Co-Occurrence of RAD21 and TNFAIP3 Mutations in Cornelia de Lange Syndrome with Pustular Psoriasis: Potential Molecular Interactions
Abstract
1. Introduction
2. Results
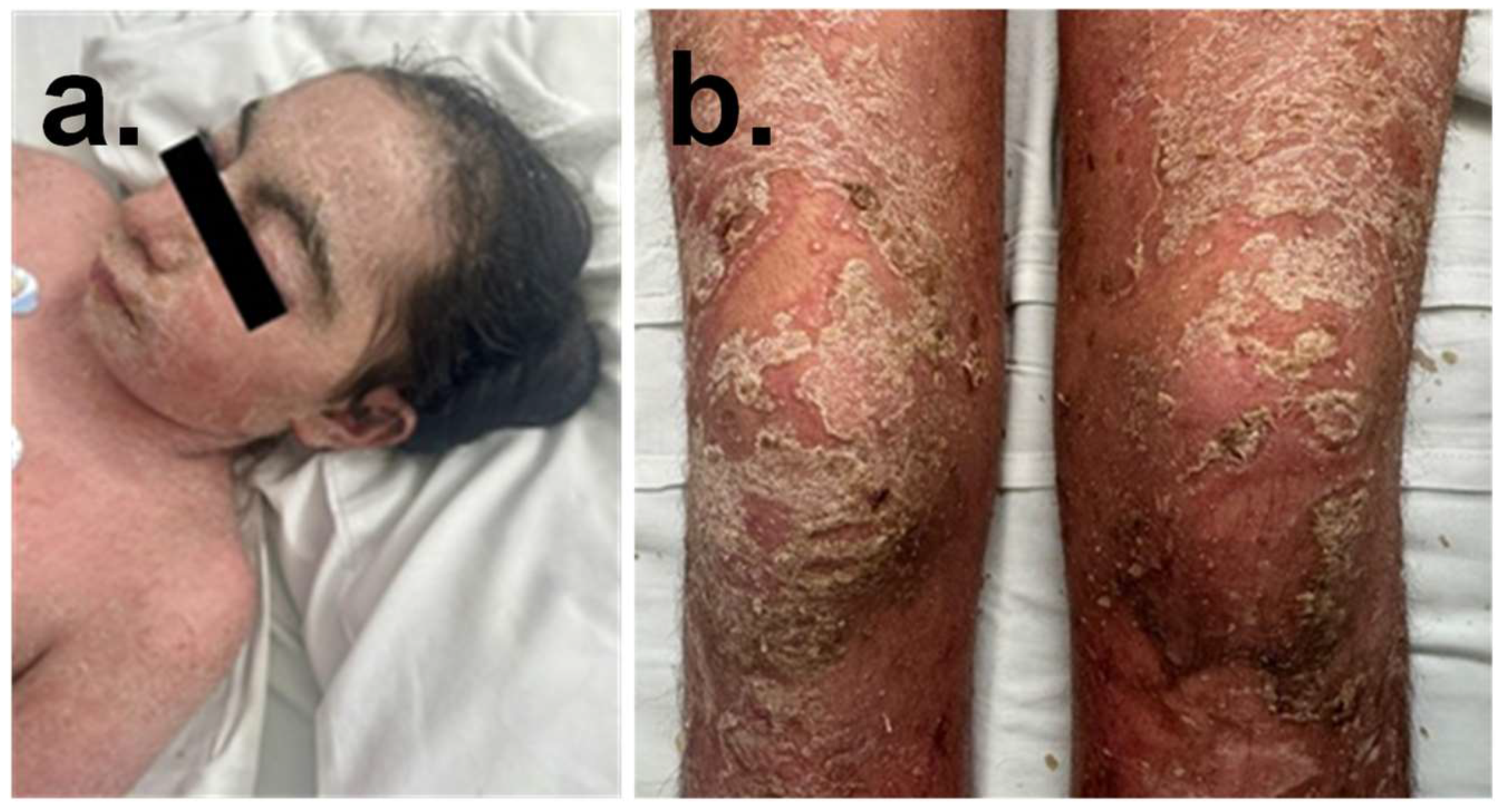
2.1. Proband Phenotype
2.2. Molecular Characterization
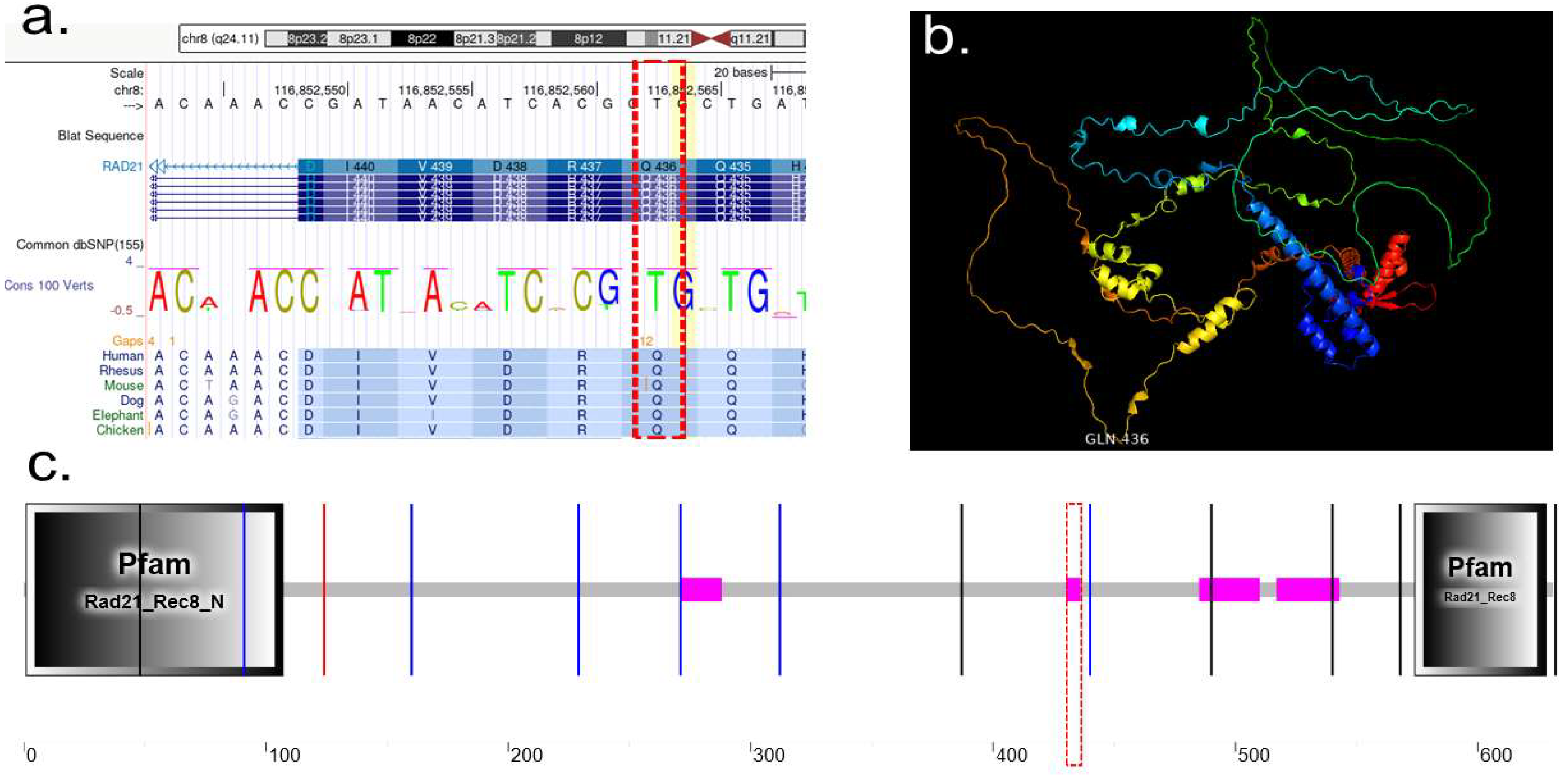
2.2.1. RAD21
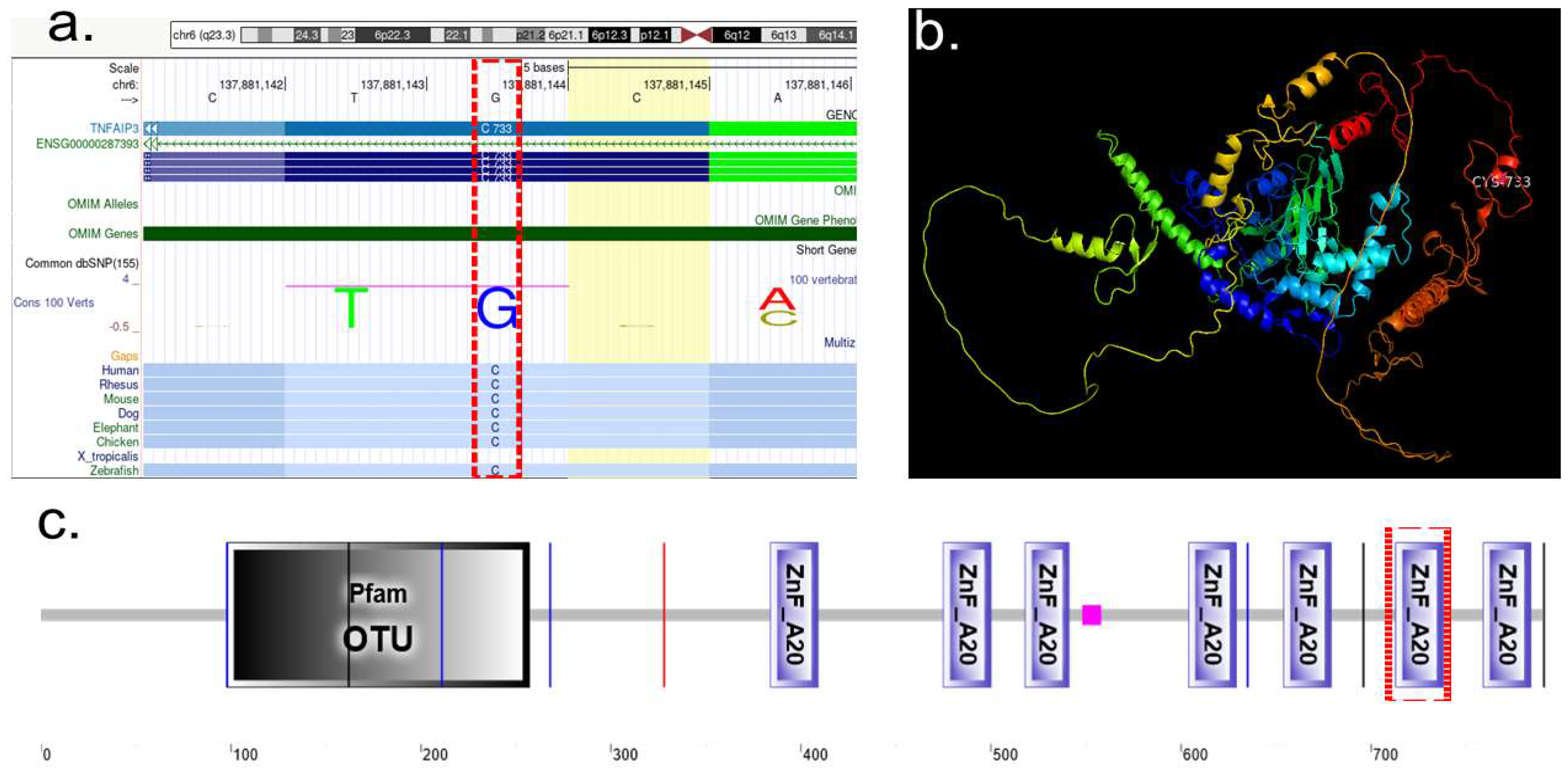
2.2.2. TNFAIP3
2.3. Protein Characterization
2.3.1. Protein Characterization of RAD21
2.3.2. Protein Characterization of TNFAIP3
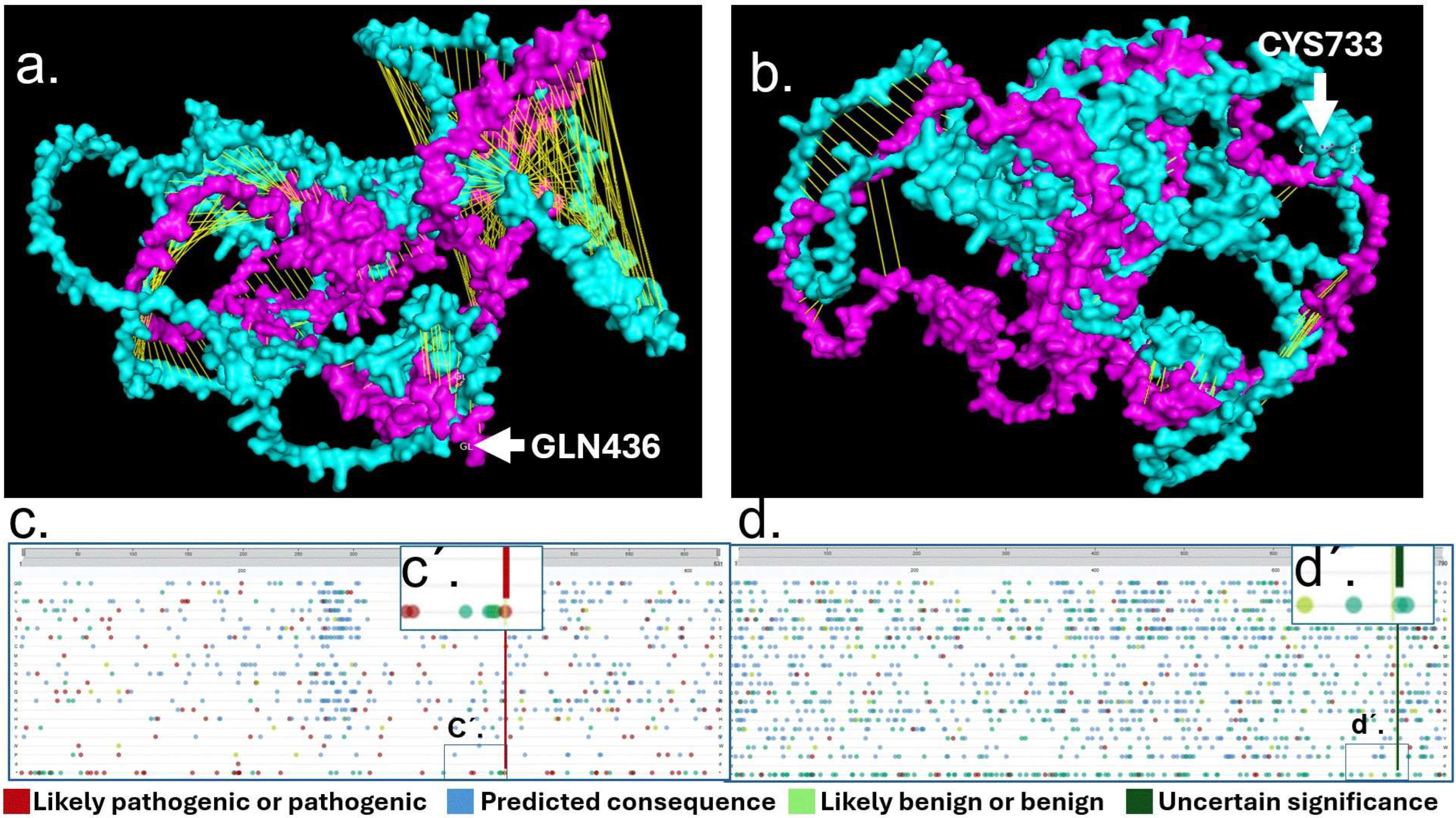
2.4. Mutational Analysis of RAD21 and TNFAIP3
2.5. Mutational Analysis of RAD21 and TNFAIP3
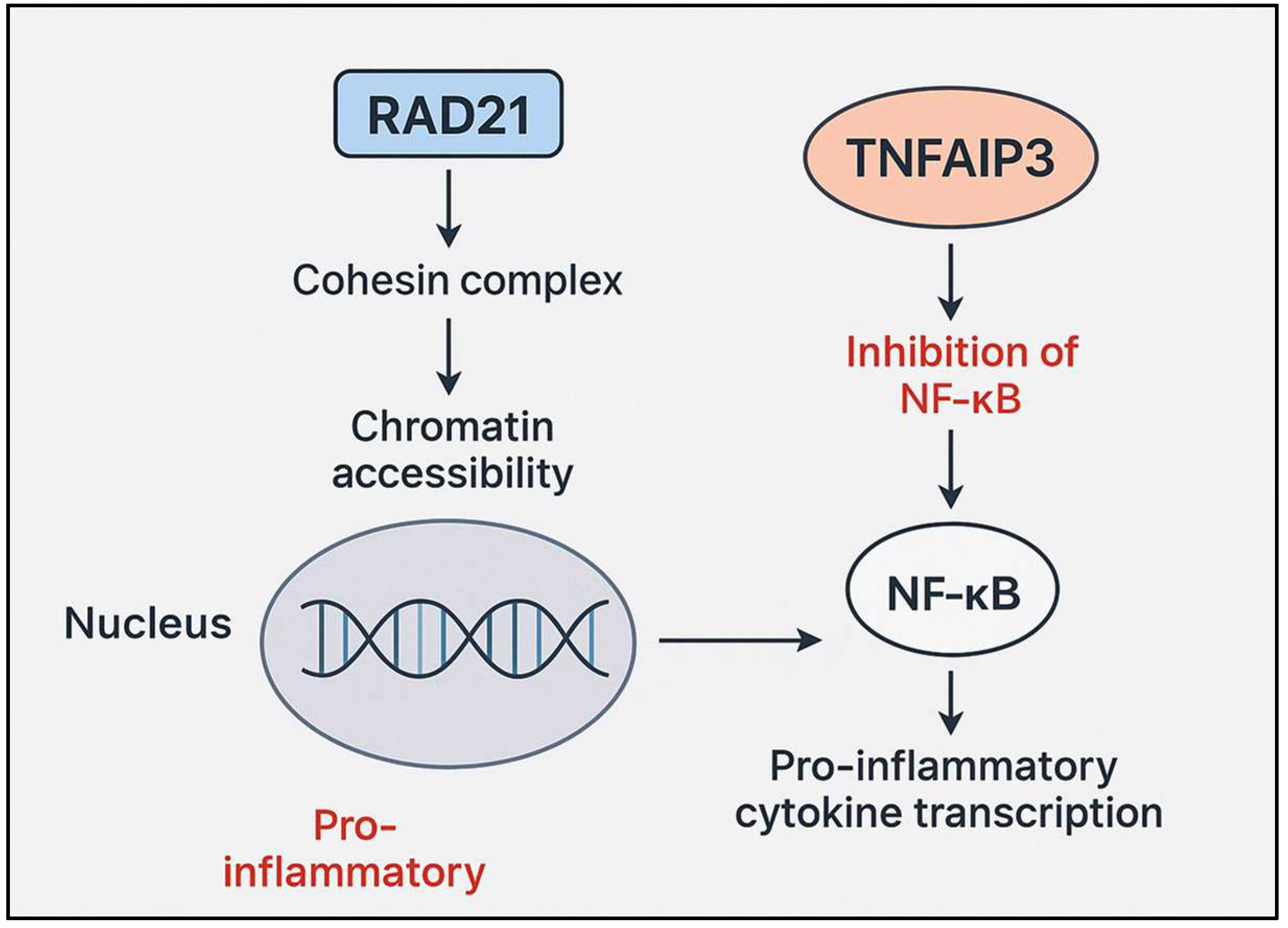
3. Discussion
4. Materials and Methods
4.1. Proband Phenotype
4.2. Molecular Characterization
4.3. Protein Characterization
4.4. Protein Mutational Analysis
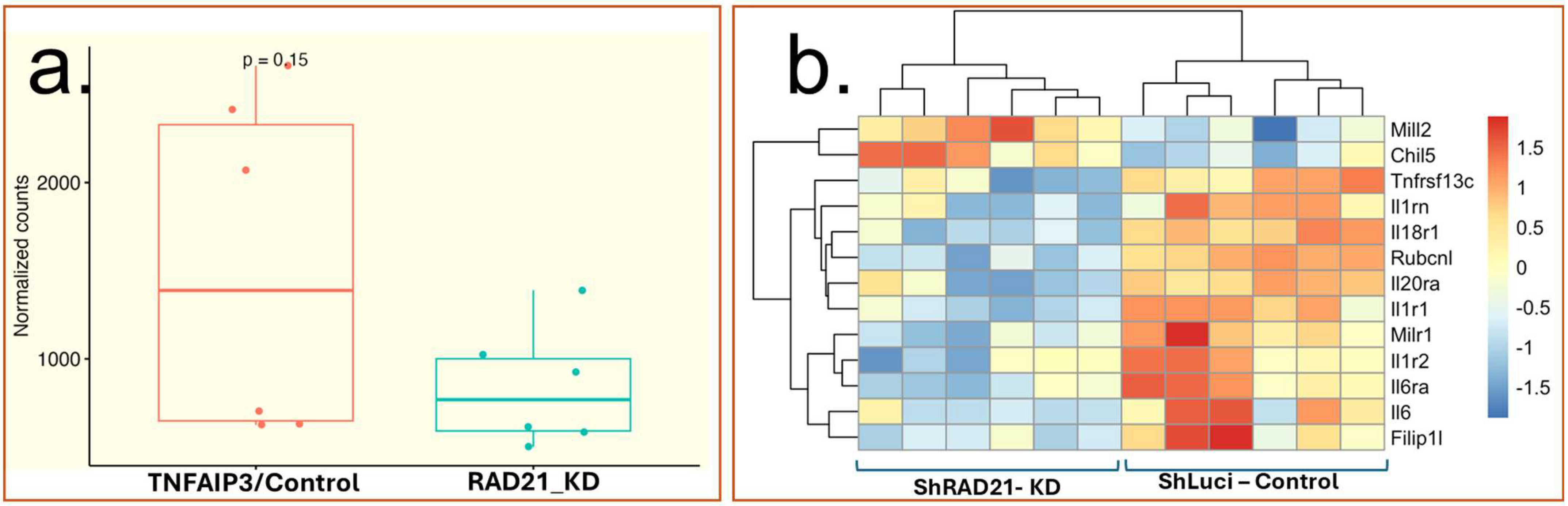
Secondary mRNA Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACMG | American College of Medical Genetics |
| AMP | Association for Molecular Pathology |
| ANKRD | Ankyrin Repeat Domain protein |
| BRD4 | Bromodomain-containing protein 4 |
| CdLS | Cornelia de Lange Syndrome |
| CNV | Copy-number variant |
| GEO | Gene Expression Omnibus |
| GPP | Generalized Pustular Psoriasis |
| HDAC8 | Histone Deacetylase 8 |
| HGMD | Human Gene Mutation Database |
| HSPCs | Hematopoietic Stem and Progenitor Cells |
| IL-1 | Interleukin 1 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NIPBL | Nipped-B-like protein |
| NMD | Nonsense-Mediated Decay |
| OMIM | Online Mendelian Inheritance in Man |
| OTU | Ovarian tumor domain |
| RAD21 | Double-strand-break repair protein rad21 homolog |
| SMC1A | Structural Maintenance of Chromosomes 1A |
| SMC3 | Structural Maintenance of Chromosomes 3 |
| TLRs | Toll-like receptors |
| TNFAIP3 | Tumor necrosis factor alpha-induced protein 3 (A20) |
| VTS | Vanishing twin syndrome |
