Dual Roles of CD147 in Regulating THP-1 Monocyte Migration and MCP-1-Induced Inflammatory Responses
Abstract
1. Introduction
2. Results
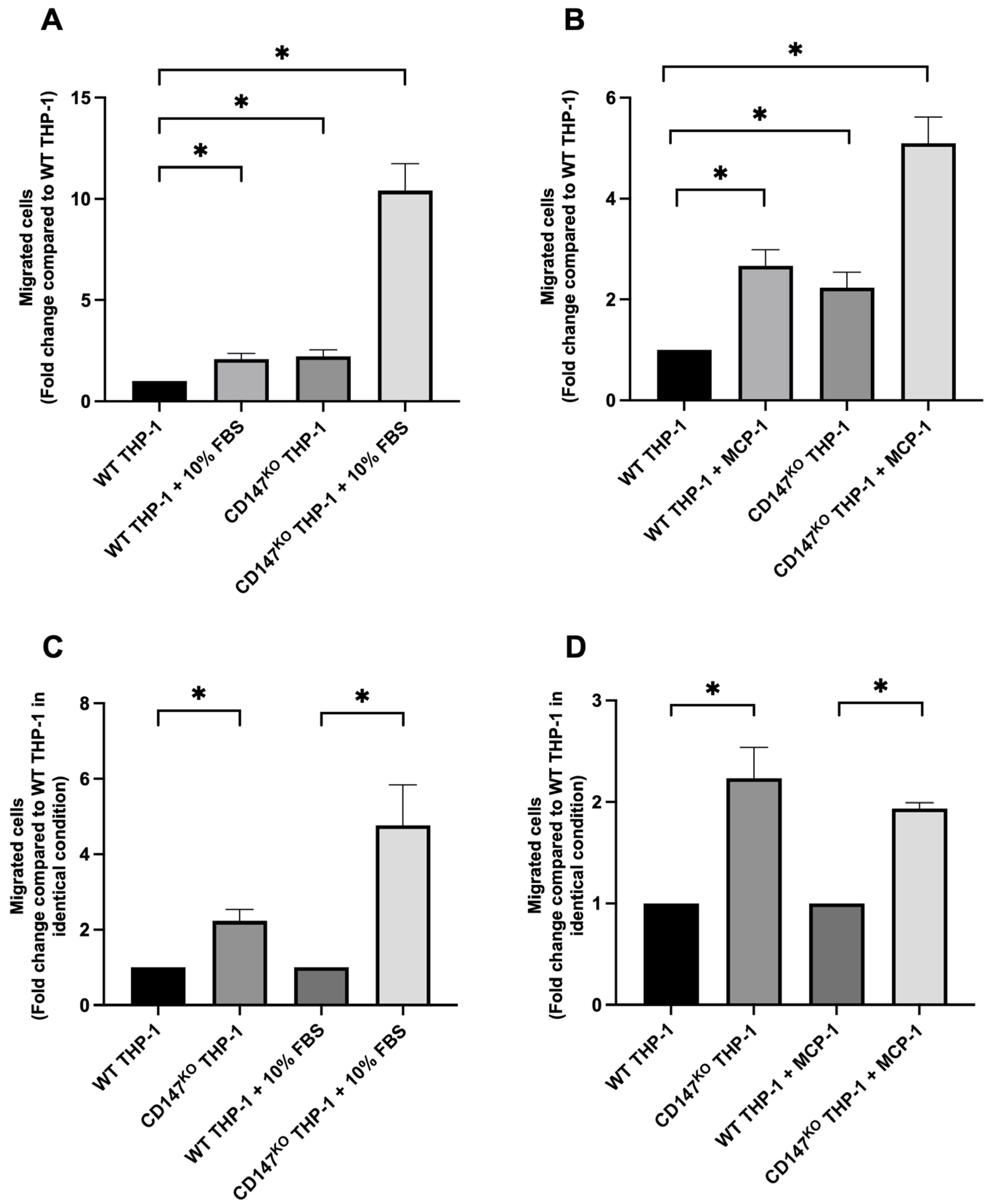
2.1. Chemotaxis Migration Assay of WT and CD147KO THP-1 Monocytes
2.2. Chemotaxis Migration Assay of WT THP-1 and CD147KO THP-1 Monocytes Toward Chemoattractants Secreted by a Breast Cancer Cell Line, MDA-MB-231
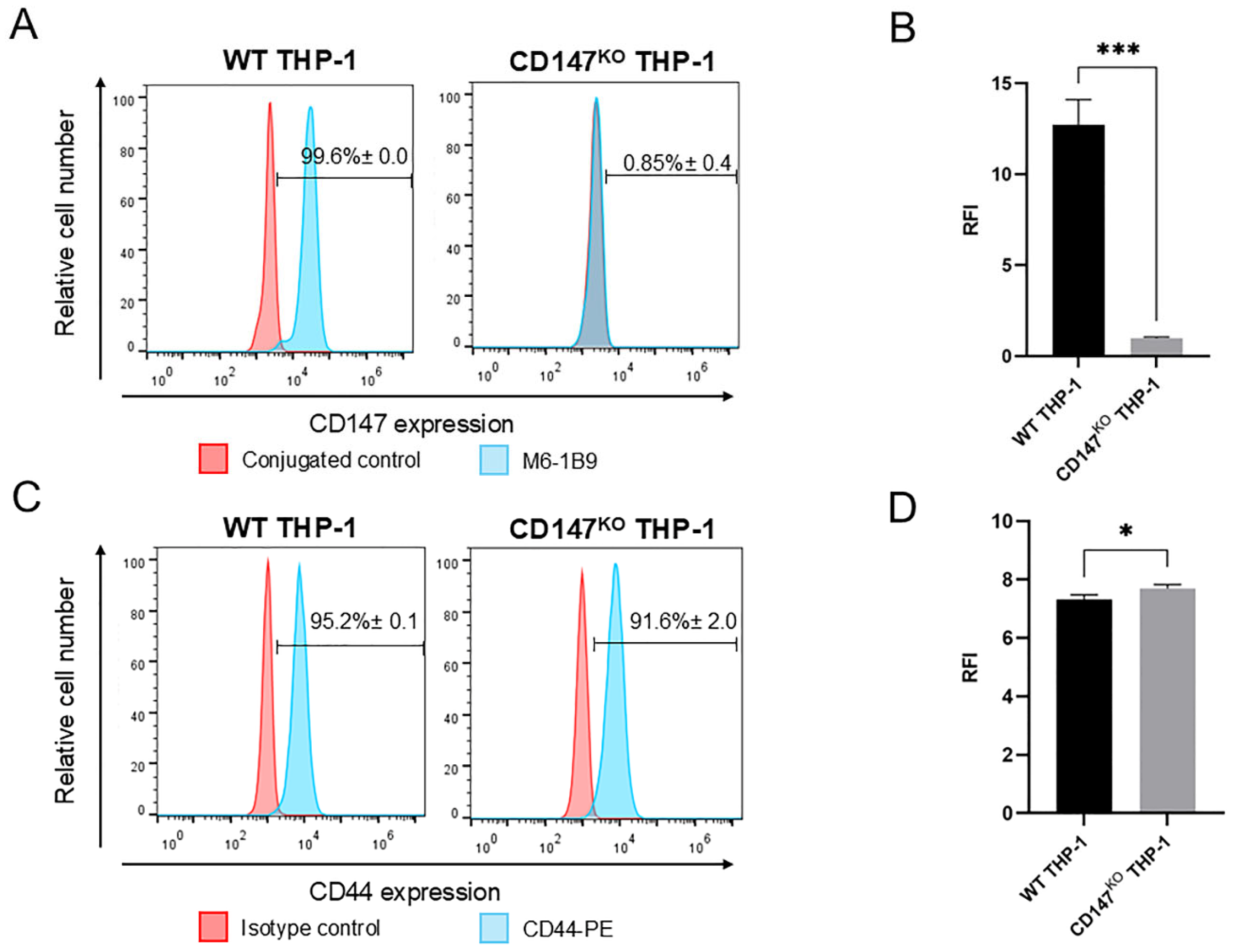
2.3. Expression of CD147 and CD44 on WT and CD147KO THP-1 Monocytes
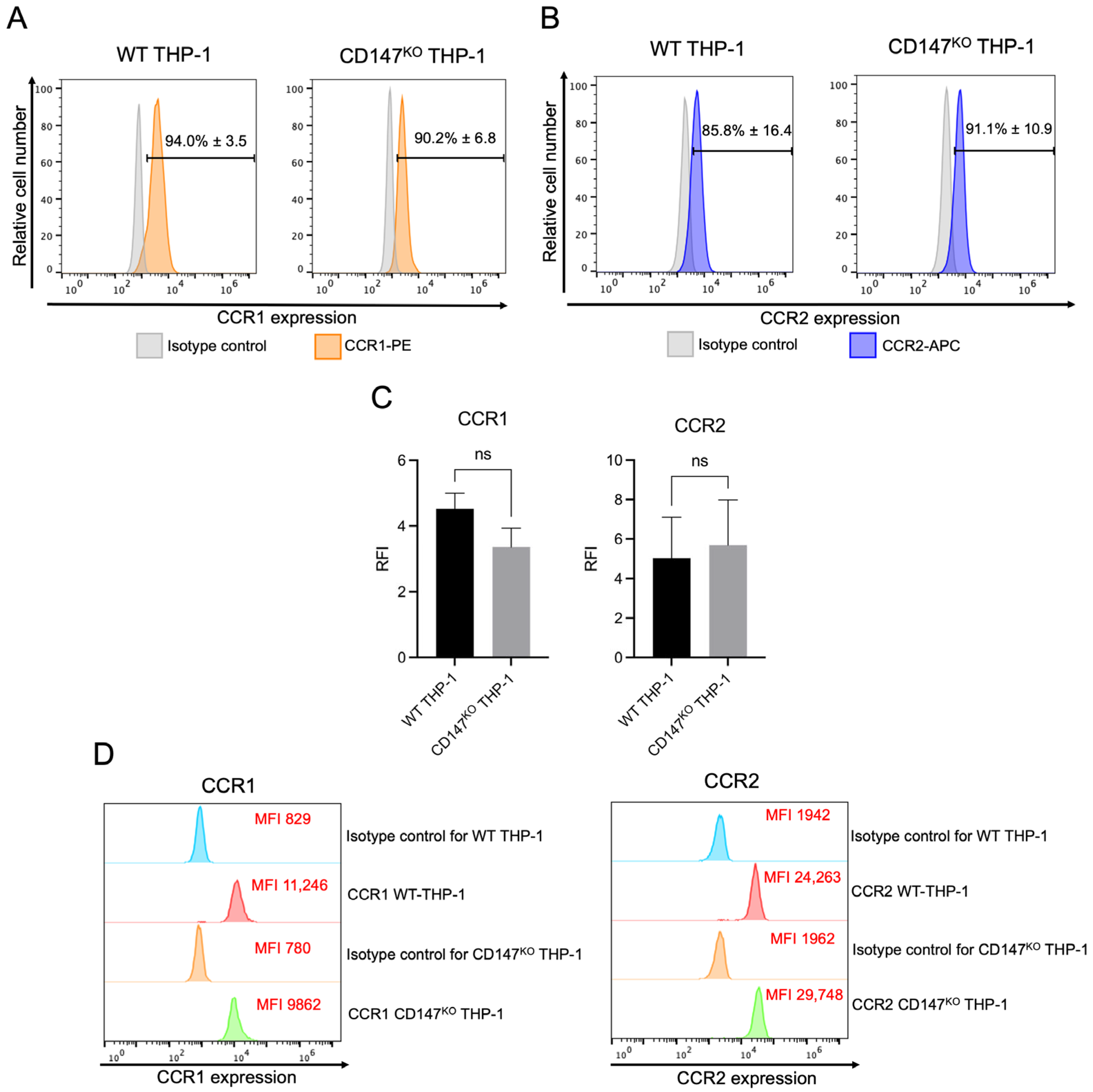
2.4. Cell Surface and Intracellular Protein Expression of CCR1 and CCR2 in WT and CD147KO THP-1 Monocytes
2.5. Expression of CCR1 and CCR2 mRNA in WT THP-1 and CD147KO THP-1 Monocytes
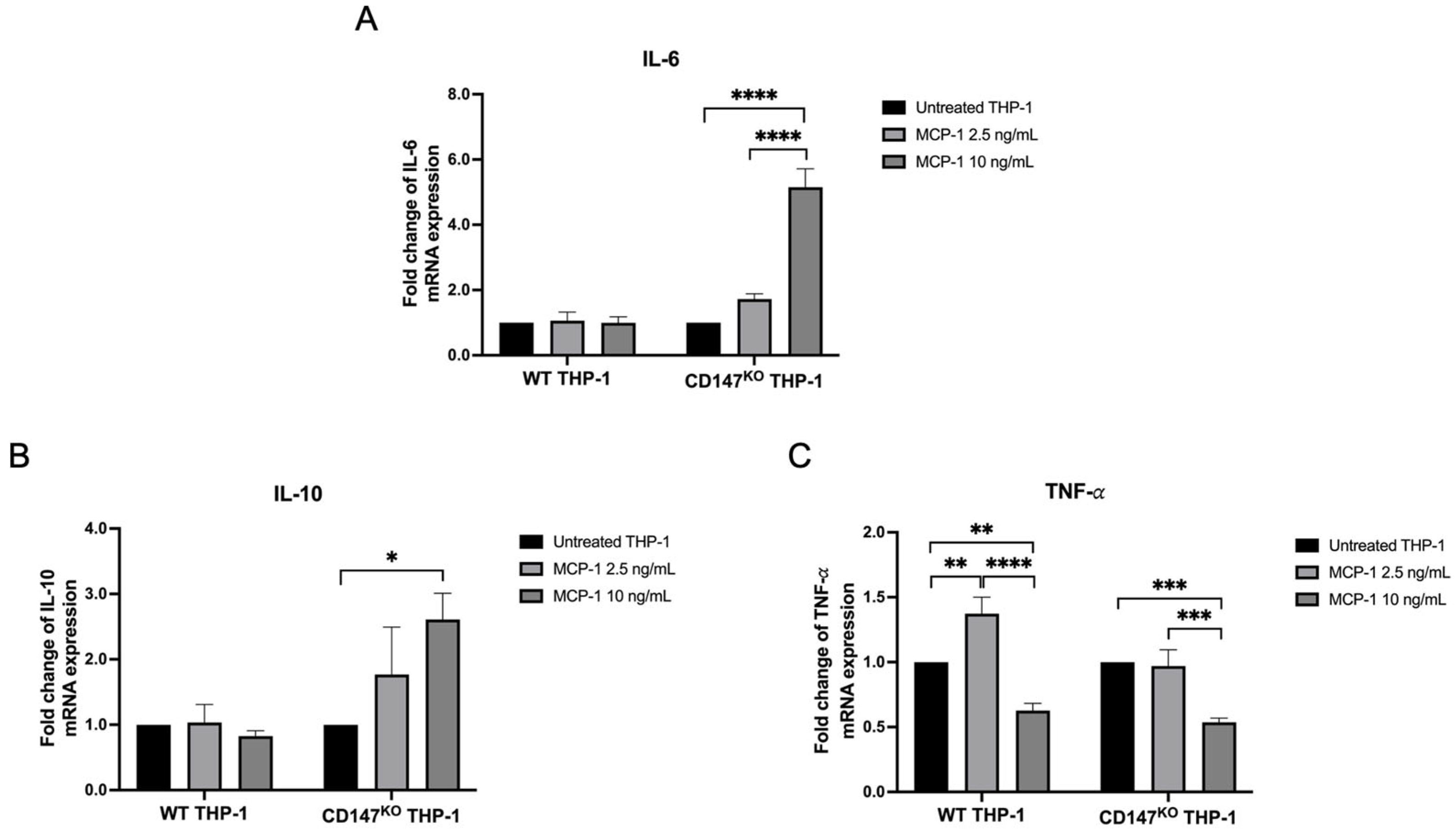
2.6. Inflammatory Cytokine Gene Expression on WT and CD147KO THP-1 Monocytes
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Transwell Migration Assay
4.3. Flow Cytometric Analysis for Expression of CD147, CD44, CCR1 and CCR2
4.4. Real-Time RT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- de la Cruz Concepcion, B.; Bartolo-Garcia, L.D.; Tizapa-Mendez, M.D.; Martinez-Velez, M.; Valerio-Diego, J.J.; Illades-Aguiar, B.; Salmeron-Barcenas, E.G.; Ortiz-Ortiz, J.; Torres-Rojas, F.I.; Mendoza-Catalan, M.A.; et al. EMMPRIN is an emerging protein capable of regulating cancer hallmarks. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6700–6724. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Yan, Y.Q.; Bai, C.Z.; Guo, J.Q. CD147 promotes breast cancer migration and invasion by inducing epithelial-mesenchymal transition via the MAPK/ERK signaling pathway. BMC Cancer 2023, 23, 1214. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Guo, J.; Jia, Y.; Han, Y.; Wang, Z. RNA interference targeting CD147 inhibits metastasis and invasion of human breast cancer MCF-7 cells by downregulating MMP-9/VEGF expression. Acta Biochim. Biophys. Sin. 2018, 50, 676–684. [Google Scholar] [CrossRef]
