Hydrogel Design Based on Bacterial Exopolysaccharides: The Biomedical Promise of Levan
Abstract
1. Introduction
- −
- Based on origin: natural or synthetic;
- −
- Based on the polymers used in synthesis: homopolymeric—a singular repetitive cross-linked structural unit; copolymeric—two different monomers arranged in a random or alternate fashion in the polymeric network; and multipolymeric—a network of two cross-linked polymers and one non-linked monomer, consisting of either natural or synthetic molecules;
- −
- Based on stimulus response: responsive at temperature, pH, ionic strength, and light- and chemical-responsive;
- −
- Based on chemical structure: amorphous, semicrystalline, or crystalline;
- −
2. Hydrophilic Gels Based on Bacterial Exopolysaccharides
3. Bacterial Levan as a Structural Biopolymer for Hydrogels
3.1. Synthesis of Levan Hydrogels
3.2. Design Strategies to Improve Levan-Based Hydrogels for Biomedical Purposes
3.3. Strategies to Improve Rheological Properties of Levan-Based Hydrogels for Biomedical Purposes
4. Current and Potential Biomedical Applications of Levan-Based Hydrogels Based on Their Properties
5. Perspectives and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef] [PubMed]
- Fu, J. Hydrogel properties and applications. J. Mater. Chem. B 2019, 7, 1523–1525. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid. Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in hydrogels: A comprehensive review of natural and synthetic innovations for biomedical applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Okay, O. General properties of hydrogels. Hydrogel sensors and actuators. In Hydrogel Sensors and Actuators: Engineering and Technology; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6, pp. 1–14. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Henderson, T.M.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, R.; Li, B.; Li, K.; Hao, Y. A controlled light-induced gas-foaming porous hydrogel with adhesion property for infected wound healing. Int. J. Biol. Macromol. 2024, 261, 129751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A comprehensive review of food hydrogels: Principles, formation mechanisms, microstructure, and its applications. Gels 2023, 9, 1. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Ali, K.; Asad, Z.; Agbna, G.H.D.; Saud, A.; Khan, A.; Zaidi, S.J. Progress and innovations in hydrogels for sustainable agriculture. Agronomy 2024, 14, 2815. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.I.; Niculescu, A.G.; Grumezescu, A.M. Advancements in wound dressing materials: Highlighting recent progress in hydrogels, foams, and antimicrobial dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozatic, P.; Chiono, V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Dsouza, A.; Constantinidou, C.; Arvanitis, T.N.; Haddleton, D.M.; Charmet, J.; Hand, R.A. Multifunctional composite hydrogels for bacterial capture, growth/elimination, and sensing applications. ACS Appl. Mater. Interfaces 2022, 14, 47323–47344. [Google Scholar] [CrossRef]
- Mukherjee, S.; Strakova, P.; Richtera, L.; Adam, V.; Ashrafi, A. Biosensors-based approaches for other viral infection detection. Advanced Biosensors for Virus Detection. In Advanced Biosensors for Virus Detection; Academic Press: Cambridge, MA, USA, 2022; pp. 391–405. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides producing bacteria: A review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef]
- Domżał-Kędzia, M.; Ostrowska, M.; Lewińska, A.; Łukaszewicz, M. Recent developments and applications of microbial Levan, a versatile polysaccharide-based biopolymer. Molecules 2023, 28, 5407. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, Y.; Lee, N.; Lee, I.; Lee, J.H. Nature-derived polysaccharide-based composite hydrogels for promoting wound healing. Int. J. Mol. Sci. 2023, 24, 16714. [Google Scholar] [CrossRef]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical applications of bacterial exopolysaccharides: A review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.A.G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent advancements in microbial polysaccharides: Synthesis and applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef]