| VUS | Variant of Uncertain Significance |
| ZnF | Zinc finger domain |
References
- De Lange, C. Sur Un Type Nouveau de Degenerescence (Typus Amstelodamensis). Arch. Med. Enfants 1933, 36, 713–719. [Google Scholar]
- Huisman, S.; Mulder, P.A.; Redeker, E.; Bader, I.; Bisgaard, A.M.; Brooks, A.; Cereda, A.; Cinca, C.; Clark, D.; Cormier-Daire, V.; et al. Phenotypes and Genotypes in Individuals with SMC1A Variants. Am. J. Med. Genet. A 2017, 173, 2108–2125. [Google Scholar] [CrossRef]
- Szyca, R.; Leksowski, K. Cornelia de Lange Syndrome—Characteristics and Laparoscopic Treatment Modalities of Reflux Based on Own Material. Videosurgery Other Miniinvasive Tech. 2011, 6, 173–177. [Google Scholar] [CrossRef]
- Liu, J.; Krantz, I.D. Cornelia de Lange Syndrome, Cohesin, and Beyond. Clin. Genet. 2009, 76, 303–314. [Google Scholar] [CrossRef]
- Deardorff, M.A.; Kaur, M.; Yaeger, D.; Rampuria, A.; Korolev, S.; Pie, J.; Gil-Rodríguez, C.; Arnedo, M.; Loeys, B.; Kline, A.D.; et al. Mutations in Cohesin Complex Members SMC3 and SMC1A Cause a Mild Variant of Cornelia de Lange Syndrome with Predominant Mental Retardation. Am. J. Hum. Genet. 2007, 80, 485–494. [Google Scholar] [CrossRef]
- Li, Q.; Chang, G.; Yin, L.; Li, J.; Huang, X.; Shen, Y.; Li, G.; Xu, Y.; Wang, J.; Wang, X. Clinical and Molecular Analysis in a Cohort of Chinese Children with Cornelia de Lange Syndrome. Sci. Rep. 2020, 10, 21224. [Google Scholar] [CrossRef]
- Kline, A.D.; Moss, J.F.; Selicorni, A.; Bisgaard, A.M.; Deardorff, M.A.; Gillett, P.M.; Ishman, S.L.; Kerr, L.M.; Levin, A.V.; Mulder, P.A.; et al. Diagnosis and Management of Cornelia de Lange Syndrome: First International Consensus Statement. Nat. Rev. Genet. 2018, 19, 649–666. [Google Scholar] [CrossRef]