- Pan, S.; Su, Y.; Sun, B.; Hao, R.; Gao, X.; Han, B. Knockout of CD147 inhibits the proliferation, invasion, and drug resistance of human oral cancer CAL27 cells In Vitro and In Vivo. Int. J. Biol. Macromol. 2021, 181, 378–389. [Google Scholar] [CrossRef]
- Hibino, T.; Sakaguchi, M.; Miyamoto, S.; Yamamoto, M.; Motoyama, A.; Hosoi, J.; Shimokata, T.; Ito, T.; Tsuboi, R.; Huh, N.H. S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res. 2013, 73, 172–183. [Google Scholar] [CrossRef]
- Alexaki, V.I.; May, A.E.; Fujii, C.; SN, V.U.-S.; Mund, C.; Gawaz, M.; Chavakis, T.; Seizer, P. S100A9 induces monocyte/macrophage migration via EMMPRIN. Thromb. Haemost. 2017, 117, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jin, R.; Zhu, X.; Yan, J.; Li, G. Function of CD147 in atherosclerosis and atherothrombosis. J. Cardiovasc. Transl. Res. 2015, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Min, D.; Twigg, S.M.; Shackel, N.A.; Warner, F.J.; Yue, D.K.; McLennan, S.V. Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: Possible role in diabetic complications. Am. J. Physiol. Cell Physiol. 2010, 299, C1212–C1219. [Google Scholar] [CrossRef]
- Zhu, P.; Ding, J.; Zhou, J.; Dong, W.J.; Fan, C.M.; Chen, Z.N. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: Its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res. Ther. 2005, 7, R1023–R1033. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Melendez, A.J. Role of cell adhesion molecules and immune-cell migration in the initiation, onset and development of atherosclerosis. Cell Adhes. Migr. 2007, 1, 171–175. [Google Scholar] [CrossRef]
