Unraveling Type 1 Diabetes: Integrating Microbiome, Metabolomics, and Immunomodulation for Next-Generation Therapies
Abstract
1. Introduction
2. Pathophysiology of Type 1 Diabetes: Immune Modulators and Therapeutic Targets
3. Alterations in the Gut Microbiome Associated with T1D
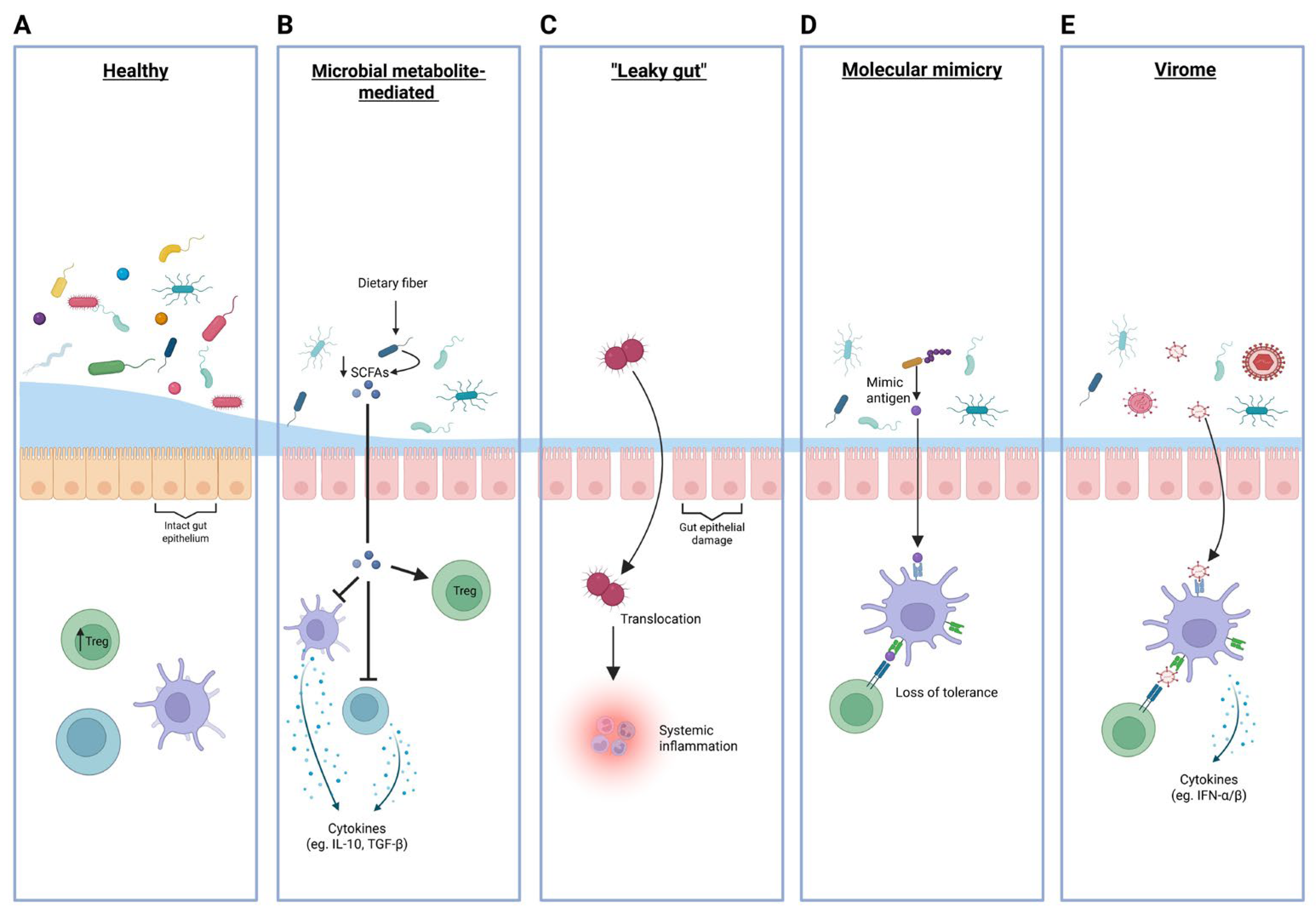
4. Potential Mechanisms Linking Gut Microbiota to T1D Pathogenesis
4.1. Microbial Metabolite-Mediated Immune Regulation
4.2. “Leaky Gut”: Microbial Product Translocation to Systemic Circulation
4.3. Molecular Mimicry and Cross-Reactive Immune Responses
4.4. Virome Contributions to T1D
5. Gut Microbiome Therapies: Fecal Microbiota Transplantations
5.1. FMT in T1D Research: Current Findings and Potential
5.2. Future of FMT in T1D: Challenges and Considerations
6. Microbial Metabolite-Driven Therapies in T1D
7. Discussion
8. Materials and Methods
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AID | Automated Insulin Delivery |
| APC | Antigen-Presenting Cell |
| CAR-Treg | Chimeric Antigen Receptor Regulatory T cells |
| CGM | Continuous Glucose Monitoring |
| DIPP | Type 1 Diabetes Prediction and Prevention |
| FMT | Fecal Microbiota Transplantation |
| FOXP3 | Forkhead Box P3 (Treg transcription factor) |
| GADA | Glutamic Acid Decarboxylase Antibodies |
| HbA1c | Hemoglobin A1c (glycemic control marker) |
| HLA | Human Leukocyte Antigen |
| IAA | Insulin Autoantibodies |
| IA-2A | Islet Antigen-2 Antibodies |
| JAK-STAT | Janus Kinase–Signal Transducer and Activator of Transcription Pathway |
| MAIT | Mucosal-Associated Invariant T cells |
| MODY | Maturity Onset Diabetes of the Young |
| MHC | Major Histocompatibility Complex |
| NOD | Non-Obese Diabetic (Mouse Model) |
| OMV | Outer Membrane Vesicles |
| RCT | Randomized Controlled Trial |
| SCFA | Short-Chain Fatty Acids |
| STAT | Signal Transducer and Activator of Transcription |
| TCR | T Cell Receptor |
| Tfh | Follicular Helper T cells |
| Th1, Th17 | T Helper Cell Subsets |
| TIGIT | T Cell Immunoreceptor with Ig and ITIM Domains |
| ZnT8 | Zinc Transporter 8 |
References
- Patterson, C.C.; Karuranga, S.; Salpea, P.; Saeedi, P.; Dahlquist, G.; Soltesz, G.; Ogle, G.D. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107842. [Google Scholar] [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Harding, J.L.; Wander, P.L.; Zhang, X.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.M.; Braffett, B.H.; Cleary, P.A.; Gubitosi-Klug, R.A.; Larkin, M.E.; DCCT/EDIC Research Group. The Long-Term Effects of Type 1 Diabetes Treatment and Complications on Health-Related Quality of Life: A 23-year follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications cohort. Diabetes Care 2013, 36, 3131–3138. [Google Scholar] [CrossRef]
- Clemens, K.K.; Woodward, M.; Neal, B.; Zinman, B. Sex Disparities in Cardiovascular Outcome Trials of Populations with Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1157–1163. [Google Scholar] [CrossRef]
- Harjutsalo, V.; Pongrac Barlovic, D.; Groop, P.-H. Long-term population-based trends in the incidence of cardiovascular disease in individuals with type 1 diabetes from Finland: A retrospective, nationwide, cohort study. Lancet Diabetes Endocrinol. 2021, 9, 575–585, Erratum in Lancet Diabetes Endocrinol. 2023, 11, e1. https://doi.org/10.1016/S2213-8587(22)00350-3. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Ilonen, J.; Hammais, A.; Laine, A.-P.; Lempainen, J.; Vaarala, O.; Veijola, R.; Simell, O.; Knip, M. Patterns of β-Cell Autoantibody Appearance and Genetic Associations During the First Years of Life. Diabetes 2013, 62, 3636–3640. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Bain, S.C.; Barnett, A.H.; Bingley, P.J.; Christie, M.R.; Gill, G.V.; Gale, E.A. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004, 364, 1699–1700. [Google Scholar] [CrossRef]
- Hermann, R.; Knip, M.; Veijola, R.; Simell, O.; Laine, A.P.; Akerblom, H.K.; Groop, P.H.; Forsblom, C.; Pettersson-Fernholm, K.; Ilonen, J. Temporal changes in the frequencies of HLA genotypes in patients with Type 1 diabetes–indication of an increased environmental pressure? Diabetologia 2003, 46, 420–425. [Google Scholar] [CrossRef]
- Ramli, R.; Reddy, M.; Oliver, N. Artificial Pancreas: Current Progress and Future Outlook in the Treatment of Type 1 Diabetes. Drugs 2019, 79, 1089–1101. [Google Scholar] [CrossRef]
- Kudva, Y.C.; Henderson, R.J.; Kanapka, L.G.; Weinstock, R.S.; Rickels, M.R.; Pratley, R.E.; Chaytor, N.; Janess, K.; Desjardins, D.; Pattan, V.; et al. Automated Insulin Delivery in Older Adults with Type 1 Diabetes. NEJM Evid. 2025, 4, EVIDoa2400200. [Google Scholar] [CrossRef]
- Limbert, C.; Kowalski, A.J.; Danne, T.P.A. Automated Insulin Delivery: A Milestone on the Road to Insulin Independence in Type 1 Diabetes. Diabetes Care 2024, 47, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.L.; Dayan, C.M.; Chatenoud, L.; Sumnik, Z.; Simmons, K.M.; Szypowska, A.; Gitelman, S.E.; Knecht, L.A.; Niemoeller, E.; Tian, W.; et al. Teplizumab and β-Cell Function in Newly Diagnosed Type 1 Diabetes. N. Engl. J. Med. 2023, 389, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.J.; Morgan, N.G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2023, 19, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018, 562, 589–594. [Google Scholar] [CrossRef]
