Hormonal Crosstalk in Melasma: Unraveling the Dual Roles of Estrogen and Progesterone in Melanogenesis
Abstract
1. Introduction
2. Mechanisms of Estrogen and Progesterone in Melasma
2.1. Mechanism of Estrogen’s Effect on Melasma
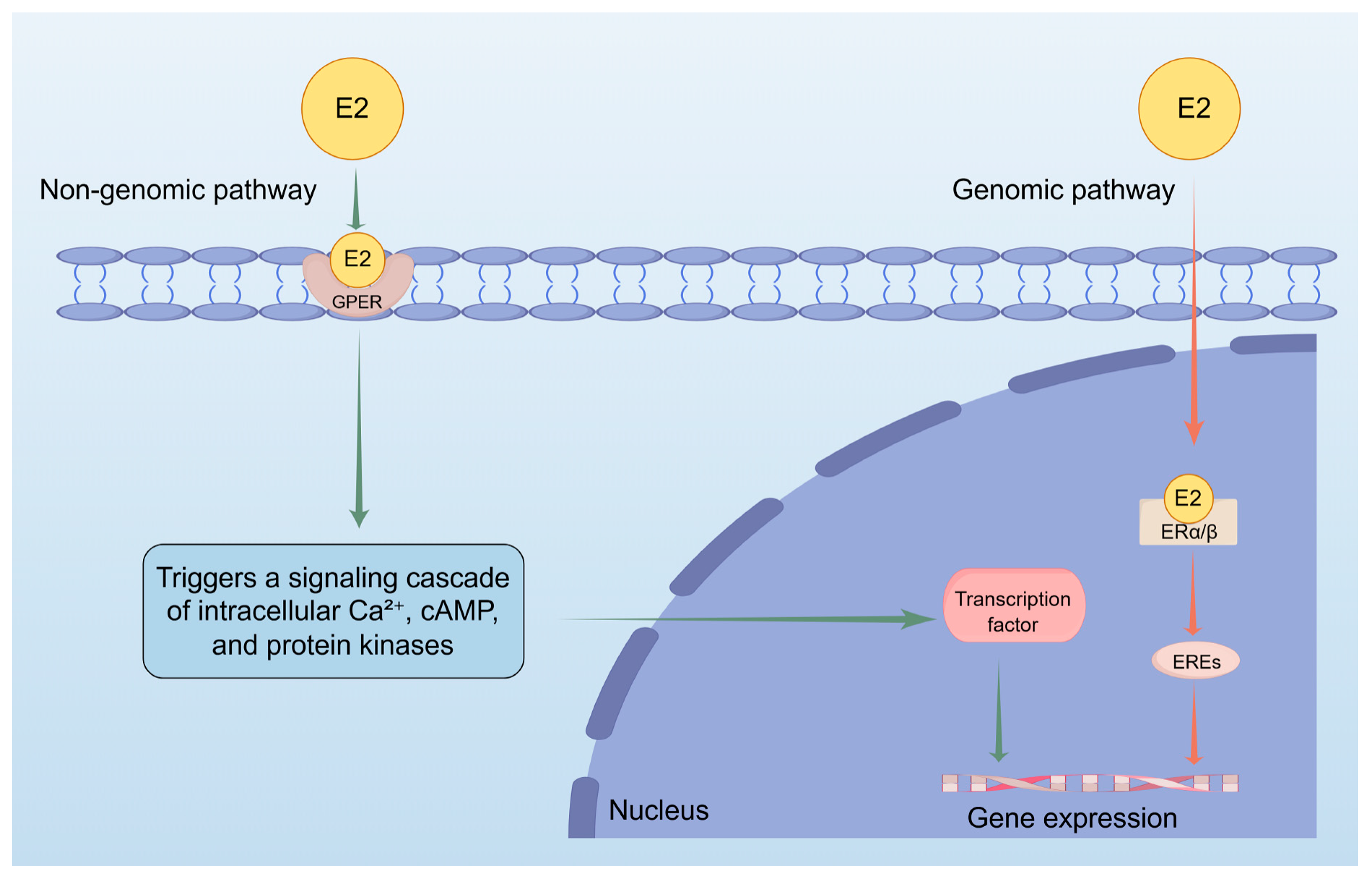
2.1.1. Estrogen Receptor-Mediated Melanin Production: The Dual Pathway Role of Estradiol (E2) in the Pathogenesis of Melasma
2.1.2. Estrone (E1) in the Pathogenesis of Melasma: Mechanisms of Upregulating Melanogenic Enzymes and Modulating Melanocyte Behavior
2.1.3. Beyond a Weak Estrogen: Unraveling Estriol (E3) Pro-Pigmentary Effects in Melasma
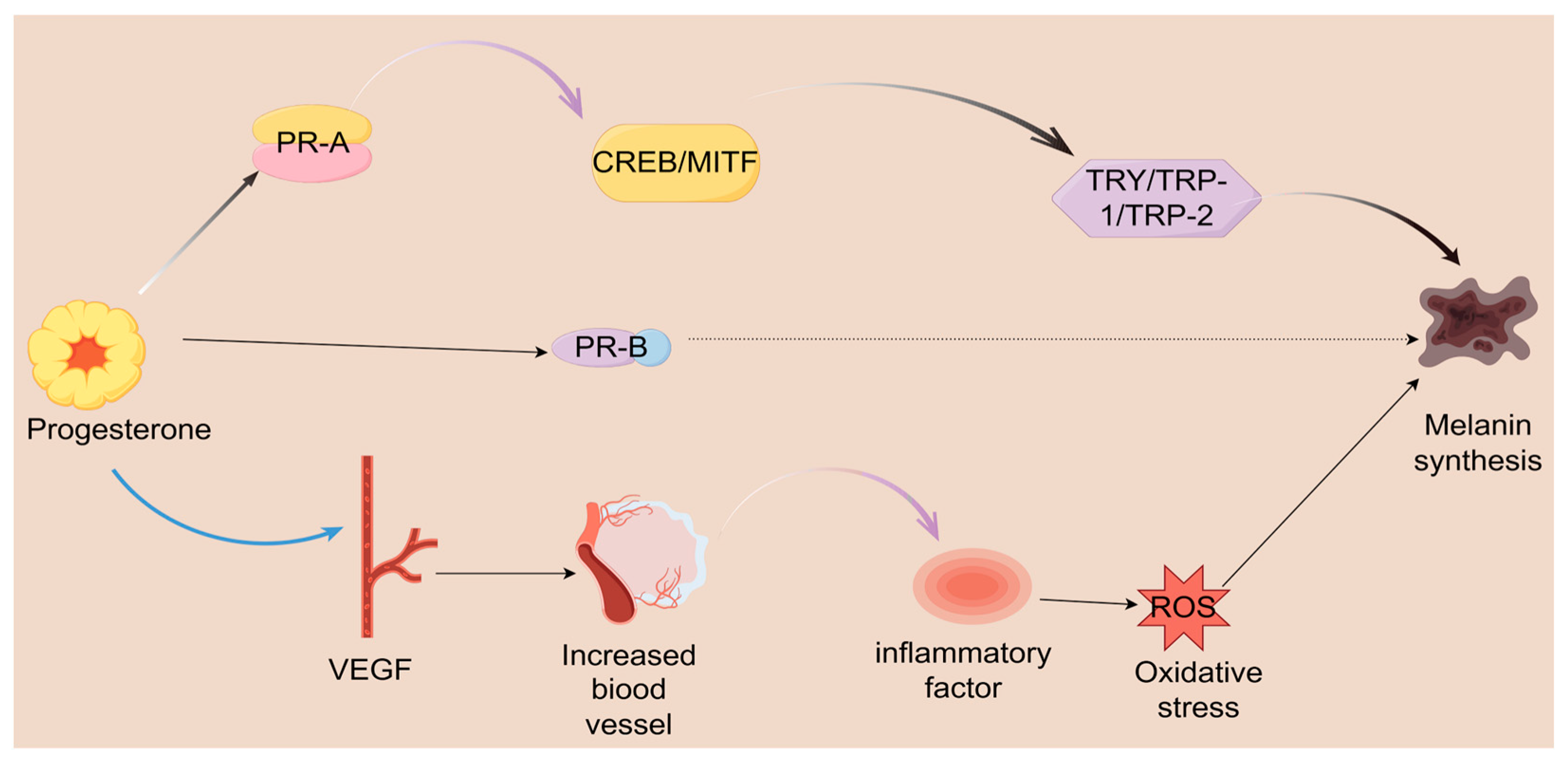
2.2. The Mechanism of Progesterone’s Effect on Melasma
2.2.1. The Role of Progesterone in Melasma: From Hormonal Fluctuations to Pigmentation Disorders
2.2.2. From Hormone to Pigment: 20α-DHP as the Link Between Steroid Metabolism and Skin Pigmentation
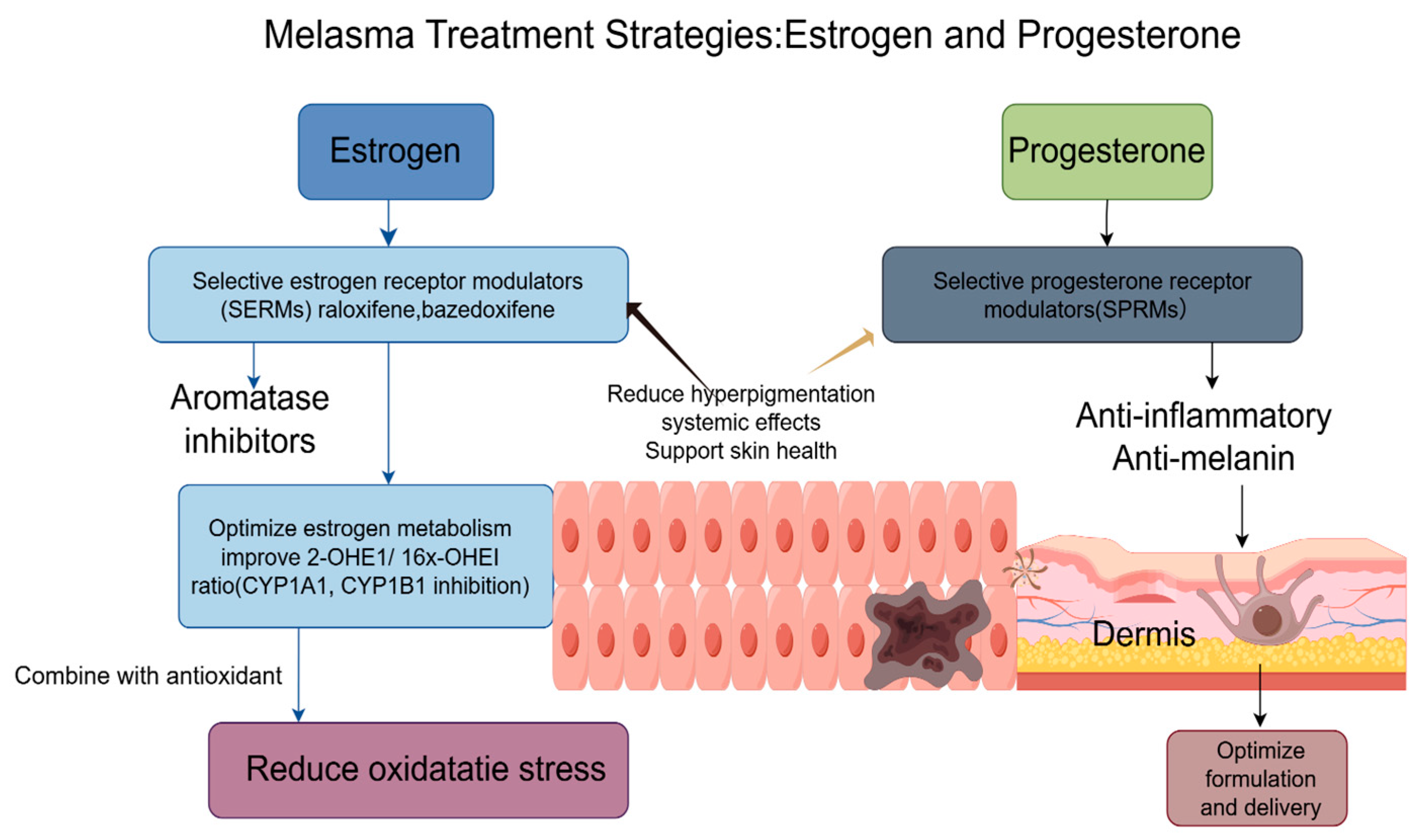
3. Treatment Strategies Based on the Hormones of Both
3.1. Treatment Strategies for Melasma Based on Estrogen
3.2. Treatment Strategies for Melasma Based on Progesterone
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| Alpha-MSH | alpha-melanocyte-stimulating hormone |
| SCF | Stem cell factor |
| Opsin-3 | OPN3 |
| OCA2 | Oculocutaneous albinism II |
| SLC24A5 | Solute carrier family 24 member 5 |
| MC1R | Melanocortin-1 receptor |
| HGF | Hepatocyte growth factor |
| ET-1 | Endothelin-1 |
| E1 | Estrone |
| E2 | Estradiol |
| E3 | Estriol |
| PR-A | Progesterone receptor-A |
| PR-B | Progesterone receptor-B |
| 17-HSD | 17-hydroxysteroid dehydrogenase |
| ERs | Estrogen receptors |
| GPER | G-protein-coupled estrogen receptor |
| cAMP | Cyclic adenosine monophosphate |
| TRP-1 | Tyrosinase-related protein-1 |
| TRP-2 | Tyrosinase-related protein-2 |
| ER-α | Estrogen receptors-α |
| ER-β | Estrogen receptors-β |
| TYR | Tyrosinase |
| EREs | Estrogen response elements |
| MAPKs | Mitogen-activated protein kinases |
| MITF | Microphthalmia-associated transcription factor |
| DHEA | Dehydroepiandrosterone |
| ROS | Reactive oxygen species |
| 20α-DHP | 20α-Hydroxyprogesterone |
| VEGF | Vascular endothelial growth factor |
| IL-6 | Interleukin-6 |
| TNF-α | TNF-alpha |
| PRs | Progesterone receptors |
| miRNAs | MicroRNAs |
| 20α-HSD | 20α-hydroxysteroid dehydrogenase |
| MC1R | Melanocortin 1 receptor |
| PR | Progesterone receptors |
| SERMs | Selective estrogen receptor modulators |
| 2-OHE1 | 2-hydroxyestrone |
| 16α-OHE1 | 16-alpha-hydroxyestrone |
| SPRMs | Selective progesterone receptor modulators |
| CREB | CAMP response element-binding protein |
| ERK | extracellular signal-regulated kinase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) |
| SOD | superoxide dismutase |
| GPx | glutathione peroxidase |
| DOPA | dihydroxyphenylalanine |
| AP | asoprisnil |
References
- Abdalla, M.A. Melasma clinical features, diagnosis, epidemiology and etiology: An update review. Siriraj Med. J. 2021, 73, 841–850. [Google Scholar] [CrossRef]
- Kang, H.Y.; Suzuki, I.; Lee, D.J.; Ha, J.; Reiniche, P.; Aubert, J.; Deret, S.; Zugaj, D.; Voegel, J.J.; Ortonne, J.-P. Transcriptional profiling shows altered expression of Wnt Pathway–and lipid Metabolism–Related genes as well as melanogenesis-related genes in melasma. J. Investig. Dermatol. 2011, 131, 1692–1700. [Google Scholar] [CrossRef]
- Melnick, S.; Lohani, S.; Alweis, R. Hyperpigmentation in a middle aged woman: A common yet underdiagnosed condition. J. Community Hosp. Intern. Med. Perspect. 2016, 6, 31544. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.C.; Miot, L.D.B.; Miot, H.A. Melasma: A clinical and epidemiological review. An. Bras. De Dermatol. 2014, 89, 771–782. [Google Scholar] [CrossRef]
- Pérez, M.; Sánchez, J.L.; Aguiló, F. Endocrinologic profile of patients with idiopathic melasma. J. Investig. Dermatol. 1983, 81, 543–545. [Google Scholar] [CrossRef]
- Vachiramon, V.; Suchonwanit, P.; Thadanipon, K. Melasma in men. J. Cosmet. Dermatol. 2012, 11, 151–157. [Google Scholar] [CrossRef]
