1. Introduction
A variety of regulatory proteins, including transcription factors such as nuclear receptors, oncogenes and tumour suppressors, are found in both the cytoplasm and the nucleus of cells. Their nuclear localisation is essential for transmitting signals to the transcriptional machinery and thereby influencing biological activity. Transcription factors can only regulate gene expression when present in the nucleus, whereas their retention in the cytoplasm prevents this function. Changes in nuclear abundance may therefore reflect critical regulatory processes in development, differentiation, and tumour transformation [
1]. Dysregulation of nucleocytoplasmic transport has been implicated in various pathological conditions, such as neurodegenerative diseases [
2] and carcinogenesis [
3]. Accurate quantification of nuclear staining is thus essential for understanding cellular functions, disease mechanisms, and therapeutic targeting, in particular: the identification of prognostic and predictive biomarkers, monitoring treatment response, drug design, and overcoming drug resistance. Therefore, assessment of this is relevant across multiple life science disciplines, including cell biology, biochemistry, histology, pathology, embryology, and pharmacology.
The nuclear/cytoplasmic distribution of proteins of interest can be determined using microscopic techniques. Common techniques include light and fluorescence microscopy. For light microscopy, the protein of interest is usually detected by two-step indirect immunohistochemistry (IHC), where the protein is detected by an unlabelled primary antibody followed by an enzymatically labelled secondary antibody. The gold standard is the use of HRP-labelled secondary antibodies and diaminobenzidine (DAB) for visualisation. For fluorescence microscopy, the target protein can also be detected with antibodies if the secondary one is labelled with fluorophore, or if the target protein can be directly labelled with GFP [
4].
However, manual scoring is time consuming and not suitable for high-throughput applications. It also introduces significant intra- and interobserver variability, leading to potential bias [
5]. When quantifying nuclear staining, the antigen of interest may be present at varying intensities in both the nucleus and the surrounding cytoplasm. Under these conditions, observers can be influenced by a phenomenon known as simultaneous contrast, where the perception of colour or intensity in one area is influenced by the surrounding context. This effect can cause regions of a slide to appear lighter or darker than the surrounding tissue, leading to inconsistent assessment [
6]. Although observer training is not critical to achieving reproducible results, experienced observers tend to perform scoring more quickly [
7]. Furthermore, interobserver variability in the interpretation of immunohistochemical staining is also influenced by observer personality traits [
8].
Although several plugins for ImageJ/Fiji exist for evaluating immunohistochemical and immunofluorescence staining, none are fully adequate for assessing nuclear localisation of antigens that also exhibit cytoplasmic positivity. IHC Profiler can evaluate DAB staining [
9] and offers cytoplasmic and nuclear modes; however, these modes differ only in whether positivity is detected automatically (cytoplasmic mode) or by user-defined thresholds (nuclear mode), and the algorithm does not distinguish subcellular localisation. The output consists of pixel counts for each category of positivity and total field intensity, without providing counts of positive cells, or moreover, the background pixels. Andy’s Algorithms [
10], suitable for automated analysis, uses the DAB_IHC macro to measure the count and area of a total selection (haematoxylin and DAB+) and positive selection (DAB+ only), as well as positive intensity. Images are analysed sequentially for total and positive selections, but the macro does not resolve subcellular localisation of the antigen. For fluorescent staining, QuantIF [
11], which was originally developed for the detection of viral infections and reports the percentage of cells with nuclear positivity, is used. The macro creates masks of nuclei and masks of signals, and the results are generated by performing an AND operation on the binary images. However, it is not possible to determine the intensity. The aim of this study is to present LoQANT, an open and freely available ImageJ plugin for reliable and efficient quantification of nuclear protein staining, and to demonstrate its applicability by analysing ligand-induced changes in the nuclear localisation of peroxisome proliferator-activated receptor alpha (PPARα) and phosphorylated p38 (phospho-p38) in HT-29 cells.
3. Discussion
Nucleocytoplasmic protein shuttling is an important regulatory mechanism in cell biology and disease. While changes in nuclear signal can reflect such processes, accurate quantification of nuclear staining itself is critical for understanding cellular function and disease mechanisms, as well as for therapeutic targeting, drug design, and overcoming drug resistance. The aim of this study was to evaluate the interobserver variability in assessing the percentage of cells with positive nuclear staining and to provide a standardised ImageJ-based approach for this type of analysis in DAB- or immunofluorescence-stained samples.
