Hydrogen Sulfide in Balneology: Physiology, Evidence, and Clinical Translation
Abstract
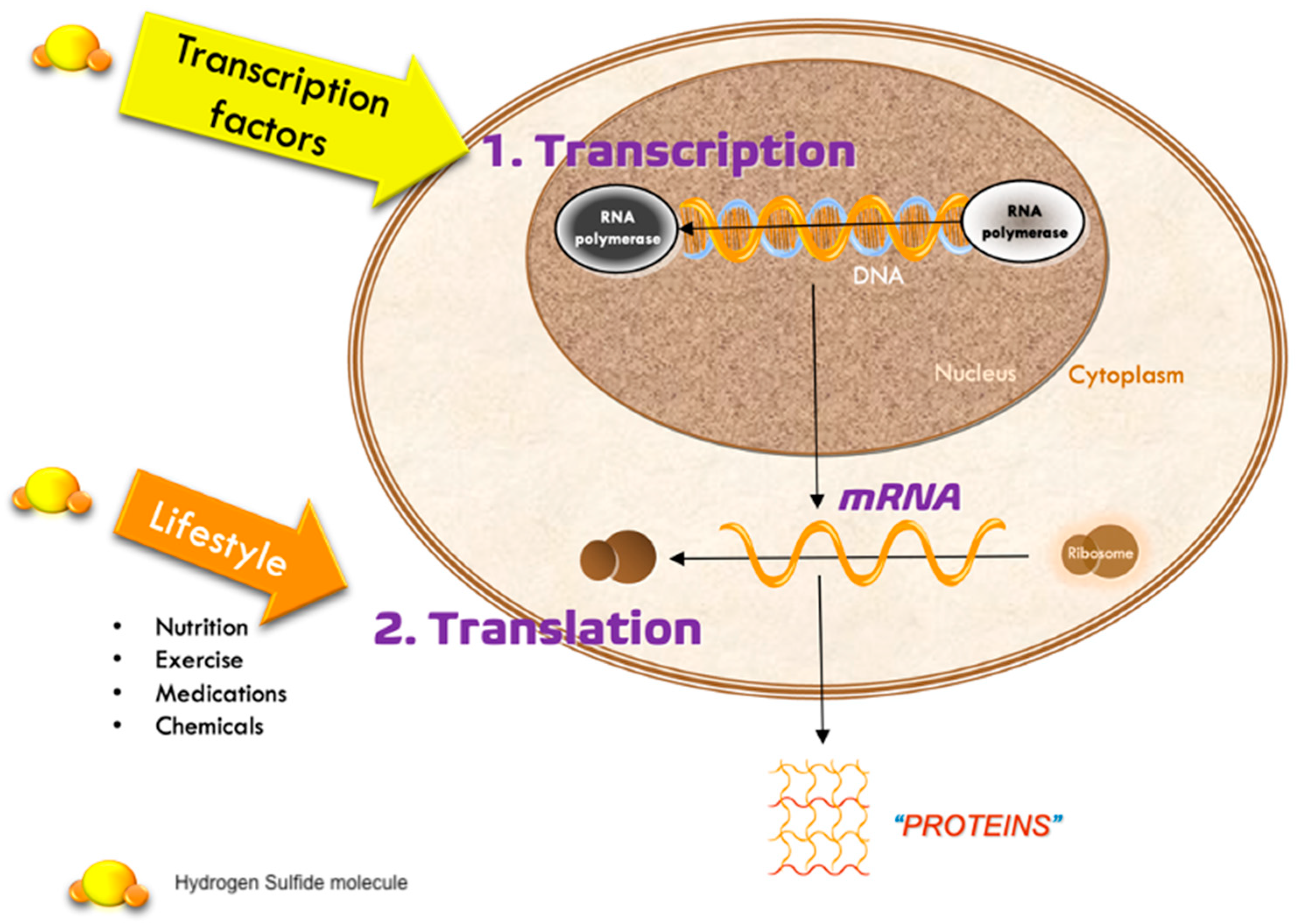
1. Introduction
2. Nature of Hydrogen Sulfide (H2S)
2.1. Physicochemical Properties
- pKa1 ≈ 6.9 → H2S ⇌ H+ + HS−.
- pKa2 ≈ 12 → HS− ⇌ H+ + S2−.
- Water pH: A pH between 5.5 and 6.5 favors the presence of molecular H2S, facilitating its absorption by passive diffusion. As pH becomes more alkaline, bioavailability decreases due to conversion into HS−, which is less bioavailable via transcutaneous or respiratory routes [10].
- Temperature: Increased temperature decreases the solubility of H2S in water, promoting its transition to the gaseous phase. This enhances its inhalation bioavailability but also accelerates volatilization, reducing its effective concentration in baths [3].
- Dissolved oxygen: H2S is rapidly oxidized to thiosulfate, sulfite, or sulfate in the presence of oxygen, reducing its biological activity, especially at elevated temperatures. Thus, hypoxic environments favor its preservation in active form, as demonstrated by water analyses and direct capture techniques in thermal environments. Therefore, the lower the dissolved oxygen content—avoiding bubbles and microbubbles—the more stable the H2S remains in its reduced and therapeutic form. In spas, water retention in pools, recirculation, or atmospheric exposure also significantly influences H2S loss. Hence, thermal circuit design should minimize aeration and turbulence to achieve the highest concentration of gaseous hydrogen sulfide [10].
- The pH of sulfurous mineral water.
- Controlled temperatures.
- Minimization of aeration and excessive recirculation.
- Use of techniques that limit volatilization losses.
2.2. Endogenous and Exogenous Sources
2.3. Transport, Catabolic Metabolism, and Excretion of Hydrogen Sulfide
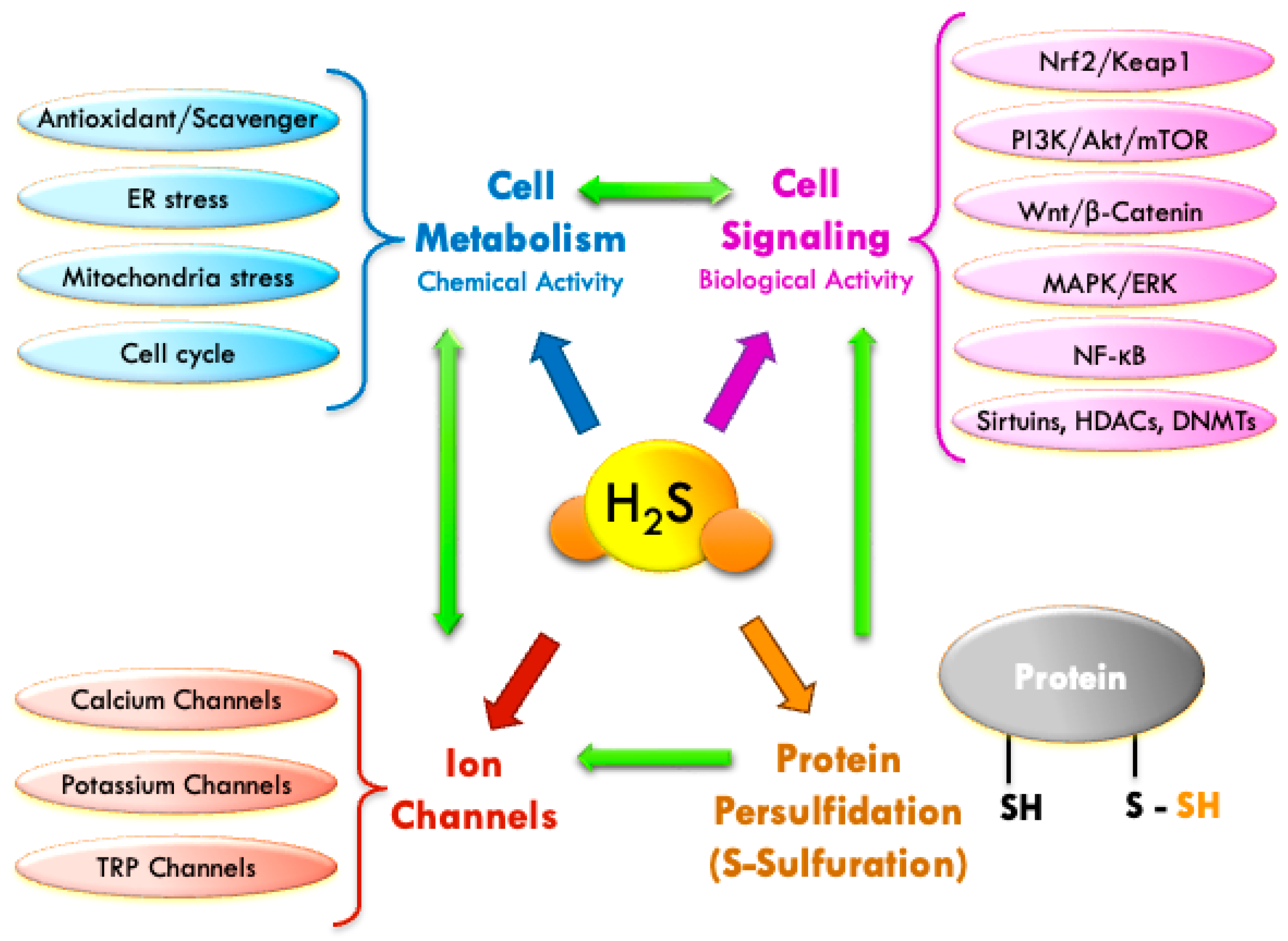
3. Physiological Mechanisms of H2S: Chemical and Biological Activity
3.1. Chemical Mechanism: Antioxidant Activity [Scavengers]
3.2. Biological Mechanisms: Cellular Signaling
3.2.1. Nrf2/Keap1 Pathway
3.2.2. PI3K/Akt/mTOR Pathway
- Under oxidative stress, H2S promotes cell survival by activating PI3K/Akt and reducing ROS via the regulation of antioxidant enzymes such as SOD, catalase, or GPx [37].
- In the cardiovascular system, H2S stimulates the PI3K/Akt pathway to protect against ischemia–reperfusion injury, decreasing apoptosis and mitochondrial damage [43].
- In mesenchymal stem cells, H2S promotes proliferation and osteogenic differentiation by activating mTOR, thereby enhancing regenerative processes [44].
- In certain tumor models, however, mTOR inhibition by H2S may produce antiproliferative and pro-autophagic effects, suggesting a biphasic action dependent on dose and cell type [45].
3.2.3. Wnt/β-Catenin Pathway
3.2.4. MAPK/ERK Pathway
3.2.5. NF-κB
- Suppression of IκBα phosphorylation, preventing nuclear translocation of the NF-κB complex (mainly p65/p50) [54].
- S-sulfuration (post-translational modification) of critical cysteine residues in key NF-κB pathway proteins, reducing their activity [54].
- Inhibition of histone deacetylases (HDACs), promoting a histone acetylation pattern that represses NF-κB-mediated pro-inflammatory genes [57].
- Suppression of pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and cyclooxygenase-2 (COX-2), while enhancing IL-10 production, exerting systemic anti-inflammatory effects [16].
- Induction of microRNAs that negatively regulate components of the NF-κB pathway [58]. At the post-transcriptional level, H2S signaling is intricately modulated by non-coding RNAs, particularly microRNAs. Among these, miR-21 has emerged as a key node in inflammatory contexts. Treatment with the slow-releasing donor GYY4137 upregulates miR-21 expression, which in turn activates the Akt pathway and contributes to endothelial protection, reduced apoptosis, and vascular regeneration [59]—mechanisms highly relevant to inflammatory and ischemic conditions that may benefit from balneotherapeutic intervention.
3.2.6. Epigenetic Modulation (Sirtuins, HDACs, DNMTs)
4. Preclinical and Clinical Evidence
4.1. Dermatological Applications in Balneotherapy
4.1.1. Mechanisms of Action on the Skin
- Effects on Barrier Function and the Cutaneous MicrobiomeH2S acts on keratinocytes and fibroblasts, regulating their differentiation, reducing pro-inflammatory cytokines, and activating the SIRT1 pathway with anti-aging effects.Clinical improvement has been observed in psoriasis, atopic dermatitis, acne, and rosacea after baths with sulfurous waters, attributed to the synergistic antioxidant, keratolytic/keratoplastic, and bacteriostatic effect [76,77,78].In addition, H2S and derived molecules have been shown to stimulate the production of type I and III collagen, with implications in dermal regeneration, supporting its use in thermal cosmetics and regenerative aesthetic medicine [79].H2S improves epidermal barrier function by stimulating keratinocyte proliferation and differentiation, with increased production of structural proteins such as filaggrin, loricrin, and claudins, which are essential for stratum corneum integrity [80].It also enhances the synthesis of epidermal lipids (ceramides, cholesterol, free fatty acids), fundamental for cutaneous impermeability [81].Moreover, H2S exhibits selective antimicrobial activity, inhibiting pathogens such as Staphylococcus aureus and Malassezia spp., without significantly altering the resident microbiota, which is especially useful in atopic dermatitis, seborrheic dermatitis, and inflammatory acne [82].