- Gauthier, Y.; Cario, M.; Pain, C.; Lepreux, S.; Benzekri, L.; Taieb, A. Oestrogen associated with ultraviolet B irradiation recapitulates the specific melanosome distribution observed in caucasoid melasma. Br. J. Dermatol. 2019, 180, 951–953. [Google Scholar] [CrossRef]
- Kheradmand, M.; Afshari, M.; Damiani, G.; Abediankenari, S.; Moosazadeh, M. Melasma and thyroid disorders: A systematic review and meta-analysis. Int. J. Dermatol. 2019, 58, 1231–1238. [Google Scholar] [CrossRef]
- Ortonne, J.; Arellano, I.; Berneburg, M.; Cestari, T.; Chan, H.; Grimes, P.; Hexsel, D.; Im, S.; Lim, J.; Lui, H. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1254–1262. [Google Scholar] [CrossRef]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B. Melanocytes sense blue light and regulate pigmentation through opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, D.; Saliba, E. The Genetics and Evolution of Human Pigmentation. Biology 2025, 14, 1026. [Google Scholar] [CrossRef]
- Khunger, N.; Kandhari, R.; Singh, A.; Ramesh, V. A clinical, dermoscopic, histopathological and immunohistochemical study of melasma and facial pigmentary demarcation lines in the skin of color. Dermatol. Ther. 2020, 33, e14515. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.; Nogara, A.; Merico, M.; Ferlin, A.; Foresta, C. Identification of functional binding sites for progesterone in rat Leydig cell plasma membrane. Steroids 1999, 64, 168–175. [Google Scholar] [CrossRef]
- Sundström-Poromaa, I.; Comasco, E.; Sumner, R.; Luders, E. Progesterone—Friend or foe? Front. Neuroendocr. 2020, 59, 100856. [Google Scholar] [CrossRef]
- Palaniappan, V.; Gopinath, H.; Murthy, A.B.; Gupta, A.; Karthikeyan, K. A narrative review of Catamenial dermatology: A glimpse into the menstrual symphony. Indian. J. Dermatol. Venereol. Leprol. 2025, 91, 616. [Google Scholar] [CrossRef]
- KrupaShankar, D.S.; Somani, V.K.; Kohli, M.; Sharad, J.; Ganjoo, A.; Kandhari, S.; Mysore, V.R.; Aurangabadkar, S.; Malakar, S.; Vedamurthy, M.; et al. A cross-sectional, multicentric clinico-epidemiological study of melasma in India. Dermatol. Ther. 2014, 4, 71–81. [Google Scholar] [CrossRef]
- Filoni, A.; Mariano, M.; Cameli, N. Melasma: How hormones can modulate skin pigmentation. J. Cosmet. Dermatol. 2019, 18, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Kruglova, L.; Ikonnikova, E. Hyperpigmentation of the skin: Modern views on etiology and pathogenesis (part 1). Russ. J. Ski. Vener. Dis. 2017, 20, 178–183. [Google Scholar] [CrossRef]
- Triplett, K.D.; Pokhrel, S.; Castleman, M.J.; Daly, S.M.; Elmore, B.O.; Joyner, J.A.; Sharma, G.; Herbert, G.; Campen, M.J.; Hathaway, H.J.; et al. GPER activation protects against epithelial barrier disruption by Staphylococcus aureus α-toxin. Sci. Rep. 2019, 9, 1343. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef]
- Cui, Y.; He, W.; Wang, Z.; Yang, H.; Zheng, M.; Li, Y. Reduced estrogenic risks of a sunscreen additive: Theoretical design and evaluation of functionally improved salicylates. J. Hazard. Mater. 2024, 477, 135371. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Oizumi, R.; Sugimoto, Y.; Aibara, H. The Potential of Exercise on Lifestyle and Skin Function: Narrative Review. JMIR Dermatol. 2024, 7, e51962. [Google Scholar] [CrossRef]
- Mandalà, M. Influence of Estrogens on Uterine Vascular Adaptation in Normal and Preeclamptic Pregnancies. Int. J. Mol. Sci. 2020, 21, 2592. [Google Scholar] [CrossRef] [PubMed]
- Mobasher, P.; Foulad, D.P.; Raffi, J.; Zachary, C.; Fackler, N.; Zohuri, N.; Juhasz, M.; Atanaskova Mesinkovska, N. Catamenial Hyperpigmentation: A Review. J. Clin. Aesthet. Dermatol. 2020, 13, 18–21. [Google Scholar] [PubMed]
- Fritz, M.; Speroff, L. Menopause and perimenopausal transition. In Clinical Gynecologic Endocrinology and Infertility; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 673–748. [Google Scholar]
- Rofiq, A.; Tantari, S.; Widiatmoko, A.; Savitri, D. Correlation of Estradiol and Estriol Serum Levels to Melasma Severity in Pregnant Women. J. Dermatol. Dis. 2018, 5, 1000268. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef] [PubMed]
- Faltas, C.L.; LeBron, K.A.; Holz, M.K. Unconventional Estrogen Signaling in Health and Disease. Endocrinology 2020, 161, bqaa030. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar]