- Wong, F.S.; Wen, L.; Tang, M.; Ramanathan, M.; Visintin, I.; Daugherty, J.; Hannum, L.G.; Janeway, C.A., Jr.; Shlomchik, M.J. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes 2004, 53, 2581–2587. [Google Scholar] [CrossRef]
- Silveira, P.A.; Johnson, E.; Chapman, H.D.; Bui, T.; Tisch, R.M.; Serreze, D.V. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur. J. Immunol. 2002, 32, 3657–3666. [Google Scholar] [CrossRef]
- Hu, C.Y.; Rodriguez-Pinto, D.; Du, W.; Ahuja, A.; Henegariu, O.; Wong, F.S.; Shlomchik, M.J.; Wen, L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Investig. 2007, 117, 3857–3867. [Google Scholar] [CrossRef]
- Willcox, A.; Richardson, S.J.; Bone, A.J.; Foulis, A.K.; Morgan, N.G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2009, 155, 173–181. [Google Scholar] [CrossRef]
- Skowera, A.; Ellis, R.J.; Varela-Calviño, R.; Arif, S.; Huang, G.C.; Van-Krinks, C.; Zaremba, A.; Rackham, C.; Allen, J.S.; Tree, T.I.; et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Investig. 2008, 118, 3390–3402. [Google Scholar] [CrossRef]
- Knight, R.R.; Kronenberg, D.; Zhao, M.; Huang, G.C.; Eichmann, M.; Bulek, A.; Wooldridge, L.; Cole, D.K.; Sewell, A.K.; Peakman, M.; et al. Human β-cell killing by autoreactive preproinsulin-specific CD8 T cells is predominantly granule-mediated with the potency dependent upon T-cell receptor avidity. Diabetes 2013, 62, 205–213. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef]
- Calderon, B.; Suri, A.; Unanue, E.R. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet beta-cell killing: Studies from an acute model. Am. J. Pathol. 2006, 169, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Marro, B.S.; Legrain, S.; Ware, B.C.; Oldstone, M.B.A. Macrophage IFN-I signaling promotes autoreactive T cell infiltration into islets in type 1 diabetes model. JCI Insight 2019, 4, e125067. [Google Scholar] [CrossRef] [PubMed]
- Kedmi, R.; Littman, D.R. Antigen-presenting cells as specialized drivers of intestinal T cell functions. Immunity 2024, 57, 2269–2279. [Google Scholar] [CrossRef]
- Khosravi-Maharlooei, M.; Madley, R.; Borsotti, C.; Ferreira, L.M.R.; Sharp, R.C.; Brehm, M.A.; Greiner, D.L.; Parent, A.V.; Anderson, M.S.; Sykes, M.; et al. Modeling human T1D-associated autoimmune processes. Mol. Metab. 2022, 56, 101417. [Google Scholar] [CrossRef] [PubMed]
- Fuhri Snethlage, C.M.; de Wit, D.; Wortelboer, K.; Rampanelli, E.; Hanssen, N.M.J.; Nieuwdorp, M. Can fecal microbiota transplantations modulate autoimmune responses in type 1 diabetes? Immunol. Rev. 2024, 325, 46–63. [Google Scholar] [CrossRef]
- Makhlouf, L.; Grey, S.T.; Dong, V.; Csizmadia, E.; Arvelo, M.B.; Auchincloss, H., Jr.; Ferran, C.; Sayegh, M.H. Depleting anti-CD4 monoclonal antibody cures new-onset diabetes, prevents recurrent autoimmune diabetes, and delays allograft rejection in nonobese diabetic mice. Transplantation 2004, 77, 990–997. [Google Scholar] [CrossRef]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef]
- Hirsch, J.S. FDA approves teplizumab: A milestone in type 1 diabetes. Lancet Diabetes Endocrinol. 2023, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Orban, T.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Marks, J.B.; Monzavi, R.; et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: A randomised, double-blind, placebo-controlled trial. Lancet 2011, 378, 412–419. [Google Scholar] [CrossRef]
- Jerram, S.T.; Yang, J.H.M.; Williams, E.; Domingo-Vila, C.; Liu, Y.F.; Peakman, M.; Leslie, R.D.; Tree, T. Subcutaneous Abatacept in New Onset Type 1 Diabetes: Clinical and Immunological Effects. Diabetes Metab. Res. Rev. 2025, 41, e70074. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.E.; Bundy, B.N.; Anderson, M.S.; Cooney, L.A.; Gitelman, S.E.; Goland, R.S.; Gottlieb, P.A.; Greenbaum, C.J.; Haller, M.J.; Krischer, J.P.; et al. Abatacept for Delay of Type 1 Diabetes Progression in Stage 1 Relatives at Risk: A Randomized, Double-Masked, Controlled Trial. Diabetes Care 2023, 46, 1005–1013. [Google Scholar] [CrossRef]
- Lin, J.; Moradi, E.; Salenius, K.; Lehtipuro, S.; Häkkinen, T.; Laiho, J.E.; Oikarinen, S.; Randelin, S.; Parikh, H.M.; Krischer, J.P.; et al. Distinct transcriptomic profiles in children prior to the appearance of type 1 diabetes-linked islet autoantibodies and following enterovirus infection. Nat. Commun. 2023, 14, 7630. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.-F. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nat. Rev. Immunol. 2018, 18, 105–120. [Google Scholar] [CrossRef]
- Ryba-Stanisławowska, M.; Rybarczyk-Kapturska, K.; Myśliwiec, M.; Myśliwska, J. Elevated levels of serum IL-12 and IL-18 are associated with lower frequencies of CD4(+)CD25 (high)FOXP3 (+) regulatory t cells in young patients with type 1 diabetes. Inflammation 2014, 37, 1513–1520. [Google Scholar] [CrossRef]
- Brusko, T.; Wasserfall, C.; McGrail, K.; Schatz, R.; Viener, H.L.; Schatz, D.; Haller, M.; Rockell, J.; Gottlieb, P.; Clare-Salzler, M.; et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes 2007, 56, 604–612. [Google Scholar] [CrossRef]