- Moh, M.C.; Shen, S. The roles of cell adhesion molecules in tumor suppression and cell migration: A new paradox. Cell Adhes. Migr. 2009, 3, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, J.W.; Li, Z.; Brass, D.M.; Garantziotis, S.; Timberlake, S.H.; Kim, A.; Hossain, I.; Savani, R.C.; Schwartz, D.A. CD44 regulates macrophage recruitment to the lung in lipopolysaccharide-induced airway disease. Am. J. Respir. Cell Mol. Biol. 2007, 37, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell. Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- White, G.E.; Iqbal, A.J.; Greaves, D.R. CC chemokine receptors and chronic inflammation--therapeutic opportunities and pharmacological challenges. Pharmacol. Rev. 2013, 65, 47–89. [Google Scholar] [CrossRef]
- Stone, M.J. Regulation of Chemokine-Receptor Interactions and Functions. Int. J. Mol. Sci. 2017, 18, 2415. [Google Scholar] [CrossRef]
- Callewaere, C.; Banisadr, G.; Rostene, W.; Parsadaniantz, S.M. Chemokines and chemokine receptors in the brain: Implication in neuroendocrine regulation. J. Mol. Endocrinol. 2007, 38, 355–363. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Bayik, D.; Tross, D.; Klinman, D.M. Factors Influencing the Differentiation of Human Monocytic Myeloid-Derived Suppressor Cells Into Inflammatory Macrophages. Front. Immunol. 2018, 9, 608. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Pamonsupornwichit, T.; Sornsuwan, K.; Juntit, O.A.; Yasamut, U.; Takheaw, N.; Kasinrerk, W.; Wanachantararak, P.; Kodchakorn, K.; Nimmanpipug, P.; Intasai, N.; et al. Engineered CD147-Deficient THP-1 Impairs Monocytic Myeloid-Derived Suppressor Cell Differentiation but Maintains Antibody-Dependent Cellular Phagocytosis Function for Jurkat T-ALL Cells with Humanized Anti-CD147 Antibody. Int. J. Mol. Sci. 2024, 25, 6626. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Staffler, G.; Huttinger, R.; Hilgert, I.; Prager, E.; Cerny, J.; Steinlein, P.; Majdic, O.; Horejsi, V.; Stockinger, H. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int. Immunol. 1999, 11, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Randolph, G.J. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006, 211, 609–618. [Google Scholar] [CrossRef]
