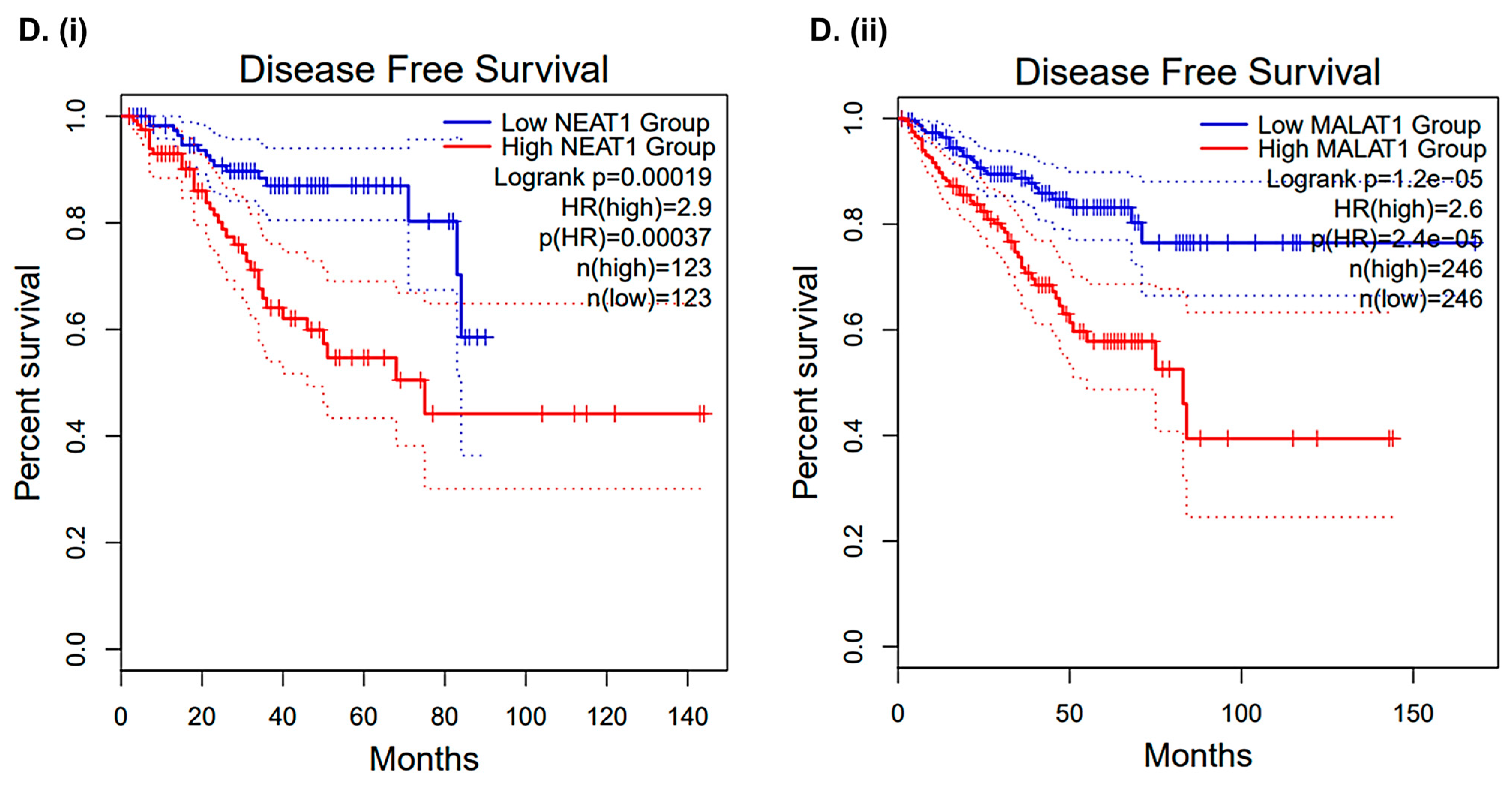
2.8. CLF Treatment-Induced Differential Regulation of Non-Coding RNAs Was Validated Using Patient Datasets
3. Discussion
4. Materials and Methods
4.1. Human Prostate Cancer Cell Lines
4.2. Drugs and Reagents
4.3. In Vitro Cytotoxicity Assays
4.4. Apoptosis Assays
4.5. Cellular Morphology Assessment
4.6. Cell Migration (Scratch) Assay
4.7. Microfluidic Cell Migration Assay
4.8. Aldeflour Activity Assay
4.9. Detection of the Mitochondrial Membrane Potential (MMP)
4.10. DCFDA Total Cellular Reactive Oxygen Species (ROS) Assay
4.11. Gene Expression Profiling (GEP) Analysis
4.12. RNAseq Data Analysis
4.13. Pathway Analysis
4.14. Single-Cell RNA Sequencing (scRNAseq)
4.15. scRNAseq Data Analysis
4.16. Patient Datasets
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sherman, R.L.; Firth, A.U.; Henley, S.J.; Siegel, R.L.; Negoita, S.; Sung, H.; Kohler, B.A.; Anderson, R.N.; Cucinelli, J.; Scott, S.; et al. Annual Report to the Nation on the Status of Cancer, featuring state-level statistics after the onset of the COVID-19 pandemic. Cancer 2025, 131, e35833. [Google Scholar] [CrossRef]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; Bianchi-Frias, D.; Dumpit, R.F.; Kaipainen, A.; Corella, A.N.; et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 2017, 32, 474–489.e476. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Manola, J.; Loffredo, M.; Renshaw, A.A.; DellaCroce, A.; Kantoff, P.W. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA 2004, 292, 821–827. [Google Scholar] [CrossRef]
- Yehya, A.; Ghamlouche, F.; Zahwe, A.; Zeid, Y.; Wakimian, K.; Mukherji, D.; Abou-Kheir, W. Drug resistance in metastatic castration-resistant prostate cancer: An update on the status quo. Cancer Drug Resist. 2022, 5, 667–690. [Google Scholar] [CrossRef] [PubMed]
- Wasim, S.; Lee, S.Y.; Kim, J. Complexities of Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 14257. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men with Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Song, X.L.; Li, X.A.; Chen, M.; Guo, J.; Yang, D.H.; Chen, Z.; Zhao, S.C. Current therapy and drug resistance in metastatic castration-resistant prostate cancer. Drug Resist. Updates 2023, 68, 100962. [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Takahashi, R.U.; Usuba, W.; Kohama, I.; Ochiya, T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Berns, A.; Ringborg, U.; Celis, J.; Jonsson, B.; Baumann, M. Strategies to reduce the cancer burden and improve access to effective and affordable cancer interventions in Europe. Mol. Oncol. 2025, 19, 1553–1560. [Google Scholar] [CrossRef]
- Xu, J.; Koval, A.; Katanaev, V.L. Clofazimine: A journey of a drug. Biomed. Pharmacother. 2023, 167, 115539. [Google Scholar] [CrossRef]