- Cooke, P.S.; Mesa, A.M.; Sirohi, V.K.; Levin, E.R. Role of nuclear and membrane estrogen signaling pathways in the male and female reproductive tract. Differentiation 2021, 118, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Madeshwaran, A.; Vijayalakshmi, P.; Umapathy, V.R.; Shanmugam, R.; Selvaraj, C. Unlocking estrogen receptor: Structural insights into agonists and antagonists for glioblastoma therapy. Adv. Protein Chem. Struct. Biol. 2024, 142, 1–24. [Google Scholar]
- Zomer, H.D.; Cooke, P.S. Targeting estrogen signaling and biosynthesis for aged skin repair. Front. Physiol. 2023, 14, 1281071. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol. Cell. Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef]
- Jee, S.H.; Lee, S.Y.; Chiu, H.C.; Chang, C.C.; Chen, T.J. Effects of estrogen and estrogen receptor in normal human melanocytes. Biochem. Biophys. Res. Commun. 1994, 199, 1407–1412. [Google Scholar] [CrossRef]
- Chang, T.-S.; Lin, V.C.-H. Melanogenesis inhibitory activity of two generic drugs: Cinnarizine and trazodone in mouse B16 melanoma cells. Int. J. Mol. Sci. 2011, 12, 8787–8796. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.H.; Hartig, R.; Weinert, S.; Haybaeck, J.; Nass, N. G-Protein-Coupled Estrogen Receptor (GPER)-Specific Agonist G1 Induces ER Stress Leading to Cell Death in MCF-7 Cells. Biomolecules 2019, 9, 503. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Chen, W.C.; Thornton, M.J.; Qin, K.; Rosenfield, R. Sexual hormones in human skin. Horm. Metab. Res. 2007, 39, 85–95. [Google Scholar] [CrossRef]
- Ranson, M.; Posen, S.; Mason, R.S. Human melanocytes as a target tissue for hormones: In vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J. Invest. Dermatol. 1988, 91, 593–598. [Google Scholar] [CrossRef]
- Jang, Y.H.; Lee, J.Y.; Kang, H.Y.; Lee, E.S.; Kim, Y.C. Oestrogen and progesterone receptor expression in melasma: An immunohistochemical analysis. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Naganuma, M.; Fukuda, M.; Matsunaga, J.; Tomita, Y. Effect of pituitary and ovarian hormones on human melanocytes in vitro. Pigment. Cell Res. 1996, 9, 204–212. [Google Scholar] [CrossRef]
- McLeod, S.D.; Ranson, M.; Mason, R.S. Effects of estrogens on human melanocytes in vitro. J. Steroid Biochem. Mol. Biol. 1994, 49, 9–14. [Google Scholar] [CrossRef]
- Kippenberger, S.; Loitsch, S.; Solano, F.; Bernd, A.; Kaufmann, R. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR--regulation by steroid hormones. J. Invest. Dermatol. 1998, 110, 364–367. [Google Scholar]
- Goandal, N.F.; Rungby, J.; Karmisholt, K.E. The role of sex hormones in the pathogenesis of melasma. Ugeskr. Laeger 2022, 184, V10210769. [Google Scholar]
- Zhou, Y.; Zeng, H.L.; Wen, X.Y.; Jiang, L.; Fu, C.H.; Hu, Y.B.; Lei, X.X.; Zhang, L.; Yu, X.; Yang, S.Y.; et al. Selaginellin Inhibits Melanogenesis via the MAPK Signaling Pathway. J. Nat. Prod. 2022, 85, 838–845. [Google Scholar] [CrossRef]
- Delgado, B.J.; Patel, P.; Lopez-Ojeda, W. Estrogen(Archived). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Baetens, D.; Güran, T.; Mendonca, B.B.; Gomes, N.L.; De Cauwer, L.; Peelman, F.; Verdin, H.; Vuylsteke, M.; Van der Linden, M.; Atay, Z.; et al. Biallelic and monoallelic ESR2 variants associated with 46,XY disorders of sex development. Genet. Med. 2018, 20, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Aromatase and estrogen receptor α deficiency. Fertil. Steril. 2014, 101, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Traj, P.; Szebeni, G.J.; Gémes, N.; Resch, V.; Paragi, G.; Mernyák, E.; Minorics, R.; Zupkó, I. Investigation of the Antineoplastic Effects of 2-(4-Chlorophenyl)-13α-Estrone Sulfamate against the HPV16-Positive Human Invasive Cervical Carcinoma Cell Line SiHa. Int. J. Mol. Sci. 2023, 24, 6625. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, T.; Wang, J.-H.; Xiong, H.; Wang, Y.-Q.; Liu, G.-L.; Qu, Y.; Zhang, N. Effects of wogonin on the mechanism of melanin synthesis in A375 cells. Exp. Ther. Med. 2017, 14, 4547–4553. [Google Scholar] [CrossRef]
