Research Status and Latest Progress in the Regulatory Mechanisms of ABCA1
Abstract
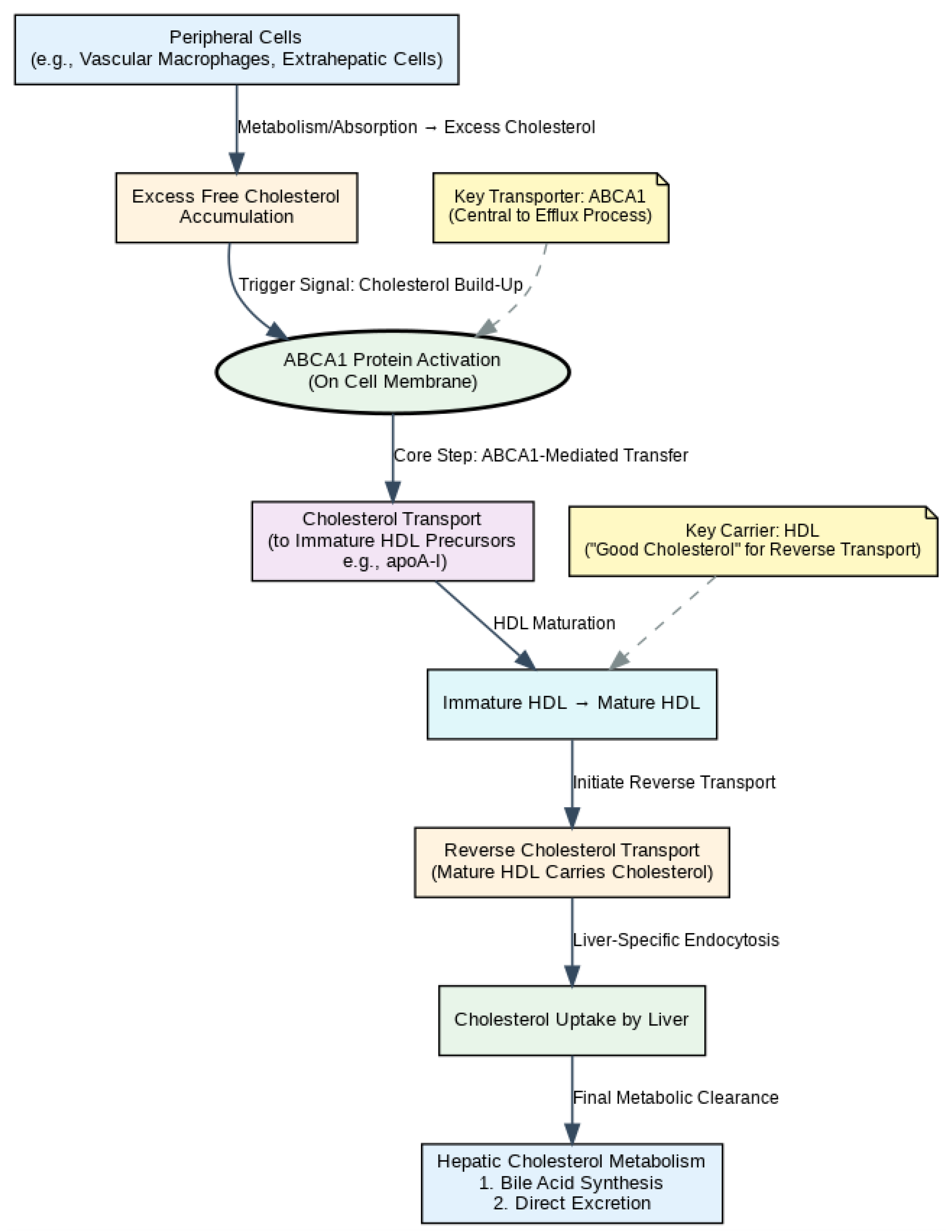
1. Introduction
2. ABCA1 Structure and Intracellular Trafficking
3. Factors Related to Affecting the Transcriptional Expression of ABCA1
3.1. LXRα/ABCA1 Pathway
3.2. MicroRNA (miR)
3.3. Non-Coding RNA (ncRNA)
3.4. Methylation
3.5. Acetylation
3.6. Other Factors
4. Factors Affecting Post-Translational Modification of ABCA1
4.1. Ubiquitin-Proteasome/Lysosome System
4.2. Calpain-Mediated Degradation Pathway
4.3. Phosphorylation
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zink, E.; Steinhäuser, J.; Blickle, P.-G.; von Meißner, W.C.G.; Strumann, C. Shift in therapeutic approaches in patients with hypercholesterolemia—A secondary data analysis. BMC Prim. Care 2025, 26, 287. [Google Scholar] [CrossRef]
- Aktar, A.; Vrieze, A.M.; Telesnicki, K.; Cox-Duvall, P.; Arbolino, M.; DeKoter, R.P.; Nagpal, A.D.; Heit, B. GATA2 induces a stem cell-like transcriptional program in macrophages that promotes an atherogenic phenotype. J. Leukocyte Biol. 2025, 117, qiaf136. [Google Scholar] [CrossRef]
- Feringa, F.M.; Koppes-den Hertog, S.J.; Wang, L.Y.; Derks, R.J.; Kruijff, I.; Erlebach, L.; Heijneman, J.; Miramontes, R.; Pömpner, N.; Blomberg, N.; et al. The neurolipid atlas: A lipidomics resource for neurodegenerative diseases. Nat. Metab. 2025, 7, 2142–2164. [Google Scholar] [CrossRef]
- Borràs, C.; Canyelles, M.; Santos, D.; Rotllan, N.; Núñez, E.; Vázquez, J.; Maspoch, D.; Cano-Sarabia, M.; Zhao, Q.; Carmona-Iragui, M.; et al. Cerebrospinal fluid lipoprotein-mediated cholesterol delivery to neurons is impaired in Alzheimer’s disease and involves APOE4. J. Lipid Res. 2025, 66, 100865. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Zou, H.; Weng, Y.; Liu, Y.F.; Li, W.; Yu, X.J.; Li, L.; Zheng, L.; Xu, J. Targeting SQLE-mediated cholesterol metabolism to enhance CD8+ T cell activation and immunotherapy efficacy in hepatocellular carcinoma. J. Immunother. Cancer 2025, 13, e012345. [Google Scholar] [CrossRef]
- Jia, H.F.; Ye, X.; Zhao, Y.; Dong, L.S. Mechanisms of Action, Medication Guidance and Adverse Reactions of Statins in the Prevention and Treatment of Strok. Chin. J. Drug Abus. Prev. Treat. 2023, 29, 1768–1770, 1779. [Google Scholar] [CrossRef]
- Tang, C.K. Targeting ABCA1 for the Prevention and Treatment of Atherosclerosis. Chin. J. Atheroscler. 2011, 19, 879–884. [Google Scholar] [CrossRef]
- An, F. ABCA1Study on the Correlation Between Gene Polymorphism, Methylation Modification and Early-Onset Coronary Heart Disease. Ph.D. Thesis, Tianjin Medical University, Tianjin, China, 2024. [Google Scholar] [CrossRef]
- Wang, S.; Smith, J.D. ABCA1 and nascent HDL biogenesis. Biofactors 2014, 40, 547–554. [Google Scholar] [CrossRef]
- Tanaka, A.R.; Kano, F.; Ueda, K.; Murata, M. The ABCA1 Q597R mutant undergoes trafficking from the ER upon ER stress. Biochem. Biophys. Res. Commun. 2008, 369, 1174–1178. [Google Scholar] [CrossRef]
- Neufeld, E.B.; Remaley, A.T.; Demosky, S.J.; Stonik, J.A.; Cooney, A.M.; Comly, M.; Dwyer, N.K.; Zhang, M.; Blanchette-Mackie, J.; Santamarina-Fojo, S.; et al. Cellular localization and trafficking of the human ABCA1 transporter*210. J. Biol. Chem. 2001, 276, 27584–27590. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.D.; Mäyränpää, M.I.; Peränen, J.; Pietilä, T.E.; Pietiäinen, V.M.; Uronen, R.-L.; Olkkonen, V.M.; Kovanen, P.T.; Ikonen, E. Rab8 regulates ABCA1 cell surface expression and facilitates cholesterol efflux in primary human macrophages. Arter. Thromb. Vasc. Biol. 2009, 29, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhou, C.; Neufeld, E.; Wang, Y.-H.; Xu, S.-W.; Lu, L.; Wang, Y.; Liu, Z.P.; Li, D.; Li, C.; et al. BIG1, a brefeldin a-inhibited guanine nucleotide-exchange protein modulates ATP-binding cassette transporter a-1 trafficking and function. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e31–e38. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Hong, L.; Jiang, H.H.; Liu, M.M.; Zhang, H.Z.; He, L.; Chen, W.D. Neuroprotective Effect of Naoluoxintong on Rats After Ischemic Stroke and Its Correlation with LXRα Activation. China Med. Her. 2025, 22, 20–28. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Zhang, J.; Liu, C.; Li, Y.; Feng, T.; Xu, Y.; Si, S. Identification of a novel partial agonist of liver X receptor α (LXRα) via screening. Biochem. Pharmacol. 2014, 92, 438–447. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, H.; Yu, S.H.; Liu, J.L.; Lu, M.Y.; Zhang, J.J. Effect of Jianpi Qushi Decoction on the PPARγ/LXRα/ABCA1 Pathway in Rats with Dyslipidemia of Spleen Deficiency and Dampness Excess Type. China J. Tradit. Chin. Med. Pharm. 2025, 40, 3088–3092. [Google Scholar]
- Singh, A.B.; Dong, B.; Kraemer, F.B.; Liu, J. FXR activation promotes intestinal cholesterol excretion and attenuates hyperlipidemia in SR-B1-deficient mice fed a high-fat and high-cholesterol diet. Physiol. Rep. 2020, 8, e14387. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Qian, T.; Wang, T.; Su, Y.; Qiu, H.; Tang, W.; Xing, Q.; Wang, L. T0901317, a liver X receptor agonist, ameliorates perinatal white matter injury induced by ischemia and hypoxia in neonatal rats. Neurosci. Lett. 2023, 793, 136994. [Google Scholar] [CrossRef]
- Zhou, E.; Ge, X.; Nakashima, H.; Li, R.; van der Zande, H.J.P.; Liu, C.; Li, Z.; Müller, C.; Bracher, F.; Mohammed, Y.; et al. Inhibition of DHCR24 activates LXRα to ameliorate hepatic steatosis and inflammation. EMBO Mol. Med. 2023, 15, e16845. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.-Y.; Byeon, H.-E.; Kim, J.-W.; Kim, H.-A.; Suh, C.-H.; Choi, S.; Linton, M.F.; Jung, J.Y. Inhibition of toll-like receptors alters macrophage cholesterol efflux and foam cell formation. Int. J. Mol. Sci. 2024, 25, 6808. [Google Scholar] [CrossRef]