- Khattak, S.; Qin, X.T.; Huang, L.H.; Xie, Y.Y.; Jia, S.R.; Zhong, C. Preparation and characterization of antibacterial bacterial cellulose/chitosan hydrogels impregnated with silver sulfadiazine. Int. J. Biol. Macromolec. 2021, 189, 483–493. [Google Scholar] [CrossRef]
- Tomulescu, C.; Stoica, R.; Sevcenco, C.; Căşărică, A.; Moscovici, M.; Vamanu, A. Levan-A mini review. Sci. Bull. Series F Biotechnol. 2016, 20, 309–317. [Google Scholar]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Moussa, T.A.; Al-Qaysi, S.A.; Thabit, Z.A.; Kadhem, S.B. Microbial levan from Brachybacterium phenoliresistens: Characterization and enhancement of production. Process Biochem. 2017, 57, 9–15. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Layegh, B.; Aminlari, L.; Hashemi, M.B. Microbial production of levan using date syrup and investigation of its properties. World Acad. Sci. Eng. Technol. 2010, 44, 1248–1254. [Google Scholar]
- Xu, L.; Wu, D.; Xu, H.; Zhao, Z.; Chen, Q.; Li, H.; Chen, L. Characterization, production optimization, and fructanogenic traits of levan in a new Microbacterium isolate. Int. J. Biol. Macromolec. 2023, 250, 126330. [Google Scholar] [CrossRef]
- Cai, G.; Liu, Y.; Li, X.; Lu, J. New levan-type exopolysaccharide from Bacillus amyloliquefaciens as an antiadhesive agent against enterotoxigenic Escherichia coli. J. Agric. Food Chem. 2019, 67, 8029–8034. [Google Scholar] [CrossRef]
- Abou-Taleb, K.; Abdel-Monem, M.; Yassin, M.; Draz, A. Production, purification and characterization of levan polymer from Bacillus lentus V8 strain. Br. Microbiol. Res. J. 2015, 5, 22–32. [Google Scholar] [CrossRef]
- Wahyuningrum, D.; Hertadi, R. Isolation and characterization of levan from moderate halophilic bacteria Bacillus licheniformis BK AG21. Procedia Chem. 2015, 16, 292–298. [Google Scholar] [CrossRef][Green Version]
- Pei, F.; Ma, Y.; Chen, X.; Liu, H. Purification and structural characterization and antioxidant activity of levan from Bacillus megaterium PFY-147. Int. J. Biol. Macromolec. 2020, 161, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, R.; Qian, H.; Mu, W.; Miao, M.; Jiang, B. Biosynthesis of levan by levansucrase from Bacillus methylotrophicus SK 21.002. Carbohydr. Polym. 2014, 101, 975–981. [Google Scholar] [CrossRef]
- Haddar, A.; Hamed, M.; Bouallegue, A.; Bastos, R.; Coelho, E.; Coimbra, M.A. Structural elucidation and interfacial properties of a levan isolated from Bacillus mojavensis. Food Chem. 2021, 343, 128456. [Google Scholar] [CrossRef]
- Nasir, A.; Ahmad, W.; Sattar, F.; Ashfaq, I.; Lindemann, S.R.; Chen, M.H.; Van den Ende, W.; Ӧner, E.T.; Kirtel, O.; Khaliq, S.; et al. Production of a high molecular weight levan by Bacillus paralicheniformis, an industrially and agriculturally important isolate from the buffalo grass rhizosphere. Antonie Van. Leeuwenhoek 2022, 115, 1101–1112. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Elattal, N.A.; Amin, M.A.; Ali, A.E.; Mansour, N.M.; Awad, G.E.; Farrag, A.R.H.; Esawy, M.A. In vivo assessment of possible probiotic properties of Bacillus subtilis and prebiotic properties of levan. Biocatal. Agri. Biotechnol. 2018, 13, 190–197. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Elattal, N.A.; Amin, M.A.; Ali, A.E.; Mansour, N.M.; Awad, G.E.; Awad, H.M.; Esawy, M.A. Possible correlation between levansucrase production and probiotic activity of Bacillus sp. isolated from honey and honey bee. World J. Microbiol. Biotechnol. 2017, 33, 69. [Google Scholar] [CrossRef] [PubMed]
