Abstract
Heart failure with preserved ejection fraction (HFpEF) currently accounts for half of the heart failure (HF) cases world-wide, affecting nearly 32 million people. HFpEF has a skewed prevalence toward females and those older than 65 years old. The pathophysiology of HFpEF is suggestive of a conglomerate of inflammatory, hypertensive, as well as metabolic dysfunction, giving rise to the syndrome. Disruptions in ceramide metabolism do occur in heart failure as well as within the HFpEF-associated risk factors, both modifiable inflammation, obesity, hypertension, diabetes, and non-modifiable-aging, and female sex. The focus of this review is to draw attention to the links between changes in female biophysiology, such as pregnancy, menopause and aging, in which ceramide is dysregulated and consequently gives rise to the same pathologies that are labeled as risk factors for HFpEF. Our objective is to highlight ceramides as potential biomarkers for prevention and initial diagnostic tools for HFpEF, especially for women later in life.
1. Introduction
Perturbations in lipid biosynthesis and metabolism are emerging as targets for biomarkers in chronic and often evasive diseases such as HFpEF (heart failure with preserved ejection fraction) [,]. The sphingolipid ceramide has gained attention in recent years for its association with increased risk for CVDs (cardiovascular diseases) including heart failure [,,,,,]. The mechanisms and relationships between ceramide and the heart failure phenotype HFpEF are paramount to understanding the pathophysiology of HFpEF. HFpEF accounts for nearly 50% of HF (heart failure) cases and has predictions of an annual increase in prevalence of 1% above HFrEF (HF with reduced ejection fraction) [,]. HFpEF risk factors include hypertension, obesity, diabetes, as well as female sex and aging. These risk factors often co-exist and confound HFpEF diagnosis and treatment. An important aspect of HFpEF is its skewed prevalence toward the female sex, accounting for 50–60% of cases [,,]. This sexual dimorphic nature of HFpEF points to the obvious physiological differences between the male and female life terms, such as the impact of pre- and post-menopausal estrogen-E2 (17β-estradiol) fluctuations and obstetric factors on female cardiovascular health [,,,]. Ceramide levels are not only known to fluctuate in line with these different stages in the female life term [,,], but also within the context of the other HFpEF risk factors [,,,,]. An event that ceramide is known to impact in the female life cycle is pregnancy. Ceramides act as stress mediators during implantation, delivery, and lactation [], and its levels are increased in pregnant versus non-pregnant women [,]. The chances of having CVDs, including HFpEF, increase with pregnancy-related complications such as GDM (gestational diabetes mellitus) and hypertensive disorders in women [,,]. Circulating ceramide levels are increased in these pregnancy complications and have been investigated as potential biomarkers for early detection or prediction [,]. Age also impacts plasma ceramide levels, with higher levels observed in post-menopausal (47–78 years old) compared to pre-menopausal women []. Since HFpEF is prevalent in females aged ≥60 years of age, understanding how these changes in ceramide over the female life term contribute to the risk factors of HFpEF may lead to ceramides being a potential biomarker for HFpEF in females. In this review, we start with a brief overview of the complexities involved in ceramide biosynthesis that can feed into ceramide dysregulation in multifactorial diseases such as HFpEF. We also evaluate and highlight how ceramide plays a role in multiple HFpEF risk factors, such as hypertension, obesity, diabetes, and aging in females, especially those with a history of multiple pregnancies and pregnancy-associated disorders. while demonstrating this we highlight the lack of female-focused research in terms of these risk factors over the life of the female.
2. Brief Overview of Ceramide Biosynthesis and Biology
Ceramide is synthesized through the de novo sphingolipid biosynthesis pathway Figure 1). Ceramide synthesis takes place at the leaflet of the ER (endoplasmic reticulum), where SPT (serine palmotyl transferase) catalyzes palmitoyl-CoA with L-serine, subsequently forming 3KS (3 ketosphinganine). 3KS is reduced to dhSph (dihydrosphingosine) by 3KS reductase. CerS1–6 (ceramide synthases 1–6) then acylate dhSph with a fatty acyl-CoA to form dhCer (dihydroceramide) with defined chain lengths (C14–C26). Ceramide chain length is important in diseases, including CVDs. The insertion of a 4–5 trans-double bond in dhCer by the enzymes DES1–2 (dihydroceramide desaturase 1 and 2) results in the formation of ceramide. Ceramide is then transported from the ER to other sites such as the trans-Golgi apparatus at membrane contact sites via vesicle and non-vesicle transport systems to produce complex sphingolipids such as glucosylceramide []. Ceramide can also be generated from enzymatic degradation of complex sphingolipids through SM (sphingomyelin) hydrolysis or the salvage pathways []. The SM hydrolysis pathway involves SMase (sphingomyelinase) converting SM to ceramide and the salvage pathway involves CerS1–6 that salvage or recycle ceramide from the catabolic breakdown of the sphingolipid backbone, resulting in production of Sph (sphingosine) which is re-acylated to ceramide. Multiple enzymes are involved in the synthesis of ceramides, and targeting these in in vivo and in vitro models has shown how ceramide can play a role in diseases. For example, targeting ceramide synthesis via ablation of genes such as Spltc2, degs1, and CerS or pharmacological inhibition of the enzymes they code for leads to reductions in ceramide accumulation and disease progression []. The CerS1–6 are crucial enzymes involved in catalyzing the final step in ceramide formation by attaching fatty acids to the sphingolipid backbone. These enzymes exhibit distinct substrate specificity for fatty acyl-CoA, leading to generation of ceramides with specific acyl chain lengths []. CerS1 primarily leads to the generation of C18 ceramides, CerS2–3 synthesizes very long-chain (C20-C26) ceramides, CerS4 synthesizes C18-C20 ceramides, while CerS5–6 synthesizes C14 and C16 ceramides []. The enzyme DES1–2 also plays a gatekeeper role in the production of ceramide from dhCer. In cellular experiments utilizing the DES1 inhibitor fenretinide, cells have a tendency to increase dhCer with significant reductions in apoptosis. Even when particular subsets of the pathway enzymes are targeted there is a compensatory increase in the other enzymes, producing biological effects that are specific to that enzyme subtype. For example, depletion of CerS2 leads to compensatory upregulation of CerS6 and therefore an increase in C16:0 ceramides.
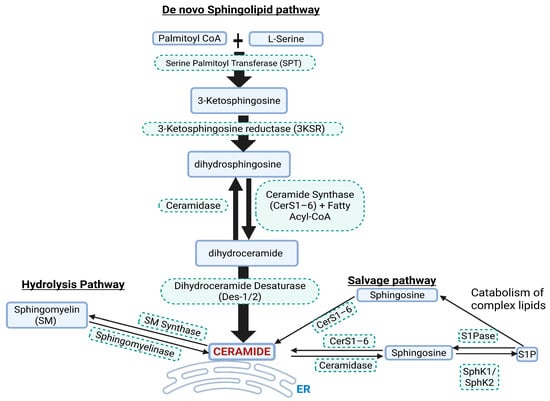
Figure 1.
Simplified pathways for Cer synthesis. ER = endoplasmic reticulum, SphK1/SphK2 = sphingosine kinase 1 and 2, S1P = sphingosine 1 phosphate, S1Pase = sphingosine 1 phosphatase.
Ceramides serve as both structural components of cell membranes and bioactive mediators in key cellular signaling pathways and homeostasis. In normal physiology, ceramides regulate a variety of essential processes including apoptosis, membrane integrity, stress adaptation, and insulin signaling modulation [,,]. Ceramides contribute to apoptosis by serving as second messengers in intrinsic and extrinsic apoptotic pathways. They accumulate in response to cellular stress and can promote mitochondrial outer membrane permeabilization, facilitating cytochrome c release and activation of caspases []. Under physiological conditions, this apoptotic role is tightly regulated and contributes to normal tissue remodeling, immune surveillance, and turnover of damaged cells. Ceramides contribute to membrane integrity through their ability to induce coalescence of lipid rafts—rigid and ordered microdomains within the cell membrane that act as platforms for numerous cellular signaling processes [,]. This structural function supports efficient cellular communication and membrane dynamics during normal cellular functions.
Ceramides also act as critical mediators of the cellular stress response to oxidative stress, UV radiation, hypoxia, and inflammatory cytokines. Moderate ceramide accumulation in this context acts as an adaptive signal to pause cell proliferation, enhance repair, or trigger controlled apoptosis when damage is irreparable []. In terms of metabolic regulation, ceramides modulate insulin signaling by acting as negative regulators of the PKB (Akt/protein kinase B) pathway. Under normal metabolic states, ceramides help fine-tune insulin sensitivity and energy storage by providing feedback inhibition during nutrient surplus []. This negative feedback is enabled through promotion of Akt dephosphorylation via activation of PP2A (protein phosphatase 2A) or by preventing Akt translocation to the cell membrane via activation of PKCζ (protein kinase C zeta) []. In states of overt obesity, diabetes, and inflammation these mechanisms can become maladaptive, contributing to IR (insulin resistance). This balancing role played by ceramide underscores its dual nature as helpful in moderation but harmful in excess or chronic elevation. Ceramide levels can rise excessively due to aging, obesity, or systemic inflammation, contributing to CVDs such as HFpEF.
3. Current Paradigm of Ceramides in HFpEF Pathophysiology
Ceramide involvement in various disease states has been reviewed recently [,,]. They have emerged as important bioactive lipids involved in HF and CVD and play crucial roles in systemic inflammation, metabolic syndrome, and diabetes [,,], all of which are indicators for HFpEF diagnosis. In the failing myocardium of human and animal HFrEF models, accumulation of ceramide in both plasma and cardiac tissue is known to be involved in cardiac remodeling and dysfunction [,]. In HFpEF, biopsies of the myocardium are rare and scarce, and data on circulating ceramide levels in HFpEF are limited. Ceramide CVD risk is related to long-chain ceramides, especially C16:0, C18:0, and C24:0 [,]. For example, increased ceramides C16:0 and C18:0 in circulation were shown to be associated with death or heart failure admission in HFpEF patients in a subset of the TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) []. An important aspect of potential ceramide involvement in HFpEF pathophysiology is ceramide’s ability to impact cellular bioenergetics. Ceramide increases mitochondrial permeability, disrupting the electron transport chain, reducing adenosine triphosphate (ATP), and increasing reactive oxygen species (ROS). These lead to disruptions of mitochondrial bioenergetics, which impacts cells that have high energy demand such as cardiac myocytes [,,]. This is important in HFpEF, where the cardiac energetic reserves are evidently reduced [,,]. Recent findings further support the cardiac myocytes’ ability to produce and use ketones as energy substrates in the setting of HFpEF []. Furthermore, HFpEF therapies targeting cardiac energetics and metabolism such as semaglutide (glucagon-like peptide-1, GLP-1, agonist) and Dapagliflozin (Sodium glucose transport protein 2 inhibitor—SGLT2i) have been shown to reduce worsening HF [,]. HFpEF is increasingly being recognized as a cardiometabolic disorder, with several publications from our group and collaborators [,,]. In addition to this, alterations in lipid metabolism due to metabolic disorders have recently being linked to HFpEF [,,].
This link between metabolic disorder and HFpEF is of relevance to HFpEF pathophysiology, where the disease prevalence has increased in line with increasing obesity and diabetes as well as an aging population []. Both diabetes and obesity are categorized as metabolic disorders and are associated with abnormalities in lipidomics []. These contribute to IR and often lead to increased flux of fatty acids to other tissues including the heart, resulting in cardio-lipotoxicity fueled by the build-up of lipid species such as DAGs (diacylglycerols) and ceramide [] and disruptions to other categories of lipids including sphingolipids. In addition, there are several unique sex-specific physiological and biological features, such as pregnancy and pregnancy-associated disorders, that can prime the heart for HF events, leading to HF phenotypes such as HFpEF as the female ages. Clinically, women with a history of multiple pregnancies have a more advanced HFpEF hemodynamic phenotype compared with women with no history of pregnancies, and those who develop HFpEF are more likely to have hypertensive disorders in pregnancy [,,]. Example, increased plasma ceramide levels have been shown to be positively correlated with pre-eclampsia and the HELLP syndrome, particularly C16:0, C18:0, C18:1, C20:0, C22:0, and C24:1 [,]. Ceramide has also been proposed as a biomarker to track the progression of pregnancy-associated complications and guide clinical interventions []. Since HFpEF is a multifactorial disease, the proceeding sections assess ceramides’ effect in the setting of its risk factors.