- Savitha, M.R.; Shervani, M.B. Cornelia de Lange Syndrome Type 4 with a Novel Heterozygous Missense Variant in RAD 21 Gene. Int. J. Contemp. Pediatr. 2024, 11, 605–608. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Vereecke, L.; Beyaert, R.; van Loo, G. The Ubiquitin-Editing Enzyme A20 (TNFAIP3) Is a Central Regulator of Immunopathology. Trends Immunol. 2009, 30, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, H.; Schwartz, D.M.; Stoffels, M.; Hwan Park, Y.; Zhang, Y.; Yang, D.; Demirkaya, E.; Takeuchi, M.; Tsai, W.L.; et al. Loss-of-Function Mutations in TNFAIP3 Leading to A20 Haploinsufficiency Cause an Early-Onset Autoinflammatory Disease. Nat. Genet. 2015, 48, 67–73. [Google Scholar] [CrossRef]
- Lee, C.C.; Huang, Y.H.; Chi, C.C.; Chung, W.H.; Chen, C.B. Generalized Pustular Psoriasis: Immunological Mechanisms, Genetics, and Emerging Therapeutics. Trends Immunol. 2025, 46, 74–89. [Google Scholar] [CrossRef]
- Bagyinszky, E.; An, S.S.A. Genetic Mutations Associated with TNFAIP3 (A20) Haploinsufficiency and Their Impact on Inflammatory Diseases. Int. J. Mol. Sci. 2024, 25, 8275. [Google Scholar] [CrossRef]
- Lubkov, A.; Uraga, V.; Briones, M.-C.; Uraga, E. Cornelia de Lange Syndrome and Psoriasis: Report of a Case. Our Dermatol. Online 2018, 9, 38–40. [Google Scholar] [CrossRef][Green Version]
- Mugheddu, C.; Dell’Antonia, M.; Sanna, S.; Agosta, D.; Atzori, L.; Rongioletti, F. Successful Guselkumab Treatment in a Psoriatic Patient Affected with Cornelia de Lange Syndrome, and Prosecution during the COVID-19 Pandemic. Dermatol. Ther. 2020, 33, e13433. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Pal, S.S.; Khurshid, K. Cornelia De Lange Syndrome with Generalized Pustular Psoriasis: A Rare Coexistence. J. Pak. Assoc. Dermatol. 2005, 15, 281–284. [Google Scholar]
- Cheng, H.; Zhang, N.; Pati, D. Cohesin Subunit RAD21: From Biology to Disease. Gene 2020, 758, 144966. [Google Scholar] [CrossRef]