- Antioxidant, Anti-inflammatory, and Anti-aging ActivityAs previously noted, H2S has potent antioxidant capacity through Nrf2 activation, which increases the expression of HO-1, GPx, and SOD, key players in protection against UV radiation and pollution [83].Furthermore, persulfidation of regulatory proteins such as Keap1 modulates adaptive stress responses. It also inhibits NF-κB activation, reducing expression of IL-1β, TNF-α, and COX-2, which are central to inflammatory skin diseases [5].Its action on the SIRT1–FoxO3a pathway promotes DNA repair, mitochondrial energy regulation, and cellular longevity, consolidating its role as a natural anti-aging agent and decreasing “silent inflammation” [84].As a summary, Table 5 lists the clinical cutaneous benefits associated with dermatological treatment based on its mechanism.
4.1.2. Dermatological Diseases Treatable with Sulfurous Waters
- Psoriasis: Elevated H2S concentrations modulate immune responses by reducing Th17/Th1 cytokines (such as IL-8 induced by IL-17/IL-22) and decreasing matrix metalloproteinases (MMP-2, MMP-13). Potential reduction of MMP-9 has also been observed, usually elevated in psoriatic patients, resulting in clinical improvement of erythema, pruritus, and scaling [86,87,88,89].
- Rosacea: High-H2S waters inhibit NF-κB activation and reduce pro-inflammatory mediators (IL-6, IL-8, TNF-α), attenuating inflammation induced by LL-37 peptide, decreasing angiogenesis, erythema, and Demodex folliculorum proliferation [90].
- Seborrheic dermatitis: High-concentration sulfurous baths decrease erythema and fungal components (Malassezia spp.) without harming resident microbiota [91].
- Wound healing: H2S supplementation accelerates healing through VEGF stimulation and oxidative stress reduction [95].
4.2. Rheumatological/Locomotor System Applications in Balneotherapy
4.2.1. Mechanisms of Action in Osteoarticular Tissues
4.2.2. Musculoskeletal Diseases Treatable with Sulfurous Waters
- Osteoarthritis (OA): Particularly in the knee, hip, and spine. H2S contributes to functional improvement, pain reduction, and slowing of structural cartilage deterioration [12]. These effects are attributed to inhibition of pro-inflammatory cytokines (TNF-α, IL-1β), suppression of metalloproteinases (MMP-13), and activation of Nrf2-dependent antioxidant pathways [101,111]. Peloids application is especially indicated in localized conditions [112,113].
- Rheumatoid Arthritis (RA): Especially in non-acute phases, H2S inhibits fibroblast-like synoviocyte proliferation, reduces IL-6 and MMP-3 production, and blocks NF-κB activation, thereby reducing joint destruction [102]. It also inhibits inflammatory mediators, particularly from T lymphocytes and macrophages [114].
- Spondyloarthropathies: Such as ankylosing spondylitis, where H2S contributes to axial pain relief and improved mobility due to anti-inflammatory and muscle-relaxant effects. Early validation came from Sukenik’s group [115], with recent studies confirming that simple balneotherapy improves outcomes [116]. Thus, sulfurous balneotherapy and muds are considered safe and effective complementary therapies, with sustained clinical effects lasting up to 12 weeks.
- Chronic Low Back and Neck Pain: Whether discogenic or due to muscle contracture, sulfurous waters or hot peloids provide clear benefits by inducing vasodilation, muscle relaxation, and improved local tissue metabolism [12]. Peloid application has shown deep local effects combining heat, sustained H2S release, and chemical action on musculoskeletal tissues [117,118,119].
- Fibromyalgia: Although evidence remains limited, some studies suggest improvement in widespread pain, sleep quality, and overall well-being, likely via modulation of oxidative stress, neuroinflammatory mediators, and autonomic tone [120].
- Chronic Tendinopathies: Such as epicondylitis, plantar fasciitis, rotator cuff tendinitis, and enthesopathies. These respond favorably to balneotherapy and local applications of sulfurous peloids, which improve microcirculation and reduce local inflammation [121].
- Sarcopenia: H2S protects against skeletal muscle aging through activation of autophagy. A recent study identified that this effect is mediated by H2S-induced deubiquitination of AMPKα1 by USP5 (ubiquitin-specific peptidase 5), modulated by S-sulfhydration. This process activates the AMPKα1–ULK1 pathway, essential for autophagy regulation [122].
- Bone healing after fractures: In vitro and animal studies suggest H2S favors bone consolidation by stimulating osteoblast proliferation and differentiation, promoting mineralization, and modulating the inflammatory microenvironment of the injured site [79].
4.3. Respiratory/ENT Applications in Balneotherapy
4.3.1. Mechanisms of Action in Respiratory Tract
- On respiratory epithelium and ciliary function. Promotes clearance, increasing production of mucin MUC5AC and enhancing electrolyte secretion, which contributes to thinner, more fluid mucus [126]. Activates key ion channels such as CFTR (cystic fibrosis transmembrane regulator) and Cl− and K+ channels, thus restoring mucociliary transport. This mechanism is particularly relevant in diseases characterized by dense mucus, such as cystic fibrosis and chronic bronchitis [127].Protects the epithelium against apoptosis induced by oxidative stress and endoplasmic reticulum (ER) stress, mainly through activation of the Nrf2 pathway. This activation enhances synthesis of endogenous antioxidants such as superoxide dismutase (SOD) and catalase, which reduces cell death under conditions of environmental aggression, including exposure to tobacco smoke and atmospheric pollutants [128]. Exerts a potent bronchodilator effect through several complementary mechanisms. Activates ATP-sensitive K+ (K_ATP) channels and large-conductance calcium-activated K+ (BK_Ca) channels, which reduces excitability and promotes bronchial relaxation [129].Inhibits Ca2+ influx or release mediated by InsP3 receptors, decreasing the frequency and amplitude of Ca2+ spikes and attenuating contractility induced by cholinergic agonists such as acetylcholine. It also reduces bronchial constriction caused by histamine and methacholine, showing a clinically relevant effect in bronchial hyperreactivity [130].
- Regulation of bronchial tone and antiasthma activity. Activates K_ATP and BK_Ca channels, producing membrane hyperpolarization and bronchial relaxation [131]. As noted above, it inhibits intracellular Ca2+ entry in smooth muscle cells, reducing contractility induced by cholinergic agonists. In asthma models, H2S has been shown to reduce the bronchoconstrictor response to histamine and methacholine [132].
- Local immunomodulation in rhinitis, COPD, and pulmonary fibrosis. Inhibits activation of M1 alveolar macrophages and favours the anti-inflammatory M2 phenotype, reducing chronic inflammation [133]. Decreases expression of IL-6, IL-8, and TNF-α in pulmonary epithelial cells and in bronchoalveolar lavage fluid [134]. In pulmonary fibrosis, H2S decreases fibroblast activation and reduces type I collagen synthesis, preventing fibrotic progression [135,136].
4.3.2. ENT and Pulmonary Diseases Treatable with Sulphurous Waters
- Allergic and non-allergic rhinitis. Inhalations with sulphurous waters significantly reduce nasal congestion, sneezing frequency, and Th2 cytokines (especially IL-5), as well as local IgE concentration. A systematic review reported improvements in mucociliary transport and a decrease in nasal epithelial infiltration and inflammation. Although some studies focus on SO2 inhalation in animal models, clinical human evidence supports symptom reduction through immune modulation [125,138,139].
- Chronic bronchitis and mild-to-moderate COPD. In COPD patients, inhalation with sulphurous waters improves respiratory and clinical parameters increases FEV1, reduces sputum volume, improves exercise tolerance, and decreases oxidative stress. A controlled trial showed a significant reduction of oxidative burst and persistent improvement after 12 days of treatment [140]. A systematic review also reported an improvement in quality of life and a reduction of airway oxidation [141].
- Chronic pharyngitis and laryngitis. Although there is less direct clinical evidence, sulfurous waters are considered to act as antiseptic, mucoregulatory, and epithelial-regenerating agents in pharyngeal and laryngeal mucosa. General studies in pulmonary hydrotherapy mention beneficial effects on inflammation and epithelial function, without distinguishing precisely these locations, but providing a plausible basis [141].
- Mild persistent or intermittent asthma. In mild asthma, inhalation techniques are used as adjuvant therapy to reduce bronchial hyperreactivity. In both human and animal models, improvements in lung function and inflammatory parameters have been observed after inhalation of mineral waters. Recent reviews report reduced inflammation and improved FEV1 and reactivity in mild asthma [141].
4.4. Activity on the Cardiovascular System
4.4.1. Mechanisms of Action on the Cardiovascular System
4.4.2. Cardiovascular Diseases Treatable with Sulfurous Waters
- Regulation of vascular tone and vasodilation. H2S induces potent vasodilation by activating ATP-sensitive potassium channels (K_ATP) in vascular smooth muscle, causing potassium efflux and membrane hyperpolarization, which relaxes the vessel. This effect contributes to lowering blood pressure and maintaining vascular tone [38].In addition, H2S interacts synergistically with NO by stimulating the expression of endothelial nitric oxide synthase (eNOS) and increasing NO bioavailability, possibly through the formation of hybrid compounds such as nitrosopersulfide [144].
- Cardioprotection in ischemia–reperfusion. In ischemia–reperfusion models, H2S exerts mitochondrial and antioxidant protection, reducing the production of reactive oxygen species (ROS) and activating the Nrf2 transcription pathway, with the consequent increase in antioxidant enzymes such as HO-1 and NQO1. At the mitochondrial level, persulfidation of cyclophilin D prevents opening of the mitochondrial permeability transition pore, a key step to avoid cell necrosis [146].
- Effects on the myocardium. H2S directly modulates cardiac function. It improves myocardial contractility, reduces post-infarction fibrosis, and contributes to repair of damaged tissue through induction of angiogenesis, mediated by increased expression of VEGF (vascular endothelial growth factor) [150].
- Anti-inflammatory and anti-atherosclerotic action. H2S inhibits activation of the NF-κB factor, leading to reduced expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, as well as vascular adhesion molecules such as VCAM-1 and ICAM-1. This action decreases endothelial inflammation, leukocyte adhesion, and formation of atherosclerotic plaques. In addition, it reduces LDL oxidation and limits the proliferation of vascular smooth muscle cells, thus slowing atherosclerosis progression [57].
- Blood pressure control. Studies in animal models with deletion of the CSE enzyme have shown that the absence of endogenous H2S production is associated with sustained elevation of basal blood pressure, reduced endothelial vasodilation, and increased peripheral vascular resistance, confirming its physiological role as a modulator of hemodynamic balance [143].
- Epigenetic mechanisms and cardiovascular longevity. Hydrogen sulfide (H2S) exerts relevant epigenetic effects in the cardiovascular system through post-translational modification of key proteins. One of the best-documented mechanisms is sulfhydration of the p65 subunit of the NF-κB transcription factor, which prevents its activation and nuclear translocation. This epigenetic modification reduces the expression of pro-inflammatory genes such as TNF-α, IL-1β, and IL-6, and diminishes the vascular inflammatory response, contributing to greater endothelial longevity and functionality [54].This effect represents a significant epigenetic pathway by which H2S protects against chronic vascular damage, regulates the cellular redox state, and contributes to long-term maintenance of hemodynamic balance and endothelial integrity [151].
4.5. Activity on Gastrointestinal Mucosa and Related Organs
4.5.1. Mechanisms of Action of the Hydropinic Cure
- Liver: one of the main H2S-producing organs, synthesized by hepatocytes, Kupffer cells, and sinusoidal endothelial cells, enabling autocrine and paracrine functions [151].
- Kidney: promotes renal vasodilation through K_ATP channel opening, regulates glomerular flow, modulates the renin–angiotensin–aldosterone axis, and participates in acid–base homeostasis via tubular ion transport [159].
4.5.2. Diseases of the Gastric Mucosa Treatable with Sulfurous Waters
- Peptic ulcer (gastric and duodenal). H2S increases mucosal blood flow, inhibits leukocyte infiltration, and decreases oxidative stress. It favors healing of ulcers induced by gastrotoxic drugs such as NSAIDs. Inhibition of endogenous synthesis reduces prostaglandins (PGE2) and COX-2, worsening damage, while H2S restitution reverses these effects [161]. H2S-NSAID derivatives show lower gastrolesivity while maintaining anti-inflammatory and analgesic efficacy.
- Chronic gastritis (inflammatory and erosive). The anti-inflammatory capacity of H2S decreases pro-inflammatory cytokines (IL-1β, TNF-α). It regulates angiogenesis and mucus/bicarbonate secretion, protecting mucosa from injurious agents.
- Gastric lesions due to stress or alcohol. H2S reduces oxidative damage and improves gastric microcirculation in experimental models of ethanol- or stress-induced injury. It favors tissue repair through stimulation of angiogenic factors and increased blood flow.