- Bouallegue, A.; Chaari, F.; Casillo, A.; Corsaro, M.M.; Bachoal, R.; Ellouz-Chaabouni, M. Levan produced by Bacillus subtilis AF17: Thermal, functional and rheological properties. J. Food Meas. Charact. 2022, 16, 440–447. [Google Scholar] [CrossRef]
- Han, Y.W.; Clarke, M.A. Production and characterization of microbial levan. J. Agricult. Food Chemistry. 1990, 38, 393–396. [Google Scholar] [CrossRef]
- Mendonça, C.M.N.; Oliveira, R.C.; Freire, R.K.B.; Piazentin, A.C.M.; Pereira, W.A.; Gudiña, E.J.; Evtuguin, D.V.; Converti, A.; Santos, J.H.P.M.; Nunes, C.; et al. Characterization of levan produced by a Paenibacillus sp. isolated from Brazilian crude oil. Int. J. Biol. Macromol. 2021, 186, 788–799. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wang, X.; Liu, Z.; Wu, Z. Levan from Leuconostoc citreum BD1707: Production optimization and changes in molecular weight distribution during cultivation. BMC Biotechnol. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Ahmad, W.; Nasir, A.; Sattar, F.; Ashfaq, I.; Chen, M.H.; Hayat, A.; Rehman, M.U.; Zhao, S.; Khaliq, S.; Ghauri, M.A.; et al. Production of bimodal molecular weight levan by a Lactobacillus reuteri isolate from fish gut. Folia Microbiol. 2022, 67, 21–31. [Google Scholar] [CrossRef]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Fermentation pH modulates the size distributions and functional properties of Gluconobacter albidus TMW 2.1191 levan. Front. Microbiol. 2017, 8, 807. [Google Scholar] [CrossRef]
- Gulitz, A.; Stadie, J.; Wenning, M.; Ehrmann, M.A.; Vogel, R.F. The microbial diversity of water kefir. Int. J. Food Microbiol. 2011, 151, 284–288. [Google Scholar] [CrossRef]
- Hövels, M.; Kosciow, K.; Kniewel, J.; Jakob, F.; Deppenmeier, U. High yield production of levan-type fructans by Gluconobacter japonicus LMG 1417. Int. J. Biol. Macromolec. 2020, 164, 295–303. [Google Scholar] [CrossRef]
- Srikanth, R.; Siddartha, G.; Reddy, C.H.S.; Harish, B.S.; Ramaiah, M.J.; Uppuluri, K.B. Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM 2526 and its statistical optimization. Carbohydr. Polym. 2015, 123, 8–16. [Google Scholar] [CrossRef]
- Silbir, S.; Dagbagli, S.; Yegin, S.; Baysal, T.; Goksungur, Y. Levan production by Zymomonas mobilis in batch and continuous fermentation systems. Carbohydr. Polym. 2014, 99, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xu, W.; Ni, D.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Preparation of a novel water-soluble gel from Erwinia amylovora levan. Int. J. Biol. Macromolec. 2019, 122, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaysi, S.A.; Al-Haideri, H.; Al-Shimmary, S.M.; Abdulhameed, J.M.; Alajrawy, O.I.; Al-Halbosiy, M.M.; Moussa, T.A.A.; Farahat, M.G. Bioactive levan-type exopolysaccharide produced by Pantoea agglomerans ZMR7: Characterization and optimization for enhanced production. J. Microbiol. Biotechnol. 2021, 31, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, S.; Zhang, T.; Jiang, B.; Mu, W. Efficient biosynthesis of levan from sucrose by a novel levansucrase from Brenneria goodwinii. Carbohydr. Polym. 2017, 157, 1732–1740. [Google Scholar] [CrossRef]
- González-Garcinuño, Á.; Tabernero, A.; Marcelo, G.; Sebastián, V.; Arruebo, M.; Santamaría, J.; Martín Del Valle, E. Differences in levan nanoparticles depending on their synthesis route: Microbial vs cell-free systems. Int. J. Biol. Macromol. 2019, 137, 62–68. [Google Scholar] [CrossRef]
- Nasir, D.Q.; Wahyuningrum, D.; Hertadi, R. Screening and characterization of levan secreted by halophilic bacterium of Halomonas and Chromohalobacter genuses originated from Bledug Kuwu mud crater. Procedia Chem. 2015, 16, 272–278. [Google Scholar] [CrossRef]