- Liu, T.; Xi, T.; Dong, X.; Xu, D. Research progress on pathogenesis of skin pigmentation in chronic liver disease. Biomol. Biomed. 2025, 25, 1218. [Google Scholar] [CrossRef]
- Cabeza, M.; Heuze, Y.; Sánchez, A.; Garrido, M.; Bratoeff, E. Recent advances in structure of progestins and their binding to progesterone receptors. J. Enzym. Inhib. Med. Chem. 2015, 30, 152–159. [Google Scholar] [CrossRef]
- González, S.L.; Coronel, M.F.; Raggio, M.C.; Labombarda, F. Progesterone receptor-mediated actions and the treatment of central nervous system disorders: An up-date of the known and the challenge of the unknown. Steroids 2020, 153, 108525. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M. The biological actions of estrogens on skin. Exp. Dermatol. 2002, 11, 487–502. [Google Scholar] [CrossRef]
- Raymond, J.H.; Aktary, Z.; Larue, L.; Delmas, V. Targeting GPCRs and their signaling as a therapeutic option in melanoma. Cancers 2022, 14, 706. [Google Scholar] [CrossRef]
- Sun, M.; Xie, H.-F.; Tang, Y.; Lin, S.-Q.; Li, J.-m.; Sun, S.-N.; Hu, X.-L.; Huang, Y.-X.; Shi, W.; Jian, D. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J. Steroid Biochem. Mol. Biol. 2017, 165, 236–246. [Google Scholar] [CrossRef]
- Yang, O. MITF-Dependent Phenotypic Plasticity Regulates Proton-Coupled Transport and Controls Lineage Resistance to Acidotic Stress. Ph.D. Thesis, University of Oxford, Oxford, UK, 2020. [Google Scholar]
- Feng, Z.; Han, C.; Zhang, N.; Wang, Y.; Luo, G.; Gao, X. An integrated strategy for deciphering the action mechanism of emplastrum: Prescription analysis-component identification-virtual screening and affinity testing in the case of Yaoshen Gao. J. Ethnopharmacol. 2025, 342, 119369. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lim, S.H.; Lee, B.; Kang, I.; Yeo, E.-J. Compound C inhibits B16-F1 tumor growth in a syngeneic mouse model via the blockage of cell cycle progression and angiogenesis. Cancers 2019, 11, 823. [Google Scholar] [CrossRef]
- Loh, H.-Y.; Norman, B.P.; Lai, K.-S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The regulatory role of microRNAs in breast cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef]
- Wang, Z. Regulation of cell cycle progression by growth factor-induced cell signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Migliano, E.; Picardo, M. A framework of major tumor-promoting signal transduction pathways implicated in melanoma-fibroblast dialogue. Cancers 2020, 12, 3400. [Google Scholar] [CrossRef] [PubMed]
- Bento-Lopes, L.; Cabaço, L.C.; Charneca, J.; Neto, M.V.; Seabra, M.C.; Barral, D.C. Melanin’s journey from melanocytes to keratinocytes: Uncovering the molecular mechanisms of melanin transfer and processing. Int. J. Mol. Sci. 2023, 24, 11289. [Google Scholar] [CrossRef]
- Wang, F.; Ma, W.; Fan, D.; Hu, J.; An, X.; Wang, Z. The biochemistry of melanogenesis: An insight into the function and mechanism of melanogenesis-related proteins. Front. Mol. Biosci. 2024, 11, 1440187. [Google Scholar] [CrossRef]
- Parisi, F.; Fenizia, C.; Introini, A.; Zavatta, A.; Scaccabarozzi, C.; Biasin, M.; Savasi, V. The pathophysiological role of estrogens in the initial stages of pregnancy: Molecular mechanisms and clinical implications for pregnancy outcome from the periconceptional period to end of the first trimester. Hum. Reprod. Update 2023, 29, 699–720. [Google Scholar] [CrossRef]
- Jackson, L.M.; Parker, R.M.; Mattison, D.R.; National Academies of Sciences, Engineering, and Medicine. Reproductive Steroid Hormones: Synthesis, Structure, and Biochemistry. In The Clinical Utility of Compounded Bioidentical Hormone Therapy: A Review of Safety, Effectiveness, and Use; National Academies Press (US): Washington, DC, USA, 2020. [Google Scholar]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin homeostasis: Mechanism and influencing factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Tang, J.; Liao, X.; Gong, Y.; Yang, Q.; Wu, Y.; Wu, G. TRIM8 inhibits breast cancer proliferation by regulating estrogen signaling. Am. J. Cancer Res. 2020, 10, 3440–3457. [Google Scholar]
- Zhou, Y.; Gu, B.; Brichant, G.; Singh, J.P.; Yang, H.; Chang, H.; Zhao, Y.; Cheng, C.; Liu, Z.W.; Alderman, M.H., 3rd; et al. The steroid hormone estriol (E(3)) regulates epigenetic programming of fetal mouse brain and reproductive tract. BMC Biol. 2022, 20, 93. [Google Scholar] [CrossRef]