4. Effect of HFpEF Risk Factors on Ceramide Biology
HFpEF is associated with a plethora of modifiable and non-modifiable risk factors. These include hypertension, diabetes, obesity, aging, and female sex. Each of these risk factors do have an impact on ceramide synthesis and signaling, which are summarized in Table 1.
4.1. Relationship of Estrogen Depletion and Ceramide
Increasing chronological age is a key non-modifiable risk factor for the development of HFpEF. In females this risk is compounded by a reduction in the sex-specific hormone estrogen due to ovarian aging or menopause. In the last two decades, several longitudinal studies have helped distinguish the effect of menopause and chronological aging effects on women’s cardiovascular health [,,,,,]. The strongest of this evidence was provided by the ongoing SWAN (Study of Women’s Health Across the Nation) heart study []. Data from these studies suggested that transitioning to menopause accelerates CVD risk factors such as shifts in body composition, increased blood pressure, worsening IR, and disruptions in lipid profile. In fact, ceramide levels within the cardiovascular system are known to increase with declining estrogen levels [] and are comparably higher in older females (>63 years old) than males [,].
In menopausal women, the major form of E2 (17β-estradiol) is reduced by 80–90%, as E1 (estrone) becomes the main type of estrogen in circulation []. E2 is predominantly synthesized by the ovaries in pre-menopausal women. Other extra-gonadal sites, such as brain, heart, skin, bone, vascular endothelium, and aortic smooth muscle cells as well as adipose tissue, produce E2 in negligibly low quantities [,]. Reduction in E2 during and post-menopause significantly affects its role in counteracting inflammatory, apoptotic, endothelial dysfunction and cellular senescence signaling within the cardiovascular system [,,]. E2 signaling can be both genomic and non-genomic through its receptors, ERα, ERβ (estrogen receptors alpha and beta), and GPER (G-protein-coupled estrogen receptor), which are encoded by ESR1, ESR2, and GPER1 genes, respectively. E2 can modulate ceramide levels affecting energy balance and cellular processes. Evidence from E2-supplemented animal models of ovarian insufficiency have shown that E2 can reduce ceramide levels in the hypothalamus by reducing the rate-limiting enzymes SPT1 and SPT2 in the de novo synthesis pathway, conferring a protective role against obesity []. The main evidence for ceramide regulation by E2 comes from studies in human MCF-7 breast cancer cells [,]. In this cell line, E2 was able to increase cancer cell survival by stimulating the sphingolipid pathway enzyme, SphK, to convert ceramide to S1P. Ceramide primarily targets non-genomic E2 signaling pathways, particularly the pathways activated through cell membrane-associated estrogen receptors for cell survival such as PI3K/Akt (Phosphatidylinositol 3-kinase/Akt) and AMPK (AMP activated protein kinase) [,] and anti-inflammation signaling via inhibition of NF-κB (Nuclear Factor Kappa B) []. In the context of both chronological and ovarian aging, increased levels of ceramide impose opposite effects on these pathways, with increased cellular stress, apoptosis, and inflammatory signaling [,].
In women, estrogen has protective effects against ceramide accumulation, and its decline may exacerbate the effects of ceramide in the aging heart, contributing to higher prevalence of HFpEF in post-menopausal women []. Reduced estrogen could also indirectly increase cardiac stiffness and reduce autophagy, impairing cardiac function [,]. In a model of cardiac aging, E2 supplementation increased autophagy through activation of the key regulator of autophagy, Beclin 1, improving cardiac function []. In cardiac myocytes, states of ceramide overload increase autophagy/mitophagy, increasing mitochondrial stress and cardio-lipotoxicity []. An interesting aspect of myocardial stiffness and estrogen is related to the key cardiac structural protein titin and its phosphorylation by PKA (protein kinase A), PKG (reduced stiffness), and PKC (increased stiffness). Estrogen through ERα modulates titin’s subunit ratio by increasing cGMP-PKG (cyclic guanosine monophosphate–PKG) activation, leading to reduced stiffness []. These indicate the inverse (increased cardiac stiffness and auto/mitophagy) is true in states of estrogen depletion.
4.2. Contribution of Ceramide to Cell Senescence and Inflammaging
In addition to diminishing E2 levels, cell senescence plays a fundamental role in aging and inflammaging. Cellular senescence occurs in response to cellular stress or damage due to chronological aging. Ceramides also accumulate with age in circulation and multiple organs across various species []. Notably, the very long-chain C24:1 ceramide increases in serum extracellular vesicles with age in both humans and non-human primates, potentially contributing to cell senescence []. Ceramide levels in the female cardiovascular system have been shown to increase up to 4-fold in senescent cells []. Ceramide’s unique biophysical properties enable it to alter cellular membrane characteristics and trigger cell senescence events []. These events include DNA damage via mitochondrial ROS (reactive oxygen species) generation, cell cycle arrest, re-enforcement of senescence SASP (senescence-associated secretory phenotype), inflammatory signaling, and lysosomal stress. For example, ceramide localizes at biological membranes such as the outer mitochondrial membrane, where it self-assembles into stable, barrel-stave channels, allowing the selective release of pro-apoptotic proteins such as cytochrome c which lead to the activation of caspases []. Increased cytochrome c in the cytosol disrupts electron-transport-chain complex III and IV, causing electron leakage, generation of ROS, and diminished ATP (adenosine triphosphate) production [,]. Subsequently, increased ROS causes DNA damage and activation of DDR (DNA damage response), which is a hallmark of senescence []. Additionally, senescent cells exhibit SASP, a complex mixture of pro-inflammatory cytokines, chemokines, growth factors, and proteases, to reinforce senescence in both an autocrine and paracrine manner []. This affects the surrounding tissue microenvironment and contributes to aging and aging-associated disorders. Ceramide can contribute to this SASP-generated microenvironment by initiating, amplifying, or maintaining the activation of NF-κB [,] and the stimulation of MAPK (mitogen activated protein kinases) and JAK/STAT (Janus kinase/Signal transducer and activator of transcription) pathways [,]. Signaling through these pathways drive the transcription of inflammatory factors such as IL6 (interleukin 6), Il8, TNF-α (tumor necrosis factor alpha), and MMPs (matrix metalloproteinases). These can then contribute to inflammaging and exacerbate the risk for HFpEF development in the aging female (Figure 2) [].
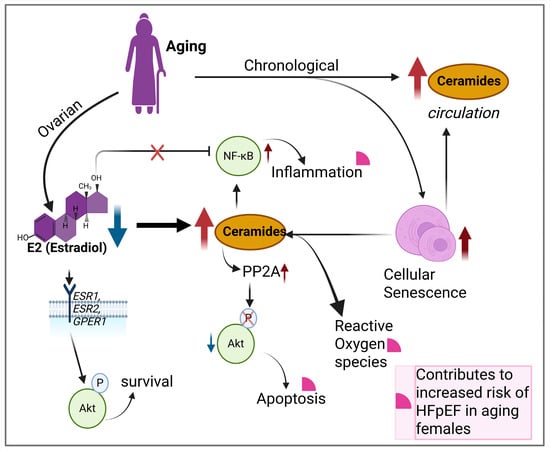
Figure 2.
Ovarian and chronological aging result in elevated ceramide levels that contribute to increased inflammation, apoptosis, ROS, and increasing risk for HFpEF development. Blue arrow = reduction, red arrow = increase or accumulation, black arrows = forward effect and pink quarter circles = indicates contributors to HFpEF.
4.3. Ceramide’s Role in Hypertension
Hypertension is the most prevalent and well-established risk for HFpEF development. It accounts for nearly 80–90% of HFpEF cases []. Other risk factors can also induce hypertension through a complex interplay of factors that affect vascular homeostasis as the disease progresses. Vascular homeostasis encompasses a balance in redox signaling, anti- or pro-inflammatory signaling, mechano-sensing, and endothelial integrity []. Ceramide is able to contribute to or disrupt vascular homeostasis. However, there is greater emphasis on the link between elevated ceramide levels and its detrimental effects on vascular health through increased inflammation, oxidative stress, endothelial dysfunction, and neurohormonal dysregulation [].
4.3.1. Ceramide Promotes Vascular Oxidative Stress and Inflammation
Redox signaling in the vascular system is crucial to maintain a plethora of physiological processes including vasoconstriction and vasocontraction. The free-radical NO (nitric oxide) plays a key role in redox signaling. NO is made available from the conversion of l-arginine by eNOS (endothelial nitric oxide synthase), which is produced in the EC (endothelial cells) in response to shear stress or agonists such as acetylcholine []. NO then diffuses into the surrounding VSMC (vascular smooth muscle cell) where it activates sGC (soluble guanylate cyclase) to increase the production of cGMP. Elevated cGMP binds to PKG, causing a conformational change that activates PKG and leading to Ca2+ (calcium) reuptake into the sarcoplasmic reticulum which lowers intracellular Ca2+ and promotes vasodilation. Ceramide has been shown to trigger Ca2+ influx through recruitment of TRPC6 (transient receptor potential canonical 6) channels to the cell membrane []. This Ca2+ influx through TRPC6 channels activates the Rho/ROCK pathway, enhancing Ca2+ signaling and contraction and leading to vascular remodeling as observed in hypoxic pulmonary vasoconstriction studies [,,]. Ceramide has also been shown to initiate the increased association of PP2A with eNOS in EC, leading to dephosphorylation of eNOS at ser1177 []. Dephosphorylation causes eNOS to uncouple, promoting the production of ROS such as superoxides instead of NO. Although short-term exposure of isolated human arteriole tissues to ceramide causes rapid switching of the mediators of flow-induced dilatation from NO to H2O2 (hydrogen peroxide) to preserve flow-induced dilatation [], this can be detrimental in the long term []. Feng and colleagues [] recently showed that ceramide 16:0 and ceramide 16:0/24:0 ratios were positively associated with cumulative coronary microvascular resistance in patients with coronary artery disease. This is not surprising since an increase in ROS in the vasculature eventually leads to oxidative stress and activation of redox sensitive transcription factors such as NF-κB, amplifying inflammatory gene expression and the activation of vasoactive proteins (ICAM-1, VCAM-1, E-selectin) within vessels. The knock-on effect of this is increased vascular stiffness and resistance, culminating in increased blood pressure or hypertension [,,]. A clear connection between ceramides and oxidative stress in aging was made by a recent study comparing cardiac tissue from young and aged donor hearts with no known cardiac-related pathologies []. It is worth noting here that 7 out of the 13 aged donors were females aged between 50 and 65 years of age.
Interestingly, ceramide has been shown to activate the NLRP3 (NOD-like receptor protein 3) inflammasome in pulmonary microvascular EC []. NLRP3 is a key component of the innate immune system []. Ceramide activates NLRP3 via upregulation of the Txnip gene (thioredoxin-interacting protein), inducing the secretion of inflammatory cytokines []. HFpEF patients have been shown to have raised levels of circulating NLRP3, which is also linked to the pathogenesis of pregnancy complications including pre-eclampsia [,,]. In women with pre-eclampsia, this inflammatory state can persist up to a median of 2 ½ years and contributes to the onset of HFpEF []. Additionally, enhanced systemic inflammation is thought of as a key player and a probable non-invasive diagnostic tool for HFpEF [,,,]. Unresolved ROS and systemic inflammation contribute to endothelial dysfunction.