- Weiss, F.D.; Calderon, L.; Wang, Y.F.; Georgieva, R.; Guo, Y.; Cvetesic, N.; Kaur, M.; Dharmalingam, G.; Krantz, I.D.; Lenhard, B.; et al. Neuronal Genes Deregulated in Cornelia de Lange Syndrome Respond to Removal and Re-Expression of Cohesin. Nat. Commun. 2021, 12, 2919. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Guo, Y.; Yang, K.; Ni, C.; Yu, H.; Kong, X.; Li, M.; Lu, Z.; Yao, Z. Coinheritance of Generalized Pustular Psoriasis and Familial Behçet-like Autoinflammatory Syndrome with Variants in IL36RN and TNFAIP3 in the Heterozygous State. J. Dermatol. 2019, 46, 907–910. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Oparina, N.; Garcia-Bonete, M.J.; Wasén, C.; Erlandsson, M.C.; Malmhäll-Bah, E.; Andersson, K.M.E.; Jensen, M.; Silfverswärd, S.T.; Katona, G.; et al. Cohesin-Mediated Chromatin Interactions and Autoimmunity. Front. Immunol. 2022, 13, 840002. [Google Scholar] [CrossRef]
- Nititham, J.; Taylor, K.E.; Gupta, R.; Chen, H.; Ahn, R.; Liu, J.; Seielstad, M.; Ma, A.; Bowcock, A.M.; Criswell, L.A.; et al. Meta-Analysis of the TNFAIP3 Region in Psoriasis Reveals a Risk Haplotype That Is Distinct from Other Autoimmune Diseases. Genes. Immun. 2015, 16, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Minor, A.; Shinawi, M.; Hogue, J.S.; Vineyard, M.; Hamlin, D.R.; Tan, C.; Donato, K.; Wysinger, L.; Botes, S.; Das, S.; et al. Two Novel RAD21 Mutations in Patients with Mild Cornelia de Lange Syndrome-like Presentation and Report of the First Familial Case. Gene 2014, 537, 279–284. [Google Scholar] [CrossRef]
- Dorval, S.; Masciadri, M.; Mathot, M.; Russo, S.; Revencu, N.; Larizza, L. A Novel RAD21 Mutation in a Boy with Mild Cornelia de Lange Presentation: Further Delineation of the Phenotype. Eur. J. Med. Genet. 2020, 63, 103620. [Google Scholar] [CrossRef] [PubMed]