- Functional gastric disorders with acid hypersecretion. By stimulating bicarbonate and prostaglandins, H2S helps buffer gastric acidity, protecting mucosa in hyperchlorhydria.
4.5.3. Intestinal Diseases Treatable with Sulfurous Waters
4.5.4. Liver Diseases Treatable with Sulfurous Waters
- Hepatic steatosis (non-alcoholic fatty liver disease, NAFLD). H2S reduces the accumulation of triglycerides and cholesterol. It inhibits lipogenic enzymes (e.g., FAS) and activates β-oxidation (CPT1). It decreases oxidative stress by enhancing SOD and GPx, reducing lipid peroxidation [166]. It also protects against oxidative stress and apoptosis in alcohol-induced liver injury. It favors cellular repair and reduces inflammation in chronic alcoholism.
- Hepatic fibrosis. It modulates oxidative stress and chronic inflammation. It regulates autophagy and glucolipid metabolism. It favors hepatic perfusion through sinusoidal vasodilation [165].
- General hepatic oxidative stress. Activation of the Nrf2/ARE pathway, which stimulates antioxidant genes (HO-1, NQO1, GCLC), provides defense against xenobiotics and hepatotoxic agents [165].
4.6. Kidney Diseases Treatable with Sulfurous Waters
4.7. Other Indications
- In rehabilitation, recent studies identify H2S as a pain modulator via activation/inhibition of TRPA1/TRPV1 and K_ATP channels, exhibiting pro- or antinociceptive effects depending on dose, chemical species, and the inflammatory context [178]. It also enhances micro perfusion through endothelium-dependent vasodilation and augmentation of the NO/cGMP pathway, which is relevant for tissue recovery and therapeutic exercise [15]. Moreover, H2S regulates cellular metabolism, autophagy, and homeostasis, with antifibrotic potential and supportive effects on muscle function and aging [122]. In selected clinical settings, benefits on pain and function have been observed in osteoarthritis, and when combined with exercise these improvements may be prolonged [123,179,180,181].
- In psychological and neurological disorders, H2S acts as a gaseous neurotransmitter synthesized by CBS, CSE, and 3-MST within the central nervous system, modulating NMDA receptors, K_ATP channels, microglial activity, and the GABA/glutamate balance [63,70,182,183]. Slow-releasing donors such as GYY4137 attenuate neuroinflammation, preserve blood–brain barrier integrity, and improve cognitive performance in animal models [184]. Along the gut–brain axis, endogenous and microbiota-derived H2S from sulfate-reducing bacteria influences intestinal permeability, immune signaling, and vagal tone, thereby linking dysbiosis to neuropsychiatric phenotypes [156].
5. Routes of Administration and Bioaccessibility of H2S
5.1. Topical Route: Waters and Peloids
5.2. Respiratory Route
5.2.1. Local Techniques
5.2.2. Inhalation Techniques
- Dry inhalations: breathing the gas or vapor released directly from the mineral-medicinal water, without entrained liquid droplets, generating fine particles of approximately 10–20 μm that can deposit in upper and middle airways; useful in rhinitis or early chronic bronchitis.
- Wet inhalations: a mixture of vapor and larger aqueous particles, 20–50 μm, with soothing and mucoregulatory action, primarily on upper airways.
- Nebulization: a very fine and abundant form of wet inhalation producing much smaller particles, 1–5 μm, which can reach bronchioles and alveoli. Indicated in mild asthma, early COPD, or chronic bronchitis.
- Atmiatric techniques: such as steam baths in a cabin, providing diffuse inhalation of particles of heterogeneous size, usually >50 μm, acting mainly on upper and middle airways and adding a beneficial thermal effect.
5.3. Hydropinic Cure
6. Safety Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-MST | 3-mercaptopyruvate sulfurtransferase |
| ACC | Acetyl-CoA carboxylase |
| ACGIH | American Conference of Governmental Industrial Hygienists |
| AE1 | Anion Exchanger 1 |
| Akt | Protein kinase B |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| ARE | Antioxidant Response Element |
| AST | Aspartate aminotransferase |
| BKCa (KCa1.1) | Large-conductance calcium-activated potassium channel |
| BMI | Body mass index |
| C. acnes | Cutibacterium acnes |
| CAT | Catalase |
| CAT (aminotransferase) | Cysteine aminotransferase |
| CBS | Cystathionine β-synthase |
| CFTR | Cystic fibrosis transmembrane regulator |
| CO | Carbon monoxide |
| COPD | Chronic obstructive pulmonary disease |
| COX-2 | Cyclooxygenase-2 |
| CPT1 | Carnitine palmitoyltransferase 1 |
| CPT2 | Carnitine palmitoyltransferase 2 |
| CRP | C-reactive protein |
| CSE | C-reactive protein |
| DLQI | Dermatology Life Quality Index |
| DNMTs | DNA methyltransferases |
| EASI | Eczema Area and Severity Index |
| eNOS | Endothelial nitric oxide synthase |
| ERK | Extracellular signal-regulated kinase |
| EU | European Union |
| FAO/WHO | Food and Agriculture Organization/World Health Organization |
| FAS | Fatty acid synthase |
| FeNO | Fractional exhaled nitric oxide |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| GABA | Gamma-aminobutyric acid |
| GCLC | Glutamate–cysteine ligase catalytic subunit |
| GCLM | Glutamate–cysteine ligase modifier subunit |
| GGT | Gamma-glutamyl transferase |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| GYY4137 | Slow-release hydrogen sulfide donor |
| H2S | Hydrogen sulfide |
| HbA1C | Glycated hemoglobin A1c |
| HDACs | Histone deacetylases |
| HDL | High-density lipoprotein cholesterol |
| HO-1 | Heme oxygenase-1 |
| HOMAR-IR | Homeostatic Model Assessment of Insulin Resistance |
| HSP(s) | Heat shock proteins |
| HS− | Hydrosulfide ion |
| IDLH | Immediately Dangerous to Life or Health |
| IgE | Immunoglobulin E |
| IL-1 β | Interleukin-1 beta |
| IL-4 | Interleukin-4 |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| IL-13 | Interleukin-13 |
| IL-17 | Interleukin-17 |
| IL-31 | Interleukin-31 |
| JAK/STAT | Janus kinase/Signal transducer and activator of transcription |
| KATP | ATP-sensitive potassium channel |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LDL | Low-density lipoprotein cholesterol |
| LL-37 | Cathelicidin antimicrobial peptide LL-37 |
| MAPK | Mitogen-activated protein kinase |
| MASLD | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| mg L−1 | Milligrams per liter |
| mg m−3 | Milligrams per cubic meter |
| miR-21 | MicroRNA-21 |
| MMP(s) | Matrix metalloproteinase(s) |
| MUC5AC | Mucin 5AC |
| mTOR | Mechanistic target of rapamycin |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced) |
| NF- κB | Nuclear factor kappa-B |
| NIOSH | National Institute for Occupational Safety and Health |
| NMDA | N-methyl-D-aspartate receptor |
| NO | Nitric oxide |
| NO/cGMP | Nitric oxide and cyclic guanosine monophosphate pathway |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NSAID(s) | Non-steroidal anti-inflammatory drugs |
| O2 | Oxygen |
| OSHA | Occupational Safety and Health Administration |
| ORAC | Oxygen Radical Absorbance Capacity |
| PEF | Peak expiratory flow |
| PEL | Permissible Exposure Limit |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K | Phosphoinositide 3-kinase |
| PPAR-α | Peroxisome proliferator-activated receptor-alpha |
| PPAR-γ | Peroxisome proliferator-activated receptor-gamma |
| ppm | Parts per million |
| RCTs | Randomized controlled trials |
| REL | Recommended Exposure Limit |
| ROS | Reactive oxygen species |
| SAA(s) | Sulfur-containing amino acids |
| SCFAs | Short-chain fatty acids |
| SCORAD | Scoring Atopic Dermatitis Index |
| SIRT1 | Sirtuin-1 |
| SIRT2 | Sirtuin-2 |
| SIRT3 | Sirtuin-3 |
| SLC26 | Solutes carrier |
| SOD | Superoxide dismutase |
| SQR | Sulfide quinone oxidoreductase |
| SRB | Sulfate-reducing bacteria |
| SREBP-1c | Sterol regulatory element-binding protein-1c |
| S2− | Sulfide ion |
| STEL | Short-Term Exposure Limit |
| TAC | Total antioxidant capacity |
| TGF-β | Transforming growth factor beta |
| TEWL | Transepidermal water loss |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| Th17 | T helper 17 |
| TLR4 | Toll-like receptor 4 |
| TLV | Threshold Limit Value |
| TRP | Transient receptor potential |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TRPV1 | Transient receptor potential vanilloid 1 |
| TSLP | Thymic stromal lymphopoietin |
| TG | Triglycerides |
| TWA | Time-Weighted Average |
| VEGF | Vascular endothelial growth factor |
| VLEP | Occupational Exposure Limit Value (EU) |
| Wnt/β-catenin | Wnt signaling/β-catenin pathway |
| °C | Degrees Celsius |
References
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Signaling Molecules: Hydrogen Sulfide and Polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C. Hydrogen Sulphide and Its Therapeutic Potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S: A Novel Gasotransmitter That Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox Regulation: Mechanisms, Biology and Therapeutic Targets in Diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Coavoy-Sánchez, S.A.; Cerqueira, A.R.A.; Teixeira, S.A.; Santagada, V.; Andreozzi, G.; Corvino, A.; Scognamiglio, A.; Sparaco, R.; Caliendo, G.; Severino, B.; et al. Beneficial Effects of Two Hydrogen Sulfide (H2S)-Releasing Derivatives of Dexamethasone with Antioxidant Activity on Atopic Dermatitis in Mice. Pharmaceutics 2023, 15, 1907. [Google Scholar] [CrossRef]
- Olson, K.R. A Practical Look at the Chemistry and Biology of Hydrogen Sulfide. Antioxid. Redox Signal. 2012, 17, 32–44. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Redox Biochemistry of Hydrogen Sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef]
- Hou, Y.; Lv, B.; Du, J.; Ye, M.; Jin, H.; Yi, Y.; Huang, Y. Sulfide Regulation and Catabolism in Health and Disease. Signal Transduct. Target. Ther. 2025, 10, 174. [Google Scholar] [CrossRef]
- Carbajo, J.M.; Maraver, F. Sulphurous Mineral Waters: New Applications for Health. Evid. Based Complement. Alternat. Med. 2017, 2017, 8034084. [Google Scholar] [CrossRef]
- Liang, X.Y.; Wang, Y.; Zhu, Y.W.; Zhang, Y.X.; Yuan, H.; Liu, Y.F.; Jin, Y.Q.; Gao, W.; Ren, Z.G.; Ji, X.Y.; et al. Role of Hydrogen Sulfide in Dermatological Diseases. Nitric Oxide 2024, 150, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Teległów, A.; Seremak, J.; Golec, J.; Marchewka, J.; Golec, P.; Marchewka, U.; Maciejczyk, M.; Golec, E. The Effect of Sulfur Baths on Hemorheological Properties of Blood in Patients with Osteoarthritis. Sci. Rep. 2023, 13, 7960. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Munteanu, C.; Popescu, C.; Vlădulescu-Trandafir, A.I.; Onose, G. Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review. Antioxidants 2024, 13, 1158. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen Sulfide-Based Therapeutics: Exploiting a Unique but Ubiquitous Gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Giuffrè, A.; Vicente, J.B. Hydrogen Sulfide Biochemistry and Interplay with Other Gaseous Mediators in Mammalian Physiology. Oxid. Med. Cell. Longev. 2018, 2018, 6290931. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Whiteman, M.; Cirino, G. Pharmacological Tools for Hydrogen Sulphide Research: A Brief, Introductory Guide for Beginners. Br. J. Pharmacol. 2015, 172, 1633–1637. [Google Scholar] [CrossRef]
