Systematic Review: Targeted Molecular Imaging of Angiogenesis and Its Mediators in Rheumatoid Arthritis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Source and Search Strategy
2.3. Study Selection
2.4. Data Extraction and Assessment
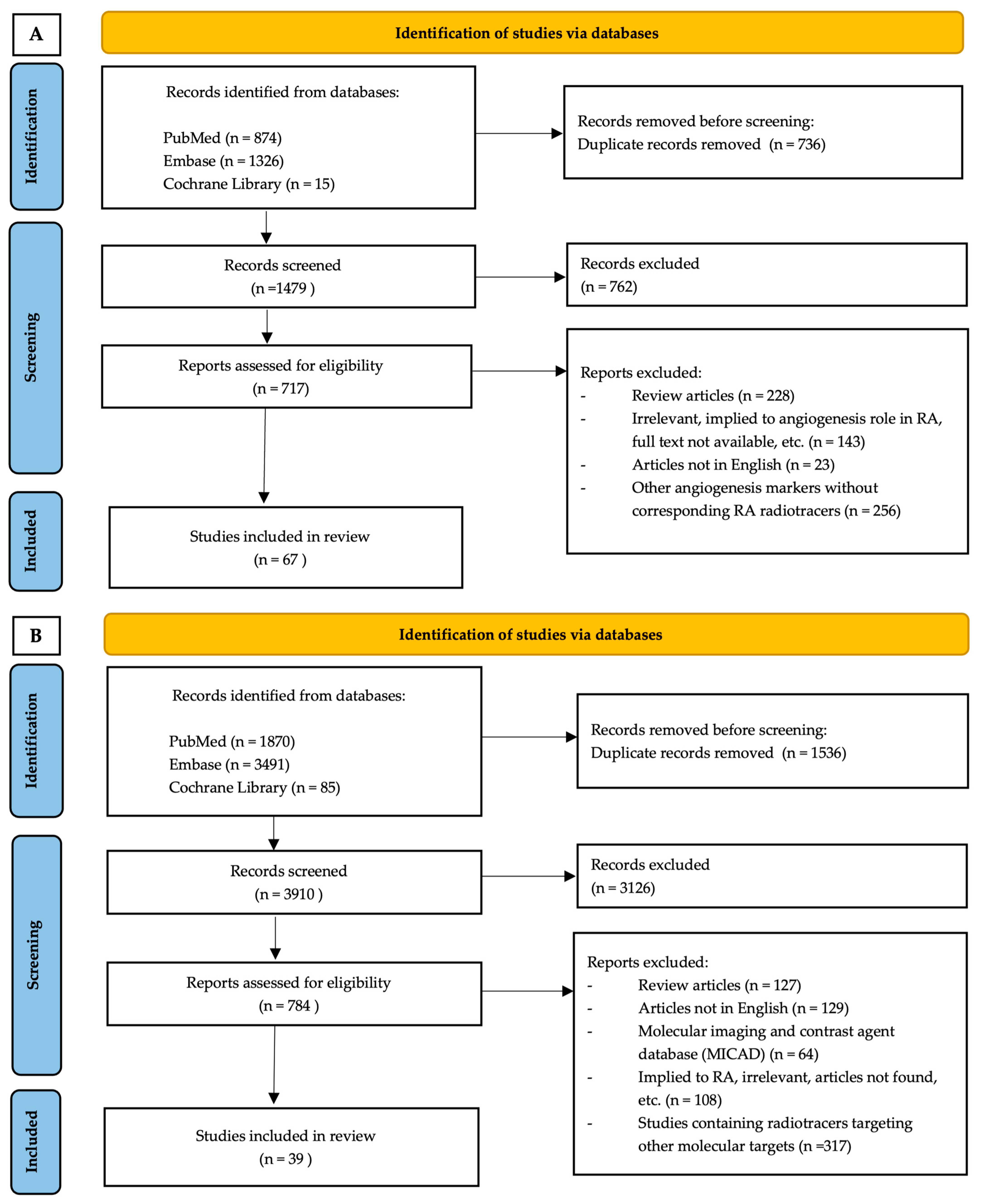
2.5. Search Results
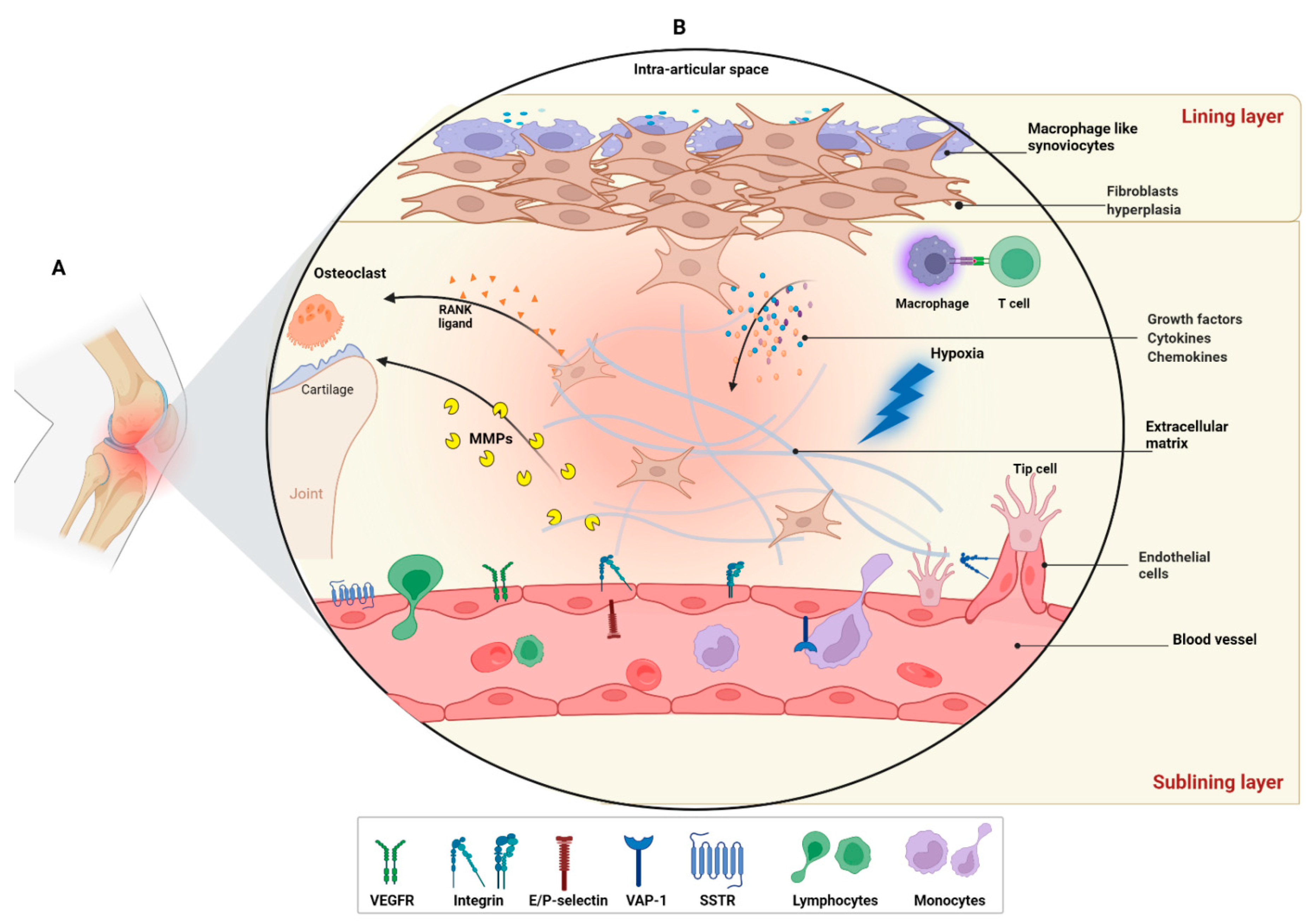
3. Results and Discussion
- Results
- Radiotracers targeting angiogenesis in RA
3.1. Environmental Factor (Hypoxia)
3.2. Somatostatin Receptors—Somatostatin
3.3. Matrix Metalloproteases
MMP-2/-9/-13
3.4. Extracellular Matrix Proteins
Extra Domain A (ED-A)
3.5. Adhesion Molecules
3.5.1. VAP-1
3.5.2. E-Selectin
3.5.3. Integrins
3.6. Cyclooxygenase Enzymes
3.7. Cytokines
3.8. Other
3.8.1. Microvascular Endothelium
3.8.2. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ang1/2 | Angiopoietin 1/2 |
| A-SSA | Acute-phase serum amyloid A |
| CAM | Cell adhesion molecules |
| CIA | Collagen-induced arthritis |
| COX | Cyclooxygenases |
| CT | Computed tomography |
| Cu-ATSM | Copper(II) diacetyl-di(N4-methylthiosemicarbazone) |
| DMARD | Disease-modifying anti-rheumatic drug |
| DOTA | 2,2′,2″,2‴-(1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid OR tetraxetan |
| ECM | Extracellular matrix |
| ED-A/-B | Extra domain of fibronectin-A/-B |
| FAP | Fibroblast activation protein |
| FAZA | Fluoroazomycinarabinoside |
| FDG | Fluorodeoxyglucose |
| FLS | Fibroblast-like synoviocyte |
| FMISO | Fluoromisonidazole) |
| HDP | Hydroxydiphosphonate |
| HIG | Human immunoglobulin |
| HUVEC | Human umbilical vein endothelial cell |
| HYNIC | Hydrazinonicotinamide |
| IAZA | 1-(α-D-5-Iodoarabinofuranosyl)-2-nitroimidazole) |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| JAK | Janus kinase |
| mAb | Monoclonal antibody |
| MAPK | Mitogen-activated protein kinase |
| MDP | Methyl diphosphonate |
| MMP | Matrix metalloproteinases |
| MRI | Magnetic resonance imaging |
| MT-MMPs | Membrane-type matrix metalloproteinases |
| NOTA | 1,4,7-Triazacyclononane-1,4,7-triacetic acid |
| OA | Osteoarthritis |
| GPI | Glucose-6-phosphate isomerase |
| PDUS | Power Doppler ultra-sonography |
| PEG | Poly(ethylene glycol) |
| PET | Positron emission tomography |
| PGE | Prostaglandin E |
| PI3K | Phosphoinositide 3-kinases |
| RA | Rheumatoid arthritis |
| RGD | Arginylglycylaspartic acid |
| SCID | Severe combined immunodeficiency disease |
| SPECT | Photon emission computed tomography |
| SRC | SRC proto-oncogene, non-receptor tyrosine kinase |
| SST | Somatostatin |
| STAT | Signal transducer and activator of transcription |
| TNF | Tumor necrosis factor |
| TRAP | Triazacyclononane-triphosphinate |
| uPAR | Urokinase plasminogen activator receptor |
| VAP-1 | Vascular adhesion protein |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Leblond, A.; Allanore, Y.; Avouac, J. Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmun. Rev. 2017, 16, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, K.W.; Kim, B.M.; Cho, M.L.; Lee, S.H. The effect of vascular endothelial growth factor on osteoclastogenesis in rheumatoid arthritis. PLoS ONE 2015, 10, e0124909. [Google Scholar] [CrossRef] [PubMed]
- Orr, C.; Vieira-Sousa, E.; Boyle, D.L.; Buch, M.H.; Buckley, C.D.; Canete, J.D.; Catrina, A.I.; Choy, E.H.S.; Emery, P.; Fearon, U.; et al. Synovial tissue research: A state-of-the-art review. Nat. Rev. Rheumatol. 2017, 13, 463–475. [Google Scholar] [CrossRef]
- Haubner, R.; Beer, A.J.; Wang, H.; Chen, X. Positron emission tomography tracers for imaging angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 86–103. [Google Scholar] [CrossRef]
- Tas, S.W.; Maracle, C.X.; Balogh, E.; Szekanecz, Z. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat. Rev. Rheumatol. 2016, 12, 111–122. [Google Scholar] [CrossRef]
- Vailhe, B.; Vittet, D.; Feige, J.J. In vitro models of vasculogenesis and angiogenesis. Lab. Investig. 2001, 81, 439–452. [Google Scholar] [CrossRef]
- Patan, S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004, 117, 3–32. [Google Scholar]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Deng, R. Angiogenesis as a potential treatment strategy for rheumatoid arthritis. Eur. J. Pharmacol. 2021, 910, 174500. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Shyni, G.L.; Raghu, K.G. Current and novel therapeutic targets in the treatment of rheumatoid arthritis. Inflammopharmacology 2020, 28, 1457–1476. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Molema, G. Angiogenesis: Potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 2000, 52, 237–268. [Google Scholar] [PubMed]
- Chen, Q.; Fan, K.; Chen, X.; Xie, X.; Huang, L.; Song, G.; Qi, W. Ezrin regulates synovial angiogenesis in rheumatoid arthritis through YAP and Akt signalling. J. Cell Mol. Med. 2021, 25, 9378–9389. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Mottaghy, F.M.; Bauwens, M. Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Status. Int J. Mol. Sci 2021, 22, 5544. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Besenyei, T.; Paragh, G.; Koch, A.E. Angiogenesis in rheumatoid arthritis. Autoimmunity 2009, 42, 563–573. [Google Scholar] [CrossRef]
- Peluzzo, A.M.; Autieri, M.V. Challenging the Paradigm: Anti-Inflammatory Interleukins and Angiogenesis. Cells 2022, 11, 587. [Google Scholar] [CrossRef]
- Clavel, G.; Bessis, N.; Lemeiter, D.; Fardellone, P.; Mejjad, O.; Menard, J.F.; Pouplin, S.; Boumier, P.; Vittecoq, O.; Le Loet, X.; et al. Angiogenesis markers (VEGF, soluble receptor of VEGF and angiopoietin-1) in very early arthritis and their association with inflammation and joint destruction. Clin. Immunol. 2007, 124, 158–164. [Google Scholar] [CrossRef]
- Lee, S.S.; Joo, Y.S.; Kim, W.U.; Min, D.J.; Min, J.K.; Park, S.H.; Cho, C.S.; Kim, H.Y. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2001, 19, 321–324. [Google Scholar]
- Ozgonenel, L.; Cetin, E.; Tutun, S.; Tonbaklar, P.; Aral, H.; Guvenen, G. The relation of serum vascular endothelial growth factor level with disease duration and activity in patients with rheumatoid arthritis. Clin. Rheumatol. 2010, 29, 473–477. [Google Scholar] [CrossRef]
- Harada, M.; Mitsuyama, K.; Yoshida, H.; Sakisaka, S.; Taniguchi, E.; Kawaguchi, T.; Ariyoshi, M.; Saiki, T.; Sakamoto, M.; Nagata, K.; et al. Vascular endothelial growth factor in patients with rheumatoid arthritis. Scand. J. Rheumatol. 1998, 27, 377–380. [Google Scholar] [PubMed]
- Sone, H.; Sakauchi, M.; Takahashi, A.; Suzuki, H.; Inoue, N.; Iida, K.; Shimano, H.; Toyoshima, H.; Kawakami, Y.; Okuda, Y.; et al. Elevated levels of vascular endothelial growth factor in the sera of patients with rheumatoid arthritis correlation with disease activity. Life Sci. 2001, 69, 1861–1869. [Google Scholar] [CrossRef]
- Leblond, A.; Pezet, S.; Trouvin, A.P.; Elhai, M.; Gonzalez, V.; Allanore, Y.; Avouac, J. Linking systemic angiogenic markers to synovial vascularization in rheumatoid arthritis. PLoS ONE 2018, 13, e0203607. [Google Scholar] [CrossRef]
