Recent Progress of Magnetic Nanomaterials with Enhanced Enzymatic Activities in Antitumor Therapy
Abstract
1. Introduction
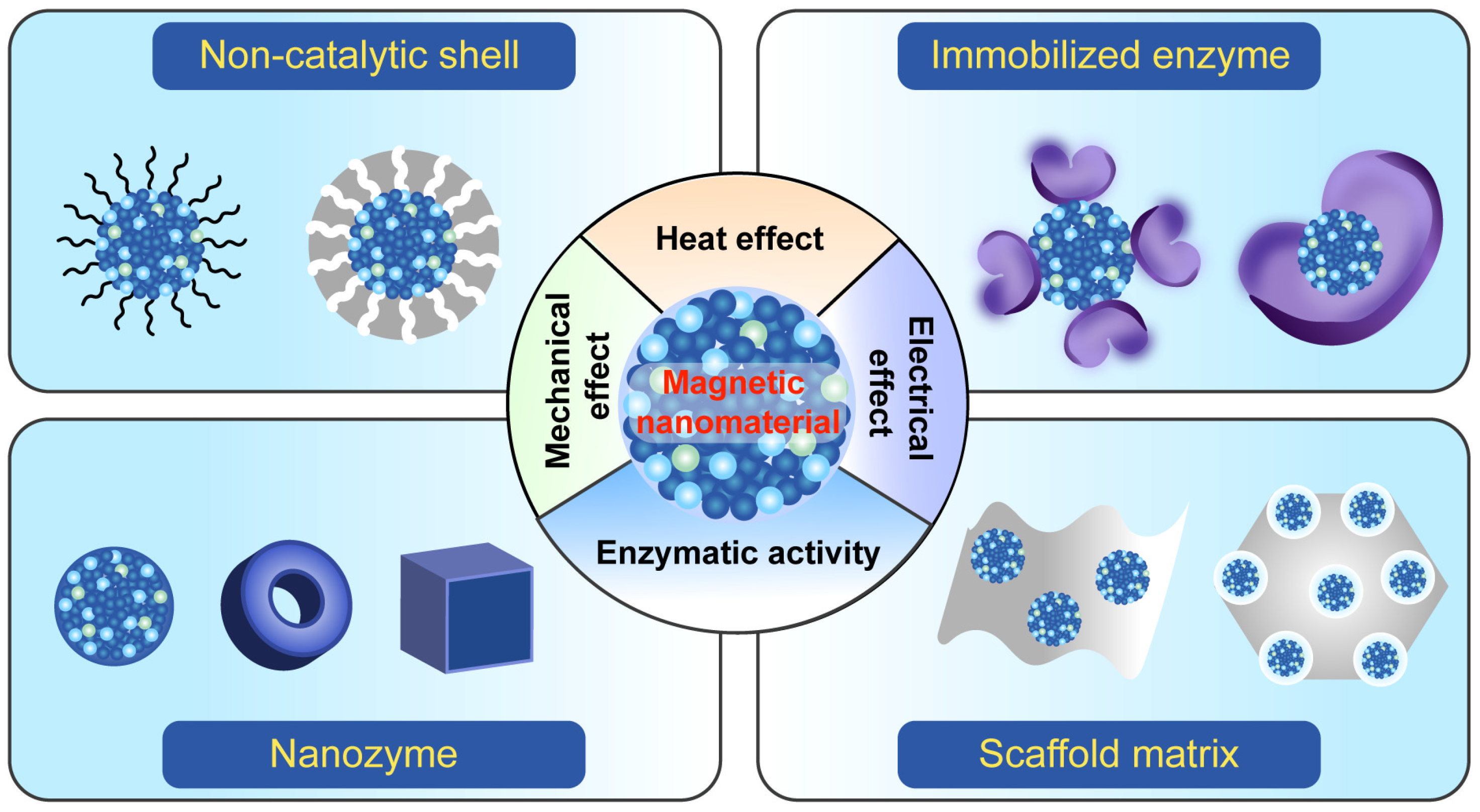
2. Various Magnetic Nanoparticles (MNPs) and Their Electromagnetic Effects
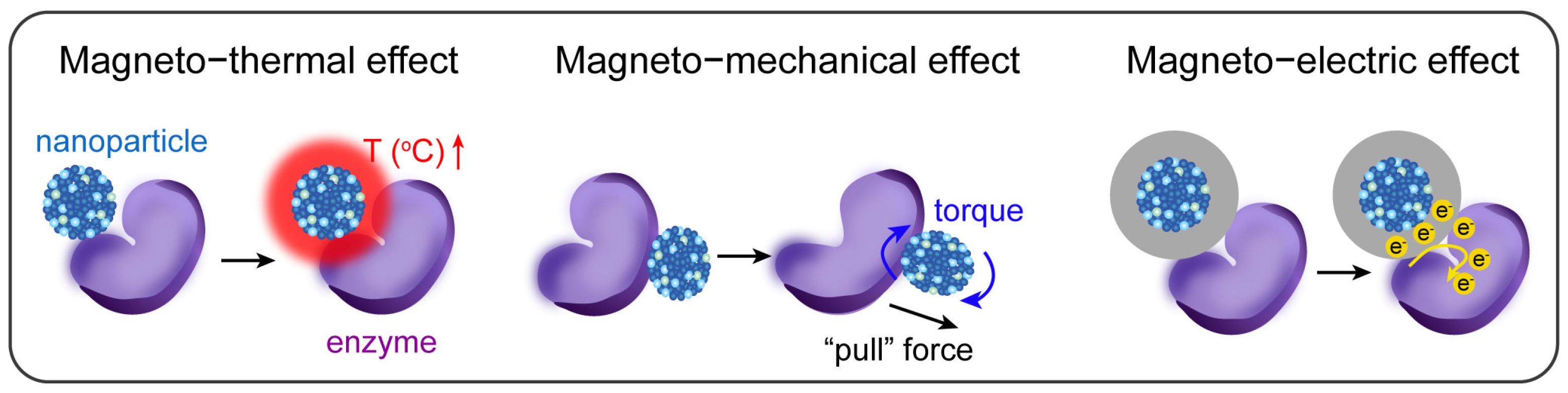
2.1. Magneto-Thermal Effect of MNPs
2.2. Magneto-Mechanical Effect of MNPs
2.3. Magneto-Electric Effect of MNPs
3. Design and Synthesis of Magnetic Nanomaterials with Enzymatic Activities
3.1. Magnetic Nanozymes
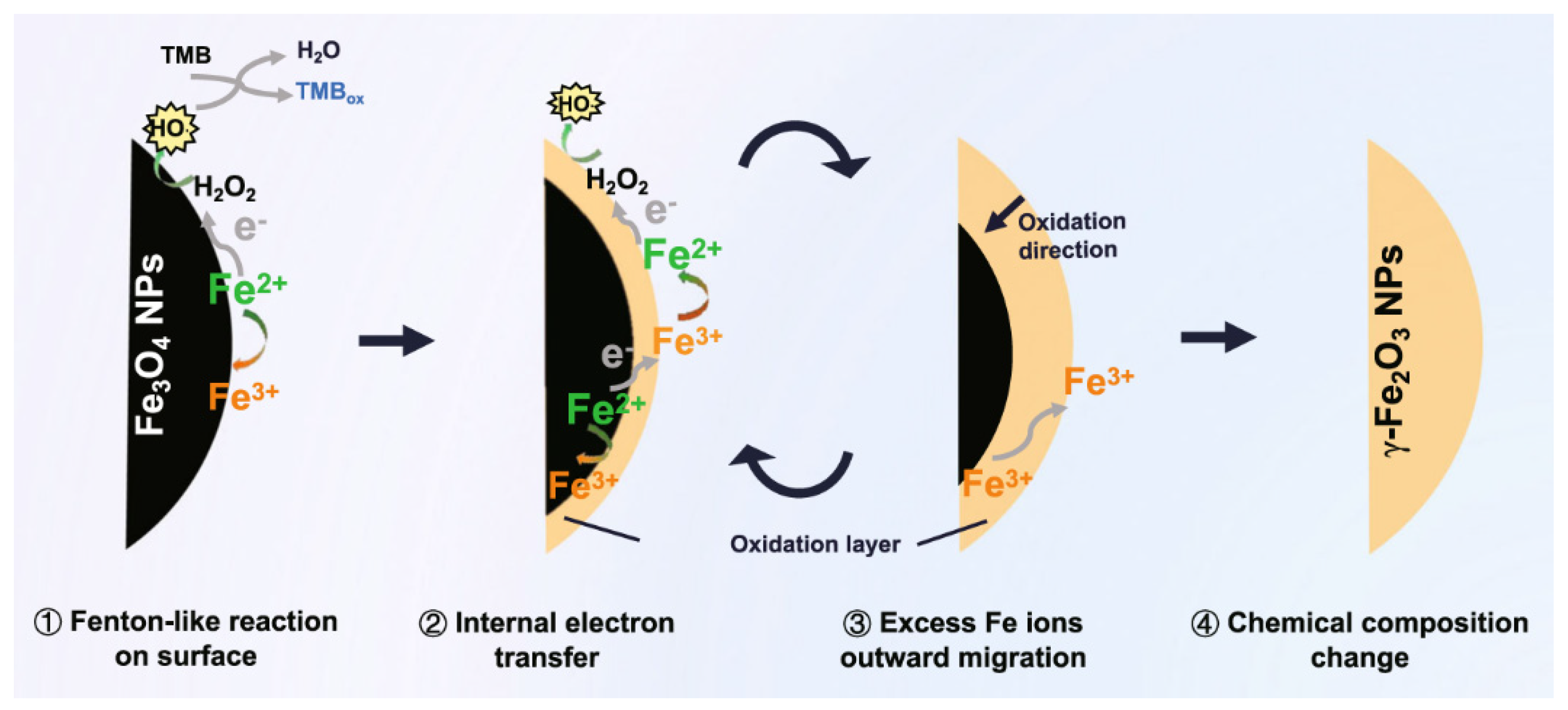
3.1.1. Fe3O4 Nanozymes with Peroxidase-like Activity
3.1.2. Magnetic Nanozymes with Catalase-like Activity
3.1.3. Magnetic Nanozymes with Oxidase-like Activity
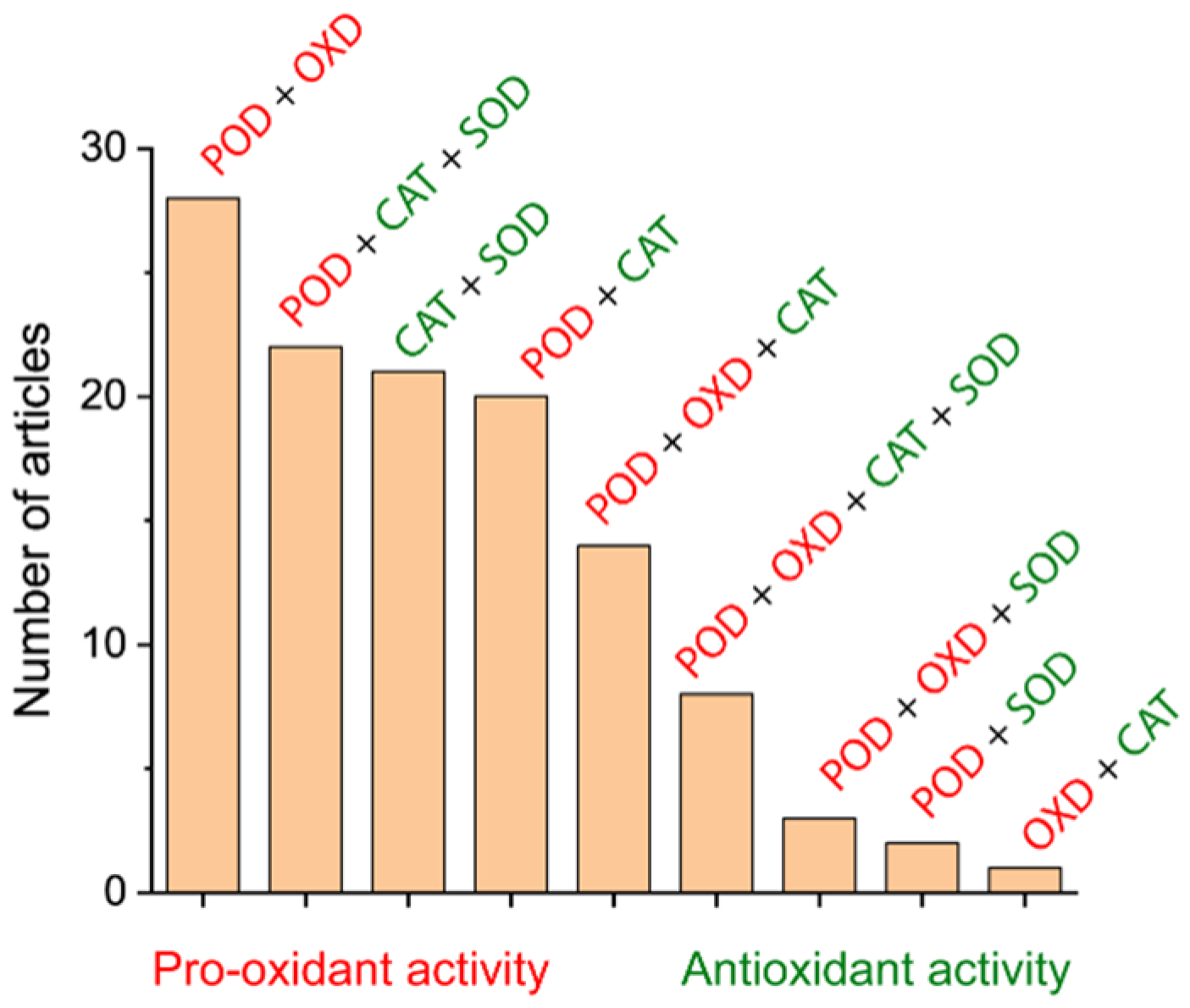
3.1.4. Magnetic Nanozymes with Multiple Enzymatic Activities
3.2. Magnetic Nanomaterial-Immobilized Enzymes
4. Enhanced Enzymatic Activities of Magnetic Nanomaterials for Efficient Antitumor Therapy
4.1. Enhanced Catalytic Activity of Magnetic Nanomaterials by Magneto-Thermal Effect
4.2. Enhanced Catalytic Activity of Magnetic Nanomaterials by Magneto-Mechanical Effect
4.3. Enhanced Catalytic Activity of Magnetic Nanomaterials by Magneto-Electrical Effect
4.4. Enhanced Catalytic Activity of Magnetic Nanomaterials by Other External Energies
5. Toxicity and Pharmacokinetics Effects of MNPs on Living Systems
6. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Q.; Smith, D.P. Global cancer burden: Progress, projections, and challenges. Lancet 2025, 406, 1536–1537. [Google Scholar] [CrossRef] [PubMed]
- The global, regional, and national burden of cancer, 1990–2023, with forecasts to 2050: A systematic analysis for the Global Burden of Disease Study 2023. Lancet 2025, 406, 1565–1586. [CrossRef]
- Wang, S.; Xu, J.; Li, W.; Sun, S.; Gao, S.; Hou, Y. Magnetic Nanostructures: Rational Design and Fabrication Strategies toward Diverse Applications. Chem. Rev. 2022, 122, 5411–5475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef]
- Shakeri-Zadeh, A.; Bulte, J.W.M. Imaging-guided precision hyperthermia with MNPs. Nat. Rev. Bioeng. 2025, 3, 245–260. [Google Scholar] [CrossRef]