- Kanjee, U.; Gruring, C.; Chaand, M.; Lin, K.M.; Egan, E.; Manzo, J.; Jones, P.L.; Yu, T.; Barker, R., Jr.; Weekes, M.P.; et al. CRISPR/Cas9 knockouts reveal genetic interaction between strain-transcendent erythrocyte determinants of Plasmodium falciparum invasion. Proc. Natl. Acad. Sci. USA 2017, 114, E9356–E9365. [Google Scholar] [CrossRef]
- Macanas-Pirard, P.; Quezada, T.; Navarrete, L.; Broekhuizen, R.; Leisewitz, A.; Nervi, B.; Ramirez, P.A. The CCL2/CCR2 Axis Affects Transmigration and Proliferation but Not Resistance to Chemotherapy of Acute Myeloid Leukemia Cells. PLoS ONE 2017, 12, e0168888. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Schulz, C.; von Bruhl, M.L.; Barocke, V.; Cullen, P.; Mayer, K.; Okrojek, R.; Steinhart, A.; Ahmad, Z.; Kremmer, E.; Nieswandt, B.; et al. EMMPRIN (CD147/basigin) mediates platelet-monocyte interactions in vivo and augments monocyte recruitment to the vascular wall. J. Thromb. Haemost. 2011, 9, 1007–1019. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cho, J.Y. Molecular association of CD98, CD29, and CD147 critically mediates monocytic U937 cell adhesion. Korean J. Physiol. Pharmacol. 2016, 20, 515–523. [Google Scholar] [CrossRef]
- Heinzmann, D.; Noethel, M.; Ungern-Sternberg, S.V.; Mitroulis, I.; Gawaz, M.; Chavakis, T.; May, A.E.; Seizer, P. CD147 is a Novel Interaction Partner of Integrin alphaMbeta2 Mediating Leukocyte and Platelet Adhesion. Biomolecules 2020, 10, 541. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Liu, Y.; He, Y.; Wang, W.; Du, Y.; Yang, C.; Gao, F. CD44 clustering is involved in monocyte differentiation. Acta Biochim. Biophys. Sin. 2014, 46, 540–547. [Google Scholar] [CrossRef]