- Wong, J.; Quinn, C.M.; Brown, A.J. SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligands for LXR. Biochem. J. 2006, 400, 485–491. [Google Scholar] [CrossRef]
- Hu, X.; Fu, Y.; Lu, X.; Zhang, Z.; Zhang, W.; Cao, Y.; Zhang, N. Protective effects of platycodin D on lipopolysaccharide-induced acute lung injury by activating LXRα-ABCA1 signaling pathway. Front. Immunol. 2016, 7, 644. [Google Scholar] [CrossRef]
- Mustra Rakic, J.; Liu, C.; Veeramachaneni, S.; Wu, D.; Paul, L.; Chen, C.-Y.O.; Ausman, L.M.; Wang, X.D. Lycopene inhibits smoke-induced chronic obstructive pulmonary disease and lung carcinogenesis by modulating reverse cholesterol transport in ferrets. Cancer Prev. Res. 2019, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-T.; Li, C.-H.; Dai, T.-T.; Tao, F.-L.; Wang, M.-W.; Wang, C.-Y.; Han, Z.L.; Sun, N.X.; Zhao, Y.N.; Wang, D.L. Effects of allyl isothiocyanate on the expression, function, and its mechanism of ABCA1 and ABCG1 in pulmonary of COPD rats. Int. Immunopharmacol. 2021, 101, 108373. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhao, Q.; Wang, X.; Chen, X.; Hou, L.; Tian, S.; Peng, Z.M.; Han, X.J.; Wang, T.; et al. PILRB potentiates the PI3K/AKT signaling pathway and reprograms cholesterol metabolism to drive gastric tumorigenesis and metastasis. Cell Death Dis. 2024, 15, 642. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, G.; Bin, Y.; Bai, R.; Liang, B.; Yang, H. C1q/tumor necrosis factor-related protein-9 enhances macrophage cholesterol efflux and improves reverse cholesterol transport via AMPK activation. Biochem. Genet. 2025, 63, 1620–1634. [Google Scholar] [CrossRef]
- Mangum, L.C.; Hou, X.; Borazjani, A.; Lee, J.H.; Ross, M.K.; Crow, J.A. Silencing carboxylesterase 1 in human THP-1 macrophages perturbs genes regulated by PPARγ/RXR and RAR/RXR: Downregulation of CYP27A1-LXRα signaling. Biochem. J. 2018, 475, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, Z.; Li, L.; He, L.; Wu, X.; Zhang, M.; Zhu, P. Neuropeptide Y regulates cholesterol uptake and efflux in macrophages and promotes foam cell formation. J. Cell Mol. Med. 2022, 26, 5391–5402. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Tang, S.-L.; Lv, Y.-C.; Ouyang, X.-P.; He, P.-P.; Yao, F.; Tang, Y.Y.; Zhang, M.; Tang, Y.L.; Tang, D.P.; et al. NF-κB suppresses the expression of ATP-binding cassette transporter A1/G1 by regulating SREBP-2 and miR-33a in mice. Int. J. Cardiol. 2014, 171, e93–e95. [Google Scholar] [CrossRef]
- Fukunaga, K.; Imachi, H.; Lyu, J.; Dong, T.; Sato, S.; Ibata, T.; Kobayashi, T.; Yoshimoto, T.; Yonezaki, K.; Matsunaga, T.; et al. IGF1 suppresses cholesterol accumulation in the liver of growth hormone-deficient mice via the activation of ABCA1. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1232–E1241. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, Y.; Peng, F.; Li, M.; Mo, Z. Midkine promote atherosclerosis by regulating the expression of ATP-binding cassette transporter A1 via activator protein-1. Cardiovasc. Drugs Ther. 2025. [Google Scholar] [CrossRef]
- Nyandwi, J.-B.; Ko, Y.S.; Jin, H.; Yun, S.P.; Park, S.W.; Kim, H.J. Rosmarinic acid increases macrophage cholesterol efflux through regulation of ABCA1 and ABCG1 in different mechanisms. Int. J. Mol. Sci. 2021, 22, 8791. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Cifuentes, D.; Xue, H.; Taylor, D.W.; Patnode, H.; Mishima, Y.; Cheloufi, S.; Ma, E.; Mane, S.; Hannon, G.J.; Lawson, N.D.; et al. A novel miRNA processing pathway independent of dicer requires Argonaute2 catalytic activity. Science 2010, 328, 1694–1698. [Google Scholar] [CrossRef]
- Chen, X.T.; Yang, J.B.; Zhou, Y.Y.; Yang, S.H.; Yang, R.H.; Fang, S.J.; Ma, Y.X.; Niu, W.Y. Research Progress on the Regulatory Mechanisms of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase. Chin. J. Pathophysiol. 2025, 41, 791–797. [Google Scholar]
- Chowdhari, S.; Deep, A.; Ahmad, B.; Samal, J.; Gupta, E.; Vivekanandan, P. HBV-miR-3 induces hepatic cholesterol accumulation by targeting ABCA1: Evidence for potential benefits of statin usage. J. Lipid Res. 2025, 66, 100866. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Ge, Z.; Shi, J.; Wang, T.; Zhang, J.; Zhang, X.; Jiang, H.; Zhou, L.; Ye, L. Effects of miR-200b-3p and miR-424–5p regulating notch signaling pathway on atherosclerosis induced by PM2.5 in vitro. Toxicology 2025, 517, 154216. [Google Scholar] [CrossRef]
- Horie, T.; Ono, K.; Horiguchi, M.; Nishi, H.; Nakamura, T.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Marusawa, H.; Iwanaga, Y.; et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 17321–17326. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, L.; Vales-Lara, F.M.; Fenstermaker, M.; Cirera-Salinas, D.; Chamorro-Jorganes, A.; Ramírez, C.M.; Mattison, J.A.; de Cabo, R.; Suárez, Y.; Fernández-Hernando, C. A regulatory role for microRNA 33* in controlling lipid metabolism gene expression. Mol. Cell Biol. 2013, 33, 2339–2352. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Pokhrel, A.; Echesabal-Chen, J.; Scott, J.; Bruce, T.; Jo, H.; Stamatikos, A. Inhibiting MiR-33a-3p expression fails to enhance ApoAI-mediated cholesterol efflux in pro-inflammatory endothelial cells. Medicina 2025, 61, 329. [Google Scholar] [CrossRef]
- Ou, Z.; Cheng, Y.; Ma, H.; Chen, K.; Lin, Q.; Chen, J.; Guo, R.; Huang, Z.; Cheng, Q.; Alaeiilkhchi, N.; et al. miR-223 accelerates lipid droplets clearance in microglia following spinal cord injury by upregulating ABCA1. J. Transl. Med. 2024, 22, 659. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Jun, J.H.; Kim, J.Y.; Park, H.J.; Cho, Y.P.; Kim, G.J. Expression of miRNAs targeting ATP binding cassette transporter 1 (ABCA1) among patients with significant carotid artery stenosis. Biomedicines 2021, 9, 920. [Google Scholar] [CrossRef]