- Bordet, A.; Leitner, W.; Chaudret, B. Magnetically Induced Catalysis: Definition, Advances, and Potential. Angew. Chem. Int. Ed. Engl. 2025, 64, e202424151. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.-P.; Gómez-Pastora, J.; et al. MNPs: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Cheng, Z.; Yang, M.; Huang, W.; Dong, X. Magnetic iron oxide nanomaterials: A key player in cancer nanomedicine. View 2020, 1, 20200046. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, H.; Liu, H. External physical field-driven nanocatalytic cancer therapy. BMEMat 2023, 1, e12010. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Y.; Gao, Y.; Zhu, Z. Rational Design Strategies for Nanozymes. ACS Nano 2023, 17, 13062–13080. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, Y.; Liu, Y. Advanced Strategies of Enzyme Activity Regulation for Biomedical Applications. Chembiochem 2022, 23, e202200358. [Google Scholar] [CrossRef] [PubMed]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. MNPs in Cancer Therapy and Diagnosis. Adv. Healthc. Mater. 2020, 9, e1901058. [Google Scholar] [CrossRef]
- Qi, A.; Wang, C.; Ni, S.; Meng, Y.; Wang, T.; Yue, Z.; Yang, K.; Li, Y.; Cheng, Z.; Guo, P.; et al. Intravesical Mucoadhesive Hydrogel Induces Chemoresistant Bladder Cancer Ferroptosis through Delivering Iron Oxide Nanoparticles in a Three-Tier Strategy. ACS Appl. Mater. Interfaces 2021, 13, 52374–52384. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Liu, H.; Chen, L.; Chen, J.; Zhang, D.; Cheng, Q.; Yang, F.; Zeng, Q.; Chen, T. Pre-clinical MRI-guided intravesical instillation theranosis of bladder cancer by tumor-selective oxygen nanogenerator. Nano Today 2021, 38, 101124. [Google Scholar] [CrossRef]
- Martins da Fonseca, J.; Trennepohl, T.; Pinheiro, L.G.; Carra Forte, G.; Campello, C.A.; Altmayer, S.; Andrade, R.G.; Hochhegger, B. Whole-body MRI for opportunistic cancer detection in asymptomatic individuals: A systematic review and meta-analysis. Eur. Radiol. 2025. [Google Scholar] [CrossRef]
- Marcuello, C.; Lim, K.; Nisini, G.; Pokrovsky, V.S.; Conde, J.; Ruggeri, F.S. Nanoscale Analysis beyond Imaging by Atomic Force Microscopy: Molecular Perspectives on Oncology and Neurodegeneration. Small Sci. 2025, e202500351. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, D.; Tian, X.; Liu, H.; Wang, X.; Li, H.; Chen, Q.; Zhang, X.; Wang, H. Synthesis of urchin-like nickel nanoparticles with enhanced rotating magnetic field-induced cell necrosis and tumor inhibition. Chem. Eng. J. 2020, 400, 125823. [Google Scholar] [CrossRef]
- Gregurec, D.; Senko, A.W.; Chuvilin, A.; Reddy, P.D.; Sankararaman, A.; Rosenfeld, D.; Chiang, P.H.; Garcia, F.; Tafel, I.; Varnavides, G.; et al. Magnetic Vortex Nanodiscs Enable Remote Magnetomechanical Neural Stimulation. ACS Nano 2020, 14, 8036–8045. [Google Scholar] [CrossRef]