- Zoller, M.; Gupta, P.; Marhaba, R.; Vitacolonna, M.; Freyschmidt-Paul, P. Anti-CD44-mediated blockade of leukocyte migration in skin-associated immune diseases. J. Leukoc. Biol. 2007, 82, 57–71. [Google Scholar] [CrossRef]
- Xu, H.; Manivannan, A.; Crane, I.; Dawson, R.; Liversidge, J. Critical but divergent roles for CD62L and CD44 in directing blood monocyte trafficking in vivo during inflammation. Blood 2008, 112, 1166–1174. [Google Scholar] [CrossRef]
- Wacker, M.; Ball, A.; Beer, H.D.; Schmitz, I.; Borucki, K.; Azizzadeh, F.; Scherner, M.; Awad, G.; Wippermann, J.; Veluswamy, P. Immunophenotyping of Monocyte Migration Markers and Therapeutic Effects of Selenium on IL-6 and IL-1beta Cytokine Axes of Blood Mononuclear Cells in Preoperative and Postoperative Coronary Artery Disease Patients. Int. J. Mol. Sci. 2023, 24, 7198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.N.; Campbell, J.J.; Salanga, C.L.; Ertl, L.S.; Wang, Y.; Yau, S.; Dang, T.; Zeng, Y.; McMahon, J.P.; Krasinski, A.; et al. CCR2-Mediated Uptake of Constitutively Produced CCL2: A Mechanism for Regulating Chemokine Levels in the Blood. J. Immunol. 2019, 203, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.; Cameroni, E.; Moepps, B.; Thelen, S.; Apuzzo, T.; Thelen, M. CCR2 acts as scavenger for CCL2 during monocyte chemotaxis. PLoS ONE 2012, 7, e37208. [Google Scholar] [CrossRef]
- Stone, M.J.; Hayward, J.A.; Huang, C.; Huma, Z.E.; Sanchez, J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int. J. Mol. Sci. 2017, 18, 342. [Google Scholar] [CrossRef] [PubMed]
- Garcia Lopez, M.A.; Aguado Martinez, A.; Lamaze, C.; Martinez, A.C.; Fischer, T. Inhibition of dynamin prevents CCL2-mediated endocytosis of CCR2 and activation of ERK1/2. Cell. Signal. 2009, 21, 1748–1757. [Google Scholar] [CrossRef]