- Chung, I.; Zhou, K.; Barrows, C.; Banyard, J.; Wilson, A.; Rummel, N.; Mizokami, A.; Basu, S.; Sengupta, P.; Shaikh, B.; et al. Unbiased Phenotype-Based Screen Identifies Therapeutic Agents Selective for Metastatic Prostate Cancer. Front. Oncol. 2021, 10, 594141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Mazumder, S.; Sharma, N.; Chakravarti, S.; Long, M.D.; Meurice, N.; Petit, J.; Liu, S.; Chesi, M.; Sanyal, S.; et al. Single-Cell Proteomics and Tumor RNAseq Identify Novel Pathways Associated with Clofazimine Sensitivity in PI- and IMiD- Resistant Myeloma, and Putative Stem-Like Cells. Front. Oncol. 2022, 12, 842200. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Paul, C.D.; Mistriotis, P.; Konstantopoulos, K. Cancer cell motility: Lessons from migration in confined spaces. Nat. Rev. Cancer 2017, 17, 131–140. [Google Scholar] [CrossRef]
- Weigelin, B.; Bakker, G.J.; Friedl, P. Intravital third harmonic generation microscopy of collective melanoma cell invasion: Principles of interface guidance and microvesicle dynamics. IntraVital 2012, 1, 32–43. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Horiguchi, M.; Koyanagi, S.; Okamoto, A.; Suzuki, S.O.; Matsunaga, N.; Ohdo, S. Stress-regulated transcription factor ATF4 promotes neoplastic transformation by suppressing expression of the INK4a/ARF cell senescence factors. Cancer Res. 2012, 72, 395–401. [Google Scholar] [CrossRef]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef]
- Korhonen, J.A.; Gaspari, M.; Falkenberg, M. TWINKLE Has 5′ -> 3′ DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. J. Biol. Chem. 2003, 278, 48627–48632. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Houry, W.A. Chemical Modulation of Human Mitochondrial ClpP: Potential Application in Cancer Therapeutics. ACS Chem. Biol. 2019, 14, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Chattopadhyay, S.; Das, N.; Shree, S.; Patel, D.; Mohapatra, J.; Gurjar, A.; Kushwaha, S.; Kumar Singh, A.; Dubey, S.; et al. Leprosy drug clofazimine activates peroxisome proliferator-activated receptor-gamma and synergizes with imatinib to inhibit chronic myeloid leukemia cells. Haematologica 2020, 105, 971. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Mitra Ghosh, T.; Mukherjee, U.K.; Chakravarti, S.; Amiri, F.; Waliagha, R.S.; Hemmati, F.; Mistriotis, P.; Ahmed, S.; Elhussin, I.; et al. Integrating Pharmacogenomics Data-Driven Computational Drug Prediction with Single-Cell RNAseq to Demonstrate the Efficacy of a NAMPT Inhibitor against Aggressive, Taxane-Resistant, and Stem-like Cells in Lethal Prostate Cancer. Cancers 2022, 14, 6009. [Google Scholar] [CrossRef]
- Long, Q.; Xu, J.; Osunkoya, A.O.; Sannigrahi, S.; Johnson, B.A.; Zhou, W.; Gillespie, T.; Park, J.Y.; Nam, R.K.; Sugar, L.; et al. Global transcriptome analysis of formalin-fixed prostate cancer specimens identifies biomarkers of disease recurrence. Cancer Res. 2014, 74, 3228–3237. [Google Scholar] [CrossRef]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. Mechanisms of Taxane Resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef]