- Lee, J.; Shin, W.; Lim, Y.; Kim, J.; Kim, W.R.; Kim, H.; Lee, J.; Cheon, J. Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals. Nat. Mater. 2021, 20, 1029–1036. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Ding, J.; Su, B.; Chen, Z.; Xiao, Z.; Wu, C.; Wei, D.; Sun, J.; Luo, F.; et al. Wireless-Powering Deep Brain Stimulation Platform Based on 1D-Structured Magnetoelectric Nanochains Applied in Antiepilepsy Treatment. ACS Nano 2023, 17, 15796–15809. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Liu, H.; Ge, S. Advancing versatile ferroelectric materials toward biomedical applications. Adv. Sci. 2021, 8, 2003074. [Google Scholar] [CrossRef]
- Ge, M.; Xu, D.; Chen, Z.; Wei, C.; Zhang, Y.; Yang, C.; Chen, Y.; Lin, H.; Shi, J. Magnetostrictive-Piezoelectric-Triggered Nanocatalytic Tumor Therapy. Nano Lett. 2021, 21, 6764–6772. [Google Scholar] [CrossRef]
- Tang, G.; He, J.; Liu, J.; Yan, X.; Fan, K. Nanozyme for tumor therapy: Surface modification matters. Exploration 2021, 1, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Dong, L.; Huang, Y.; Wang, B.; Dong, J.; Lu, Y. Honeycomb-like active microswarms for magnetically tunable cascade enzyme catalysis. Nanoscale 2022, 14, 6535–6542. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dubbu, S.; Kumari, N.; Kumar, A.; Lim, J.; Kim, S.; Lee, I.S. Magnetothermia-Induced Catalytic Hollow Nanoreactor for Bioorthogonal Organic Synthesis in Living Cells. Nano Lett. 2020, 20, 6981–6988. [Google Scholar] [CrossRef]
- Zhou, P.; Dai, Y.; Lin, X.; Song, Y.; Pang, Y.; Chen, R.; Xiao, R. Specific and Magnetic Covalent Organic Framework Confined Os Nanoclusterzyme for Interference-Free and Ultrasensitive Biosensing. Adv. Funct. Mater. 2024, 34, 2400875. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, B.; An, X.; Guo, Z.; Liu, X.; Lu, H.; Hu, F.; Chen, Z.; Guo, C.; Li, C.M. MOFs-Based Magnetic Nanozyme to Boost Cascade ROS Accumulation for Augmented Tumor Ferroptosis. Adv. Healthc. Mater. 2024, 13, 2304591. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Wang, L.; Lu, N. Tetrahedral DNA Framework-Engineered Magnetic Nanozyme for Visual Biomarker Sensing via Alkaline Phosphatase-Triggered Signal Switching. Anal. Chem. 2025, 97, 19505–19516. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferroMNPs. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Du, W.; Liu, T.; Xue, F.; Cai, X.; Chen, Q.; Zheng, Y.; Chen, H. Fe3O4 Mesocrystals with Distinctive Magnetothermal and Nanoenzyme Activity Enabling Self-Reinforcing Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 19285–19294. [Google Scholar] [CrossRef]