- Erdal Altıntaş, Ö.; Toksoy Öner, E.; Çabuk, A.; Aytar Çelik, P. Biosynthesis of Levan by Halomonas elongata 153B: Optimization for enhanced production and potential Biological activities for Pharmaceutical Field. J. Polym. Environ. 2023, 31, 440–1455. [Google Scholar] [CrossRef]
- Tohme, S.; Hacıosmanoğlu, G.G.; Eroğlu, M.S.; Kasavi, C.; Genç, S.; Can, Z.S.; Öner, E.T. Halomonas smyrnensis as a cell factory for co-production of PHB and levan. Int. J. Biol. Macromolec. 2018, 118, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Jathore, N.R.; Bule, M.V.; Tilay, A.V.; Annapure, U.S. Microbial levan from Pseudomonas fluorescens: Characterization and medium optimization for enhanced production. Food Sci. Biotechnol. 2012, 21, 1045–1053. [Google Scholar] [CrossRef]
- Abaramak, G.; Kırtel, O.; Öner, E.T. Fructanogenic halophiles: A new perspective on extremophiles. In Physiological and Biotechnological Aspects of Extremophiles; Academic Press: Cambridge, MA, USA, 2020; pp. 123–130. [Google Scholar] [CrossRef]
- Miranda-Molina, A.; Castrejón-Carrillo, S.; Zavala-Padilla, G.T.; Antúnez-Mojica, M.; Alvarez, L.; Rodríguez-Alegría, M.E.; Munguía, A.L. Branching and molecular weight in levan: A detailed analysis of structural variability and enzymatic hydrolysis susceptibility. Carbohydr. Polym. 2025, 352, 123236. [Google Scholar] [CrossRef]
- Srikanth, R.; Reddy, C.H.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Hundschell, C.S.; Jakob, F.; Wagemans, A.M. Molecular weight dependent structure of the exopolysaccharide levan. Int. J. Biol. Macromol. 2020, 161, 398–405. [Google Scholar] [CrossRef] [PubMed]
- González-Garcinuño, Á.; Tabernero, A.; Sánchez-Álvarez, J.M.; Galán, M.A.; Martin Del Valle, E.M. Effect of bacteria type and sucrose concentration on levan yield and its molecular weight. Microb. Cell Fact. 2017, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Daguer, J.P.; Geissmann, T.; Petit-Glatron, M.F.; Chambert, R. Autogenous modulation of the Bacillus subtilis sacB–levB–yveA levansucrase operon by the levB transcript. Microbiology 2004, 150, 3669–3679. [Google Scholar] [CrossRef][Green Version]
- Kunst, F.; Ogasawara, N.; Moszer, I.; Albertini, A.M.; Alloni, G.; Azevedo, V.; Bertero, M.G.; Bessières, P.; Bolotin, A.; Borchert, S.; et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef]
- Velázquez-Hernández, M.L.; Baizabal-Aguirre, V.M.; Bravo-Patiño, A.; Cajero-Juárez, M.; Chávez-Moctezuma, M.P.; Valdez-Alarcón, J.J. Microbial fructosyltransferases and the role of fructans. J. Appl. Microbiol. 2009, 106, 1763–1778. [Google Scholar] [CrossRef]
- Kumar, C.G.; Sripada, S.; Poornachandra, Y. Status and future prospects of fructooligosaccharides as nutraceuticals. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 451–503. [Google Scholar] [CrossRef]
- Wang, S.; Wu, B.; Todhanakasem, T. Expanding the horizons of levan: From microbial biosynthesis to applications and advanced detection methods. World J. Microbiol. Biotechnol. 2024, 40, 214. [Google Scholar] [CrossRef]
- Runyon, J.R.; Nilsson, L.; Ulmius, M.; Castro, A.; Ionescu, R.; Andersson, C.; Schmidt, C. Characterizing changes in levan physicochemical properties in different pH environments using asymmetric flow field-flow fractionation. Anal. Bioanal. Chem. 2014, 406, 1597–1605. [Google Scholar] [CrossRef]
- Arvidson, S.A.; Rinehart, B.T.; Gadala-Maria, F. Concentration regimes of solutions of levan polysaccharide from Bacillus sp. Carbohydr. Polym. 2006, 65, 144–149. [Google Scholar] [CrossRef]
- Kim, S.J.; Bae, P.K.; Chung, B.H. Self-assembled levan nanoparticles for targeted breast cancer imaging. Chem. Commun. 2015, 51, 107–110. [Google Scholar] [CrossRef]
- Xie, C.; Liu, G.; Wang, L.; Yang, Q.; Liao, F.; Yang, X.; Xiao, B.; Duan, L. Synthesis and properties of injectable hydrogel for tissue filling. Pharmaceutics 2024, 16, 430. [Google Scholar] [CrossRef]