- Van Rensburg, C.E.; Van Staden, A.M.; Anderson, R. The riminophenazine agents clofazimine and B669 inhibit the proliferation of cancer cell lines in vitro by phospholipase A2-mediated oxidative and nonoxidative mechanisms. Cancer Res. 1993, 53, 318–323. [Google Scholar]
- Sri-Pathmanathan, R.M.; Plumb, J.A.; Fearon, K.C. Clofazimine alters the energy metabolism and inhibits the growth rate of a human lung-cancer cell line in vitro and in vivo. Int. J. Cancer 1994, 56, 900–905. [Google Scholar] [CrossRef]
- Xu, J.; Koval, A.; Katanaev, V.L. Beyond TNBC: Repositioning of Clofazimine Against a Broad Range of Wnt-Dependent Cancers. Front. Oncol. 2020, 10, 602817. [Google Scholar] [CrossRef]
- Xue, G.; Li, X.; Kalim, M.; Fang, J.; Jiang, Z.; Zheng, N.; Wang, Z.; Li, X.; Abdelrahim, M.; He, Z.; et al. Clinical drug screening reveals clofazimine potentiates the efficacy while reducing the toxicity of anti-PD-1 and CTLA-4 immunotherapy. Cancer Cell 2024, 42, 780–796.e786. [Google Scholar] [CrossRef]
- Zeng, Z.; Fu, M.; Hu, Y.; Wei, Y.; Wei, X.; Luo, M. Regulation and signaling pathways in cancer stem cells: Implications for targeted therapy for cancer. Mol. Cancer 2023, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Mitra Ghosh, T.; Mazumder, S.; Davis, J.; Yadav, J.; Akinpelu, A.; Alnaim, A.; Kumar, H.; Waliagha, R.; Church Bird, A.E.; Rais-Bahrami, S.; et al. Metronomic Administration of Topotecan Alone and in Combination with Docetaxel Inhibits Epithelial-mesenchymal Transition in Aggressive Variant Prostate Cancers. Cancer Res. Commun. 2023, 3, 1286–1311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lee, S.J.; Park, S.; Konstantopoulos, K.; Glunde, K.; Chen, Y.; Barman, I. Cancer cells display increased migration and deformability in pace with metastatic progression. FASEB J. 2020, 34, 9307–9315. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Wisniewski, E.O.; Mistriotis, P.; Bera, K.; Law, R.A.; Zhang, J.; Nikolic, M.; Weiger, M.; Parlani, M.; Tuntithavornwat, S.; Afthinos, A.; et al. Dorsoventral polarity directs cell responses to migration track geometries. Sci. Adv. 2020, 6, eaba6505. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- Mungrue, I.N.; Pagnon, J.; Kohannim, O.; Gargalovic, P.S.; Lusis, A.J. CHAC1/MGC4505 Is a Novel Proapoptotic Component of the Unfolded Protein Response, Downstream of the ATF4-ATF3-CHOP Cascasde. J. Immunol. 2009, 182, 466–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Lai, S.; Wang, Z.; Yang, Y.; Liu, W.; Wang, H.; Tang, B. The m6A demethylase ALKBH5-mediated upregulation of DDIT4-AS1 maintains pancreatic cancer stemness and suppresses chemosensitivity by activating the mTOR pathway. Mol. Cancer 2022, 21, 174. [Google Scholar] [CrossRef]