4.3.2. Ceramide and Endothelial Dysfunction
Endothelial dysfunction is a key disease mechanism for hypertension development and a feature of HFpEF, ultimately contributing to its progression []. ECs line blood and lymphatic vessels in the body and are an essential interface between blood and vessels in maintaining tissue homeostasis. Both a lack and accumulation of ceramide can cause endothelial dysfunction. A recent animal study showed that suppression of ceramide and S1P in the EC of high-fat-diet-fed mice underlies vascular and metabolic dysfunction []. Whether this depletion of ceramide in EC was a counter-regulatory mechanism of elevated ceramide levels in circulation or within the tissue microenvironment (EC isolated from heart and lung tissues) was not resolved in this study. This aspect is important since ECs have similar basic functions but show heterogeneity in terms of tissue microenvironment []. Human studies show that elevated plasma ceramides are associated with endothelial dysfunction in coronary and peripheral arteries [,]. Endothelial dysfunction is evident in aging and in women with HDP (hypertensive disorders of pregnancy) such as pre-eclampsia. In the latter, ceramide accumulation in the placenta leads to endothelial dysfunction, causing reduced tubular formation and increased trophoblast cell death []. Women with a history of HDP have a 2.09 times higher risk of hospitalization with HFpEF and an increased short- and long-term risk of incident HFpEF [,]. Diastolic dysfunction and LV remodeling are well-established features of pre-eclampsia.
Clinical studies have indicated specific ceramide long-chain species (d18:1/18:0, d18:1/24:1, ratio of (d18:1/18:0)/(d18:1/16:0)) as novel predictors of new onset hypertension [] or as predictors of MACE (major adverse cardiac events) for hypertensive patients (CERT-HBP) identified to be at high CV risk []. In the myocardium, hypertension-induced ceramide accumulation could contribute to myocardial fibrosis and impaired diastolic relaxation, both of which are critical components of HFpEF. This is most likely through the activation of pathways such as MAPK and NF-κB [,], which promote inflammation and fibrosis in the heart, worsening the condition. Additionally, correlating the relationship between circulating ceramide and NLRP3 in pre-eclamptic or hypertensive female patients as a risk for HFpEF could provide diagnostic tools for HFpEF prevention in women.
4.4. Ceramide Biology in Metabolic Disorders
Metabolic disorders such as obesity and T2DM (type 2 diabetes mellitus) are strongly linked to the development and progression of HFpEF, with a higher prevalence in women than men [,]. Both disorders have prominent features of lipotoxicity and IR []. Lipotoxicity or the accumulation of lipids (including ceramides) within the circulation system due to increases in fat have deleterious effects on tissue glucose metabolism. Increased fat in the body occurs through the expansion (hypertrophy) or generation of new adipocytes (hyperplasia), which leads to an increase in abdominal, subcutaneous, and visceral adipocytes []. In post-menopausal women, abdominal fat distribution increases, possibly influenced by reduced E2 levels which have a negative correlation with ceramide levels [,]. Specific ceramide species such as d18:1/16:0, d18:1/18:0, and d18:1/24:1 accumulate in the visceral and subcutaneous tissue of diabetic or pre-diabetic females but not males []. Dysregulation of ceramide synthesis and degradation involving key enzymes in the sphingolipid synthesis pathways leads to ceramide accumulation and disrupts glucose metabolism in tissues such as the skeletal muscles, liver, and possibly the heart.
4.4.1. Ceramides Influence on IR in Liver and Muscle
The skeletal muscle and liver play key roles in postprandial glucose handling [,]. Disruption of glucose metabolism in skeletal muscle and liver leading to the development of IR due to elevated ceramide levels is well characterized and reviewed []. IR develops prior to the onset of T2DM (type 2 diabetes mellitus) and can increase in severity as the disease progresses, with declining β-cell function and hyperglycemia. Recently, the co-existence of MASLD (metabolic-associated fatty liver disease) and HFpEF and their shared comorbidities, obesity and diabetes, have gained attention [,,]. MASLD was found to be associated with a higher risk of developing HFpEF [].
The type of ceramide species in circulation/tissue, which is linked to the fatty acyl-chain length preferences of the CerS1–6 enzymes, impacts insulin sensitivity. For example, animal models show that C16 and C18 ceramides modulate insulin sensitivity [], while C24 and C24:1 have a protective role []. These studies targeted complete or partial depletion of CerS6 and CerS2 in the liver, respectively. The antagonistic role of ceramide C16 against insulin signaling and mitochondrial respiration has been attributed to its ability to regulate mitochondrial fission factor, which leads to the development of the insulin resistant phenotype []. Increased C16 ceramide levels in plasma have been linked to increased death and hospitalization in HFpEF patients []. Ceramide also interferes directly with insulin receptor signaling []. In normal physiology, insulin binds to insulin receptors, leading to the phosphorylation of insulin receptor substrates that in-turn activate the PI3K/Akt pathway. Ceramide targets AKT phosphorylation by increasing PP2A, reducing AKT phosphorylation [] or directing PKCζ to caveolin-enriched microdomains to sequester AKT in a repressed state []. Ceramide’s blockade of AKT activity also impacts the plasma membrane translocation and fusion of GLUT4 (glucose transporter 4), thereby dysregulating insulin-dependent glycemic control [].
In terms of skeletal muscles, two studies have shown evidence for muscular mitochondrial dysfunction in HFpEF patients aged >59 years old, and over 60% of patients were female [,]. Aging is known to shift mitochondrial dynamics towards fission and could further enhance IR []. Skeletal muscle dysfunction is also associated with exercise intolerance in HFpEF patients, apart from cardiac insufficiency [,,,]. Improved insulin sensitivity and glucose tolerance have been noted in skeletal muscles of mice lacking CerS1 []. Targeting genes of other enzymes in the de novo sphingolipid synthesis pathway, such as Degs1 in other organs such as the liver, lowered skeletal muscle ceramide levels and improved insulin sensitivity []. This implies that circulating ceramides do have an impact on skeletal muscle glucose regulation. High concentrations of C16 and C18 ceramide produced by the ceramide synthase isoforms (CerS1, CerS5, and CerS6) play an important role in fat-induced skeletal muscle and hepatic IR [,]. Whether or not there is sexual dimorphism in the mechanisms of ceramide regulation within the liver and skeletal muscle in the absence of estrogen is currently unclear.
4.4.2. Ceramides and IR in Obesity
Obesity impairs relaxation and filling of the heart, the key features of HFpEF []. Obesity also exacerbates vascular dysfunction and promotes pericardial fat accumulation, which enhances molecular and cellular inflammatory signals that drive HFpEF. An increase in fat depots increases recruitment of macrophages to the site, creating an inflammatory state characterized by increased cytokines such as Il-6, TNFα, and Il-1β [,,]. Serum levels of TNFα often correlate with levels of ceramide or IR. In cultured cells TNFα stimulates ceramide biogenesis by inducing ceramide synthesis and sphingomyelin hydrolysis enzyme genes, which creates a vicious cycle of ceramide production and inflammatory signaling [,]. Ceramide-induced IR is partly attributed to the adipokine adiponectin and its receptors AdipoR1/AdipoR2 which improve insulin sensitivity by catabolism of tissue ceramide [,]. AdipoR1/AdipoR2 have intrinsic ceramidase activity and catalyze ceramide into S1P, improving insulin sensitivity and metabolic health. Adiponectin is encoded by the ADIPOQ gene and secreted in three known isoforms by adipocytes, with the high-molecular-weight (HWM) multimeric adiponectin being the most bioactive and negatively correlated to obesity []. This relationship has been observed in a study in post-menopausal women with BMI > 24 []. Furthermore, reduced adiponectin levels in pregnancy contribute to IR, as seen in gestational diabetes []. Increasing IR in HFpEF is also associated with worsened LV strain and MACE [,]. It is not clear if the changes during gestation, such as increased IR and reduced adiponectin, contribute to the reduced adiponectin and increased ceramide post-menopause, exacerbating obesity-related effects on the heart.


Table 1.
Main triggers and mechanisms affecting ceramide biology in HFpEF risk factors.
Table 1.
Main triggers and mechanisms affecting ceramide biology in HFpEF risk factors.
| Risk Factors | Main Triggers | Mechanisms | References |
|---|---|---|---|
| Aging + Female sex | Estrogen depletion | Increased ceramide due to reduced E2 effect on SPT1 and 2, reduced protection against obesity | [] |
| Increased inflammation, cellular stress, and apoptosis | [,,] | ||
| Increased cardiac stiffness through titin subunit modulation | [] | ||
| Cellular Senescence | 4-fold elevation of ceramides in circulation | [] | |
| Ceramide increases mitochondrial ROS generation and DNA damage | [] | ||
| Amplifying the inflammatory effects of senescence-associated secretory proteins (SASPs) through NF-κB MAPK and JAK/STAT pathways | [,,,] | ||
| Hypertension | Reduced NO availability | Ceramide triggers Ca2+ influx through TRPC6 channels | [] |
| Dephosphorylation of eNOS increasing ROS | [] | ||
| EC dysfunction | Activation of NLRP3 inflammasome | [] | |
| Elevated ceramide associated with endothelial dysfunction in coronary and peripheral arteries | [] | ||
| Metabolic Disorders | Insulin resistance +/− Obesity | Ceramide regulates mitochondrial fission factor | [] |
| Direct interference of insulin receptor signaling in liver and muscle through Akt modulation | [,] | ||
| Reduced adiponectin levels due to obesity or pregnancy increases tissue ceramides | [,,] |
5. Ceramide Effects on Cardiac Metabolism
Metabolic dysfunction in HF is widely known. In HFpEF, this is an area of evolving interest. The current trajectory of research indicates HFpEF as a metabolic syndrome that is pronounced in females and has an interdependence of lipid use with cardiac hemodynamics [], perhaps highlighting the potential mechanisms through which the SGLT2 inhibitors were improving cardiac function in HFpEF patients []. In the failing myocardium, ceramide levels are elevated, and this has been described as a contributor to clinical HF in the Framingham Heart Cohort [,]. Evidence from a recent in vitro model of ischemia/reperfusion injury in mice and hiPSC (human induced pluripotent stem cells) cardiomyocytes supports ceramide accumulation in myocytes when the enzyme CerS2 was over-expressed []. Ceramide’s effects on cardiac metabolism can be ascribed to its role in IR, inflammation, and arthrosclerosis. These effects can be conferred through ceramide’s downstream bioactive intermediate metabolites such as S1P and dhS1P (dihydrosphingosine 1 phosphate). We have reported hypertrophic and fibrotic effects of these metabolites on ventricular fibroblasts and myocytes isolated from neonatal rats that were comparable to angiotensin II [,]. However, in ischemia/reperfusion injury animal models (leads to HFrEF phenotype) these metabolites have a more protective effect [,], implying a disease-etiology-driven response. In fact, metabolic dysfunction in the heart is evidenced by the heart switching substrate type to maintain homeostasis in normal physiological and disease states []. Ceramide impacts mitochondrial function (as described earlier), which plays a vital role in this substrate switching response.
6. Current Recommended HFpEF Therapies and Their Effect on Ceramides
Several diabetes drugs have been trialed as management strategies for HFpEF, including the GIP (glucose-dependent insulinotropic polypeptide) and GLP1 agonists—semaglutide and tirzepatide—and SGLT inhibitors—dapagliflozin and empagliflozin. GLP-1 agonists, currently being trialed for HFpEF, have been found to be effective in reducing hospitalisations, including in those with obesity-induced IR [,]. Several lines of evidence show GLP-1 receptor agonists can inhibit ceramide generation, leading to reductions in serum levels of C16 and C24:1 ceramide species [,]. This reduction in ceramide may be a result of GLP-1R-enhanced metabolism of ceramide through alkaline ceramidase 2 (Acer2), and attenuation of TNFα induction by toll-like receptors, leading to a reduction in inflammation-induced ceramide accumulation or an increase in adiponectin and resulting in increased ceramide catabolism [,,]. A clinical study reported reduced ceramide and its precursor metabolite, DhCer, in type 2 diabetes patients receiving liraglutide (GLP-1R agonist) and it was recommended as a CVD prevention therapy in a post hoc analysis of a randomized clinical trial showing reduced ceramides [,]. This implies that this therapy has either a direct or indirect effect on the de novo sphingolipid synthesis pathway. Despite this clinical evidence, there is a lack of mechanistic studies on the effects of current recommended therapies on ceramide biology in the context of HFpEF. This highlights a major need for more research in this area.