- Lindley, S.; Dayan, C.M.; Bishop, A.; Roep, B.O.; Peakman, M.; Tree, T.I. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes 2005, 54, 92–99. [Google Scholar] [CrossRef]
- Haseda, F.; Imagawa, A.; Murase-Mishiba, Y.; Terasaki, J.; Hanafusa, T. CD4+ CD45RA− FoxP3high activated regulatory T cells are functionally impaired and related to residual insulin-secreting capacity in patients with type 1 diabetes. Clin. Exp. Immunol. 2013, 173, 207–216. [Google Scholar] [CrossRef]
- Brusko, T.M.; Wasserfall, C.H.; Clare-Salzler, M.J.; Schatz, D.A.; Atkinson, M.A. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes 2005, 54, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Herman, A.E.; Matos, M.; Mathis, D.; Benoist, C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 2005, 202, 1387–1397. [Google Scholar] [CrossRef]
- Mellanby, R.J.; Thomas, D.; Phillips, J.M.; Cooke, A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology 2007, 121, 15–28. [Google Scholar] [CrossRef]
- Tang, Q.; Henriksen, K.J.; Bi, M.; Finger, E.B.; Szot, G.; Ye, J.; Masteller, E.L.; McDevitt, H.; Bonyhadi, M.; Bluestone, J.A. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Marek-Trzonkowska, N.; Mysliwiec, M.; Dobyszuk, A.; Grabowska, M.; Techmanska, I.; Juscinska, J.; Wujtewicz, M.A.; Witkowski, P.; Mlynarski, W.; Balcerska, A.; et al. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 2012, 35, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Rosenzwajg, M.; Salet, R.; Lorenzon, R.; Tchitchek, N.; Roux, A.; Bernard, C.; Carel, J.C.; Storey, C.; Polak, M.; Beltrand, J.; et al. Low-dose IL-2 in children with recently diagnosed type 1 diabetes: A Phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia 2020, 63, 1808–1821. [Google Scholar] [CrossRef]
- Dong, S.; Hiam-Galvez, K.J.; Mowery, C.T.; Herold, K.C.; Gitelman, S.E.; Esensten, J.H.; Liu, W.; Lares, A.P.; Leinbach, A.S.; Lee, M.; et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 2021, 6, e147474. [Google Scholar] [CrossRef]
- Spanier, J.A.; Fung, V.; Wardell, C.M.; Alkhatib, M.H.; Chen, Y.; Swanson, L.A.; Dwyer, A.J.; Weno, M.E.; Silva, N.; Mitchell, J.S.; et al. Tregs with an MHC class II peptide-specific chimeric antigen receptor prevent autoimmune diabetes in mice. J. Clin. Investig. 2023, 133, e168601. [Google Scholar] [CrossRef]
- Uenishi, G.I.; Repic, M.; Yam, J.Y.; Landuyt, A.; Saikumar-Lakshmi, P.; Guo, T.; Zarin, P.; Sassone-Corsi, M.; Chicoine, A.; Kellogg, H.; et al. GNTI-122: An autologous antigen-specific engineered Treg cell therapy for type 1 diabetes. JCI Insight 2024, 9, e171844. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, J. Regulatory T cell-based therapy in type 1 diabetes: Latest breakthroughs and evidence. Int. Immunopharmacol. 2024, 140, 112724. [Google Scholar] [CrossRef]
- Russell, M.A.; Richardson, S.J.; Morgan, N.G. The role of the interferon/JAK-STAT axis in driving islet HLA-I hyperexpression in type 1 diabetes. Front. Endocrinol. 2023, 14, 1270325. [Google Scholar] [CrossRef]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546, Erratum in Drugs 2017, 77, 939. https://doi.org/10.1007/s40265-017-0736-y. [Google Scholar] [CrossRef]
- Waibel, M.; Wentworth, J.M.; So, M.; Couper, J.J.; Cameron, F.J.; MacIsaac, R.J.; Atlas, G.; Gorelik, A.; Litwak, S.; Sanz-Villanueva, L.; et al. Baricitinib and β-Cell Function in Patients with New-Onset Type 1 Diabetes. N. Engl. J. Med. 2023, 389, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Chaimowitz, N.S.; Ebenezer, S.J.; Hanson, I.C.; Anderson, M.; Forbes, L.R. STAT1 Gain of Function, Type 1 Diabetes, and Reversal with JAK Inhibition. N. Engl. J. Med. 2020, 383, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Mannering, S.I.; Harrison, L.C.; Williamson, N.A.; Morris, J.S.; Thearle, D.J.; Jensen, K.P.; Kay, T.W.; Rossjohn, J.; Falk, B.A.; Nepom, G.T.; et al. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J. Exp. Med. 2005, 202, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T.; Faridi, P.; Lim, J.J.; Ting, Y.T.; Onwukwe, G.; Bhattacharjee, P.; Jones, C.M.; Tresoldi, E.; Cameron, F.J.; La Gruta, N.L.; et al. T cell receptor recognition of hybrid insulin peptides bound to HLA-DQ8. Nat. Commun. 2021, 12, 5110. [Google Scholar] [CrossRef]
- Wondafrash, D.Z.; Nire’a, A.T.; Tafere, G.G.; Desta, D.M.; Berhe, D.A.; Zewdie, K.A. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab. Syndr. Obes. 2020, 13, 43–51. [Google Scholar] [CrossRef]
- Mitchell, J.S.; Spanier, J.A.; Dwyer, A.J.; Knutson, T.P.; Alkhatib, M.H.; Qian, G.; Weno, M.E.; Chen, Y.; Shaheen, Z.R.; Tucker, C.G.; et al. CD4(+) T cells reactive to a hybrid peptide from insulin-chromogranin A adopt a distinct effector fate and are pathogenic in autoimmune diabetes. Immunity 2024, 57, 2399–2415.e2398. [Google Scholar] [CrossRef]
- Delong, T.; Wiles, T.A.; Baker, R.L.; Bradley, B.; Barbour, G.; Reisdorph, R.; Armstrong, M.; Powell, R.L.; Reisdorph, N.; Kumar, N.; et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 2016, 351, 711–714. [Google Scholar] [CrossRef]