- Goyal, B.; Yadav, S.R.M.; Awasthee, N.; Gupta, S.; Kunnumakkara, A.B.; Gupta, S.C. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188502. [Google Scholar] [CrossRef]
- Kim, J.; Piao, H.L.; Kim, B.J.; Yao, F.; Han, Z.; Wang, Y.; Xiao, Z.; Siverly, A.N.; Lawhon, S.E.; Ton, B.N.; et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat. Genet. 2018, 50, 1705–1715. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Y.; Tan, D.; Meng, H.; Wang, K.; Bai, Y.; Yang, K. Up-regulation of long noncoding RNA MALAT1 contributes to proliferation and metastasis in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2015, 3, 7. [Google Scholar] [CrossRef]
- Gutschner, T.; Hammerle, M.; Eissmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Gross, M.; et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013, 73, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.H.; Spieker, T.; Koschmieder, S.; Schaffers, S.; Humberg, J.; Jungen, D.; Bulk, E.; Hascher, A.; Wittmer, D.; Marra, A.; et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. Thorac. Oncol. 2011, 6, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Chen, Q.; Wang, Y.; Zhou, Z.; Huang, Y.; Qiu, F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol. Biosyst. 2012, 8, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Liu, Y.; Xu, W.; Sun, Y.; Lu, J.; Wang, F.; Wei, M.; Shen, J.; Hou, J.; Gao, X.; et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J. Urol. 2013, 190, 2278–2287. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, B.; An, R.; Qian, W.; Han, L.; Duan, W.; Wang, Z.; Ma, Q. Molecular Interactions of the Long Noncoding RNA NEAT1 in Cancer. Cancers 2022, 14, 4009. [Google Scholar] [CrossRef]
- Chakravarty, D.; Sboner, A.; Nair, S.S.; Giannopoulou, E.; Li, R.; Hennig, S.; Mosquera, J.M.; Pauwels, J.; Park, K.; Kossai, M.; et al. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat. Commun. 2014, 5, 5383. [Google Scholar] [CrossRef]
- Wen, S.; Wei, Y.; Zen, C.; Xiong, W.; Niu, Y.; Zhao, Y. Long non-coding RNA NEAT1 promotes bone metastasis of prostate cancer through N6-methyladenosine. Mol. Cancer 2020, 19, 171. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, L.; Long, H. Single-cell sequencing analysis revealed that NEAT1 was a potential biomarker and therapeutic target of prostate cancer. BMC Cancer 2024, 24, 1242. [Google Scholar] [CrossRef]
- Han, S.; Chen, L.L. Long non-coding RNAs in the nucleolus: Biogenesis, regulation, and function. Curr. Opin. Struct. Biol. 2024, 87, 102866. [Google Scholar] [CrossRef]
- Arun, G.; Aggarwal, D.; Spector, D.L. MALAT1 Long Non-Coding RNA: Functional Implications. Non-Coding RNA 2020, 6, 22. [Google Scholar] [CrossRef]
- Jiang, Q.; Xue, D.; Shi, F.; Qiu, J. Prognostic significance of an autophagy-related long non-coding RNA signature in patients with oral and oropharyngeal squamous cell carcinoma. Oncol. Lett. 2021, 21, 29. [Google Scholar] [CrossRef]