Interventions targeting ceramide metabolism show promise in improving health-span and potentially life-span []. For instance, inhibiting ceramide synthesis with myriocin enhances glucose homeostasis and grip strength in mice [], and dietary interventions targeting older adults (55 to 80 years, >50% females) lead to a reduction in ceramide levels and CVD risk score []. It is understood that the prevalence of HFpEF increases in aging due to cellular aging, myocardial stiffness, and multiple comorbidities. At the cellular level, ceramides interfere with the function of EC, smooth muscle cells, and cardiomyocytes, leading to impairment of NO signaling and promotion of fibrosis which increases myocardial stiffness [,,]. Ceramide is also known to activate stress kinases such as JNK (c-Jun N-terminal kinase) and p38 MAPK (mitogen-activated protein kinase) which increase the oxidative stress response in aging and can exacerbate myocardial stiffness [,,], a hallmark of HFpEF. Therefore, interventions targeting ceramide could be beneficial for CVD health in the female aging population. However, there are limitations to targeting ceramides for therapy. The most obvious of these include ceramides’ complex biology and the heterogeneity of HFpEF pathophysiology, both of which have the possibility of introducing off-target effects and exacerbating the condition.
7. Pregnancy and Its Influence on Ceramide Biology
In addition to the risk factors mentioned above, recent data suggests a history of multiple pregnancies could be a risk for HFpEF development [,]. Ceramides play a significant role in pregnancy, with levels increasing towards the end of gestation in conditions such as pre-term labor and pre-eclampsia [,,,]. These unique temporal gestation patterns of ceramide levels highlight a potential unique role in gestation []. Ceramides also act as stress mediators during implantation, delivery, and lactation []. Elevated plasma ceramides during pregnancy are associated with IR and metabolic changes [,]. These changes may have implications for endothelial dysfunction, gestation diabetes mellitus, and fetal development []. Hormonal fluctuations in pregnancy are fundamental to successful pregnancy and birth of the child. Ceramides play a multifaceted role in metabolism and the signaling of steroid hormones. Ceramides can inhibit progesterone biosynthesis in rat luteal cells and granulosa cells, potentially contributing to luteal regression [,]. For example, in granulosa cells, ceramides mediate the inhibitory effects of IL-1β on progesterone production and enhance prostaglandin E2 biosynthesis. Ceramides are also involved in estrogen-modulated processes, as indicated earlier. What is yet to be researched is the genetic and epigenetic effects these changes may have and how they influence age-dependent disease development and progression as it relates to the pathogenesis of HFpEF in an aging female. This is especially important in terms of the effects of multiple pregnancies on heart health post-menopause, since a single pregnancy has tremendous cardiac and hemodynamic effects on the mother. Women with a history of multiple pregnancies (>3 pregnancies) tended to have an exaggerated hemodynamic phenotype than those who did not in female HFpEF patients []. Others have found multiparity to be associated with poorer cardiovascular health, even when adjusted for confounders such as job strain [,]. This association was later supported by a meta-analysis study showing multiparity has a dose-response relationship to CVD risk compared to nulliparity []. The analysis included 10 cohort studies involving over 3 million participants, with 150,512 incident cases of CVD. This is further supported by recent findings from analysis of HF risk within the UK Biobank showing multiparity to be associated with increased risk of HF []. This study emphasized the importance of female reproductive history in the assessment of HF risk. Additionally, we have published evidence of persistent transcriptomic changes in the myocardium of aged (24 month old) mice with a history of multiple pregnancies versus age-matched virgin mice []. Despite these recent efforts showing increasing risk of CVD with parity, there is a lack of comparable data in terms of differences in ceramide levels over the reproductive history between women with a history of multiparity and no pregnancy to clearly understand the potential contributions of ceramide to HFpEF in multiparous women. This lack of evidence supports the need to stratify studies well to capture such effects, if any. Such efforts would help determine whether measuring ceramide levels can enhance current HFpEF diagnostic methods for females.
8. Conclusions
As the evidence for ceramide in HFpEF slowly builds, its role in the pathophysiology of HFpEF, particularly in women, is important to decipher. However, when considering the HFpEF risk factor-based evidence currently available that show ceramide dysfunction, and involvement in molecular processes such as inflammation, metabolic dysfunction, and endothelial dysfunction influenced by declining levels of estrogen with age, it should be noted that ceramide has the potential to be a biomarker for early detection and risk assessment for HFpEF in women. This assessment is currently hampered by the lack of clinical and population studies targeting women at different life-stages and obstetric conditions. Future research into ceramide’s role in HFpEF should account for sex differences as well as stratifying for life-stages and obstetric factors to inform targeted therapies for women. These considerations should be incorporated even in basic mechanistic studies in cells and animals, where possible. This could lead to targeted strategies for early diagnosis or intervention, especially for women with a history of multiple pregnancies, pregnancy complications, or post-menopausal changes who have developed hypertension and–or obesity as they age.
Author Contributions
R.R.M.—concept for manuscript, the literature search, and writing and editing; D.M.K. and B.H.W. contributed to forming and framing the themes of the manuscript and writing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable. This is only appropriate if no new data is generated or the article describes entirely theoretical research. No new data were created or analyzed in this study.
Conflicts of Interest
The authors have no conflict of interests.
References
- Leggat, J.; Bidault, G.; Vidal-Puig, A. Lipotoxicity: A driver of heart failure with preserved ejection fraction? Clin. Sci. 2021, 135, 2265–2283. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Mantovani, A.; Bonapace, S.; Morandin, R.; Sani, E.; Lunardi, G.; Salgarello, M.; Molon, G.; Targher, G. A new plasma ceramide 24-based risk score predicts overall mortality and nonfatal myocardial infarction in patients with suspected or known coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2025, in press. [CrossRef]
- Klingenberg, R.; Leiherer, A.; Dobrev, D.; Kaski, J.C.; Levkau, B.; März, W.; Sossalla, S.; von Eckardstein, A.; Drexel, H. Ceramides in cardiovascular disease: Emerging role as independent risk predictors and novel therapeutic targets. Cardiovasc. Res. 2025, 121, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre Rozenn, N.; Jensen Paul, N.; Hoofnagle, A.; McKnight, B.; Fretts Amanda, M.; King Irena, B.; Siscovick David, S.; Psaty Bruce, M.; Heckbert Susan, R.; Mozaffarian, D.; et al. Plasma Ceramides and Sphingomyelins in Relation to Heart Failure Risk. Circ. Heart Fail. 2019, 12, e005708. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Völzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J. Am. Heart Assoc. 2018, 7, e007931. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.C.; Duncan, M.; Xanthakis, V.; Peterson, L.R.; Mitchell, G.F.; McManus, D.; Cheng, S.; Vasan, R.S. Association of Circulating Ceramides with Cardiac Structure and Function in the Community: The Framingham Heart Study. J. Am. Heart Assoc. 2019, 8, e013050. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection Fraction. JACC 2023, 81, 1810–1834. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Sotomi, Y.; Hikoso, S.; Nakatani, D.; Mizuno, H.; Okada, K.; Dohi, T.; Kitamura, T.; Sunaga, A.; Kida, H.; Oeun, B.; et al. Sex Differences in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e018574. [Google Scholar] [CrossRef] [PubMed]
- van Essen, B.J.; Emmens, J.E.; Tromp, J.; Ouwerkerk, W.; Smit, M.D.; Geluk, C.A.; Baumhove, L.; Suthahar, N.; Gansevoort, R.T.; Bakker, S.J.L.; et al. Sex-specific risk factors for new-onset heart failure: The PREVEND study at 25 years. Eur. Heart J. 2025, 46, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D. Methods in a longitudinal cohort study of late reproductive age women: The Penn Ovarian Aging Study (POAS). Womens Midlife Health 2016, 2, 1. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Nasr, A. Cardiovascular Disease in Women: Does Menopause Matter? Curr. Opin. Endocr. Metab. Res. 2022, 27, 100419. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, A.C.; Michos, E.D.; Shufelt, C.L.; Vermunt, J.V.; Minissian, M.B.; Quesada, O.; Smith, G.N.; Rich-Edwards, J.W.; Garovic, V.D.; Khoudary, S.R.E.; et al. Pregnancy and Reproductive Risk Factors for Cardiovascular Disease in Women. Circ. Res. 2022, 130, 652–672. [Google Scholar] [CrossRef]
- Williams, D.; Stout, M.J.; Rosenbloom, J.I.; Olsen, M.A.; Joynt Maddox, K.E.; Deych, E.; Davila-Roman, V.G.; Lindley, K.J. Preeclampsia Predicts Risk of Hospitalization for Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2021, 78, 2281–2290. [Google Scholar] [CrossRef]
- Vozella, V.; Basit, A.; Piras, F.; Realini, N.; Armirotti, A.; Bossù, P.; Assogna, F.; Sensi, S.L.; Spalletta, G.; Piomelli, D. Elevated plasma ceramide levels in post-menopausal women: A cross-sectional study. Aging 2019, 11, 73–88. [Google Scholar] [CrossRef]
- Ermini, L.; Farrell, A.; Alahari, S.; Ausman, J.; Park, C.; Sallais, J.; Melland-Smith, M.; Porter, T.; Edson, M.; Nevo, O.; et al. Ceramide-Induced Lysosomal Biogenesis and Exocytosis in Early-Onset Preeclampsia Promotes Exosomal Release of SMPD1 Causing Endothelial Dysfunction. Front. Cell Dev. Biol. 2021, 9, 652651. [Google Scholar] [CrossRef]
- Lantzanaki, M.; Vavilis, T.; Harizopoulou, V.C.; Bili, H.; Goulis, D.G.; Vavilis, D. Ceramides during Pregnancy and Obstetrical Adverse Outcomes. Metabolites 2023, 13, 1136. [Google Scholar] [CrossRef]
- Venable, M.E.; Lee, J.Y.; Smyth, M.J.; Bielawska, A.; Obeid, L.M. Role of Ceramide in Cellular Senescence. J. Biol. Chem. 1995, 270, 30701–30708. [Google Scholar] [CrossRef]