- Kim, J.W.; Kong, J.S.; Lee, S.; Yoo, S.A.; Koh, J.H.; Jin, J.; Kim, W.U. Angiogenic cytokines can reflect the synovitis severity and treatment response to biologics in rheumatoid arthritis. Exp. Mol. Med. 2020, 52, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Wauke, K.; Hirano, D.; Ishigami, S.; Aono, H.; Takai, M.; Sasano, M.; Yoshino, S. Effects of combinations of anti-rheumatic drugs on the production of vascular endothelial growth factor and basic fibroblast growth factor in cultured synoviocytes and patients with rheumatoid arthritis. Rheumatology 2000, 39, 1255–1262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paleolog, E.M.; Young, S.; Stark, A.C.; McCloskey, R.V.; Feldmann, M.; Maini, R.N. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998, 41, 1258–1265. [Google Scholar] [CrossRef]
- Nagashima, M.; Yoshino, S.; Aono, H.; Takai, M.; Sasano, M. Inhibitory effects of anti-rheumatic drugs on vascular endothelial growth factor in cultured rheumatoid synovial cells. Clin. Exp. Immunol. 1999, 116, 360–365. [Google Scholar] [CrossRef]
- Macias, I.; Garcia-Perez, S.; Ruiz-Tudela, M.; Medina, F.; Chozas, N.; Giron-Gonzalez, J.A. Modification of pro- and antiinflammatory cytokines and vascular-related molecules by tumor necrosis factor-a blockade in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 2102–2108. [Google Scholar]
- Umemura, M.; Isozaki, T.; Ishii, S.; Seki, S.; Oguro, N.; Miura, Y.; Miwa, Y.; Nakamura, M.; Inagaki, K.; Kasama, T. Reduction of Serum ADAM17 Level Accompanied with Decreased Cytokines after Abatacept Therapy in Patients with Rheumatoid Arthritis. Int. J. Biomed. Sci. 2014, 10, 229–235. [Google Scholar]
- Hirohata, S.; Abe, A.; Murasawa, A.; Kanamono, T.; Tomita, T.; Yoshikawa, H. Differential effects of IL-6 blockade tocilizumab and TNF inhibitors on angiogenesis in synovial tissues from patients with rheumatoid arthritis. Mod. Rheumatol. 2017, 27, 766–772. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Ruscitti, P.; Berardicurti, O.; Panzera, N.; Grazia, N.; Di Vito Nolfi, M.; Di Francesco, B.; Navarini, L.; Maurizi, A.; Rucci, N.; et al. Blocking Jak/STAT signalling using tofacitinib inhibits angiogenesis in experimental arthritis. Arthritis Res. Ther. 2021, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Zisman, D.; Safieh, M.; Simanovich, E.; Feld, J.; Kinarty, A.; Zisman, L.; Gazitt, T.; Haddad, A.; Elias, M.; Rosner, I.; et al. Tocilizumab (TCZ) Decreases Angiogenesis in Rheumatoid Arthritis Through Its Regulatory Effect on miR-146a-5p and EMMPRIN/CD147. Front. Immunol. 2021, 12, 739592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, F.; Gao, W. Tripterygium wilfordii Inhibiting Angiogenesis for Rheumatoid Arthritis Treatment. J. Natl. Med. Assoc. 2017, 109, 142–148. [Google Scholar] [CrossRef]
- Bainbridge, J.; Sivakumar, B.; Paleolog, E. Angiogenesis as a therapeutic target in arthritis: Lessons from oncology. Curr. Pharm. Des. 2006, 12, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.K.; Conaghan, P.G. Imaging in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Haupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Kgoebane, K.; Ally, M.; Duim-Beytell, M.C.; Suleman, F.E. The role of imaging in rheumatoid arthritis. SA J. Radiol. 2018, 22, 1316. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Gassert, F.T.; Steiger, K.; Sommer, P.; Weichert, W.; Rummeny, E.J.; Schwaiger, M.; Kessler, H.; Meier, R.; Kimm, M.A. In vivo imaging of early stages of rheumatoid arthritis by alpha5beta1-integrin-targeted positron emission tomography. EJNMMI Res. 2019, 9, 87. [Google Scholar] [CrossRef]
- Rosado-de-Castro, P.H.; Lopes de Souza, S.A.; Alexandre, D.; Barbosa da Fonseca, L.M.; Gutfilen, B. Rheumatoid arthritis: Nuclear Medicine state-of-the-art imaging. World J. Orthop. 2014, 5, 312. [Google Scholar] [CrossRef]
- van der Meulen, N.P.; Strobel, K.; Lima, T.V.M. New Radionuclides and Technological Advances in SPECT and PET Scanners. Cancers 2021, 13, 6183. [Google Scholar] [CrossRef] [PubMed]
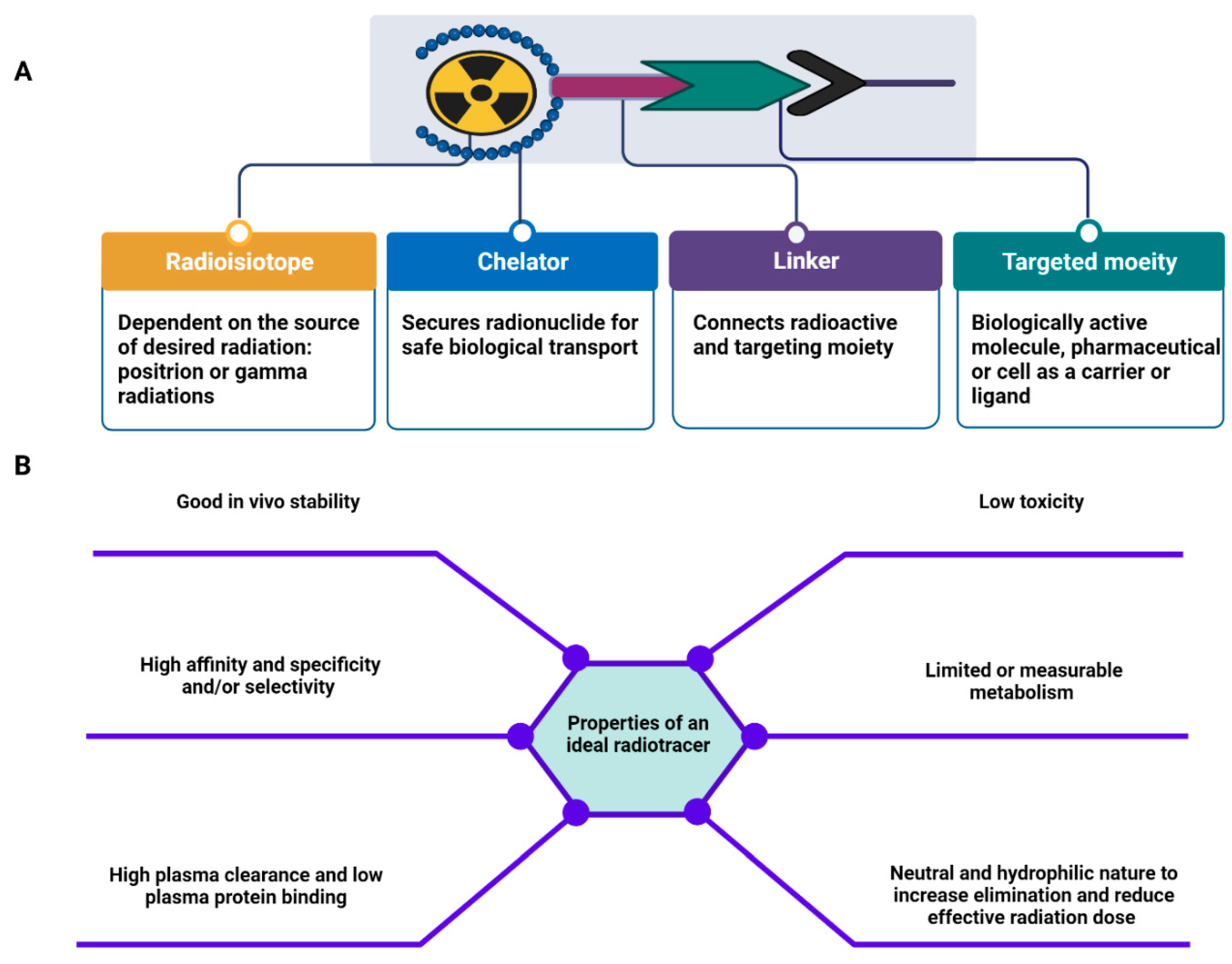
- Stacy, M.R.; Maxfield, M.W.; Sinusas, A.J. Targeted molecular imaging of angiogenesis in PET and SPECT: A review. Yale J. Biol. Med. 2012, 85, 75–86. [Google Scholar] [PubMed]
- Shaw, R.C.; Tamagnan, G.D.; Tavares, A.A.S. Rapidly (and Successfully) Translating Novel Brain Radiotracers From Animal Research Into Clinical Use. Front. Neurosci. 2020, 14, 871. [Google Scholar] [CrossRef]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Chen, F.; Zhang, Y.; Cai, W. New radiotracers for imaging of vascular targets in angiogenesis-related diseases. Adv. Drug Deliv. Rev. 2014, 76, 2–20. [Google Scholar] [CrossRef]
- Okoye, N.C.; Baumeister, J.E.; Khosroshahi, F.N.; Hennkens, H.M.; Jurisson, S.S. Chelators and metal complex stability for radiopharmaceutical applications. Radiochim. Acta 2019, 107, 1087–1120. [Google Scholar] [CrossRef]
- Evans, B.J.; King, A.T.; Katsifis, A.; Matesic, L.; Jamie, J.F. Methods to Enhance the Metabolic Stability of Peptide-Based PET Radiopharmaceuticals. Molecules 2020, 25, 2314. [Google Scholar] [CrossRef]
- Sharma, R.; Aboagye, E. Development of radiotracers for oncology--the interface with pharmacology. Br. J. Pharmacol. 2011, 163, 1565–1585. [Google Scholar] [CrossRef]
- Gotthardt, M.; Bleeker-Rovers, C.P.; Boerman, O.C.; Oyen, W.J. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J. Nucl. Med. Technol. 2013, 41, 157–169. [Google Scholar] [CrossRef]
- Nadig, V.; Herrmann, K.; Mottaghy, F.M.; Schulz, V. Hybrid total-body pet scanners-current status and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Bowden, D.J.; Barrett, T. Angiogenesis imaging in neoplasia. J. Clin. Imaging Sci. 2011, 1, 38. [Google Scholar] [CrossRef] [PubMed]
- Niccoli Asabella, A.; Di Palo, A.; Altini, C.; Ferrari, C.; Rubini, G. Multimodality Imaging in Tumor Angiogenesis: Present Status and Perspectives. Int J. Mol. Sci. 2017, 18, 1864. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical Imaging Modalities: Principles and Applications in Preclinical Research and Clinical Settings. J. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Gambhir, S.S.; Willmann, J.K. Molecular imaging: Current status and emerging strategies. Clin. Radiol. 2010, 65, 500–516. [Google Scholar] [CrossRef]
- Kubota, K.; Yamashita, H.; Mimori, A. Clinical Value of FDG-PET/CT for the Evaluation of Rheumatic Diseases: Rheumatoid Arthritis, Polymyalgia Rheumatica, and Relapsing Polychondritis. Semin. Nucl. Med. 2017, 47, 408–424. [Google Scholar] [CrossRef]
- Narayan, N.; Owen, D.R.; Taylor, P.C. Advances in positron emission tomography for the imaging of rheumatoid arthritis. Rheumatology 2017, 56, 1837–1846. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Guenthoer, P.; Fuchs, K.; Reischl, G.; Quintanilla-Martinez, L.; Gonzalez-Menendez, I.; Laufer, S.; Pichler, B.J.; Kneilling, M. Evaluation of the therapeutic potential of the selective p38 MAPK inhibitor Skepinone-L and the dual p38/JNK 3 inhibitor LN 950 in experimental K/BxN serum transfer arthritis. Inflammopharmacology 2019, 27, 1217–1227. [Google Scholar] [CrossRef]
- Fuchs, K.; Kuehn, A.; Mahling, M.; Guenthoer, P.; Hector, A.; Schwenck, J.; Hartl, D.; Laufer, S.; Kohlhofer, U.; Quintanilla-Martinez, L.; et al. In Vivo Hypoxia PET Imaging Quantifies the Severity of Arthritic Joint Inflammation in Line with Overexpression of Hypoxia-Inducible Factor and Enhanced Reactive Oxygen Species Generation. J. Nucl. Med. 2017, 58, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.T.; Hanaoka, H.; Nakajima, T.; Yamaguchi, A.; Okamura, K.; Chikuda, H.; Tsushima, Y. (64)Cu-ATSM and (99m)Tc(CO)3-DCM20 potential in the early detection of rheumatoid arthritis. Mod. Rheumatol. 2021, 31, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Wiebe, L.I.; Atrazheva, E.; Tandon, M. An improved synthesis of a-AZA, a-AZP and a-AZG, the precursors to clinical markers of tissue hypoxia. Tetrahedron Lett. 2001, 42, 2077–2088. [Google Scholar] [CrossRef]