- Cirino, G.; Szabó, C.; Papapetropoulos, A. Physiological Roles of Hydrogen Sulfide in Mammalian Cells, Tissues, and Organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Blachier, F.; Andriamihaja, M.; Larraufie, P.; Ahn, E.; Lan, A.; Kim, E. Production of Hydrogen Sulfide by the Intestinal Microbiota and Epithelial Cells and Consequences for the Colonic and Rectal Mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G125–G135. [Google Scholar] [CrossRef]
- Birg, A.; Hu, S.; Lin, H.C. Reevaluating our understanding of lactulose breath tests by incorporating hydrogen sulfide measurements. JGH Open 2019, 3, 228–233. [Google Scholar] [CrossRef]
- Suriano, F.; Nyström, E.E.L.; Sergi, D.; Gustafsson, J.K. Diet, Microbiota, and the Mucus Layer: The Guardians of Our Health. Front. Immunol. 2022, 13, 953196. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Louis, P. Gut Microbiota-Derived Short-Chain Fatty Acids and Their Role in Human Health and Disease. Nat. Rev. Microbiol. 2025, 23, 635–651. [Google Scholar] [CrossRef]
- Xu, C.; Marques, F.Z. How Dietary Fibre, Acting via the Gut Microbiome, Lowers Blood Pressure. Curr. Hypertens. Rep. 2022, 24, 509–521. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, Y.; Sawa, T.; Akaike, T.; Matsunaga, T. Supersulfide Donors and Their Therapeutic Targets in Inflammatory Diseases. Front. Immunol. 2025, 16, 1581385. [Google Scholar] [CrossRef]
- Shoveller, A.K.; Stoll, B.; Ball, R.O.; Burrin, D.G. Nutritional and Functional Importance of Intestinal Sulfur Amino Acid Metabolism. J. Nutr. 2005, 135, 1609–1612. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen Sulfide Chemical Biology: Pathophysiological Roles and Detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef]
- Yenchitsomanus, P.T. Human Anion Exchanger 1 Mutations and Distal Renal Tubular Acidosis. Southeast Asian J. Trop. Med. Public Health 2003, 34, 651–658. [Google Scholar]
- Chen, L.; Han, L.; Lian, G. Recent Advances in Predicting Skin Permeability of Hydrophilic Solutes. Adv. Drug Deliv. Rev. 2013, 65, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the Human Mitochondrial Hydrogen Sulfide Oxidation Pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Melideo, S.L.; Jorns, M.S. Human Sulfide:Quinone Oxidoreductase Catalyzes the First Step in Hydrogen Sulfide Metabolism and Produces a Sulfane Sulfur Metabolite. Biochemistry 2012, 51, 6804–6815. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Hydrogen Sulfide as an Oxygen Sensor. Antioxid. Redox Signal. 2015, 22, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A. A Critical Review of the Literature on Hydrogen Sulfide Toxicity. Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive Cysteine Persulfides and S-Polythiolation Regulate Oxidative Stress and Redox Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S Signals through Protein S-Sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen Sulfide Protects against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The Vasorelaxant Effect of H2S as a Novel Endogenous Gaseous KATP Channel Opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Peers, C.; Bauer, C.C.; Boyle, J.P.; Scragg, J.L.; Dallas, M.L. Modulation of Ion Channels by Hydrogen Sulfide. Antioxid. Redox Signal. 2012, 17, 95–105. [Google Scholar] [CrossRef]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides Are Possible H2S-Derived Signaling Molecules in Rat Brain. FASEB J. 2013, 27, 2451–2457. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor–Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-Sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef]
- Cai, W.J.; Wang, M.J.; Moore, P.K.; Jin, H.M.; Yao, T.; Zhu, Y.C. The Novel Proangiogenic Effect of Hydrogen Sulfide Is Dependent on Akt Phosphorylation. Cardiovasc. Res. 2007, 76, 29–40. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR–Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, Y.; Shi, S. Hydrogen Sulfide Regulates Homeostasis of Mesenchymal Stem Cells and Regulatory T Cells. J. Dent. Res. 2016, 95, 1445–1451. [Google Scholar] [CrossRef]
- Guo, L.; Peng, W.; Tao, J.; Lan, Z.; Hei, H.; Tian, L.; Pan, W.; Wang, L.; Zhang, X. Hydrogen Sulfide Inhibits Transforming Growth Factor-β1-Induced EMT via Wnt/β-Catenin Pathway. PLoS ONE 2016, 11, e0147018. [Google Scholar] [CrossRef]
- Duan, S.F.; Zhang, M.M.; Dong, Q.; Yang, B.; Liu, W.; Zhang, X.; Yu, H.L.; Zhang, S.H.; Khan, N.H.; Wu, D.D.; et al. A Water-Soluble Hydrogen Sulfide Donor Suppresses the Growth of Hepatocellular Carcinoma via Inhibiting the AKT/GSK-3β/β-Catenin and TGF-β/Smad2/3 Signaling Pathways. J. Oncol. 2023, 2023, 8456852. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, H.; Cai, Y.; Shen, M.; Li, X.; Han, Y.; Deng, X.; Cao, H.; Liu, J.; Li, H.; et al. Hydrogen Sulfide-Mediated Persulfidation Regulates Homocysteine Metabolism and Enhances Ferroptosis in Non-Small Cell Lung Cancer. Mol. Cell 2024, 84, 4016–4030.e6. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, L.; Yu, S.; Ren, Z.; Wang, W.; Jia, Y.; Liu, M.; Wang, P.; Ji, D.; Yu, Y.; et al. GYY4137, a H2S Donor, Ameliorates Kidney Injuries in Diabetic Mice by Modifying Renal ROS-Associated Enzymes. Biomed. Pharmacother. 2023, 162, 114694. [Google Scholar] [CrossRef]
- Meng, G.; Wang, J.; Xiao, Y.; Bai, W.; Xie, L.; Shan, L.; Moore, P.K.; Ji, Y. GYY4137 Protects against Myocardial Ischemia and Reperfusion Injury by Attenuating Oxidative Stress and Apoptosis in Rats. J. Biomed. Res. 2015, 29, 203–213. [Google Scholar] [CrossRef]
- Ban, T.; Hamada, D.; Hasegawa, K.; Naiki, H.; Goto, Y. Direct Observation of Amyloid Fibril Growth Monitored by Thioflavin T Fluorescence. J. Biol. Chem. 2003, 278, 16462–16465. [Google Scholar] [CrossRef]
- Yang, G.; Sun, X.; Wang, R. Hydrogen Sulfide-Induced Apoptosis of Human Aorta Smooth Muscle Cells via the Activation of Mitogen-Activated Protein Kinases and Caspase-3. FASEB J. 2004, 18, 1782–1784. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen Sulfide-Linked Sulfhydration of NF-κB Mediates Its Antiapoptotic Actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of Hydrogen Sulfide in NRF2- and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, H.; Liang, X.; Zhang, Z.; Wang, X.; Shi, N.; Bian, W.; Di, Q.; Huang, H. Hydrogen Sulfide Attenuates Lipopolysaccharide-Induced Inflammation via the P-Glycoprotein and NF-κB Pathway in Astrocytes. Neurochem. Res. 2023, 48, 1424–1437. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Zhuo, Y.; Zhang, Y.; Wang, Z.; Qiu, Y.; Chen, K.; Ding, Q.; Qi, W.; Zhu, M.; et al. Sp1 S-Sulfhydration Induced by Hydrogen Sulfide Inhibits Inflammation via HDAC6/MyD88/NF-κB Signaling Pathway in Adjuvant-Induced Arthritis. Antioxidants 2022, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Zhou, W.; Guo, Y.; Hu, G.; Chu, J.; Xie, F.; Li, Y.; Wang, W. H2S Attenuates Oxidative Stress via Nrf2/NF-κB Signaling to Regulate Restenosis after Percutaneous Transluminal Angioplasty. Exp. Biol. Med. 2020, 246, 226–239. [Google Scholar] [CrossRef]
- Youness, R.A.; Habashy, D.A.; Khater, N.; Elsayed, K.; Dawoud, A.; Hakim, S.; Nafea, H.; Bourquin, C.; Abdel-Kader, R.M.; Gad, M.Z. Role of Hydrogen Sulfide in Oncological and Non-Oncological Disorders and Its Regulation by Non-Coding RNAs: A Comprehensive Review. Noncoding RNA 2024, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-Dependent Transcription and Cell Survival by the SIRT1 Deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Sun, H.; Li, D.; Wei, C.; Liu, L.; Xin, Z.; Gao, H.; Gao, R. The Relationship between SIRT1 and Inflammation: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1465849. [Google Scholar] [CrossRef]
- Sulen, A.; Gullaksen, S.E.; Bader, L.; McClymont, D.W.; Skavland, J.; Gavasso, S.; Gjertsen, B.T. Signaling Effects of Sodium Hydrosulfide in Healthy Donor Peripheral Blood Mononuclear Cells. Pharmacol. Res. 2016, 113, 216–227. [Google Scholar] [CrossRef]
- Dogaru, B.G.; Munteanu, C. The Role of Hydrogen Sulfide (H2S) in Epigenetic Regulation of Neurodegenerative Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12555. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Lin, X.; Xu, W.; Zheng, F.; Cai, J.; Yang, J.; Cui, Q.; Tang, C.; Cai, J.; Xu, G.; et al. Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetic Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxid. Redox Signal. 2019, 30, 184–197. [Google Scholar] [CrossRef]
- Liu, M.; Lin, X.; Xiao, L. Hydrogen Sulfide Attenuates TMAO-Induced Macrophage Inflammation through Increased SIRT1 Sulfhydration. Mol. Med. Rep. 2023, 28, 129. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Zheng, H.; Xu, Q.; Zhou, K.; Liu, H.; Xia, Y.; Wei, D.H.; Jiang, M.; Tang, Z.H.; et al. Hydrogen Sulfide Upregulates SIRT1 to Inhibit ox-HDL-Induced Endothelial Cell Damage and Mitochondrial Dysfunction. Nitric Oxide 2024, 152, 78–89. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ding, H.; Li, D.; Shen, W.; Zhang, X. The Current State of Research on Sirtuin-Mediated Autophagy in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Spezzini, J.; Piragine, E.; d’Emmanuele di Villa Bianca, R.; Bucci, M.; Martelli, A.; Calderone, V. Hydrogen Sulfide and Epigenetics: Novel Insights into the Cardiovascular Effects of This Gasotransmitter. Br. J. Pharmacol. 2023, 180, 1793–1802. [Google Scholar] [CrossRef]
- Ding, Q.; Song, W.; Zhu, M.; Yu, Y.; Lin, Z.; Hu, W.; Cai, J.; Zhang, Z.; Zhang, H.; Zhou, J.; et al. Hydrogen Sulfide and Functional Therapy: Novel Mechanisms from Epigenetics. Antioxid. Redox Signal. 2024, 40, 110–121. [Google Scholar] [CrossRef]
- Fioravanti, A.; Antonelli, M.; Vitale, M. Advances in Modern Balneology: New Evidence-Based Indications from Recent Studies. Int. J. Biometeorol. 2024, 68, 2447–2452. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Gontova, T.; Gheorghe, E.; Kassym, L.; Kussainova, A.; Voloshyn, V.; Semenova, Y.; Lysiuk, R.; Voloshyn, O.; et al. Minerals and Trace Elements: Key Protectors of Skin Health and Defenders Against Skin Disorders. Curr. Med. Chem. 2025; in press. [Google Scholar] [CrossRef]
- Gálvez, I.; Torres-Piles, S.; Ortega, E. Effect of Mud-Bath Therapy on the Innate/Inflammatory Responses in Elderly Patients with Osteoarthritis: A Discussion of Recent Results and a Pilot Study on the Role of the Innate Function of Monocytes. Int. J. Biometeorol. 2020, 64, 927–935. [Google Scholar] [CrossRef]
- Gálvez, I.; Torres-Piles, S.; Ortega-Rincón, E. Balneotherapy, Immune System, and Stress Response: A Hormetic Strategy? Int. J. Mol. Sci. 2018, 19, 1687. [Google Scholar] [CrossRef]