- Zhu, M.; Jia, L.; Jia, J. Inhibition of miR-96-5p may reduce Aβ42/Aβ40 ratio via regulating ATP-binding cassette transporter A1. J. Alzheimer’s Dis. JAD 2021, 83, 367–377. [Google Scholar] [CrossRef]
- Ma, L.; He, S.; Li, H.; Zhang, S.; Yin, Y. HAND2-AS1 targeting miR-1208/SIRT1 axis alleviates foam cell formation in atherosclerosis. Int. J. Cardiol. 2022, 346, 53–61. [Google Scholar] [CrossRef]
- Torres-Paz, Y.E.; Gamboa, R.; Fuentevilla-Álvarez, G.; Cardoso-Saldaña, G.; Martínez-Alvarado, R.; Soto, M.E.; Huesca-Gómez, C. Involvement of expression of miR33-5p and ABCA1 in human peripheral blood mononuclear cells in coronary artery disease. Int. J. Mol. Sci. 2024, 25, 8605. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, X.; Hu, J.; Liu, X.; Guo, Z.; Wu, J.; Shao, Y.; Hao, M.; Zhang, S.; Hu, W.; et al. The translational potential of miR-26 in atherosclerosis and development of agents for its target genes ACC1/2, COL1A1, CPT1A, FBP1, DGAT2, and SMAD7. Cardiovasc. Diabetol. 2024, 23, 1–21. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Shao, X.; Huang, S.; Wang, J.; Zhou, S.; Liu, H.; Lin, Y.; Yu, P. GLP-1RA improves diabetic renal injury by alleviating glomerular endothelial cells pyrotosis via RXRα/circ8411/miR-23a-5p/ABCA1 pathway. PLoS ONE 2024, 19, e0314628. [Google Scholar] [CrossRef]
- Xu, F.; Shen, L.; Chen, H.; Wang, R.; Zang, T.; Qian, J.; Ge, J. circDENND1B participates in the antiatherosclerotic effect of IL-1β monoclonal antibody in mouse by promoting cholesterol efflux via miR-17-5p/Abca1 axis. Front. Cell Dev. Biol. 2021, 9, 652032. [Google Scholar] [CrossRef] [PubMed]
- Sallam, T.; Jones, M.; Thomas, B.J.; Wu, X.; Gilliland, T.; Qian, K.; Eskin, A.; Casero, D.; Zhang, Z.; Sandhu, J.; et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat. Med. 2018, 24, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Ren, S.; Ji, H.; Ding, X.; Zou, P.; Lu, J. The lncRNA DAPK-IT1 regulates cholesterol metabolism and inflammatory response in macrophages and promotes atherogenesis. Biochem. Biophys. Res. Commun. 2019, 516, 1234–1241. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Wang, Y.; An, J.-H.; Tang, W.-Y.; Xu, X.-D.; Ren, K. LncRNA DANCR promotes macrophage lipid accumulation through modulation of membrane cholesterol transporters. Aging 2024, 16, 12510–12524. [Google Scholar] [CrossRef]
- Fu, C.L.; Lin, Y.M.; Tu, H.S.; Ye, J.X.; Huang, Y.F.; Ma, D.Z.; Zheng, C.S. Mechanism of Tougu Xiaotong Capsules in delaying degeneration of osteoarthritis by regulating cholesterol metabolism in chondrocytes through lncRNA MALAT1. Zhongguo Zhong Yao Za Zhi 2024, 49, 1785–1792. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, Y.; Chen, H.; Tang, X.; Zhao, H.; Meng, Z.; Jia, X.; Liu, W.; Li, X.; Wang, L.; et al. Genetic dissection of the impact of lncRNA AI662270 during the development of atherosclerosis. J. Transl. Med. 2023, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, R.; Jiang, Y.; Li, Y.; Lin, L.; Liu, Z.; Zhao, Y.; Shen, H.; Hu, Z.; Wei, Y.; et al. Comprehensive analyses of m6A regulators and interactive coding and non-coding RNAs across 32 cancer types. Mol. Cancer 2021, 20, 67. [Google Scholar] [CrossRef]
- Lin, Y.; Li, J.; Liang, S.; Chen, Y.; Li, Y.; Cun, Y.; Tian, L.; Zhou, Y.; Chen, Y.; Chu, J.; et al. Pan-cancer analysis reveals m6A variation and cell-specific regulatory network in different cancer types. Genom. Proteom. Bioinform. 2024, 22, qzae052. [Google Scholar] [CrossRef]
- Zhou, E.Y.; Yang, C.; Li, W.M.; Ran, D.C.; Xu, J.M.; Wang, C.Q. m6A Methylation and Osteoporosis. Chin. J. Osteoporos. 2025, 31, 1377–1382. [Google Scholar]
- Zhang, W.H.; Jia, W.Y.; Wang, C.R.; Jiang, Y.J.; Yi, D.; Gong, Y.B. Research Progress of N6-Methyladenine Methylation in Type 2 Diabetes Mellitus. Chin. J. Diabetes 2025, 33, 314–316. [Google Scholar]
- Wang, T.; Kong, S.; Tao, M.; Ju, S. The potential role of RNA N6-methyladenosine in cancer progression. Mol. Cancer 2020, 19, 88. [Google Scholar] [CrossRef]
- Xu, S.; Liu, K.; Chen, Z.; Tang, W.; Chen, Z. IGF2BP1-mediated methylation of ABCA1 facilitates tumor progression by affecting cholesterol metabolism in lung adenocarcinoma. Amino Acids 2025, 57, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, S.; Liu, H.; Liu, Y.; Zhang, Z.; Zhou, Z.; Wang, P.; Qi, S.; Xie, J. ALKBH5 facilitates the progression of skin cutaneous melanoma via mediating ABCA1 demethylation and modulating autophagy in an m6A-dependent manner. Int. J. Biol. Sci. 2024, 20, 1729–1743. [Google Scholar] [CrossRef]
- Zhang, Y.K. Valeric Acid Regulates MUC2, Downregulates ALKBH5, Promotes m6A Modification of ABCA1 mRNA, and Alleviates Atherosclerosis. Master’s Thesis, University of South China, Hengyang, China, 2025. [Google Scholar] [CrossRef]
- Zhou, T. Study on the Relationship Between Arsenic Exposure and DNA Methylation in the Promoter Region of ABCA1 Gene. Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2019. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201901&filename=1018799099.nh (accessed on 1 October 2025).
- Wang, H.Y.; Zhang, Z.J.; Yuan, F.Y.; Zeng, W.Q.; Ma, X.; Zhai, X.J.; Gao, S.L. Effect of Homocysteine on Promoter Methylation Level and Expression of ATP-Binding Cassette Transporter A1 in Human Aortic Smooth Muscle Cells. China Med. Her. 2018, 15, 19–22. [Google Scholar]
- Tang, Y.Y. EZH2 Mediates DNA and Histone Methylation in the ABCA1 Promoter Region to Promote the Progression of Atherosclerosis. Master’s Thesis, University of South China, Hengyang, China, 2015. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201501&filename=1014410781.nh (accessed on 1 October 2025).