- Panferov, V.G.; Zhang, X.; Wong, K.Y.; Lee, J.H.; Liu, J. Biomedical Applications of Nanozymes: An Enzymology Perspective. Angew. Chem. Int. Ed. Engl. 2025, 137, e202512409. [Google Scholar] [CrossRef]
- Dong, H.; Du, W.; Dong, J.; Che, R.; Kong, F.; Cheng, W.; Ma, M.; Gu, N.; Zhang, Y. Depletable peroxidase-like activity of Fe3O4 nanozymes accompanied with separate migration of electrons and iron ions. Nat. Commun. 2022, 13, 5365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Chu, C.; Zhou, Z.; Chen, B.; Pang, X.; Lin, G.; Lin, H.; Guo, Y.; Ren, E.; et al. Genetically engineered magnetic nanocages for cancer magneto-catalytic theranostics. Nat. Commun. 2020, 11, 5421. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Baile, M.; Pérez-Valenciano, E.; García-Morales, P.; de Juan Romero, C.; Bello-Gil, D.; Barberá, V.M.; Rodríguez-Lescure, Á.; Sanz, J.M.; Alenda, C.; Saceda, M. CLytA-DAAO Chimeric Enzyme Bound to MNPs. A New Therapeutical Approach for Cancer Patients? Int. J. Mol. Sci. 2021, 22, 1477. [Google Scholar] [CrossRef]
- Riskowski, R.A. Nanoscale Temperature Increases Are Not Required for Molecular Stimulation Using Nanoparticles under Radiofrequency Magnetic Fields: A Thermal Energy Flux Mechanism of Remote Enzyme Stimulation. Nano Lett. 2025, 25, 15848–15853. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, L.; Chen, Y.; Shi, J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017, 8, 357. [Google Scholar] [CrossRef]
- Sun, C.; Wang, W.; Sun, X.; Chu, W.; Yang, J.; Dai, J.; Ju, Y. An intrinsically thermogenic nanozyme for synergistic antibacterial therapy. Biomater. Sci. 2021, 9, 8323–8334. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Zhang, Y.; Wang, Y.; Cui, M.; Li, G.; Liu, X.; Fan, H. Magnetoresponsive nanozyme: Magnetic stimulation on the nanozyme activity of iron oxide nanoparticles. Sci. China Life Sci. 2022, 65, 184–192. [Google Scholar] [CrossRef]
- Liu, X.; Yan, B.; Li, Y.; Ma, X.; Jiao, W.; Shi, K.; Zhang, T.; Chen, S.; He, Y.; Liang, X.-J.; et al. Graphene Oxide-Grafted Magnetic Nanorings Mediated Magnetothermodynamic Therapy Favoring Reactive Oxygen Species-Related Immune Response for Enhanced Antitumor Efficacy. ACS Nano 2020, 14, 1936–1950. [Google Scholar] [CrossRef]