- De Falco, A.; De Brasi, D.; Della Monica, M.; Cesario, C.; Petrocchi, S.; Novelli, A.; D’Alterio, G.; Iolascon, A.; Capasso, M.; Piscopo, C. A Novel Variant in RAD21 in Cornelia De Lange Syndrome Type 4: Case Report and Bioinformatic Analysis. Genes 2023, 14, 119. [Google Scholar] [CrossRef]
- Sarogni, P.; Pallotta, M.M.; Musio, A. Cornelia de Lange Syndrome: From Molecular Diagnosis to Therapeutic Approach. J. Med. Genet. 2020, 57, 289–295. [Google Scholar] [CrossRef]
- Deardorff, M.A.; Wilde, J.J.; Albrecht, M.; Dickinson, E.; Tennstedt, S.; Braunholz, D.; Mönnich, M.; Yan, Y.; Xu, W.; Gil-Rodríguez, M.C.; et al. RAD21 Mutations Cause a Human Cohesinopathy. Am. J. Hum. Genet. 2012, 90, 1014–1027. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving Access to Variant Interpretations and Supporting Evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Scott, A.F.; Hamosh, A. OMIM.Org: Leveraging Knowledge across Phenotype-Gene Relationships. Nucleic Acids Res. 2019, 47, D1038–D1043. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.Org: Online Mendelian Inheritance in Man (OMIM®), an Online Catalog of Human Genes and Genetic Disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, L.; Wang, Y.; Yang, X.; Wang, W.; Lin, L.; Sun, B.; Hou, J.; Ying, W.; Hui, X.; et al. Novel Heterogeneous Mutation of TNFAIP3 in a Chinese Patient with Behçet-Like Phenotype and Persistent EBV Viremia. J. Clin. Immunol. 2019, 39, 188–194. [Google Scholar] [CrossRef]
- Hauf, S.; Waizenegger, I.C.; Peters, J.M. Cohesin Cleavage by Separase Required for Anaphase and Cytokinesis in Human Cells. Science 2001, 293, 1320–1323. [Google Scholar] [CrossRef]
- Zinc-Finger Protein A20, a Regulator of Inflammation and Cell Survival, Has de-Ubiquitinating Activity | Biochemical Journal | Portland Press. Available online: https://portlandpress.com/biochemj/article-abstract/378/3/727/41489/Zinc-finger-protein-A20-a-regulator-of?redirectedFrom=fulltext (accessed on 10 September 2025).