As expected, manual scoring produced highly variable results. It was far from achieving good reliability between any two observers. Even the expert observers achieved good but not excellent reliability. Interobserver variability is a well-known phenomenon in manual scoring. The previous study performed by Jaraj et al. showed that subjective assessment of intensity can be performed with a high level of reproducibility, while estimation of staining extent is less reliable [
7]. The other study performed by Varghese et al. comparing the scores of three pathologists showed that the scores may vary significantly [
9]. Our study also showed that observer agreement, as well as time expenditure, is not dependent on the level of experience of the observers. These findings are partially consistent with a previous study that concluded that level of training was not critical for obtaining reproducible results, although experienced observers were faster [
7]. Interestingly, interobserver variability in assessment of immunohistochemical staining is also influenced by observer personality [
8]. Pathologists with high conscientiousness scores had the lowest interobserver variability, the highest diagnostic accuracy, and reported fewer tumours as positive. In contrast, those with high neuroticism scores had higher variability, lower accuracy, and reported more tumours as positive [
8]. All of the above demonstrates the need for standardisation.
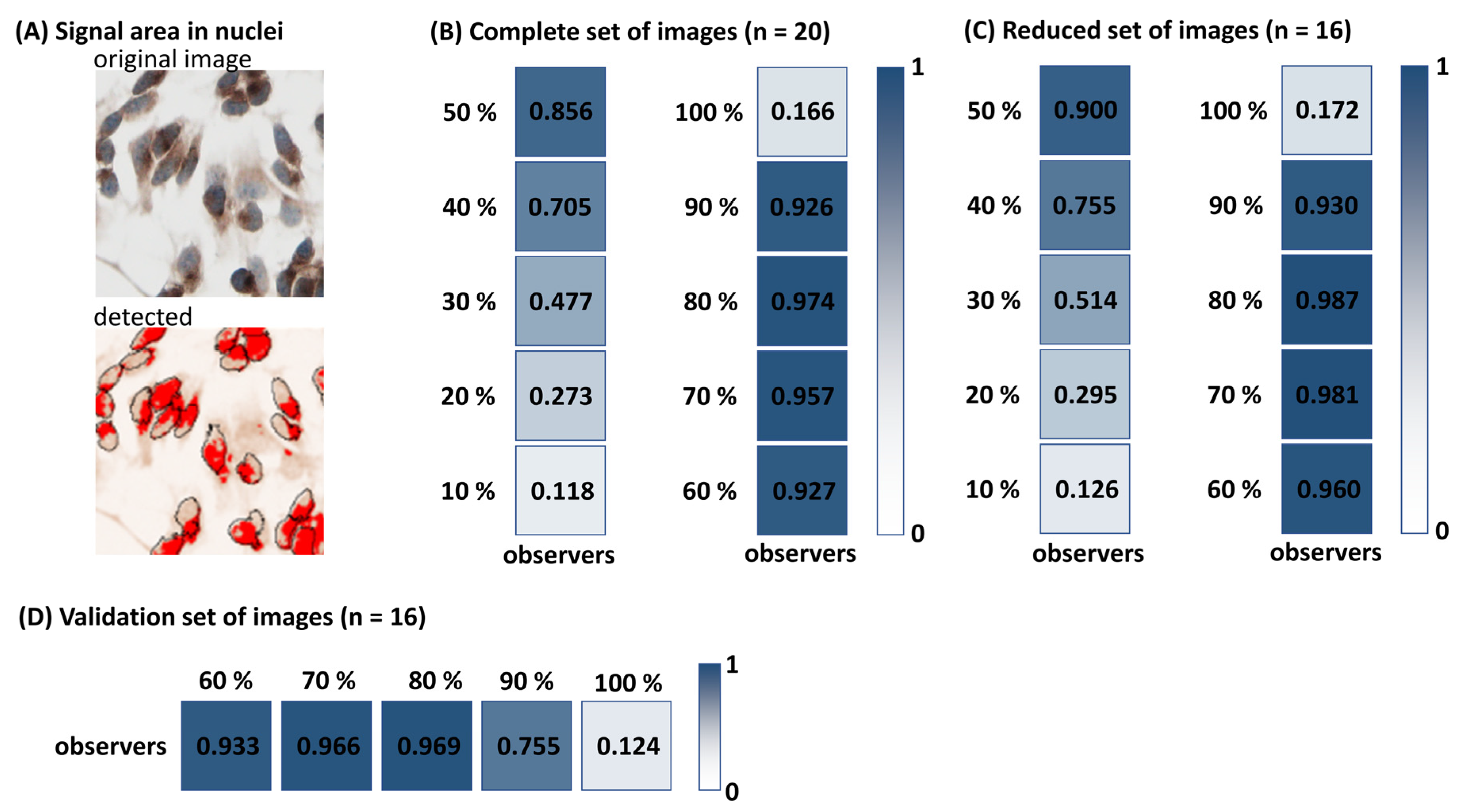
LoQANT evaluates the positivity of the nuclei based on the positive nuclear area. The 80% nuclear area coverage threshold was identified as the optimal criterion for classifying nuclear positivity in LoQANT, yielding the highest concordance with human consensus. It is acknowledged, however, that this validation was conducted using the same cell line and antigen. Extension of the evaluation to independent cohorts and biologically diverse tissue types is therefore recommended to further substantiate the generalizability of this parameter. Importantly, LoQANT allows users to modify the nuclear area coverage threshold according to their experimental needs. For low-abundance nuclear antigens or weakly expressed targets, applying a lower threshold may provide a more accurate representation of nuclear positivity, as the default 80% cut-off could lead to underestimation. Users are thus encouraged to empirically determine and validate the most appropriate threshold for their specific datasets.