- Crawford, S.A.; Wiles, T.A.; Wenzlau, J.M.; Powell, R.L.; Barbour, G.; Dang, M.; Groegler, J.; Barra, J.M.; Burnette, K.S.; Hohenstein, A.C.; et al. Cathepsin D Drives the Formation of Hybrid Insulin Peptides Relevant to the Pathogenesis of Type 1 Diabetes. Diabetes 2022, 71, 2793–2803. [Google Scholar] [CrossRef]
- Hyöty, H. Viruses in type 1 diabetes. Pediatr. Diabetes 2016, 17, 56–64. [Google Scholar] [CrossRef]
- Krogvold, L.; Mynarek, I.M.; Ponzi, E.; Mørk, F.B.; Hessel, T.W.; Roald, T.; Lindblom, N.; Westman, J.; Barker, P.; Hyöty, H.; et al. Pleconaril and ribavirin in new-onset type 1 diabetes: A phase 2 randomized trial. Nat. Med. 2023, 29, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Parviainen, A.; But, A.; Sund, R.; Arffman, M.; Siljander, H.; Knip, M. Incidence of Type 1 Diabetes in Relation to Exposure to Rotavirus Infections in Pre- and Postvaccine Birth Cohorts in Finland. Diabetes Care 2023, 47, 97–100. [Google Scholar] [CrossRef]
- Yu, J.; Sharma, P.; Girgis, C.M.; Gunton, J.E. Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14434. [Google Scholar] [CrossRef]
- Miranda, M.C.G.; Oliveira, R.P.; Torres, L.; Aguiar, S.L.F.; Pinheiro-Rosa, N.; Lemos, L.; Guimarães, M.A.; Reis, D.; Silveira, T.; Ferreira, Ê.; et al. Frontline Science: Abnormalities in the gut mucosa of non-obese diabetic mice precede the onset of type 1 diabetes. J. Leukoc. Biol. 2019, 106, 513–529. [Google Scholar] [CrossRef]
- Yang, J.; Wen, X.; Xu, H.; Torres-Chinn, N.; Speake, C.; Greenbaum, C.J.; Nepom, G.T.; Kwok, W.W. Antigen-Specific T Cell Analysis Reveals That Active Immune Responses to β Cell Antigens Are Focused on a Unique Set of Epitopes. J. Immunol. 2017, 199, 91–96. [Google Scholar] [CrossRef]
- Rui, J.; Deng, S.; Perdigoto, A.L.; Ponath, G.; Kursawe, R.; Lawlor, N.; Sumida, T.; Levine-Ritterman, M.; Stitzel, M.L.; Pitt, D.; et al. Tet2 Controls the Responses of β cells to Inflammation in Autoimmune Diabetes. Nat. Commun. 2021, 12, 5074. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.S.; Harenda, Q.; Pietrzak, S.; Oktay, H.Z.; Schreiber, S.; Liao, Y.; Sonthalia, S.; Ciecko, A.E.; Chen, Y.G.; et al. Beta Cell Dedifferentiation Induced by IRE1α Deletion Prevents Type 1 Diabetes. Cell Metab. 2020, 31, 822–836.e825. [Google Scholar] [CrossRef]
- Thompson, P.J.; Shah, A.; Ntranos, V.; Van Gool, F.; Atkinson, M.; Bhushan, A. Targeted Elimination of Senescent Beta Cells Prevents Type 1 Diabetes. Cell Metab. 2019, 29, 1045–1060.e1010. [Google Scholar] [CrossRef] [PubMed]
- Rubin de Celis, M.F.; Garcia-Martin, R.; Syed, I.; Lee, J.; Aguayo-Mazzucato, C.; Bonner-Weir, S.; Kahn, B.B. PAHSAs reduce cellular senescence and protect pancreatic beta cells from metabolic stress through regulation of Mdm2/p53. Proc. Natl. Acad. Sci. USA 2022, 119, e2206923119. [Google Scholar] [CrossRef]
- Redondo, M.J.; Jeffrey, J.; Fain, P.R.; Eisenbarth, G.S.; Orban, T. Concordance for islet autoimmunity among monozygotic twins. N. Engl. J. Med. 2008, 359, 2849–2850. [Google Scholar] [CrossRef]
- Redondo, M.J.; Rewers, M.; Yu, L.; Garg, S.; Pilcher, C.C.; Elliott, R.B.; Eisenbarth, G.S. Genetic determination of islet cell autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of patients with type 1 diabetes: Prospective twin study. Bmj 1999, 318, 698–702. [Google Scholar] [CrossRef]
- Ogle, G.D.; Wang, F.; Haynes, A.; Gregory, G.A.; King, T.W.; Deng, K.; Dabelea, D.; James, S.; Jenkins, A.J.; Li, X.; et al. Global type 1 diabetes prevalence, incidence, and mortality estimates 2025: Results from the International diabetes Federation Atlas, 11th Edition, and the T1D Index Version 3.0. Diabetes Res. Clin. Pract. 2025, 225, 112277. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.A.; McNulty, N.P.; Guruge, J.L.; Gordon, J.I. IgA Response to Symbiotic Bacteria as a Mediator of Gut Homeostasis. Cell Host Microbe 2007, 2, 328–339. [Google Scholar] [CrossRef]
- Fenneman, A.C.; Weidner, M.; Chen, L.A.; Nieuwdorp, M.; Blaser, M.J. Antibiotics in the pathogenesis of diabetes and inflammatory diseases of the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Yin, Y.S.; Wang, J.; Battaglia, T.; Krautkramer, K.; Li, W.V.; Li, J.; Brown, M.; Zhang, M.; Badri, M.H.; et al. Maternal cecal microbiota transfer rescues early-life antibiotic-induced enhancement of type 1 diabetes in mice. Cell Host Microbe 2021, 29, 1249–1265.e1249. [Google Scholar] [CrossRef]
- Livanos, A.E.; Greiner, T.U.; Vangay, P.; Pathmasiri, W.; Stewart, D.; McRitchie, S.; Li, H.; Chung, J.; Sohn, J.; Kim, S.; et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 2016, 1, 16140. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021, 70, 92–105. [Google Scholar] [CrossRef]
- Schmidt, T.S.B.; Li, S.S.; Maistrenko, O.M.; Akanni, W.; Coelho, L.P.; Dolai, S.; Fullam, A.; Glazek, A.M.; Hercog, R.; Herrema, H.; et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nat. Med. 2022, 28, 1902–1912. [Google Scholar] [CrossRef]
- Rosell-Mases, E.; Santiago, A.; Corral-Pujol, M.; Yáñez, F.; Varela, E.; Egia-Mendikute, L.; Arpa, B.; Cosovanu, C.; Panosa, A.; Serrano-Gómez, G.; et al. Mutual modulation of gut microbiota and the immune system in type 1 diabetes models. Nat. Commun. 2023, 14, 7770. [Google Scholar] [CrossRef]