- Meade, R.; Chao, Y.; Harroun, N.; Li, C.; Hafezi, S.; Hsu, F.F.; Semenkovich, C.F.; Zayed, M.A. Ceramides in peripheral arterial plaque lead to endothelial cell dysfunction. JVS Vasc. Sci. 2023, 4, 100181. [Google Scholar] [CrossRef] [PubMed]
- Shoghli, M.; Lokki, A.I.; Lääperi, M.; Sinisalo, J.; Lokki, M.L.; Hilvo, M.; Jylhä, A.; Tuomilehto, J.; Laaksonen, R. The Novel Ceramide- and Phosphatidylcholine-Based Risk Score for the Prediction of New-Onset of Hypertension. J. Clin. Med. 2023, 12, 7524. [Google Scholar] [CrossRef] [PubMed]
- Brusatori, M.; Wood, M.H.; Tucker, S.C.; Maddipati, K.R.; Koya, S.K.; Auner, G.W.; Honn, K.V.; Seyoum, B. Ceramide changes in abdominal subcutaneous and visceral adipose tissue among diabetic and nondiabetic patients. J. Diabetes 2022, 14, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, J.L.; Tatum, S.M.; Holland, W.L.; Summers, S.A. Ceramides are fuel gauges on the drive to cardiometabolic disease. Physiol. Rev. 2024, 104, 1061–1119. [Google Scholar] [CrossRef]
- Fakhr, Y.; Brindley, D.N.; Hemmings, D.G. Physiological and pathological functions of sphingolipids in pregnancy. Cell. Signal. 2021, 85, 110041. [Google Scholar] [CrossRef]
- Enthoven, L.F.; Shi, Y.; Fay, E.; Kim, A.; Moreni, S.; Mao, J.; Isoherranen, N.; Totah, R.A.; Hebert, M.F. Effects of Pregnancy on Plasma Sphingolipids Using a Metabolomic and Quantitative Analysis Approach. Metabolites 2023, 13, 1026. [Google Scholar] [CrossRef]
- Pabón, M.A.; Misra, A.; Honigberg, M.C. Adverse pregnancy outcomes and future risk of heart failure. Curr. Opin. Cardiol. 2023, 38, 215–222. [Google Scholar] [CrossRef]
- Mao, Y.; Hu, W.; Xia, B.; Liu, L.; Han, X.; Liu, Q. Association Between Gestational Diabetes Mellitus and the Risks of Type-Specific Cardiovascular Diseases. Front. Public. Health 2022, 10, 940335. [Google Scholar] [CrossRef]
- Khosla, K.; Heimberger, S.; Nieman, K.M.; Tung, A.; Shahul, S.; Staff, A.C.; Rana, S. Long-Term Cardiovascular Disease Risk in Women After Hypertensive Disorders of Pregnancy: Recent Advances in Hypertension. Hypertension 2021, 78, 927–935. [Google Scholar] [CrossRef]
- Juchnicka, I.; Kuźmicki, M.; Zabielski, P.; Krętowski, A.; Błachnio-Zabielska, A.; Szamatowicz, J. Serum C18:1-Cer as a Potential Biomarker for Early Detection of Gestational Diabetes. J. Clin. Med. 2022, 11, 384. [Google Scholar] [CrossRef]
- Goto, A.; Mizuike, A.; Hanada, K. Sphingolipid Metabolism at the ER-Golgi Contact Zone and Its Impact on Membrane Trafficking. Contact 2020, 3, 2515256420959514. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.A.; Hanun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. In Sphingolipids as Signaling and Regulatory Molecules; Chalfant, C., De Poeta, M., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; pp. 1–23. [Google Scholar]
- Ruangsiriluk, W.; Grosskurth, S.E.; Ziemek, D.; Kuhn, M.; des Etages, S.G.; Francone, O.L. Silencing of enzymes involved in ceramide biosynthesis causes distinct global alterations of lipid homeostasis and gene expression. J. Lipid Res. 2012, 53, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Zelnik, I.D.; Volpert, G.; Viiri, L.E.; Kauhanen, D.; Arazi, T.; Aalto-Setälä, K.; Laaksonen, R.; Futerman, A.H. Different rates of flux through the biosynthetic pathway for long-chain versus very-long-chain sphingolipids. J. Lipid Res. 2020, 61, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.W.C.; Zheng, X.; Ali, Y. Ceramide Acyl Chain Length and Its Relevance to Intracellular Lipid Regulation. Int. J. Mol. Sci. 2022, 23, 9697. [Google Scholar] [CrossRef]
- Pilátová, M.B.; Solárová, Z.; Mezencev, R.; Solár, P. Ceramides and their roles in programmed cell death. Adv. Med. Sci. 2023, 68, 417–425. [Google Scholar] [CrossRef]
- Ding, S.; Li, G.; Fu, T.; Zhang, T.; Lu, X.; Li, N.; Geng, Q. Ceramides and mitochondrial homeostasis. Cell. Signal. 2024, 117, 111099. [Google Scholar] [CrossRef]
- Sokolowska, E.; Blachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191, Erratum in Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef]
- Kinoshita, M.; Matsumori, N. Inimitable Impacts of Ceramides on Lipid Rafts Formed in Artificial and Natural Cell Membranes. Membranes 2022, 12, 727. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Chaurasia, B.; Kaddai, V.A.; Lancaster, G.I.; Henstridge, D.C.; Sriram, S.; Galam, D.L.; Gopalan, V.; Prakash, K.N.; Velan, S.S.; Bulchand, S.; et al. Adipocyte Ceramides Regulate Subcutaneous Adipose Browning, Inflammation, and Metabolism. Cell Metab. 2016, 24, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.E.; Houck, K.L.; O’Neill, S.M.; Nagarajan, M.; Stover, T.C.; Pomianowski, P.T.; Unal, O.; Yun, J.K.; Naides, S.J.; Kester, M. Ceramide recruits and activates protein kinase C zeta (PKCζ) within structured membrane microdomains. J. Biol. Chem. 2007, 282, 12450–12457. [Google Scholar] [CrossRef]
- Shen, X.; Feng, R.; Zhou, R.; Zhang, Z.; Liu, K.; Wang, S. Ceramide as a Promising Tool for Diagnosis and Treatment of Clinical Diseases: A Review of Recent Advances. Metabolites 2025, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front. Endocrinol. 2020, 11, 570628. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K. Ceramides predict CV death in stable CAD and ACS. Nat. Rev. Cardiol. 2016, 13, 381. [Google Scholar] [CrossRef]
- Spaggiari, R.; Angelini, S.; Di Vincenzo, A.; Scaglione, G.; Morrone, S.; Finello, V.; Fagioli, S.; Castaldo, F.; Sanz, J.M.; Sergi, D.; et al. Ceramides as Emerging Players in Cardiovascular Disease: Focus on Their Pathogenetic Effects and Regulation by Diet. Adv. Nutr. 2024, 15, 100252. [Google Scholar] [CrossRef]
- Beale, A.L.; Nanayakkara, S.; Segan, L.; Mariani, J.A.; Maeder, M.T.; van Empel, V.; Vizi, D.; Evans, S.; Lam, C.S.P.; Kaye, D.M. Sex Differences in Heart Failure with Preserved Ejection Fraction Pathophysiology: A Detailed Invasive Hemodynamic and Echocardiographic Analysis. JACC Heart Fail. 2019, 7, 239–249. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Ji, R.; Akashi, H.; Drosatos, K.; Liao, X.; Jiang, H.; Kennel, P.J.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Deng, L.Y.; et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017, 2, e82922. [Google Scholar] [CrossRef]
- Park, T.-S.; Hu, Y.; Noh, H.-L.; Drosatos, K.; Okajima, K.; Buchanan, J.; Tuinei, J.; Homma, S.; Jiang, X.-C.; Abel, E.D.; et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 2008, 49, 2101–2112. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040, Erratum in Circulation 2019, 140, e726. [Google Scholar] [CrossRef]
- Vasile, V.C.; Meeusen, J.W.; Medina Inojosa, J.R.; Donato, L.J.; Scott, C.G.; Hyun, M.S.; Vinciguerra, M.; Rodeheffer, R.R.; Lopez-Jimenez, F.; Jaffe, A.S. Ceramide Scores Predict Cardiovascular Risk in the Community. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1558–1569. [Google Scholar] [CrossRef]
- Javaheri, A.; Allegood Jeremy, C.; Cowart, L.A.; Chirinos Julio, A. Circulating Ceramide 16:0 in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2020, 75, 2273–2275. [Google Scholar] [CrossRef]
- Colombini, M. Ceramide channels and mitochondrial outer membrane permeability. J. Bioenerg. Biomembr. 2017, 49, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Vegas, A.; Madsen, S.; Cooke, K.C.; Carroll, L.; Khor, J.X.Y.; Turner, N.; Lim, X.Y.; Astore, M.A.; Morris, J.C.; Don, A.S.; et al. Mitochondrial electron transport chain, ceramide, and coenzyme Q are linked in a pathway that drives insulin resistance in skeletal muscle. eLife 2023, 12, RP87340. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Dulovic-Mahlow, M.; Mandik, F.; Frese, L.; Kanana, Y.; Haissatou Diaw, S.; Depperschmidt, J.; Böhm, C.; Rohr, J.; Lohnau, T.; et al. Ceramide accumulation induces mitophagy and impairs β-oxidation in PINK1 deficiency. Proc. Natl. Acad. Sci. USA 2021, 118, e2025347118. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.F.; Li, M.; Koay, Y.C.; Wang, X.S.; Guglielmi, G.; Marques, F.Z.; Nanayakkara, S.; Mariani, J.; Slaughter, E.; Kaye, D.M. Cardiac Substrate Utilization and Relationship to Invasive Exercise Hemodynamic Parameters in HFpEF. JACC Basic. Transl. Sci. 2024, 9, 281–299. [Google Scholar] [CrossRef]
- Henry, J.A.; Couch, L.S.; Rider, O.J. Myocardial Metabolism in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2024, 13, 1195. [Google Scholar] [CrossRef]
- Phan, T.T.; Abozguia, K.; Nallur Shivu, G.; Mahadevan, G.; Ahmed, I.; Williams, L.; Dwivedi, G.; Patel, K.; Steendijk, P.; Ashrafian, H.; et al. Heart Failure with Preserved Ejection Fraction Is Characterized by Dynamic Impairment of Active Relaxation and Contraction of the Left Ventricle on Exercise and Associated with Myocardial Energy Deficiency. J. Am. Coll. Cardiol. 2009, 54, 402–409. [Google Scholar] [CrossRef]
- Koay, Y.C.; McIntosh, B.; Ng, Y.H.; Cao, Y.; Wang, X.S.; Han, Y.; Tomita, S.; Bai, A.Y.; Hunter, B.; Misra, A.; et al. The Heart Has Intrinsic Ketogenic Capacity That Mediates NAD+ Therapy in HFpEF. Circ. Res. 2025, 136, 1113–1130. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Kaye, D.M.; Wang, X.S.; Koay, Y.C.; Li, M.; McIntosh, B.; Ng, Y.H.; Rahman, M.; Cao, Y.; Marques, F.Z.; Malecki, C.; et al. Mechanical unloading is accompanied by reverse metabolic remodelling in the failing heart: Identification of a novel citraconate-mediated pathway. Eur. J. Heart Fail. 2025, 27, 1342–1352. [Google Scholar] [CrossRef]
- De Jong, K.A.; Lopaschuk, G.D. Complex Energy Metabolic Changes in Heart Failure with Preserved Ejection Fraction and Heart Failure with Reduced Ejection Fraction. Can. J. Cardiol. 2017, 33, 860–871. [Google Scholar] [CrossRef]
- Cohen, J.B.; Schrauben, S.J.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Yarde, M.; Wang, Z.; Bhattacharya, P.T.; Chirinos, D.A.; et al. Clinical Phenogroups in Heart Failure with Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020, 8, 172–184. [Google Scholar] [CrossRef]
- Sindone, A.P.; Abhayaratna, W.P.; Chan, A.; Leung, M.; Hopper, I.; Amerena, J.; De Pasquale, C.G.; Burdeniuk, C.; Coats, A.J.S.; Atherton, J.J. Heart Failure with Preserved Ejection Fraction in Patients with Obesity: A Growing Cardiometabolic Concern. Heart Lung Circ. 2025, 34, 1033–1040. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Drosatos, K.; Schulze, P.C. Cardiac lipotoxicity: Molecular pathways and therapeutic implications. Curr. Heart Fail. Rep. 2013, 10, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; Cosentino, C.; Segan, L.; Mariani, J.A.; Vizi, D.; Evans, S.; Nanayakkara, S.; Kaye, D.M. The effect of parity on exercise physiology in women with heart failure with preserved ejection fraction. ESC Heart Fail. 2020, 7, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Smits, P.D.; Rodriguez, P.; Cartwright, B.M.G.; Gratzl, S.; Brar, R.; Garrett, E.; Stucky, N.L. The temporal relationship between preeclampsia and heart failure. medRxiv 2023. [Google Scholar] [CrossRef]