- Van Scott, E.J.; Flesch, P. Sulfhydryl Disulfide in Keratinization. Science 1954, 119, 70–71. [Google Scholar] [CrossRef]
- Fukuyama, K.; Epstein, W.L. Sulfur-Containing Proteins and Epidermal Keratinization. J. Cell Biol. 1969, 40, 830–838. [Google Scholar] [CrossRef]
- Kanwal, S.; Osman, E.Y.; Khiari, I. Comprehensive Review of Dermatological and Cosmeceutical Manifestations of Thermal Water and Future Insights. Int. J. Biometeorol. 2025, 69, 1783–1817. [Google Scholar] [CrossRef]
- Moini Jazani, A.; Ayati, M.H.; Nadiri, A.A.; Nasimi Doost Azgomi, R. Efficacy of Hydrotherapy, Spa Therapy, and Balneotherapy for Psoriasis and Atopic Dermatitis: A Systematic Review. Int. J. Dermatol. 2023, 62, 177–189. [Google Scholar] [CrossRef]
- Protano, C.; Vitali, M.; De Giorgi, A.; Marotta, D.; Crucianelli, S.; Fontana, M. Balneotherapy Using Thermal Mineral Water Baths and Dermatological Diseases: A Systematic Review. Int. J. Biometeorol. 2024, 68, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Gambari, L.; Grigolo, B.; Grassi, F. Hydrogen Sulfide in Bone Tissue Regeneration and Repair: State of the Art and New Perspectives. Int. J. Mol. Sci. 2019, 20, 5231. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Roop, D.R. Loricrin at the Boundary between Inside and Outside. Biomolecules 2022, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Carballal, S.; Vitvitsky, V.; Kumar, R.; Hanna, D.A.; Libiad, M.; Gupta, A.; Jones, J.W.; Banerjee, R. Hydrogen Sulfide Stimulates Lipid Biogenesis from Glutamine That Is Dependent on the Mitochondrial NAD(P)H Pool. J. Biol. Chem. 2021, 297, 100950. [Google Scholar] [CrossRef]
- Kulisch, Á.; Mándó, Z.; Sándor, E.; Lengyel, Z.; Illés, A.; Kósa, J.; Árvai, K.; Lakatos, P.; Tóbiás, B.; Papp, M.; et al. Evaluation of the Effects of Lake Hévíz Sulfur Thermal Water on Skin Microbiome in Plaque Psoriasis: An Open Label, Pilot Study. Int. J. Biometeorol. 2023, 67, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Lohakul, J.; Jeayeng, S.; Chaiprasongsuk, A.; Torregrossa, R.; Wood, M.E.; Saelim, M.; Thangboonjit, W.; Whiteman, M.; Panich, U. Mitochondria-Targeted Hydrogen Sulfide Delivery Molecules Protect against UVA-Induced Photoaging in Human Dermal Fibroblasts, and in Mouse Skin In Vivo. Antioxid. Redox Signal. 2022, 36, 1268–1288. [Google Scholar] [CrossRef]
- Qin, X.; Lu, F.; Wan, J.; Teng, X.; Jin, S.; Xiao, L.; Xue, H.; Guo, Q.; Tian, D.; Wu, Y. Hydrogen Sulfide Preserves the Function of Senescent Endothelium through SIRT2-Mediated Inflammatory Inhibition. J. Mol. Cell. Cardiol. 2025, 203, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Coavoy-Sánchez, S.A.; Costa, S.K.P.; Muscará, M.N. Hydrogen Sulfide and Dermatological Diseases. Br. J. Pharmacol. 2020, 177, 857–865. [Google Scholar] [CrossRef]
- Mirandola, P.; Gobbi, G.; Micheloni, C.; Vaccarezza, M.; Di Marcantonio, D.; Ruscitti, F.; de Panfilis, G.; Vitale, M. Hydrogen Sulfide Inhibits IL-8 Expression in Human Keratinocytes via MAP Kinase Signaling. Lab. Investig. 2011, 91, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, L.; Song, S.; Pan, L.; Muhammad Arslan, I.; Chen, Y.; Yang, S. Hydrogen Sulfide: Recent Progress and Perspectives for the Treatment of Dermatological Diseases. J. Adv. Res. 2020, 27, 11–17. [Google Scholar] [CrossRef]
- Beylot-Barry, M.; Mahé, E.; Rolland, C.; de la Bretèque, M.A.; Eychenne, C.; Charles, J.; Payen, C.; Machet, L.; Vermorel, C.; Foote, A.; et al. Evaluation of the Benefit of Thermal Spa Therapy in Plaque Psoriasis: The PSOTHERMES Randomized Clinical Trial. Int. J. Biometeorol. 2022, 66, 1247–1256. [Google Scholar] [CrossRef]
- Costantino, M.; Conti, V.; Corbi, G.; Giudice, V.; Caro, F.; Filippelli, A. Marked Reduction of Oxidant Species after Sulfureous Crenotherapy in Females with Joint Diseases and Psoriasis: A Retrospective Real-Life Study. J. Clin. Med. 2023, 12, 5731. [Google Scholar] [CrossRef]
- Joura, M.I.; Jobbágy, A.; Dunai, Z.A.; Makra, N.; Bánvölgyi, A.; Kiss, N.; Sárdy, M.; Sándor, S.E.; Holló, P.; Ostorházi, E. Characteristics of the Stool, Blood and Skin Microbiome in Rosacea Patients. Microorganisms 2024, 12, 2667. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The Role of Thermal Water in Chronic Skin Diseases Management: A Review of the Literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Hydrobiome of Thermal Waters: Potential Use in Dermocosmetics. Cosmetics 2023, 10, 94. [Google Scholar] [CrossRef]
- Edslev, S.M.; Olesen, C.M.; Nørreslet, L.N.; Ingham, A.C.; Iversen, S.; Lilje, B.; Clausen, M.L.; Jensen, J.S.; Stegger, M.; Agner, T.; et al. Staphylococcal Communities on Skin Are Associated with Atopic Dermatitis and Disease Severity. Microorganisms 2021, 9, 432. [Google Scholar] [CrossRef]
- Rodrigues, L.; Ekundi-Valentim, E.; Florenzano, J.; Cerqueira, A.R.; Soares, A.G.; Schmidt, T.P.; Santos, K.T.; Teixeira, S.A.; Ribela, M.T.; Rodrigues, S.F.; et al. Protective Effects of Exogenous and Endogenous Hydrogen Sulfide in Mast Cell-Mediated Pruritus and Cutaneous Acute Inflammation in Mice. Pharmacol. Res. 2017, 115, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hua, Y.; Qi, Y.; Meng, G.; Yang, S. Exogenous Hydrogen Sulphide Supplement Accelerates Skin Wound Healing via Oxidative Stress Inhibition and Vascular Endothelial Growth Factor Enhancement. Exp. Dermatol. 2019, 28, 776–785. [Google Scholar] [CrossRef]
- Xiao, Q.; Xiong, L.; Tang, J.; Li, L.; Li, L. Hydrogen Sulfide in Skin Diseases: A Novel Mediator and Therapeutic Target. Oxid. Med. Cell. Longev. 2021, 2021, 6652086. [Google Scholar] [CrossRef]
- Kieronska-Rudek, A.; Ascencao, K.; Chlopicki, S.; Szabo, C. Increased Hydrogen Sulfide Turnover Serves a Cytoprotective Role during the Development of Replicative Senescence. Biochem. Pharmacol. 2024, 230, 116595. [Google Scholar] [CrossRef]
- Fioravanti, A. Foreword: Balneotherapy in Rheumatic Diseases. Int. J. Biometeorol. 2020, 64, 903–904. [Google Scholar] [CrossRef]
- Karagülle, M.; Karagülle, M.Z. Effectiveness of Balneotherapy and Spa Therapy for the Treatment of Chronic Low Back Pain: A Review on Latest Evidence. Clin. Rheumatol. 2015, 34, 207–214. [Google Scholar] [CrossRef]
- Tefner, I.K.; Gaál, R.; Koroknai, A.; Ráthonyi, A.; Gáti, T.; Monduk, P.; Kiss, E.; Kovács, C.; Bálint, G.; Bender, T. The Effect of Neydharting Mud-Pack Therapy on Knee Osteoarthritis: A Randomized, Controlled, Double-Blind Follow-Up Pilot Study. Rheumatol. Int. 2013, 33, 2569–2576. [Google Scholar] [CrossRef]
- Burguera, E.F.; Meijide-Failde, R.; Blanco, F.J. Hydrogen Sulfide and Inflammatory Joint Diseases. Curr. Drug Targets 2017, 18, 1641–1652. [Google Scholar] [CrossRef]
- Song, Y.; Wu, S.; Zhang, R.; Zhong, Q.; Zhang, X.; Sun, X. Therapeutic Potential of Hydrogen Sulfide in Osteoarthritis Development. Front. Pharmacol. 2024, 15, 1336693. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen Sulfide: An Endogenous Regulator of the Immune System. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Cunha, T.M.; Dal-Secco, D.; Verri, W.A., Jr.; Guerrero, A.T.; Souza, G.R.; Vieira, S.M.; Lotufo, C.M.; Neto, A.F.; Ferreira, S.H.; Cunha, F.Q. Dual Role of Hydrogen Sulfide in Mechanical Inflammatory Hypernociception. Eur. J. Pharmacol. 2008, 590, 127–135. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, N.; Liu, H.Z.; Wang, J.L.; Zhang, Y.B.; Su, D.D.; Miao, J. H2S Alleviates Neuropathic Pain in Mice by Nrf2 Signaling Pathway Activation. J. Mol. Neurosci. 2023, 73, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Xu, A.J.; Cai, W.W.; Han, Z.J.; Zhang, S.J.; Hou, B.; Wen, Y.Y.; Cao, X.Y.; Li, H.D.; Du, Y.Q.; et al. NaHS@Cy5@MS@SP Nanoparticles Improve Rheumatoid Arthritis by Inactivating the Hedgehog Signaling Pathway through Sustained and Targeted Release of H2S into the Synovium. J. Nanobiotechnol. 2025, 23, 192. [Google Scholar] [CrossRef]
- Nasi, S.; Ehirchiou, D.; Bertrand, J.; Castelblanco, M.; Mitchell, J.; Ishii, I.; So, A.; Busso, N. The Gasotransmitter Hydrogen Sulfide (H2S) Prevents Pathologic Calcification (PC) in Cartilage. Antioxidants 2021, 10, 1433. [Google Scholar] [CrossRef] [PubMed]
- Batallè, G.; Cabarga, L.; Pol, O. The Inhibitory Effects of Slow-Releasing Hydrogen Sulfide Donors in the Mechanical Allodynia, Grip Strength Deficits, and Depressive-Like Behaviours Associated with Chronic Osteoarthritis Pain. Antioxidants 2020, 9, 31. [Google Scholar] [CrossRef]
- Gambari, L.; Grigolo, B.; Filardo, G.; Grassi, F. Sulfurous Thermal Waters Stimulate the Osteogenic Differentiation of Human Mesenchymal Stromal Cells—An In Vitro Study. Biomed. Pharmacother. 2020, 129, 110344. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, Y.X.; Zhu, Y.W.; Tang, A.Q.; Liang, H.B.; Yang, Y.L.; Zhai, Y.K.; Ji, X.Y.; Wu, D.D. Hydrogen Sulfide in Musculoskeletal Diseases: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2025, 42, 321–340. [Google Scholar] [CrossRef]
- Burguera, E.F.; Vela-Anero, Á.; Gato-Calvo, L.; Vaamonde-García, C.; Meijide-Faílde, R.; Blanco, F.J. Hydrogen Sulfide Biosynthesis Is Impaired in the Osteoarthritic Joint. Int. J. Biometeorol. 2020, 64, 997–1010. [Google Scholar] [CrossRef]
- Forestier, R.; Desfour, H.; Tessier, J.M.; Françon, A.; Foote, A.M.; Genty, C.; Rolland, C.; Roques, C.F.; Bosson, J.L. Spa Therapy in the Treatment of Knee Osteoarthritis: A Large Randomised Multicentre Trial. Ann. Rheum. Dis. 2010, 69, 660–665. [Google Scholar] [CrossRef]
- Cantista, P.; Maraver, F. Balneotherapy for Knee Osteoarthritis in S. Jorge: A Randomized Controlled Trial. Int. J. Biometeorol. 2020, 64, 1027–1038. [Google Scholar] [CrossRef]
- Li, M.; Mao, J.C.; Zhu, Y.Z. Hydrogen Sulfide: A Novel Immunoinflammatory Regulator in Rheumatoid Arthritis. Adv. Exp. Med. Biol. 2021, 1315, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Codish, S.; Dobrovinsky, S.; Abu Shakra, M.; Flusser, D.; Sukenik, S. Spa Therapy for Ankylosing Spondylitis at the Dead Sea. Isr. Med. Assoc. J. 2005, 7, 443–446. [Google Scholar]
- Bestaş, E.; Dündar, Ü.; Köken, T.; Koca, B.; Yeşil, H. The Comparison of Effects of Balneotherapy, Water-Based and Land-Based Exercises on Disease Activity, Symptoms, Sleep Quality, Quality of Life and Serum Sclerostin Level in Patients with Ankylosing Spondylitis: A Prospective, Randomized Study. Arch. Rheumatol. 2021, 37, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.; Conti, V.; Corbi, G.; Marongiu, F.; Marongiu, M.B.; Filippelli, A. Sulphurous Mud-Bath Therapy for Treatment of Chronic Low Back Pain Caused by Lumbar Spine Osteoarthritis. Intern. Emerg. Med. 2019, 14, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Forestier, R.; Suehs, C.; Françon, A.; Marty, M.; Genevay, S.; Sellam, J.; Chauveton, C.; Erol Forestier, F.B.; Molinari, N. Usual Care Including Home Exercise with versus without Spa Therapy for Chronic Low Back Pain: Protocol for the LOMBATHERM’ Study, a Multicentric Randomised Controlled Trial. Trials 2020, 21, 392. [Google Scholar] [CrossRef]