- Shilo, S.; Godneva, A.; Rachmiel, M.; Korem, T.; Bussi, Y.; Kolobkov, D.; Karady, T.; Bar, N.; Wolf, B.C.; Glantz-Gashai, Y.; et al. The Gut Microbiome of Adults with Type 1 Diabetes and Its Association with the Host Glycemic Control. Diabetes Care 2022, 45, 555–563. [Google Scholar] [CrossRef]
- Anderson, M.S.; Bluestone, J.A. The NOD mouse: A model of immune dysregulation. Annu. Rev. Immunol. 2005, 23, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Godovannyi, A.; Ma, C.; Zhang, Y.; Ahmadi-Vand, Z.; Dai, C.; Gorzelak, M.A.; Chan, Y.; Chan, J.M.; Lochner, A.; et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. Isme J. 2016, 10, 321–332. [Google Scholar] [CrossRef]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, X.; Sun, L.; Liu, Y.; Lv, Y.; Gang, X.; Wang, G. Gut Microbiota Profile in Patients with Type 1 Diabetes Based on 16S rRNA Gene Sequencing: A Systematic Review. Dis. Markers 2020, 2020, 3936247. [Google Scholar] [CrossRef] [PubMed]
- Roesch, L.F.; Lorca, G.L.; Casella, G.; Giongo, A.; Naranjo, A.; Pionzio, A.M.; Li, N.; Mai, V.; Wasserfall, C.H.; Schatz, D.; et al. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. Isme J. 2009, 3, 536–548. [Google Scholar] [CrossRef]
- Higuchi, B.S.; Rodrigues, N.; Gonzaga, M.I.; Paiolo, J.C.C.; Stefanutto, N.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci, E., Jr.; Mariano, V.S.; et al. Intestinal Dysbiosis in Autoimmune Diabetes Is Correlated with Poor Glycemic Control and Increased Interleukin-6: A Pilot Study. Front. Immunol. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality Between Children with Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef]
- Valladares, R.; Sankar, D.; Li, N.; Williams, E.; Lai, K.K.; Abdelgeliel, A.S.; Gonzalez, C.F.; Wasserfall, C.H.; Larkin, J.; Schatz, D.; et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS ONE 2010, 5, e10507. [Google Scholar] [CrossRef]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal Mucosa of Patients with Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.C.; Hu, J.; Ruan, H.B.; Guo, H.M.; Zhang, H.H.; Wang, X.; Pei, Y.F.; Pan, Y.; Fang, C. Gut microbiota profiling in Han Chinese with type 1 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 256–263. [Google Scholar] [CrossRef]
- Cinek, O.; Kramna, L.; Mazankova, K.; Odeh, R.; Alassaf, A.; Ibekwe, M.U.; Ahmadov, G.; Elmahi, B.M.E.; Mekki, H.; Lebl, J.; et al. The bacteriome at the onset of type 1 diabetes: A study from four geographically distant African and Asian countries. Diabetes Res. Clin. Pract. 2018, 144, 51–62. [Google Scholar] [CrossRef]
- de Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; van Raalte, D.H.; Scheithauer, T.P.; et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.J.; Zhang, Q.; Yu, M.; Xu, J.P.; Zheng, J.; Wang, T.; Xiao, X.H. Imbalance of Fecal Microbiota at Newly Diagnosed Type 1 Diabetes in Chinese Children. Chin. Med. J. 2016, 129, 1298–1304. [Google Scholar] [CrossRef]
- Salamon, D.; Sroka-Oleksiak, A.; Kapusta, P.; Szopa, M.; Mrozińska, S.; Ludwig-Słomczyńska, A.H.; Wołkow, P.P.; Bulanda, M.; Klupa, T.; Małecki, M.T.; et al. Characteristics of gut microbiota in adult patients with type 1 and type 2 diabetes based on next-generation sequencing of the 16S rRNA gene fragment. Pol. Arch. Intern. Med. 2018, 128, 336–343. [Google Scholar] [CrossRef]
- Stewart, C.J.; Nelson, A.; Campbell, M.D.; Walker, M.; Stevenson, E.J.; Shaw, J.A.; Cummings, S.P.; West, D.J. Gut microbiota of Type 1 diabetes patients with good glycaemic control and high physical fitness is similar to people without diabetes: An observational study. Diabet. Med. 2017, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mejía-León, M.E.; Petrosino, J.F.; Ajami, N.J.; Domínguez-Bello, M.G.; de la Barca, A.M.C. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 2014, 4, 3814. [Google Scholar] [CrossRef]
- Vatanen, T.; de Beaufort, C.; Marcovecchio, M.L.; Overbergh, L.; Brunak, S.; Peakman, M.; Mathieu, C.; Knip, M.; INNODIA consortium. Gut microbiome shifts in people with type 1 diabetes are associated with glycaemic control: An INNODIA study. Diabetologia 2024, 67, 1930–1942. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef]
- Bishai, J.D.; Palm, N.W. Small Molecule Metabolites at the Host-Microbiota Interface. J. Immunol. 2021, 207, 1725–1733. [Google Scholar] [CrossRef]
- Millard, A.L.; Mertes, P.M.; Ittelet, D.; Villard, F.; Jeannesson, P.; Bernard, J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin. Exp. Immunol. 2002, 130, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450, Erratum in Nature 2014, 506, 254. https://doi.org/10.1038/nature13041. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- de Groot, P.F.; Nikolic, T.; Imangaliyev, S.; Bekkering, S.; Duinkerken, G.; Keij, F.M.; Herrema, H.; Winkelmeijer, M.; Kroon, J.; Levin, E.; et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: A randomised controlled trial. Diabetologia 2020, 63, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nieuwdorp, M.; de Vos, W.M.; Rampanelli, E. Microbial Tryptophan Metabolism Tunes Host Immunity, Metabolism, and Extraintestinal Disorders. Metabolites 2022, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pu, A.; Sheng, B.; Zhang, Z.; Li, L.; Liu, Z.; Wang, Q.; Li, X.; Ma, Y.; Yu, M.; et al. Aryl hydrocarbon receptor activation modulates CD8αα(+)TCRαβ(+) IELs and suppression of colitis manifestations in mice. Biomed. Pharmacother. 