- Selvi, S.S.; Hasköylü, M.E.; Genç, S.; Toksoy Öner, E. Synthesis and characterization of levan hydrogels and their use for resveratrol release. J. Bioact. Compat. Polym. 2021, 36, 464–480. [Google Scholar] [CrossRef]
- Berg, A.; Oner, E.T.; Combie, J.; Schneider, B.; Ellinger, R.; Weisser, J.; Wirwa, R.; Schnabelrauch, M. Formation of new, cytocompatible hydrogels based on photochemically crosslinkable levan methacrylates. Int. J. Biol. Macromolec. 2018, 107, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Hwang, Y.; Sahu, A.; Min, K.; Sung, D.; Tae, G.; Chang, J.H. An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomat. Sci. 2018, 6, 2627–2638. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.S.; An, H.; Oh, H.; Sung, D.; Tae, G.; Choi, W.I. Hydroxyapatite-embedded levan composite hydrogel as an injectable dermal filler for considerable enhancement of biological efficacy. J. Ind. Eng. Chem. 2021, 104, 491–499. [Google Scholar] [CrossRef]
- Tekin, A.; Tornacı, S.; Boyacı, D.; Li, S.; Calligaris, S.; Maalej, H.; Toksoy Öner, E. Hydrogels of levan polysaccharide: A systematic review. Int. J. Biol. Macromol. 2025, 315, 144430. [Google Scholar] [CrossRef]
- Srivastava, N.; Choudhury, A.R. Recent advances in composite hydrogels prepared solely from polysaccharides. Colloids Surf. B Biointerfaces 2021, 205, 111891. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.M.; Gamal-Eldeen, A.M.; Helmy, W.A.; Esawy, M.A. Antitumor and antioxidant activities of levan and its derivative from the isolate Bacillus subtilis NRC1aza. Carbohydr. Polym. 2012, 89, 314–322. [Google Scholar] [CrossRef]
- Leibovici, J.; Stark, Y. Increase in cell permeability to a cytotoxic agent by the polysaccharide levan. Cell Molec Biol. 1985, 31, 337–341. [Google Scholar]
- Taylan, O.; Yilmaz, M.T.; Dertli, E. Partial characterization of a levan type exopolysaccharide (EPS) produced by Leuconostoc mesenteroides showing immunostimulatory and antioxidant activities. Int. J. Biol. Macromol. 2019, 136, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Su, Q.; Ma, Y.; Zhao, S.; Zhang, H.; Gao, X. Research progress on the polysaccharide extraction and antibacterial activity. Ann. Microbiol. 2024, 74, 17. [Google Scholar] [CrossRef]
- Qamar, S.A.; Riasat, A.; Jahangeer, M.; Fatima, R.; Bilal, M.; Iqbal, H.M.; Mu, B.Z. Prospects of microbial polysaccharides-based hybrid constructs for biomimicking applications. J. Basic Microbiol. 2022, 62, 1319–1336. [Google Scholar] [CrossRef]
- Veerapandian, B.; Selvaraj, T.K.R.; Shanmugam, S.R.; Sarwareddy, K.K.; Mani, K.P.; Venkatachala, P. In-vitro drug release and stability assessment of tailored levan–chitosan biocomposite hydrogel. Iran. Polym. J. 2024, 33, 11–23. [Google Scholar] [CrossRef]
- Kim, S.J.; Bae, P.K.; Choi, M.; Keem, J.O.; Chung, W.; Shin, Y.B. Fabrication and application of Levan–PVA hydrogel for effective influenza virus capture. ACS Appl. Mater. Interfaces 2020, 12, 29103–29109. [Google Scholar] [CrossRef]
- Osman, A.; Oner, E.T.; Eroglu, M.S. Novel levan and pNIPA temperature sensitive hydrogels for 5-ASA controlled release. Carbohydr. Polym. 2017, 165, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Choudhury, A.R. Synthesis and rheological characterization of a novel shear thinning levan gellan hydrogel. Int. J. Biol. Macromolec. 2020, 159, 922–930. [Google Scholar] [CrossRef]
- González-Garcinuño, Á.; Tabernero, A.; del Valle, E.M.M. Hydrogels based on levan. In Polysaccharide Hydrogels for Drug Delivery and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2024; pp. 175–186. [Google Scholar] [CrossRef]
- Demirci, T.; Hasköylü, M.E.; Eroğlu, M.S.; Hemberger, J.; Öner, E.T. Levan-based hydrogels for controlled release of Amphotericin B for dermal local antifungal therapy of Candidiasis. Eur. J. Pharma. Sci. 2020, 145, 105255. [Google Scholar] [CrossRef]