- Luo, W.; Meng, J.; Yu, X.H.; Zhang, Z.Z.; Wang, G.; He, J. Indole-3-carboxaldehyde inhibits inflammatory response and lipid accumulation in macrophages through the miR-1271-5p/HDAC9 pathway. J. Cell Mol. Med. 2024, 28, e70263. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yi, K.; Tu, J.; Li, F.J.; Chen, W.J.; Xiao, X.H.; Jiang, Z.S.; Tang, C.K. Berberine Regulates Deacetylation of Liver X Receptor α to Promote ATP-Binding Cassette Transporter A1 Expression and Cholesterol Efflux in THP-1 Macrophages. Chin. J. Arterioscler. 2012, 20, 1074–1078. [Google Scholar] [CrossRef]
- Zhang, L.H.; Kamanna, V.S.; Ganji, S.H.; Xiong, X.M.; Kashyap, M.L. Niacin increases HDL biogenesis by enhancing DR4-dependent transcription of ABCA1 and lipidation of apolipoprotein a-I in HepG2 cells. J. Lipid Res. 2012, 53, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Mäyränpää, M.I.; Wong, J.; Perttilä, J.; Lehto, M.; Jauhiainen, M.; Kovanen, P.T.; Ehnholm, C.; Brown, A.J.; Olkkonen, V.M. OSBP-related protein 8 (ORP8) suppresses ABCA1 expression and cholesterol efflux from macrophages. J. Biol. Chem. 2008, 283, 332–340. [Google Scholar] [CrossRef]
- Langmann, T.; Schumacher, C.; Morham, S.G.; Honer, C.; Heimerl, S.; Moehle, C.; Schmitz, G. ZNF202 is inversely regulated with its target genes ABCA1 and apoE during macrophage differentiation and foam cell formation. J. Lipid Res. 2003, 44, 968–977. [Google Scholar] [CrossRef][Green Version]
- Langmann, T.; Porsch-Ozcürümez, M.; Heimerl, S.; Probst, M.; Moehle, C.; Taher, M.; Borsukova, H.; Kielar, D.; Kaminski, W.E.; Dittrich-Wengenroth, E.; et al. Identification of sterol-independent regulatory elements in the human ATP-binding cassette transporter A1 promoter: Role of Sp1/3, E-box binding factors, and an oncostatin M-responsive element. J. Biol. Chem. 2002, 277, 14443–14450. [Google Scholar] [CrossRef]
- Wang, D.; Yeung, A.W.K.; Atanasov, A.G. A review: Molecular mechanism of regulation of ABCA1 expression. Curr. Protein Pept. Sci. 2022, 23, 170–191. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Wu, J.; Xia, C.; Lala, D.S. Liver X receptors interact with corepressors to regulate gene expression. Mol. Endocrinol. 2003, 17, 1019–1026. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Moser, A.H.; Shigenaga, J.K.; Grunfeld, C.; Feingold, K.R. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: Differential role of LXR. J. Lipid Res. 2003, 44, 1728–1736. [Google Scholar] [CrossRef]
- Thottakkattumana Parameswaran, V.; Hild, C.; Eichner, G.; Ishaque, B.; Rickert, M.; Steinmeyer, J. Interleukin-1 induces the release of lubricating phospholipids from human osteoarthritic fibroblast-like synoviocytes. Int. J. Mol. Sci. 2022, 23, 2409. [Google Scholar] [CrossRef]
- Schmitz, G.; Langmann, T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta. 2005, 1735, 1–19. [Google Scholar] [CrossRef]
- Kato, T.; Kawahito, H.; Kishida, S.; Irie, D.; Wakana, N.; Kikai, M.; Takata, H.; Ogata, T.; Ueyama, T.; Matoba, S.; et al. Bone marrow angiotensin AT2 receptor deficiency aggravates atherosclerosis development by eliminating macrophage liver X receptor-mediated anti-atherogenic actions. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Q.; Yang, J.; Ma, Y.; Ding, G. Angiotensin II induces cholesterol accumulation and injury in podocytes. Sci. Rep. 2017, 7, 10672. [Google Scholar] [CrossRef]
- Huuskonen, J.; Vishnu, M.; Pullinger, C.R.; Fielding, P.E.; Fielding, C.J. Regulation of ATP-binding cassette transporter A1 transcription by thyroid hormone receptor. Biochemistry 2004, 43, 1626–1632. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Yang, Y. Roles of Insulin-Like Growth Factor-1 and Inflammatory Factors in Diabetic Peripheral Neuropathy of Type 2 Diabetes Mellitus. Chin. J. Microcirc. 2025, 35, 101–105. [Google Scholar]
- Lyu, J.; Imachi, H.; Iwama, H.; Zhang, H.; Murao, K. Insulin-like growth factor 1 regulates the expression of ATP-binding cassette transporter A1 in pancreatic beta cells. Horm. Metab. Res. 2016, 48, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.; Zhou, Y.; Wang, Q.; Xue, S.; Zhang, Y.; Niu, W. Research progress and prospects of flavonoids in the treatment of hyperlipidemia: A narrative review. Molecules 2025, 30, 3103. [Google Scholar] [CrossRef]
- Liu, R.; Liu, P.; Li, Z.; Luo, C.Y.; Li, Z.T.; Kang, Y. Effect of Zhige Baogan Jiangzhi Formula on Cholesterol Metabolism in Rats with Alcoholic Liver Disease by Regulating the PPARα-LXRα-ABCA1 Signaling Pathway. Chin. Tradit. Pat. Med. 2024, 46, 4164–4169. [Google Scholar]
- Borràs, C.; Rotllan, N.; Griñán, R.; Santos, D.; Solé, A.; Dong, C.; Zhao, Q.; Llorente-Cortes, V.; Mourín, M.; Soto, B.; et al. Restoring cholesterol efflux in vascular smooth muscle cells transitioning into foam cells through liver X receptor activation. Biomed. Pharmacother. 2025, 188, 118178. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Su, X.; Cao, A.; Chen, F.; Chen, P.; Yan, F.; Hu, H. SREBP2 promotes the viability, proliferation, and migration and inhibits apoptosis in TGF-β1-induced airway smooth muscle cells by regulating TLR2/NF-κB/NFATc1/ABCA1 regulatory network. Bioengineered 2022, 13, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Hank, E.; Giera, M.; Bracher, F. Dehydrocholesterol reductase 24 (DHCR24): Medicinal chemistry, pharmacology and novel therapeutic options. Curr. Med. Chem. 2022, 29, 4005–4025. [Google Scholar] [CrossRef]