- Forestier, R.; Fioravanti, A.; Bender, T.; Santos, I.; Erol Forestier, F.B.; Muela Garcia, A.; Françon, A. Crenobalneotherapy for Low Back Pain: Systematic Review of Clinical Trials. Int. J. Biometeorol. 2022, 66, 13–23. [Google Scholar] [CrossRef]
- García-López, H.; García-Giménez, M.T.; Obrero-Gaitán, E.; Lara-Palomo, I.C.; Castro-Sánchez, A.M.; Rey, R.R.; Cortés-Pérez, I. Effectiveness of Balneotherapy in Reducing Pain, Disability, and Depression in Patients with Fibromyalgia Syndrome: A Systematic Review with Meta-Analysis. Int. J. Biometeorol. 2024, 68, 1935–1951. [Google Scholar] [CrossRef]
- Koç, C.; Kurt, E.E.; Koçak, F.A.; Erdem, H.R.; Konar, N.M. Does Balneotherapy Provide Additive Effects to Physical Therapy in Patients with Subacute Supraspinatus Tendinopathy? A Randomized, Controlled, Single-Blind Study. Int. J. Biometeorol. 2021, 65, 301–310. [Google Scholar] [CrossRef]
- Yang, J.H.; Gao, J.; E, Y.Q.; Jiao, L.J.; Wu, R.; Yan, Q.Y.; Wei, Z.Y.; Yan, G.L.; Liang, J.L.; Li, H.Z. Hydrogen Sulfide Inhibits Skeletal Muscle Ageing by Up-Regulating Autophagy through Promoting Deubiquitination of Adenosine 5′-Monophosphate (AMP)-Activated Protein Kinase α1 via Ubiquitin Specific Peptidase 5. J. Cachexia Sarcopenia Muscle 2024, 15, 2118–2133. [Google Scholar] [CrossRef]
- Munteanu, C.; Munteanu, D.; Onose, G. Hydrogen Sulfide (H2S)—Therapeutic Relevance in Rehabilitation and Balneotherapy. Systematic Literature Review and Meta-Analysis Based on the PRISMA Paradigm. Balneo PRM Res. J. 2021, 12, 176–195. [Google Scholar] [CrossRef]
- Franz, L.; Manica, P.; Claudatus, J.; Frigo, A.C.; Marioni, G.; Staffieri, A. Sulfurous-Arsenical-Ferruginous Thermal Water Nasal Inhalation and Irrigation in Children with Recurrent Upper Respiratory Tract Infections: Clinical Outcomes and Predictive Factors. Am. J. Otolaryngol. 2021, 42, 103083. [Google Scholar] [CrossRef]
- Khaltaev, N.; Solimene, U.; Vitale, F.; Zanasi, A. Balneotherapy and Hydrotherapy in Chronic Respiratory Disease. J. Thorac. Dis. 2020, 12, 4459–4468. [Google Scholar] [CrossRef] [PubMed]
- Bazhanov, N.; Ansar, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Hydrogen Sulfide: A Novel Player in Airway Development, Pathophysiology of Respiratory Diseases, and Antiviral Defenses. Am. J. Respir. Cell Mol. Biol. 2017, 57, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Shaffer, K.M.; Ehre, C. Mucins and CFTR: Their Close Relationship. Int. J. Mol. Sci. 2022, 23, 10232. [Google Scholar] [CrossRef]
- Audousset, C.; McGovern, T.; Martin, J.G. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches—Pulmonary Disease/Asthma. Front. Physiol. 2021, 12, 727806. [Google Scholar] [CrossRef] [PubMed]
- Viegas, J.; Esteves, A.F.; Cardoso, E.M.; Arosa, F.A.; Vitale, M.; Taborda-Barata, L. Biological Effects of Thermal Water-Associated Hydrogen Sulfide on Human Airways and Associated Immune Cells: Implications for Respiratory Diseases. Front. Public Health 2019, 7, 128. [Google Scholar] [CrossRef]
- Castro-Piedras, I.; Perez-Zoghbi, J.F. Hydrogen Sulphide Inhibits Ca2⁺ Release through InsP3 Receptors and Relaxes Airway Smooth Muscle. J. Physiol. 2013, 591, 5999–6015. [Google Scholar] [CrossRef]
- Dunn, W.R.; Alexander, S.P.H.; Ralevic, V.; Roberts, R.E. Effects of Hydrogen Sulphide in Smooth Muscle. Pharmacol. Ther. 2016, 158, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Davis, B.E.; Blais, C.M. Comparison of Methacholine and Mannitol Challenges: Importance of Method of Methacholine Inhalation. Allergy Asthma Clin. Immunol. 2020, 16, 14. [Google Scholar] [CrossRef]
- Lee, J.-W.; Chun, W.; Lee, H.J.; Min, J.-H.; Kim, S.-M.; Seo, J.-Y.; Ahn, K.-S.; Oh, S.-R. The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells 2021, 10, 897. [Google Scholar] [CrossRef]
- Li, T.; Zhao, B.; Wang, C.; Wang, H.; Liu, Z.; Li, W.; Jin, H.; Tang, C.; Du, J. Regulatory Effects of Hydrogen Sulfide on IL-6, IL-8 and IL-10 Levels in the Plasma and Pulmonary Tissue of Rats with Acute Lung Injury. Exp. Biol. Med. 2008, 233, 1081–1087. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, C.; Zhou, F.; Yuan, Z.; Wang, H.; Cui, W.; Zhang, G. Hydrogen Sulfide as a Potential Therapeutic Target in Fibrosis. Oxid. Med. Cell. Longev. 2015, 2015, 593407. [Google Scholar] [CrossRef]
- Pozzi, G.; Gobbi, G.; Masselli, E.; Carubbi, C.; Presta, V.; Ambrosini, L.; Vitale, M.; Mirandola, P. Buffering Adaptive Immunity by Hydrogen Sulfide. Cells 2022, 11, 325. [Google Scholar] [CrossRef]
- Fontana, M.; Vitali, M.; Del Prete, J.; Borzì, S.; Pozzoli, A.; Vitale, K.; De Giorgi, A.; Zanni, S.; Crucianelli, S.; Protano, C. Beneficial Effects of Thermal Waters on Respiratory Diseases: A Systematic Review. Int. J. Biometeorol. 2025, 69, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Viegas, J.; Cardoso, E.M.; Bonneau, L.; Esteves, A.F.; Ferreira, C.L.; Alves, G.; Santos-Silva, A.J.; Vitale, M.; Arosa, F.A.; Taborda-Barata, L. A Novel Bionebulizer Approach to Study the Effects of Natural Mineral Water on a 3D In Vitro Nasal Model from Allergic Rhinitis Patients. Biomedicines 2024, 12, 408. [Google Scholar] [CrossRef]
- Antonelli, M.; Pennacchi, A.; Pasquarella, G.; Moscoloni, M.; Mariani, G.; Borioni, B. Inhalation Therapy with Sulfur-Rich Thermal Water for Rhinogenic Deafness: A Series of Case Reports. Int. J. Biometeorol. 2025, 69, 703–707. [Google Scholar] [CrossRef]
- Contoli, M.; Gnesini, G.; Forini, G.; Marku, B.; Pauletti, A.; Padovani, A.; Casolari, P.; Taurino, L.; Ferraro, A.; Chicca, M.; et al. Reducing Agents Decrease the Oxidative Burst and Improve Clinical Outcomes in COPD Patients: A Randomised Controlled Trial on the Effects of Sulphurous Thermal Water Inhalation. Sci. World J. 2013, 2013, 927835. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Daniele, N.; Chetta, A.; Vitale, M.; Gholamalishahi, S.; Cazzola, M.; Rogliani, P. The Impact of Thermal Water in Asthma and COPD: A Systematic Review According to the PRISMA Statement. J. Clin. Med. 2024, 13, 1071. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mesfin, F.M.; Hunter, C.E.; Olson, K.R.; Shelley, W.C.; Brokaw, J.P.; Manohar, K.; Markel, T.A. Recent Development of the Molecular and Cellular Mechanisms of Hydrogen Sulfide Gasotransmitter. Antioxidants 2022, 11, 1788. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine Gamma-Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Altaany, Z.; Ju, Y.; Yang, G.; Wang, R. The Coordination of S-Sulfhydration, S-Nitrosylation, and Phosphorylation of Endothelial Nitric Oxide Synthase by Hydrogen Sulfide. Sci. Signal. 2014, 7, ra87. [Google Scholar] [CrossRef]
- Piragine, E.; Citi, V.; Lawson, K.; Calderone, V.; Martelli, A. Regulation of Blood Pressure by Natural Sulfur Compounds: Focus on Their Mechanisms of Action. Biochem. Pharmacol. 2022, 206, 115302. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Rios, E.C.; Szczesny, B.; Soriano, F.G.; Olah, G.; Szabo, C. Hydrogen Sulfide Attenuates Cytokine Production through the Modulation of Chromatin Remodeling. Int. J. Mol. Med. 2015, 35, 1741–1746. [Google Scholar] [CrossRef]
- Kimura, Y.; Goto, Y.; Kimura, H. Hydrogen Sulfide Increases Glutathione Production and Suppresses Oxidative Stress in Mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Surdu, T.-V.; Surdu, M.; Surdu, O.; Franciuc, I.; Tucmeanu, E.-R.; Tucmeanu, A.-I.; Serbanescu, L.; Tica, V.I. Microvascular Responses in the Dermis and Muscles after Balneotherapy: Results from a Prospective Pilot Histological Study. Water 2025, 17, 1830. [Google Scholar] [CrossRef]
- Łoboda, A.; Dulak, J. Cardioprotective Effects of Hydrogen Sulfide and Its Potential Therapeutic Implications in the Amelioration of Duchenne Muscular Dystrophy Cardiomyopathy. Cells 2024, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.H.; Ren, Z.; Qu, S.L.; Liu, M.H.; Liu, L.S.; Jiang, Z.S. Hydrogen Sulfide, the Next Potent Preventive and Therapeutic Agent in Aging and Age-Associated Diseases. Mol. Cell. Biol. 2013, 33, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Ianaro, A.; de Nucci, G. Gaseous Mediators in Gastrointestinal Mucosal Defense and Injury. Dig. Dis. Sci. 2017, 62, 2223–2230. [Google Scholar] [CrossRef]
- Wallace, J.L.; Motta, J.P.; Buret, A.G. Hydrogen Sulfide: An Agent of Stability at the Microbiome–Mucosa Interface. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G143–G149. [Google Scholar] [CrossRef]
- Linden, D.R. Hydrogen Sulfide Signaling in the Gastrointestinal Tract. Antioxid. Redox Signal. 2014, 20, 818–830. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen Sulfide Toxicity in the Gut Environment: Meta-Analysis of Sulfate-Reducing and Lactic Acid Bacteria in Inflammatory Processes. J. Adv. Res. 2020, 27, 55–69. [Google Scholar] [CrossRef]
- Munteanu, C.; Onose, G.; Rotariu, M.; Poștaru, M.; Turnea, M.; Galaction, A.I. Role of Microbiota-Derived Hydrogen Sulfide (H2S) in Modulating the Gut–Brain Axis: Implications for Alzheimer’s and Parkinson’s Disease Pathogenesis. Biomedicines 2024, 12, 2670. [Google Scholar] [CrossRef]
- Kumar, R.; Sykes, D.J.; Band, V.I.; Schaller, M.L.; Patel, R.; Vitvitsky, V.; Sajjakulnukit, P.; Singhal, R.; Wong, H.K.A.; Hourigan, S.K.; et al. Gut Sulfide Metabolism Modulates Behavior and Brain Bioenergetics. Proc. Natl. Acad. Sci. USA 2025, 122, e2503677122. [Google Scholar] [CrossRef] [PubMed]
- Birg, A.; Lin, H.C. The Role of Bacteria-Derived Hydrogen Sulfide in Multiple Axes of Disease. Int. J. Mol. Sci. 2025, 26, 3340. [Google Scholar] [CrossRef]
- Dugbartey, G.J. Physiological Role of Hydrogen Sulfide in the Kidney and Its Therapeutic Implications for Kidney Diseases. Biomed. Pharmacother. 2023, 166, 115396. [Google Scholar] [CrossRef]
- Stoeva, S.; Vankova, D.; Sokrateva, T.; Nashar, M. The Potential of Sulfur-Containing Mineral Water and Sulfur-Containing Bioactive Compounds to Modulate Inflammatory Markers in Human Intestinal Epithelial Cells: A Comparative Study. Preprints 2025, 2025061946. [Google Scholar] [CrossRef]
- Shen, F.; Zhao, C.S.; Shen, M.F.; Wang, Z.; Chen, G. The Role of Hydrogen Sulfide in Gastric Mucosal Damage. Med. Gas Res. 2019, 9, 88–92. [Google Scholar] [CrossRef]
- Dumitrescu, M.; Iliescu, M.G.; Mazilu, L.; Micu, S.I.; Suceveanu, A.P.; Voinea, F.; Voinea, C.; Stoian, A.P.; Suceveanu, A.I. Benefits of Crenotherapy in Digestive Tract Pathology (Review). Exp. Ther. Med. 2022, 23, 122. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Dwidar, M.; Gao, Y.; Xiang, L.; Wu, X.; Medema, M.H.; Xu, S.; Li, X.; Schäfer, H.; et al. Microbial Assimilatory Sulfate Reduction-Mediated H2S: An Overlooked Role in Crohn’s Disease Development. Microbiome 2024, 12, 152. [Google Scholar] [CrossRef]