- Wiebe, I.L.; McEwan, A.J. Scintigraphic imaging of focal hypoxic tissue: Development and clinical applications of 123IAZA. Braz. Arch. Biol. Techology 2002, 45, 69–81. [Google Scholar] [CrossRef]
- Shamim, S.A.; Arora, G.; Kumar, N.; Behera, A.; Hussain, J.; Gupta, R.; Kumar, R.; Bal, C. Comparison of 99mTc-methyl diphosphonate bone scintigraphy and 68Ga-DOTANOC PET/computed tomography in articular manifestation of rheumatoid arthritis. Nucl. Med. Commun. 2022, 43, 428–432. [Google Scholar] [CrossRef]
- Anzola-Fuentes, L.K.; Chianelli, M.; Galli, F.; Glaudemans, A.W.; Martin Martin, L.; Todino, V.; Migliore, A.; Signore, A. Somatostatin receptor scintigraphy in patients with rheumatoid arthritis and secondary Sjogren’s syndrome treated with Infliximab: A pilot study. EJNMMI Res. 2016, 6, 49. [Google Scholar] [CrossRef][Green Version]
- Vanhagen, P.M.; Markusse, H.M.; Lamberts, S.W.; Kwekkeboom, D.J.; Reubi, J.C.; Krenning, E.P. Somatostatin receptor imaging. The presence of somatostatin receptors in rheumatoid arthritis. Arthritis Rheum. 1994, 37, 1521–1527. [Google Scholar] [CrossRef]
- Papathanasiou, N.D.; Rondogianni, P.E.; Pianou, N.K.; Karampina, P.A.; Vlontzou, E.A.; Datseris, I.E. 99mTc-depreotide in the evaluation of bone infection and inflammation. Nucl. Med. Commun. 2008, 29, 239–246. [Google Scholar] [CrossRef]
- Schrigten, D.; Breyholz, H.J.; Wagner, S.; Hermann, S.; Schober, O.; Schafers, M.; Haufe, G.; Kopka, K. A new generation of radiofluorinated pyrimidine-2,4,6-triones as MMP-targeted radiotracers for positron emission tomography. J. Med. Chem. 2012, 55, 223–232. [Google Scholar] [CrossRef]
- Hugenberg, V.; Wagner, S.; Kopka, K.; Schafers, M.; Schuit, R.C.; Windhorst, A.D.; Hermann, S. Radiolabeled Selective Matrix Metalloproteinase 13 (MMP-13) Inhibitors: (Radio)Syntheses and in Vitro and First in Vivo Evaluation. J. Med. Chem. 2017, 60, 307–321. [Google Scholar] [CrossRef]
- Rangasamy, L.; Geronimo, B.D.; Ortin, I.; Coderch, C.; Zapico, J.M.; Ramos, A.; de Pascual-Teresa, B. Molecular Imaging Probes Based on Matrix Metalloproteinase Inhibitors (MMPIs). Molecules 2019, 24, 2982. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, S.T.G.; Chandrupatla, D.; Giovanonni, L.; Neri, D.; Vugts, D.J.; Huisman, M.C.; Hoekstra, O.S.; Musters, R.J.P.; Lammertsma, A.A.; van Dongen, G.; et al. F8-IL10: A New Potential Antirheumatic Drug Evaluated by a PET-Guided Translational Approach. Mol. Pharm. 2019, 16, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Viitanen, R.; Moisio, O.; Lankinen, P.; Li, X.G.; Koivumaki, M.; Suilamo, S.; Tolvanen, T.; Taimen, K.; Mali, M.; Kohonen, I.; et al. First-in-Humans Study of (68)Ga-DOTA-Siglec-9, a PET Ligand Targeting Vascular Adhesion Protein 1. J. Nucl. Med. 2021, 62, 577–583. [Google Scholar] [CrossRef]
- Virtanen, H.; Autio, A.; Siitonen, R.; Liljenback, H.; Saanijoki, T.; Lankinen, P.; Makila, J.; Kakela, M.; Teuho, J.; Savisto, N.; et al. 68Ga-DOTA-Siglec-9--a new imaging tool to detect synovitis. Arthritis Res. Ther. 2015, 17, 308. [Google Scholar] [CrossRef] [PubMed]
- Autio, A.; Vainio, P.J.; Suilamo, S.; Mali, A.; Vainio, J.; Saanijoki, T.; Noponen, T.; Ahtinen, H.; Luoto, P.; Teras, M.; et al. Preclinical evaluation of a radioiodinated fully human antibody for in vivo imaging of vascular adhesion protein-1-positive vasculature in inflammation. J. Nucl. Med. 2013, 54, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Garrood, T.; Blades, M.; Haskard, D.O.; Mather, S.; Pitzalis, C. A novel model for the pre-clinical imaging of inflamed human synovial vasculature. Rheumatology 2009, 48, 926–931. [Google Scholar] [CrossRef]
- Jamar, F.; Houssiau, F.A.; Devogelaer, J.P.; Chapman, P.T.; Haskard, D.O.; Beaujean, V.; Beckers, C.; Manicourt, D.H.; Peters, A.M. Scintigraphy using a technetium 99m-labelled anti-E-selectin Fab fragment in rheumatoid arthritis. Rheumatology 2002, 41, 53–61. [Google Scholar] [CrossRef]
- Jamar, F.; Chapman, P.T.; Manicourt, D.H.; Glass, D.M.; Haskard, D.O.; Peters, A.M. A comparison between 111In-anti-E-selectin mAb and 99Tcm-labelled human non-specific immunoglobulin in radionuclide imaging of rheumatoid arthritis. Br. J. Radiol. 1997, 70, 473–481. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, Y.; Zheng, K.; Li, F.; Chen, X.; Zhang, F.; Zhang, X. Evaluation of synovial angiogenesis in patients with rheumatoid arthritis using (6)(8)Ga-PRGD2 PET/CT: A prospective proof-of-concept cohort study. Ann. Rheum. Dis. 2014, 73, 1269–1272. [Google Scholar] [CrossRef]
- Imberti, C.; Terry, S.Y.; Cullinane, C.; Clarke, F.; Cornish, G.H.; Ramakrishnan, N.K.; Roselt, P.; Cope, A.P.; Hicks, R.J.; Blower, P.J.; et al. Enhancing PET Signal at Target Tissue in Vivo: Dendritic and Multimeric Tris(hydroxypyridinone) Conjugates for Molecular Imaging of alphavbeta3 Integrin Expression with Gallium-68. Bioconjug. Chem. 2017, 28, 481–495. [Google Scholar] [CrossRef]
- Kneilling, M.; Hultner, L.; Pichler, B.J.; Mailhammer, R.; Morawietz, L.; Solomon, S.; Eichner, M.; Sabatino, J.; Biedermann, T.; Krenn, V.; et al. Targeted mast cell silencing protects against joint destruction and angiogenesis in experimental arthritis in mice. Arthritis Rheum. 2007, 56, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.; Nicod Lalonde, M.; Omoumi, P.; Testart Dardel, N.; Hügle, T.; Prior, J.O. Imaging of ανβ3 integrin expression in rheumatoid arthritis with [68Ga]Ga-NODAGA-RGDyk PET/CT in comparison to [18F]FDG PET/CT. Med. Nucl. 2021, 45, 293–295. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, Q.; Miao, W. Study of novel molecular probe 99mTc-3PRGD2 in the diagnosis of rheumatoid arthritis. Nucl. Med. Commun. 2015, 36, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, G.; Wang, X.; Zhao, Z.; Wang, T.; Wang, X.; Li, X.F. Early detection of rheumatoid arthritis in rats and humans with 99mTc-3PRGD2 scintigraphy: Imaging synovial neoangiogenesis. Oncotarget 2017, 8, 5753–5760. [Google Scholar] [CrossRef]
- Attipoe, L.; Chaabo, K.; Wajed, J.; Hassan, F.U.; Shivapatham, D.; Morrison, M.; Ballinger, J.; Cook, G.; Cope, A.P.; Garrood, T. Imaging neoangiogenesis in rheumatoid arthritis (INIRA): Whole-body synovial uptake of a (99m)Tc-labelled RGD peptide is highly correlated with power Doppler ultrasound. Ann. Rheum. Dis. 2020, 79, 1254–1255. [Google Scholar] [CrossRef]
- Terry, S.Y.; Koenders, M.I.; Franssen, G.M.; Nayak, T.K.; Freimoser-Grundschober, A.; Klein, C.; Oyen, W.J.; Boerman, O.C.; Laverman, P. Monitoring Therapy Response of Experimental Arthritis with Radiolabeled Tracers Targeting Fibroblasts, Macrophages, or Integrin alphavbeta3. J. Nucl. Med. 2016, 57, 467–472. [Google Scholar] [CrossRef]
- Wang, Y.; Da, G.; Li, H.; Zheng, Y. Avastin exhibits therapeutic effects on collagen-induced arthritis in rat model. Inflammation 2013, 36, 1460–1467. [Google Scholar] [CrossRef]
- Hungnes, I.N.; Al-Salemee, F.; Gawne, P.J.; Eykyn, T.; Atkinson, R.A.; Terry, S.Y.A.; Clarke, F.; Blower, P.J.; Pringle, P.G.; Ma, M.T. One-step, kit-based radiopharmaceuticals for molecular SPECT imaging: A versatile diphosphine chelator for (99m)Tc radiolabelling of peptides. Dalton Trans. 2021, 50, 16156–16165. [Google Scholar] [CrossRef]