2017, 87, 127–134. [Google Scholar] [CrossRef]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248.e231. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Alberto González-Regueiro, J.; Moreno-Castañeda, L.; Uribe, M.; Carlos Chávez-Tapia, N. The Role of Bile Acids in Glucose Metabolism and Their Relation with Diabetes. Ann. Hepatol. 2017, 16 (Suppl. S1), S15–S20. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Alves, M.A.; Härkönen, T.; Honkanen, J.; Vatanen, T.; Xavier, R.J.; Hyötyläinen, T.; Knip, M.; et al. Dysregulation of secondary bile acid metabolism precedes islet autoimmunity and type 1 diabetes. Cell Rep. Med. 2022, 3, 100762. [Google Scholar] [CrossRef] [PubMed]
- Erttmann, S.F.; Swacha, P.; Aung, K.M.; Brindefalk, B.; Jiang, H.; Härtlova, A.; Uhlin, B.E.; Wai, S.N.; Gekara, N.O. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity 2022, 55, 847–861.e810. [Google Scholar] [CrossRef]
- Gilmore, W.J.; Johnston, E.L.; Bitto, N.J.; Zavan, L.; O’Brien-Simpson, N.; Hill, A.F.; Kaparakis-Liaskos, M. Bacteroides fragilis outer membrane vesicles preferentially activate innate immune receptors compared to their parent bacteria. Front. Immunol. 2022, 13, 970725. [Google Scholar] [CrossRef]
- Girdhar, K.; Huang, Q.; Chow, I.-T.; Vatanen, T.; Brady, C.; Raisingani, A.; Autissier, P.; Atkinson, M.A.; Kwok, W.W.; Kahn, C.R.; et al. A gut microbial peptide and molecular mimicry in the pathogenesis of type 1 diabetes. Proc. Natl. Acad. Sci. USA 2022, 119, e2120028119, Correction in Proc. Natl. Acad. Sci. USA 2023, 120, e2309963120. https://doi.org/10.1073/pnas.2309963120. [Google Scholar] [CrossRef] [PubMed]
- Tai, N.; Peng, J.; Liu, F.; Gulden, E.; Hu, Y.; Zhang, X.; Chen, L.; Wong, F.S.; Wen, L. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J. Exp. Med. 2016, 213, 2129–2146. [Google Scholar] [CrossRef]
- Hebbandi Nanjundappa, R.; Ronchi, F.; Wang, J.; Clemente-Casares, X.; Yamanouchi, J.; Sokke Umeshappa, C.; Yang, Y.; Blanco, J.; Bassolas-Molina, H.; Salas, A.; et al. A Gut Microbial Mimic that Hijacks Diabetogenic Autoreactivity to Suppress Colitis. Cell 2017, 171, 655–667.e617. [Google Scholar] [CrossRef]
- Vehik, K.; Lynch, K.F.; Wong, M.C.; Tian, X.; Ross, M.C.; Gibbs, R.A.; Ajami, N.J.; Petrosino, J.F.; Rewers, M.; Toppari, J.; et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med. 2019, 25, 1865–1872. [Google Scholar] [CrossRef]
- Zhao, G.; Vatanen, T.; Droit, L.; Park, A.; Kostic, A.D.; Poon, T.W.; Vlamakis, H.; Siljander, H.; Härkönen, T.; Hämäläinen, A.-M.; et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc. Natl. Acad. Sci. USA 2017, 114, E6166–E6175. [Google Scholar] [CrossRef]
- Faulkner, C.L.; Luo, Y.X.; Isaacs, S.; Rawlinson, W.D.; Craig, M.E.; Kim, K.W. The virome in early life and childhood and development of islet autoimmunity and type 1 diabetes: A systematic review and meta-analysis of observational studies. Rev. Med. Virol. 2021, 31, e2209. [Google Scholar] [CrossRef]
- Mynarek, I.M.; Krogvold, L.; Mørk, F.B.; Lawaetz, T.W.H.; Roald, T.; Fagerland, M.W.; Lindblom, N.; Westman, J.; Barker, P.; Hyöty, H.; et al. Three-Year Follow-up After Antiviral Treatment in New-Onset Type 1 Diabetes: Results from the Diabetes Virus Detection and Intervention Trial. Diabetes Care 2025, 48, 481–488. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M. Fame and future of faecal transplantations–developing next-generation therapies with synthetic microbiomes. Microb. Biotechnol. 2013, 6, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Bojanova, D.P.; Bordenstein, S.R. Fecal Transplants: What Is Being Transferred? PLoS Biol. 2016, 14, e1002503. [Google Scholar] [CrossRef]
- Zheng, L.; Ji, Y.Y.; Wen, X.L.; Duan, S.L. Fecal microbiota transplantation in the metabolic diseases: Current status and perspectives. World J. Gastroenterol. 2022, 28, 2546–2560. [Google Scholar] [CrossRef]
- Bénard, M.V.; de Bruijn, C.M.A.; Fenneman, A.C.; Wortelboer, K.; Zeevenhoven, J.; Rethans, B.; Herrema, H.J.; van Gool, T.; Nieuwdorp, M.; Benninga, M.A.; et al. Challenges and costs of donor screening for fecal microbiota transplantations. PLoS ONE 2022, 17, e0276323. [Google Scholar] [CrossRef]
- Basson, A.R.; Zhou, Y.; Seo, B.; Rodriguez-Palacios, A.; Cominelli, F. Autologous fecal microbiota transplantation for the treatment of inflammatory bowel disease. Transl. Res. 2020, 226, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10, eaap9489. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Singh, S.K.; Corrie, L.; Kaur, I.P.; Chandwani, L. Delivery routes for faecal microbiota transplants: Available, anticipated and aspired. Pharmacol. Res. 2020, 159, 104954. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Jing, X.-B.; Chen, X.; Chen, L.-Z.; Zhang, S.-H.; Cai, X.-B. Fecal microbiota transplantation treatment for type 1 diabetes mellitus with malnutrition: A case report. Ther. Adv. Chronic Dis. 2022, 13, 20406223221117449. [Google Scholar] [CrossRef]