- Fu, Y.; Xin, Z.; Liu, B.; Wang, J.; Wang, J.; Zhang, X.; Wang, Y.; Li, F. Platycodin D inhibits inflammatory response in LPS-stimulated primary rat microglia cells through activating LXRα-ABCA1 signaling pathway. Front. Immunol. 2017, 8, 1929. [Google Scholar] [CrossRef]
- Bao, R.K. Study on Lycopene Antagonizing DEHP-Induced Hepatotoxicity by Regulating Lipid Metabolism. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2022. [Google Scholar] [CrossRef]
- Lei, S.Y. Study on the Role and Mechanism of CTRP9 in Macrophage Apoptosis and Cholesterol Reverse Transport. Ph.D. Thesis, Shandong University, Jinan, China, 2021. [Google Scholar] [CrossRef]
- Fu, H.; Tang, Y.Y.; Ouyang, X.P.; Tang, S.L.; Su, H.; Li, X.; Huang, L.P.; He, M.; Lv, Y.C.; He, P.P.; et al. Interleukin-27 inhibits foam cell formation by promoting macrophage ABCA1 expression through JAK2/STAT3 pathway. Biochem. Biophys. Res. Commun. 2014, 452, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Jiang, X.; Jiang, C.R.; Gong, H.Q. Peroxiredoxin 2 Inhibits Macrophage Lipid Accumulation via the ROS-NF-κB-miR-33a-ABCA1 Pathway. Chin. J. Biochem. Mol. Biol. 2020, 36, 1446–1454. [Google Scholar] [CrossRef]
- Oladosu, O.; Chin, E.; Barksdale, C.; Powell, R.R.; Bruce, T.; Stamatikos, A. Inhibition of miR-33a-5p in macrophage-like cells In vitro promotes apoAI-mediated cholesterol efflux. Pathophysiology. 2024, 31, 117–126. [Google Scholar] [CrossRef]
- Hu, Y.W.; Hu, Y.R.; Zhao, J.Y.; Li, S.F.; Ma, X.; Wu, S.G.; Lu, J.B.; Qiu, Y.R.; Sha, Y.H.; Wang, Y.C.; et al. An agomir of miR-144-3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production. PLoS ONE 2014, 9, e94997. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernández-Hernando, C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kinoo, S.M.; Chuturgoon, A.A.; Singh, B.; Nagiah, S. Hepatic expression of cholesterol regulating genes favour increased circulating low-density lipoprotein in HIV infected patients with gallstone disease: A preliminary study. BMC Infect. Dis. 2021, 21, 294. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Mo, L.; Ou, J.; Fang, Q.; Wu, H.; Wu, Y.; Nandakumar, K.S. Proteus mirabilis vesicles induce mitochondrial apoptosis by regulating miR96-5p/Abca1 to inhibit osteoclastogenesis and bone loss. Front. Immunol. 2022, 13, 833040. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Chen, J.J.; Deng, W.Y.; Yu, X.H.; Tan, W.H. CTRP1 decreases ABCA1 expression and promotes lipid accumulation through the miR-424-5p/FoxO1 pathway in THP-1 macrophage-derived foam cells. Cell Biol. Int. 2021, 45, 2226–2237. [Google Scholar] [CrossRef]
- Wu, Y.T.; Guo, Z.J.; Li, J.B.; Ye, Z.R.; Zhang, G.X.; Hong, C.M.; Li, M.; Wang, X.W.; Xu, W.F.; Liang, G.T.; et al. Role of MEG3 in cellular physiology of atherosclerosis. Ann. Clin. Lab. Sci. 2023, 53, 619–629. [Google Scholar]
- Zhang, S.; Li, L.; Wang, J.; Zhang, T.; Ye, T.; Wang, S.; Xing, D.; Chen, W. Recent advances in the regulation of ABCA1 and ABCG1 by lncRNAs. Clin. Chim. Acta; Int. J. Clin. Chem. 2021, 516, 100–110. [Google Scholar] [CrossRef]
- Li, M.Q. Study on Long Non-Coding RNA DANCR Regulating Macrophage Lipid Accumulation via miR-33a. Master’s Thesis, Guilin Medical University, Guilin, China, 2021. [Google Scholar] [CrossRef]
- Liu, L.; Tan, L.; Yao, J.; Yang, L. Long non-coding RNA MALAT1 regulates cholesterol accumulation in ox-LDL-induced macrophages via the microRNA-17-5p/ABCA1 axis. Mol. Med. Rep. 2020, 21, 1761–1770. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Z.Y.; Ai, W.M.; Cai, J.X. miR-193a-5p Inhibits Lipid Accumulation in Macrophages via the HDAC9-ABCA1/G1 Pathway. Acta Biochim. Et Biophys. Sin. 2024, 40, 1751–1760. [Google Scholar] [CrossRef]
- Gaidarov, I.; Chen, X.; Anthony, T.; Maciejewski-Lenoir, D.; Liaw, C.; Unett, D.J. Differential tissue and ligand-dependent signaling of GPR109A receptor: Implications for anti-atherosclerotic therapeutic potential. Cell Signal. 2013, 25, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, Z.; Zhang, M.; Yu, X.; Yao, F.; Tan, Y.; Liu, D.; Gong, D.; Chong, H.; Liu, X.; et al. Macrophage-activating lipopeptide-2 downregulates the expression of ATP-binding cassette transporter A1 by activating the TLR2/NF-κB/ZNF202 pathway in THP-1 macrophages. Acta Biochim. Biophys. Sin. 2016, 48, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jung, Y.H.; Lee, H.J.; Chae, C.W.; Choi, G.E.; Lim, J.R.; Kim, S.Y.; Lee, J.E.; Han, H.J. Melatonin activates ABCA1 via the BiP/NRF1 pathway to suppress high-cholesterol-induced apoptosis of mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-A.; Hoang, H.-D.; Rasheed, A.; Duchez, A.-C.; Wyatt, H.; Cottee, M.L.; Graber, T.E.; Susser, L.; Robichaud, S.; Berber, İ.; et al. miR-223 exerts translational control of proatherogenic genes in macrophages. Circ. Res. 2022, 131, 42–58. [Google Scholar] [CrossRef]
- Wagner, B.L.; Valledor, A.F.; Shao, G.; Daige, C.L.; Bischoff, E.D.; Petrowski, M.; Jepsen, K.; Baek, S.H.; Heyman, R.A.; Rosenfeld, M.G.; et al. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol. Cell Biol. 2003, 23, 5780–5789. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Li, Z.; Gao, H.; Yue, Z.; Liu, Z.; Liu, X.; Feng, X.; Liu, P. RIP140 triggers foam-cell formation by repressing ABCA1/G1 expression and cholesterol efflux via liver X receptor. FEBS Lett. 2015, 589, 455–460. [Google Scholar] [CrossRef]