- Kikuchi, T.; Okada, M.; Nengaki, N.; Furutsuka, K.; Wakizaka, H.; Okamura, T.; Zhang, M.R.; Kato, K. Efficient synthesis and chiral separation of 11C-labeled ibuprofen assisted by DMSO for imaging of in vivo behavior of the individual isomers by positron emission tomography. Bioorg. Med. Chem. 2011, 19, 3265–3273. [Google Scholar] [CrossRef]
- Nozaki, S.; Ozaki, N.; Suzuki, S.; Goto, M.; Mawatari, A.; Nakatani, Y.; Hayashinaka, E.; Wada, Y.; Doi, H.; Watanabe, Y. Development of Diagnostic Techniques for Early Rheumatoid Arthritis Using Positron Emission Tomography with [(11)C]PK11195 and [(11)C]Ketoprofen Tracers. Mol. Imaging Biol. 2017, 19, 746–753. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, M.J.; Eldridge, M.; Lehmann, M.L.; Frankland, M.; Liow, J.S.; Yu, Z.X.; Cortes-Salva, M.; Telu, S.; Henter, I.D.; et al. PET measurement of cyclooxygenase-2 using a novel radioligand: Upregulation in primate neuroinflammation and first-in-human study. J. Neuroinflamm. 2020, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Beckford-Vera, D.R.; Gonzalez-Junca, A.; Janneck, J.S.; Huynh, T.L.; Blecha, J.E.; Seo, Y.; Li, X.; VanBrocklin, H.F.; Franc, B.L. PET/CT Imaging of Human TNFalpha Using [(89)Zr]Certolizumab Pegol in a Transgenic Preclinical Model of Rheumatoid Arthritis. Mol. Imaging Biol. 2020, 22, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Carron, P.; Lambert, B.; Van Praet, L.; De Vos, F.; Varkas, G.; Jans, L.; Elewaut, D.; Van den Bosch, F. Scintigraphic detection of TNF-driven inflammation by radiolabelled certolizumab pegol in patients with rheumatoid arthritis and spondyloarthritis. RMD Open 2016, 2, e000265. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Carron, P.; D’Asseler, Y.; Bacher, K.; Van den Bosch, F.; Elewaut, D.; Verbruggen, G.; Beyaert, R.; Dumolyn, C.; De Vos, F. (99m)Tc-labelled S-HYNIC certolizumab pegol in rheumatoid arthritis and spondyloarthritis patients: A biodistribution and dosimetry study. EJNMMI Res. 2016, 6, 88. [Google Scholar] [CrossRef]
- Alexandre, D.J.A.; Carmo, C.C.M.; Romeiro, L.D.; Gutfilen-Schlesinger, G.; Amarante, J.L., Jr.; de Souza, S.A.L.; Gutfilen, B. 99mTc-antitumor necrosis factor-alpha scintigraphy for the detection of inflammatory activity in rheumatoid arthritis. Nucl. Med. Commun. 2021, 42, 389–395. [Google Scholar] [CrossRef]
- Conti, F.; Malviya, G.; Ceccarelli, F.; Priori, R.; Iagnocco, A.; Valesini, G.; Signore, A. Role of scintigraphy with (9)(9)mTc-infliximab in predicting the response of intraarticular infliximab treatment in patients with refractory monoarthritis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1339–1347. [Google Scholar] [CrossRef]
- van der Laken, C.J.; Voskuyl, A.E.; Roos, J.C.; Stigter van Walsum, M.; de Groot, E.R.; Wolbink, G.; Dijkmans, B.A.; Aarden, L.A. Imaging and serum analysis of immune complex formation of radiolabelled infliximab and anti-infliximab in responders and non-responders to therapy for rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 253–256. [Google Scholar] [CrossRef]
- Wythe, S.E.; DiCara, D.; Taher, T.E.; Finucane, C.M.; Jones, R.; Bombardieri, M.; Man, Y.K.; Nissim, A.; Mather, S.J.; Chernajovsky, Y.; et al. Targeted delivery of cytokine therapy to rheumatoid tissue by a synovial targeting peptide. Ann. Rheum. Dis. 2013, 72, 129–135. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Maltezos, E.; Athanassou, N.; Papazoglou, D.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. Upregulated hypoxia inducible factor-1alpha and -2alpha pathway in rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2003, 5, R193. [Google Scholar] [CrossRef]
- Hitchon, C.; Wong, K.; Ma, G.; Reed, J.; Lyttle, D.; El-Gabalawy, H. Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum. 2002, 46, 2587–2597. [Google Scholar] [CrossRef]
- Wang, C.H.; Yao, H.; Chen, L.N.; Jia, J.F.; Wang, L.; Dai, J.Y.; Zheng, Z.H.; Chen, Z.N.; Zhu, P. CD147 induces angiogenesis through a vascular endothelial growth factor and hypoxia-inducible transcription factor 1 alpha-mediated pathway in rheumatoid arthritis. Arthritis Rheum. 2012, 64, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Westra, J.; Brouwer, E.; van Roosmalen, I.A.; Doornbos-van der Meer, B.; van Leeuwen, M.A.; Posthumus, M.D.; Kallenberg, C.G. Expression and regulation of HIF-1alpha in macrophages under inflammatory conditions; significant reduction of VEGF by CaMKII inhibitor. BMC Musculoskelet. Disord. 2010, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Westra, J.; Molema, G.; Kallenberg, C.G. Hypoxia-inducible factor-1 as regulator of angiogenesis in rheumatoid arthritis-therapeutic implications. Curr. Med. Chem. 2010, 17, 254–263. [Google Scholar] [CrossRef]
- Konisti, S.; Kiriakidis, S.; Paleolog, E.M. Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat. Rev. Rheumatol. 2012, 8, 153–162. [Google Scholar] [CrossRef]
- Liu, J.; Hajibeigi, A.; Ren, G.; Lin, M.; Siyambalapitiyage, W.; Liu, Z.; Simpson, E.; Parkey, R.W.; Sun, X.; Oz, O.K. Retention of the radiotracers 64Cu-ATSM and 64Cu-PTSM in human and murine tumors is influenced by MDR1 protein expression. J. Nucl. Med. 2009, 50, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.L.; Adams, I.P.; Lindow, S.W.; Zhong, W.; Atkin, S.L. Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium. Br. J. Cancer 2005, 92, 1493–1498. [Google Scholar] [CrossRef]
- Casnici, C.; Lattuada, D.; Crotta, K.; Truzzi, M.C.; Corradini, C.; Ingegnoli, F.; Tonna, N.; Bianco, F.; Marelli, O. Anti-inflammatory Effect of Somatostatin Analogue Octreotide on Rheumatoid Arthritis Synoviocytes. Inflammation 2018, 41, 1648–1660. [Google Scholar] [CrossRef]
- Pacilio, M.; Lauri, C.; Prosperi, D.; Petitti, A.; Signore, A. New SPECT and PET Radiopharmaceuticals for Imaging Inflammatory Diseases: A Meta-analysis of the Last 10 Years. Semin. Nucl. Med. 2018, 48, 261–276. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Markusse, H.M.; Krenning, E.P.; VanHagen, M.; Laissue, J.A. Vascular somatostatin receptors in synovium from patients with rheumatoid arthritis. Eur. J. Pharmacol. 1994, 271, 371–378. [Google Scholar] [CrossRef]
- Watson, J.C.; Balster, D.A.; Gebhardt, B.M.; O’Dorisio, T.M.; O’Dorisio, M.S.; Espenan, G.D.; Drouant, G.J.; Woltering, E.A. Growing vascular endothelial cells express somatostatin subtype 2 receptors. Br. J. Cancer 2001, 85, 266–272. [Google Scholar] [CrossRef]
- Danesi, R.; Agen, C.; Benelli, U.; Paolo, A.D.; Nardini, D.; Bocci, G.; Basolo, F.; Campagni, A.; Tacca, M.D. Inhibition of experimental angiogenesis by the somatostatin analogue octreotide acetate (SMS 201–995). Clin. Cancer Res. 1997, 3, 265–272. [Google Scholar] [PubMed]
- Dasgupta, P. Somatostatin analogues: Multiple roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol. Therapeut. 2004, 102, 61–85. [Google Scholar] [CrossRef]
- Takeba, Y.; Suzuki, N.; Takeno, M.; Asai, T.; Tsuboi, S.; Hoshino, T.; Sakane, T. Modulation of synovial cell function by somatostatin in patients with rheumatoid arthritis. Arthritis Rheum. 1997, 40, 2128–2138. [Google Scholar] [CrossRef]