- Amraoui, F.; Hassani Lahsinoui, H.; Spijkers, L.J.A.; Vogt, L.; Peters, S.L.M.; Wijesinghe, D.S.; Warncke, U.O.; Chalfant, C.E.; Ris-Stalpers, C.; van den Born, B.-J.H.; et al. Plasma ceramide is increased and associated with proteinuria in women with pre-eclampsia and HELLP syndrome. Pregnancy Hypertens. 2020, 19, 100–105. [Google Scholar] [CrossRef]
- Szoeke, C.; Coulson, M.; Campbell, S.; Dennerstein, L. Cohort profile: Women’s Healthy Ageing Project (WHAP)—A longitudinal prospective study of Australian women since 1990. Womens Midlife Health 2016, 2, 5. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Wildman, R.P.; Matthews, K.; Thurston, R.C.; Bromberger, J.T.; Sutton-Tyrrell, K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis 2012, 225, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Samargandy, S.; Matthews, K.A.; Brooks, M.M.; Barinas-Mitchell, E.; Magnani, J.W.; Janssen, I.; Kazlauskaite, R.; El Khoudary, S.R. Abdominal visceral adipose tissue over the menopause transition and carotid atherosclerosis: The SWAN heart study. Menopause 2021, 28, 626–633. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Ruotsalainen, S.; Ottensmann, L.; Gerl, M.J.; Klose, C.; Tukiainen, T.; Pirinen, M.; Simons, K.; Widén, E.; Ripatti, S. Lipidome- and Genome-Wide Study to Understand Sex Differences in Circulatory Lipids. J. Am. Heart Assoc. 2022, 11, e027103. [Google Scholar] [CrossRef]
- Muilwijk, M.; Callender, N.; Goorden, S.; Vaz, F.M.; van Valkengoed, I.G.M. Sex differences in the association of sphingolipids with age in Dutch and South-Asian Surinamese living in Amsterdam, the Netherlands. Biol. Sex Differ. 2021, 12, 13. [Google Scholar] [CrossRef]
- Xu, X.L.; Huang, Z.Y.; Yu, K.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front. Endocrinol. 2022, 13, 827032. [Google Scholar] [CrossRef]
- Simpson, E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–496. [Google Scholar] [CrossRef]
- Adu-Amankwaah, J.; Bushi, A.; Tan, R.; Adekunle, A.O.; Adzika, G.K.; Ndzie Noah, M.L.; Nadeem, I.; Adzraku, S.Y.; Koda, S.; Mprah, R.; et al. Estradiol mitigates stress-induced cardiac injury and inflammation by downregulating ADAM17 via the GPER-1/PI3K signaling pathway. Cell Mol. Life Sci. 2023, 80, 246. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.D.; Pourati, I.; Aronovitz, M.J.; Baur, J.; Celestin, F.; Chen, X.; Michael, A.; Haq, S.; Nuedling, S.; Grohe, C.; et al. 17β-Estradiol Reduces Cardiomyocyte Apoptosis In Vivo and In Vitro via Activation of Phospho-Inositide-3 Kinase/Akt Signaling. Circ. Res. 2004, 95, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Liu, J.; Liu, H. Targeting estrogen receptor signaling for treating heart failure. Heart Fail. Rev. 2024, 29, 125–131. [Google Scholar] [CrossRef] [PubMed]
- González-García, I.; Contreras, C.; Estévez-Salguero, Á.; Ruíz-Pino, F.; Colsh, B.; Pensado, I.; Liñares-Pose, L.; Rial-Pensado, E.; Martínez de Morentin, P.B.; Fernø, J.; et al. Estradiol Regulates Energy Balance by Ameliorating Hypothalamic Ceramide-Induced ER Stress. Cell Rep. 2018, 25, 413–423.e415. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Wang, L.; Albanese, N.; Pitson, S.M.; Vadas, M.A.; Xia, P. Sphingosine Kinase Transmits Estrogen Signaling in Human Breast Cancer Cells. Mol. Endocrinol. 2003, 17, 2002–2012. [Google Scholar] [CrossRef]
- García-González, V.; Díaz-Villanueva, J.F.; Galindo-Hernández, O.; Martínez-Navarro, I.; Hurtado-Ureta, G.; Pérez-Arias, A.A. Ceramide Metabolism Balance, a Multifaceted Factor in Critical Steps of Breast Cancer Development. Int. J. Mol. Sci. 2018, 19, 2527. [Google Scholar] [CrossRef]
- Ishii, T.; Warabi, E. Mechanism of Rapid Nuclear Factor-E2-Related Factor 2 (Nrf2) Activation via Membrane-Associated Estrogen Receptors: Roles of NADPH Oxidase 1, Neutral Sphingomyelinase 2 and Epidermal Growth Factor Receptor (EGFR). Antioxidants 2019, 8, 69. [Google Scholar] [CrossRef]
- Wade, C.B.; Dorsa, D.M. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology 2003, 144, 832–838. [Google Scholar] [CrossRef]
- Levy, M.V.; Fandl, H.K.; Hijmans, J.G.; Stockelman, K.A.; Ruzzene, S.T.; Reiakvam, W.R.; Goldthwaite, Z.A.; Greiner, J.J.; DeSouza, C.A.; Garcia, V.P. Effect of 17β-Estradiol on Endothelial Cell Expression of Inflammation- Related MicroRNA. Microrna 2025, 14, 3–8. [Google Scholar] [CrossRef]
- Dilber, Y.; Çeker, H.T.; Öztüzün, A.; Çırçırlı, B.; Kırımlıoğlu, E.; Barut, Z.; Aslan, M. Sparstolonin B Reduces Estrogen-Dependent Proliferation in Cancer Cells: Possible Role of Ceramide and PI3K/AKT/mTOR Inhibition. Pharmaceuticals 2024, 17, 1564. [Google Scholar] [CrossRef]
- Song, Y.; Hou, M.; Li, Z.; Luo, C.; Ou, J.S.; Yu, H.; Yan, J.; Lu, L. TLR4/NF-κB/Ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells. Eur. J. Pharmacol. 2017, 794, 45–51. [Google Scholar] [CrossRef]
- Fukuma, N.; Takimoto, E.; Ueda, K.; Liu, P.; Tajima, M.; Otsu, Y.; Kariya, T.; Harada, M.; Toko, H.; Koga, K.; et al. Estrogen Receptor-α Non-Nuclear Signaling Confers Cardioprotection and Is Essential to cGMP-PDE5 Inhibition Efficacy. JACC Basic Transl. Sci. 2020, 5, 282–295. [Google Scholar] [CrossRef]
- LeWinter, M.M.; Granzier, H. Cardiac Titin. Circulation 2010, 121, 2137–2145. [Google Scholar] [CrossRef]
- Ye, L.; Wang, R.; Zhao, J.; Chen, J.; Wang, F. 17β-estradiol delays cardiac aging through suppressing the methylation of Beclin1 in a murine model. Steroids 2025, 216, 109587. [Google Scholar] [CrossRef] [PubMed]
- Bekhite, M.; González-Delgado, A.; Hübner, S.; Haxhikadrija, P.; Kretzschmar, T.; Müller, T.; Wu, J.M.F.; Bekfani, T.; Franz, M.; Wartenberg, M.; et al. The role of ceramide accumulation in human induced pluripotent stem cell-derived cardiomyocytes on mitochondrial oxidative stress and mitophagy. Free Radic. Biol. Med. 2021, 167, 66–80. [Google Scholar] [CrossRef]
- Scroggins, L.; Echevarria, K.; Gutierrez, E.; Palavicini, J.; Quiles, P. Evidence Supporting Ceramide Accumulation as a Novel Hallmark of Aging. Innov. Aging 2024, 8 (Suppl. S1), 689–690. [Google Scholar] [CrossRef]
- Khayrullin, A.; Krishnan, P.; Martinez-Nater, L.; Mendhe, B.; Fulzele, S.; Liu, Y.; Mattison, J.A.; Hamrick, M.W. Very Long-Chain C24:1 Ceramide Is Increased in Serum Extracellular Vesicles with Aging and Can Induce Senescence in Bone-Derived Mesenchymal Stem Cells. Cells 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef]
- Gudz, T.I.; Tserng, K.Y.; Hoppel, C.L. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 1997, 272, 24154–24158. [Google Scholar] [CrossRef]
- Zigdon, H.; Kogot-Levin, A.; Park, J.W.; Goldschmidt, R.; Kelly, S.; Merrill, A.H., Jr.; Scherz, A.; Pewzner-Jung, Y.; Saada, A.; Futerman, A.H. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 2013, 288, 4947–4956. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Demarchi, F.; Bertoli, C.; Greer, P.A.; Schneider, C. Ceramide triggers an NF-κB-dependent survival pathway through calpain. Cell Death Differ. 2005, 12, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Chen, C.-L.; Lin, C.-F.; Chang, W.-T.; Huang, W.-C.; Teng, C.-F.; Lin, Y.-S. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood 2008, 111, 4365–4374. [Google Scholar] [CrossRef]
- Mazière, C.; Conte, M.A.; Mazière, J.C. Activation of the JAK/STAT pathway by ceramide in cultured human fibroblasts. FEBS Lett. 2001, 507, 163–168. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Grillari, J.; Balistreri, C.R. Cellular Senescence and Inflammaging in Age-Related Diseases. Mediat. Inflamm. 2018, 2018, 9076485. [Google Scholar] [CrossRef]
- Muthusamy, V.V. Hypertension and Heart Failure with Preserved Ejection Fraction. Hypertens 2018, 4, 183–184. [Google Scholar] [CrossRef]
- SenthilKumar, G.; Zirgibel, Z.; Cohen, K.E.; Katunaric, B.; Jobe, A.M.; Shult, C.G.; Limpert, R.H.; Freed, J.K. Ying and Yang of Ceramide in the Vascular Endothelium. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1725–1736. [Google Scholar] [CrossRef]
- Harrison, D.G.; Coffman, T.M.; Wilcox, C.S. Pathophysiology of Hypertension: The Mosaic Theory and Beyond. Circ. Res. 2021, 128, 847–863. [Google Scholar] [CrossRef]
- Pasut, A.; Lama, E.; Van Craenenbroeck, A.H.; Kroon, J.; Carmeliet, P. Endothelial cell metabolism in cardiovascular physiology and disease. Nat. Rev. Cardiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Thatte, H.S.; Prabhakar, P.; Golan, D.E.; Michel, T. Calcium-independent activation of endothelial nitric oxide synthase by ceramide. Proc. Natl. Acad. Sci. USA 1999, 96, 12583–12588. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ouyang, H.; Zhou, Q.; Tang, S.; Fang, P.; Xie, G.; Yang, J.; Sun, G. LPS induces pulmonary microvascular endothelial cell barrier dysfunction by upregulating ceramide production. Cell Signal 2022, 92, 110250, Erratum in Cell Signal 2022, 95, 110349. [Google Scholar] [CrossRef] [PubMed]
- Cogolludo, A.; Moreno, L.; Frazziano, G.; Moral-Sanz, J.; Menendez, C.; Castañeda, J.; González, C.; Villamor, E.; Perez-Vizcaino, F. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc. Res. 2008, 82, 296–302. [Google Scholar] [CrossRef]
- Tabeling, C.; Yu, H.; Wang, L.; Ranke, H.; Goldenberg, N.M.; Zabini, D.; Noe, E.; Krauszman, A.; Gutbier, B.; Yin, J.; et al. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc. Natl. Acad. Sci. USA 2015, 112, E1614–E1623. [Google Scholar] [CrossRef]
- Bharath, L.P.; Ruan, T.; Li, Y.; Ravindran, A.; Wan, X.; Nhan, J.K.; Walker, M.L.; Deeter, L.; Goodrich, R.; Johnson, E.; et al. Ceramide-Initiated Protein Phosphatase 2A Activation Contributes to Arterial Dysfunction In Vivo. Diabetes 2015, 64, 3914–3926. [Google Scholar] [CrossRef]
- Freed, J.K.; Beyer, A.M.; LoGiudice, J.A.; Hockenberry, J.C.; Gutterman, D.D. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ. Res. 2014, 115, 525–532. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, X.; Song, J.; Yang, S.; Xiang, J.; Zhang, M.; Tu, C.; Song, X. Association between the plasma ceramide and coronary microvascular resistance. Cardiovasc. Diabetol. 2024, 23, 395. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef]
- Zhang, D.X.; Zou, A.P.; Li, P.L. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H605–H612. [Google Scholar] [CrossRef]