- Karpov, D.S.; Spirin, P.V.; Zheltukhin, A.O.; Tutyaeva, V.V.; Zinovieva, O.L.; Grineva, E.N.; Matrosova, V.A.; Krasnov, G.S.; Snezhkina, A.V.; Kudryavtseva, A.V.; et al. LINC00973 Induces Proliferation Arrest of Drug-Treated Cancer Cells by Preventing p21 Degradation. Int. J. Mol. Sci. 2020, 21, 8322. [Google Scholar] [CrossRef]
- An, J.; Wang, H.; Wei, M.; Yu, X.; Liao, Y.; Tan, X.; Hu, C.; Li, S.; Luo, Y.; Gui, Y.; et al. Identification of chemical inhibitors targeting long noncoding RNA through gene signature-based high throughput screening. Int. J. Biol. Macromol. 2025, 292, 139119. [Google Scholar] [CrossRef]
- Xu, X.; Ma, L.; Zhang, X.; Guo, S.; Guo, W.; Wang, Y.; Qiu, S.; Tian, X.; Miao, Y.; Yu, Y.; et al. A positive feedback circuit between RN7SK snRNA and m(6)A readers is essential for tumorigenesis. Mol. Ther. 2023, 31, 1615–1635. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Wu, L.; Yin, X.; Tang, Q.; Yin, J.; Zhou, Z.; Yu, H.; Yan, S. Prognostic implications of necroptosis-related long noncoding RNA signatures in muscle-invasive bladder cancer. Front. Genet. 2022, 13, 1036098. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Rong, Y.; Liu, S.; Wu, Z.; Ma, G.; Li, X.; Cai, H. Potential role of lncRNA LOXL1-AS1 in human cancer development: A narrative review. Transl. Cancer Res. 2024, 13, 1997. [Google Scholar] [CrossRef] [PubMed]
- Yousefnia, S. A comprehensive review on lncRNA LOXL1-AS1: Molecular mechanistic pathways of lncRNA LOXL1-AS1 in tumorigenicity of cancer cells. Front. Oncol. 2024, 14, 1384342. [Google Scholar] [CrossRef]
- Fu, X.P.; Ji, C.Y.; Tang, W.Q.; Yu, T.T.; Luo, L. Long non-coding RNA LOXL1-AS1: A potential biomarker and therapeutic target in human malignant tumors. Clin. Exp. Med. 2024, 24, 93. [Google Scholar] [CrossRef]
- Rysz, J.; Konecki, T.; Franczyk, B.; Lawinski, J.; Gluba-Brzozka, A. The Role of Long Noncoding RNA (lncRNAs) Biomarkers in Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 24, 643. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Panda, A.C.; Kang, M.J.; Guo, R.; Kim, J.; Grammatikakis, I.; Yoon, J.H.; Dudekula, D.B.; Noh, J.H.; Yang, X.; et al. 7SL RNA represses p53 translation by competing with HuR. Nucleic Acids Res. 2014, 42, 10099–10111. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Meng, S.; Tsuji, Y.; Arao, Y.; Saito, Y.; Sato, H.; Motooka, D.; Uchida, S.; Ishii, H. RN7SL1 may be translated under oncogenic conditions. Proc. Natl. Acad. Sci USA 2024, 121, e2312322121. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Ravan, M.; Karimi-Sani, I.; Aria, H.; Hasan-Abad, A.M.; Banasaz, B.; Atapour, A.; Sarab, G.A. Screening and identification of potential biomarkers for pancreatic cancer: An integrated bioinformatics analysis. Pathol. Res. Pract. 2023, 249, 154726. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.; Akinpelu, A.A.; Keith, W.C.; Hemmati, F.; Vaghasiya, R.S.; Bowen, D.; Waliagha, R.S.; Wang, C.; Chen, P.; Mitra, A.K.; et al. Confinement controls the directional cell responses to fluid forces. Cell Rep. 2024, 43, 114692. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Agrawal, A.; Balci, H.; Hanspers, K.; Coort, S.L.; Martens, M.; Slenter, D.N.; Ehrhart, F.; Digles, D.; Waagmeester, A.; Wassink, I.; et al. WikiPathways 2024: Next generation pathway database. Nucleic Acids Res. 2024, 52, D679–D689. [Google Scholar] [CrossRef]
- Mitra, A.K.; Mukherjee, U.K.; Harding, T.; Jang, J.S.; Stessman, H.; Li, Y.; Abyzov, A.; Jen, J.; Kumar, S.; Rajkumar, V.; et al. Single-cell analysis of targeted transcriptome predicts drug sensitivity of single cells within human myeloma tumors. Leukemia 2016, 30, 1094–1102. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]

| Gene Name | Gene ID | Gene Biotype | p-Value (CLF vs. No Treatment) | Fold Change (CLF vs. No Treatment) |
|---|---|---|---|---|
| MALAT1 | ENSG00000251562 | lncRNA | 1.07 × 10−170 | −18.84 |
| RN7SK | ENSG00000283293 | snRNA | 1.30 × 10−5 | −13.20 |
| NEAT1 | ENSG00000245532 | lncRNA | 4.06 × 10−159 | −10.36 |
| TUBA1B-AS1 | ENSG00000258017 | lncRNA | 1.88 × 10−4 | −8.32 |
| LINC00342 | ENSG00000232931 | lncRNA | 2.69 × 10−11 | −7.91 |
| RN7SL1 | ENSG00000276168 | misc_RNA | 6.84 × 10−97 | −7.83 |
| MT-RNR1 | ENSG00000211459 | Mt_rRNA | 0.00 × 100 | −6.54 |
| RN7SL2 | ENSG00000274012 | misc_RNA | 1.40 × 10−92 | −6.17 |
| MBNL1-AS1 | ENSG00000229619 | lncRNA | 2.03 × 10−4 | −4.86 |
| TGFB2-OT1 | ENSG00000281453 | lncRNA | 1.93 × 10−2 | −4.63 |
| NSUN5P1 | ENSG00000291151 | lncRNA | 1.21 × 10−6 | −4.12 |
| WASH5P | ENSG00000292982 | lncRNA | 2.18 × 10−7 | −3.93 |
| 7SK | ENSG00000202198 | misc_RNA | 1.32 × 10−3 | −3.89 |
| TALAM1 | ENSG00000289740 | lncRNA | 2.11 × 10−2 | −3.76 |
| HERC2P9 | ENSG00000291082 | lncRNA | 7.76 × 10−4 | −3.48 |
| FLNB-AS1 | ENSG00000244161 | lncRNA | 2.36 × 10−2 | −3.35 |
| FKBP9P1 | ENSG00000291029 | lncRNA | 5.83 × 10−3 | −3.31 |
| MIR23AHG | ENSG00000267519 | lncRNA | 3.20 × 10−7 | −3.27 |
| STAG3L5P-PVRIG2P-PILRB | ENSG00000272752 | lncRNA | 2.96 × 10−4 | −3.17 |
| MUC20-OT1 | ENSG00000242086 | lncRNA | 2.16 × 10−5 | −3.13 |
| MIR222HG | ENSG00000270069 | lncRNA | 1.28 × 10−2 | −3.12 |
| PDCD6IP-DT | ENSG00000271643 | lncRNA | 2.12 × 10−3 | −3.03 |
| RNF213-AS1 | ENSG00000263069 | lncRNA | 3.89 × 10−2 | −3.03 |
| GOLGA2P5 | ENSG00000290571 | lncRNA | 1.88 × 10−2 | −2.89 |
| RTCA-AS1 | ENSG00000224616 | lncRNA | 1.87 × 10−2 | −2.89 |
| KLC1-AS1 | ENSG00000246451 | lncRNA | 4.07 × 10−2 | −2.77 |
| USP34-DT | ENSG00000270820 | lncRNA | 1.75 × 10−2 | −2.70 |
| MIRLET7BHG | ENSG00000197182 | lncRNA | 4.02 × 10−2 | −2.69 |
| MT-RNR2 | ENSG00000210082 | Mt_rRNA | 0.00 × 100 | −2.60 |