- Leite, G.; Rezaie, A.; Mathur, R.; Barlow, G.M.; Rashid, M.; Hosseini, A.; Wang, J.; Parodi, G.; Villanueva-Millan, M.J.; Sanchez, M.; et al. Defining Small Intestinal Bacterial Overgrowth by Culture and High Throughput Sequencing. Clin. Gastroenterol. Hepatol. 2024, 22, 259–270. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Xu, M.; Ma, Y.; Sun, R.; Ding, H.; Li, L. The Double-Edged Role of Hydrogen Sulfide in the Pathomechanism of Multiple Liver Diseases. Front. Pharmacol. 2022, 13, 899859. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, N.; Qi, K.; Cheng, H.; Sun, Z.; Gao, B.; Zhang, Y.; Pang, W.; Huangfu, C.; Ji, S.; et al. Exogenous Hydrogen Sulfide Mitigates the Fatty Liver in Obese Mice through Improving Lipid Metabolism and Antioxidant Potential. Med. Gas Res. 2015, 5, 1. [Google Scholar] [CrossRef]
- Pichette, J.; Gagnon, J. Implications of Hydrogen Sulfide in Glucose Regulation: How H2S Can Alter Glucose Homeostasis through Metabolic Hormones. Oxid. Med. Cell. Longev. 2016, 2016, 3285074. [Google Scholar] [CrossRef]
- Costantino, M.; Conti, V.; Corbi, G.; Filippelli, A. Hydropinotherapy with Sulphurous Mineral Water as Complementary Treatment to Improve Glucose Metabolism, Oxidative Status, and Quality of Life. Antioxidants 2021, 10, 1773. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Rotariu, M.; Turnea, M.; Dogaru, G.; Popescu, C.; Spînu, A.; Andone, I.; Postoiu, R.; Ionescu, E.V.; Oprea, C.; et al. Recent Advances in Molecular Research on Hydrogen Sulfide (H2S) Role in Diabetes Mellitus (DM)—A Systematic Review. Int. J. Mol. Sci. 2022, 23, 6720. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Rotariu, M.; Turnea, M.A.; Anghelescu, A.; Albadi, I.; Dogaru, G.; Silișteanu, S.C.; Ionescu, E.V.; Firan, F.C.; Ionescu, A.M.; et al. Topical Reappraisal of Molecular Pharmacological Approaches to Endothelial Dysfunction in Diabetes Mellitus Angiopathy. Curr. Issues Mol. Biol. 2022, 44, 3378–3397. [Google Scholar] [CrossRef] [PubMed]
- Fauste, E.; Rodrigo, S.; Aguirre, R.; Donis, C.; Rodríguez, L.; Álvarez-Millán, J.J.; Panadero, M.I.; Otero, P.; Bocos, C. Maternal Fructose Intake Increases Liver H2S Synthesis but Exacerbates Its Fructose-Induced Decrease in Female Progeny. Mol. Nutr. Food Res. 2020, 64, 2000628. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Nguyen, P.L.; Park, S.H.; Jung, C.H.; Jeon, T.I. Hydrogen Sulfide and Liver Health: Insights into Liver Diseases. Antioxid. Redox Signal. 2024, 40, 122–144. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Yang, Y.; Lan, T.; Wang, H.; Wu, D. Hydrogen Sulfide Plays an Important Role by Regulating Endoplasmic Reticulum Stress in Diabetes-Related Diseases. Int. J. Mol. Sci. 2022, 23, 7170. [Google Scholar] [CrossRef]
- Beck, K.F.; Pfeilschifter, J. The Pathophysiology of H2S in Renal Glomerular Diseases. Biomolecules 2022, 12, 207. [Google Scholar] [CrossRef]
- Song, K.; Wang, F.; Li, Q.; Shi, Y.B.; Zheng, H.F.; Peng, H.; Shen, H.Y.; Liu, C.F.; Hu, L.F. Hydrogen Sulfide Inhibits the Renal Fibrosis of Obstructive Nephropathy. Kidney Int. 2014, 85, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Ngowi, E.E.; Sarfraz, M.; Afzal, A.; Khan, N.H.; Khattak, S.; Zhang, X.; Li, T.; Duan, S.F.; Ji, X.Y.; Wu, D.D. Roles of Hydrogen Sulfide Donors in Common Kidney Diseases. Front. Pharmacol. 2020, 11, 564281. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Guo, N. Protective Effect of Hydrogen Sulfide on the Kidney (Review). Mol. Med. Rep. 2021, 24, 696. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, K.; Mustafina, A.; Yakovlev, A.; Hermann, A.; Giniatullin, R.; Sitdikova, G. Receptor Mechanisms Mediating the Pro-Nociceptive Action of Hydrogen Sulfide in Rat Trigeminal Neurons and Meningeal Afferents. Front. Cell. Neurosci. 2017, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Fontana, M.; De Giorgi, A.; Marotta, D.; Cocomello, N.; Crucianelli, S.; Del Cimmuto, A.; Vitali, M. Balneotherapy for Osteoarthritis: A Systematic Review. Rheumatol. Int. 2023, 43, 1597–1610. [Google Scholar] [CrossRef]
- Maccarone, M.C.; Magro, G.; Albertin, C.; Barbetta, G.; Barone, S.; Castaldelli, C.; Manica, P.; Marcoli, S.; Mediati, M.; Minuto, D.; et al. Short-Time Effects of Spa Rehabilitation on Pain, Mood and Quality of Life among Patients with Degenerative or Post-Surgery Musculoskeletal Disorders. Int. J. Biometeorol. 2023, 67, 29–36. [Google Scholar] [CrossRef]
- Maccarone, M.C.; Regazzo, G.; Contessa, P.; Scanu, A.; Masiero, S. Healing with Thermal Mineral-Rich Waters: The Role of Spa Therapy in Post-Surgical Rehabilitation. Int. J. Biometeorol. 2025, 69, 2125–2129. [Google Scholar] [CrossRef]
- Layal, H.; Rajbongshi, J.; Kumar, R.; Pandey, S.; Mishra, R.; Yadav, P.K. Hydrogen Sulfide in the Brain as a Silent Neuroprotector in Alzheimer’s Disease. Neuroscience 2025, 585, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Mahdi, A.A.; Alhmadi, H.B.; Medvedev, O. Unveiling Hydrogen Sulfide: A New Frontier in Neuroprotection and Neuromodulation. Indian J. Clin. Biochem. 2025, 40, 540–550. [Google Scholar] [CrossRef]
- Cui, W.; Chen, J.; Yu, F.; Liu, W.; He, M. GYY4137 Protected the Integrity of the Blood–Brain Barrier via Activation of the Nrf2/ARE Pathway in Mice with Sepsis. FASEB J. 2021, 35, e21710. [Google Scholar] [CrossRef]
- Cheleschi, S.; Gallo, I.; Tenti, S. A Comprehensive Analysis to Understand the Mechanism of Action of Balneotherapy: Why, How, and Where They Can Be Used? Evidence from In Vitro Studies Performed on Human and Animal Samples. Int. J. Biometeorol. 2020, 64, 1247–1261. [Google Scholar] [CrossRef]
- Morer, C.; Roques, C.F.; Françon, A.; Forestier, R.; Maraver, F. The Role of Mineral Elements and Other Chemical Compounds Used in Balneology: Data from Double-Blind Randomized Clinical Trials. Int. J. Biometeorol. 2017, 61, 2159–2173. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Unveiling the Role of Minerals and Trace Elements of Thermal Waters in Skin Health. Appl. Sci. 2024, 14, 6291. [Google Scholar] [CrossRef]
- Yamazaki, T.; Ushikoshi-Nakayama, R.; Shakya, S.; Omagari, D.; Matsumoto, N.; Nukuzuma, C.; Komatsu, T.; Lee, M.C.; Inoue, H.; Saito, I. The Effects of Bathing in Neutral Bicarbonate Ion Water. Sci. Rep. 2021, 11, 21789. [Google Scholar] [CrossRef]
- Gomes, C.; Carretero, M.I.; Pozo, M.; Maraver, F.; Cantista, P.; Armijo, F.; Legido, J.L.; Teixeira, F.; Rautureau, M.; Delgado, R. Peloids and Pelotherapy: Historical Evolution, Classification and Glossary. Appl. Clay Sci. 2013, 75–76, 28–38. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in Pelotherapy. A Review. Part II: Organic Compounds, Microbiology and Medical Applications. Appl. Clay Sci. 2020, 189, 105531. [Google Scholar] [CrossRef]
- Ortega-Collazos, E.; Otero, E.; López-Jurado, C.; Navarro, C.; Martín-Cordero, L.; Gálvez, I.; Torres-Piles, S.; Ortega, E.; Hinchado, M.D. Neuroimmunomodulation Induced by Mud-Bath Therapy: Clinical Benefits and Bioregulation of the Innate/Inflammatory Responses Induced by a Peloid Enriched with Rosmarinic Acid in Elderly Patients with Osteoarthritis. Int. J. Biometeorol. 2025, 69, 2115–2124. [Google Scholar] [CrossRef]
- Güneri, F.D.; Karaarslan, F.; Özen, H.; Odabaşi, E. Medical Mud-Pack Treatment with Different Temperatures in Patients with Knee Osteoarthritis. Int. J. Biometeorol. 2025, 69, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Maraver, F.; Armijo, F.; Fernández-Torán, M.A.; Armijo, O.; Ejeda, J.M.; Vázquez, I.; Corvillo, I.; Torres-Piles, S. Peloids as Thermotherapeutic Agents. Int. J. Environ. Res. Public Health 2021, 18, 1965. [Google Scholar] [CrossRef] [PubMed]
- Shoubir, M.; Kasior, I.; Zasadzka, E. The Effects of Peloid and Balneotherapy on Arthritis: A Systematic Review. J. Med. Case Rep. 2024, 6, 1–8. [Google Scholar] [CrossRef]
- Stanciu, L.E.; Iliescu, M.G.; Petcu, L.; Uzun, A.B.; Ungureanu, A.E.; Ungur, R.A.; Ciortea, V.M.; Irsay, L.; Ionescu, E.V.; Oprea, C.; et al. The Analyse of the Antioxidant Effect of Natural Peloidotherapy in Aging Process. Balneo PRM Res. J. 2023, 14, 541. [Google Scholar] [CrossRef]
- Hernández Torres, A. Técnicas y Tecnologías en Hidrología Médica e Hidroterapia; AETS–Instituto de Salud Carlos III: Madrid, Spain, 2006. [Google Scholar]
- Zajac, D. Inhalations with Thermal Waters in Respiratory Diseases. J. Ethnopharmacol. 2021, 281, 114505. [Google Scholar] [CrossRef]
- Karagülle, M.Z.; Karagülle, M. Effects of Drinking Natural Hydrogen Sulfide (H2S) Waters: A Systematic Review of In Vivo Animal Studies. Int. J. Biometeorol. 2020, 64, 1011–1022. [Google Scholar] [CrossRef]
- Tiganescu, E.; Lämmermann, M.A.; Ney, Y.; Abdin, A.Y.; Nasim, M.J.; Jacob, C. A Whiff of Sulfur: One Wind a Day Keeps the Doctor Away. Antioxidants 2022, 11, 1036. [Google Scholar] [CrossRef]

| Variable (Range) | Predominant Chemical Species | Bioavailable Fraction | Main Absorption Route in Spa Practice | Therapeutic Implication |
|---|---|---|---|---|
| pH 4.5–6.5 (acidic) | H2S (gas) | High (lipophilic) | Topical (diffusion through skin) | Favorable for dermatological applications |
| Physio pH 7.2–7.4 | HS− + H2S (≈4:1) | Moderate | Inhalation (alveolar uptake of H2S gas) | Useful for respiratory indications; monitor exposure limits |
| Alkaline pH > 8.0 | HS− ≫ H2S | Low (ionized) | Limited | Reduced activity |
| Tª < 30 °C | Higher solubility of H2S in water | Moderate | Topical (slow volatilization) | Longer bath retention; mild inhalation |
| Tª 30–40 °C | Equilibrium shift to gas phase | High near surface | Combined topical + inhalation | Optimal spa range; increases systemic delivery |
| Tª > 40 °C | Rapid H2S volatilization | Variable (declines in water) | Predominantly inhalation (short exposure) | Requires ventilation to avoid toxic peaks |
| Low O2 (<2 mg L−1) | H2S preserved, minimal oxidation | High | Topical/inhalation (stable gas) | Maximisers therapeutic fraction |
| High O2 (>6 mg L−1) | Oxidation to thiosulfate/sulfate | Very low | Negligible | Loss of activity; avoid aeration |
| Organ/Tissue | Dominant Enzyme | Functional Relevance |
|---|---|---|
| Brain | CBS | Neuroprotection, neurogenesis |
| Heart/Vessels | CSE | Vasodilation, blood pressure regulation |
| Liver/Kidney | CBS/CSE | Redox metabolism, fibrosis protection |
| Mitochondria (various) | 3-MST | Energy homeostasis, cellular bioenergetics |
| Mechanism | H2S Action | Reference |
|---|---|---|
| Direct neutralization | Scavenging of H2O2 and •OH | Kimura, 2015 [2] |
| Antioxidant enzymes | ↑ SOD, GPx, GSH | Paul, 2015 [4] |
| Nrf2 activation | Genetic transcription of HO-1, NQO1 | Yang et al., 2013 [37] |
| Persulfidation | Protein protection | Mustafa et al., 2009 [35] |
| Mitochondrial redox | ↑ mitochondrial efficiency ↓ ROS production | Kabil & Banerjee, 2010 [8] |
| Mechanism | Cellular Target | Physiological Consequence | Therapeutic Implication |
|---|---|---|---|
| Persulfidation (S-sulfuration) | Proteins with free cysteine groups | Enzymatic and structural modulation | Cytoprotection, metabolic regulation |