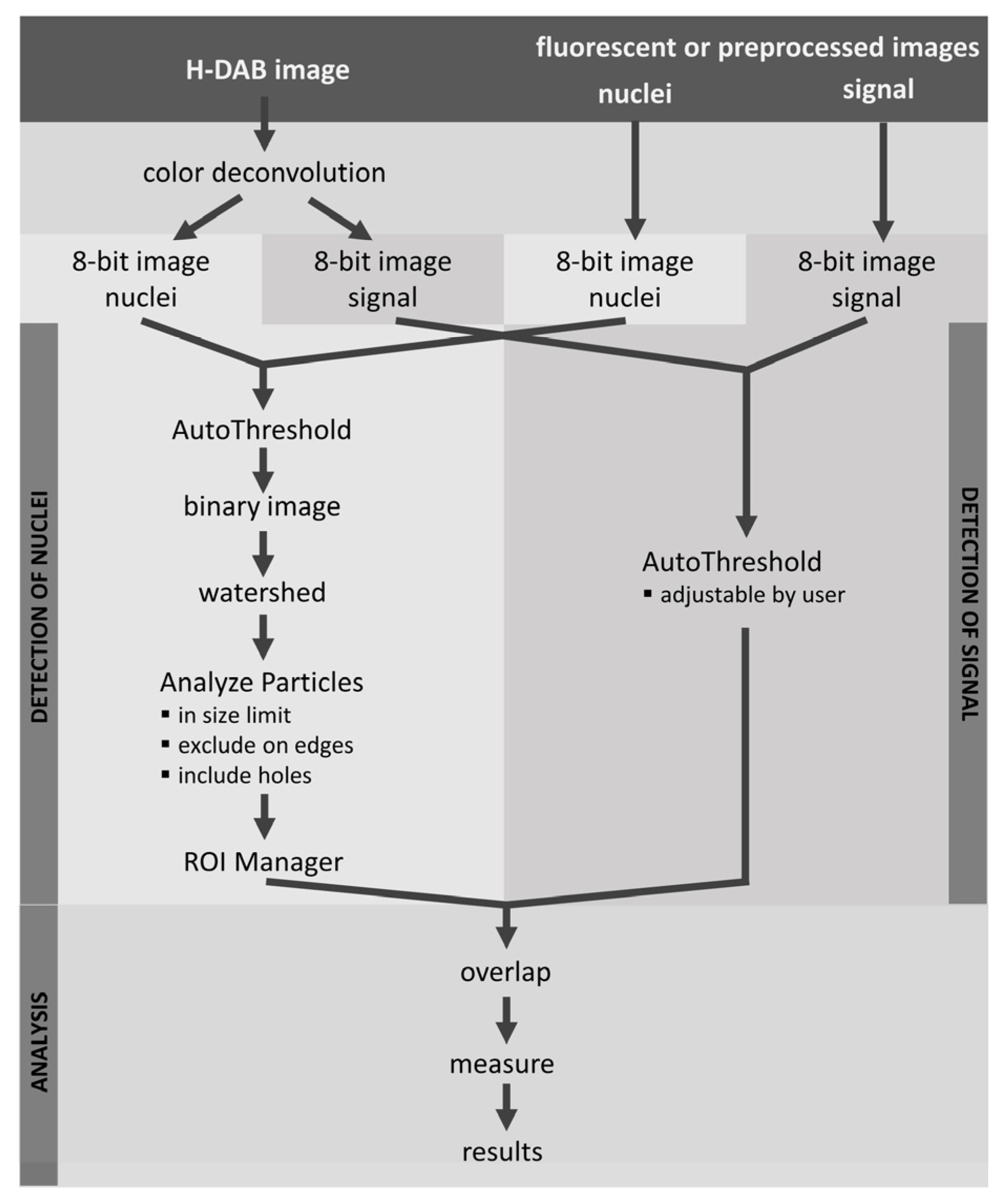
A major advantage of LoQANT is that it exclusively evaluates the positive signal in the nucleus, independent of cytoplasmic staining. To the best of the author’s knowledge there is currently no straightforward way to perform this type of dedicated nuclear positivity analysis in ImageJ/Fiji. The essential step for successful LoQANT analysis is accurate detection of nuclei. By default, LoQANT employs the AutoThreshold method provided by ImageJ/Fiji to segment nuclei and signals. However, in some cases this approach may not provide optimal results. To address this limitation, LoQANT provides an option to analyse preprocessed images (“preprocessed images: H-DAB” method) allowing segmentation of nuclei by external tools prior to analysis. This feature supports flexible integration of different segmentation strategies, including AI-based approaches available for ImageJ such as StarDist [
28], Trainable WEKA [
29], or CellPose [
30]. In the present study, this functionality is illustrated by comparing nuclear masks generated with StarDist (deep learning) and Trainable WEKA (classical machine learning), followed by analysis in LoQANT when both approaches produced highly consistent results.