- He, Q.; Yuan, J.; Yang, H.; Du, T.; Hu, S.; Ding, L.; Yan, W.; Chen, P.; Li, J.; Huang, Z. Maternal exposure to fullerenols impairs placental development in mice by inhibiting estriol synthesis and reducing ERα. J. Nanobiotechnology 2025, 23, 30. [Google Scholar] [CrossRef]
- Ozougwu, J.C. The role of reactive oxygen species and antioxidants in oxidative stress. Int. J. Res. 2016, 1, 1–8. [Google Scholar]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.Z. Free radicals and oxidative stress: Signaling mechanisms, redox basis for human diseases, and cell cycle regulation. Curr. Mol. Med. 2023, 23, 13–35. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W.; Li, M.; Yang, Y.; Tian, C.; Zhang, D.; Chang, Z.; Zhang, Y.; Zhao, Z.J.; Chen, Y.; et al. SHP2 participates in decidualization by activating ERK to maintain normal nuclear localization of progesterone receptor. Reproduction 2023, 166, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Gong, J.; Zang, L.; Song, D.; Ran, X.; Li, J.; Jiang, B.; Xu, J.; Wu, Q. Mechanism of Progesterone in Treatment of Traumatic Brain Injury Based on Network Pharmacology and Molecular Docking Technology. Med. Sci. Monit. 2022, 28, e937564. [Google Scholar] [CrossRef] [PubMed]
- Banov, D.; Song, G.; Ip, K.; Seeley, E.H.; Linehan, S.T.; Bassani, I.; Ferron, G.; Bassani, A.S.; Valdez, B.C. In vitro evaluation of the percutaneous absorption of progesterone in anhydrous permeation-enhancing base using the Franz skin finite dose model and mass spectrometry. Arch. Dermatol. Res. 2024, 316, 291. [Google Scholar] [CrossRef]
- Gratton, R.; Del Vecchio, C.; Zupin, L.; Crovella, S. Unraveling the Role of Sex Hormones on Keratinocyte Functions in Human Inflammatory Skin Diseases. Int. J. Mol. Sci. 2022, 23, 3132. [Google Scholar] [CrossRef]
- Henderson, V.W. Progesterone and human cognition. Climacteric 2018, 21, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nadeau, M.; Faucher, F.; Lescelleur, O.; Biron, S.; Daris, M.; Rhéaume, C.; Luu-The, V.; Tchernof, A. Progesterone metabolism in adipose cells. Mol. Cell Endocrinol. 2009, 298, 76–83. [Google Scholar] [CrossRef]
- Stoffel-Wagner, B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001, 145, 669–679. [Google Scholar] [CrossRef]
- Arevalo, M.-A.; Azcoitia, I.; Garcia-Segura, L.M. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2015, 16, 17–29. [Google Scholar] [CrossRef]
- Long, H.; Yu, W.; Yu, S.; Yin, M.; Wu, L.; Chen, Q.; Cai, R.; Suo, L.; Wang, L.; Lyu, Q.; et al. Progesterone affects clinic oocyte yields by coordinating with follicle stimulating hormone via PI3K/AKT and MAPK pathways. J. Adv. Res. 2021, 33, 189–199. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Binderwala, F.; Powers, R.; McFee, R.M.; Cupp, A.S.; Wood, J.R.; Davis, J.S. Central Role for Glycolysis and Fatty Acids in LH-responsive Progesterone Synthesis. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, S.; Sharifi, M.; Rashidi, B. Ovarian stimulation and exogenous progesterone affect the endometrial miR-16-5p, VEGF protein expression, and angiogenesis. Microvasc. Res. 2021, 133, 104074. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Ghaffarizadeh, F.; Mojdeganlou, H. Prognostic Significance of Microvessel Density in Invasive Ductal Carcinoma of Breast. Int. J. Hematol. Oncol. Stem Cell Res. 2023, 17, 100–105. [Google Scholar] [CrossRef]
- Alawadhi, M.; Kilarkaje, N.; Mouihate, A.; Al-Bader, M.D. Role of progesterone on dexamethasone-induced alterations in placental vascularization and progesterone receptors in rats. Biol. Reprod. 2023, 108, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, G. Progesterone Resistance in Endometriosis: Current Evidence and Putative Mechanisms. Int. J. Mol. Sci. 2023, 24, 6992. [Google Scholar] [CrossRef]
- Matsuyama, S.; Whiteside, S.; Li, S.Y. Implantation and Decidualization in PCOS: Unraveling the Complexities of Pregnancy. Int. J. Mol. Sci. 2024, 25, 1203. [Google Scholar] [CrossRef]