- Shen, J.; Rees, T.W.; Zhou, Z.; Yang, S.; Ji, L.; Chao, H. A mitochondria-targeting magnetothermogenic nanozyme for magnet-induced synergistic cancer therapy. Biomaterials 2020, 251, 120079. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhou, Q.; Chen, X.; Jiao, W.; Li, G.; Peng, M.; Liu, X.; He, Y.; Fan, H. Precise Regulation of Enzyme–Nanozyme Cascade Reaction Kinetics by Magnetic Actuation toward Efficient Tumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 52395–52405. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Lin, J.; Li, Y.; Zhang, K.; Li, H.; Fu, Q.; Jiang, Y. Engineering nanozyme immunomodulator with magnetic targeting effect for cascade-enzyodynamic and ultrasound-reinforced metallo-immunotherapy in prostate carcinoma. Nat. Commun. 2025, 16, 1876. [Google Scholar] [CrossRef]
- Torres-Herrero, B.; Armenia, I.; Alleva, M.; Asín, L.; Correa, S.; Ortiz, C.; Fernández-Afonso, Y.; Gutiérrez, L.; de la Fuente, J.M.; Betancor, L.; et al. Remote Activation of Enzyme Nanohybrids for Cancer Prodrug Therapy Controlled by Magnetic Heating. ACS Nano 2023, 17, 12358–12373. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Chen, Y.; Wu, J.; Wang, K.; Chen, L.; Wang, Y.; Jiang, X.; Liu, Y.; Wu, Y.; et al. Magneto-Electrically Enhanced Intracellular Catalysis of FePt-FeC Heterostructures for Chemodynamic Therapy. Adv. Mater. 2021, 33, 2100472. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, C.; Zheng, F.; Qian, Y.; Zhang, X.; Wang, H. Electron Density Modulation-Enhanced Magnetic Nanocatalysis for Anti-Tumor Therapy. Adv. Funct. Mater. 2025, 35, 2422270. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Dong, X.; Mei, L.; Wu, X.; Gu, Z.; Zhao, Y. X-ray-facilitated redox cycling of nanozyme possessing peroxidase-mimicking activity for reactive oxygen species-enhanced cancer therapy. Biomaterials 2021, 276, 121023. [Google Scholar] [CrossRef]
- Li, G. Ferrimagnetic Vortex Nanorings Facilitate Efficient and Safe Deep-Brain Magnetothermal Stimulation in Freely Moving Mice. Exploration 2024. [Google Scholar] [CrossRef]
- Veselov, M.M.; Uporov, I.V.; Efremova, M.V.; Le-Deygen, I.M.; Prusov, A.N.; Shchetinin, I.V.; Savchenko, A.G.; Golovin, Y.I.; Kabanov, A.V.; Klyachko, N.L. Modulation of α-Chymotrypsin Conjugated to MNPs by the Non-Heating Low-Frequency Magnetic Field: Molecular Dynamics, Reaction Kinetics, and Spectroscopy Analysis. ACS Omega 2022, 7, 20644–20655. [Google Scholar] [CrossRef]