LoQANT can be used across a wide range of sample types. It has been successfully applied to a variety of biological images, including cells grown directly on slides, smears, and formalin-fixed, paraffin-embedded tissue samples. Other advantages of the provided algorithm include elimination of interobserver visual perception bias and requiring minimal supervision for analysis; thus, it significantly eliminates the dependence on a trained pathologist/histologist, reducing the time burden for analysis, requiring only a few steps to follow the analysis and measurement of staining intensity.
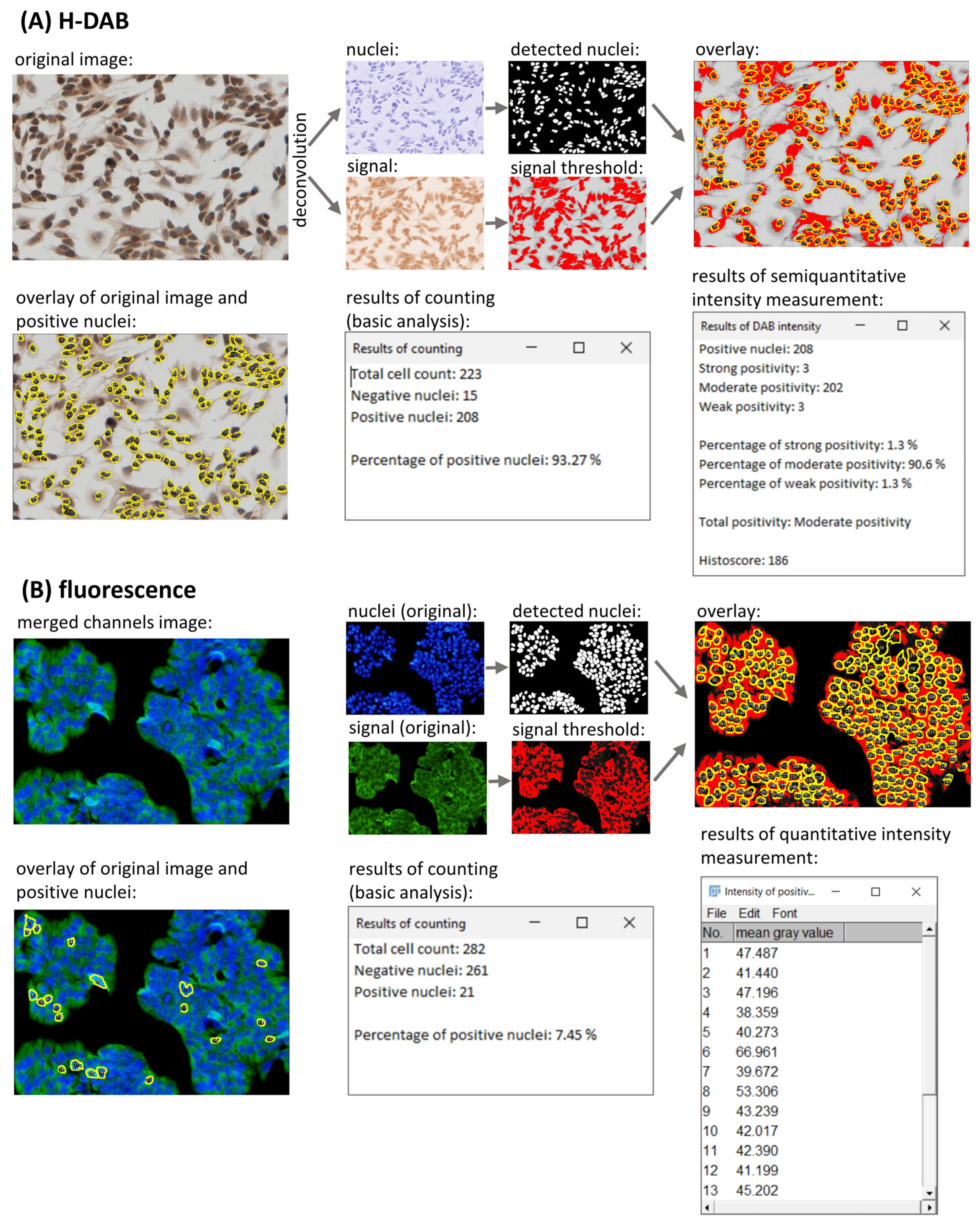
As an option, LoQANT provides measurement of staining intensity using two methods: semiquantitative and quantitative. The semiquantitative measurement provides the number of positive nuclei in three discrete categories: weak, moderate, and strong intensity [
25]. The determination of the category is based on the mean gray value using the previously described category threshold [
9]. In addition, the histoscore and overall positivity category for a given field of view are also determined [
25,
26]. This semiquantitative approach is used for H-DAB-stained specimens. DAB staining is not stochiometric, therefore the darkness of the stain does not equate to the expression of a particular antigen. It does not follow the Beer–Lambert law, which describes a linear relationship between the concentration of a compound and its absorbance or optical density. As a result, dark-stained DAB has a different spectral shape than light-stained DAB. In addition, H-DAB staining also uses a series of amplification steps to visualise the results [
31,
32,
33]. Although IHC is a qualitative laboratory assay and the extent of linearity of the assay is unknown, some linearity is assumed; lower staining intensity is expected to represent weaker expression of the antigen of interest and vice versa, at least in most clinically used IHC biomarker assays. The histoscore has been widely used in pathology research and has also been shown to be potentially useful for some predictive biomarkers [
27]. In contrast to enzymatic detection by DAB, fluorescence is fully quantifiable and LoQANT provides the option to measure intensity of each nucleus.