- Ibata, T.; Lyu, J.; Imachi, H.; Fukunaga, K.; Sato, S.; Kobayashi, T.; Saheki, T.; Yoshimura, T.; Murao, K. Effects of 2-methoxyestradiol, a main metabolite of estradiol on hepatic ABCA1 expression in HepG2 cells. Nutrients 2022, 14, 288. [Google Scholar] [CrossRef]
- Neggazi, S.; Canaple, L.; Hamlat, N.; Gauthier, K.; Samarut, J.; Bricca, G.; Aouichat-Bouguerra, S.; Beylot, M. Thyroid hormone receptor alpha deletion in ApoE-/- mice alters the arterial renin-angiotensin system and vascular smooth muscular cell cholesterol metabolism. J. Vasc. Res. 2018, 55, 224–234. [Google Scholar] [CrossRef]
- Loix, M.; Vanherle, S.; Bolkaerts, L.; Verberk, S.G.S.; Punt, M.; Wouters, F.; Moonen, B.; Verhagen, R.; Van Wouw, S.A.E.; Jongejan, A.; et al. UBE3A promotes foam cell formation and counters remyelination by targeting ABCA1 for proteasomal degradation. Nat. Commun. 2025, 16, 8077. [Google Scholar] [CrossRef] [PubMed]
- Boro, M.; Govatati, S.; Kumar, R.; Singh, N.K.; Pichavaram, P.; Traylor, J.G.; Orr, A.W.; Rao, G.N. Thrombin-Par1 signaling axis disrupts COP9 signalosome subunit 3-mediated ABCA1 stabilization in inducing foam cell formation and atherogenesis. Cell Death Differ. 2021, 28, 780–798. [Google Scholar] [CrossRef]
- Yin, J.; Xu, J.; Chen, C.; Ma, X.; Zhu, H.; Xie, L.; Wang, B.; Shao, Y.; Zhao, Y.; Wei, Y.; et al. HECT, UBA and WWE domain containing 1 represses cholesterol efflux during CD4+ T cell activation in sjögren’s syndrome. Frontiers 2023, 14, 1191692. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Chen, W.; Li, X.; Xia, Q.; Zheng, L.; Duan, Q.; Zhang, H.; Zhao, Y. Reduced annexin A1 secretion by ABCA1 causes retinal inflammation and ganglion cell apoptosis in a murine glaucoma model. Front. Cell Neurosci. 2018, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, X.; Dai, S.; Liu, H.; Yan, D.; Zhou, Y.; Gu, J.; Shi, H. Cadmium induced redistribution of cholesterol by upregulating ABCA1 and downregulating OSBP. J. Inorg. Biochem. 2018, 189, 199–207. [Google Scholar] [CrossRef]
- Filbeck, S.; Cerullo, F.; Pfeffer, S.; Joazeiro, C.A.P. Ribosome-associated quality-control mechanisms from bacteria to humans. Mol. Cell 2022, 82, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, J.; Yu, L.; Yang, W.; Qi, W.; Ren, R.; Liu, Y.; Hou, Y.; Cao, Y.; Li, Q.; et al. E3 ubiquitin ligase listerin regulates macrophage cholesterol efflux and atherosclerosis by targeting ABCA1. J. Clin. Investig. 2025, 135, e186509. [Google Scholar] [CrossRef]
- Iborra, R.T.; Machado-Lima, A.; Okuda, L.S.; Pinto, P.R.; Nakandakare, E.R.; Machado, U.F.; Correa-Giannella, M.L.; Pickford, R.; Woods, T.; Brimble, M.A.; et al. AGE-albumin enhances ABCA1 degradation by ubiquitin-proteasome and lysosomal pathways in macrophages. J. Diabetes Complicat. 2018, 32, 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Li, J.; Cai, Z.; Hugo, C.; Sun, Y.; Qian, L.; Tcw, J.; Chui, H.C.; Dikeman, D.; et al. Cellular senescence induced by cholesterol accumulation is mediated by lysosomal ABCA1 in APOE4 and AD. Mol. Neurodegener. 2025, 20, 15. [Google Scholar] [CrossRef]
- Raghavan, S.; Singh, N.K.; Mani, A.M.; Rao, G.N. Protease-activated receptor 1 inhibits cholesterol efflux and promotes atherogenesis via cullin 3-mediated degradation of the ABCA1 transporter. J. Biol. Chem. 2018, 293, 10574–10589. [Google Scholar] [CrossRef]
- Aleidi, S.M.; Yang, A.; Sharpe, L.J.; Rao, G.; Cochran, B.J.; Rye, K.-A.; Kockx, M.; Brown, A.J.; Gelissen, I.C. The E3 ubiquitin ligase, HECTD1, is involved in ABCA1-mediated cholesterol export from macrophages. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2018, 1863, 359–368. [Google Scholar] [CrossRef]
- Aleidi, S.M.; Howe, V.; Sharpe, L.J.; Yang, A.; Rao, G.; Brown, A.J.; Gelissen, I.C. The E3 ubiquitin ligases, HUWE1 and NEDD4-1, are involved in the post-translational regulation of the ABCG1 and ABCG4 lipid transporters. J. Biol. Chem. 2015, 290, 24604–24613. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Koya, T.; Kigawa, Y.; Oguchi, T.; Lei, X.-F.; Kim-Kaneyama, J.; Miyazaki, A. Calpain and atherosclerosis. J. Atheroscler. Thromb. 2013, 20, 228–237. [Google Scholar] [CrossRef]
- Yokoyama, S.; Arakawa, R.; Wu, C.-A.; Iwamoto, N.; Lu, R.; Tsujita, M.; Abe-Dohmae, S. Calpain-mediated ABCA1 degradation: Post-translational regulation of ABCA1 for HDL biogenesis. Biochim. Biophys. Acta. 2012, 1821, 547–551. [Google Scholar] [CrossRef]
- Martinez, L.O.; Agerholm-Larsen, B.; Wang, N.; Chen, W.; Tall, A.R. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J Biol Chem. 2003, 278, 37368–37374. [Google Scholar] [CrossRef]
- Cheng, H.; Cheng, Q.; Bao, X.; Luo, Y.; Zhou, Y.; Li, Y.; Hua, Q.; Liu, W.; Tang, S.; Feng, D.; et al. Over-activation of NMDA receptors promotes ABCA1 degradation and foam cell formation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020, 1865, 158778. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Palme, V.; Rotter, S.; Schilcher, N.; Cukaj, M.; Wang, D.; Ladurner, A.; Heiss, E.H.; Stangl, H.; Dirsch, V.M.; et al. Piperine inhibits ABCA1 degradation and promotes cholesterol efflux from THP-1-derived macrophages. Mol. Nutr. Food Res. 2017, 61, 1500960. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Ishikawa, T.; Tanaka, M.; Tsuboi, T.; Yokoyama, S. Zinc increases ABCA1 by attenuating its clearance through the modulation of calmodulin activity. J. Atheroscler. Thromb. 2021, 28, 261–270. [Google Scholar] [CrossRef]