- Malecki, C.; Guglielmi, G.; Hunter, B.; Harney, D.; Koay, Y.C.; Don, A.S.; Han, O.; Khor, J.; Nguyen, L.; Pan, M.; et al. The Human Cardiac “Age-OME”: Age-Specific Changes in Myocardial Molecular Expression. Aging Cell 2025, e70219. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, Y.; Wu, J.; Ji, Y. Mechanisms of pulmonary microvascular endothelial cells barrier dysfunction induced by LPS: The roles of ceramides and the Txnip/NLRP3 inflammasome. Microvasc. Res. 2023, 147, 104491. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, H.; Wen, X.; Li, G.; Guo, S.; Zhang, D. NLRP3-inflammasome inhibition by MCC950 attenuates cardiac and pulmonary artery remodelling in heart failure with preserved ejection fraction. Life Sci. 2023, 333, 122185. [Google Scholar] [CrossRef] [PubMed]
- Balci, C.N.; Acar, N. NLRP3 inflammasome pathway, the hidden balance in pregnancy: A comprehensive review. J. Reprod. Immunol. 2024, 161, 104173. [Google Scholar] [CrossRef]
- C.Weel, I.; Romão-Veiga, M.; Matias, M.L.; Fioratti, E.G.; Peraçoli, J.C.; Borges, V.T.; Araujo, J.P.; Peraçoli, M.T. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol. 2017, 123, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Peh, Z.H.; Dihoum, A.; Hutton, D.; Arthur, J.S.C.; Rena, G.; Khan, F.; Lang, C.C.; Mordi, I.R. Inflammation as a therapeutic target in heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 2023, 10, 1125687. [Google Scholar] [CrossRef]
- Liu, H.; Magaye, R.; Kaye, D.M.; Wang, B.H. Heart failure with preserved ejection fraction: The role of inflammation. Eur. J. Pharmacol. 2024, 980, 176858. [Google Scholar] [CrossRef]
- Zhou, R.; Xia, Y.-Y.; Li, Z.; Wu, L.-D.; Shi, Y.; Ling, Z.-Y.; Zhang, J.-X. HFpEF as systemic disease, insight from a diagnostic prediction model reminiscent of systemic inflammation and organ interaction in HFpEF patients. Sci. Rep. 2024, 14, 5386. [Google Scholar] [CrossRef]
- De Luca, M.; Crisci, G.; Armentaro, G.; Cicco, S.; Talerico, G.; Bobbio, E.; Lanzafame, L.; Green, C.G.; McLellan, A.G.; Debiec, R.; et al. Endothelial Dysfunction and Heart Failure with Preserved Ejection Fraction-An Updated Review of the Literature. Life 2023, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Rubinelli, L.; Manzo, O.L.; Sungho, J.; Del Gaudio, I.; Bareja, R.; Marino, A.; Palikhe, S.; Di Mauro, V.; Bucci, M.; Falcone, D.J.; et al. Suppression of endothelial ceramide de novo biosynthesis by Nogo-B contributes to cardiometabolic diseases. Nat. Commun. 2025, 16, 1968. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Akhiyat, N.; Vasile, V.; Ahmad, A.; Sara, J.D.; Nardi, V.; Lerman, L.O.; Jaffe, A.; Lerman, A. Plasma Ceramide Levels Are Elevated in Patients with Early Coronary Atherosclerosis and Endothelial Dysfunction. J. Am. Heart Assoc. 2022, 11, e022852. [Google Scholar] [CrossRef]
- Mantel, Ä.; Sandström, A.; Faxén, J.; Andersson, D.C.; Razaz, N.; Cnattingius, S.; Stephansson, O. Pregnancy-Induced Hypertensive Disorder and Risks of Future Ischemic and Nonischemic Heart Failure. JACC Heart Fail. 2023, 11, 1216–1228. [Google Scholar] [CrossRef]
- Yin, W.; Li, F.; Tan, X.; Wang, H.; Jiang, W.; Wang, X.; Li, S.; Zhang, Y.; Han, Q.; Wang, Y.; et al. Plasma Ceramides and Cardiovascular Events in Hypertensive Patients at High Cardiovascular Risk. Am. J. Hypertens. 2021, 34, 1209–1216. [Google Scholar] [CrossRef]
- Prausmüller, S.; Weidenhammer, A.; Heitzinger, G.; Spinka, G.; Goliasch, G.; Arfsten, H.; Abdel Mawgoud, R.; Gabler, C.; Strunk, G.; Hengstenberg, C.; et al. Obesity in heart failure with preserved ejection fraction with and without diabetes: Risk factor or innocent bystander? Eur. J. Prev. Cardiol. 2023, 30, 1247–1254. [Google Scholar] [CrossRef]
- Raaijmakers, A.; Curl, C.; Janssens, J.; Varma, U.; Harrap, S.; Weeks, K.; Mellor, K.; Bell, J.; Delbridge, L. HFpEF with diabetic comorbidity is linked to cardiac lipid accumulation with severe diastolic dysfunction in female but not male hearts. J. Mol. Cell. Cardiol. 2022, 173, S77–S78. [Google Scholar] [CrossRef]
- Yazıcı, D.; Demir, S.Ç.; Sezer, H. Insulin Resistance, Obesity, and Lipotoxicity. In Obesity and Lipotoxicit; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 391–430. [Google Scholar]
- O’Connell, J.; Lynch, L.; Cawood, T.J.; Kwasnik, A.; Nolan, N.; Geoghegan, J.; McCormick, A.; O’Farrelly, C.; O’Shea, D. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS ONE 2010, 5, e9997. [Google Scholar] [CrossRef]
- Opoku, A.A.; Abushama, M.; Konje, J.C. Obesity and menopause. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 88, 102348. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Li, J.; Sun, Y.; Yang, Q.; Wang, S.; Luo, X.; Wang, W.; Wang, K.; Bai, W.; et al. The imbalance in the aortic ceramide/sphingosine-1-phosphate rheostat in ovariectomized rats and the preventive effect of estrogen. Lipids Health Dis. 2020, 19, 95. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Gastaldelli, A. The role of the liver in the modulation of glucose and insulin in non alcoholic fatty liver disease and type 2 diabetes. Curr. Opin. Pharmacol. 2020, 55, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Capone, F.; Vettor, R.; Schiattarella, G.G. Cardiometabolic HFpEF: NASH of the Heart. Circulation 2023, 147, 451–453. [Google Scholar] [CrossRef]
- Kucsera, D.; Ruppert, M.; Sayour, N.V.; Tóth, V.E.; Kovács, T.; Hegedűs, Z.I.; Onódi, Z.; Fábián, A.; Kovács, A.; Radovits, T.; et al. NASH triggers cardiometabolic HFpEF in aging mice. Geroscience 2024, 46, 4517–4531. [Google Scholar] [CrossRef]
- Chang, K.C.; Su, T.H.; Wu, C.K.; Huang, S.C.; Tseng, T.C.; Hong, C.M.; Hsu, S.J.; Liu, C.H.; Yang, H.C.; Liu, C.J.; et al. Metabolic dysfunction-associated steatotic liver disease is associated with increased risks of heart failure. Eur. J. Heart Fail. 2025, 27, 512–520. [Google Scholar] [CrossRef]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Bronneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Ohman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Ostkotte, D.; Nolte, H.; Gerl, M.J.; Jais, A.; Brunner, H.L.; Sprenger, H.G.; Awazawa, M.; Nicholls, H.T.; Turpin-Nolan, S.M.; et al. CerS6-Derived Sphingolipids Interact with Mff and Promote Mitochondrial Fragmentation in Obesity. Cell 2019, 177, 1536–1552.e1523. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. A ceramide-centric view of insulin resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef]
- Hajduch, E.; Turban, S.; Le Liepvre, X.; Le Lay, S.; Lipina, C.; Dimopoulos, N.; Dugail, I.; Hundal, H.S. Targeting of PKCζ and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem. J. 2008, 410, 369–379. [Google Scholar] [CrossRef]
- Blouin, C.M.; Prado, C.; Takane, K.K.; Lasnier, F.; Garcia-Ocana, A.; Ferré, P.; Dugail, I.; Hajduch, E. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 2010, 59, 600–610. [Google Scholar] [CrossRef]
- Summers, S.A.; Garza, L.A.; Zhou, H.; Birnbaum, M.J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell Biol. 1998, 18, 5457–5464. [Google Scholar] [CrossRef]
- Molina, A.J.A.; Bharadwaj, M.S.; Horn, C.V.; Nicklas, B.J.; Lyles, M.F.; Eggebeen, J.; Haykowsky, M.J.; Brubaker, P.H.; Kitzman, D.W. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients with Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016, 4, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Scandalis, L.; Kitzman, D.W.; Nicklas, B.J.; Lyles, M.; Brubaker, P.; Nelson, M.B.; Gordon, M.; Stone, J.; Bergstrom, J.; Neufer, P.D.; et al. Skeletal Muscle Mitochondrial Respiration and Exercise Intolerance in Patients with Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2023, 8, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Amartuvshin, O.; Lin, C.H.; Hsu, S.C.; Kao, S.H.; Chen, A.; Tang, W.C.; Chou, H.L.; Chang, D.L.; Hsu, Y.Y.; Hsiao, B.S.; et al. Aging shifts mitochondrial dynamics toward fission to promote germline stem cell loss. Aging Cell 2020, 19, e13191. [Google Scholar] [CrossRef] [PubMed]
- Bekfani, T.; Bekhite Elsaied, M.; Derlien, S.; Nisser, J.; Westermann, M.; Nietzsche, S.; Hamadanchi, A.; Fröb, E.; Westphal, J.; Haase, D.; et al. Skeletal Muscle Function, Structure, and Metabolism in Patients with Heart Failure with Reduced Ejection Fraction and Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2020, 13, e007198. [Google Scholar] [CrossRef]
- Saw, E.L.; Ramachandran, S.; Valero-Muñoz, M.; Sam, F. Skeletal muscle (dys)function in heart failure with preserved ejection fraction. Curr. Opin. Cardiol. 2021, 36, 219–226. [Google Scholar] [CrossRef]
- Turpin-Nolan, S.M.; Hammerschmidt, P.; Chen, W.; Jais, A.; Timper, K.; Awazawa, M.; Brodesser, S.; Brüning, J.C. CerS1-Derived C(18:0) Ceramide in Skeletal Muscle Promotes Obesity-Induced Insulin Resistance. Cell Rep. 2019, 26, 1–10.e17. [Google Scholar] [CrossRef]
- Chaurasia, B.; Tippetts, T.S.; Mayoral Monibas, R.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Chacinska, M.; Vendelbo, M.H.; Zabielski, P. The Crucial Role of C18-Cer in Fat-Induced Skeletal Muscle Insulin Resistance. Cell Physiol. Biochem. 2016, 40, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, P.; Sjöblom, P.; Nilsson, M.; Wijkman, M.; Engvall, M.; Länne, T.; Nyström, F.H.; Östgren, C.J.; Engvall, J. Overweight and obesity impair left ventricular systolic function as measured by left ventricular ejection fraction and global longitudinal strain. Cardiovasc. Diabetol. 2018, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Wang, Y.; Ma, C.; Zhao, Q.; Yin, H.; Li, L.; Wang, D.; Huang, Y.; Zhao, Y.; et al. Interleukin-6 promotes visceral adipose tissue accumulation during aging via inhibiting fat lipolysis. Int. Immunopharmacol. 2024, 132, 111906. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Hofwimmer, K.; de Paula Souza, J.; Subramanian, N.; Vujičić, M.; Rachid, L.; Méreau, H.; Zhao, C.; Dror, E.; Barreby, E.; Björkström, N.K.; et al. IL-1β promotes adipogenesis by directly targeting adipocyte precursors. Nat. Commun. 2024, 15, 7957. [Google Scholar] [CrossRef]
- Plomgaard, P.; Nielsen, A.R.; Fischer, C.P.; Mortensen, O.H.; Broholm, C.; Penkowa, M.; Krogh-Madsen, R.; Erikstrup, C.; Lindegaard, B.; Petersen, A.M.; et al. Associations between insulin resistance and TNF-α in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia 2007, 50, 2562–2571. [Google Scholar] [CrossRef]
- Brindley, D.N.; Wang, C.N.; Mei, J.; Xu, J.; Hanna, A.N. Tumor necrosis factor-alpha and ceramides in insulin resistance. Lipids 1999, 34 (Suppl. S1), S85–S88. [Google Scholar] [CrossRef]