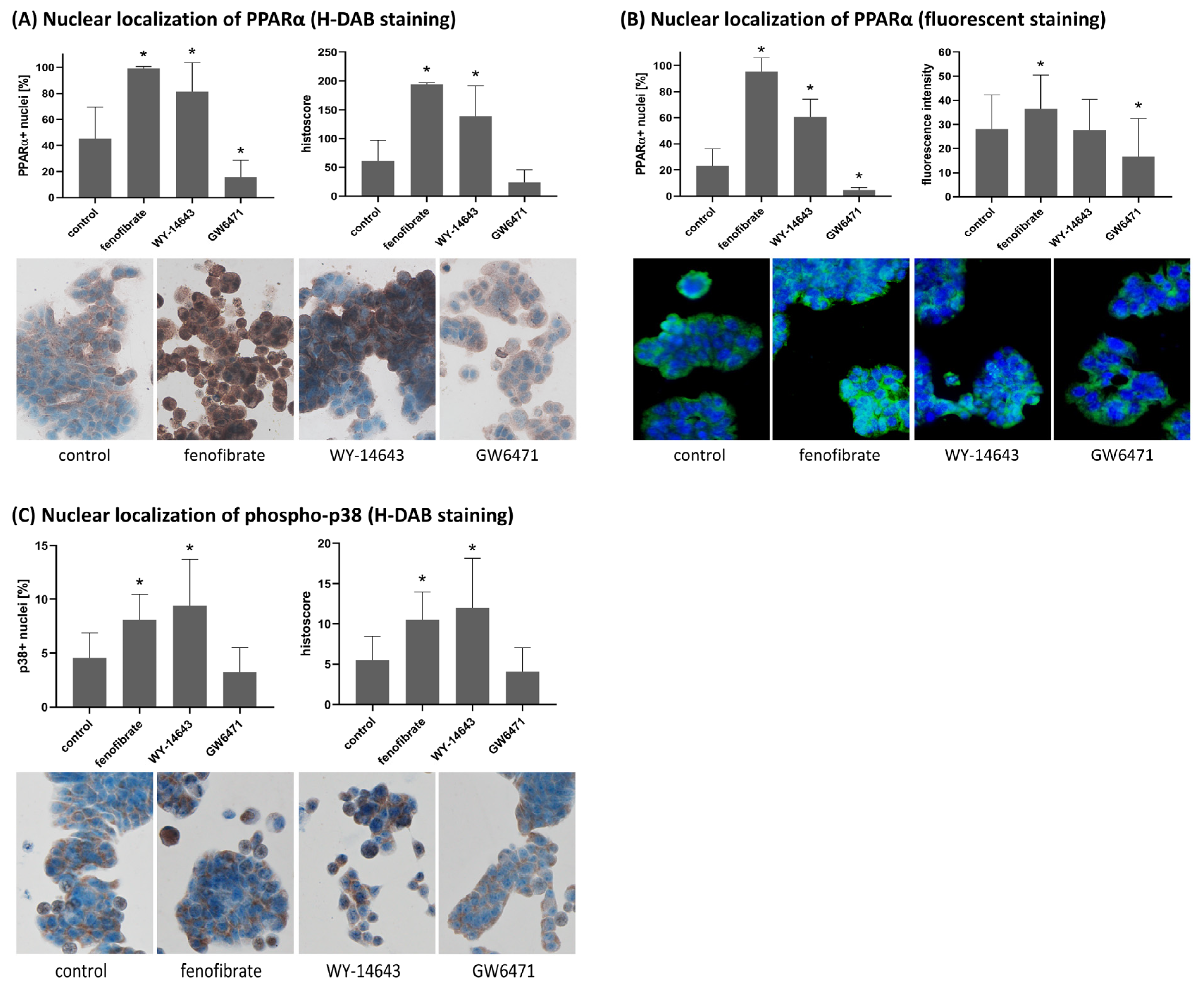
The biological examples included in this study demonstrate the practical usefulness of LoQANT in quantifying nuclear staining of PPARα and phospho-p38 in colorectal adenocarcinoma-derived HT-29 cells. Both proteins are known to undergo nucleocytoplasmic shuttling, and changes in their nuclear abundance are functionally relevant. PPARα is a nuclear receptor that regulates genes involved in lipid metabolism, energy homeostasis, and inflammation. In HT29 cells, fenofibrate and WY-14643 increased nuclear PPARα, whereas GW6471 decreased it, consistent with their expected effects [
34,
35,
36,
37]. p38 is a stress-activated kinase that regulates gene expression, cell cycle checkpoints, and apoptosis. Given its critical role in colorectal cancer progression and therapy resistance, targeting the nuclear translocation or activity of p38α emerges as a promising therapeutic strategy. In colorectal cancer, nuclear p38α activity has been linked to chemoresistance, whereas its inhibition can sensitise cancer cells to chemotherapeutic agents such as 5-fluorouracil. Moreover, blocking nuclear p38 has been shown to inhibit tumour growth in inflammation-associated colon cancer models and to improve responses to chemotherapy [
38,
39,
40]. LoQANT analysis showed that nuclear phospho-p38 increased after fenofibrate and WY-14643 treatment but was unaffected by GW6471, suggesting a link between PPARα activation and p38 nuclear translocation. This highlights a potential link between PPARα activation and p38 nuclear translocation [
41,
42] which should be confirmed in follow-up studies or orthogonal assays. These results emphasise that automated, high-throughput quantification of nuclear positivity can reveal subtle yet biologically significant changes in protein localisation. LoQANT is therefore a powerful tool for quantifying nuclear staining, providing objective and reproducible measurements. By enabling precise and reliable assessment of nuclear signals, the plugin complements traditional manual methods and can support studies of dynamic protein localisation, including processes involving nucleocytoplasmic shuttling.