- Li, H.; Wu, M.; Zhao, X. Role of chemokine systems in cancer and inflammatory diseases. MedComm 2022, 3, e147. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Hiraoka, M.; Nitta, N.; Nagai, M.; Shimokado, K.; Yoshida, M. MCP-1-induced enhancement of THP-1 adhesion to vascular endothelium was modulated by HMG-CoA reductase inhibitor through RhoA GTPase-, but not ERK1/2-dependent pathway. Life Sci. 2004, 75, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Israelsson, P.; Dehlin, E.; Nagaev, I.; Lundin, E.; Ottander, U.; Mincheva-Nilsson, L. Cytokine mRNA and protein expression by cell cultures of epithelial ovarian cancer-Methodological considerations on the choice of analytical method for cytokine analyses. Am. J. Reprod. Immunol. 2020, 84, e13249. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, S.; Kamohara, H.; Yoshimura, T. MCP-1 is selectively expressed in the late phase by cytokine-stimulated human neutrophils: TNF-alpha plays a role in maximal MCP-1 mRNA expression. J. Leukoc. Biol. 1999, 65, 671–679. [Google Scholar] [CrossRef]
- Yamamoto, T.; Eckes, B.; Mauch, C.; Hartmann, K.; Krieg, T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J. Immunol. 2000, 164, 6174–6179. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; Arefanian, H.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. ROS/TNF-alpha Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-kappaB and ERK1/2 Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 10519. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Biswas, P.; Delfanti, F.; Bernasconi, S.; Mengozzi, M.; Cota, M.; Polentarutti, N.; Mantovani, A.; Lazzarin, A.; Sozzani, S.; Poli, G. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood 1998, 91, 258–265. [Google Scholar] [CrossRef][Green Version]