- Parkes, E.; Al Samad, A.; Mazzotti, G.; Newell, C.; Ng, B.; Radford, A.; Booth, M.J. Magnetic activation of spherical nucleic acids enables the remote control of synthetic cells. Nat. Chem. 2025, 17, 1505–1513. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Yang, S.; Isak, A.N.; Song, Y.; Zhang, F.; Mao, D.; Zhu, X. Magnetism-Controllable Catalytic Activity of DNAzyme. Anal. Chem. 2022, 94, 2827–2834. [Google Scholar] [CrossRef]
- Szekeres, K.; Bollella, P.; Kim, Y.; Minko, S.; Melman, A.; Katz, E. Magneto-Controlled Enzyme Activity with Locally Produced pH Changes. J. Phys. Chem. Lett. 2021, 12, 2523–2527. [Google Scholar] [CrossRef]
- Jang, J.; Park, C.B. Magnetoelectric dissociation of Alzheimer’s β-amyloid aggregates. Sci. Adv. 2022, 8, eabn1675. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Xue, Y.; Zhang, J.; Bai, H.; Pan, S.; Peng, B.; Li, L.; Voelcker, N.H. X-Ray-Induced Drug Release for Cancer Therapy. Angew. Chem. Int. Ed. 2023, 62, e202306100. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Chen, Z.; Chen, Q.; Yang, H. Advancing X-ray Luminescence for Imaging, Biosensing, and Theragnostics. Acc. Chem. Res. 2023, 56, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kim, S.-J.; Wu, X.; Cui, H.; Hahn, S.K.; Hong, G. Principles and applications of sono-optogenetics. Adv. Drug Deliv. Rev. 2023, 194, 114711. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of magnetic iron oxide nanoparticles for medical applications. J. Nanobiotechnol. 2022, 20, 305. [Google Scholar] [CrossRef]

| Nanomaterial | Enzymatic Activity | Mechanism | Application |
|---|---|---|---|
| Fe3O4-GOx in silica nanoparticles [37] | Showed rapid glucose consumption and abundant •OH production | Cascade catalytic reactions and reduced pH | Resulting in both “starvation” and oxidative antitumor effects in mice bearing 4T1 breast cancer |
| Magnetic covalent organic framework confined Os nanoclusterzyme Fe3O4@COF@Os [27] | Displayed superior POD-specific activity | Regulated by deoxidizers and the functional groups | Analyze serum cancer biomarker prostate-specific antigen, with a detection limit of 3.83 pg mL-1 |
| Porphyrin-Zr-MOF-zinc ferrite-based magnetic nanozyme [28] | Showed multienzyme-like cascade activity | Magneto-electrical effect | MOF-based magnetic nanozyme exhibits significantly enhanced ferroptosis in tumor-bearing mice |
| Fe3O4@ZIF-67@Pt nanozyme [29] | Showed significantly enhanced peroxidase-like activity | Synergistic effect between ZIF-67 and Pt | Achieved a highly accurate detection of PSA in human serum samples and distinguished prostate cancer patients from healthy individuals |