- Pelletier, G.; Ren, L. Localization of sex steroid receptors in human skin. Histol. Histopathol. 2004, 19, 629–636. [Google Scholar] [PubMed]
- Xiao, Y.; Tao, W.; Shan, X.; Li, D.; Tao, W.; Qian, H.; Zhao, Y.; Zhang, C. Components analysis of San-Bai decoction, and its pharmacodynamics and mechanism on preventing and treating melasma. J. Ethnopharmacol. 2024, 332, 118388. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. Faseb J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Neuffer, S.J. Pigment Genetics as Told by Crasher: Characterization of a Novel Zebrafish Albinism Model Through Genetic Engineering and Gene Expression Analysis; Washington State University: Pullman, WA, USA, 2021. [Google Scholar]
- Yu, X.; Xu, J.; Song, B.; Zhu, R.; Liu, J.; Liu, Y.F.; Ma, Y.J. The role of epigenetics in women’s reproductive health: The impact of environmental factors. Front. Endocrinol. 2024, 15, 1399757. [Google Scholar] [CrossRef]
- Nasreen, A.; Chowdhury, S.; Selvam, D.A.; Natarajan, V.T. Epigenetic Echoes: Decoding the Acetylation Journey from Neural Crest to Melanocyte. Results Probl. Cell Differ. 2025, 75, 189–209. [Google Scholar]
- Sarkar, D.; Leung, E.Y.; Baguley, B.C.; Finlay, G.J.; Askarian-Amiri, M.E. Epigenetic regulation in human melanoma: Past and future. Epigenetics 2015, 10, 103–121. [Google Scholar] [CrossRef]
- Chen, Y.; Vellaichamy, G.; Schneider, S.L.; Kong, W.; Liu, Z. Exposure factors in the occurrence and development of melasma. Exp. Ther. Med. 2024, 27, 131. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Yoon, J.H.; Jo, C.S.; Hwang, J.S. Comprehensive Analysis of Exosomal MicroRNAs Derived from UVB-Irradiated Keratinocytes as Potential Melanogenesis Regulators. Int. J. Mol. Sci. 2024, 25, 3095. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, Y.; Li, Y.; Li, P.; Cheng, Z.; Li, H.; Zhang, L.; Tang, Z. The comprehensive detection of miRNA, lncRNA, and circRNA in regulation of mouse melanocyte and skin development. Biol. Res. 2020, 53, 4. [Google Scholar] [CrossRef]
- Cheng, Y.; Yan, S.; Li, L.; Du, S.; Zhong, C.; Gao, X.; Chen, C. Study on 20-hydroxyprogesterone: Chiral resolution, content determination and progesterone-like activity. J. Steroid Biochem. Mol. Biol. 2024, 244, 106592. [Google Scholar] [CrossRef]
- Li, L.; Yan, S.; Cheng, Y.; Zhong, C.; Chen, C.; Gao, X. Advances in Sources, Content Determination, and Bioactivity of 20α-Hydroxyprogesterone. Endocr. Metab. Immune Disord.-Drug Targets 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, S.; Cheng, Y.; Aisan, M.; Du, S.; Zhong, C.; Chen, C.; Gao, X. Synthesis of 20α/β-hydroxyprogesterone-trimethyl lock-triglyceride-mimetic prodrugs and its bioavailability study in rats. Bioorg Med. Chem. 2025, 128, 118285. [Google Scholar] [CrossRef] [PubMed]
- Niepoth, N.; Merritt, J.R.; Uminski, M.; Lei, E.; Esquibies, V.S.; Bando, I.B.; Hernandez, K.; Gebhardt, C.; Wacker, S.A.; Lutzu, S.; et al. Evolution of a novel adrenal cell type that promotes parental care. Nature 2024, 629, 1082–1090. [Google Scholar] [CrossRef]
- Sharma, K.; Joshi, N.; Goyal, C. Critical review of Ayurvedic Varṇya herbs and their tyrosinase inhibition effect. Anc. Sci. Life 2015, 35, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, T.J.; Alver, T.N.; Heintz, K.M.; Wernhoff, P.; Nygaard, V.; Nakken, S.; Øy, G.F.; Bøe, S.L.; Urbanucci, A.; Hovig, E. Dysregulation of MITF Leads to Transformation in MC1R-Defective Melanocytes. Cancers 2020, 12, 1719. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Bulletti, C.; Bulletti, F.M.; Sciorio, R.; Guido, M. Progesterone: The Key Factor of the Beginning of Life. Int. J. Mol. Sci. 2022, 23, 14138. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Wang, Y. Melanocytes, Melanin-Synthesis, and Related Signaling Pathway Mélanocytes et Synthèse de Mélanine, Voies Privilégiées Pour Activer la Synthèse. Ph.D. Thesis, Université Bourgogne Franche-Comté, Dijon, France, 2017. [Google Scholar]
- Yoo, H.; Lee, H.-R.; Kim, K.-H.; Kim, M.-A.; Bang, S.; Kang, Y.-H.; Kim, W.-h.; Song, Y.; Chang, S.E. CRTC3, a sensor and key regulator for melanogenesis, as a tunable therapeutic target for pigmentary disorders. Theranostics 2021, 11, 9918. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Marino, M. Steroid hormones: Synthesis, secretion, and transport. In Principles of Endocrinology and Hormone Action; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–72. [Google Scholar]