- He, L.; Chen, R.; Zhang, B.; Zhang, S.; Khan, B.A.; Zhu, D.; Wu, Z.; Xiao, C.; Chen, B.; Chen, F.; et al. Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes mellitus. Front. Immunol. 2022, 13, 930872. [Google Scholar] [CrossRef] [PubMed]
- Høyer, K.L.; Dahl Baunwall, S.M.; Kornum, D.S.; Klinge, M.W.; Drewes, A.M.; Yderstræde, K.B.; Thingholm, L.B.; Mortensen, M.S.; Mikkelsen, S.; Erikstrup, C.; et al. Faecal microbiota transplantation for patients with diabetes type 1 and severe gastrointestinal neuropathy (FADIGAS): A randomised, double-blinded, placebo-controlled trial. eClinicalMedicine 2025, 79, 103000. [Google Scholar] [CrossRef]
- de Groen, P.; Fuhri Snethlage, C.M.; Wortelboer, K.; Tokgöz, S.; Davids, M.; Verdoes, X.; Westerbeke, F.H.M.; Meijer, R.I.; Gotthardt, M.; de Vos, W.M.; et al. Autologous fecal microbiota capsules are safe and potentially preserve beta-cell function in individuals with type 1 diabetes. Gut Microbes 2025, 17, 2563155. [Google Scholar] [CrossRef]
- Halaweish, H.F.; Boatman, S.; Staley, C. Encapsulated Fecal Microbiota Transplantation: Development, Efficacy, and Clinical Application. Front. Cell Infect. Microbiol. 2022, 12, 826114. [Google Scholar] [CrossRef]
- Vaughn, B.P.; Fischer, M.; Kelly, C.R.; Allegretti, J.R.; Graiziger, C.; Thomas, J.; McClure, E.; Kabage, A.J.; Khoruts, A. Effectiveness and Safety of Colonic and Capsule Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection. Clin. Gastroenterol. Hepatol. 2023, 21, 1330–1337.e1332. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Soto, M.T.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, C.; Zellmer, C.; Panchal, P.; Budree, S.; Osman, M.; Alm, E.J. Framework for rational donor selection in fecal microbiota transplant clinical trials. PLoS ONE 2019, 14, e0222881. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Chen, Y.; Yao, Q.; Zhou, J.; Wang, X.; Meng, Q.; Ji, J.; Yu, Z.; Chen, X. Fecal microbiota transplantation: Application scenarios, efficacy prediction, and factors impacting donor-recipient interplay. Front. Microbiol. 2025, 16, 1556827. [Google Scholar] [CrossRef] [PubMed]
- Petrof, E.O.; Gloor, G.B.; Vanner, S.J.; Weese, S.J.; Carter, D.; Daigneault, M.C.; Brown, E.M.; Schroeter, K.; Allen-Vercoe, E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013, 1, 3. [Google Scholar] [CrossRef]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Tougaard, N.H.; Frimodt-Møller, M.; Salmenkari, H.; Stougaard, E.B.; Zawadzki, A.D.; Mattila, I.M.; Hansen, T.W.; Legido-Quigley, C.; Hörkkö, S.; Forsblom, C.; et al. Effects of Butyrate Supplementation on Inflammation and Kidney Parameters in Type 1 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Med. 2022, 11, 3573. [Google Scholar] [CrossRef]
- de Wit, D.F.; Fuhri Snethlage, C.M.; Rampanelli, E.; Maasen, K.; Walpot, N.; van Raalte, D.H.; Nieuwdorp, M.; Soeters, M.R.; Hanssen, N.M.J. Higher fibre and lower carbohydrate intake are associated with favourable CGM metrics in a cross-sectional cohort of 470 individuals with type 1 diabetes. Diabetologia 2024, 67, 2199–2209. [Google Scholar] [CrossRef]
- Nansel, T.R.; Lipsky, L.M.; Liu, A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am. J. Clin. Nutr. 2016, 104, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Giacco, R.; Parillo, M.; Rivellese, A.A.; Lasorella, G.; Giacco, A.; D’Episcopo, L.; Riccardi, G. Long-term dietary treatment with increased amounts of fiber-rich low-glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care 2000, 23, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Nicolucci, A.C.; Virtanen, H.; Schick, A.; Meddings, J.; Reimer, R.A.; Huang, C. Effect of Prebiotic on Microbiota, Intestinal Permeability, and Glycemic Control in Children with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4427–4440. [Google Scholar] [CrossRef]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562, Erratum in Nat. Immunol. 2017, 18, 1271. https://doi.org/10.1038/ni1117-1271c. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Saad, S.; Tillett, B.J.; McGuire, H.M.; Bordbar, S.; Yap, Y.A.; Nguyen, L.T.; Wilkins, M.R.; Corley, S.; Brodie, S.; et al. Metabolite-based dietary supplementation in human type 1 diabetes is associated with microbiota and immune modulation. Microbiome 2022, 10, 9. [Google Scholar] [CrossRef]
- Ismail, H.M.; Liu, J.; Netherland, M., Jr.; Evans-Molina, C.; DiMeglio, L.A. Safety and effects of acetylated and butyrylated high amylose maize starch in recently diagnosed youths with type 1 diabetes; a Pilot Study. medRxiv 2024. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef]
- Hakola, L.; Miettinen, M.E.; Syrjälä, E.; Åkerlund, M.; Takkinen, H.M.; Korhonen, T.E.; Ahonen, S.; Ilonen, J.; Toppari, J.; Veijola, R.; et al. Association of Cereal, Gluten, and Dietary Fiber Intake with Islet Autoimmunity and Type 1 Diabetes. JAMA Pediatr. 2019, 173, 953–960. [Google Scholar] [CrossRef]
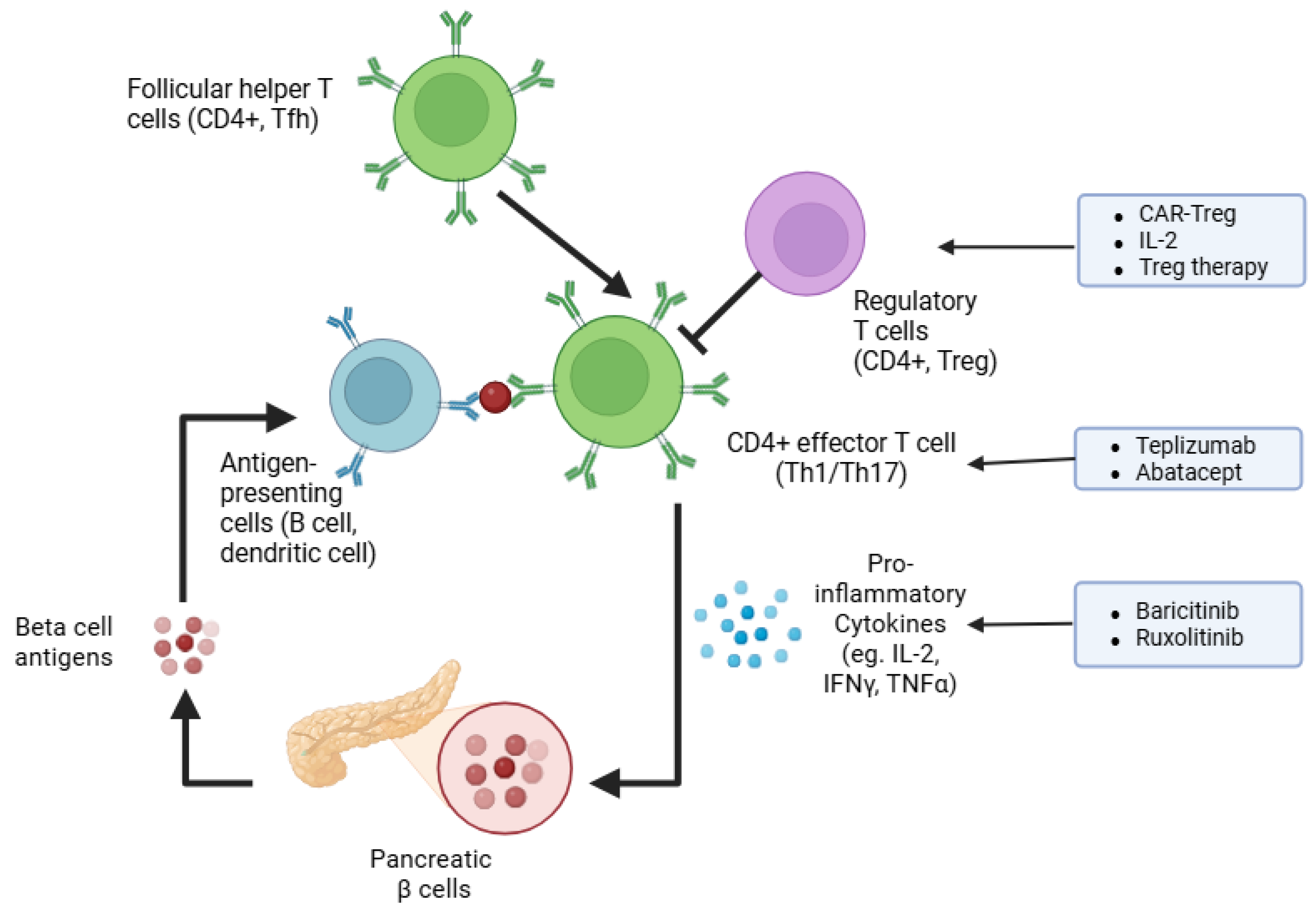
| Intervention | Population Description | Main Outcomes | References |
|---|---|---|---|