| Hollow Fe3O4 mesocrystals [31] | Exhibited increased peroxidase-like activity | High ratio of Fe2+/Fe3+, many oxygen defects, and magneto-thermal effect | Achieved a self-augmented synergistic effect between hyperthermia and catalytic therapy of 4T1-tumor-bearing nude mice |
| Magnetic Fe3O4 nanocomposites [34] | Showed enhanced catalase-like activity | Magneto-thermal effect | Achieved tumor angiogenesis inhibition in BALB/c nude mice bearing fLuc-LM3 orthotopic HCC |
| Yolk–shell Fe2C@Fe3O4-PEG nanozymes [38] | Showed high magnetothermal-enhanced peroxidase-like activities | Magneto-thermal effect | Exhibited superior synergistic antibacterial efficacy in in vitro and in vivo experiments |
| Fe3O4 nanoparticles [39] | First reported magneto-thermal regulation on nanozyme activity | Local magneto-thermal effect | - |
| Fe3O4 nanoring and graphene oxide hybrid nanoparticles [40] | Induced a significantly amplified ROS level | Magneto-thermal effect | Attained ROS-related immune response and impressive systemic therapeutic efficacy of 4T1-tumor-bearing mice |
| Ir@MnFe2O4 nanozyme [41] | Increased the rate of conversion of both Fe(III) to Fe(II) and H2O2 to •OH | Magneto-thermal effect | Mediated cellular redox homeostasis disruption and led to efficient treatment in mice bearing xenografted HeLa tumors |
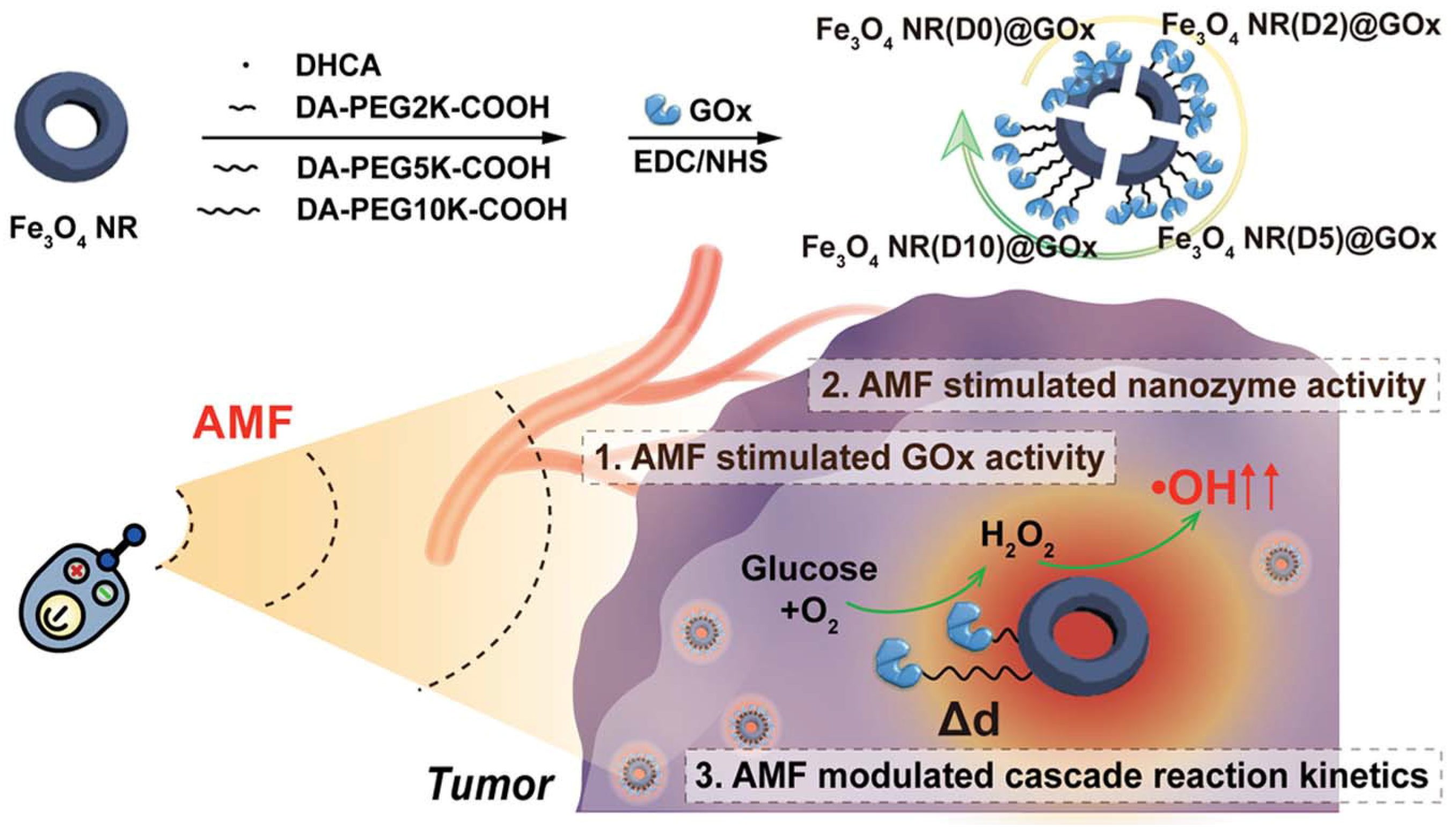
| GOx-Fe3O4 nanoring [42] | Exhibited a superior kinetic match between GOx and Fe3O4 nanozymes and over a 400-fold higher cascade activity | Magneto-thermal effect, cascade catalytic reaction | Significantly improved tumor inhibition in 4T1 tumor-bearing mice. |
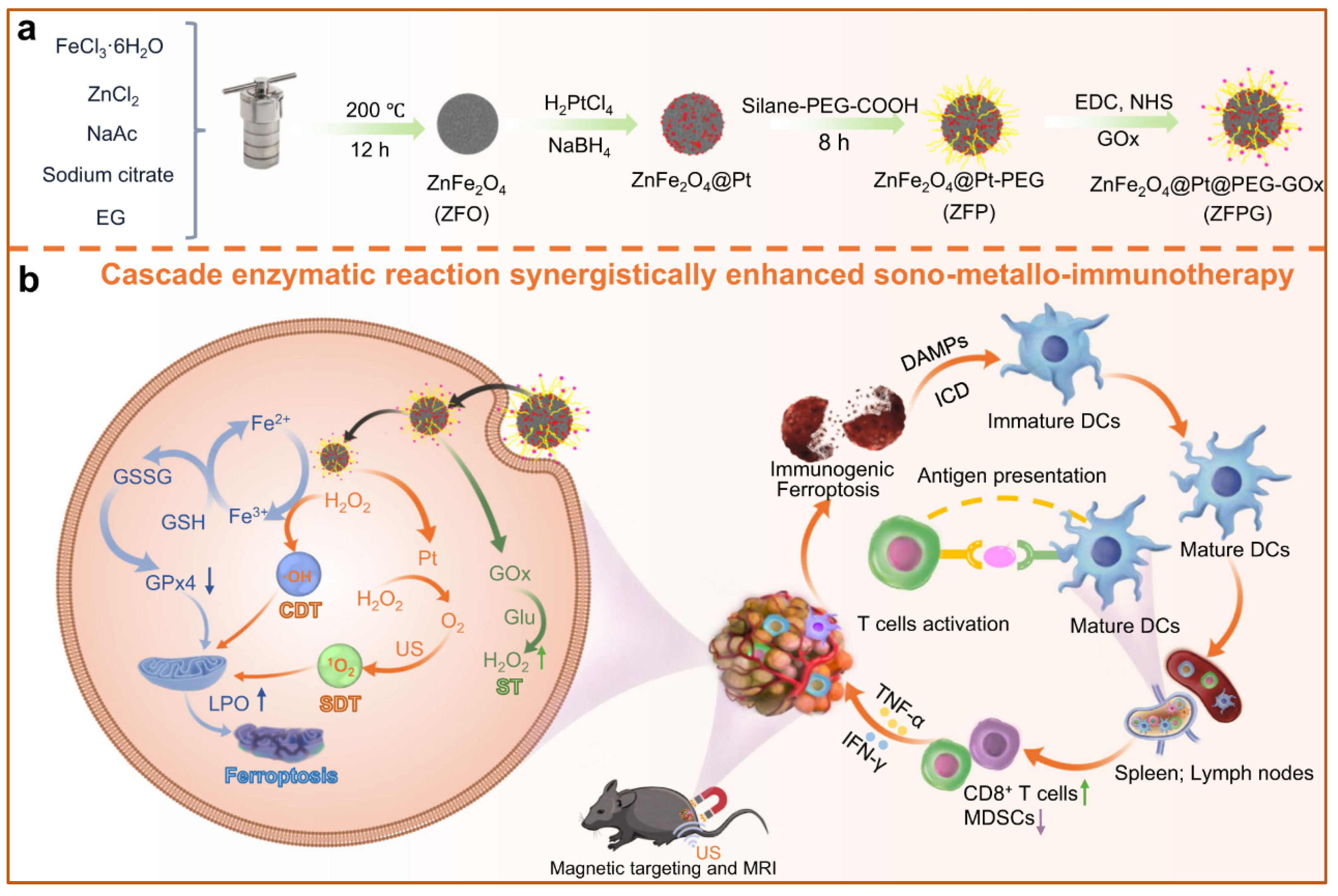
| ZnFe2O4@Pt-GOx core-shell nanoparticles [43] | Showed five-enzyme activities | Ultrasound activation, cascade catalytic reaction | Facilitated sono-metallo-immunotherapy for prostate cancer treatment in male mice. |
| Magnetic nanoparticle-HRP-silica nanohybrids [44] | Increased HRP-catalyzed conversion rate from indole-3-acetic acid into peroxylated radicals | Magneto-thermal effect, cascade catalytic reaction | Showed higher reductions in the tumor volume growth in mice using a human pancreatic cancer cell line |
| FePt-FeC heterostructures [45] | Enhanced catalytic hydrogenation reaction of FePt-FeC heterostructures | Magneto-electrical effect | Killed senescent cancer cells efficiently with catalytic therapy |