- Mauro, C.; Pacifico, F.; Lavorgna, A.; Mellone, S.; Iannetti, A.; Acquaviva, R.; Formisano, S.; Vito, P.; Leonardi, A. ABIN-1 Binds to NEMO/IKKγ and Co-Operates with A20 in Inhibiting NF-ΚB. J. Biol. Chem. 2006, 281, 18482–18488. [Google Scholar] [CrossRef]
- Wartz, I.E.; O’Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-Ubiquitination and Ubiquitin Ligase Domains of A20 Downregulate NF-ΚB Signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing Structure Coverage for over 214 Million Protein Sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef]
- Watrin, E.; Schleiffer, A.; Tanaka, K.; Eisenhaber, F.; Nasmyth, K.; Peters, J.M. Human Scc4 Is Required for Cohesin Binding to Chromatin, Sister-Chromatid Cohesion, and Mitotic Progression. Curr. Biol. 2006, 16, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Nasmyth, K.; Haering, C.H. Cohesin: Its Roles and Mechanisms. Annu. Rev. Genet. 2009, 43, 525–558. [Google Scholar] [CrossRef]
- Ma, A.; Malynn, B.A. A20: Linking a Complex Regulator of Ubiquitylation to Immunity and Human Disease. Nat. Rev. Immunol. 2012, 12, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.L.; Turer, E.E.; Lee, E.G.; Ahmad, R.C.; Wheeler, M.T.; Tsui, C.; Hurley, P.; Chien, M.; Chai, S.; Hitotsumatsu, O.; et al. The Ubiquitin-Modifying Enzyme A20 Is Required for Termination of Toll-like Receptor Responses. Nat. Immunol. 2004, 5, 1052–1060, Erratum in: Nat. Immunol. 2005, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Luck, K.; Kim, D.K.; Lambourne, L.; Spirohn, K.; Begg, B.E.; Bian, W.; Brignall, R.; Cafarelli, T.; Campos-Laborie, F.J.; Charloteaux, B.; et al. A Reference Map of the Human Binary Protein Interactome. Nature 2020, 580, 402–408. [Google Scholar] [CrossRef]
- Chen, Z.; Amro, E.M.; Becker, F.; Hölzer, M.; Rasa, S.M.M.; Njeru, S.N.; Han, B.; Sanzo, S.D.; Chen, Y.; Tang, D.; et al. Cohesin-Mediated NF-ΚB Signaling Limits Hematopoietic Stem Cell Self-Renewal in Aging and Inflammation. J. Exp. Med. 2019, 216, 152–175. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Ghaderi, S.; Morken, N.H.; Magnus, P.; Romundstad, L.B.; Skjærven, R.; Wilcox, A.J.; Håberg, S.E. Vanishing Twin Syndrome among ART Singletons and Pregnancy Outcomes. Hum. Reprod. 2017, 32, 2298–2304. [Google Scholar] [CrossRef]
- Solovyeva, N.A.; Kurmaeva, E.A.; Kulakova, G.A.; Volgina, S.Y.; Rudnitskaya, A.A.; Samigullina, R.R.; Danilaeva, N.M. Cornelia de Lange Syndrome. Ross. Vestn. Perinatol. I Pediatr. 2023, 67, 211–215. [Google Scholar] [CrossRef]
- Tejasvi, T.; Stuart, P.E.; Chandran, V.; Voorhees, J.J.; Gladman, D.D.; Rahman, P.; Elder, J.T.; Nair, R.P. TNFAIP3 Gene Polymorphisms Are Associated with Response to TNF Blockade in Psoriasis. J. Investig. Dermatol. 2012, 132, 593–600. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Zheng, X.; Jin, H. Immune Regulation of TNFAIP3 in Psoriasis through Its Association with Th1 and Th17 Cell Differentiation and P38 Activation. J. Immunol. Res. 2020, 2020, 5980190. [Google Scholar] [CrossRef] [PubMed]
- Sahlol, N.Y.; Mostafa, M.S.; El-Fattah Madkour, L.A.; Salama, D.M. Low TNFAIP3 Expression in Psoriatic Skin Promotes Disease Susceptibility and Severity. PLoS ONE 2019, 14, e0217352. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, H.; Fan, Y.; Chen, J.; Song, Y.; Wang, S.; Zhu, F.; Guo, C.; Zhang, L.; Shi, Y. Expression of Tumor Necrosis Factor Alpha-Induced Protein 3 MRNA in Peripheral Blood Mononuclear Cells Negatively Correlates with Disease Severity in Psoriasis Vulgaris. Clin. Vaccine Immunol. 2012, 19, 1938–1942. [Google Scholar] [CrossRef]
- Malik, L.M. Cornelia de Lange Syndrome—A Cause of Hypertrichosis in Children: Case Report and Review of Literature. J. Pak. Assoc. Dermatol. 2016, 21, 211–214. [Google Scholar]
- Xiu, Z.; Sun, L.; Liu, K.; Cao, H.; Qu, H.Q.; Glessner, J.T.; Ding, Z.; Zheng, G.; Wang, N.; Xia, Q.; et al. Shared Molecular Mechanisms and Transdiagnostic Potential of Neurodevelopmental Disorders and Immune Disorders. Brain Behav. Immun. 2024, 119, 767–780. [Google Scholar] [CrossRef]
- Park, J.; Wick, H.C.; Kee, D.E.; Noto, K.; Maron, J.L.; Slonim, D.K. Finding Novel Molecular Connections between Developmental Processes and Disease. PLoS Comput. Biol. 2014, 10, e1003578. [Google Scholar] [CrossRef][Green Version]
- National Center for Biotechnology Information ClinVar: NM_006265.3(RAD21):C.1306C>T (p.Gln436Ter). Available online: https://www.ncbi.nlm.nih.gov/clinvar/RCV001215062/ (accessed on 10 September 2025).