- Anzola, L.K.; Glaudemans, A.; Dierckx, R.; Martinez, F.A.; Moreno, S.; Signore, A. Somatostatin receptor imaging by SPECT and PET in patients with chronic inflammatory disorders: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2496–2513. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Feng, S.B.; Cao, Z.W.; Bei, J.J.; Chen, Q.; Zhao, W.B.; Xu, X.J.; Zhou, Z.; Yu, Z.P.; Hu, H.Y. Up-Regulated Expression of Matrix Metalloproteinases in Endothelial Cells Mediates Platelet Microvesicle-Induced Angiogenesis. Cell Physiol. Biochem. 2017, 41, 2319–2332. [Google Scholar] [CrossRef]
- Middleton, J.; Americh, L.; Gayon, R.; Julien, D.; Aguilar, L.; Amalric, F.; Girard, J.P. Endothelial cell phenotypes in the rheumatoid synovium: Activated, angiogenic, apoptotic and leaky. Arthritis Res. Ther. 2004, 6, 60–72. [Google Scholar] [CrossRef]
- Jungel, A.; Ospelt, C.; Lesch, M.; Thiel, M.; Sunyer, T.; Schorr, O.; Michel, B.A.; Gay, R.E.; Kolling, C.; Flory, C.; et al. Effect of the oral application of a highly selective MMP-13 inhibitor in three different animal models of rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 898–902. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases, angiogenesis, and cancer: Commentary re: A. C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res. 2003, 9, 551–554. [Google Scholar]
- Zhou, M.; Qin, S.; Chu, Y.; Wang, F.; Chen, L.; Lu, Y. Immunolocalization of MMP-2 and MMP-9 in human rheumatoid synovium. Int. J. Clin. Exp. Pathol. 2014, 7, 3048–3056. [Google Scholar] [PubMed]
- Jackson, C.; Nguyen, M.; Arkell, J.; Sambrook, P. Selective matrix metalloproteinase (MMP) inhibition in rheumatoid arthritis--targetting gelatinase A activation. Inflamm. Res. 2001, 50, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Choi, H.M.; Lee, Y.A.; Choi, I.A.; Lee, S.H.; Hong, S.J.; Yang, H.I.; Yoo, M.C. Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatol. Int. 2011, 31, 543–547. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, Y.A.; Choi, H.M.; Yoo, M.C.; Yang, H.I. Implication of MMP-9 and urokinase plasminogen activator (uPA) in the activation of pro-matrix metalloproteinase (MMP)-13. Rheumatol. Int. 2012, 32, 3069–3075. [Google Scholar] [CrossRef]
- Itoh, T.; Matsuda, H.; Tanioka, M.; Kuwabara, K.; Itohara, S.; Suzuki, R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J. Immunol. 2002, 169, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Mengshol, J.A.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases as therapeutic targets in arthritic diseases-Bull’s-eye or missing the mark? Arthritis Rheum. 2002, 46, 13–20. [Google Scholar] [CrossRef]
- Breyholz, H.J.; Schafers, M.; Wagner, S.; Holtke, C.; Faust, A.; Rabeneck, H.; Levkau, B.; Schober, O.; Kopka, K. C-5-disubstituted barbiturates as potential molecular probes for noninvasive matrix metalloproteinase imaging. J. Med. Chem. 2005, 48, 3400–3409. [Google Scholar] [CrossRef]
- Breyholz, H.J.; Wagner, S.; Faust, A.; Riemann, B.; Holtke, C.; Hermann, S.; Schober, O.; Schafers, M.; Kopka, K. Radiofluorinated pyrimidine-2,4,6-triones as molecular probes for noninvasive MMP-targeted imaging. ChemMedChem 2010, 5, 777–789. [Google Scholar] [CrossRef]
- Eming, S.A.; Hubbell, J.A. Extracellular matrix in angiogenesis: Dynamic structures with translational potential. Exp. Dermatol 2011, 20, 605–613. [Google Scholar] [CrossRef]
- Bencharit, S.; Bin Cui, C.; Siddiqui, A.; Howard-Williams, E.L.; Sondek, J.; Zuobi-Hasona, K.; Aukhil, I. Structural insights into fibronectin type III domain-mediated signaling. J. Mol. Biol. 2007, 367, 303–309. [Google Scholar] [CrossRef]
- Kumra, H.; Reinhardt, D.P. Fibronectin-targeted drug delivery in cancer. Adv. Drug Deliv. Rev. 2016, 97, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kriegsmann, J.; Berndt, A.; Hansen, T.; Borsi, L.; Zardi, L.; Brauer, R.; Petrow, P.K.; Otto, M.; Kirkpatrick, C.J.; Gay, S.; et al. Expression of fibronectin splice variants and oncofetal glycosylated fibronectin in the synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Rheumatol. Int. 2004, 24, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Belloni, E.; Pradella, D.; Cappelletto, A.; Volf, N.; Zacchigna, S.; Ghigna, C. Alternative splicing in endothelial cells: Novel therapeutic opportunities in cancer angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 275. [Google Scholar] [CrossRef] [PubMed]
- Schwager, K.; Kaspar, M.; Bootz, F.; Marcolongo, R.; Paresce, E.; Neri, D.; Trachsel, E. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res. Ther. 2009, 11, R142. [Google Scholar] [CrossRef] [PubMed]
- Hemmerle, T.; Doll, F.; Neri, D. Antibody-based delivery of IL4 to the neovasculature cures mice with arthritis. Proc. Natl. Acad. Sci. USA 2014, 111, 12008–12012. [Google Scholar] [CrossRef]
- Berndt, A.; Borsi, L.; Luo, X.; Zardi, L.; Katenkamp, D.; Kosmehl, H. Evidence of ED-B+ fibronectin synthesis in human tissues by non-radioactive RNA in situ hybridization. Investigations on carcinoma (oral squamous cell and breast carcinoma), chronic inflammation (rheumatoid synovitis) and fibromatosis (Morbus Dupuytren). Histochem. Cell Biol. 1998, 109, 249–255. [Google Scholar] [CrossRef]
- Vollmer, S.; Vater, A.; Licha, K.; Gemeinhardt, I.; Gemeinhardt, O.; Voigt, J.; Ebert, B.; Schnorr, J.; Taupitz, M.; Macdonald, R.; et al. Extra domain B fibronectin as a target for near-infrared fluorescence imaging of rheumatoid arthritis affected joints in vivo. Mol. Imaging 2009, 8, 330–340. [Google Scholar] [CrossRef]
- Galeazzi, M.; Bazzichi, L.; Sebastiani, G.D.; Neri, D.; Garcia, E.; Ravenni, N.; Giovannoni, L.; Wilton, J.; Bardelli, M.; Baldi, C.; et al. A phase IB clinical trial with Dekavil (F8-IL10), an immunoregulatory ’armed antibody’ for the treatment of rheumatoid arthritis, used in combination wiIh methotrexate. Isr. Med. Assoc. J. 2014, 16, 666. [Google Scholar]
- Harjunpaa, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Bischoff, J. Cell adhesion and angiogenesis. J. Clin. Investig. 1997, 99, 373–376. [Google Scholar] [CrossRef]
- Malemud, C.J.; Reddy, S.K. Targeting Cytokines, Chemokines and Adhesion Molecules in Rheumatoid Arthritis. Curr. Rheumatol. Rev. 2008, 4, 219–234. [Google Scholar] [CrossRef]
- Jalkanen, S.; Karikoski, M.; Mercier, N.; Koskinen, K.; Henttinen, T.; Elima, K.; Salmivirta, K.; Salmi, M. The oxidase activity of vascular adhesion protein-1 (VAP-1) induces endothelial E- and P-selectins and leukocyte binding. Blood 2007, 110, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Eliceiri, B.P.; Cheresh, D.A. The role of alphav integrins during angiogenesis. Mol. Med. 1998, 4, 741–750. [Google Scholar] [CrossRef]
- Francavilla, C.; Maddaluno, L.; Cavallaro, U. The functional role of cell adhesion molecules in tumor angiogenesis. Semin. Cancer Biol. 2009, 19, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Aalto, K.; Autio, A.; Kiss, E.A.; Elima, K.; Nymalm, Y.; Veres, T.Z.; Marttila-Ichihara, F.; Elovaara, H.; Saanijoki, T.; Crocker, P.R.; et al. Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in PET imaging of inflammation and cancer. Blood 2011, 118, 3725–3733. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid. Redox. 2019, 30, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Marttila-Ichihara, F.; Smith, D.J.; Stolen, C.; Yegutkin, G.G.; Elima, K.; Mercier, N.; Kiviranta, R.; Pihlavisto, M.; Alaranta, S.; Pentikainen, U.; et al. Vascular amine oxidases are needed for leukocyte extravasation into inflamed joints in vivo. Arthritis Rheum. 2006, 54, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Menghis, A.; Botz, B.; Borbely, E.; Kemeny, A.; Tekus, V.; Csepregi, J.Z.; Mocsai, A.; Juhasz, T.; Zakany, R.; et al. Analgesic and Anti-Inflammatory Effects of the Novel Semicarbazide-Sensitive Amine-Oxidase Inhibitor SzV-1287 in Chronic Arthritis Models of the Mouse. Sci. Rep. 2017, 7, 39863. [Google Scholar] [CrossRef]