| Carbon-coated NiFe2O4 nanocatalysts [46] | Increased Fenton reaction activity | Localized electron distribution at octahedral Fe active sites | Mediated efficient photothermal and catalytic therapy in 4T1 tumor-bearing mice |
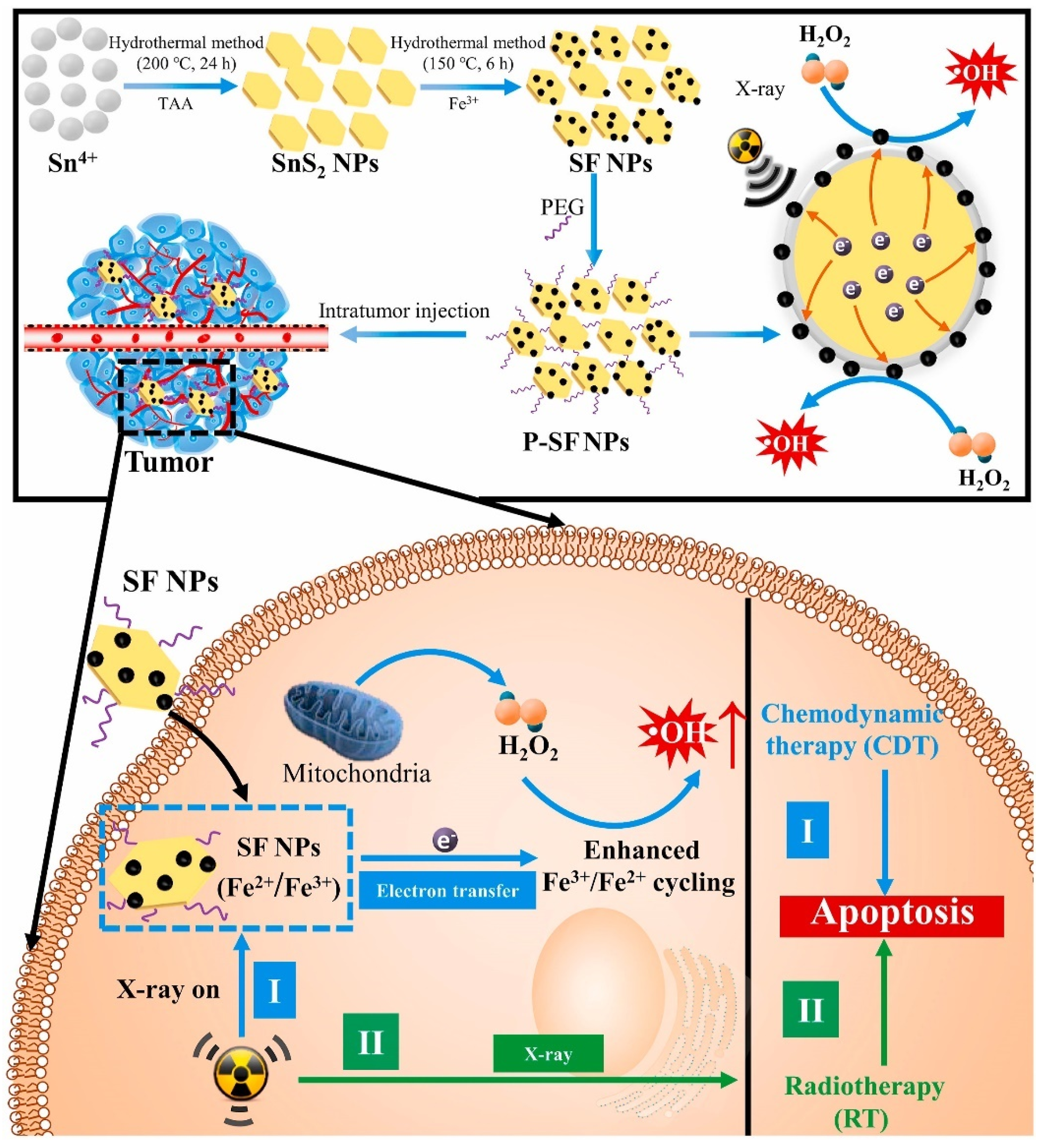
| SnS2-Fe3O4 nanocomposites [47] | Enhanced catalytic reaction of overexpressed H2O2 to ROS | X-ray-mediated electron transfer. | Demonstrated synergistic effects of radiotherapy and catalytic therapy in HeLa tumor-bearing nude mice |
| Method | Advantage | Limitation |
|---|---|---|
| Magneto-thermal effect | Catalytic therapy combined with magnetic hyperthermia | Enzyme inactivation is possible |
| Overheating damage is possible | ||
| Magneto-mechanical effect | Low field strength is possible Can be used for drug delivery | Very large gradients are needed |
| Magneto-electrical effect | Effective for enhancing the redox reaction | Working distance is usually short |
| Force/torque may be insufficient | ||
| X-rays | No limit on penetration depth | Inevitable radiation damage |
| Long working distance | ||
| Ultrasound | Acoustic wave induced pressure and heat | Working distance is short |
| Hard to penetrate bone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, D.; Liang, H.; Lan, B.; Chang, P.; Yang, Y.; Cheng, Y.; Li, G.; Lu, H. Recent Progress of Magnetic Nanomaterials with Enhanced Enzymatic Activities in Antitumor Therapy. Int. J. Mol. Sci. 2025, 26, 10890. https://doi.org/10.3390/ijms262210890
Zhang Y, Li D, Liang H, Lan B, Chang P, Yang Y, Cheng Y, Li G, Lu H. Recent Progress of Magnetic Nanomaterials with Enhanced Enzymatic Activities in Antitumor Therapy. International Journal of Molecular Sciences. 2025; 26(22):10890. https://doi.org/10.3390/ijms262210890
Chicago/Turabian StyleZhang, Yifan, Dongyan Li, Hongxia Liang, Bin Lan, Peidan Chang, Yaoxin Yang, Yuanyuan Cheng, Galong Li, and Hongbing Lu. 2025. "Recent Progress of Magnetic Nanomaterials with Enhanced Enzymatic Activities in Antitumor Therapy" International Journal of Molecular Sciences 26, no. 22: 10890. https://doi.org/10.3390/ijms262210890
APA StyleZhang, Y., Li, D., Liang, H., Lan, B., Chang, P., Yang, Y., Cheng, Y., Li, G., & Lu, H. (2025). Recent Progress of Magnetic Nanomaterials with Enhanced Enzymatic Activities in Antitumor Therapy. International Journal of Molecular Sciences, 26(22), 10890. https://doi.org/10.3390/ijms262210890