- Kadowaki, S.; Hashimoto, K.; Nishimura, T.; Kashimada, K.; Kadowaki, T.; Kawamoto, N.; Imai, K.; Okada, S.; Kanegane, H.; Ohnishi, H. Functional Analysis of Novel A20 Variants in Patients with Atypical Inflammatory Diseases. Arthritis Res. Ther. 2021, 23, 52. [Google Scholar] [CrossRef]
- Shiraki, M.; Kadowaki, S.; Miwa, Y.; Nishimura, K.; Maruyama, Y.; Kishida, D.; Imagawa, K.; Kobayashi, C.; Takada, H.; Mitsunaga, K.; et al. Clinical Characteristics and Treatment Strategies for A20 Haploinsufficiency in Japan: A National Epidemiological Survey. Front. Immunol. 2025, 16, 1548042. [Google Scholar] [CrossRef]
- Karolak, J.A.; Szafranski, P.; Kilner, D.; Patel, C.; Scurry, B.; Kinning, E.; Chandler, K.; Jhangiani, S.N.; Coban Akdemir, Z.H.; Lupski, J.R.; et al. Heterozygous CTNNB1 and TBX4 Variants in a Patient with Abnormal Lung Growth, Pulmonary Hypertension, Microcephaly, and Spasticity. Clin. Genet. 2019, 96, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.S.; Appenheimer, M.M. A Fever-Th17 Cell Immune Axis: Some SMADs Like It Hot. Immunity 2020, 52, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.P.; Huang, S.C.; Glenn St Hilaire, B.; Engreitz, J.M.; Perez, E.M.; Kieffer-Kwon, K.R.; Sanborn, A.L.; Johnstone, S.E.; Bascom, G.D.; Bochkov, I.D.; et al. Cohesin Loss Eliminates All Loop Domains. Cell 2017, 171, 305–320.e24. [Google Scholar] [CrossRef]
- Perea-Resa, C.; Wattendorf, L.; Marzouk, S.; Blower, M.D. Cohesin: Behind Dynamic Genome Topology and Gene Expression Reprogramming. Trends Cell Biol. 2021, 31, 760–773. [Google Scholar] [CrossRef]
- Richterova, J.; Huraiova, B.; Gregan, J. Genome Organization: Cohesin on the Move. Mol. Cell 2017, 66, 444–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Potjewijd, J.; Koenen, H.J.P.M.; van der Made, C.I.; van Rijssen, E.; He, X.; Ysermans, R.; Ungethum, L.; Theunissen, R.; Schurgers, L.J.; Damoiseaux, J.; et al. A Novel Missense Variant in TNFAIP3 Associated with Autoimmunity Reveals the Contribution of STAT1/ MTOR Pathways. Clin. Exp. Immunol. 2025, 219, uxaf048. [Google Scholar] [CrossRef]
- Yan, M.; Li, D.; Aknai, S.; Zhu, H.; Abudureyim, M. Mutation Analysis of the TNFAIP3 in A20 Haploinsufficiency: A Case Report. Medicine 2021, 100, E25954. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, X.; Zhao, W.; Zhang, Y.; Chen, K.; Li, Y.; Wang, X.; Zhang, M.; Xue, B.; Yu, W.; et al. RAD21 Is the Core Subunit of the Cohesin Complex Involved in Directing Genome Organization. Genome Biol. 2023, 24, 155. [Google Scholar] [CrossRef]
- Xiang, J.; Lai, Y.; He, Z. The Functions and Mechanisms of the Cohesin Complex in Regulating the Fate Determinations of Stem Cells. Research 2025, 8, 0757. [Google Scholar] [CrossRef]
- Yun, J.; Song, S.; Kim, H.; Han, S.; Yi, E.C.; Kim, T. Dynamic Cohesin-mediated Chromatin Architecture Controls Epithelial–Mesenchymal Plasticity in Cancer. EMBO Rep. 2016, 17, 1343. [Google Scholar] [CrossRef]
- Adams, N.M.; Galitsyna, A.; Tiniakou, I.; Esteva, E.; Lau, C.M.; Reyes, J.; Abdennur, N.; Shkolikov, A.; Yap, G.S.; Khodadadi-Jamayran, A.; et al. Cohesin-Mediated Chromatin Remodeling Controls the Differentiation and Function of Conventional Dendritic Cells. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Horsfield, J.A. Full Circle: A Brief History of Cohesin and the Regulation of Gene Expression. FEBS J. 2023, 290, 1670–1687. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, I.; Ahuja, V.; Paul, J. Altered Expression of Tumor Necrosis Factor Alpha-Induced Protein 3 Correlates with Disease Severity in Ulcerative Colitis. Sci. Rep. 2017, 7, 9420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, L.; Luo, H.; Li, Q.Z.; Zuo, X. TNFAIP3 Downregulation Mediated by Histone Modification Contributes to T-Cell Dysfunction in Systemic Lupus Erythematosus. Rheumatology 2017, 56, 835–843. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, B.; Zhao, M.; Lu, Q. An Update on Epigenetic Regulation in Autoimmune Diseases. J. Transl. Autoimmun. 2022, 5, 100176. [Google Scholar] [CrossRef]
- Robles-Rebollo, I.; Cuartero, S.; Canellas-Socias, A.; Wells, S.; Karimi, M.M.; Mereu, E.; Chivu, A.G.; Heyn, H.; Whilding, C.; Dormann, D.; et al. Cohesin Couples Transcriptional Bursting Probabilities of Inducible Enhancers and Promoters. Nat. Commun. 2022, 13, 4342. [Google Scholar] [CrossRef]
- Bonora, E.; Bianco, F.; Cordeddu, L.; Bamshad, M.; Francescatto, L.; Dowless, D.; Stanghellini, V.; Cogliandro, R.F.; Lindberg, G.; Mungan, Z.; et al. Mutations in RAD21 Disrupt Regulation of APOB in Patients with Chronic Intestinal Pseudo-Obstruction. Gastroenterology 2015, 148, 771. [Google Scholar] [CrossRef]
- Horsfield, J.A.; Print, C.G.; Mönnich, M. Diverse Developmental Disorders Fromthe One Ring: Distinct Molecular Pathways Underlie the Cohesinopathies. Front. Genet. 2012, 3, 32472. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Karri, U.; Luo, Y.; Gedik, K.C.; Carpio Tumba, M.; Chhibbar, P.; Roy, P.; Falduto, G.; Das, J.; Schwartz, D. Rare TNFAIP3 Hypomorphic Variants Are a Massively Underestimated Driver of Human Autoinflammatory Disease. Available online: https://acrabstracts.org/abstract/rare-tnfaip3-hypomorphic-variants-are-a-massively-underestimated-driver-of-human-autoinflammatory-disease/ (accessed on 28 September 2025).