- Danby, W. Acne, dairy and cancer: The 5α-P link. Derm.-Endocrinol. 2009, 1, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Kably, A.; Barroso, G. Introduction to anatomy and physiology of human conception. Reprod. Biomed. Online 2000, 1, 109–121. [Google Scholar] [CrossRef]
- Goldstein, S.R. Selective estrogen receptor modulators and bone health. Climacteric 2022, 25, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Khater, S.I.; Shalabi, M.; Alammash, B.B.; Alrais, A.I.; Al-Ahmadi, D.; Alqahtani, L.S.; Khamis, T.; Abdelaziz, S.; Aldawy, K. Autophagy characteristics of phytoestrogens in management and prevention of diseases: A narrative review of in-vivo and in-vitro studies. J. Adv. Vet. Anim. Res. 2023, 10, 308–320. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Conner, E.A. Beyond estrogen: Advances in tissue selective estrogen complexes and selective estrogen receptor modulators. Climacteric 2019, 22, 140–147. [Google Scholar] [CrossRef]
- Ferro, A.; Generali, D.; Caffo, O.; Caldara, A.; De Lisi, D.; Dipasquale, M.; Lorenzi, M.; Monteverdi, S.; Fedele, P.; Ciribilli, Y. Oral selective estrogen receptor degraders (SERDs): The new emperors in breast cancer clinical practice? Semin. Oncol. 2023, 50, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shen, L.; Contreras, J.M.; Liu, Z.; Ma, K.; Ma, B.; Liu, X.; Wang, D.O. New potential selective estrogen receptor modulators in traditional Chinese medicine for treating menopausal syndrome. Phytother. Res. 2024, 38, 4736–4756. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.S.; Bongiovanni, B.; Bralley, J.A. Estrogen metabolism and the diet-cancer connection: Rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern. Med. Rev. 2002, 7, 112–129. [Google Scholar]
- Shan, X.; Li, D.; Yin, H.; Tao, W.; Zhou, L.; Gao, Y.; Xing, C.; Zhang, C. Recent Insights on the Role of Nuclear Receptors in Alzheimer’s Disease: Mechanisms and Therapeutic Application. Int. J. Mol. Sci. 2025, 26, 1207. [Google Scholar] [CrossRef]
- Islam, M.S.; Afrin, S.; Jones, S.I.; Segars, J. Selective progesterone receptor modulators—Mechanisms and therapeutic utility. Endocr. Rev. 2020, 41, bnaa012. [Google Scholar] [CrossRef]
- Simons, N.E.; Leeuw, M.; Van’t Hooft, J.; Limpens, J.; Roseboom, T.J.; Oudijk, M.A.; Pajkrt, E.; Finken, M.; Painter, R.C. The long-term effect of prenatal progesterone treatment on child development, behaviour and health: A systematic review. BJOG 2021, 128, 964–974. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Asoprisnil, a Selective Progesterone Receptor Modulator (SPRM), Inhibits Melanosome Export in B16F10 Cells and HEMn-DP Melanocytes. Molecules 2020, 25, 3581. [Google Scholar] [CrossRef]
- Jung, H.J.; Noh, S.G.; Ryu, I.Y.; Park, C.; Lee, J.Y.; Chun, P.; Moon, H.R.; Chung, H.Y. (E)-1-(Furan-2-yl)-(substituted phenyl)prop-2-en-1-one Derivatives as Tyrosinase Inhibitors and Melanogenesis Inhibition: An In Vitro and In Silico Study. Molecules 2020, 25, 5460. [Google Scholar] [CrossRef]
- Kim, O.; Park, E.Y.; Kwon, S.Y.; Shin, S.; Emerson, R.E.; Shin, Y.H.; DeMayo, F.J.; Lydon, J.P.; Coffey, D.M.; Hawkins, S.M.; et al. Targeting progesterone signaling prevents metastatic ovarian cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 31993–32004. [Google Scholar] [CrossRef]
- Addor, F.A.S.; Barcaui, C.B.; Gomes, E.E.; Lupi, O.; Marçon, C.R.; Miot, H.A. Sunscreen lotions in the dermatological prescription: Review of concepts and controversies. Bras. Dermatol. 2022, 97, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Hawwam, S.A.; Ismail, M.; El-Attar, Y.A. Split-face comparative study between intradermal tranexamic acid injection alone versus intradermal tranexamic acid injection combined with Q-switched Nd: YAG laser in melasma treatment: Dermoscopic and clinical evaluation. Lasers Med. Sci. 2022, 37, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Chuang, A.D.-C.; Lin, E.-T.; Chang, C.-C.; Huang, Y.-H.; Lu, M.-E.; Chuang, C.-F.; Chiang, H.-M. Comparison between the efficacy of cysteamine cream and combined hydroquinone cream in the treatment of melasma using skin analytical systems: An open-label quasi-randomized controlled trial in Asia patients. Dermatol. Sin. 2024, 42, 194–201. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, T.; Shen, N. Effect of platelet-rich plasma combined with tranexamic acid in the treatment of melasma and its effect on the serum levels of vascular endothelial growth factor, endothelin-1 and melatonin. Pak. J. Med. Sci. 2022, 38, 2163. [Google Scholar] [CrossRef] [PubMed]