- Peng, H.; Tang, J.; Zhao, S.; Shen, L.; Xu, D. Inhibition of soluble epoxide hydrolase in macrophages ameliorates the formation of foam cells—Role of heme oxygenase-1. Circ. J. 2019, 83, 2555–2566. [Google Scholar] [CrossRef]
- Ku, C.S.; Rasmussen, H.E.; Park, Y.; Jesch, E.D.; Lee, J. Unsaturated fatty acids repress the expression of ATP-binding cassette transporter A1 in HepG2 and FHs 74 int cells. Nutr. Res. 2011, 31, 278–285. [Google Scholar] [CrossRef][Green Version]
- Yamauchi, Y.; Hayashi, M.; Abe-Dohmae, S.; Yokoyama, S. Apolipoprotein a-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J. Biol. Chem. 2003, 278, 47890–47897. [Google Scholar] [CrossRef]
- Accacha, S.; Voloshyna, I.; Kasselman, L.J.; Mejia-Corletto, J.; Srivastava, A.; Renna, H.A.; De Leon, J.; Levine, R.L.; Reiss, A.B. Plasma from type 1 diabetes patients promotes pro-atherogenic cholesterol transport in human macrophages. J. Investig. Med. 2025, 73, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Yang, Y.L.; Yu, J.X.; Jia, L.H.; Huang, H.-C. APOE4-driven lipid metabolic dysregulation in alzheimer’s disease: Multi-pathway mechanisms and therapeutic perspectives. Biochem. Biophys. Res. Commun. 2025, 778, 152335. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Qu, K.; Xu, R.; Zhu, H. MP allosterically activates AMPK to enhance ABCA1 stability by retarding the calpain-mediated degradation pathway. Int. J. Mol. Sci. 2023, 24, 17280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Oram, J.F. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J. Lipid Res. 2007, 48, 1062–1068. [Google Scholar] [CrossRef]
- Moon, H.R.; Yun, J.M. Protective effect of allium hookeri water extract and its main compound, cycloalliin, on foam cell formation in THP-1-derived macrophages. Food Nutr. Res. 2025, 69, 10763. [Google Scholar] [CrossRef] [PubMed]
- Moumou, M.; Mokhtari, I.; Harnafi, M.; Alrugaibah, M.; Aljutaily, T.; Alharbi, H.F.; Alhuwaymil, A.; Almutairi, A.S.; Barakat, H.; Milenkovic, D.; et al. Argan fruit polyphenols regulate lipid homeostasis, prevent liver fat accumulation, and improve antioxidant defense in high-calorie diet fed mice: In vivo study and In silico prediction of possible underlying mechanisms. Metabolites 2025, 15, 234. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Lv, Q.; Gao, K.; Li, Z.; Miao, Q.; Shen, L. Gegen qinlian decoction modulates atherosclerosis and lipid metabolism through cellular interplay and signaling pathways. Comb. Chem. High. Throughput Screen. 2024, 27, 2609–2621. [Google Scholar] [CrossRef]
- Roh, J.W.; Park, M.-H.; Son, J.-W.; Bae, S. HM-ROS-OS-2102 Study Investigator Group. Effectiveness and safety of very-low-dose rosuvastatin-ezetimibe therapy in korean patients with dyslipidaemia: A multicentre prospective observational study. Clin. Drug Investig. 2025, 45, 803–813. [Google Scholar] [CrossRef] [PubMed]
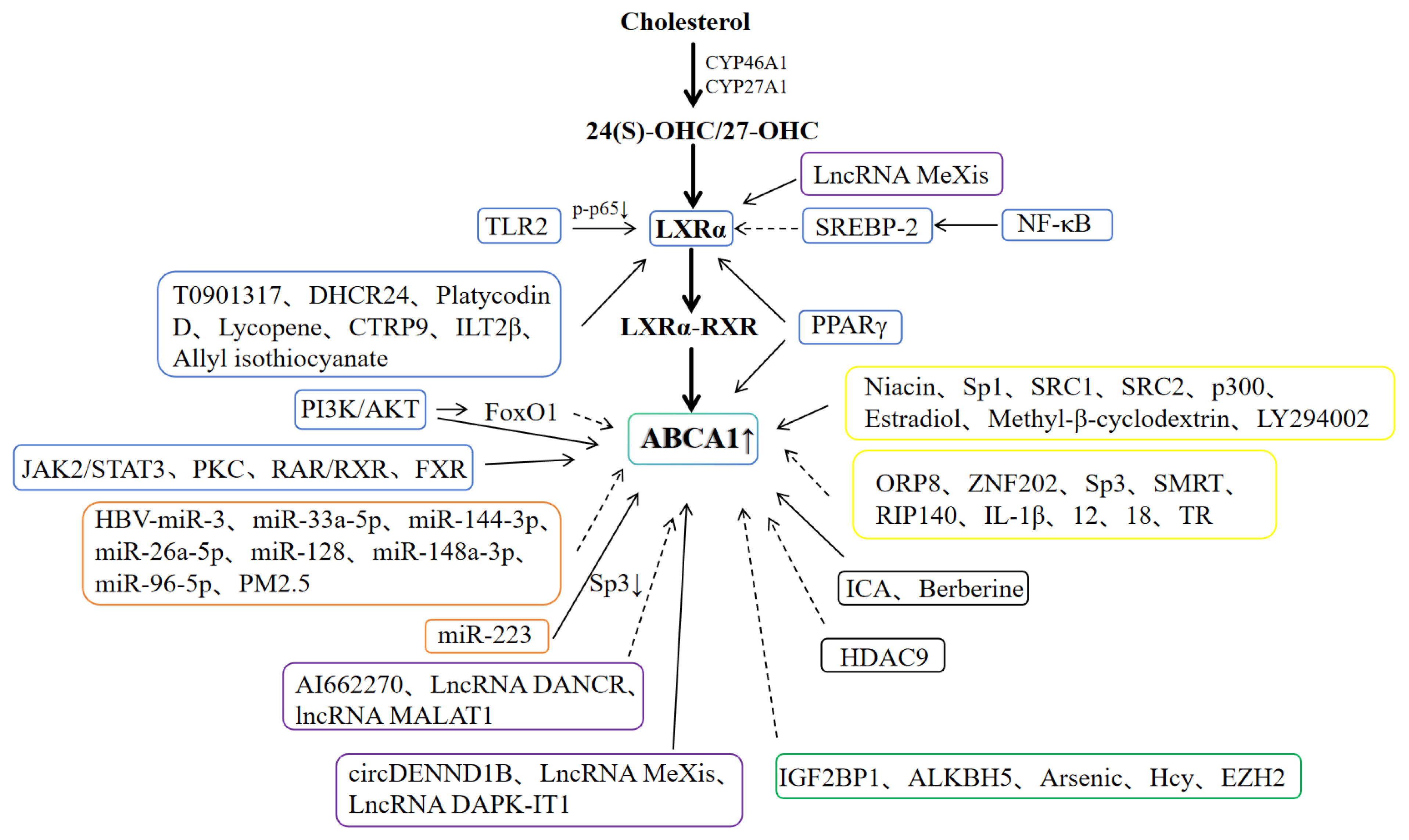
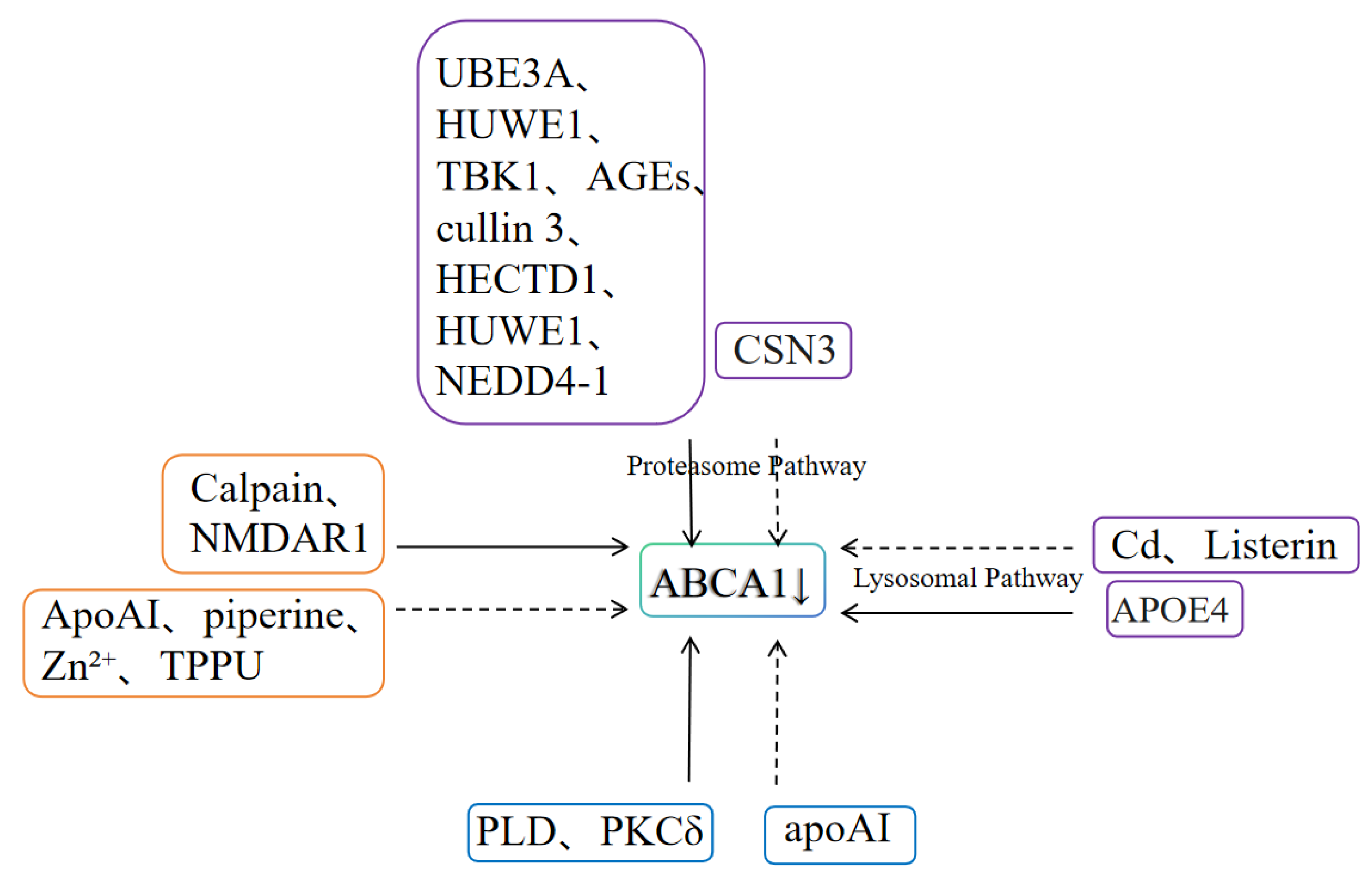
| Types of Regulation | Direct/Indirect | Regulatory Factors | Transactivation/Inhibition | Effects | References |
|---|---|---|---|---|---|
| Pathway | Direct | LXRα | Transactivation | Promote Cholesterol Efflux and Treat Hyperlipidemia | [81] |
| Direct/Indirect | PPARγ | Transactivation | Promote Cholesterol Metabolism and Treat Alcoholic Fatty Liver | [82] | |
| Indirect | T0901317 | Transactivation | Improve Cholesterol Efflux in Vascular Smooth Muscle Cells | [83] | |
| TLR2 | Transactivation | Promote Airway Smooth Muscle Cell Proliferation and Migration and Inhibit Cell Apoptosis | [84] | ||
| DHCR24 | Transactivation | Regulate Lipid Metabolism and Inflammation, and Ameliorate Diseases Such as Atherosclerosis and Cancer | [85] | ||
| SREBP-2 | Transactivation | Positively Regulate Cholesterol Efflux | [21] | ||
| Platycodin D | Transactivation | Inhibit the Inflammatory Response of Primary Rat Microglia Stimulated by LPS | [86] | ||
| Lycopene | Transactivation | Regulate Lipid Metabolism to Antagonize DEHP-Induced Hepatotoxicity | [87] | ||
| Allyl isothiocyanate | Transactivation | Activate the LXR Pathway to Reduce Inflammatory Responses and Improve COPD | [24] | ||
| CTRP9 | Transactivation | Regulate Macrophage Apoptosis and Cholesterol Reverse Transport | [88] | ||
| LILRB2 | Inhibition | Reprogram Cholesterol Metabolism to Drive Gastric Tumorigenesis and Metastasis | [25] | ||
| JAK2/STAT3 | Transactivation | Promote ABCA1 Expression in Macrophages to Inhibit Foam Cell Formation | [89] | ||
| NF-κB | Transactivation | Inhibit Lipid Accumulation in Macrophages | [90] | ||
| PI3K/AKT | Inhibition/Transactivation | Regulate Lipid Metabolism | [30,31] | ||
| FXR | Transactivation | Increase Ileal Cholesterol Transport | [17] | ||
| MicroRNA | Direct | HBV-miR-3 | Inhibition | Promote Cholesterol Accumulation and Facilitate Hepatocellular Carcinoma Progression | [36] |
| miR-33a | Inhibition | Promote the Progression of Atherosclerosis | [91] | ||
| miR-144-3p, miR-26a-5p, miR-128, miR-148a-3p, miR-96-5p | Inhibition | Affect Cholesterol Metabolism, Promote the Progression of Atherosclerosis, and Aggravate Bone Loss in Collagen-Induced Arthritis | [40,92,93,94,95] | ||
| Direct/Indirect | miR-223 | Inhibition/Transactivation | Regulate Lipid Droplet Clearance in Microglia After Spinal Cord Injury | [41] | |
| Indirect | miR-200b-3p, miR-424-5p | Inhibition | Promote the Progression of Atherosclerosis | [96,97] | |