- Akkulah, C.Y.; Erginer, M.; Cumbul, A.; Kirtel, O.; Bayram, F.; Öner, E.T. Enhanced effects of levan hydrogels and bovine grafts on guided bone regeneration: In-vitro and in-vivo analysis. Int. J. Biol. Macromolec. 2025, 292, 139129. [Google Scholar] [CrossRef] [PubMed]
- Waidi, Y.O.; Wagh, V.S.; Mishra, S.; Jhunjhunwala, S.; Dastager, S.G.; Chatterjee, K. Vat-Based 3D-Bioprinted Scaffolds from Photocurable Bacterial Levan for Osteogenesis and Immunomodulation. Biomacromolecules 2025, 26, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Combie, J.; Öner, E.T. From healing wounds to resorbable electronics, levan can fill bioadhesive roles in scores of markets. Bioinspir. Biomim. 2018, 14, 011001. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.D.; Caridade, S.G.; Sousa, M.P.; Azevedo, S.; Kandur, M.Y.; Öner, E.T.; Alves, N.M.; Mano, J.F. Adhesive free-standing multilayer films containing sulfated levan for biomedical applications. Acta Biomater. 2018, 69, 183–195. [Google Scholar] [CrossRef]
- de Siqueira, E.C.; Rebouças, J.S.; Pinheiro, I.O.; Formiga, F.R. Levan-based nanostructured systems: An overview. Int. J. Pharm. 2020, 580, 119242. [Google Scholar] [CrossRef]
- Sezer, A.D.; Kazak Sarılmışer, H.; Rayaman, E.; Çevikbaş, A.; Öner, E.T.; Akbuğa, J. Development and characterization of vancomycin-loaded levan-based microparticular system for drug delivery. Pharm. Dev. Technol. 2015, 22, 627–634. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-assembled hydrogel nanoparticles for drug delivery applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef]
- Sezer, A.D.; Kazak, H.; Öner, E.T.; Akbuğa, J. Levan-based nanocarrier system for peptide and protein drug delivery: Optimization and influence of experimental parameters on the nanoparticle characteristics. Carbohydr. Polym. 2011, 84, 358–363. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, E.; Oh, H.; Choi, W.I.; Koo, H. Levan nanoparticles with intrinsic CD44-targeting ability for tumor-targeted drug delivery. Int. J. Biol. Macromolec. 2023, 234, 123634. [Google Scholar] [CrossRef]
- Bahadori, F.; Eskandari, Z.; Ebrahimi, N.; Bostan, M.S.; Eroğlu, M.S.; Oner, E.T. Development and optimization of a novel PLGA-Levan based drug delivery system for curcumin, using a quality-by-design approach. Eur. J. Pharm. Sci. 2019, 138, 105037. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Methacanon, P.; Lloyd, L.L.; Paterson, M.; Knill, C.J. Carbohydrate polymers as wound management aids. Carbohydr. Polym. 1997, 4, 422. [Google Scholar] [CrossRef]
- Hamada, M.A.; Hassan, R.A.; Abdou, A.M.; Elsaba, Y.M.; Aloufi, A.S.; Sonbol, H.; Korany, S.M. Bio_fabricated levan polymer from Bacillus subtilis MZ292983. 1 with antibacterial, antibiofilm, and burn healing properties. Appl. Sci. 2022, 12, 6413. [Google Scholar] [CrossRef]
- Upadhyay, R. Use of polysaccharide hydrogels in drug delivery and tissue engineering. Adv. Tissue Eng. Regen. Med. 2017, 2, 145–151. [Google Scholar] [CrossRef]
- Erginer, M.; Akcay, A.; Coskunkan, B.; Morova, T.; Rende, D.; Bucak, S.; Baysal, N.; Ozisik, R.; Eroglu, M.S.; Agirbasli, M.; et al. Sulfated levan from Halomonas smyrnensis as a bioactive, heparin-mimetic glycan for cardiac tissue engineering applications. Carbohydr. Polym. 2016, 149, 289–296. [Google Scholar] [CrossRef]
- Bostan, M.S.; Mutlu, E.C.; Kazak, H.; Sinan Keskin, S.; Oner, E.T.; Eroglu, M.S. Comprehensive characterization of chitosan/PEO/levan ternary blend films. Carbohydr Polym. 2014, 102, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, J.; Qi, X.; Zhong, Y.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Synthesis and characterization of a multi-sensitive polysaccharide hydrogel for drug delivery. Carbohydr. Polym. 2017, 177, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Pap, M.M.; Jaroch, D.B.; Morrison, F.A.; Gilbert, R.J. Fabrication and characterization of tunable polysaccharide hydrogel blends for neural repair. Acta Biomater. 2011, 7, 1634–1643. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.A. An improved injectable polysaccharide hydrogel: Modified gellan gum for long-term cartilage regeneration in vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines 2025, 13, 731. [Google Scholar] [CrossRef]