When using LoQANT, it is recommended that good image analysis practices are followed to obtain valuable results, e.g., fixation, staining, image acquisition settings, exposure, contrast, and brightness are consistent across a cohort of samples [
43]. For LoQANT to work properly, the size of the nuclei in pixels must be specified. This will filter out artefacts or overlapping nuclei. Furthermore, it is recommended to test which of the available algorithms for automatic thresholding is suitable for the measured samples.
LoQANT is a dedicated ImageJ/Fiji plugin designed for the targeted quantification of nuclear protein localisation. Other open-source platforms for image analysis, such as QuPath [
44] and CellProfiler [
45], are also available, in addition to Fiji. Both QuPath and CellProfiler are general-purpose image analysis tools. QuPath is optimised primarily for whole-slide and tissue-level studies, providing advanced algorithms for cell detection and intensity-based classification. It can quantify nuclear staining intensity in both H-DAB and fluorescent images, and compute parameters such as the percentage of positive cells, H-score and Allred score. However, batch analysis in QuPath is usually carried out using user-customised scripts [
44]. CellProfiler, on the other hand, enables flexible, high-throughput image quantification through modular, customisable pipelines, but it requires extensive parameter optimisation and is not specifically optimised for the assessment of nuclear localisation [
45]. By contrast, LoQANT offers a lightweight, task-specific tool that is seamlessly integrated within the ImageJ/Fiji environment. This facilitates robust and user-friendly quantification of nuclear positivity.
In summary, LoQANT is a computational image processing tool that provides an unbiased and reproducible evaluation of nuclear staining. It assesses the positive signal in the nucleus, independent of cytoplasmic staining, in both H-DAB- and fluorescence-stained samples across multiple sample types. LoQANT significantly reduces analysis time and eliminates the need for highly trained observers, offering a robust and efficient alternative to traditional manual scoring methods.