- Jayaraman, S.; Balmuri, N. Case Report: Exploring the Spectrum of A20 Haploinsufficiency in Children. Front. Pediatr. 2025, 13, 1624715. [Google Scholar] [CrossRef]
- Aksentijevich, I.; McDermott, M.F. Lessons from Characterization and Treatment of the Inflammatory Syndromes. Curr. Opin. Rheumatol. 2017, 29, 187. [Google Scholar] [CrossRef]
- Boltsis, I.; Grosveld, F.; Giraud, G.; Kolovos, P. Chromatin Conformation in Development and Disease. Front. Cell Dev. Biol. 2021, 9, 723859. [Google Scholar] [CrossRef]
- Nissen, T.; Wynn, R. The Clinical Case Report: A Review of Its Merits and Limitations. BMC Res. Notes 2014, 7, 264. [Google Scholar] [CrossRef]
- Casanova, J.L.; Conley, M.E.; Seligman, S.J.; Abel, L.; Notarangelo, L.D. Guidelines for Genetic Studies in Single Patients: Lessons from Primary Immunodeficiencies. J. Exp. Med. 2014, 211, 2137. [Google Scholar] [CrossRef]
- Nakamura, T.; Igarashi, H.; Ito, T.; Jensen, R.T. Important of Case-Reports/Series, in Rare Diseases: Using Neuroendocrine Tumors as an Example. World J. Clin. Cases WJCC 2014, 2, 608. [Google Scholar] [CrossRef] [PubMed]
- Liévin, V.; Hansen, J.M.; Lund, A.; Elstein, D.; Matthiesen, M.E.; Elomaa, K.; Zarakowska, K.; Himmelhan, I.; Botha, J.; Borgeskov, H.; et al. FindZebra Online Search Delving into Rare Disease Case Reports Using Natural Language Processing. PLoS Digit. Health 2023, 2, e0000269. [Google Scholar] [CrossRef]
- Ernst, M.A.; Draghi, B.N.; Cimino, J.J.; Patel, V.L.; Zhou, Y.; Shubrook, J.H.; De Lacalle, S.; Weaver, A.; Liu, C.; Jing, X. A Secondary Data Analysis on Hypotheses Generated by Inexperienced Clinical Researchers: Cases from a Randomized Controlled Study. Health Inform. J. 2025, 31, 14604582251353587. [Google Scholar] [CrossRef]
- Radaschin, D.S.; Iancu, A.V.; Ionescu, A.M.; Gurau, G.; Niculet, E.; Bujoreanu, F.C.; Beiu, C.; Tatu, A.L.; Popa, L.G. Comparative Analysis of the Cutaneous Microbiome in Psoriasis Patients and Healthy Individuals-Insights into Microbial Dysbiosis: Final Results. Int. J. Mol. Sci. 2024, 25, 10583. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Zhu, W.; Kuang, Y.; Liu, T.; Zhang, W.; Chen, X.; Peng, C. Skin and Gut Microbiome in Psoriasis: Gaining Insight Into the Pathophysiology of It and Finding Novel Therapeutic Strategies. Front. Microbiol. 2020, 11, 589726. [Google Scholar] [CrossRef]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Chapman, M.; Evans, K.; Azevedo, L.; Hayden, M.; Heywood, S.; Millar, D.S.; Phillips, A.D.; et al. The Human Gene Mutation Database (HGMD®): Optimizing Its Use in a Clinical Diagnostic or Research Setting. Hum. Genet. 2020, 139, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Rehm, H.L.; Berg, J.S.; Brooks, L.D.; Bustamante, C.D.; Evans, J.P.; Landrum, M.J.; Ledbetter, D.H.; Maglott, D.R.; Martin, C.L.; Nussbaum, R.L.; et al. ClinGen--the Clinical Genome Resource. N. Engl. J. Med. 2015, 372, 2235–2242. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Adesina, A.; Ahmad, S.; Bowler-Barnett, E.H.; Bye-A-Jee, H.; Carpentier, D.; Denny, P.; et al. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent Updates, New Developments and Status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The Reactome Pathway Knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI Gene Expression and Hybridization Array Data Repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.; Falcon, S.; Pages, H.; Li, N. org. Hs. eg. db: Genome wide annotation for Human. R package version, 2023, 3, 3. Available online: https://scholar.google.com/citations?user=uO7FAgkAAAAJ&hl=en (accessed on 17 September 2025).
- Wickham, H. Data Analysis. In ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco, B.E.; Orozco, C.V.; Meléndez, E.; Mangones, M.F.; Valderrama, J.; Lobato, A.; Garavito-Galofre, P.; Vélez, J.I.; Vidal, O.M. Co-Occurrence of RAD21 and TNFAIP3 Mutations in Cornelia de Lange Syndrome with Pustular Psoriasis: Potential Molecular Interactions. Int. J. Mol. Sci. 2025, 26, 10783. https://doi.org/10.3390/ijms262110783
Orozco BE, Orozco CV, Meléndez E, Mangones MF, Valderrama J, Lobato A, Garavito-Galofre P, Vélez JI, Vidal OM. Co-Occurrence of RAD21 and TNFAIP3 Mutations in Cornelia de Lange Syndrome with Pustular Psoriasis: Potential Molecular Interactions. International Journal of Molecular Sciences. 2025; 26(21):10783. https://doi.org/10.3390/ijms262110783
Chicago/Turabian StyleOrozco, Beatriz E., Cindy V. Orozco, Esperanza Meléndez, María F. Mangones, José Valderrama, Adalberto Lobato, Pilar Garavito-Galofre, Jorge I. Vélez, and Oscar M. Vidal. 2025. "Co-Occurrence of RAD21 and TNFAIP3 Mutations in Cornelia de Lange Syndrome with Pustular Psoriasis: Potential Molecular Interactions" International Journal of Molecular Sciences 26, no. 21: 10783. https://doi.org/10.3390/ijms262110783
APA StyleOrozco, B. E., Orozco, C. V., Meléndez, E., Mangones, M. F., Valderrama, J., Lobato, A., Garavito-Galofre, P., Vélez, J. I., & Vidal, O. M. (2025). Co-Occurrence of RAD21 and TNFAIP3 Mutations in Cornelia de Lange Syndrome with Pustular Psoriasis: Potential Molecular Interactions. International Journal of Molecular Sciences, 26(21), 10783. https://doi.org/10.3390/ijms262110783