- Bournazou, E.; Samuels, J.; Zhou, H.; Krasnokutsky, S.; Patel, J.; Han, T.Z.; Bencardino, J.; Rybak, L.; Abramson, S.B.; Junker, U.; et al. Vascular Adhesion Protein-1 (VAP-1) as Predictor of Radiographic Severity in Symptomatic Knee Osteoarthritis in the New York University Cohort. Int. J. Mol. Sci. 2019, 20, 2642. [Google Scholar] [CrossRef]
- Tabi, T.; Szoko, E.; Merey, A.; Toth, V.; Matyus, P.; Gyires, K. Study on SSAO enzyme activity and anti-inflammatory effect of SSAO inhibitors in animal model of inflammation. Neural Transm. 2013, 120, 963–967. [Google Scholar] [CrossRef]
- Davis, L.S.; Sackler, M.; Brezinschek, R.I.; Lightfoot, E.; Bailey, J.L.; Oppenheimer-Marks, N.; Lipsky, P.E. Inflammation, immune reactivity, and angiogenesis in a severe combined immunodeficiency model of rheumatoid arthritis. Am. J. Pathol. 2002, 160, 357–367. [Google Scholar] [CrossRef]
- Li, H.; Du, S.; Niu, P.; Gu, X.; Wang, J.; Zhao, Y. Vascular Adhesion Protein-1 (VAP-1)/Semicarbazide-Sensitive Amine Oxidase (SSAO): A Potential Therapeutic Target for Atherosclerotic Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 679707. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Jalkanen, S. Ectoenzymes in leukocyte migration and their therapeutic potential. Semin. Immunopathol. 2014, 36, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Kivi, E.; Elima, K.; Aalto, K.; Nymalm, Y.; Auvinen, K.; Koivunen, E.; Otto, D.M.; Crocker, P.R.; Salminen, T.A.; Salmi, M.; et al. Human Siglec-10 can bind to vascular adhesion protein-1 and serves as its substrate. Blood 2009, 114, 5385–5392. [Google Scholar] [CrossRef]
- Ley, K. The role of selectins in inflammation and disease. Trends Mol. Med. 2003, 9, 263–268. [Google Scholar] [CrossRef]
- Koch, A.E.; Halloran, M.M.; Haskell, C.J.; Shah, M.R.; Polverini, P.J. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 1995, 376, 517–519. [Google Scholar] [CrossRef]
- Firestein, G.S. Starving the synovium: Angiogenesis and inflammation in rheumatoid arthritis. J. Clin. Investig. 1999, 103, 3–4. [Google Scholar] [CrossRef]
- Sakurai, D.; Tsuchiya, N.; Yamaguchi, A.; Okaji, Y.; Tsuno, N.H.; Kobata, T.; Takahashi, K.; Tokunaga, K. Crucial role of inhibitor of DNA binding/differentiation in the vascular endothelial growth factor-induced activation and angiogenic processes of human endothelial cells. J. Immunol. 2004, 173, 5801–5809. [Google Scholar] [CrossRef]
- Sharaki, O.A.; El-Guiziry, D.A.; Abou-Zeid, A.A.; El-Noueam, K.I.; Helal, A.E.; Gaballah, A.E. Clinical usefulness of basic fibroblast growth factor and E-selectin in patients with rheumatoid arthritis. Egypt. Immunol. 2004, 11, 91–100. [Google Scholar]
- Chapman, P.T.; Jamar, F.; Keelan, E.T.; Peters, A.M.; Haskard, D.O. Use of a radiolabeled monoclonal antibody against E-selectin for imaging of endothelial activation in rheumatoid arthritis. Arthritis Rheum. 1996, 39, 1371–1375. [Google Scholar] [CrossRef]
- Morshed, A.; Abbas, A.B.; Hu, J.; Xu, H. Shedding New Light on The Role of alphanubeta3 and alpha5beta1 Integrins in Rheumatoid Arthritis. Molecules 2019, 24, 1537. [Google Scholar] [CrossRef] [PubMed]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.J.; Mas-Moruno, C.; et al. Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [PubMed]
- Lowin, T.; Straub, R.H. Integrins and their ligands in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Shahrara, S.; Castro-Rueda, H.P.; Haines, G.K.; Koch, A.E. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res. Ther. 2007, 9, R112. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.L. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann. Rheum. Dis. 2002, 61 (Suppl. S2), ii96–ii99. [Google Scholar] [CrossRef]
- Hao, J.; Wu, X.; Setrerrahmane, S.; Qian, K.; Hou, Y.; Yu, L.; Lin, C.; Wu, Q.; Xu, H. Combination Therapy of PEG-HM-3 and Methotrexate Retards Adjuvant-Induced Arthritis. Int J. Mol. Sci. 2017, 18, 1538. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.F.; Xue, Y.Q.; Fang, L.K.; Guo, X.H.; Guo, X.; Liu, M.; Mo, B.Y.; Yang, M.R.; Liu, F.; et al. uPAR promotes tumor-like biologic behaviors of fibroblast-like synoviocytes through PI3K/Akt signaling pathway in patients with rheumatoid arthritis. Cell Mol. Immunol. 2018, 15, 171–181. [Google Scholar] [CrossRef]
- Connolly, M.; Veale, D.J.; Fearon, U. Acute serum amyloid A regulates cytoskeletal rearrangement, cell matrix interactions and promotes cell migration in rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 1296–1303. [Google Scholar] [CrossRef]
- Park, C.C.; Morel, J.C.M.; Amin, M.A.; Connors, M.A.; Harlow, L.A.; Koch, A.E. Evidence of IL-18 as a novel angiogenic mediator. J. Immunol. 2001, 167, 1644–1653. [Google Scholar] [CrossRef]
- Colman, R.W. Regulation of angiogenesis by the kallikrein-kinin system. Curr. Pharm. Des. 2006, 12, 2599–2607. [Google Scholar] [CrossRef]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin alphavbeta3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Steiger, K.; Hoffmann, F.; Reich, D.; Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Kessler, H.; Wester, H.J. Complementary, Selective PET Imaging of Integrin Subtypes alpha5beta1 and alphavbeta3 Using 68Ga-Aquibeprin and 68Ga-Avebetrin. J. Nucl. Med. 2016, 57, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Claria, J.; Romano, M. Pharmacological intervention of cyclooxygenase-2 and 5-lipoxygenase pathways. Impact on inflammation and cancer. Curr. Pharm. Des. 2005, 11, 3431–3447. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hong, F.F.; Yang, S.L. Role of prostaglandins in rheumatoid arthritis. Clin. Exp. Rheumatol. 2021, 39, 162–172. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Pelletier, J.P.; Fahmi, H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin. Arthritis Rheum. 2003, 33, 155–167. [Google Scholar] [CrossRef]
- Ben-Av, P.; Crofford, L.J.; Wilder, R.L.; Hla, T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: A potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995, 372, 83–87. [Google Scholar] [CrossRef]
- Lainer, D.T.; Brahn, E. New antiangiogenic strategies for the treatment of proliferative synovitis. Expert Opin. Investig. Drugs 2005, 14, 1–17. [Google Scholar] [CrossRef]
- El-Sayed, R.M.; Moustafa, Y.M.; El-Azab, M.F. Evening primrose oil and celecoxib inhibited pathological angiogenesis, inflammation, and oxidative stress in adjuvant-induced arthritis: Novel role of angiopoietin-1. Inflammopharmacology 2014, 22, 305–317. [Google Scholar] [CrossRef]
- Fikry, E.M.; Gad, A.M.; Eid, A.H.; Arab, H.H. Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Pharmacother 2019, 110, 878–886. [Google Scholar] [CrossRef]
- Pakneshan, P.; Birsner, A.E.; Adini, I.; Becker, C.M.; D’Amato, R.J. Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3909–3913. [Google Scholar] [CrossRef]