4. Material and Methods
4.1. Cell Samples
SH-SY5Y and HT-29 cell lines were obtained from the American Type Culture Collection (ATCC Numbers: CRL-2266 and HTB-38, respectively) and authenticated by STR profiles prior to the experiment by the Department of Clinical Genetics, Palacky University, Olomouc. Cells were routinely cultured in DMEM (HT-29; Sigma Aldrich, St. Louis, MI, USA, Cat. No. D6171) and DMEM/F12 (SH-SY5Y; Sigma Aldrich, D8437) supplemented with 10% FBS (HyClone, Marlborough, MA, USA, Cat. No. SV30160.03), penicillin (100 U/mL), and streptomycin (100 mg/L). Cells were incubated at 37 °C in 5% CO2 and passaged twice a week.
Cells were seeded on 8-well cell culture slides (SPL Life Sciences, Gyeonggi-do, Republic of Korea, Cat. No. 30108) at a density of 18,000 cells/well, adhered overnight and treated with the following PPARα ligands: 150 µM fenofibrate (Cayman Chemicals, Ann Arbor, MI, USA, Cat. No. 10005368), 200 µM WY-14643 (Sigma-Aldrich, Cat. No. C7081), or 10 µM GW6471 (Cayman Chemicals, Cat. No. 11697) to achieve a broad spectrum of nucleocytoplasmic distribution of the antigen of interest. The cells were incubated with ligands for 72 h. Stock solutions of PPARα ligands were prepared by dissolving in DMSO. The control cells were treated by an appropriate concentration of DMSO (0.5%).
After incubation period, the cells were fixed in 4% paraformaldehyde for 10 min in RT and stained for the nuclear receptor PPARα (GeneTex, San Antonio, TX, USA, Cat. No. GTX28934, dilution 1:200) and phospho-p38α (dilution 1:1000, Invitrogen, Waltham, MA, USA, Cat. No. MA-5-15177). For H-DAB and fluorescent staining method, the slides were rehydrated, cell membranes were permeabilized with 0.01% Triton-X100, and heat-induced antigen retrieval was performed in citrate buffer pH 6 for phosphor-p38α or EDTA pH9 for PPARα. In both cases, this step was performed at 120 °C for 15 min in Histos device. After pre-treatment with PolyDetector Peroxidase Blocker (Bio SB, Santa Barbara, CA, USA part of the detection kit) for 5 min and ProteinBlock (Dako, Glostrup, Denmark) for 30 min, samples were incubated with primary antibodies for 1 h at RT. For H-DAB method, visualisation was performed using the Mouse/Rabbit PolyDetector DAB HRP Brown Kit (Bio SB, Santa Barbara, CA, USA, Cat. No. BSB 0205). For fluorescent method, Opal520 fluorophore from Opal 3-Plex Manual Detection Kit (Akoya bioscience, Marlborough, MA, USANEL810001KT) was used according to vendor protocol. Briefly, the slides were incubated with Opal Anti-Ms + Rb HRP for 10 min (part of the kit) and then with Opal520 for 10 min. Finally, heat-induced antigen retrieval was performed again. The nuclei were counterstained with haematoxylin or DAPI depending on the method of visualisation and cover slipped. Tris buffer with TWEEN 20 (pH 7.6) was used for washing between different steps.
Images were captured using an Olympus BX40 microscope equipped with an Olympus DP71 camera (Olympus, Shinjuku, Japan) at 200× magnification. All images were taken at a resolution of 1920 × 1200 pixels and saved as .tif files. Twenty images with different intensity of immunostaining in the nucleus and cytoplasm of each method were selected for interobserver variability testing and LoQANT settings. Moreover, the validation set of different images (n = 15) of SH-SY5Y cells stained for PPARα was collected.
4.2. Manual Samples Evaluation of PPARα
The selected H-DAB-stained images were manually scored by six observers with different levels of experience: two experts (O1 and O2), two trained observers (O3 and O4), and two observers with no previous experience (O5 and O6). Both O5 and O6 underwent identical training provided by the same expert shortly before the analysis which included scoring of different images under supervision. A session was conducted prior to the main analysis where observers received identical instructions and reference examples of nuclear positivity. All of them used the manual counting tool provided in ImageJ/Fiji. The evaluation resulted in the percentage of cells with nuclear positivity for each image. In addition, the observers were asked to measure the time taken to score three pre-selected images. The reliability between each pair of observers was evaluated using the intraclass correlation coefficient (ICC). Moreover, each observer’s percentage score against consensus defined as the mean of all other observers for each image was evaluated. Mean difference, standard deviation (SD), 95% confidence interval (CI) of the mean difference, intraclass correlation coefficient (ICC), and Pearson correlation coefficient (r) between each observer and the consensus were calculated.