| Non-Coding RNA | Direct | circ8411 | Transactivation | Alleviate Pyroptosis of Glomerular Endothelial Cells and Improve Diabetic Renal Injury | [47] |
| AI662270 | Inhibition | Reduce Cholesterol Efflux and Promote Atherosclerosis | [53] | ||
| Indirect | circDENND1B | Transactivation | Promote Cholesterol Efflux and Treat Atherosclerosis | [48] | |
| LncRNA MeXis | Transactivation | Promote Cholesterol Efflux and Treat Atherosclerosis | [98] | ||
| LncRNA DAPK-IT1 | Transactivation | Reduce the Formation of Foam Cells Derived from THP-1 Macrophages | [50] | ||
| LncRNA DANCR | Inhibition | Regulate Lipid Accumulation in Macrophages | [99] | ||
| lncRNA MALAT1 | Inhibition | Inhibit Cholesterol Efflux | [100] | ||
| Methylation | Direct | IGF2BP | Inhibition | Inhibit Cholesterol Efflux and Promote Lung Adenocarcinoma | [59] |
| ALKBH5 | Inhibition | Promote Cutaneous Melanoma | [60] | ||
| Indirect | MUC2 | Transactivation | Reduce Intracellular Cholesterol Accumulation and Exert Anti-Atherosclerotic Effects | [61] | |
| Arsenic | Inhibition | Inhibit the Expression of ABCA1 Gene and Protein in Macrophages | [62] | ||
| Hcy | Inhibition | Reduce Cholesterol Efflux and Promote Atherosclerosis | [63] | ||
| EZH2 | Inhibition | Reduce Intracellular Cholesterol Efflux, Promote Intracellular Lipid Accumulation and the Progression of Atherosclerosis | [64] | ||
| Acetylation | Direct | HDAC9 | Inhibition | Inhibit Cholesterol Efflux | [101] |
| Indirect | ICA | Transactivation | Promote Cholesterol Efflux | [65] | |
| Berberine | Transactivation | Promote Cholesterol Efflux | [66] | ||
| Other Factors | Direct | Niacin | Transactivation | Promote Cholesterol Efflux | [102] |
| Direct | ORP8 | Inhibition | Inhibit Cholesterol Efflux | [68] | |
| Direct | ZNF202 | Inhibition | Inhibit Cholesterol Efflux and Promote Atherosclerosis | [103] | |
| Direct | Sp1/Sp3 | Transactivation/Inhibition | Regulate Cholesterol Efflux | [104,105] | |
| Direct | SRC1, SRC2, p300 | Transactivation | Promote Cholesterol Efflux | [71] | |
| Direct | SMRT, RIP140 | Inhibition | Inhibit Cholesterol Efflux | [106,107] | |
| Indirect | IL-1β, 12, 18 | Inhibition | Inhibit Cholesterol Efflux | [73] | |
| Indirect | Estradiol | Transactivation | Reduce Lipid Content in Hepatocytes | [108] | |
| Indirect | Methyl-β-cyclodextrin | Transactivation | Reduce Cholesterol Accumulation in Podocytes | [77] | |
| Indirect | TR | Inhibition | Inhibit Cholesterol Efflux | [109] | |
| Indirect | LY294002 | Transactivation | Promote Cholesterol Efflux | [80] |
| Types of Regulation | Direct/Indirect | Regulatory Factors | Transactivation/Inhibition | Effects | References |
|---|---|---|---|---|---|
| Ubiquitin-Proteasome/Lysosome System | Direct | UBE3A | Inhibition | Promote Foam Cell Formation and Oppose Myelin Regeneration | [110] |
| HUWE1 | Inhibition | Regulate Cholesterol Efflux and the Development of Sjögren’s Syndrome | [112] | ||
| TBK1 | Inhibition | Retinal Inflammation and Retinal Ganglion Cell Apoptosis | [113] | ||
| cullin 3 | Inhibition | Inhibit Cholesterol Efflux and Promote Atherosclerosis | [119] | ||
| HECTD1 | Inhibition | Inhibit Cholesterol Efflux | [120] | ||
| NEDD4-1 | Inhibition | Inhibit Cholesterol Efflux | [121] | ||
| Listerin | Transactivation | Inhibit Atherosclerosis | [116] | ||
| Indirect | CSN3 | Transactivation | Inhibit Foam Cell Formation | [111] | |
| Cd | Transactivation | Promote Cholesterol Efflux | [114] | ||
| Advanced glycation end products | Inhibition | Inhibit Cholesterol Efflux and Promote Atherosclerosis | [131] | ||
| APOE4 | Inhibition | Lipid Metabolism Dysregulation in Alzheimer’s Disease | [132] | ||
| Calpain-Mediated Degradation Pathway | Direct | Calpain | Inhibition | Inhibit Cholesterol Efflux and Promote Atherosclerosis | [133] |
| ApoA I | Transactivation | Promote Cholesterol Efflux | [124] | ||
| Indirect | NMDAR | Inhibition | Promote Foam Cell Formation | [125] | |
| Piperine | Inhibition | Promote Foam Cell Formation | [126] | ||
| Zn2+ | Inhibition | Promote Foam Cell Formation | [127] | ||
| TPPU | Inhibition | Promote Foam Cell Formation | [128] | ||
| Phosphorylation | Direct | PKCδ | Inhibition | Inhibit Cholesterol Efflux | [134] |
| Indirect | PLD | Inhibition | Inhibit Cholesterol Efflux | [129] | |
| apoA-I | Transactivation | Promote Cholesterol Efflux | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhou, Y.; Yang, J.; Xue, S.; Wang, Q.; Guo, X.; Zhang, Y.; Niu, W. Research Status and Latest Progress in the Regulatory Mechanisms of ABCA1. Int. J. Mol. Sci. 2025, 26, 10855. https://doi.org/10.3390/ijms262210855
Chen X, Zhou Y, Yang J, Xue S, Wang Q, Guo X, Zhang Y, Niu W. Research Status and Latest Progress in the Regulatory Mechanisms of ABCA1. International Journal of Molecular Sciences. 2025; 26(22):10855. https://doi.org/10.3390/ijms262210855
Chicago/Turabian StyleChen, Xingtong, Yunyue Zhou, Jinbiao Yang, Shuang Xue, Qiao Wang, Xuan Guo, Yukun Zhang, and Wenying Niu. 2025. "Research Status and Latest Progress in the Regulatory Mechanisms of ABCA1" International Journal of Molecular Sciences 26, no. 22: 10855. https://doi.org/10.3390/ijms262210855
APA StyleChen, X., Zhou, Y., Yang, J., Xue, S., Wang, Q., Guo, X., Zhang, Y., & Niu, W. (2025). Research Status and Latest Progress in the Regulatory Mechanisms of ABCA1. International Journal of Molecular Sciences, 26(22), 10855. https://doi.org/10.3390/ijms262210855