| Nrf2 activation | ARE (Antioxidant Response Elements) in cell nucleus | Induction of endogenous antioxidants | Anti-aging, oxidative defense |
| Inhibition of HDACs and DNMTs | Epigenetic enzymes | Re-expression of silenced genes | Skin repair, longevity |
| Activation of sirtuins | SIRT1/SIRT3 in nucleus and mitochondria | Energy regulation and cellular repair | Epigenetics, tissue protection |
| Activation of K_ATP channels | Cell membrane | Membrane hyperpolarization and vasodilation | Muscle relaxation, analgesia, peripheral circulation |
| Inhibition of NADPH oxidase | Inflammatory cells | Reduction of ROS and free radicals | Anti-inflammatory, neuroprotection |
| NF-κB modulation | Pro-inflammatory pathway | Decrease in pro-inflammatory cytokines | Immunomodulation, pain treatment |
| Effect | Molecular Mechanism | Clinical Benefit |
|---|---|---|
| Antioxidant | Nrf2 activation; persulfidation; inhibition of NADPHox | Protection against photoaging and oxidative stress |
| Anti-inflammatory | NF-κB inhibition; ↓ IL-1β, TNF-α | Relief of pruritus, psoriasis, eczema |
| Epigenetic repair | Activation of SIRT1/SIR2/SIRT3; inhibition of HDACs & DNMTs | Skin regeneration, cellular longevity |
| Microbiome modulation | Selective action against pathogens | Reduction of dysbiosis in dermatitis and seborrhea |
| Barrier restoration | Stimulation of filaggrin, loricrin, and lipids | Re-epithelialization, hydration, and barrier repair |
| Angiogenesis & tissue repair | VEGF activation and fibroblast migration | Healing of ulcers, wounds, and chronic erosions |
| Condition | Main Effects | Mechanisms | Evidence |
|---|---|---|---|
| Psoriasis | ↓ Inflammation, ↓ MMPs | Keratolysis; Th1/Th17 cytokine inhibition; ↓ MMP-9; normalized proliferation | Clinical + preclinical |
| Atopic Dermatitis | ↓ S. aureus; barrier restoration | Keratoplasia; microbiota rebalancing; lipid improvement; ↓ IL-4/IL-13 | Clinical + spa use |
| Seborrheic Dermatitis | ↓ Malassezia spp.; ↓ erythema | Selective antifungal; localized anti-inflammatory | Clinical-observational |
| Rosacea | ↓ LL-37; ↓ erythema; ↓ Demodex | Neurovascular inhibition; local immune modulation | Preclinical + empirical |
| Inflammatory Acne | ↓ C. acnes | Seboregulation; keratolysis; antimicrobial & lipid regulation | Case reports + observational |
| Chronic Eczema | Barrier restoration; ↓ microbial colonization | Lipid improvement; ↑ filaggrin & loricrin | Clinical experience + studies |
| Pruritus | ↓ IL-31; ↓ mast cell activation | Neuroimmune modulation; ↓ pruritogenic cytokines | Preclinical + clinical |
| Wound Healing | ↑ VEGF; ↓ oxidative stress | Angiogenesis & fibroblast migration | Preclinical + observational |
| Well-being | Improved microcirculation; anti-aging | Sirtuin & NO pathway activation | In vitro + clinical |
| Condition | Main Effects | Mechanisms | Evidence |
|---|---|---|---|
| Osteoarthritis | ↓ Inflammation, ↓ oxidative stress, ↓ apoptosis, ↓ pain | Inhibits NF-κB/MAPK/PI3K; activates Nrf2/HO-1/K+ channels | Preclinical + balneotherapy |
| Rheumatoid Arthritis | ↓ Synovial inflammation, ↓ FLS proliferation, ↓ erosion | Inhibits cytokines & NF-κB/MAPK; restores H2S via nano-carriers | Preclinical + biomarker data |
| Skeletal Muscle Aging (Sarcopenia) | ↑ Autophagy, ↓ muscle atrophy markers | USP5-mediated AMPKα1 deubiquitination; activation of AMPKα1–ULK1 | Preclinical in vitro & in vivo |
| Spondyloarthropathies | ↓ Axial pain, ↑ mobility | Anti-inflammatory & myorelaxant effects | Clinical + observational |
| Chronic Low Back Pain | ↓ Pain, ↑ local metabolism | Vasodilation, heat effect, H2S release | Clinical |
| Fibromyalgia | ↓ Generalized pain, ↑ sleep, ↑ well-being | Oxidative stress & neuroinflammation modulation | Observational |
| Chronic Tendinopathies | ↓ Inflammation, ↑ microcirculation | Local peloid application, sustained H2S release | Clinical |
| Bone Healing | ↑ Osteogenesis, ↑ mineralization | Osteoblast proliferation, VEGF activation | Preclinical |
| Warnings | Fast-release H2S may exacerbate inflammation | Dose- and release- dependent effects on immune cells | Mechanistic studies |
| Condition | Main Effects | Mechanisms | Evidence |
|---|---|---|---|
| Allergic & non-allergic rhinitis | ↓ Nasal congestion, ↓ sneezing, ↓ IL-5 and local Ig E | Inhibits Th2 cytokines (IL-5), ↓ IgE; improves epithelial barrier & mucociliary clearance | RCTs + clinical studies |
| Chronic pharyngitis/laryngitis | ↓ Inflammation, ↓ dysphonia, ↑ epithelial regeneration | Mucoregulatory, antiseptic, and epithelial-regenerating action | Observational + spa studies |
| Chronic bronchitis/mild-to- moderate COPD | ↑ FEV1, ↓ sputum, ↑ exercise tolerance, ↓ oxidants in exhaled air | ↓ ROS; activates Nrf2/HO-1; modulates microbiota & local cytokines | RCTs + systematic review |
| Mild persistent/intermittent asthma | ↓ Bronchial hyperreactivity, ↓ inflammation, ↑ lung function | Th2 modulation; ↓ eosinophils; ↓ IL-4/IL-13; activates K+ channels & Nrf2 | Preclinical + observational spa studies |
| Subacute/mild chronic sinusitis | ↑ Mucociliary clearance, ↓ mucus secretion, ↓ inflammation | Improves ciliary transport; ↓ MUC5AC; ↓ microbial biofilm | Observational + physiopathological basis |
| Vasomotor/non-allergic rhinitis | ↓ Rhinorrhea, ↑ vascular tone, ↑ ciliary transport | Regulation of neurovegetative tone and nasal secretion | Small clinical studies |
| Condition | Main Effects | Mechanisms | Evidence |
|---|---|---|---|
| Vascular tone regulation (NO interaction) | Vasodilation and reduced vascular resistance | K_ATP channel activation → hyperpolarization; ↑ eNOS; nitrosopersulfide formation | Observational + pathophysiological basis |
| Ischemia–reperfusion injury | Mitochondrial protection and antioxidant activity | ↑ Nrf2; ↑ HO-1/NQO1; ↓ ROS; persulfidation of CypD | Preclinical + balneary observational |
| Myocardial repair | Increased contractility and angiogenesis | ↑ VEGF expression; myocardial angiogenesis | Observational + balneary studies |
| Atherosclerosis and inflammation | Reduced inflammation and LDL oxidation | ↓ TNF-α, IL-1β, IL-6, ICAM-1, VCAM-1; ↓ VSMC proliferation | RCT + clinical studies |
| Blood pressure control | Baseline hypertension in CSE−/− models | Loss of CSE → ↓ H2S → ↑ BP; ↓ endothelial relaxation | Preclinical + balneary observational |
| Endothelial epigenetic longevity | Reduced NF-κB activity and delayed vascular aging | S-sulfhydration of p65 NF-κB → ↓ transcriptional activity | Small clinical studies |
| Condition | Main Effects | Mechanisms | Evidence |
|---|---|---|---|
| Uncomplicated chronic gastritis | ↓ epigastric pain- better gastric tolerance | Local anti-inflammatory effect via H2S (↓ NF-κB,↑ Nrf2/SIRT1), mucin stimulation, ↑ mucosal blood flow | Observational clinical studies + solid preclinical |
| Peptic ulcer (adjuvant) | Faster healing, ↓ recurrence | ↑ prostaglandins and NO, angiogenesis Activation, ↓ oxidative stress, epithelial repair via H2S signaling | Robust preclinical + clinical series |
| Functional dyspepsia | ↓ Heartburn, ↓ postprandial fullness | Motility modulation, protection of epithelial tight junctions, local antioxidant action | Small trials + observational |
| Functional constipation | Improved intestinal transit | Stimulation of colonic motility via K_ATP channels and smooth-muscle activation by H2S | Preclinical + clinical experience |
| General liver function | Improvement of liver markers (↓ ALT, AST),support for detoxification processes | Activation of endogenous antioxidants (↑ SOD, GPx), reduction of hepatic inflammation and fibrosis via H2S | Preclinical studies + observational |
| Mild/moderate NAFLD (adjuvant) | ↓ transaminases- slight ultrasound improvement | Systemic antioxidant effect- improved lipid metabolism and insulin sensitivity via H2S signaling | Animal studies + human observational |
| Metabolic syndrome/Diabetes | ↓ fasting and postprandial glucose- improved insulin sensitivity↓ triglycerides | Activation of redox and metabolic pathways (↑ AMPK), modulation of systemic inflammation | Preclinical + pilot studies |
| Hyperuricemia (secondary prevention) | ↓ uricemia, ↓ attacks | ↑ renal urate excretion via vasodilatory and natriuretic action of H2S | Observational; high plausibility |
| Uric acid urolithiasis | ↓ recurrence | Urinary alkalinization by suitable mineral composition- renal vasodilation mediated by H2S | Observational |
| Characteristics | Sulfurous Peloids | Sulfurous Baths |
|---|---|---|
| Release profile | Slow and sustained | Rapid and immediate |
| Local concentration | High | More uniform distribution |
| Duration of effect | Prolonged | Limited |
| Depth of thermal action | High | Moderate |
| Application temperature | 38–46 °C | 34–38 °C |
| Additional mineral supply | Water and peloid | Water |
| Scope of action | Localized | Generalized |
| Organization | Value Type | Notes | Limit (ppm) |
|---|---|---|---|
| OSHA (Washington, DC, USA) | PEL–TWA | Up to 50 ppm allowed if it does not exceed 10 min and never exceeds 20 ppm on average. | 20 ppm (ceiling) |
| NIOSH (Washington, DC, USA) | REL–TWA | Recommended limit (8 h/day, 40 h/week). | 10 ppm |
| NIOSH (Washington, DC, USA) | REL–STEL | Maximum permitted exposure for 15 min. | 15 ppm |
| NIOSH (Washington, DC, USA) | IDLH | Immediately dangerous to life or health. | 100 ppm |
| ACGIH (Sharonville, OH, USA) | TLV–TWA | Threshold limit value, 8 h time-weighted average. Revised in 2010. | 1 ppm |
| ACGIH (Sharonville, OH, USA) | TLV–STEL | Short-term exposure limit (15 min). | 5 ppm |
| EU (Brussels, Belgium) | VLEP–TWA | Occupational exposure limit value (8 h). | 5 ppm (7 mg/m3) |
| EU (Brussels, Belgium) | VLEP–STEL | Short-term exposure limit value (15 min). | 10 ppm (14 mg/m3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbajo, J.M.; Maraver, F.; Vela, L.; Munteanu, C. Hydrogen Sulfide in Balneology: Physiology, Evidence, and Clinical Translation. Int. J. Mol. Sci. 2025, 26, 10790. https://doi.org/10.3390/ijms262110790
Carbajo JM, Maraver F, Vela L, Munteanu C. Hydrogen Sulfide in Balneology: Physiology, Evidence, and Clinical Translation. International Journal of Molecular Sciences. 2025; 26(21):10790. https://doi.org/10.3390/ijms262110790
Chicago/Turabian StyleCarbajo, Jose Manuel, Francisco Maraver, Lorena Vela, and Constantin Munteanu. 2025. "Hydrogen Sulfide in Balneology: Physiology, Evidence, and Clinical Translation" International Journal of Molecular Sciences 26, no. 21: 10790. https://doi.org/10.3390/ijms262110790
APA StyleCarbajo, J. M., Maraver, F., Vela, L., & Munteanu, C. (2025). Hydrogen Sulfide in Balneology: Physiology, Evidence, and Clinical Translation. International Journal of Molecular Sciences, 26(21), 10790. https://doi.org/10.3390/ijms262110790