- Shokrani, H.; Shokrani, A.; Saeb, M.R. Methods for biomaterials printing: A short review and perspective. Methods 2022, 206, 1–7. [Google Scholar] [CrossRef]
- Qiu, J.; Ma, S.; Qu, X. Challenges and Innovative Strategies in 3D Printing of Natural Biomolecular Hydrogels. Nano Sel. 2025, 6, e202400149. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Liu, Y.; Musuc, A.M.; Fajardo, A.R.; Gowda, B.H.J.; Vora, L.K.; Shavandi, A.; Okoro, O.V. Recent advances in 3D bioprinted polysaccharide hydrogels for biomedical applications: A comprehensive review. Carbohydr. Polym. 2025, 348, 122845. [Google Scholar] [CrossRef]
- Lapomarda, A.; Pulidori, E.; Cerqueni, G.; Chiesa, I.; De Blasi, M.; Geven, M.A.; Montemurro, F.; Duce, C.; Mattioli-Belmonte, M.; Tiné, M.R.; et al. Pectin as rheology modifier of a gelatin-based biomaterial ink. Materials 2021, 14, 3109. [Google Scholar] [CrossRef] [PubMed]
- Congur, G.; Eksin, E.; Erdem, A. Levan modified DNA biosensor for voltammetric detection of daunorubicin-DNA interaction. Sens. Actuators B Chem. 2021, 326, 128818. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Lee, J.S.; Ko, G.J.; Sunwoo, S.H.; Lee, S.; Jo, Y.J.; Choi, C.H.; Hwang, S.W.; Kim, T.I. Biosafe, eco-friendly levan polysaccharide toward transient electronics. Small 2018, 14, 1801332. [Google Scholar] [CrossRef]
- Wani, S.; Shaikh, S.; Sayyed, R. Microbial biopolymers in biomedical field. MedCrave Online J. Cell Sci. Rep. 2016, 3, 65–67. [Google Scholar] [CrossRef][Green Version]
- De France, K.J.; Xu, F.; Hoare, T. Structured macroporous hydrogels: Progress, challenges, and opportunities. Adv. Healthcare Mat. 2018, 7, 1700927. [Google Scholar] [CrossRef]
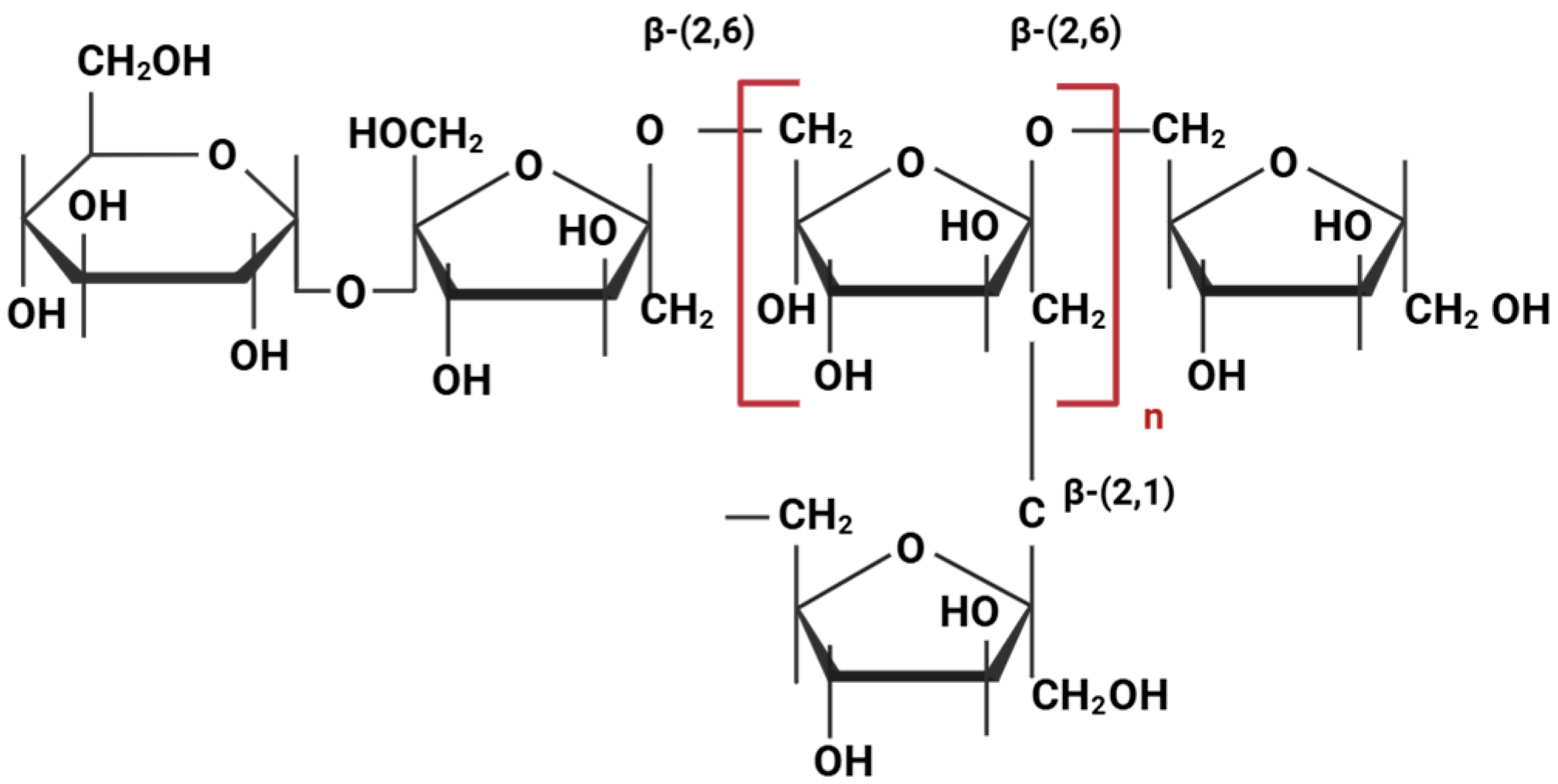
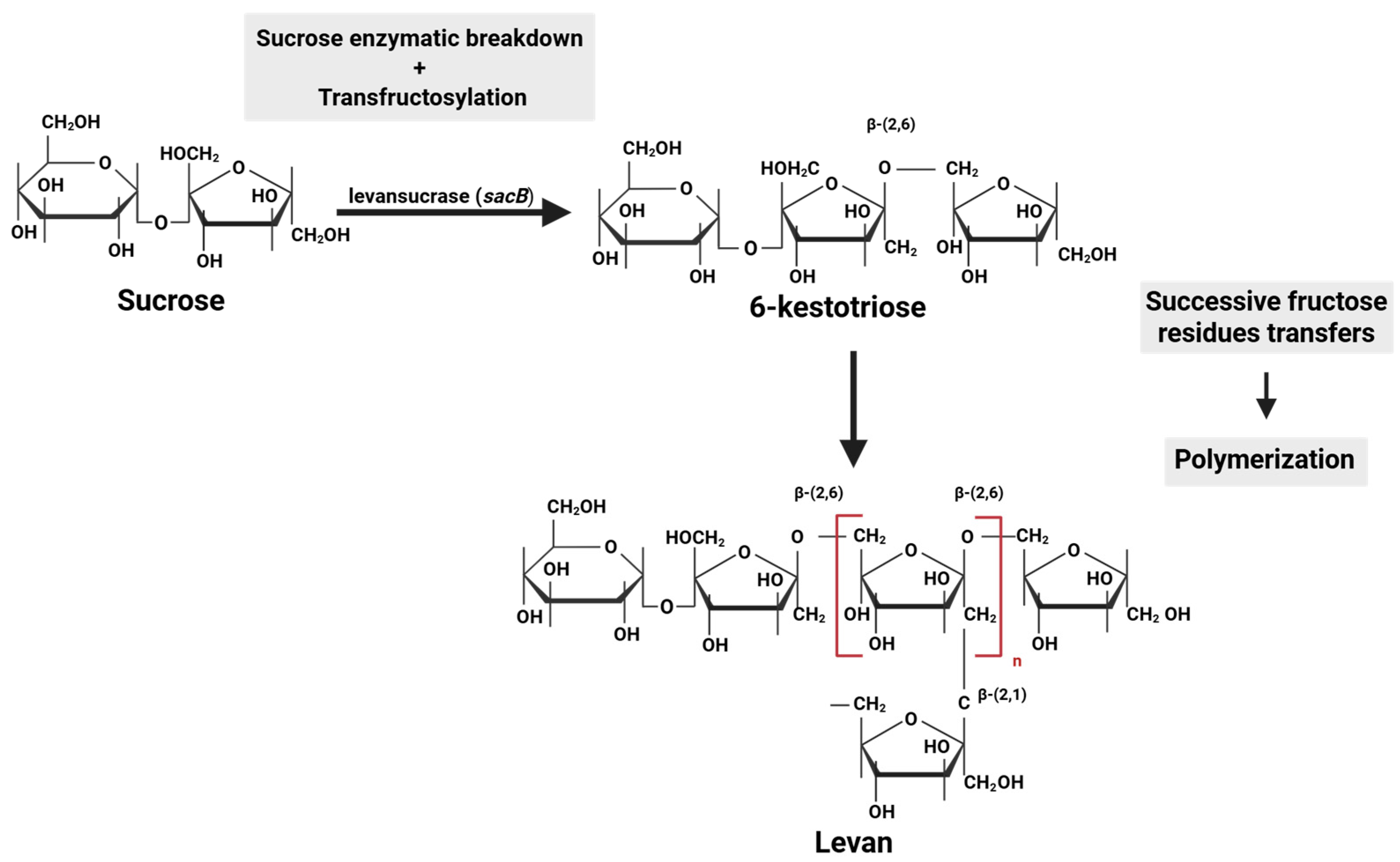


| Lineage: Phylum, Class, Order, Family | Species | Strain | Source | References |
|---|---|---|---|---|
| Actinomycetota; Actinomycetes; Micrococcales; Dermabacteraceae | Brachybacterium phenoliresistens | n.a. | Rhizosphere | [36] |
| Actinomycetota; Actinomycetes; Micrococcales; Microbacteriaceae | Microbacterium laevaniformans | PTCC 1406 | Active sludge | [37] |
| Actinomycetota; Actinomycetes; Micrococcales; Microbacteriaceae | Microbacterium sp. | XL1 | Soil | [38] |
| Bacillota; Bacilli; Bacillales; Bacillaceae | Bacillus amyloliquefaciens | n.a. | n.a. | [39] |
| Bacillota; Bacilli; Bacillales; Bacillaceae | Bacillus lentus | V8 | Rhizosphere | [40] |
| Bacillota; Bacilli; Bacillales; Bacillaceae | Bacillus licheniformis | BK AG21 | Mud crater | [41] |
| Bacillota; Bacilli; Bacillales; Bacillaceae | Bacillus megaterium | PFY-147 | Rhizosphere | [42] |
| Bacillota; Bacilli; Bacillales; Bacillaceae | Bacillus methylotrophicus | SK 21.002 | Soil | [43] |
| Bacillota; Bacilli; Bacillales; Bacillaceae | Bacillus mojavensis | n.a. | Soil | [44] |
| Bacillota; Bacilli; Bacillales; Bacillaceae | Bacillus paralicheniformis | n.a. | Rhizosphere | [45] |
| Bacillota; Bacilli; Bacillales; Bacillaceae | Bacillus subtilis | HMNig-2; MENO2 AF17 | Honey Honey bee gut Kefir | [46] [47] [48] |
| Bacillota; Bacilli; Bacillales; Paenibacillaceae | Paenibacillus polymyxa (formerly Bacillus polymyxa) | n.a. | Soil | [49] |
| Bacillota; Bacilli; Bacillales; Paenibacillaceae | Paenibacillus sp. | #210 | Crude oil | [50] |
| Bacillota; Bacilli; Lactobacillales; Lactobacillaceae | Leuconostoc citreum | BD1707 | Kefir | [51] |
| Bacillota; Bacilli; Lactobacillales; Lactobacillaceae | Limosilactobacillus reuteri (formerly Lactobacillus reuteri) | FW2 | Fish gut | [52] |