| Hormone | Main Source(s) | Receptor(s) Involved | Key Signaling Pathways Activated | Effects on Melanogenesis | Additional Contributions (Oxidative Stress, Inflammation, Vascularization) |
|---|---|---|---|---|---|
| Estradiol (E2) | Ovaries, peripheral conversion | ER-α, ER-β, GPER | Genomic (ERE-mediated), Non-genomic (MAPK, cAMP, Ca2+) | ↑ TYR, TRP-1/2 expression; ↑ melanocyte proliferation; ↑ melanin deposition | Enhances skin thickness & moisture; paradoxically promotes hyperpigmentation |
| Estrone (E1) | Ovaries, adipose tissue (postmenopause) | ER-α, ER-β | cAMP/PKA, MAPK, MITF | ↑ TYR, TRP-1/2 expression; concentration-dependent effects on proliferation/migration | Modulates cell cycle (Cyclin D1, p21); ↑ melanocyte migration |
| Estriol (E3) | Placenta (during pregnancy) | ER (low affinity) | NF-κB, ROS-related pathways | Mild estrogenic effect; modulates proliferation/differentiation | ↑ ROS generation, aggravates oxidative stress contributing to melasma |
| Progesterone | Corpus luteum, placenta, adrenal cortex | PR-A, PR-B, membrane PRs | PI3K/Akt/GSK3β, CREB/MITF, Nrf2/Keap1 | ↑ TYR, TRP-1/2 expression; ↑ melanocyte proliferation & melanin synthesis | ↑ VEGF-mediated angiogenesis; ↑ IL-6/TNF-α inflammation; redox imbalance |
| 20α-DHP | Progesterone metabolite (ovary, placenta) | PR, MC1R-MITF axis | MITF nuclear translocation, cAMP/PKA | ↑ TYR, TYR1 expression; ↑ melanin biosynthesis | Pregnancy-associated elevation linked to melasma onset |
| Therapeutic Category | Example Agents | Efficacy | Safety Considerations |
|---|---|---|---|
| SERMs | Raloxifene, Bazedoxifene | Significant improvement in pigmentation scores after 12 weeks in clinical trials. | Generally well-tolerated; long-term safety in melasma treatment requires further study. |
| Aromatase Inhibitors | - | Effective in reducing hyperpigmentation by decreasing estrogenic activity. | Risk of skin dryness, thinning, or atrophy with excessive estrogen suppression. |
| SPRMs | Asoprisnil (AP) | Suppresses extracellular melanin levels in vitro; reduces melanocyte activity. | Local application minimizes systemic exposure; long-term dermatological safety under investigation. |
| 20-Hydroxyprogesterone Analogues | - | Potential to normalize pigmentation and reduce inflammation in melasma. | Topical formulations (e.g., nanocarriers) enhance safety and efficacy; minimal systemic absorption. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, T.; Li, Z.; Qin, C.; Dai, J.; Zhao, Y.; Wu, S.; Jia, Z. Hormonal Crosstalk in Melasma: Unraveling the Dual Roles of Estrogen and Progesterone in Melanogenesis. Int. J. Mol. Sci. 2025, 26, 10856. https://doi.org/10.3390/ijms262210856
Zhang J, Wang T, Li Z, Qin C, Dai J, Zhao Y, Wu S, Jia Z. Hormonal Crosstalk in Melasma: Unraveling the Dual Roles of Estrogen and Progesterone in Melanogenesis. International Journal of Molecular Sciences. 2025; 26(22):10856. https://doi.org/10.3390/ijms262210856
Chicago/Turabian StyleZhang, Jian, Tao Wang, Zhixian Li, Chuntang Qin, Jinjin Dai, Yihan Zhao, Shiguo Wu, and Zhuangzhuang Jia. 2025. "Hormonal Crosstalk in Melasma: Unraveling the Dual Roles of Estrogen and Progesterone in Melanogenesis" International Journal of Molecular Sciences 26, no. 22: 10856. https://doi.org/10.3390/ijms262210856
APA StyleZhang, J., Wang, T., Li, Z., Qin, C., Dai, J., Zhao, Y., Wu, S., & Jia, Z. (2025). Hormonal Crosstalk in Melasma: Unraveling the Dual Roles of Estrogen and Progesterone in Melanogenesis. International Journal of Molecular Sciences, 26(22), 10856. https://doi.org/10.3390/ijms262210856