| ZNF767P | ENSG00000291118 | lncRNA | 1.24 × 10−2 | −2.58 |
| ERVK13-1 | ENSG00000260565 | lncRNA | 9.18 × 10−3 | −2.34 |
| SDHAP1 | ENSG00000290763 | lncRNA | 2.81 × 10−2 | −2.28 |
| LINC-PINT | ENSG00000231721 | lncRNA | 9.68 × 10−4 | −2.28 |
| GUSBP11 | ENSG00000228315 | lncRNA | 2.25 × 10−2 | −2.17 |
| WAC-AS1 | ENSG00000254635 | lncRNA | 7.46 × 10−5 | −2.06 |
| LINC03072 | ENSG00000273270 | lncRNA | 3.62 × 10−2 | −2.05 |
| TRIM16L | ENSG00000291110 | lncRNA | 4.08 × 10−3 | 1.88 |
| MIR100HG | ENSG00000255248 | lncRNA | 4.34 × 10−3 | 1.95 |
| KTN1-AS1 | ENSG00000186615 | lncRNA | 1.68 × 10−3 | 2.02 |
| LINC00886 | ENSG00000240875 | lncRNA | 2.20 × 10−2 | 2.22 |
| MIR1915HG | ENSG00000204682 | lncRNA | 3.40 × 10−2 | 2.26 |
| ZNF503-AS2 | ENSG00000237149 | lncRNA | 1.00 × 10−3 | 2.44 |
| TBX2-AS1 | ENSG00000267280 | lncRNA | 4.39 × 10−2 | 2.75 |
| SMARCA5-AS1 | ENSG00000245112 | lncRNA | 4.45 × 10−4 | 2.93 |
| APCDD1L-DT | ENSG00000231290 | lncRNA | 7.17 × 10−3 | 2.98 |
| MYG1-AS1 | ENSG00000257605 | lncRNA | 2.26 × 10−2 | 3.00 |
| PCAT1 | ENSG00000253438 | lncRNA | 7.60 × 10−5 | 3.84 |
| LINC00973 | ENSG00000240476 | lncRNA | 9.36 × 10−7 | 3.92 |
| TMEM202-AS1 | ENSG00000261423 | lncRNA | 7.54 × 10−5 | 4.79 |
| PHLDA1-AS1 | ENSG00000257453 | lncRNA | 4.28 × 10−5 | 7.63 |
| MIF-AS1 | ENSG00000218537 | lncRNA | 3.38 × 10−6 | 7.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batten, S.; Kumar, H.; Pfitzer, J.; Nweze, D.C.; Mazumder, S.; Arnold, R.D.; Mistriotis, P.; Mitra Ghosh, T.; Mitra, A.K. Clofazimine Treatment Modulates Key Non-Coding RNAs Associated with Tumor Progression and Drug Resistance in Lethal Prostate Cancer. Int. J. Mol. Sci. 2025, 26, 10892. https://doi.org/10.3390/ijms262210892
Batten S, Kumar H, Pfitzer J, Nweze DC, Mazumder S, Arnold RD, Mistriotis P, Mitra Ghosh T, Mitra AK. Clofazimine Treatment Modulates Key Non-Coding RNAs Associated with Tumor Progression and Drug Resistance in Lethal Prostate Cancer. International Journal of Molecular Sciences. 2025; 26(22):10892. https://doi.org/10.3390/ijms262210892
Chicago/Turabian StyleBatten, Sarah, Harish Kumar, Jeremiah Pfitzer, Daniel Chinedu Nweze, Suman Mazumder, Robert D. Arnold, Panagiotis Mistriotis, Taraswi Mitra Ghosh, and Amit Kumar Mitra. 2025. "Clofazimine Treatment Modulates Key Non-Coding RNAs Associated with Tumor Progression and Drug Resistance in Lethal Prostate Cancer" International Journal of Molecular Sciences 26, no. 22: 10892. https://doi.org/10.3390/ijms262210892
APA StyleBatten, S., Kumar, H., Pfitzer, J., Nweze, D. C., Mazumder, S., Arnold, R. D., Mistriotis, P., Mitra Ghosh, T., & Mitra, A. K. (2025). Clofazimine Treatment Modulates Key Non-Coding RNAs Associated with Tumor Progression and Drug Resistance in Lethal Prostate Cancer. International Journal of Molecular Sciences, 26(22), 10892. https://doi.org/10.3390/ijms262210892