| Pseudomonadota; Alphaproteobacteria; Acetobacterales; Acetobacteraceae | Gluconobacter albidus | TMW 2.1191 | Water kefir | [53,54] |
| Pseudomonadota; Alphaproteobacteria; Acetobacterales; Acetobacteraceae | Gluconobacter japonicus | LMG 1417 | n.a. | [55] |
| Pseudomonadota; Alphaproteobacteria; Acetobacterales; Acetobacteraceae | Komagataeibacter xylinus (formerly Acetobacter xylinum) | NCIM 2526 | n.a. | [56] |
| Pseudomonadota; Alphaproteobacteria; Sphingomonadales; Zymomonadaceae | Zymomonas mobilis | NRRL B-14023 | Sugarcane fermentations | [57] |
| Pseudomonadota; Gammaproteobacteria; Enterobacterales; Erwiniaceae | Erwinia amylovora | n.a. | n.a. | [58] |
| Pseudomonadota; Gammaproteobacteria; Enterobacterales; Erwiniaceae | Pantoea agglomerans | ZMR7 | Rhizosphere | [59] |
| Pseudomonadota; Gammaproteobacteria; Enterobacterales; Pectobacteriaceae | Brenneria goodwinii | OBR1 | n.a | [60] |
| Pseudomonadota; Gammaproteobacteria; Moraxellales; Moraxellaceae | Acinetobacter nectaris | CECT 8127 | Floral nectar | [61] |
| Pseudomonadota; Gammaproteobacteria; Oceanospirillales; Halomonadaceae | Chromohalobacter japonicus | BK-AB18 | Mud crater | [62] |
| Pseudomonadota; Gammaproteobacteria; Oceanospirillales; Halomonadaceae | Halomonas elongata | BK-AB8; BK-AG18; 153B | Mud crater Saltern | [62] [63] |
| Pseudomonadota; Gammaproteobacteria; Oceanospirillales; Halomonadaceae | Halomonas eurihalina | BK-AB15 | Mud crater | [62] |
| Pseudomonadota; Gammaproteobacteria; Oceanospirillales; Halomonadaceae | Vreelandella meridiana (formerly Halomonas meridiana) | BK-AB4 | Mud crater | [62] |
| Pseudomonadota; Gammaproteobacteria; Oceanospirillales; Halomonadaceae | Halomonas smyrnensis | AAD6T | Saltern | [64] |
| Pseudomonadota; Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae | Pseudomonas fluorescens | NCIM 2059 | n.a. | [65] |
| Cross-Linking Agent/Co-Polymer | Cross-Linkage Type | Levan Source | Applications | Ref. |
|---|---|---|---|---|
| PF127/CMC | Physical (thermo-cross-linking) | X—not mentioned, commercially available levan powder |
| [82] |
| GA/PVA | Chemical | X—not mentioned, commercially available levan powder |
| [91] |
| PF127/CMC/Hydroxyapatite composite | Physical (thermo-cross-linking) | Erwinia herbicola |
| [83] |
| BDDE | Chemical | Halomonas smyrnensis AAD6T |
| [96] |
| Levan/pNIPA | Physical (thermo-cross-linking) | Halomonas smyrnensis (from bioreactor cultures) |
| [93] |
| BDDE | Chemical | Halomonas smyrnensis (from bioreactor cultures) |
| [80,97] |
| UV photo-initiators (I 2959/LAP) | Physical (photo-cross-linking) | Bacillus subtilis |
| [81] |
| Chitosan/oxidized levan composite | Chemical (Schiff’s base reaction) | Bacillus subtilis MTCC 441 |
| [91] |
| Levan–gellan composite | Chemical | Erwinia herbicola L8647 |
| [94] |
| UV photo-initiator (LAP) | Physical (photo-cross-linking) | Bacillus sp. SGD-03 |
| [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.I.; Carpa, R.; Farkas, A. Hydrogel Design Based on Bacterial Exopolysaccharides: The Biomedical Promise of Levan. Int. J. Mol. Sci. 2025, 26, 10828. https://doi.org/10.3390/ijms262210828
Popa AI, Carpa R, Farkas A. Hydrogel Design Based on Bacterial Exopolysaccharides: The Biomedical Promise of Levan. International Journal of Molecular Sciences. 2025; 26(22):10828. https://doi.org/10.3390/ijms262210828
Chicago/Turabian StylePopa, Andrada Ioana, Rahela Carpa, and Anca Farkas. 2025. "Hydrogel Design Based on Bacterial Exopolysaccharides: The Biomedical Promise of Levan" International Journal of Molecular Sciences 26, no. 22: 10828. https://doi.org/10.3390/ijms262210828
APA StylePopa, A. I., Carpa, R., & Farkas, A. (2025). Hydrogel Design Based on Bacterial Exopolysaccharides: The Biomedical Promise of Levan. International Journal of Molecular Sciences, 26(22), 10828. https://doi.org/10.3390/ijms262210828