- Ha, M.K.; Song, Y.H.; Jeong, S.J.; Lee, H.J.; Jung, J.H.; Kim, B.; Song, H.S.; Huh, J.E.; Kim, S.H. Emodin inhibits proinflammatory responses and inactivates histone deacetylase 1 in hypoxic rheumatoid synoviocytes. Biol. Pharm. Bull. 2011, 34, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Jantan, I.; Bukhari, S.N.A. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed. Pharmacother. 2017, 92, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Krausz, S.; Ambarus, C.A.; Fernandez, B.M.; Hartkamp, L.M.; van Es, I.E.; Hamann, J.; Baeten, D.L.; Tak, P.P.; Reedquist, K.A. Tie2 signaling cooperates with TNF to promote the pro-inflammatory activation of human macrophages independently of macrophage functional phenotype. PLoS ONE 2014, 9, e82088. [Google Scholar] [CrossRef]
- DeBusk, L.M.; Chen, Y.; Nishishita, T.; Chen, J.; Thomas, J.W.; Lin, P.C. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.B.; Zaratin, P.F.; Gilmartin, A.G.; Hansbury, M.J.; Colombo, A.; Belpasso, C.; Winkler, J.D.; Jackson, J.R. TNF-alpha modulates angiopoietin-1 expression in rheumatoid synovial fibroblasts via the NF-kappa B signalling pathway. Biochem. Biophys. Res. Commun. 2005, 328, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Tsunemi, S.; Kitano, S.; Sekiguchi, M.; Sano, H.; Iwasaki, T. Differential regulation of c-Met signaling pathways for synovial cell function. Springerplus 2014, 3, 554. [Google Scholar] [CrossRef]
- Maini, R.N.; Taylor, P.C.; Paleolog, E.; Charles, P.; Ballara, S.; Brennan, F.M.; Feldmann, M. Anti-tumour necrosis factor specific antibody (infliximab) treatment provides insights into the pathophysiology of rheumatoid arthritis. Ann. Rheum. Dis. 1999, 58, I56–I60. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N. Anti-TNF alpha therapy of rheumatoid arthritis: What have we learned? Annu. Rev. Immunol. 2001, 19, 163–196. [Google Scholar] [CrossRef]
- Kanakaraj, P.; Puffer, B.A.; Yao, X.T.; Kankanala, S.; Boyd, E.; Shah, R.R.; Wang, G.; Patel, D.; Krishnamurthy, R.; Kaithamana, S.; et al. Simultaneous targeting of TNF and Ang2 with a novel bispecific antibody enhances efficacy in an in vivo model of arthritis. MAbs 2012, 4, 600–613. [Google Scholar] [CrossRef]
- Lupia, E.; Montrucchio, G.; Battaglia, E.; Modena, V.; Camussi, G. Role of tumor necrosis factor-alpha and platelet-activating factor in neoangiogenesis induced by synovial fluids of patients with rheumatoid arthritis. Eur. J. Immunol. 1996, 26, 1690–1694. [Google Scholar] [CrossRef]
- Tak, P.P.; Taylor, P.C.; Breedveld, F.C.; Smeets, T.J.; Daha, M.R.; Kluin, P.M.; Meinders, A.E.; Maini, R.N. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1996, 39, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Lupia, E. The future role of anti-tumour necrosis factor (TNF) products in the treatment of rheumatoid arthritis. Drugs 1998, 55, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Sidibe, A.; Mannic, T.; Arboleas, M.; Subileau, M.; Gulino-Debrac, D.; Bouillet, L.; Jan, M.; Vandhuick, T.; Le Loet, X.; Vittecoq, O.; et al. Soluble VE-cadherin in rheumatoid arthritis patients correlates with disease activity: Evidence for tumor necrosis factor alpha-induced VE-cadherin cleavage. Arthritis Rheum. 2012, 64, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Amin, M.A.; Ruth, J.H.; Campbell, P.L.; Koch, A.E. Suppression of endothelial cell activity by inhibition of TNFalpha. Arthritis Res. Ther. 2012, 14, R88. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Ng, C.T.; Chang, T.C.; Biniecka, M.; O’Sulliv’n, J.N.; Heffernan, E.; Fearon, U.; Veale, D.J. Tumor necrosis factor blocking therapy alters joint inflammation and hypoxia. Arthritis Rheum. 2011, 63, 923–932. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Liu, C.; Guo, W.; Li, X.; Su, X.; Wan, H.; Sun, Y.; Lin, N. Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS ONE 2013, 8, e77513. [Google Scholar] [CrossRef]
- Liu, C.; Kong, X.; Li, X.; Guo, W.; Zhang, C.; Sun, Y.; Su, X.; Liu, X.; Lu, A.; Lin, N. Wen Luo Yin inhibits angiogenesis in collagen-induced arthritis rat model and in vitro. J. Ethnopharmacol. 2013, 149, 478–489. [Google Scholar] [CrossRef]
- Paleolog, E. Target effector role of vascular endothelium in the inflammatory response: Insights from the clinical trial of anti-TNF alpha antibody in rheumatoid arthritis. Mol. Pathol. 1997, 50, 225–233. [Google Scholar] [CrossRef]
- Huang, M.; Qiu, Q.; Zeng, S.; Xiao, Y.; Shi, M.; Zou, Y.; Ye, Y.; Liang, L.; Yang, X.; Xu, H. Niclosamide inhibits the inflammatory and angiogenic activation of human umbilical vein endothelial cells. Inflamm. Res. 2015, 64, 1023–1032. [Google Scholar] [CrossRef]
- Barrera, P.; Oyen, W.J.; Boerman, O.C.; van Riel, P.L. Scintigraphic detection of tumour necrosis factor in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 825–828. [Google Scholar] [CrossRef]
- Chapman, A.P. PEGylated antibodies and antibody fragments for improved therapy: A review. Adv. Drug Deliv. Rev. 2002, 54, 531–545. [Google Scholar] [CrossRef]
- Zhang, T.; Ouyang, X.; Gou, S.; Zhang, Y.; Yan, N.; Chang, L.; Li, B.; Zhang, F.; Liu, H.; Ni, J. Novel synovial targeting peptide-sinomenine conjugates as a potential strategy for the treatment of rheumatoid arthritis. Int. J. Pharm. 2022, 617, 121628. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Buckley, C.; Blades, M.C.; Panayi, G.; George, A.J.; Pitzalis, C. Identification of synovium-specific homing peptides by in vivo phage display selection. Arthritis Rheum. 2002, 46, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Swidrowska-Jaros, J.; Smolewska, E. A fresh look at angiogenesis in juvenile idiopathic arthritis. Cent. Eur. J. Immunol. 2018, 43, 325–330. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Rodriguez, E.; Boelaars, K.; Brown, K.; Eveline Li, R.J.; Kruijssen, L.; Bruijns, S.C.M.; van Ee, T.; Schetters, S.T.T.; Crommentuijn, M.H.W.; van der Horst, J.C.; et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat. Commun. 2021, 12, 1270. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Yu, J.; Yuan, S. Preliminary Clinical Application of RGD-Containing Peptides as PET Radiotracers for Imaging Tumors. Front. Oncol. 2022, 12, 837952. [Google Scholar] [CrossRef]
- Steiger, K.; Quigley, N.G.; Groll, T.; Richter, F.; Zierke, M.A.; Beer, A.J.; Weichert, W.; Schwaiger, M.; Kossatz, S.; Notni, J. There is a world beyond alphavbeta3-integrin: Multimeric ligands for imaging of the integrin subtypes alphavbeta6, alphavbeta8, alphavbeta3, and alpha5beta1 by positron emission tomography. EJNMMI Res. 2021, 11, 106. [Google Scholar] [CrossRef]
- Bernhagen, D.; Jungbluth, V.; Gisbert Quilis, N.; Dostalek, J.; White, P.B.; Jalink, K.; Timmerman, P. High-Affinity alpha5beta1-Integrin-Selective Bicyclic RGD Peptides Identified via Screening of Designed Random Libraries. ACS Comb. Sci. 2019, 21, 598–607. [Google Scholar] [CrossRef]
- Lopes, S.; Ferreira, S.; Caetano, M. PET/CT in the Evaluation of Hypoxia for Radiotherapy Planning in Head and Neck Tumors: Systematic Literature Review. J. Nucl. Med. Technol. 2021, 49, 107–113. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Postel, R.; Brandwijk, R.J.; Dings, R.P.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, L.; Sun, J.; Sui, Y.; Li, D.; Li, G.; Liu, J.; Shu, Q. Expression of Galectin-9 and correlation with disease activity and vascular endothelial growth factor in rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 654–661. [Google Scholar] [PubMed]