- Holland, W.L.; Adams, A.C.; Brozinick, J.T.; Bui, H.H.; Miyauchi, Y.; Kusminski, C.M.; Bauer, S.M.; Wade, M.; Singhal, E.; Cheng, C.C.; et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013, 17, 790–797. [Google Scholar] [CrossRef]
- Sharma, A.X.; Holland, W.L. Adiponectin and its Hydrolase-Activated Receptors. J. Nat. Sci. 2017, 3, e396. [Google Scholar]
- Błażejewska, W.; Dąbrowska, J.; Michałowska, J.; Bogdański, P. The Role of Adiponectin and ADIPOQ Variation in Metabolic Syndrome: A Narrative Review. Genes 2025, 16, 699. [Google Scholar] [CrossRef]
- Guo, M.-M.; Duan, X.-N.; Cui, S.-D.; Tian, F.-G.; Cao, X.-C.; Geng, C.-Z.; Fan, Z.-M.; Wang, X.; Wang, S.; Jiang, H.-C.; et al. Circulating High-Molecular-Weight (HMW) Adiponectin Level Is Related with Breast Cancer Risk Better than Total Adiponectin: A Case-Control Study. PLoS ONE 2015, 10, e0129246. [Google Scholar] [CrossRef] [PubMed]
- Moyce Gruber, B.L.; Dolinsky, V.W. The Role of Adiponectin During Pregnancy and Gestational Diabetes. Life 2023, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Gudenkauf, B.; Shaya, G.; Mukherjee, M.; Michos, E.D.; Madrazo, J.; Mathews, L.; Shah, S.J.; Sharma, K.; Hays, A.G. Insulin resistance is associated with subclinical myocardial dysfunction and reduced functional capacity in heart failure with preserved ejection fraction. J. Cardiol. 2024, 83, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Jiang, R.; Xu, D.; Zhu, J.; Chen, J.; Lin, Y.; Zhou, H. Association between insulin resistance indices and outcomes in patients with heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 2025, 24, 32. [Google Scholar] [CrossRef]
- Colombini, M. Ceramide channels and their role in mitochondria-mediated apoptosis. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 1239–1244. [Google Scholar] [CrossRef]
- Cinti, F.; Laborante, R.; Cappannoli, L.; Morciano, C.; Gugliandolo, S.; Pontecorvi, A.; Burzotta, F.; Donniacuo, M.; Cappetta, D.; Patti, G.; et al. The effects of SGLT2i on cardiac metabolism in patients with HFpEF: Fact or fiction? Cardiovasc. Diabetol. 2025, 24, 208. [Google Scholar] [CrossRef]
- Haxhikadrija, P.; Wu, J.M.F.; Hübner, S.; Grün, K.; Kretzschmar, T.; Müller, T.; Gräler, M.H.; Backsch, C.; Weise, A.; Klein, E.; et al. Inhibition of ceramide synthesis improves the outcome of ischemia/reperfusion injury in cardiomyocytes derived from human induced pluripotent stem cell. Stem Cell Res. Ther. 2025, 16, 190. [Google Scholar] [CrossRef]
- Magaye, R.R.; Savira, F.; Hua, Y.; Xiong, X.; Huang, L.; Reid, C.; Flynn, B.; Kaye, D.; Liew, D.; Wang, B.H. Exogenous dihydrosphingosine 1 phosphate mediates collagen synthesis in cardiac fibroblasts through JAK/STAT signalling and regulation of TIMP1. Cell. Signal. 2020, 72, 109629. [Google Scholar] [CrossRef]
- Magaye, R.R.; Savira, F.; Hua, Y.; Xiong, X.; Huang, L.; Reid, C.; Flynn, B.L.; Kaye, D.; Liew, D.; Wang, B.H. Attenuating PI3K/Akt- mTOR pathway reduces dihydrosphingosine 1 phosphate mediated collagen synthesis and hypertrophy in primary cardiac cells. Int. J. Biochem. Cell Biol. 2021, 134, 105952. [Google Scholar] [CrossRef]
- Frias, M.A.; James, R.W.; Gerber-Wicht, C.; Lang, U. Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: Role of sphingosine-1-phosphate. Cardiovasc. Res. 2009, 82, 313–323. [Google Scholar] [CrossRef]
- Fang, R.; Zhang, L.-L.; Zhang, L.-Z.; Li, W.; Li, M.; Wen, K. Sphingosine 1-Phosphate Postconditioning Protects Against Myocardial Ischemia/reperfusion Injury in Rats via Mitochondrial Signaling and Akt-Gsk3β Phosphorylation. Arch. Med. Res. 2017, 48, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.H.; Koay, Y.C.; Marques, F.Z.; Kaye, D.M.; O’Sullivan, J.F. Leveraging metabolism for better outcomes in heart failure. Cardiovasc. Res. 2024, 120, 1835–1850. [Google Scholar] [CrossRef]
- Cimino, G.; Vaduganathan, M.; Lombardi, C.M.; Pagnesi, M.; Vizzardi, E.; Tomasoni, D.; Adamo, M.; Metra, M.; Inciardi, R.M. Obesity, heart failure with preserved ejection fraction, and the role of glucagon-like peptide-1 receptor agonists. ESC Heart Fail. 2024, 11, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Wadid, M.; Makwana, B.; Kumar, A.; Khadke, S.; Bhatti, A.; Banker, A.; Husami, Z.; Labib, S.; Venesy, D.; et al. GLP-1 Receptor Agonists Among Patients with Overweight or Obesity, Diabetes, and HFpEF on SGLT2 Inhibitors. JACC Heart Fail. 2024, 12, 1814–1826. [Google Scholar] [CrossRef]
- Lin, K.; Dong, C.; Zhao, B.; Zhou, B.; Yang, L. Glucagon-like peptide-1 receptor agonist regulates fat browning by altering the gut microbiota and ceramide metabolism. MedComm 2023, 4, e416. [Google Scholar] [CrossRef] [PubMed]
- Wretlind, A.; Curovic, V.R.; de Zawadzki, A.; Suvitaival, T.; Xu, J.; Zobel, E.H.; von Scholten, B.J.; Ripa, R.S.; Kjaer, A.; Hansen, T.W.; et al. Ceramides are decreased after liraglutide treatment in people with type 2 diabetes: A post hoc analysis of two randomized clinical trials. Lipids Health Dis. 2023, 22, 160. [Google Scholar] [CrossRef]
- Wong, C.K.; McLean, B.A.; Baggio, L.L.; Koehler, J.A.; Hammoud, R.; Rittig, N.; Yabut, J.M.; Seeley, R.J.; Brown, T.J.; Drucker, D.J. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metab. 2024, 36, 130–143.e135. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Sánchez-García, A.; Linden-Torres, E.; Simental-Mendía, M. Impact of glucagon-like peptide-1 receptor agonists on adiponectin concentrations: A meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2021, 87, 4140–4149. [Google Scholar] [CrossRef]
- Denimal, D.; Bergas, V.; Pais-de-Barros, J.-P.; Simoneau, I.; Demizieux, L.; Passilly-Degrace, P.; Bouillet, B.; Petit, J.-M.; Rouland, A.; Bataille, A.; et al. Liraglutide reduces plasma dihydroceramide levels in patients with type 2 diabetes. Cardiovasc. Diabetol. 2023, 22, 104. [Google Scholar] [CrossRef]
- Zobel, E.H.; Wretlind, A.; Ripa, R.S.; Rotbain Curovic, V.; von Scholten, B.J.; Suvitaival, T.; Hansen, T.W.; Kjær, A.; Legido-Quigley, C.; Rossing, P. Ceramides and phospholipids are downregulated with liraglutide treatment: Results from the LiraFlame randomized controlled trial. BMJ Open Diabetes Res. Care 2021, 9, e002395. [Google Scholar] [CrossRef]
- Wang, S.; Jin, Z.; Wu, B.; Morris, A.J.; Deng, P. Role of dietary and nutritional interventions in ceramide-associated diseases. J. Lipid Res. 2025, 66, 100726. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Düsing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network with High Therapeutic Potential. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, C.; Liao, J.; Dong, M.; Xiao, Z.; Lei, M. Ceramide mediates inhibition of the Akt/eNOS pathway by high levels of glucose in human vascular endothelial cells. J. Pediatr. Endocrinol. Metab. 2013, 26, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Luo, Y.; Huang, F.; Xie, Y.; Zheng, S.; Jia, B.; Xiao, Z. Sphingolipids: Drivers of cardiac fibrosis and atrial fibrillation. J. Mol. Med. 2024, 102, 149–165. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Gayathri, S.; Bhaskar, J.P.; Krishnan, V.; Balamurugan, K. Understanding the role of p38 and JNK mediated MAPK pathway in response to UV-A induced photoaging in Caenorhabditis elegans. J. Photochem. Photobiol. B 2020, 205, 111844. [Google Scholar] [CrossRef]
- Yabu, T.; Shiba, H.; Shibasaki, Y.; Nakanishi, T.; Imamura, S.; Touhata, K.; Yamashita, M. Stress-induced ceramide generation and apoptosis via the phosphorylation and activation of nSMase1 by JNK signaling. Cell Death Differ. 2015, 22, 258–273. [Google Scholar] [CrossRef]
- Magaye, R.R.; Wang, B.H.; Liu, H.R.; Bailey, S.; Nuon, R.; Sutano, S.; Nyugen, M.; Kiriazis, H.; Grigolon, K.; Shan, L.; et al. Multiparity induces persistent myocardial structural, functional and transcriptomic remodelling in mice. Sci. Rep. 2025, 15, 24254. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Blachnio-Zabielska, A.; Raba, G.; Sakowicz, A.; Kalinka, J.; Chabowski, A.; Laudanski, P. Sphingolipids as a new factor in the pathomechanism of preeclampsia—Mass spectrometry analysis. PLoS ONE 2017, 12, e0177601. [Google Scholar] [CrossRef]
- Laudanski, P.; Charkiewicz, K.; Kisielewski, R.; Kuc, P.; Koc-Zorawska, E.; Raba, G.; Kraczkowski, J.; Dymicka-Piekarska, V.; Chabowski, A.; Kacerovsky, M.; et al. Plasma C16-Cer levels are increased in patients with preterm labor. Prostaglandins Other Lipid Mediat. 2016, 123, 40–45. [Google Scholar] [CrossRef]
- Huang, Q.; Hao, S.; Yao, X.; You, J.; Li, X.; Lai, D.; Han, C.; Schilling, J.; Hwa, K.Y.; Thyparambil, S.; et al. High-throughput quantitation of serological ceramides/dihydroceramides by LC/MS/MS: Pregnancy baseline biomarkers and potential metabolic messengers. J. Pharm. Biomed. Anal. 2021, 192, 113639. [Google Scholar] [CrossRef]
- Rico, J.E.; Specker, B.; Perry, C.A.; McFadden, J.W. Plasma Ceramides and Triglycerides Are Elevated during Pregnancy in Association with Markers of Insulin Resistance in Hutterite Women. Lipids 2020, 55, 375–386. [Google Scholar] [CrossRef]
- Li, Q.; Ni, J.; Sl, B.; Lc, Y.; Zhu, H.; Zhang, W. Inhibition of steroidogenesis and induction of apoptosis in rat luteal cells by cell-permeable ceramide in vitro. Sheng Li Xue Bao [Acta Physiol. Sin.] 2001, 53, 142–146. [Google Scholar] [PubMed]
- Santana, P.; Llanes, L.; Hernández, I.; Gonzalez-Robayna, I.J.; Tabraue, C.; González-Reyes, J.A.; Quintana, J.M.; Estévez, F.; Galarreta, C.M.R.d.; Fanjul, L.F. Interleukin-1 beta stimulates sphingomyelin hydrolysis in cultured granulosa cells: Evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology 1996, 137, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Ogunmoroti, O.; Osibogun, O.; Kolade, O.B.; Ying, W.; Sharma, G.; Vaidya, D.; Michos, E.D. Multiparity is associated with poorer cardiovascular health among women from the Multi-Ethnic Study of Atherosclerosis. Am. J. Obstet. Gynecol. 2019, 221, 631.e1–631.e16. [Google Scholar] [CrossRef] [PubMed]
- Durazo, E.M.; Cabeza de Baca, T.; Slopen, N.; Parikh, N.I.; Buring, J.E.; Glynn, R.J.; Albert, M.A. Parity, Job Strain, and Cardiovascular Risk in the Women’s Health Study. Curr. Cardiovasc. Risk Rep. 2018, 12, 8. [Google Scholar] [CrossRef]
- Li, W.; Ruan, W.; Lu, Z.; Wang, D. Parity and risk of maternal cardiovascular disease: A dose-response meta-analysis of cohort studies. Eur. J. Prev. Cardiol. 2019, 26, 592–602. [Google Scholar] [CrossRef]
- Zhu, F.; Qi, H.; Bos, M.; Boersma, E.; Kavousi, M. Female Reproductive Factors and Risk of New-Onset Heart Failure: Findings from UK Biobank. JACC Heart Fail. 2023, 11, 1203–1212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).