| Teplizumab (anti-CD3 mAb) | At-risk individuals with familial/genetic risk; newly diagnosed T1D patients (within weeks to months of onset) | Delayed T1D onset by ~2 years; preserved beta cell function; no HbA1c improvement | [14,31,32,36] |
| Abatacept (CD80/CD86 blockade) | New-onset T1D patients, typically <3 months post-diagnosis | Slowed beta cell decline over 2 years; reduced memory and follicular helper T cells; no delay in progression to glucose intolerance in at-risk individuals | [33,34,35,37] |
| Regulatory T cell (Treg) therapies | New-onset T1D children and NOD mouse models | Defective function in Treg from T1D patients; adoptive transfer reversed diabetes in mice; low-dose IL-2 expanded Tregs with mixed effects | [38,39,40,41,42,43,44,45,46,47,48] |
| CAR-Treg therapy | Preclinical murine models (NOD mice) | Antigen-specific CAR-Tregs prevented diabetes onset; induced broad immunosuppression against islet-specific T cells | [49,50,51] |
| JAK-STAT pathway inhibitors | New-onset T1D patients (first 12 weeks); rare monogenic autoimmune T1D cases | Baricitinib preserved beta cell function and reduced inflammation; ruxolitinib reversed autoimmune diabetes in a mutation case | [52,53,54,55] |
| Beta cell neoantigens | Human pancreatic tissues; T1D patient samples | Post-translational modifications create neoantigens that trigger autoreactive T cells | [56,57,58,59,60,61] |
| Environmental triggers | Birth cohorts; genetically at-risk children; general population | Viral infections, diet (gluten, vitamin D), and hygiene hypothesis influence T1D risk | [17,36,37,62,63,64,65,66] |
| Intervention | Population | Main Changes | Reference |
|---|---|---|---|
| Allogeneic FMT | Twenty-year-old T1D patient with malnutrition and GI symptoms (n = 1) | Shift in gut microbiome toward donor; improved glycemia (possibly due to symptom relief) | [135] |
| Allogeneic and Autologous FMT | Adults, newly diagnosed T1D (within 6 weeks) (n = 21) | Autologous group maintained stable C-peptide, with changes in microbiome, metabolites, and immune cells; allogeneic group did not | [81] |
| Autologous Encapsulated FMT | Adolescents with 1-year T1D (n = 2) | Improved glycemia and increased gut microbial diversity | [136] |
| Encapsulated Allogeneic FMT | T1D patients with gastroenteropathy | Reduction in GI symptoms; increased gut microbial diversity; improved quality of life | [137] |
| Autologous Encapsulated FMT (Ongoing) | Recently diagnosed T1D (0.5–3.5 years) | No significant decrease in beta cell function, suggesting the treatment may stabilize beta cell function | [138] |
| Intervention | Population | Main Changes | Reference |
|---|---|---|---|
| Oral sodium butyrate 4 g/day for 1 month | Individuals with long-standing T1D (n = 30) | Minor microbiota changes; no improvement in beta cell function or glycemic control; reduced IA2+ CD8+ T cells | [110] |
| Oral sodium butyrate 3.6 g/day for 12 weeks | Individuals with long-standing T1D (n = 53) | No changes in inflammation or glycemic markers | [149] |
| HAMSAB, 6-week + 6-week washout | Adults with long-standing T1D | Increased fecal and plasma SCFA; microbial shifts; immune modulation; no glycemic improvement | [147] |
| HAMSAB, 4-week pilot crossover study | Adolescents with recent-onset T1D | Immune changes (reduced MAIT activation); no glycemic change | [148] |
| Diabetic diet rich in inulin, FOS, GOS (prebiotics) | T1D patients (n = 470) | Associated with better glycemic control | [150] |
| Increased dietary fiber intake (inulin, FOS, GOS) | T1D patients (n = 136) | Improved glycemic control by CGM and other metrics | [151] |
| High fiber natural foods diet, 24 weeks | T1D patients (n = 63) | Reduced blood glucose and hypoglycemic episodes | [152] |
| Oligofructose-enriched inulin 12 weeks | Children with T1D (n = 38) | Preserved beta cell function; microbial changes; improved gut permeability; no HbA1c changes | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Groen, P.; Blok, L.C.H.M.; Fuhri Snethlage, C.M.; Hanssen, N.M.J.; Rampanelli, E.; Nieuwdorp, M. Unraveling Type 1 Diabetes: Integrating Microbiome, Metabolomics, and Immunomodulation for Next-Generation Therapies. Int. J. Mol. Sci. 2025, 26, 10788. https://doi.org/10.3390/ijms262110788
de Groen P, Blok LCHM, Fuhri Snethlage CM, Hanssen NMJ, Rampanelli E, Nieuwdorp M. Unraveling Type 1 Diabetes: Integrating Microbiome, Metabolomics, and Immunomodulation for Next-Generation Therapies. International Journal of Molecular Sciences. 2025; 26(21):10788. https://doi.org/10.3390/ijms262110788
Chicago/Turabian Stylede Groen, Pleun, Lente C. H. M. Blok, Coco M. Fuhri Snethlage, Nordin M. J. Hanssen, Elena Rampanelli, and Max Nieuwdorp. 2025. "Unraveling Type 1 Diabetes: Integrating Microbiome, Metabolomics, and Immunomodulation for Next-Generation Therapies" International Journal of Molecular Sciences 26, no. 21: 10788. https://doi.org/10.3390/ijms262110788
APA Stylede Groen, P., Blok, L. C. H. M., Fuhri Snethlage, C. M., Hanssen, N. M. J., Rampanelli, E., & Nieuwdorp, M. (2025). Unraveling Type 1 Diabetes: Integrating Microbiome, Metabolomics, and Immunomodulation for Next-Generation Therapies. International Journal of Molecular Sciences, 26(21), 10788. https://doi.org/10.3390/ijms262110788