- Weng, Y.S.; Tseng, H.Y.; Chen, Y.A.; Shen, P.C.; Al Haq, A.T.; Chen, L.M.; Tung, Y.C.; Hsu, H.L. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer 2019, 18, 42. [Google Scholar] [CrossRef]
- Zhao, X.; Rong, L.; Zhao, X.; Li, X.; Liu, X.; Deng, J.; Wu, H.; Xu, X.; Erben, U.; Wu, P.; et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J. Clin. Investig. 2012, 122, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.; Jauch-Speer, S.L.; Gandhi, S.; Vogl, T.; Roth, J.; Fehler, O. The roles of toll-like receptor 4, CD33, CD68, CD69, or CD147/EMMPRIN for monocyte activation by the DAMP S100A8/S100A9. Front. Immunol. 2023, 14, 1110185. [Google Scholar] [CrossRef]
- Zhu, C.; Xiong, Z.; Chen, X.; Lu, Z.; Zhou, G.; Wang, D.; Bao, J.; Hu, X. Soluble vascular endothelial growth factor (VEGF) receptor-1 inhibits migration of human monocytic THP-1 cells in response to VEGF. Inflamm. Res. 2011, 60, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.Q.; Chen, L.H.; Li, X.J.; Lin, Z.B.; Li, W.D. Sinomenine influences capacity for invasion and migration in activated human monocytic THP-1 cells by inhibiting the expression of MMP-2, MMP-9, and CD147. Acta Pharmacol. Sin. 2009, 30, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Campwala, H.; Sexton, D.W.; Crossman, D.C.; Fountain, S.J. P2Y(6) receptor inhibition perturbs CCL2-evoked signalling in human monocytic and peripheral blood mononuclear cells. J. Cell Sci. 2014, 127, 4964–4973. [Google Scholar] [CrossRef]
- Yoshimura, T.; Li, C.; Wang, Y.; Matsukawa, A. The chemokine monocyte chemoattractant protein-1/CCL2 is a promoter of breast cancer metastasis. Cell. Mol. Immunol. 2023, 20, 714–738. [Google Scholar] [CrossRef]
- Juntit, O.A.; Sornsuwan, K.; Yasamut, U.; Tayapiwatana, C. Integration of Image Pattern Recognition and Photon Sensor for Analyzing Cytokine Gene Expression Using piCode MicroDisc. Biosensors 2024, 14, 306. [Google Scholar] [CrossRef]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef]


| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| CCR1 | TGCTCTGCTCACACTCATGG | TCCAAAGCTGTCCGTTTGAT |
| CCR2 | TGACAGGCACAGATGAATGG | ATCATCTCCTGGCTGAATGC |
| IL-6 | AGACAGCCACTCACCTCTTC | AGTGCCTCTTTGCTGCTTTC |
| IL-10 | CCTGCCTAACATGCTTCGAG | GGCAACCCAGGTAACCCTTA |
| TNF-α | CTGCACTTTGGAGTGATCGG | TACAACATGGGCTACAGGCT |
| GAPDH | ACCCAGAAGACTGTGGATGG | CAGCTCAGGGATGACCTTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intasai, N.; Sornsuwan, K.; Juntit, O.-a.; Pamonsupornwichit, T.; Thongheang, K.; Jantaree, P.; Tayapiwatana, C. Dual Roles of CD147 in Regulating THP-1 Monocyte Migration and MCP-1-Induced Inflammatory Responses. Int. J. Mol. Sci. 2025, 26, 10850. https://doi.org/10.3390/ijms262210850
Intasai N, Sornsuwan K, Juntit O-a, Pamonsupornwichit T, Thongheang K, Jantaree P, Tayapiwatana C. Dual Roles of CD147 in Regulating THP-1 Monocyte Migration and MCP-1-Induced Inflammatory Responses. International Journal of Molecular Sciences. 2025; 26(22):10850. https://doi.org/10.3390/ijms262210850
Chicago/Turabian StyleIntasai, Nutjeera, Kanokporn Sornsuwan, On-anong Juntit, Thanathat Pamonsupornwichit, Kanyarat Thongheang, Phatcharida Jantaree, and Chatchai Tayapiwatana. 2025. "Dual Roles of CD147 in Regulating THP-1 Monocyte Migration and MCP-1-Induced Inflammatory Responses" International Journal of Molecular Sciences 26, no. 22: 10850. https://doi.org/10.3390/ijms262210850
APA StyleIntasai, N., Sornsuwan, K., Juntit, O.-a., Pamonsupornwichit, T., Thongheang, K., Jantaree, P., & Tayapiwatana, C. (2025). Dual Roles of CD147 in Regulating THP-1 Monocyte Migration and MCP-1-Induced Inflammatory Responses. International Journal of Molecular Sciences, 26(22), 10850. https://doi.org/10.3390/ijms262210850