4.3. Agreement Between Observers and LoQANT
The results obtained from the observers were used to determine which nuclei would be classified as positive by LoQANT. The same image set was analysed with LoQANT with varying portions of positive nuclei in the range from 0.1 to 1.0, with 0.1 increments. The nuclei and signal segmentation algorithms were set to “Default”. The size of nuclei was limited to 300–1200 based on pre-measurement areas of 50 cells of the cell line tested (may vary for other cell lines). The measured results (% of positive nuclei) for each overlap fraction were compared with the user results. For each image, the human consensus defined as mean % of cells with positive nuclei were calculated from the values obtained by observers O1, O2, O3, O4, and O6. Observer O5 was excluded (see
Section 2.1). Then, the reliability (ICC) between human observers and LoQANT was calculated.
4.4. Validation of the 80% Threshold for Nuclear Positivity
To validate the 80% nuclear area coverage threshold used to define nuclear positivity, a validation set of 15 different images was evaluated by observers O1, O2, O3, and O4 (observers O5 and O6 were unavailable). The same validation sample set was then processed in LoQANT using nuclear coverage thresholds of 60%, 70%, 80%, 90%, and 100%. The size of the nuclei was limited to 300–1200, the nuclei and signal segmentation algorithm were set to “Default” (as described previously). The reliability (ICC) between human consensus (mean of human observers for each image) and LoQANT was calculated. Moreover, mean difference, standard deviation (SD), 95% confidence interval (CI) of the mean difference, and Pearson correlation coefficient (r) between human consensus and LoQANT were also evaluated.
4.5. Incorporation of Existing AI-Based Segmentation Fiji Plugins to the Analysis Workflow
AI-based plugins for Fiji can be readily integrated into the LoQANT workflow by supplying pre-segmented images as input through the “preprocessed images: H-DAB” option of LoQANT. To demonstrate this, nuclear segmentation of the same set of 20 H-DAB-stained SH-SY5Y cell images was performed using the StarDist 2D [
28] and Trainable WEKA Segmentation [
29] plugins. For deep learning-based segmentation, nuclei were detected using StarDist with the pretrained 2D “versatile (H&E nuclei)” model on original images with default StarDist settings (probability/score threshold = 0.5, overlap threshold = 0.4). These settings were surprisingly very accurate for the tested sample set (see Results section). Prior to classical machine learning-based segmentation, all images were processed with Color Deconvolution available in Fiji. For this approach, Trainable WEKA Segmentation was trained on representative subsets of nuclei and background from the deconvoluted images. The resulting classifier was saved and applied to the entire dataset. The nuclei masks were obtained from both segmentation approaches. After segmentation, the complete set of images was analysed using LoQANT. The input for analysis were the nuclei masks and corresponding DAB signal images.
To validate the AI-based segmentation, the number of nuclei detected per image by StarDist, Trainable WEKA, and human consensus (mean of all observers O1–O4) was compared using mean difference, standard deviation (SD), 95% confidence interval (CI) of the mean difference, intraclass correlation coefficient (ICC), and Pearson correlation coefficient (r). In addition, visual inspection was performed to assess the accuracy of nuclear boundary delineation across the tested sample set.
4.6. Application Examples of the Nuclear Positivity Analysis Using LoQANT
To illustrate the biological applicability of LoQANT, the nuclear staining of two proteins that are known to undergo nucleocytoplasmic shuttling, PPARα, and phospho-p38 in HT29 cells was evaluated. The cells were treated and stained using H-DAB and fluorescence method as mentioned above. Images were captured using an Olympus BX40 microscope equipped with an Olympus DP71 camera at 200× magnification. All images were taken at a resolution of 1920 × 1200 pixels and saved as .tif files. Automated analysis with LoQANT was performed at 20 fields of vision per group using the batch version of the script with default settings. In all cases, the % of positive cells were detected. Moreover, the histoscore for H-DAB staining and intensity measurement for immunofluorescence were also used (intensity measurement method: “quantitative: all nuclei (rec for fluorescence)”). The nuclear localisation of PPARα and p-p38 in comparison to control was assessed using Student’s t-test (two-tailed).
4.7. Statistic Evaluation
Reliability between each pair of observers, between observers and LoQANT, and between LoQANT results after incorporating StarDist and Trainable WEKA Segmentation to the workflow was assessed as intraclass correlation coefficient (ICC), random mixed model, and absolute agreement using IBM SPSS Statistics (ver. 29.0.1.0). Values greater than 0.75 indicate good reliability, while values greater than 0.9 indicate excellent reliability [
46]. Moreover, additional parameters such as mean difference, standard deviation (SD), 95% confidence interval (CI) of the mean difference, and Pearson correlation coefficient (r) between each observer and consensus were calculated. A Pearson r greater than 0.7 indicates strong correlation, while values greater than 0.9 indicate very strong correlation [
47]. The nuclear localisation of PPARα and p-p38 in comparison to control was assessed using Student’s
t-test (two-tailed). The sample size required to achieve a statistical power of 80% (α = 0.05) based on the observed standard deviations was calculated using G*Power software (version 3.1.9.7). Graphs were created by GraphPad Prism 8 software.