- O’Brien, M.J.; Shu, Q.; Stinson, W.A.; Tsou, P.S.; Ruth, J.H.; Isozaki, T.; Campbell, P.L.; Ohara, R.A.; Koch, A.E.; Fox, D.A.; et al. A unique role for galectin-9 in angiogenesis and inflammatory arthritis. Arthritis Res. Ther. 2018, 20, 31. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Lu, D.; Zhou, H.; Li, N.; Wang, Y.; Zhang, T.; Wang, F.; Liu, N.; Zhu, H.; Zhang, J.; Yang, Z.; et al. Galectin expression detected by (68)Ga-galectracer PET as a predictive biomarker of radiotherapy resistance. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2746–2760. [Google Scholar] [CrossRef] [PubMed]
- Duet, M.; Liote, F. Somatostatin and somatostatin analog scintigraphy: Any benefits for rheumatology patients? Jt. Bone Spine 2004, 71, 530–535. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Sun, L.; Lan, X.; Zeng, D.; Cai, W. Dual-Targeted Molecular Imaging of Cancer. J. Nucl. Med. 2018, 59, 390–395. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, H.; Tan, H.; Cui, X.; Yao, S.; Zang, J.; Zhang, L.; Zhu, Z. Evaluation of Lung Cancer and Neuroendocrine Neoplasm in a Single Scan by Targeting Both Somatostatin Receptor and Integrin alphavbeta3. Clin. Nucl. Med. 2019, 44, 687–694. [Google Scholar] [CrossRef]
- Baart, V.M.; Houvast, R.D.; de Geus-Oei, L.F.; Quax, P.H.A.; Kuppen, P.J.K.; Vahrmeijer, A.L.; Sier, C.F.M. Molecular imaging of the urokinase plasminogen activator receptor: Opportunities beyond cancer. EJNMMI Res. 2020, 10, 87. [Google Scholar] [CrossRef]
- Huang, C.C.; Tseng, T.T.; Liu, S.C.; Lin, Y.Y.; Law, Y.Y.; Hu, S.L.; Wang, S.W.; Tsai, C.H.; Tang, C.H. S1P Increases VEGF Production in Osteoblasts and Facilitates Endothelial Progenitor Cell Angiogenesis by Inhibiting miR-16–5p Expression via the c-Src/FAK Signaling Pathway in Rheumatoid Arthritis. Cells 2021, 10, 2168. [Google Scholar] [CrossRef]
- Sun, M.; Deng, R.; Wang, Y.; Wu, H.; Zhang, Z.; Bu, Y.; Zhang, H. Sphingosine kinase 1/sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 pathway: A novel target of geniposide to inhibit angiogenesis. Life Sci. 2020, 256, 117988. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.P.; Wagner, S.; Keul, P.; Hermann, S.; Levkau, B.; Schafers, M.; Haufe, G. Synthesis of fluorinated analogues of sphingosine-1-phosphate antagonists as potential radiotracers for molecular imaging using positron emission tomography. Bioorgan. Med. Chem. 2014, 22, 5168–5181. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jin, H.J.; Yue, X.Y.; Han, J.B.; Baum, P.; Abendschein, D.R.; Tu, Z.D. PET Study of Sphingosine-1-Phosphate Receptor 1 Expression in Response to Vascular Inflammation in a Rat Model of Carotid Injury. Mol. Imaging 2017, 16, 1536012116689770. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Gai, Y.; Ji, H.; Jiang, Y.; Qiao, P.; Wang, W.; Zhang, Y.; Xia, X.; Lan, X. A Novel Radioimmune (99m)Tc-Labeled Tracer for Imaging Sphingosine 1-Phosphate Receptor 1 in Tumor Xenografts: An In Vitro and In Vivo Study. Front. Immunol. 2021, 12, 660842. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.S.; Schilson, S.S.; Wagner, S.; Hermann, S.; Keul, P.; Levkau, B.; Schafers, M.; Haufe, G. Synthesis and evaluation of fluorinated fingolimod (FTY720) analogues for sphingosine-1-phosphate receptor molecular imaging by positron emission tomography. J. Med. Chem. 2015, 58, 3471–3484. [Google Scholar] [CrossRef]
| Type | Target | Radiotracer Name | Imaging Mode | Developmental Phase | Year | Ref. |
|---|---|---|---|---|---|---|
| Environmental factor | Hypoxia | 18F-FMISO | PET | Animal | 2017/2019 | [60,61] |
| 18F-FAZA | PET | Animal | 2017 | [61] | ||
| 67Cu-ATSM | PET | Animal | 2021 | [62] | ||
| α-[123I]-IAZA α-[123I]-IAZP | Scintigraphy | Human | 2002/2001 | [63] [64] | ||
| Somatostatin | SST2 | 68Ga- DOTANOC | PET | Human | 2022 | [65] |
| 99mTc-HYNIC-TOC | Scintigraphy | Human | 2016 | [66] | ||
| 111In-DTPA-D-Phe-octreotide (pentetreotide) | Scintigraphy | Human | 1994 | [67] | ||
| 99mTc-EDDA/HYNIC-TOC | Scintigraphy | Human | 2012 | [66] | ||
| 99mTc-depreotide | Scintigraphy | Human | 2017 | [68] | ||
| Matrix Proteases | MMP-2/-9 | 18F-2,4,6-trione-30 | PET | Animal | 2012 | [69] |
| MMP-13 | 11C-MMP-13 inhibitor (2d) | PET | Animal * | 2017 | [70,71] | |
| 68Ga-MMP13 inhibitor (2i) | PET | Animal * | 2017 | [70,71] | ||
| 18F-MMP-13 inhibitor (2a) | PET | Animal * | 2017 | [70,71] | ||
| ECM | ED-A fibronectin | 124I-F8-IL10 | PET | Animal/Human | 2019 | [72] |
| Adhesion molecules | VAP-1 | 68Ga-DOTA-Siglec-9 | PET | Animal/Human | 2021/2015 | [73,74] |
| 124I-BTT-1023 | SPECT | Animal | 2013 | [75] | ||
| E-selectin | 111In-anti-E-selectin | Scintigraphy | Animal | 2009 | [76] | |
| 99mTc-1.2B6-Fab | Scintigraphy | Human | 1997/2002 | [77,78] | ||
| Integrin a5B1 | 68Ga-Aquibeprin | PET | Animal | 2019 | [39] | |
| Integrin avB3 | 68Ga-Avebretin | PET | Animal | 2019 | [39] | |
| 68Ga-PRGD2 | PET | Human | 2014 | [79] | ||
| 68Ga-HP(3)-RGD(3) | PET | Animal | 2017 | [80] | ||
| 18F-galacto-RGD | PET | Animal | 2007 | [81] | ||
| 68Ga-NODAGA-RGDyk | PET | Human | 2021 | [82] | ||
| 99mTc-HYNIC-3PRGD2 | Scintigraphy | Animal | 2015 | [83] | ||
| 99mTc-3PRGD2 | Scintigraphy | Animal/Human | 2017 | [84] | ||
| 99mTc-maraciclatide | Scintigraphy | Human | 2020 | [85] | ||
| 111In-DOTA-E-[c(RGDfK)]2 | SPECT | Animal | 2016 | [86] | ||
| 99mTc-3P4-RGD2 | SPECT | Animal | 2013 | [87] | ||
| 99mTcO2(DP-RGD)2 | SPECT | Animal | 2021 | [88] | ||
| Enzymes | COX1–3 | 11C-Ibuprofen | PET | Animal | 2011 | [89] |
| 11C-Ketoprofen | PET | Animal | 2017 | [90] | ||
| COX-2 | 11C-MC1 | PET | Human | 2020 | [91] | |
| Cytokines | TNF-α | 89Zr-Certolizumab Pegol | PET | Animal | 2020 | [92] |
| 99mTc- certolizumab pegol | Scintigraphy | Human | 2016 | [93] | ||
| 99mTc-S-HYNIC-certolizumab pegol | Scintigraphy | Human | 2016 | [94] | ||
| 99mTc-adalimumab | Scintigraphy | Human | 2021 | [95] | ||
| 99mTc-Infliximab | Scintigraphy | Human | 2007/2012 | [96,97] | ||
| Other | IL-4- ECs of inflamed tissue | 125I-IL-4-SyETP | SPECT | Animal | 2013 | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodadust, F.; Ezdoglian, A.; Steinz, M.M.; van Beijnum, J.R.; Zwezerijnen, G.J.C.; Jansen, G.; Tas, S.W.; van der Laken, C.J. Systematic Review: Targeted Molecular Imaging of Angiogenesis and Its Mediators in Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 7071. https://doi.org/10.3390/ijms23137071
Khodadust F, Ezdoglian A, Steinz MM, van Beijnum JR, Zwezerijnen GJC, Jansen G, Tas SW, van der Laken CJ. Systematic Review: Targeted Molecular Imaging of Angiogenesis and Its Mediators in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2022; 23(13):7071. https://doi.org/10.3390/ijms23137071
Chicago/Turabian StyleKhodadust, Fatemeh, Aiarpi Ezdoglian, Maarten M. Steinz, Judy R. van Beijnum, Gerben J. C. Zwezerijnen, Gerrit Jansen, Sander W. Tas, and Conny J. van der Laken. 2022. "Systematic Review: Targeted Molecular Imaging of Angiogenesis and Its Mediators in Rheumatoid Arthritis" International Journal of Molecular Sciences 23, no. 13: 7071. https://doi.org/10.3390/ijms23137071
APA StyleKhodadust, F., Ezdoglian, A., Steinz, M. M., van Beijnum, J. R., Zwezerijnen, G. J. C., Jansen, G., Tas, S. W., & van der Laken, C. J. (2022). Systematic Review: Targeted Molecular Imaging of Angiogenesis and Its Mediators in Rheumatoid Arthritis. International Journal of Molecular Sciences, 23(13), 7071. https://doi.org/10.3390/ijms23137071






